Abstract
Objectives
Middle ear mucins are associated with otitis media (OM), contribute to hearing loss and are regulated by cytokines. This work investigates the regulation of mucin secretion from human middle ear epithelial cells (HMEEC) by inflammatory cytokines interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α) and cytokine inhibitors interleukin-1 receptor antagonist (IL-1ra) and anti-tumor necrosis factor-α antibody (TNFab).
Methods
HMEEC were exposed to IL-1β and TNF-α in a dose- and time-dependent manner. Cytokine stimulated HMEEC were also exposed to IL-1ra and TNFab in a dose-dependent manner. Mucin secretion was characterized by exclusion chromatography and liquid scintillation.
Results
HMEEC exposed to IL-1β and TNF-α demonstrated significant upregulation of mucin secretion in a dose-dependent fashion. Cultures exposed to IL-1β at 100ng/ml and TNF-α at 200ng/ml showed increased mucin secretion in time-dependent experiments at 16 hours (P=0.00008) for TNF-α and 8 (P=0.028) and 16 hours (P=0.00001) for IL-1 β. IL-1ra and TNFab inhibited the effects of increased mucin secretion by IL-1β and TNF-α.
Conclusions
IL-1β and TNF-α upregulate mucin secretion from HMEEC in a dose- and time-dependant manner and these effects can be inhibited by cytokine blockade. Improved understanding of these mechanisms has the potential to alter the approach and management of OM and lead to novel therapeutic interventions.
Keywords: otitis media, mucin, interleukin-1 β, tumor necrosis factor - α
1.0 Introduction
Otitis media (OM) is the most common diagnosis in children who visit physicians for illness in the United States [1]. Along with this disease prevalence is an economic burden of treatment in the billions of dollars per year in the US [2]. A more thorough understanding of the pathophysiology and the inflammatory events that occur during this disease process is needed to provide novel solutions in the treatment of OM. In particular, although antibiotic treatment has been the mainstay of otitis media treatment, the increasing prevalence of microbial resistance will make the singular use of antibiotics challenging. Additionally, evidence has been generated suggesting that chronic inflammatory responses to middle ear pathogens may be important in the development of otitis media with effusion (OME) and raises the possibility that control of this response may reduce otitis media related morbidity [3].
The persistence of middle ear effusion (MEE) following OM has the potential for significant morbidity and hearing loss for the developing child. The effusion contains mucins, which are responsible for the high-viscosity fluid that can prevent normal mucociliary clearance [4, 5]. Several cytokines have been found in a high percentage of these effusions, including TNF-α and IL -1β [6]. In addition to the general inflammatory regulatory mechanisms associated with these cytokines evidence exists that TNF-α and IL-1β are also integral in the inflammatory response in otitis media and regulate mucin production and secretion from middle ear epithelium [7-11]. Previous investigations in our laboratory using primary culture chinchilla middle ear epithelial (CMEE) cells have laid the ground work for the current investigations [12]. In these studies, CMEE exposed to both IL-1β and TNF-α responded with an increase in mucin secretion in a time and dose dependent manner. The present study was designed to investigate the response of human middle ear epithelial cells (HMEE) to inflammatory cytokines.
2.0 Methods
2.1 Establishment of Cultures
Cells used in the cultures were human middle ear epithelial (HMEE) cells from a transformed cell line stored in liquid nitrogen. (These cells were generously provided by Dr D. Lim, House Ear Institute). From the stock supply of HMEE cells, aliquots were thawed and grown in a 50/50 mixture of Bronchial Epithelial Cell Basal Medium (Gibco, Carlsbad, California) and Dulbecco’s Modified Eagle Medium (Gibco, Carlsbad, California). Each 500 ml portion of media was supplemented with antibiotic/antimycotic solution (1000 U/ml of penicillin G sulfate, 100 μg/ml of streptomycin sulfate, and 250 ng/ml of amphotericin B; Invitrogen, Carlsbad, California) and BEGM aliquots (Clonetics, San Diego, California) which contain 2 ml 13 mg/ml bovine pituitary extract, 0.5 ml 5 mg/ml insulin, 0.5 ml 0.5 mg/ml hydrocortisone, 0.5 ml 0.1 mg/ml retinoic acid, 0.5 ml 10 mg/ml transferrin, 0.5 ml 6.5 mg/ml triiodothyronine, 0.5 ml 0.5 mg/ml epinephrine, 0.5 ml 0.5 mg/ml epinephrine, and 0.5 ml 0.5 mg/ml human epidermal growth factor. When the cells reached approximately 70% confluency, they were removed from the flask using Trypsin/EDTA passaging.
The cells were counted by hemocytometer and plated in 12 well plates at approximately 1 × 105 cells per cm2. The cells were grown in a humidified atmosphere at 37° C containing 95% air and 5% carbon dioxide. Cells were grown to confluency and prepared for experimentation.
Using methods well-characterized in our laboratory [14, 15], tritiated glucosamine was used to radioactively label the mucin within the samples for analysis. The radioactivity was applied to the cells when 70% confluent. The cells were incubated 5 μCi of tritiated glucosamine per 1 ml of growth media for 24 hours before being with removed to allow for experimentation.
2.2 Experimental Conditions
2.21 Dose Dependency
After metabolic labeling, cell cultures were incubated with media containing scaled concentrations of TNF-α or IL-1β (R&D Systems, Minneapolis, MN). Past experiments conducted in our lab, using chinchilla middle ear epithelial (CMEE) cells treated with TNF-α or IL-1β guided the dose treatments for the present study [12-14]. The dose found to maximally stimulate CMEE mucin secretion was 200 ng/ml of TNF-α and of 100 μg/ml IL-1β. Concentrations of 0, 5, 25, 50, 100, 200, 300 ng/ml of TNF-α and 0, 2.5, 5, 10, 50, 100, and 200 ng/ml of IL-1β were used. Each plate of treated cells was incubated for 16 hours at 37° C in 95% air - 5% carbon dioxide. A minimum of 4 data points were performed for each experimental condition. Controls were labeled and incubated with growth media only. Cell viability was assessed by Trypan blue exclusion.
2.22 Time Dependency
After metabolic labeling, paired cell cultures were incubated with and without TNF-α at 200 ng/ml or IL-1β at 100 ng/ml for 4, 8, 12, 16, 24, and 48 hours. Past experiments conducted in our lab using CMEE cells treated with TNF-α or IL-1β guided the time treatments for the present study [12-14]. The time found to maximally stimulate CMEE mucin secretion was 16 hours for both TNF-α and IL-1β. A minimum of 4 data points were found for each experimental condition. Control wells were not treated with IL-1β or TNF-α and were used to demonstrate a base-line secretion of mucin. Cell viability was assessed using Trypan blue exclusion.
2.23 Inhibitors of TNF-α and IL-1β
After metabolic labeling, cell cultures were incubated in media containing increasing doses of anti TNF-α antibody or IL-1 receptor antagonist (R&D Systems, Minneapolis, MN) with and without 200 ng/ml TNF-α or 100 ng/ml IL-1β. IL-1ra is the recombinant form of the naturally occurring IL-1ra and binds to both IL-1 receptor type I and II. TNF-α ab is a monoclonal antibody that is specific for TNF-α. Concentrations of 0, 2, 20 and 40 μg/mL of antiTNF-α antibody and 0, 100, 200, and 400 ng/mL of IL-1 receptor antagonist were used. Controls were labeled and incubated with growth media only, TNF-α or IL-1β only, and antiTNF-α antibody or IL-1 receptor antagonist only. Each plate of treated cells was incubated for 16 hours at 37° C in 95% air - 5% carbon dioxide. A minimum of 4 data points were found for each experimental condition. Cell viability was assessed by Trypan blue exclusion.
2.3 Quantification of Mucin Secreted
Mucin was quantified using exclusion chromatography as previously described [14, 15,]. Briefly, after exposure to the experimental conditions 0.9 ml media/cytokine mixture was drawn from each well of cells. Aspirates were stored at -80° C for no more than 2 weeks. Upon thawing of the aspirate, 100 μl of testicular chondroitinase ABC (Sigma-Aldrich Corp) was added to the aspirate to digest the proteoglycans. This digestion mixture was incubated for 5 hours at 37° C. After incubation the mixture was applied to a Sepharose CL-4B column (0.7 × 50 cm). The column was eluted with phosphate-buffered saline solution containing 0.02% (wt/vol) sodium azide (Sigma-Aldrich Corp), 0.9% sodium chloride, and 5 mM dithiothreitol (Sigma-Aldrich Corp) at a constant flow rate of 0.5 ml/min to collect 2-ml fractions. These fractions were mixed with 8 ml of scintillation fluid (Ecosint A; National Diagnostics, Atlanta, GA). Radioactivity was counted using a liquid scintillation system (Tri-Carb 4530; Perkin Elmer, Inc, Downers Grove, IL). The radioactivity was compared to the appropriate control to determine the amount of mucin produced.
2.4 Statistical Analysis
ANOVA models were used to assess concentration dependence data and Tukey adjustments were used for multiple comparisons, but not presenting all pair wised comparisons. Dose dependent and cytokine inhibitor data was analyzed with paired t-tests. Means with standard error of the mean were calculated for statistical analysis. The analysis was carried out using SigmaStat statistical software and in consultation with Biostatistics Department of the Medical College of Wisconsin.
2.5 Funding Source
The funding source, National Institutes of Health, reviewed the initial grant which assisted in the ability to perform this work but played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication
3.0 Results
3.1 Cell Culture Morphology
Cell culture morphology was consistent with homogenous pure middle ear epithelial cultures assessed with histologic staining as previously described [14, 15].
3.2 Concentration Dependency
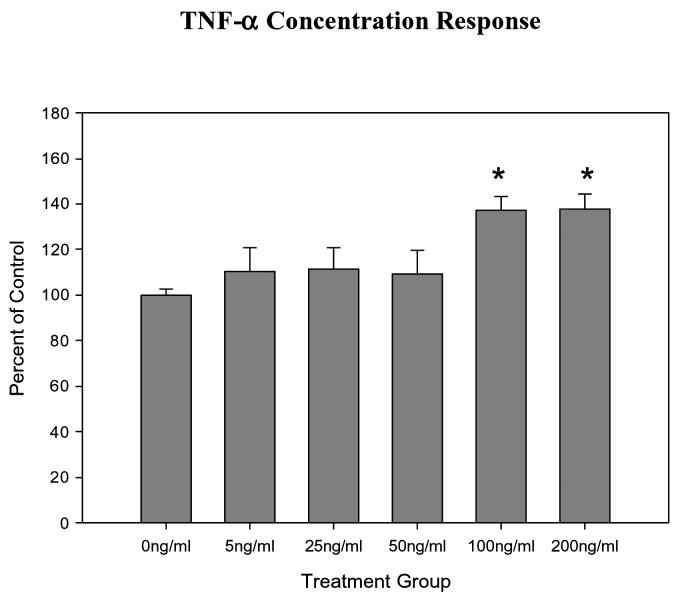
IL-1β and TNF-α stimulated mucin secretion in a concentration dependent manner, with an increase in mucin secretion observed with increased concentrations of IL-1β or TNF-α in the culture media. For IL-1 β this was statistically significant at concentrations of 5, 10, 50 and 100ng/ml (all P≤0.001). For TNF- α this was statistically significant at concentrations of 100 and 200ng/ml (all P≤0.001) The greatest mucin secretion was stimulated by a concentration of 100 ng/ml for IL-1β and 200 ng/ml TNF-α (Figures 1 and 2).
Figure 1.
Graphical representation of the dose-dependent effects of TNF-a on mucin secretion in HMEE. TNF-a concentrations at 100 and 200ng/ml were significantly greater than controls.
Figure 2.
Graphical representation of the dose-dependent effects of IL-1ß on mucin secretion in human middle ear epithelial cells. IL-1ß concentrations at 5, 10 50 and 100ng/ml were significantly greater than controls.
3.3 Time Dependency
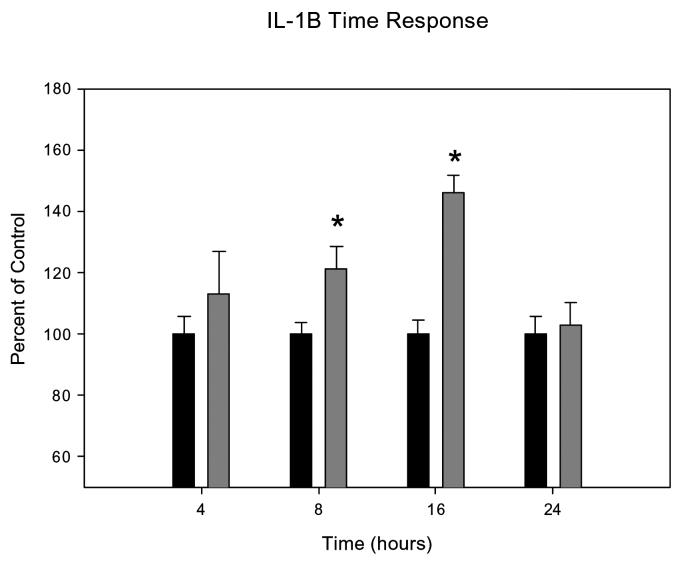
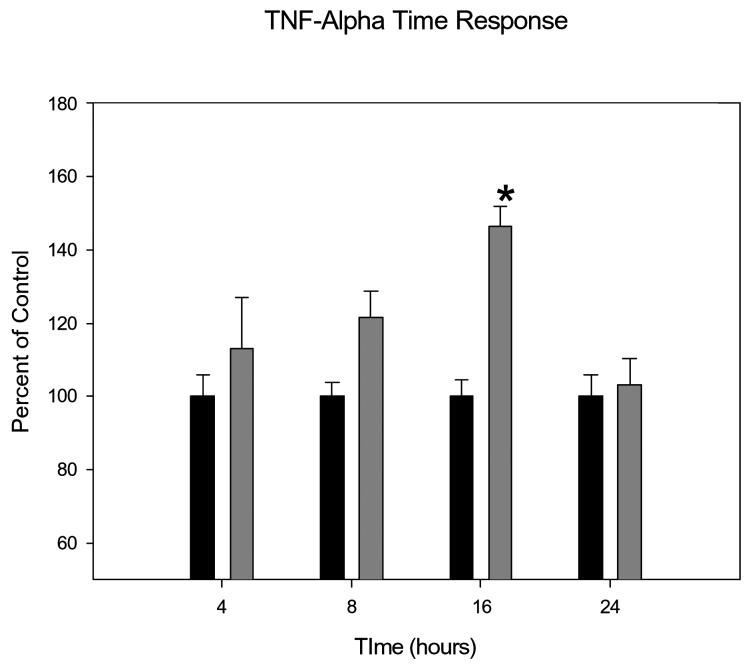
IL-1β and TNF-α stimulated mucin secretion in a time dependent manner, with an increase in mucin secretion observed with increased concentrations of IL-1β or TNF-α in the culture media. There was a trend towards and increase of mucin secretion at all time points, but the greatest mucin secretion was stimulated by incubation with IL-1β for 16 hours (P=0.00008) and TNF-α for 16 hours (P=0.00001). By 24 hours the effects dissipated (Figures 3 and 4).
Figure 3.
Graphical representation of the time-dependent effects of IL-1ß on mucin secretion in HMEE cells. Incubations of 100ng/ml IL-1ß with HMEEC for 8 and 16 hours were significantly greater than controls.
Figure 4.
Graphical representation of time-dependent effects of TNF-a on HMEE cell mucin secretion. Incubations of 200ng/ml TNF-a with HMEEC for 16 hours was significantly greater than control.
3.4 Inhibitors of IL-1β and TNF-α
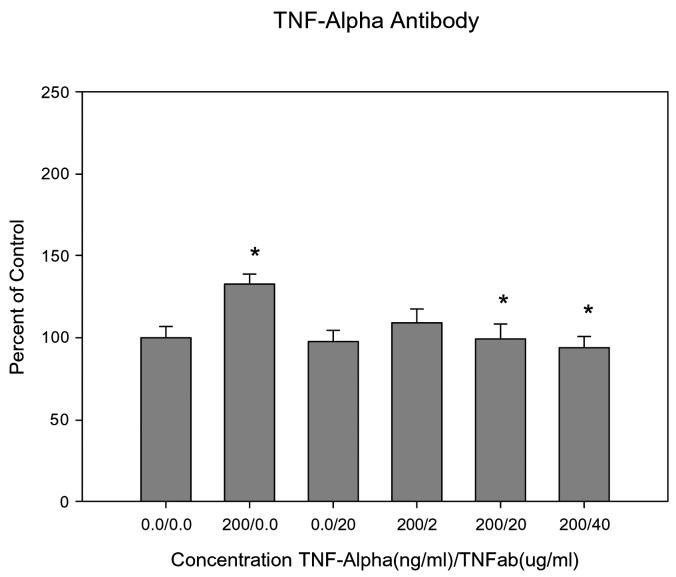
IL-1β receptor antagonist inhibited mucin secretion in a dose dependent manner. IL-1βra induced a significant reduction in mucin secretion when compared to stimulation of mucin by 100ng/ml IL-1β (all P≤0.04) (Figure 5). IL-1βra alone did not block the basal mucin secretion from the HMEE. TNFab inhibited mucin secretion in a dose dependent manner (Figure 6). TNFab induced a significant reduction in mucin secretion at 20μg (P=0.014) and 40μg (P=0.0014) when compared to stimulation of mucin by 200ng/ml TNF-α alone. TNFab did not block the basal mucin secretion from HMEE.
Figure 5.
Graphical representation of the dose-dependent effects of IL-1ßra on mucin secretion in human middle ear epithelial cells. IL-1ß induces a significant increase compared with control. IL-1ßra concentrations at 100, 200, and 400ng/ml significantly reduced mucin secretion compared with IL-1ß induced mucin secretion.
Figure 6.
Graphical representation of the dose-dependent effects of TNFab on mucin secretion in human middle ear epithelial cells. TNF-a induces a significant increase compared with control. TNFab concentrations at 20 and 400ug/ml significantly reduced mucin secretion compared with TNF-a induced mucin secretion.
4.0 Discussion
Mucins are high-molecular-weight glycoproteins with a variety of functions in the middle ear space including protecting the underlying mucosa and assisting with mucociliary clearance. The mucins in MEE are the components that are responsible for determining the viscosity of the effusion [5, 12]. In patients with OME mucins are not removed from the middle ear space and contribute to hearing loss and other potential developmental delays. In addition, mucins interact with middle ear pathogens to limit their adherence and invasion of the middle ear mucosa; necessary steps in the development of acute otitis media [12, 16]. 20 different human mucins have been identified; most of which are expressed in MEE [17]. These mucins vary in size and can be membrane bound or secreted. The process of defining the specific functions of each of these mucin products in the middle ear is still ongoing.
There is currently a growing body of evidence demonstrating that a cytokine mediated inflammatory response is important in the pathophysiology of acute and chronic otitis media [8-15]. This study, along with similar studies conducted in our laboratory using chinchilla middle ear epithelial cells, shows clear evidence of this cytokine mediated inflammatory response particularly as it relates to mucin pathophysiology and regulation. The dose and time dependent responses of HMEE to TNF-α and IL-1β in these experiments corroborate our findings in CMEE and lend further weight to the argument that mucin secretion in the middle ear is regulated by the inflammatory cytokines TNF-α and IL-1β, which in turn can lead to mucin hypersecretion in chronic otitis media (Figures 1-4).
Cytokine inhibitors establish the possibility of inhibiting the undesired effects of inflammatory cytokines, such as increased mucin secretion. This study demonstrates that interleukin-1 receptor antagonist (IL-1ra) and anti-TNF antibody (TNF ab) significantly inhibited TNF-α and IL-1β stimulated mucin secretion. TNF-α and IL-1β blockade has been used in animal models to reduce shock and mortality in sepsis, reduce the inflammatory response in arthritis, reduce mortality and inflammation in pancreatitis and reduce the inflammatory response in inflammatory bowel disease [18-24]. In our laboratory, cytokine inhibition has also been employed in a chinchilla model to effect a more rapid and complete resolution from Haemophilus influenzae induced otitis media compared with antibiotic treatment alone [13]. More recently, cytokine inhibition has also been used clinically to successfully manage rheumatoid arthritis and inflammatory bowel disease [25-27]. The results of this current investigation would suggest that cytokine inhibition may be a fertile ground for investigations examining novel methods to regulate mucin hypersecretion in chronic otitis media.
These current experiments also continue to develop and characterize human models to study otitis media on a cellular and molecular level, specifically in regards to inflammatory mediators and mucin responses. Attempts at creating primary cell cultures using human material have been hampered by lack of availability of human middle ear tissue for the primary cells and when this tissue is available, difficulty in initiating, and maintaining uniform cultures for experimentation. Other respiratory epithelial tissues that closely resemble the epithelium of the middle ear cytologically, such as tracheal and bronchial epithelium, have been used as surrogates for middle ear epithelium, but obviously lacked the specificity of being from middle ear origin [28]. However, recently, a method for specifically studying human middle ear epithelium in culture has been developed [29]. This immortalized human middle ear epithelial cell line (HMEE), used in these experiments, provides significant advantages over primary cell culture techniques. The need for tissue harvest is eliminated, a large amount of cell culture material can easily be generated for experimentation, cell uniformity is ensured to allow for comparative results over multiple experiments, reagents to allow for investigations are compatible with the human tissue and applicability of transferring ex vivo findings from human middle ear tissue to human pathophysiology is enhanced.
The findings in this investigation and their close resemblance to our previous work in the chinchilla animal model with primary middle ear cell [14] provides further evidence that the HMEE cell model is a reliable and worthwhile model in the study of middle ear inflammatory and mucin physiology and pathophysiology on a cellular and molecular level.
The experiments performed in this investigation establish a framework for additional studies ongoing to further our understanding of the relationship of inflammatory cytokines, mucins and otitis media pathophysiology. Importantly, it is clear that inflammatory cytokines have a differential effect on the various mucin types expressed in any given tissue [12]. The expression profile of each of the 20 identified human mucin genes in the middle ear space has recently been characterized [17]. Ongoing experiments will more specifically elucidate the responses of each of these mucin genes in HMEE to inflammatory cytokines as well as the secretory responses of these specific mucins. Additionally, investigations into the linkage between specific mucins and middle ear epithelial defenses, mucociliary clearance, immune surveillance, mucosal integrity, epithelial-pathogen adherence and biofilm formation are ongoing in an effort to provide a more thorough understanding of the role mucins and inflammatory cytokines play in otitis media pathophysiology.
Conclusion
The prevalence, cost, and morbidity of otitis media coupled with the declining efficacy of current treatment methods mandate a better understanding of the pathophysiology of otitis media and subsequent generation of new treatment strategies. This study provides further evidence of the importance of IL-1β and TNF-α in the pathophysiology of otitis media. Both the stimulation of mucin by these cytokines and the inhibition of mucin secretion through use of specific inhibitors of these cytokines aids in the understanding of the inflammation associated with otitis media. Understanding the relationship between mucin production and inflammatory cytokine stimulation may present novel treatments for otitis media.
Acknowledgment
The corresponding author (JEK) served as the Principal Investigator and was awarded funding from the NIH/NIDCD, Grants DC007903 and DC00192, to assist in the completion of this work. Additional funding was provided through the Department of Otolaryngology and Communication Sciences, Medical College of Wisconsin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bluestone CD, Klein JO. Otitis media, atelectasis, and eustacian tube dysfunction. In: Bluestone CD, Stool SE, Kenna MA, editors. Pediatric Otolaryngology. WB Saunders Co; Philadelphia, Pa: 1996. pp. 388–582. [Google Scholar]
- 2.Seiden AM, Tami TA, Pensak ML, Cotton RT. Otolaryngology: The Essentials. Thieme Medical Publishers; 2000. pp. 44–59. [Google Scholar]
- 3.Hall-Stoodley L, Hu FZ, Gieseke A, Nistico L, Nguyen D, Hayes J, Forbes M, Greenberg DP, Dice B, Burrows A, Wackym PA, Stoodley P, Post JC, Ehrlich GD, Kerschner JE. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA. 2006;296(2):202–211. doi: 10.1001/jama.296.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fitzgerald JE, Green GG, Stafford FW, Birchall JP, Pearson JP. Characterization of human middle ear mucus glycoprotein in chronic secretory otitis media. Clin Chim Acta. 1987;169:281–297. doi: 10.1016/0009-8981(87)90328-7. [DOI] [PubMed] [Google Scholar]
- 5.Hutton DA, Fogg FJ, Murty G, Birchall JP, Pearson JP. Preliminary characterization of mucin from effusions of cleft palate patients. Otolaryngology Head and Neck Surgery. 1993;109:1000–1006. doi: 10.1177/019459989310900605. [DOI] [PubMed] [Google Scholar]
- 6.Smirnova MG, Birchall JP, Pearson JP. In vitro study of IL-8 and goblet cells: possible role of IL-8 in the aetiology of otitis media with effusion. Acta Otolaryngology. 2002;122:146–152. doi: 10.1080/00016480252814144. [DOI] [PubMed] [Google Scholar]
- 7.Dinarello CA. Interleukin-1 and its biologically related cytokines. Advances in Immunology. 1989;44:153–205. doi: 10.1016/s0065-2776(08)60642-2. [DOI] [PubMed] [Google Scholar]
- 8.Chonmaitree T, Patel JA, Garofalo R, et al. Role of leukotrienne B4 and interleukin-8 in acute bacterial and viral otitis media. Annals of Otology, Rhinology and Laryngology. 1996;105:968–974. doi: 10.1177/000348949610501207. [DOI] [PubMed] [Google Scholar]
- 9.Juhn SK, Garvis WJ, Lees CJ, Le CT, Kim CS. Determining otitis media severity from middle ear fluid analysis. Annals of Otology, Rhinology and Laryngology. 1994;163:43–45. doi: 10.1177/00034894941030s512. [DOI] [PubMed] [Google Scholar]
- 10.Maxwell KS, Fitzgerald JE, Burleson JA, Leonard G, Carpenter R, Kreutzer DL. Interleukin-8 expression in otitis media. Laryngoscope. 1994;104:989–995. doi: 10.1288/00005537-199408000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Yellon RF, Doyle WJ, Whiteside TL, Diven WF, March AR, Fireman P. Cytokines, immunoglobulins, and bacterial pathogens in middle ear effusions. Archives of Otolaryngology-Head and Neck Surgery. 1995;121:865–869. doi: 10.1001/archotol.1995.01890080033006. [DOI] [PubMed] [Google Scholar]
- 12.Kerschner JE, Burrows A, Meyer TK. Chinchilla middle ear epithelial mucin gene expression in response to inflammatory cytokines. Archives of Otolaryngology - HNS. 2004;130:1163–1167. doi: 10.1001/archotol.130.10.1163. [DOI] [PubMed] [Google Scholar]
- 13.Kerschner JE, Beste DJ, Lynch JB, Fox MC, Kehl MS. Interleukin-1 receptor antagonist as an adjunct in the treatment of otitis media. Laryngoscope. 2000;110:1457–1461. doi: 10.1097/00005537-200009000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Kerschner JE, Meyer TK, Wohlfeill E. Middle ear epithelial mucin production in response to interleukin 1beta exposure in vitro. Otolaryngology - Head & Neck Surgery. 2003;129(1):128–135. doi: 10.1016/S0194-59980300532-1. [DOI] [PubMed] [Google Scholar]
- 15.Kerschner JE, Meyer TK, Burrows A, Yang C. Middle ear epithelial mucin production in response to interleukin-6 exposure in vitro. Cytokine. 2004;26:30–36. doi: 10.1016/j.cyto.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Diamond G, Legarda D, Ryan LK. The innate immune response of the respiratory epithelium. Immunological Reviews. 2000;173:27–38. doi: 10.1034/j.1600-065x.2000.917304.x. [DOI] [PubMed] [Google Scholar]
- 17.Kerschner JE. Mucin gene expression in human middle ear epithelium. American Laryngological, Rhinological and Otological Society Thesis. Laryngoscope. doi: 10.1097/MLG.0b013e31806db531. In Press. [DOI] [PubMed] [Google Scholar]
- 18.Norman J, Franz M, Messina J, et al. Interleukin-1 receptor antagonist decreases severity of experimental acute pancreatitis. Surgery. 1995;117:648–655. doi: 10.1016/s0039-6060(95)80008-5. [DOI] [PubMed] [Google Scholar]
- 19.Arend WP. Interleukin-1 receptor antagonist: a new member of the interleukin-1 family. Journal of Clinical Investigation. 1991;88:1445–1451. doi: 10.1172/JCI115453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dinarello CA, Thompson RC. Blocking IL-1: interleukin-1 receptor antagonist in vivo and in vitro. Immunology Today. 1991;12:404–410. doi: 10.1016/0167-5699(91)90142-G. [DOI] [PubMed] [Google Scholar]
- 21.Dinarello CA. Interleukin-1 and interleukin-1 antagonism. Blood. 1991;77:1627–1652. [PubMed] [Google Scholar]
- 22.Feldmann M, Brennan FM, Williams RO, Elliot MJ, Maini RN. Cytokine expression and networks in rheumatoid arthritis: rationale for anti-TN F alpha antibody therapy and its mechanism of action. Journal of Inflammation. 19951996;47:90–96. [PubMed] [Google Scholar]
- 23.O’Riordan MG, O’Riordan DS, Molloy RG, Mannick JA, Rodrick ML. Dosage and timing of anti-TNF-alpha antibody treatment determines its effect of resistance to sepsis after injury. Journal of Surgical Research. 1996;64:95–101. doi: 10.1006/jsre.1996.0312. [DOI] [PubMed] [Google Scholar]
- 24.Wakabayashi G, Gelfand JA, Burke JF, Thompson RC, Dinarello CA. A specific receptor antagonist for interleukin-1 prevents Escherichia coli-induced shock in rabbits. FASEB Journal. 1991;5:338–343. doi: 10.1096/fasebj.5.3.1825816. [DOI] [PubMed] [Google Scholar]
- 25.Bennet AN, Peterson P, Zain A, Grumley J, Ranayi G, Kirkham B. Adalimumab in clinical practice. Outcome in 70 rheumatoid arthritis patients, including comparison of patients with and without previous anti-TNF exposure. Rheumatology. doi: 10.1093/rheumatology/keh673. Epub 2005 May 3. [DOI] [PubMed] [Google Scholar]
- 26.First DE. Anakinra: review of recombinant human interleukin-1 receptor antagonist in treatment of rheumatoid arthritis. Clinical Therapeutics. 2004;26(12):1960–75. doi: 10.1016/j.clinthera.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 27.Travassos WJ, Cheifetz AS. Infliximab: use in inflammatory bowel disease. Current Treatment Options in Gastroenterology. 2005;8:187–196. doi: 10.1007/s11938-005-0011-2. [DOI] [PubMed] [Google Scholar]
- 28.Ars B, Ars-Piret N. Morpho-functional partition of the middle ear cleft. Acta Oto-Rhino-Laryngologica Belgica. 1997;51(3):181–184. [PubMed] [Google Scholar]
- 29.Chun YM, Moon SK, Lee HY, Webster P, Brackmann DE, Rhim JS, Lim DJ. Immortalization of normal adult human middle ear epithelial cells using a retrovirus containing the E6/E7 genes of human papillomavirus type 16. Annals of Otology, Rhinology & Laryngology. 2002;111(6):507–517. doi: 10.1177/000348940211100606. [DOI] [PubMed] [Google Scholar]