Abstract
Individuals with disorders marked by antisocial behavior frequently show deficits in recognizing displays of facial affect. Antisociality may be associated with specific deficits in identifying fearful expressions, which would implicate dysfunction in neural structures that subserve fearful expression processing. A meta-analysis of 20 studies was conducted to assess: (a) if antisocial populations show any consistent deficits in recognizing six emotional expressions; (b) beyond any generalized impairment, whether specific fear recognition deficits are apparent; and (c) if deficits in fear recognition are a function of task difficulty. Results show a robust link between antisocial behavior and specific deficits in recognizing fearful expressions. This impairment cannot be attributed solely to task difficulty. These results suggest dysfunction among antisocial individuals in specified neural substrates, namely the amygdala, involved in processing fearful facial affect.
Keywords: Facial affect, fearful expression, antisocial, amygdala, psychopathy, emotion, meta-analysis
Processing facial affect is crucial for socialization and normal social interaction (Corden et al. 2006; Fridlund, 1991). Aggression and other maladaptive antisocial behaviors may result from failure to be appropriately guided by the social cues of others (Blair, 2003a; Montagne et al. 2005; Walker and Leister, 1994). Some have suggested that distress-related cues, particularly fearful expressions, play an important role in inhibiting antisocial behavior (Blair, 2001; Nichols, 2001; Price, 2004). Accordingly, many studies find impairments in processing distress-related cues among antisocial populations. Not all studies find this impairment, however. It is unclear whether these inconsistencies result from disparate methodologies, sample populations, or analytic techniques, or, alternately, from the absence of a strong relationship between fearful affect processing and antisociality. Better understanding of facial affect recognition deficits associated with antisociality would permit more precise hypotheses to be formulated regarding neurocognitive correlates of antisocial behavior. We thus conducted this meta-analysis to assess associations between antisociality and facial affect recognition deficits. We hypothesized that, beyond any general facial affect recognition deficits, antisocial individuals show specific deficits in processing fearful expressions. We also hypothesized that such deficits are not solely attributable to task difficulty.
Emotional facial expressions play an important role in modulating interpersonal behavior. Given this, extensive research has assessed the relationship between facial affect recognition and psychiatric disorders characterized by interpersonal deficits. Generalized impairments in processing facial affect have been found in antisocial populations (Kropp and Haynes, 1987; Woodbury-Smith et al. 2005; Zabel, 1979). But such impairments have also been found in disorders like autism, schizophrenia and social anxiety disorder (Gross, 2004; Singh et al. 1998; Tremeau, 2006; Easter et al. 2005). Myriad factors, including general intelligence, age, attention, verbal ability, and task-specific motivation can be associated with reductions in facial affect recognition scores (Herba and Phillips, 2004; Moore, 2001). It is thus unsurprising that many clinical populations suffer facial affect recognition deficits. However, it means that little clinically or neuropsychologically specific information regarding a particular population's impairments can be extracted from general facial affect processing deficits.
More informative would be evidence for an association between antisocial behavior and a deficit in recognizing one or more specific expressions. Likely candidates include distress cues like fear and sadness because these expressions may inhibit or avert inappropriate behavior like aggression (Blair, 2003b; Blair et al. 1997; Marsh et al. 2005; Walker and Leister, 1994). Ethologists find similar aggression-inhibiting effects of distress cues in other primates and have speculated that distress cues evolved for this purpose (Preuschoft, 2000). Developmental, behavioral, and clinical research shows that distress cues elicit empathy in those who see them (Hoffman, 1987; Marsh & Ambady, 2007; Nichols, 2001; Preston & de Waal, 2002). Empathy is generally associated with decreased antisocial behavior (Eisenberg, 2000). It has been proposed that distress cues possess perceptual properties that elicit empathy and inhibit aggression (Marsh et al. 2005). It has also been proposed that fearful and sad expressions serve as social reinforcers that condition developing children to avoid engaging in the antisocial behaviors that elicit these expressions. This process is specified by the Integrated Emotion Systems (IES) model (Blair, 2005).
Facial affect processing relies on a distributed network of structures that includes occipitotemporal cortex (particularly fusiform gyrus and superior temporal gyrus), anterior cingulate cortex, amygdala, and ventromedial prefrontal cortex (Adolphs, 2006; Murphy et al. 2003). Evidence is accumulating, however, that beyond this general network partially separable neural systems process different expressions. Detection of fearful expressions relies disproportionately on the amygdala, and the detection of disgust on the insula and basal ganglia (Adolphs, 2002; Phillips et al. 2004; Murphy et al, 2003). Evidence that antisocial individuals are impaired in processing specific expressions like fear or disgust could facilitate targeted research into neurocognitive deficits underlying antisocial behavior.
Antisocial behaviors are those that violate the rights or welfare of other individuals. Multiple studies have shown specific impairments in fearful expression processing in populations who engage in these behaviors (e.g., Blair et al. 2004; Blair and Cipolotti, 2000; Carr et al. 2005; Walker et al. 1994; Woodbury-Smith et al. 2005). These populations include those primarily classified by the presence of antisocial behaviors (e.g., aggressive, criminal, externalizing, abusive), and those classified on the basis of both antisocial behaviors and personality traits such as a lack of empathy and remorse (e.g., psychopaths). However, not all of these studies find fearful expression recognition impairments (Kosson et al. 2002). Many of those that do assess children (Blair and Coles, 2000; Blair et al. 2001; Stevens et al. 2001). This leaves questions remaining, such as whether apparently specific deficits in recognizing fearful expressions stem from task difficulty rather than fear-specific neuropsychological deficits.
Even in healthy populations, fearful expressions are more often misidentified than other expressions. It is important to determine whether task difficulty can fully account for the link between fear recognition deficits and antisociality. If it can, it suggests the deficits can be accounted for by general facial affect processing impairments. Such deficits may simply be more obvious for expressions that are more difficult to recognize. If, however, fear-recognition deficits cannot be explained by task difficulty, this suggests dysfunction in neural structures or systems, such as the amygdala, that are particularly important to fearful expression processing (Adolphs, 2002; Rapcsak et al. 2000).
Prior research supports the contention that fearful expression recognition deficits may be associated with both amygdala dysfunction and antisocial behavior. Patients with amygdala lesions show deficits in recognizing fearful expressions (Adolphs et al. 1999; Papps et al. 2003). Neuroimaging studies also support the role of the amygdala in processing fearful expressions (Corden et al. 2006; Murphy et al. 2003; Phillips et al. 2004; Whalen et al. 1998). Considerable recent work has indicated impaired amygdala dysfunctioning in antisocial populations during affective tasks (Levenston et al. 2000; Patrick, 1994; Blair, 2003a, 2003b). And recent neuroimaging research has supported the existence of amygdala dysfunction in antisocial individuals (Birbaumer et al. 2005; Kiehl et al. 2001; Sterzer et al. 2005). This body of evidence is consistent with the hypothesis that across studies, antisocial individuals show a consistent and specific deficit in the recognition of fearful expressions. However, this hypothesis has not yet been tested.
The goal of this meta-analysis was to assess the existing literature that has measured facial expression recognition abilities in individuals with disorders or behaviors that are defined by antisociality. Such individuals include those classified as psychopathic, aggressive, criminal, delinquent, or externalizing. We investigated whether (a) individuals with antisocial behavior show impairments in recognition of each of the six expressions, (b) whether this deficit is greater for fearful expressions than for other emotional expressions, and (c) whether deficits in fear recognition are attributable to task difficulty.
Method
A variety of strategies were used to find relevant articles. Once located, several criteria were applied to determine whether a study could be included in the meta-analysis:
Literature search
Methods used to locate relevant studies included:
First, initial computer searches of PsycINFO and PubMed were conducted to retrieve articles; searched contained terms such as facial expression, facial affect, emotion recognition, face, antisocial, conduct disorder, psychopath, callous, aggression, violence, empathy, and sympathy.
All articles referenced by usable articles acquired via these searches were examined.
The Social Science Citation Index was used to check citations for any usable article that had been found through other methods.
Unpublished manuscripts were directly solicited. Requests for such manuscripts were placed via the e-mail list for a psychology research listserve and were made directly to several investigators with published research pertaining to the topic. Our own files were also reviewed for preprints and unpublished manuscripts.
Studies that were included satisfied the following criteria:
Participants must have been objectively selected, classified, or assessed on the basis of a pathology, trait, or behavior that is primarily defined by antisocial traits or behaviors. Under this criteria, participants clinically diagnosed with antisocial personality disorder, conduct disorder, or classified as having externalizing behavior disorders or psychopathy were included. Also included were participants selected for showing high levels of violence and/or aggression. These included participants in forensic settings. Participants were also included who were assessed or classed using trait measures of antisociality, such as psychopathy scales. Not included were participants primarily classed or assessed on traits or disorderes that are not primarily defined by the presence of antisociality, such as ADHD, autism, or bipolar disorder.
Judgments of facial affect must have been made wherein there was an objective criterion for accuracy (i.e., the stimuli must have been appropriately normed or validated). Only judgments of the six emotions most frequently assessed in facial affect studies (anger, disgust, fear, happiness, sadness, and surprise) were included in the analyses.
Participants in the study must otherwise have been relatively well matched in terms of other relevant variables (e.g., age, gender). When multiple comparison groups were included in a study, the most homogenous sample or samples were selected in order to be conservative (e.g., in a study comparing psychopathic inmates with non-psychopathic inmates and healthy adults from the community, comparisons between only the former two groups were used).
Information regarding the accuracy with which individual expressions were identified must be available.
Participants presenting with brain injuries or Axis I psychiatric disorders were excluded.
Study Characteristics
Twenty articles were identified that met our criteria (Table 1). These articles included 38 independent samples of groups differing in antisocial behavior, incorporating 1244 research participants. The mean number of participants per study was 62.2 (SD=97.3), with a median of 38.5. Fourteen of the twenty studies used the Pictures of Facial Affect stimulus set (Ekman and Friesen, 1976). Two studies (Carr and Lutjemeier, 2005; Stevens et al. 2001) used the Diagnostic Assessment of Nonverbal Accuracy (DANVA) (Nowicki and Duke, 1994). The remaining studies (Dadds et al. 2006; Kropp & Haynes, 1987; Montagne et al. 2005; Walker, 1981) used other validated stimulus sets. Eighteen studies employed a multiple-choice response format, and two (Matheson et al. 2005; Walz and Benson, 1996) employed a free-response format (Table 2).
Table 1.
Characteristics and results of of studies included in the meta-analysis
| Study | N | M age | % Female | Recognition Accuracy | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti | C | Anti | C | Anti | C | A | D | F | H | Sa | Su | |
| Percent accuracy data |
Proportion correct (C — Anti) |
|||||||||||
| Psychopathic | ||||||||||||
| Blair, Cipolotti 2000 | 5 | 5 | 48 | 49 | 0 | 0 | −.20 | .01 | .17 | −.02 | .01 | .01 |
| Blair, Coles 2000 | 11 | 10 | 12.4 | 12.6 | 18 | 40 | .26 | .09 | .22 | .00 | .35 | .04 |
| Blair et al. 2001 | 20 | 31 | 12.9 | 12.8 | 0 | 0 | .06 | .03 | .26 | .00 | .12 | −.03 |
| Blair et al. 2004 | 19 | 19 | 33.6 | 30.6 | 0 | 0 | −.02 | .06 | .25 | .02 | .00 | .11 |
| Dolan et al. 2006 | 22 | 27 | 35.2 | 35.2 | 0 | 0 | .06 | −.01 | .11 | .06 | .19 | .08 |
| Kosson et al. 2002 | 27 | 27 | 34 | 33 | 0 | 0 | −.11 | .20 | .00 | .01 | .01 | .03 |
| Montagne et al. 2005 | 16 | 16 | — | — | 50 | 44 | −.07 | .11 | .24 | .00 | .06 | −.02 |
| Stevens et al. 2001 | 9 | 9 | 11.7 | 11.4 | 0 | 0 | .16 | — | .28 | .00 | .30 | — |
| Antisocial, not psychopathic | ||||||||||||
| Kropp et al. 1987 | 20 | 20 | 29.3 | 29.3 | 100 | 100 | .33 | — | .36 | .02 | −.01 | .23 |
| Lough et al. 2005 | 18 | 13 | 61 | 57 | 11 | 31 | .38 | .34 | .26 | .06 | .26 | .14 |
| Matheson et al. 2005 | 19 | 15 | 40.1 | 32.1 | 42 | 53 | .08 | −.05 | .01 | .07 | .18 | .21 |
| McCown et al. 1986 | 40 | 40 | 15.4 | 14.9 | 0 | 0 | .11 | .11 | .03 | .07 | .13 | .18 |
| Walker et al. 1994 | 191 | 273 | 15.1 | 15.2 | 15 | 21 | .08 | .02 | .29 | .09 | .07 | .05 |
| Walker 1981 | 15 | 15 | 11.6 | 11.7 | 40 | 53 | .02 | −.04 | .11 | .03 | .10 | .04 |
| Walz et al. 1996 | 18 | 21 | 38.6 | 40.3 | 0 | 0 | .00 | −.09 | .15 | −.02 | .09 | .10 |
| Data available only as effect size (r) |
Effect size r |
|||||||||||
| Psychopathic | ||||||||||||
| Dadds et al. 2006** | 76 | — | 12.1 | — | 43 | — | .29 | .01 | .28 | −.11 | −.07 | — |
| Mitchell et al. 2006 | 5 | 5 | 41.4 | 43.6 | 0 | 0 | .3 | .13 | .74 | .12 | .00 | .35 |
| Antisocial, not psychopathic | ||||||||||||
| Carr et al. 2005* | 19 | — | 15.3 | — | 0 | — | −.10 | — | .43 | −.01 | .19 | — |
| Woodbury-Smith et al. 2005 | 21 | 23 | 35.4 | 29.7 | 14 | 13 | −.06 | −.21 | .31 | −.04 | .06 | −.11 |
| Zabel, 1979 | 40 | 51 | 11.5 | 11.5 | 14.1 | 18 | 33 | — | .34 | .34 | — | .22 |
Abbreviations: Anti = Antisocial; C = Comparison.
r's represent means of the following correlations: a) child DANVA emotion recognition and empathy, b) child DANVA emotion recognition and violent behavior, c) adult DANVA emotion recognition and empathy, and d) adult DANVA emotion recognition and violent behavior.
Mean represent means of child-rated antisocial behavior and callous-unemotional subscales.
Table 2.
Subject selection criteria and task variables across studies
|
Study |
Subject selection |
Task variables |
|||||
|---|---|---|---|---|---|---|---|
| |
Selection basis |
Criterion |
Stimulus duration |
Expression intensity |
Stimuli randomized |
Multiple choice |
Response Time Limit |
| Blair, Cipolotti, 2000 | PCL-R | ≤20 / ≥30 | 3 s | morphs | √ | √ | √ |
| Blair, Coles 2000 | PSD | ≤4 / ≥13 | 3 s | hexagon | √ | √ | √ |
| Blair et al. 2001 | PSD | <20 / >28 | 3 s | morphs | √ | √ | √ |
| Blair et al. 2004 | PCL-R | <20 / ≥30 | 3 s | morphs | √ | √ | √ |
| Carr et al. 2005 | SDRQ, Empathy | All | no limit | full intensity | √ | ||
| Dadds et al. 2006 | APSD | All | 2 s | morphs | √ | √ | |
| Dolan et al. 2006 | PCL-SV | <17 / ≥17 | no limit | morphs | √ | √ | |
| Kosson et al. 2002 | PCL-R | ≤20 / ≥30 | 1 s | full intensity | √ | √ | |
| Kropp et al. 1987 | Abusiveness | History/no history of child abuse | 30 s | full intensity | √ | √ | |
| Lough et al. 2005 | FTD Diagnosis, IRI | Diagnosis/ Healthy | 23 s | morphs | √ | ||
| Matheson et al. 2005 | CCB | 0 / ≥4 incidents | no limit | full intensity | √ | ||
| McCown et al. 1986 | Incarceration | Incarcerated/ Community | 5 s | full intensity | √ | ||
| Mitchell et al. 2006 | PCL-R | ≤20 / ≥30 | 3 s | morphs | √ | √ | √ |
| Montagne et al. 2005 | BIS/BAS | <10th / >90th percentile | not specified | morphs | √ | √ | √ |
| Stevens et al. 2001 | PSD | <20 / >25 | 2 s | full intensity | √ | ||
| Walker et al. 1994 | Externalizing | Diagnosis/ Healthy | 20 s | full intensity | √ | √ | √ |
| Walker 1981 | Diagnosis | Aggressive-unsocialized/Healthy | no limit | full intensity | √ | √ | |
| Walz et al. 1996 | RBPC CD subscale | ≤2 / ≥13 | 10 s | full intensity | |||
| Woodbury-Smith et al. 2005 | Criminal record | 0 / ≥1 conviction | 5 s | hexagon | √ | √ | |
| Zabel, 1979 | School setting | Emotionally disturbed/ Normal | no limit | full intensity | √ | ||
Abbreviations: PCL-R = Psychopathy Checklist-Revised. PSD = Psychopathy Screening Device an alternate abbreviation for this scale is APSD. PCL-SV = Psychopathy Checklist: Screening Version. FTD = Frontotemporal dementia. IRI = Interpersonal Reactivity Index. CCB = Checklist of Challenging Behaviors. BIS/BAS = Behavioral Approach System/Behavioral Inhibition System. RBPC = Revised Behavior Problem Checklist. CD = Conduct Disorder. SRDQ = Self-Reported Delinquency Questionnaire.
Statistical analyses
The results of these studies were analyzed with a goal of determining for which expressions recognition deficits are associated with antisocial behavior tendencies; whether observed deficits are greater for the fearful expressions or extend equally to other expressions; and whether fear recognition deficits can be attributed to task difficulty.
To allow all studies to factor into a common analysis, we first computed non-parametric tests addressing questions (a) and (b). Although lower powered, non-parametric tests are not disrupted by non-normality of variance and can compare different types of data. We next conducted parametric analyses to compare group differences in percent accuracy and to compare effect sizes (r) for those studies that provided data sufficient to calculate each. (Most of the studies were included in all analyses. However, some studies report percent accuracy or means that can be converted to percent accuracy, but no measures of variance, e.g., standard deviation. Other studies report Pearson's r or values that could be converted to r, e.g., F-values, but not mean or percent accuracy scores.) It will be noted that degrees of freedom differ across expressions within some analyses when relevant data were not provided for every expression. In all cases, threshold significance was set at P<0.05. All P-values are two-tailed unless otherwise indicated.
Prior to calculating parametric tests, percent accuracy and r scores were weighted by the sample size of the studies. Skewness (Z=7.97, P<0.001) in the distribution of sample sizes was corrected via log transformation. This transformation successfully reduced skewness to non-significance (Z=1.89, P=0.06). Transformed sample sizes were then multiplied by the relevant percent accuracy scores and effect sizes, and the products divided by the total of all log-transformed sample sizes to create weighted estimates of effect size (Doria, 2005).
We also conducted follow-up analyses to determine whether any detected deficits varied as a function of the samples' classification, age, or gender distribution. Finally, we conducted a file-drawer analysis to determine the number of null results required to counteract our findings.
Results
Do antisocial populations show deficits in recognizing the six emotional expressions?
For each analysis addressing this question, we compared accuracy for recognizing the six basic expressions among antisocial individuals to accuracy for controls. We used both single-sample t-tests and their non-parametric equivalent to calculate these comparisons. In all cases, threshold significance was set at P<0.05; only results that survive correction for multiple comparisons via the Bonferroni method are presented.
Non-parametric analysis
The binomial distribution test is the non-parametric equivalent of a single-sample t-test. We calculated six such tests; each assessed whether the proportion of studies in which antisocial individuals showed deficits for recognizing an expression was greater than the proportion of studies that did not show deficits for that expression. These analyses incorporated all 20 studies. The results (Ps one-tailed) indicated deficits in recognizing fear, Z=4.25, P<0.0000001, and sadness, Z=2.46, P<0.006. No significant group differences for anger, disgust, happiness, or surprise were found (Table 2).
Parametric analysis: Percent accuracy
Sixteen of the 20 studies reported percentage accuracy or data that could be converted to percentage accuracy and were included in this analysis. Of these, for the 14 studies incorporating a multiple-choice format, we corrected for the degree of accuracy to be expected from chance guessing to compare studies with different numbers of response options using the formula: (proportion correct – (1/number of choices))/(1 – (1/number of choices)) (Elfenbein and Ambady, 2002). Then for each expression, we calculated a single-sample t-test to compare average percent accuracy in antisocial and comparison groups. The results indicated lower percent accuracy scores in antisocial populations for fear, t(15)=5.65, P<0.001; sadness, t(14)=4.77 P<0.001; and surprise t(13)=3.81, P<0.005. No group differences for anger, disgust, or happiness were found.
Parametric analysis: Effect size
We calculated effect sizes for the 17 studies that provided sufficient data. This calculation indexed the magnitude of the effect (r) of antisocial tendencies on the recognition of various emotional expressions. For 15 studies, r represents the magnitude of the difference between two samples: r = square root (t2 / (t2 + df)). For 2 studies (Carr and Lutjemeier, 2005; Dadds et al. 2006), r represents the correlation between accuracy and antisocial scores in a single sample: r = (∑(Zaccuracy * Zantisociality)) / N. All scores were normalized using a Fisher's Z transformation (prior to weighting by sample size). Then we calculated single-sample t-tests to compare Zr scores in antisocial and comparison groups. The results indicated significant effect sizes associated with deficits in recognizing fear, t(16)=7.15, P<0.001; sadness, t(15)=4.39, P=0.001; and surprise t(11)=3.24, P=0.008. No group differences for anger, disgust, or happiness were found.
Thus, the results of the three types of analysis showed consistent deficits in the recognition fear and sadness in antisocial samples (two of the three analyses found differences for surprise). No consistent deficits were found in the recognition of the remaining three expressions. Deficits were greater for fear than for all other expressions across all analyses.
Are observed deficits greater for the fear facial expression than for other emotional expressions?
We next assessed whether antisocial samples' fear recognition deficits were significantly greater than deficits for the remaining 5 expressions. For each analysis, we assessed whether accuracy deficits among antisocial individuals were greater for fear expressions than for other expressions. We used repeated-measures ANOVAs and the non-parametric equivalent of this analysis (the Friedman test) to calculate these comparisons.
Non-parametric analysis
The Friedman test is analogous to the one-way ANOVA. We computed the rank order of expression recognition deficits for each study in our analysis. For example, if in a study antisocial individuals showed the greatest deficit for fear, fear would be ranked 1 for that study. If deficits were second-greatest for sadness, sadness would be ranked 2. The result of the Friedman test comparing the distribution of ranks across expressions was significant, χ2(5)=16.67, P=0.005, suggesting that recognition deficits were not evenly distributed among the 6 expressions. With five paired-samples Wilcoxon's tests we compared fear recognition deficits to deficits for each other expression. These tests were again computed on the rank ordered data. The results showed that fear recognition deficits were significantly greater than deficits for happiness, Z=3.479, P<0.001; disgust, Z=2.592, P=0.005; anger, Z=2.371, P=0.009; surprise, Z=1.694, P=0.045; and sadness, Z=1.666, P=0.048 expressions (all Ps one-tailed).
Parametric analysis: Percent accuracy
We next calculated a 6-level (emotion) repeated-measures ANOVA to assess whether percent accuracy deficits in antisocial individuals significantly vary among the 6 emotions. This ANOVA, calculated on weighted percent accuracy difference scores, revealed a main effect of expression category, F(5, 60)=3.86, P<0.01. Five paired-sample t-tests comparing fear recognition deficits to deficits for each of the remaining emotional expressions showed greater percent accuracy deficits for fear than for happiness, t(14)=4.68, P<0.001; anger, t(14)=2.82, P=0.014; disgust, t(13)=2.58, P=0.023; and surprise, t(13)=2.20, P= 0.046. Deficits for fear were also greater than for sadness, but this difference was not significant, t(14)=1.53, P=0.15.
Parametric analysis: Effect size
We conducted a second 6-level (emotion) repeated-measures ANOVA on the weighted Fisher's Zr scores to assess whether effect sizes associated with recognition deficits varied among the 6 expressions. The results indicated a significant main effect of emotion category, F(5, 55)=2.44, P<.05. Five follow-up paired-sample t-tests showed that recognition deficits in antisocial individuals were associated with greater effect sizes for fear than for happiness, t(13)=3.14, P=0.007; anger, t(14)=3.11, P=0.008; disgust, t(13)=2.60, P=0.022; surprise, t(11)=2.40, P=0.035; and sadness, t(15)=2.35 P=0.033.
These results indicated that antisocial individuals show consistently greater deficits in recognizing fear expressions than in recognizing the remaining 5 expressions (two of the three analyses found greater deficits for fear than sadness). This suggests a specific deficit rather than a global expression processing deficit distributed evenly across the six expressions (Figure 1). We next calculated whether this specific deficit is attributable to the general difficulty of recognizing fearful expressions.
Figure 1.
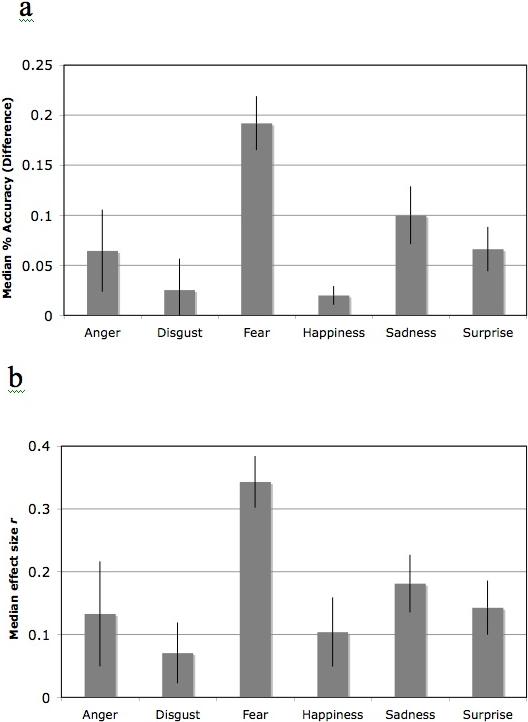
Median differences in recognizing six facial expressions in antisocial and comparison populations. Differences expressed as percent accuracy (a) and r (b). Error bars show standard error of the mean (SEM).
Are fear recognition deficits among antisocial populations attributable to task difficulty?
We used the method described by Rapcsak and colleagues (2000) to assess whether fear recognition deficits remained significantly greater in antisocial populations than comparisons after accounting for task difficulty. We created Z scores for each antisocial and comparison sample. These were created using each sample's percent accuracy scores for fear recognition and the means and standard deviation of percent accuracy scores for all 6 expressions. Using a paired-samples t-test, we found Z scores for fear expressions to be significantly lower in antisocial samples (Z=−0.94) than comparison samples (Z=−0.54), t(16)=2.19, P<0.05. This shows that antisocial samples show proportionally more difficulty recognizing fearful expressions than do comparisons, indicating that fear recognition deficits among antisocial individuals do not simply result from task difficulty.
Assessments of moderator variables
The studies we assessed varied widely in terms of their samples' age and gender distributions and primary classification. We thus computed follow-up correlations to assess whether any of these variables moderated the relationship between antisociality, measured as either percent accuracy difference scores (comparison minus antisocial group) or Zr scores. The results showed no consistent relationship between deficits for recognition of any of the 6 expressions and whether the antisocial population was classified as psychopathic or not (binomial coding), the average age of participants, and the percentage of female participants in the sample (all Ps > .05).
File drawer analysis
We calculated six file drawer analyses to determine, for each expression, how many unpublished studies with effect sizes of zero would be required to render the obtained effects nonsignificant. These analyses were based on effect sizes (r) for each expression in each study for which effect sizes were available. Using the formula provided by Rosenthal (1979), we compared the values to a standard tolerance level (5k + 15), the value of which indicates the minimum acceptable number of file drawer studies, with k equal to the number of studies included in the meta-analysis. This equation sets the minimum possible tolerance level at 20. Only the file drawer statistics for fear and sadness expressions exceeded the tolerance level, with, respectively, 241 and 106 null findings required to render the results of the meta-analysis non-significant.
Discussion
We found a consistent, robust link between antisocial behavior and impaired recognition of fearful facial affect. Relative to comparison groups, antisocial populations showed significant impairments in recognizing fearful, sad, and surprised expressions. They were not reliably impaired in recognizing happiness, anger, or disgust expressions. Deficits for recognizing fear were significantly greater than deficits for any of the other expressions. This specificity suggests that fear recognition deficits in antisocial samples do not simply reflect differences in factors that alter facial affect recognition broadly, such as general intelligence, attention, task-specific motivation, or perceptual processing deficits. Rather, they suggest that antisocial behavior may be associated with deficits in neurocognitive mechanisms that specifically underlie processing of fearful expressions. These results reinforce that meta-analyses can reveal latent but consistent data patterns across multiple studies (Rosenthal and DiMatteo, 2001). Although the results of many of the studies we assessed showed fear recognition deficits, often the authors did not emphasize or discuss these deficits. And in aggregate, they yielded a consistent relationship between fear deficits and antisociality, although they contained samples with heterogeneous age and gender distributions and diverse specific classifications.
Fear is indisputably more difficult for even healthy individuals to recognize than expressions such as happiness and sadness (Russell, 1994; Elfenbein and Ambady, 2002). Accuracy rates for this expression are typically around sixty to seventy percent in healthy populations. It has been suggested that fear recognition deficits in some clinical populations may result from the general difficulty of recognizing fearful expressions. Rapcsak and colleagues (2000) found that fear recognition deficits in individuals with large temporal or parietal cortex lesions were statistically related to healthy individuals' difficulty in processing fearful expressions. When task difficulty was accounted for, subjects with lesions were not disproportionately impaired in fear recognition. By contrast, our results show that fear recognition deficits in antisocial populations are proportionally greater than in comparison populations, indicating that these deficits are not attributable solely to task difficulty (see Adolphs, 2002). It should also be noted that our comparison samples showed nearly equivalent accuracy for fear and disgust expression recognition, as has been found previously (Calder et al. 2003; Camras and Allison, 1985; Ekman et al. 1987). However, antisocial individuals showed no recognition deficits for disgust.
Fear recognition deficits may instead indicate consistent neurocognitive dysfunctions among antisocial populations. Recent reviews indicate that fear recognition relies disproportionately on the amygdala (Adolphs, 2006; Murphy et al. 2003). Clinical studies find that amygdala lesions lead to specific impairments in recognizing fearful expressions (Adolphs et al. 1999; Papps et al. 2003). Neuroimaging studies find larger increases in amygdala activation to fearful expressions than other expressions. The results of a recent meta-analysis (Murphy et al. 2003) indicated that amygdala activity is “remarkably selective” (p. 225) for fear-related processing. This conclusion was based on results showing that over 60% of studies assessing fear expression processing show enhanced amygdala activation, compared to fewer than 20% of studies assessing emotional processing relevant to disgust, anger, happiness, or sadness. Several studies have shown enhanced amygdala activity across multiple expressions; however, the largest increase in amygdala activity still may be found to fearful expressions (Winston et al. 2003; Fitzgerald et al. 2006). Finally, sampling participants on the basis of their fear recognition ability shows that reduced ability to identify fearful expressions is associated with amygdala hyporesponsivity (Corden et al. 2006). Together, this evidence supports the association between specific fear recognition deficits and amygdala dysfunction.
Extensive indirect and direct evidence supports the existence of amygdala dysfunction in antisocial populations. Patrick was the first to consider that amygdala dysfunction might be linked to the development of psychopathy (Patrick, 1994). Subsequent neurocognitive testing to probe functions subserved by the amygdala has found consistent deficits in antisocial individuals. Individuals with psychopathy show impairment on tasks that assess the generation of autonomic responses to anticipated threat (Hare, 1982; Ogloff and Wong, 1990), the augmentation of the startle reflex following visually presented threat primes (Levenston et al. 2000), passive avoidance learning (Lykken, 1957; Newman and Kosson, 1986), and affective priming (Blair et al. 2006). Individuals with antisocial personality disorder and children with conduct disorder have similarly shown reduced autonomic responding to aversive stimuli (Dinn and Harris, 2000; Herpertz et al. 2001). Children who show antisocial behavior report reduced arousal to aversive stimuli (Sharp et al. 2006). The results of more recent imaging studies have confirmed that amygdala activation is reduced during affective processing tasks in psychopathic adults (Birbaumer et al. 2005; Kiehl et al. 2001) and children with conduct disorder (Sterzer et al. 2005). Behavioral data cannot conclusively settle questions relevant to neuroanatomical substrates. However, the current data are consistent with existing neuroanatomical data that suggest amygdala impairment to be associated with both antisociality and specific impairments in fearful expression processing.
The role that the amygdala plays in the correct identification of fearful expressions is not yet clear. Several possibilities have been proposed. One is that perception of an emotion causes the viewer to simulate the perceived emotional experience. A viewer may draw on information from an amygdala-based fear simulation to reconstruct and identify the expresser's emotional state (Goldman & Sripada, 2005; Lawrence & Calder, 2004). This explanation specifies that reduced fear responding will be associated with impaired fearful expression recognition. As has been discussed, some antisocial populations (particularly psychopaths) show just this pattern: both limited fearful responding (Patrick, 1994) and limited fearful expression recognition (Blair et al. 2004). Another proposed mechanism is that activity in the amygdala facilitates retrieval of semantic labels for facial expressions like fear. In this view, expression recognition impairments represent impairments in the ability to generate a verbal label for an expression like fear (Adolphs, 2006; Blair et al. 2005). Empirical support exists for both of these models of facial affect recognition; this meta-analysis cannot provide conclusive evidence in support of one over the other.
This meta-analysis also cannot clarify whether deficits in the recognition of fear expressions are specific to antisocial populations or extend to other clinical populations. However, the available evidence does not suggest that specific deficits in fear expression recognition are characteristic of all clinical samples. There is no theoretical support of which we are aware for fear recognition deficits in disorders such as depression, anxiety, or borderline personality disorder. Some data even suggest that individuals with these disorders show enhanced sensitivity to fearful expressions (Bhagwagar et al. 2004; Le Masurier et al. 2007; Surcinelli et al. 2006; Wagner and Linehan, 1999). Indications of amygdala dysfunction in autism have led to suggestions of fear recognition impairment in this population (Howard et al. 2000). However, individuals with autism are typically found to show equivalent impairments in the recognition of multiple expressions (Castelli, 2005; Humphreys et al. 2007; Sasson, 2006). This suggests broader face processing deficits in autism in accordance with the broad social dysfunction characteristic of this disorder.
It should be emphasized that identifying any emotional expression, including fearful expressions, is a complex task that requires visual scanning, perceptual processing, effortful attention, working memory, and semantic processing. Accordingly, such processing relies on a large, distributed network of neural structures. At the most basic level, intact functioning of occipitotemporal visual cortex is required to process the geometric configuration of the features of the face (Allison et al. 1999). The superior temporal gyrus and fusiform gyrus (part of inferior temporal cortex) play central roles in processing faces (LaBar et al. 2003). Once configural features have been assessed, intact functioning of structures in the temporal lobes is required to link the configural properties of facial expressions with stored knowledge about what those properties represent (Haxby et al. 2002). Dysfunction in any of these structures may characterize individuals who show generalized impairments in processing facial emotion.
Low levels of impairment were found across expressions in the antisocial samples in this meta-analysis. This suggests that dysfunction in one or more structures in this network may be associated with antisocial behavior. Dysfunction in superior temporal sulcus has been implicated in antisocial populations (Kiehl, 2006). In addition, regions involved in general emotional processing that are functionally connected to the amygdala may be associated with deficits in processing facial expressions, particularly fearful expressions (Murphy et al. 2003). Such regions include the dorsal anterior cingulate cortex (Stein et al. 2007) and the ventromedial prefrontal cortex, a region that has extensive functional connections with the amygdala and that is often found to be impaired in antisocial individuals (Birbaumer et al. 2005; Kiehl et al. 2001). Lesions to these regions, and disrupted connections between these regions and the amygdala, appear to affect processing of multiple emotional expressions rather than of fearful expression specifically (Hornak et al. 2003). Finally, subcortical routes that involve the thalamus and superior colliculus appear to be involved in processing emotional expressions, particularly fearful expressions (Luo et al. 2007; Morris, et al. 1999). Dysfunction in these regions could also exist among antisocial populations.
Most of the populations in the studies included in this meta-analysis had clinical diagnoses, were incarcerated, or were in other secure settings (e.g., camps or schools for children with behavior problems). These populations presumably posessed serious functional impairments. Only three studies (Blair & Coles, 2000; Dadds, 2006; Montagne et al, 2005) drew from community samples. It is thus difficult to infer how the observed relationship between antisociality and fear recognition would vary as a function of functional impairments. We speculate that increasingly severe antisocial behaviors and traits such as callousness would be associated with increasingly impoverished fear recognition abilities. Thus, any two samples that differ in antisociality will also differ in fear recognition skills, but the greater the disparity in antisocial traits and behavior, the greater the disparity in facial affect recognition.
This meta-analysis included populations characterized as psychopathic, conduct disordered, aggressive, unsocialized, abusive, and criminal. These classifications all are primarily defined by persistent behavior that violates the rights and welfare of others or breaks important normative rules—i.e., antisocial behavior. Unlike the other classifications, a classification of psychopathy indicates particular antisocial personality traits (e.g., lack of empathy and remorse) in addition to antisocial behaviors such as criminal offenses. The remaining classifications do not specify what personality or environmental variables (such as impulsiveness, substance abuse, physical abuse, or trauma) are associated with observed antisocial behavior. Psychopathic individuals thus represent a more homogenous group, and evidence of impaired amygdala functioning in this group is accordingly more consistent (Birbaumer et al. 2005; Blair et al. 2006; Herpertz et al. 2001; Kiehl et al. 2001; Mitchell et al. 2006). This may be because amygdala dysfunction is most pertinent to callous and unemotional personality traits and instrumental antisocial behavior, which are characteristic of psychopaths but may or may not be present in other antisocial individuals (Blair, 2001). However, the consistency of our results among psychopathic and non-psychopathic samples, as well as frequent findings of reduced amygdala functioning among other antisocial samples, suggest a high degree of overlap among these samples.
Our analyses found sadness recognition deficits were also associated with antisociality. This is theoretically consistent in that sadness, like fear, is a distress cue (Blair et al. 1997; Decety and Chaminade, 2003). In addition, similar neural structures, including the amygdala, are associated with processing sadness expressions (Adolphs and Tranel, 2004; Blair et al. 1999). It is unclear why antisociality is more reliably linked to fear recognition than sadness recognition; perhaps recognition deficits are less evident for expressions that possess identifiable markers such as the downturned mouth of sadness. Comparing correlates of fear and sadness recognition might be facilitated by examining patterns of errors during identification of these expressions. Only three studies we assessed provided error information (Blair et al. 2004; Matheson et al. 2005; Walz and Benson, 1996), and examination of these data did not reveal clear error patterns for fear or sadness. More consistent provision of such data might improve the interpretation of the results of future studies.
Distress cues such as fearful or sad expressions have been theorized to stabilize social interactions among healthy individuals by eliciting affective responses that reduce the likelihood of continued aggression against the victim (Blair, 1995, 2001; Marsh and Ambady, 2007). Such affective response may include empathy and remorse. Both of these responses are elicited by the perception and correct interpretation of distress cues such as fear expressions (Blair, 1995; Hoffman, 1987; Marsh and Ambady, 2007). That antisociality is associated with a lack of empathy and remorse again supports the conclusions that antisocial individuals do not respond appropriately to distress cues (Eisenberg, 2000). The Integrated Emotion Systems model provides one explanation for this process, specifying that distress cues are social reinforcers that enable conspecifics to convey the societal valence of objects and behaviors to the developing individual (Blair, 2001; 2005). Because the amygdala is involved in the formation of stimulus-reinforcement associations, it is theorized that amygdala dysfunction impairs the learning that typically results following distress cues. This prevents moral socialization and increases the likelihood of antisocial behavior (Blair, 2005). Another mechanism by which fearful expressions may elicit empathy is via infantile appearance cues (e.g., wide, round eyes) that stimulate caring, non-aggressive responses in perceivers (Marsh et al. 2005).
Relatively few studies were included in this analysis. However, the consistency of the results of the various analyses we conducted strengthens our conclusions. Our file-drawer analysis indicates that our findings did not likely result from sampling bias, which in meta-analyses usually results from the exclusion of unpublished or “file drawer” studies that do not show significant results. The nature of the meta-analysis we conducted minimized such biases because facial affect recognition studies do not typically test a single effect relevant to the null hypothesis. Rather, they test the recognition of multiple separate expressions individually and in aggregate. Thus, a study need not have demonstrated fear recognition deficits in order to be published (and indeed, several did not).
Conclusions
Antisocial populations across 20 studies showed specific deficits in recognizing fearful facial affect. The perception and recognition of fearful expressions have been previously linked to intact amygdala function, which may be impaired in individuals who exhibit persistent antisocial behaviors. The results of this meta-analysis may assist researchers in developing more precise hypotheses regarding the neural correlates and causes of antisocial behavior.
Table 3.
Comparisons of deficits in recognizing six emotional facial expressions in antisocial populations
| Expression | Corrected % Accuracy (Mean and SD) | M Diff (Antisocial — Compar.) | M Diff from Fear | |||
|---|---|---|---|---|---|---|
| Antisocial | Control | % Accuracy | r | % Accuracy | r | |
| Fear | 48.4 (18.6) | 63.8 (20.7) | 18.09** | .34** | — | — |
| Sadness | 63.9 (14.5) | 74.4 (8.7) | 12.38** | .19** | 6.05** | .16** |
| Surprise | 67.1 (25.0) | 74.5 (20.5) | 8.37** | .15** | 9.34** | .20** |
| Disgust | 57.8 (24.7) | 61.6 (28.2) | 4.97** | .12** | 11.09** | .23** |
| Anger | 65.7 (21.0) | 72.1 (13.7) | 7.61** | .10** | 10.81** | .28** |
| Happiness | 90.5 (10.1) | 92.7 (8.7) | 2.60** | .11** | 15.82** | .25** |
Acknowledgments
This research was supported by the Intramural Research Program of the NIH:NIMH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R. Neural systems for recognizing emotion. Curr. Opin. Neurobiol. 2002;12:169–77. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Perception and emotion: How we recognize facial expressions. Curr. Dir. Psychol. Sci. 2006;15:222–226. [Google Scholar]
- Adolphs R, Tranel D. Impaired judgments of sadness but not happiness following bilateral amygdala damage. J. Cogn. Neurosci. 2004;16:453–62. doi: 10.1162/089892904322926782. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Hamann S, Young AW, Calder AJ, Phelps EA, et al. Recognition of facial emotion in nine individuals with bilateral amygdala damage. Neuropsychologia. 1999;37:1111–17. doi: 10.1016/s0028-3932(99)00039-1. [DOI] [PubMed] [Google Scholar]
- Allison T, Puce A, Spencer DD, McCarthy G. Electrophysiological studies of human face perception. I: Potentials generated in occipitotemporal cortex by face and non-face stimuli. Cereb. Cortex. 1999;9:415–430. doi: 10.1093/cercor/9.5.415. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Cowen PJ, Goodwin GM, Harmer CJ. Normalization of enhanced fear recognition by acute SSRI treatment in subjects with a previous history of depression. Am. J. Psychiatry. 2004;161:166–68. doi: 10.1176/appi.ajp.161.1.166. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Veit R, Lotze M, Erb M, Hermann C, Grodd W, et al. Deficient fear conditioning in psychopathy: a functional magnetic resonance imaging study. Arch. Gen. Psychiatry. 2005;62:799–805. doi: 10.1001/archpsyc.62.7.799. [DOI] [PubMed] [Google Scholar]
- Blair KS, Richell RA, Mitchell DG, Leonard A, Morton J, Blair RJ. They know the words, but not the music: affective and semantic priming in individuals with psychopathy. Biol. Psychol. 2006;73:114–23. doi: 10.1016/j.biopsycho.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Blair RJ. A cognitive developmental approach to morality: investigating the psychopath. Cognition. 1995;57:1–29. doi: 10.1016/0010-0277(95)00676-p. [DOI] [PubMed] [Google Scholar]
- Blair RJ. Neurocognitive models of aggression, the antisocial personality disorders, and psychopathy. J. Neurol. Neurosurg. Psychiatry. 2001;71:727–31. doi: 10.1136/jnnp.71.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ. Neurobiological basis of psychopathy. Br. J. Psychiatry. 2003a;182:5–7. doi: 10.1192/bjp.182.1.5. [DOI] [PubMed] [Google Scholar]
- Blair RJ. Facial expressions, their communicatory functions and neurocognitive substrates. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2003b;358:561–72. doi: 10.1098/rstb.2002.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ. Applying a cognitive neuroscience perspective to the disorder of psychopathy. Dev. Psychopathol. 2005;17:865–91. doi: 10.1017/S0954579405050418. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Budhani S, Colledge E, Scott S. Deafness to fear in boys with psychopathic tendencies. J. Child. Psychol. Psychiatry. 2005;46:327–336. doi: 10.1111/j.1469-7610.2004.00356.x. [DOI] [PubMed] [Google Scholar]
- Blair RJR, Coles M. Expression recognition and behavioural problems in early adolescence. Cogn. Devel. 2000;15:421–34. [Google Scholar]
- Blair RJR, Mitchell DGV, Peschardt KS, Colledge E, Leonard RA, Shine JH, et al. Reduced sensitivity to others' fearful expressions in psychopathic individuals. Pers. Indiv. Dif. 2004;37:1111–22. [Google Scholar]
- Blair RJ, Cipolotti L. Impaired social response reversal. A case of ‘acquired sociopathy’. Brain. 2000;123:1122–41. doi: 10.1093/brain/123.6.1122. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Colledge E, Murray L, Mitchell DG. A selective impairment in the processing of sad and fearful expressions in children with psychopathic tendencies. J. Abnorm. Child Psychol. 2001;29:491–98. doi: 10.1023/a:1012225108281. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Jones L, Clark F, Smith M. The psychopathic individual: a lack of responsiveness to distress cues? Psychophysiology. 1997;34:192–98. doi: 10.1111/j.1469-8986.1997.tb02131.x. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Morris JS, Frith CD, Perrett DI, Dolan RJ. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122:883–93. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Keane J, Manly T, Sprengelmeyer R, Scott S, Nimmo-Smith I, et al. Facial expression recognition across the adult life span. Neuropsychologia. 2003;41:195–202. doi: 10.1016/s0028-3932(02)00149-5. [DOI] [PubMed] [Google Scholar]
- Camras LA, Allison K. Children's understanding of emotional expressions and verbal labels. J. Nonverbal Behav. 1985;9:84–94. [Google Scholar]
- Carr MB, Lutjemeier JA. The relation of facial affect recognition and empathy to delinquency in youth offenders. Adolescence. 2005;40:601–19. [PubMed] [Google Scholar]
- Castelli F. Understanding emotions from standardized facial expressions in autism and normal development. Autism. 2005;9:428–449. doi: 10.1177/1362361305056082. [DOI] [PubMed] [Google Scholar]
- Corden B, Critchley HD, Skuse D, Dolan RJ. Fear recognition ability predicts differences in social cognitive and neural functioning in men. J. Cogn. Neurosci. 2006;18:889–97. doi: 10.1162/jocn.2006.18.6.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadds MR, Perry Y, Hawes DJ, Merz S, Riddell AC, Haines DJ, et al. Look at the eyes! Overcoming impaired fear recognition in boys with psychopathic traits. Br. J. Psychiatry. 2006;189:280–281. doi: 10.1192/bjp.bp.105.018150. [DOI] [PubMed] [Google Scholar]
- Decety J, Chaminade T. Neural correlates of feeling sympathy. Neuropsychologia. 2003;41:127–38. doi: 10.1016/s0028-3932(02)00143-4. [DOI] [PubMed] [Google Scholar]
- Dinn WM, Harris CL. Neurocognitive function in antisocial personality disorder. Psychiatry Res. 2000;97:173–90. doi: 10.1016/s0165-1781(00)00224-9. [DOI] [PubMed] [Google Scholar]
- Dolan M, Fullam R. Face affect recognition deficits in personality-disordered offenders: association with psychopathy. Psychol. Med. 2006;36:1563–1569. doi: 10.1017/S0033291706008634. [DOI] [PubMed] [Google Scholar]
- Doria AS. Meta-analysis and structured literature review in radiology. Acad. Radiol. 2005;12:399–408. doi: 10.1016/j.acra.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Easter J, McClure EB, Monk CS, Dhanani M, Hodgdon H, Leibenluft E, et al. Emotion recognition deficits in pediatric anxiety disorders: implications for amygdala research. J. Child Adolesc. Psychopharmacol. 2005;15:563–70. doi: 10.1089/cap.2005.15.563. [DOI] [PubMed] [Google Scholar]
- Eisenberg N. Emotion, regulation, and moral development. Annu. Rev. Psychol. 2000;51:665–97. doi: 10.1146/annurev.psych.51.1.665. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of facial affect. Consulting Psychologists; Palo Alto, CA: 1976. [Google Scholar]
- Ekman P, Friesen WV, O'Sullivan M, Chan A, Diacoyanni-Tarlatzis I, Heider K, et al. Universals and cultural differences in the judgments of facial expressions of emotion. J. Pers. Soc. Psychol. 1987;53:712–17. doi: 10.1037//0022-3514.53.4.712. [DOI] [PubMed] [Google Scholar]
- Elfenbein HA, Ambady N. On the universality and cultural specificity of emotion recognition: a meta-analysis. Psychol. Bull. 2002;128:203–35. doi: 10.1037/0033-2909.128.2.203. [DOI] [PubMed] [Google Scholar]
- Fitzgerald DA, Angstadt M, Jelsone LM, Nathan PJ, Phan KL. Beyond threat: amygdala reactivity across multiple expressions of facial affect. Neuroimage. 2006;30:1441–1448. doi: 10.1016/j.neuroimage.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Fridlund AJ. Evolution and facial action in reflex, social motive, and paralanguage. Biol. Psychol. 1991;32:3–100. doi: 10.1016/0301-0511(91)90003-y. [DOI] [PubMed] [Google Scholar]
- Goldman AI, Sripada CS. Simulationist models of face-based emotion recognition. Cognition. 2005;94:193–213. doi: 10.1016/j.cognition.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Gross TF. The perception of four basic emotions in human and nonhuman faces by children with autism and other developmental disabilities. J. Abnorm. Child Psychol. 2004;32:469–80. doi: 10.1023/b:jacp.0000037777.17698.01. [DOI] [PubMed] [Google Scholar]
- Hare RD. Psychopathy and physiological activity during anticipation of an adversive stimulus in a distraction paradigm. Psychophysiology. 1982;19:266–71. doi: 10.1111/j.1469-8986.1982.tb02559.x. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. Human neural systems for face recognition and social communication. Biol Psychiatry. 2002;51:59–67. doi: 10.1016/s0006-3223(01)01330-0. [DOI] [PubMed] [Google Scholar]
- Herba C, Phillips M. Annotation: Development of facial expression recognition from childhood to adolescence: behavioural and neurological perspectives. J. Child Psychol. Psychiatry. 2004;45:1185–98. doi: 10.1111/j.1469-7610.2004.00316.x. [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Werth U, Lukas G, Qunaibi M, Schuerkens A, Kunert HJ, et al. Emotion in criminal offenders with psychopathy and borderline personality disorder. Arch. Gen. Psychiatry. 2001;58:737–45. doi: 10.1001/archpsyc.58.8.737. [DOI] [PubMed] [Google Scholar]
- Hoffman ML. The contribution of empathy to justice and moral judgment. In: Eisenberg N, Strayer J, editors. Empathy and its development. Cambridge University Press; Cambridge: 1987. pp. 47–80. [Google Scholar]
- Hornak J, Bramham J, Rolls ET, Morris RG, O'Doherty J, Bullock PR, Polkey CE. Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain. 2003;126:1691–1712. doi: 10.1093/brain/awg168. [DOI] [PubMed] [Google Scholar]
- Howard MA, Cowell PE, Boucher J, Broks P, Mayes A, Farrant A, Roberts N. Convergent neuroanatomical and behavioural evidence of an amygdala hypothesis of autism. Neuroreport. 2000;11:2931–2935. doi: 10.1097/00001756-200009110-00020. [DOI] [PubMed] [Google Scholar]
- Humphreys K, Minshew N, Leonard GL, Behrmann M. A fine-grained analysis of facial expression processing in high-functioning adults with autism. Neuropsychologia. 2007;45:685–695. doi: 10.1016/j.neuropsychologia.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Kiehl KA. A cognitive neuroscience perspective on psychopathy: Evidence for paralimbic system dysfunction. Psychiatry Res. 2006;142:107–128. doi: 10.1016/j.psychres.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl KA, Smith AM, Hare RD, Mendrek A, Forster BB, Brink J, et al. Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biol. Psychiatry. 2001;50:677–84. doi: 10.1016/s0006-3223(01)01222-7. [DOI] [PubMed] [Google Scholar]
- Kosson DS, Suchy Y, Mayer AR, Libby J. Facial affect recognition in criminal psychopaths. Emotion. 2002;2:398–411. doi: 10.1037/1528-3542.2.4.398. [DOI] [PubMed] [Google Scholar]
- Kropp JP, Haynes OM. Abusive and nonabusive mothers' ability to identify general and specific emotion signals of infants. Child Dev. 1987;58:187–90. [PubMed] [Google Scholar]
- LaBar KS, Crupain MJ, Voyvodic JT, McCarthy G. Dynamic perception of facial affect and identity in the human brain. Cereb. Cortex. 2003;13:1023–1033. doi: 10.1093/cercor/13.10.1023. [DOI] [PubMed] [Google Scholar]
- Lawrence AD, Calder AJ. Homologizing human emotions. In: Evans DA, Cruse P, editors. Emotions, evolution and rationality. Oxford University Press; Oxford: 2004. [Google Scholar]
- Le Masurier M, Cowen PJ, Harmer CJ. Emotional bias and waking salivary cortisol in relatives of patients with major depression. Psychol. Med. 2007;37:403–10. doi: 10.1017/S0033291706009184. [DOI] [PubMed] [Google Scholar]
- Levenston GK, Patrick CJ, Bradley MM, Lang PJ. The psychopath as observer: emotion and attention in picture processing. J. Abnorm. Psychol. 2000;109:373–85. [PubMed] [Google Scholar]
- Lough S, Kipps CM, Treise C, Watson P, Blair JR, Hodges JR. Social reasoning, emotion and empathy in frontotemporal dementia. Neuropsychologia. 2006;44:950–58. doi: 10.1016/j.neuropsychologia.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Luo Q, Holroyd T, Jones M, Hendler T, Blair J. Neural dynamics for facial threat processing as revealed by gamma band synchronization using MEG. Neuroimage. 2007;34:839–847. doi: 10.1016/j.neuroimage.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykken DT. A study of anxiety in the sociopathic personality. J. Abnorm. Psychol. 1957;55:6–10. doi: 10.1037/h0047232. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Adams RB, Jr., Kleck RE. Why do fear and anger look the way they do? Form and social function in facial expressions. Pers. Soc. Psychol. Bull. 2005;31:73–86. doi: 10.1177/0146167204271306. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Ambady N. The influence of the fear facial expression on prosocial responding. Cogn. Emot. 2007;21:225–247. [Google Scholar]
- Matheson E, Jahoda A. Emotional understanding in aggressive and nonaggressive individuals with mild or moderate mental retardation. Am. J. Ment. Retard. 2005;110:57–67. doi: 10.1352/0895-8017(2005)110<57:EUIAAN>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ. A subcortical pathway to the right amygdala mediating “unseen” fear. Proc. Natl. Acad. Sci. 1999;96:1680–1685. doi: 10.1073/pnas.96.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCown W, Johnson J, Austin S. Inability of delinquents to recognize facial affects. J. Soc. Behav. Pers. 1986;1:489–96. [Google Scholar]
- Mitchell DG, Avny SB, Blair RJ. Divergent patterns of aggressive and neurocognitive characteristics in acquired versus developmental psychopathy. Neurocase. 2006;12:164–78. doi: 10.1080/13554790600611288. [DOI] [PubMed] [Google Scholar]
- Montagne B, van Honk J, Kessels RPC, Frigerio E, Burt M, van Zandvoort MJE, et al. Reduced efficiency in recognising fear in subjects scoring high on psychopathic personality characteristics. Pers. Indiv. Dif. 2005;38:5–11. [Google Scholar]
- Moore DG. Reassessing emotion recognition performance in people with mental retardation: a review. Am. J. Ment. Retard. 2001;106:481–502. doi: 10.1352/0895-8017(2001)106<0481:RERPIP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cogn. Affect. Behav. Neurosci. 2003;3:207–233. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- Newman JP, Kosson DS. Passive avoidance learning in psychopathic and nonpsychopathic offenders. J. Abnorm. Psychol. 1986;95:252–56. [PubMed] [Google Scholar]
- Nichols S. Mindreading and the cognitive architecture underlying altruistic motivation. Mind Lang. 2001;16:425–455. [Google Scholar]
- Nowicki S, Duke MP. Individual differences in the nonverbal communication of affect. The Diagnostic Analysis of Nonverbal Accuracy (DANVA) Scale. J. Nonverbal Commun. 1994;18:9–18. [Google Scholar]
- Ogloff JR, Wong S. Electrodermal and cardiovascular evidence of a coping response in psychopaths. Crim. Justice Behav. 1990;17:231–245. [Google Scholar]
- Papps BP, Calder AJ, Young AW, O'Carroll RE. Dissociation of affective modulation of recollective and perceptual experience following amygdala damage. J. Neurol. Neurosurg. Psychiatry. 2003;74:253–54. doi: 10.1136/jnnp.74.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ. Emotion and psychopathy: startling new insights. Psychophysiology. 1994;31:319–30. doi: 10.1111/j.1469-8986.1994.tb02440.x. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Williams LM, Heining M, Herba CM, Russell T, Andrew C, et al. Differential neural responses to overt and covert presentations of facial expressions of fear and disgust. Neuroimage. 2004;21:1484–96. doi: 10.1016/j.neuroimage.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Preston SD, de Waal FBM. Empathy: Its ultimate and proximate bases. Behav. Brain Sci. 2002;25:1–72. doi: 10.1017/s0140525x02000018. [DOI] [PubMed] [Google Scholar]
- Preuschoft S. Primate faces and facial expressions. Soc. Res. 2000;67:245–271. [Google Scholar]
- Price JS, Gardner RJ, Erickson M. Can depression, anxiety and somatization be understood as appeasement displays? J. Affect. Disord. 2004;79:1–11. doi: 10.1016/S0165-0327(02)00452-4. [DOI] [PubMed] [Google Scholar]
- Rapcsak SZ, Galper SR, Comer JF, Reminger SL, Nielsen L, Kaszniak AW, et al. Fear recognition deficits after focal brain damage: a cautionary note. Neurology. 2000;54:575–81. doi: 10.1212/wnl.54.3.575. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. The “file drawer problem” and tolerance for null results. Psychol. Bull. 1979;36:638–641. [Google Scholar]
- Rosenthal R, DiMatteo MR. Meta-analysis: recent developments in quantitative methods for literature reviews. Annu. Rev. Psychol. 2001;52:59–82. doi: 10.1146/annurev.psych.52.1.59. [DOI] [PubMed] [Google Scholar]
- Russell JA. Is there universal recognition of emotion from facial expression? A review of the cross-cultural studies. Psychol. Bull. 1994;115:102–141. doi: 10.1037/0033-2909.115.1.102. [DOI] [PubMed] [Google Scholar]
- Sasson NJ. The development of face processing in autism. J. Autism Dev. Disord. 2006;36:381–394. doi: 10.1007/s10803-006-0076-3. [DOI] [PubMed] [Google Scholar]
- Sharp C, van Goozen S, Goodyer I. Children's subjective emotional reactivity to affective pictures: gender differences and their antisocial correlates in an unselected sample of 7−11-year-olds. J. Child Psychol, Psychiatry. 2006;47:143–50. doi: 10.1111/j.1469-7610.2005.01464.x. [DOI] [PubMed] [Google Scholar]
- Singh SD, Ellis CR, Winton AS, Singh NN, Leung JP, Oswald DP. Recognition of facial expressions of emotion by children with attention-deficit hyperactivity disorder. Behav. Modif. 1998;22:128–42. doi: 10.1177/01454455980222002. [DOI] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, Meyer-Lindenberg A. A validated network of effective amygdala connectivity. Neuroimage. 2007;36:736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Sterzer P, Stadler C, Krebs A, Kleinschmidt A, Poustka F. Abnormal neural responses to emotional visual stimuli in adolescents with conduct disorder. Biol Psychiatry. 2005;57:7–15. doi: 10.1016/j.biopsych.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Stevens D, Charman T, Blair RJ. Recognition of emotion in facial expressions and vocal tones in children with psychopathic tendencies. J. Genet. Psychol. 2001;162:201–11. doi: 10.1080/00221320109597961. [DOI] [PubMed] [Google Scholar]
- Surcinelli P, Codispoti M, Montebarocci O, Rossi N, Baldaro B. Facial emotion recognition in trait anxiety. J. Anxiety Disord. 2006;20:110–17. doi: 10.1016/j.janxdis.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Tremeau F. A review of emotion deficits in schizophrenia. Dialogues Clin. Neuroscience. 2006;8:59–70. doi: 10.31887/DCNS.2006.8.1/ftremeau. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AW, Linehan MM. Facial expression recognition ability among women with borderline personality disorder: implications for emotion regulation? J. Pers. Disord. 1999;13:329–44. doi: 10.1521/pedi.1999.13.4.329. [DOI] [PubMed] [Google Scholar]
- Walker DW, Leister C. Recognition of facial affect cues by adolescents with emotional and behavioral disorders. Behav. Disord. 1994;19:269–76. [Google Scholar]
- Walker E. Emotion recognition in disturbed and normal children: A research note. J. Child Psychol. Psychiatry. 1981;22:263–68. doi: 10.1111/j.1469-7610.1981.tb00551.x. [DOI] [PubMed] [Google Scholar]
- Walz NC, Benson BA. Labeling and discrimination of facial expressions by aggressive and nonaggressive men with mental retardation. Am. J. Ment. Retard. 1996;101:282–91. [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J. Neurosci. 1998;18:411–18. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston JS, O'Doherty J, Dolan RJ. Common and distinct neural responses during direct and incidental processing of multiple facial emotions. Neuroimage. 2003;20:84–97. doi: 10.1016/s1053-8119(03)00303-3. [DOI] [PubMed] [Google Scholar]
- Woodbury-Smith MR, Clare ICH, Holland AJ, Kearns A, Staufenberg E, Watson P. A case-control study of offenders with high functioning autistic spectrum disorders. J. Forensic Psychiatry Psychol. 2005;16:747–763. [Google Scholar]
- Zabel RH. Recognition of emotions in facial expressions by emotionally disturbed and nondisturbed children. Psychol. Sch. 1979;16:119–26. [Google Scholar]


