Abstract
From early in the AIDS epidemic, psychosocial stressors have been proposed as contributors to the variation in disease course. To test this hypothesis, rhesus macaques were assigned to stable or unstable social conditions and were inoculated with the simian immunodeficiency virus. Animals in the unstable condition displayed more agonism and less affiliation, shorter survival, and lower basal concentrations of plasma cortisol compared with stable animals. Early after inoculation, but before the emergence of group differences in cortisol levels, animals receiving social threats had higher concentrations of simian immunodeficiency virus RNA in plasma, and those engaging in affiliation had lower concentrations. The results indicate that social factors can have a significant impact on the course of immunodeficiency disease. Socially induced changes in pituitary–adrenal hormones may be one mechanism mediating this relationship.
The rate of progression of HIV disease is quite variable. Median durations of ≈10 years have been reported for the period of clinical latency following infection with HIV; a second variable period, which can last beyond 2 years, may follow a diagnosis of AIDS (1, 2). The sources of this variation in HIV disease progression are unclear, although from early in the epidemic, psychosocial factors have been suggested as one source (3, 4).
Psychoneuroimmunological research has shown that stressors can influence immune functioning in ways that might be relevant to HIV disease progression (5), and there is strong evidence that HIV disease, both in the U.S. and worldwide, is associated with considerable psychosocial stress. Likely sources of this stress include: experience of multiple bereavements; social isolation, because support is withdrawn by family and friends because of fear of infection or widespread negative attitudes; and threats from institutional sources that can result in loss of one’s housing, employment, and health care (6–8). Understanding the consequences of psychosocial stress for HIV disease progression in human populations is complicated by a variety of factors such as the number, nature, and timing of stressors experienced by different individuals and by differences in how individuals cope with stressors, such as by engaging in unhealthy behaviors (drug or alcohol use and unprotected sex) that might further compromise immune function (e.g., ref. 9). Nevertheless, there is good evidence that one psychosocial stressor—bereavement—has negative consequences for immune and psychological functioning among HIV-infected people (10).
Studies with animals afford a better opportunity to control both the agent of the disease and the context in which the disease develops. A particularly useful model for the study of social processes and disease is provided by simian immunodeficiency virus (SIV) disease in rhesus macaques (11). Like humans, rhesus monkeys live in complex societies in which relationships are formed and maintained, sometimes for years, but regular emigration and immigration require that old associations must be changed and new associations must be forged. This situation is particularly true for the subjects of our research, young adult male monkeys, for which intergroup transfers are normal, but highly stressful, features of their life history (12, 13). Considerable data from nonhuman primates indicate that social instability can result in alterations in hypothalamic–pituitary–adrenal (HPA) hormone concentrations and immune function (14–17). In the present study, we hypothesized that instability in rhesus monkey social groups would have implications for immunodeficiency disease progression as well.
METHODS
Subjects.
Subjects were 36 adult male rhesus macaques (Macaca mulatta), born and raised in one-half-acre cages at the California Regional Primate Research Center. Animals were selected based on good physical health, no prior participation in invasive research, negative serostatus for the retroviruses simian retrovirus type D, SIV, and simian t-lymphotropic virus type 1, and intermediate social rank in their natal cages. At a mean age of 6.2 (range 5.0–8.3) years, animals were relocated from their natal cages to indoor housing in standard individual laboratory cages. SIV- and saline-inoculated animals were housed in identical, adjacent rooms. Animals in the same social condition were housed so as to prevent visual contact. The present experiment began when the monkeys were a mean age of 7.6 years.
Apparatus.
Social groups were formed in a 1.8 (depth) × 3.1 (width) × 2.2- (height) m cage constructed of cyclone fencing and pipe, with a floor constructed of 2.5-cm2 mesh. Two cages, situated 0.7 m apart and separated by an opaque barrier, were located in each of two identical test rooms; one room was used for group formations of SIV-inoculated monkeys and the other for control animal social groups. Monkeys were transported to test rooms via individual transport boxes (0.5 × 0.3 × 0.4 m) constructed of aluminum.
Design.
Eighteen animals were inoculated with SIV, and 18 animals served as saline-inoculated controls. Nine animals from each inoculation condition were formed daily into stable social groups, and nine animals experienced unstable social conditions, resulting in a 2 (inoculation condition) × 2 (social condition) factorial design with nine subjects per cell. Stable social groups comprised three animals, with unvarying membership in each group. Unstable social groups met for an equivalent period of time each day, although the group size and membership varied: Two-, three-, and four-member unstable groups were formed each day from among the nine animals assigned to a particular inoculation condition. Thus, the opportunity for social interactions for monkeys in the stable and unstable conditions was equal; the principal difference between the conditions was the extent to which the development of stable social relationships was facilitated by consistency of group composition. All of the social exposures occurred within inoculation condition.
Groups were formed 3–5 days/wk, and each exposure session was 100 min in duration. One stable and one unstable social group were formed at each of three daily time periods (0800, 1115, and 1345 hr) in each test room. Insofar as possible, scheduling of groups was done to balance time period, cage within the test rooms, and identity of the groups being tested at the same time, within each 2-wk period of the study. Because the effects of initial encounters with unfamiliar animals would be comparable for monkeys in the stable and unstable conditions, we inoculated all animals after they had already experienced three formations of their social groups to insure that the experiences among them were different at the point of inoculation.
Procedures.
SIV inoculation. All animals were immobilized with ketamine hydrochloride (10 mg/kg). Two cryopreserved aliquots of SIVmac251 virus (grown in rhesus peripheral blood mononuclear cells) were thawed and diluted 1:10 in phosphate buffered saline. Nineteen syringes were prepared. Following i.v. inoculation of the 18 animals on the same day, the contents of the 19th syringe were cultured with CEMX174 cells to reveal a final dosage of 103.68 tissue culture 50% infective dose. The 18 control animals were inoculated i.v. with an equivalent volume (1 ml) of saline.
Behavioral testing.
Behavioral data were collected during all social sessions by trained observers using the observer (18) software. Behavior categories used were those commonly used for this species (19). Frequency data were collected during the first 30 min and last 10 min of each observation session, and durations of social interactions were obtained between minutes 55 and 90. Observers alternated 5-min sessions of data collection for the two cages in a test room.
Physical examinations.
Biweekly, all animals were immobilized with ketamine and SIV-inoculated animals were given complete physical examinations. Five ml of blood was drawn via femoral venipuncture. Immediately following sample collection, 3 ml of blood was transferred to a tube containing EDTA, and the remaining 2 ml was transferred to a second EDTA tube. Both tubes were spun at 2,200 rpm for 20 min in a refrigerated centrifuge, after which plasma was removed. Plasma from the 2-ml tube was stored at −70°C for subsequent analysis of anti-SIV IgG, and plasma from the 3-ml tube was centrifuged for a second time at 2,800 rpm for 15 min before being aliquoted and stored at −70°C for assay of SIV RNA. Animals did not receive social exposures on days when physicals were scheduled.
Basal blood sampling.
Baseline blood samples were drawn between 1500 and 1530 hr every 4 wk from antecubital veins of conscious animals via arm pulls; ketamine was not used. Virtually all of the blood samples were drawn within 3 min of contacting each animal. Immediately following the sample collection, 1.5 ml of blood was transferred to a tube containing EDTA. Samples were centrifuged at 3,000 rpm for 20 min, after which plasma was removed and stored at −70°C for analysis of plasma concentrations of cortisol. The remainder of the blood was aliquoted for additional assays not reported here. Animals did not receive social exposures on days when basal blood sampling was performed.
Assessment of pituitary–adrenal regulation.
Animals were removed from their living cages via a pole and collar procedure (20) and were placed in a primate chair (Primate Products, Redwood City, CA) for two 2-hr sessions. For one session, animals received an i.m. injection of dexamethasone (50 μg/kg) 6 hr before placement in the chair, and for a second session, animals received an i.m. injection of saline of equivalent volume (≈0.15 ml). Order of sessions was counterbalanced, and a minimum of 10 days intervened between sessions. Three animals, all from the same inoculation and social condition, were tested each day. On test days, animals did not experience social group formations.
When placed in the chair, a 5-ml blood sample was drawn from an antecubital vein. Subsequent samples were drawn at 15, 30, 60, and 120 min later. Blood was transferred immediately to tubes containing EDTA; tubes were centrifuged at 3,000 rpm for 20 min, and plasma was banked for later corticotropin and cortisol assays. Only data from the time 0 and time 120 samples are reported here. One unstable, inoculated animal had missing data; consequently, his data were dropped. Testing began 12 wk after inoculation and took 6 wk to complete.
Assessment of immune functioning.
To assess humoral immunity, animals received a booster immunization with tetanus toxoid at 21-wk postinoculation (p.i.; the animals had last been boosted 7 wk before inoculation). Animals were immobilized with ketamine, and 3 ml of blood was drawn, after which animals were immunized i.m. with 0.5 ml of tetanus toxoid (Connaught Laboratories). Additional 3-ml blood samples were drawn at 10- and 24-days postimmunization during physical exams. Blood was drawn into unheparinized syringes, transferred to sterile tubes, and allowed to clot. Serum was extracted and stored at −70°C for later assay of antitetanus IgG.
To assess cellular immunity in SIV-inoculated animals, the proliferative response of lymphocytes to mitogen stimulation was measured. Three days after tetanus toxoid immunization (week 21 p.i.), 8 ml of blood was drawn after ketamine immobilization. Blood was transferred to cell preparation tubes (Becton Dickinson), and lymphocytes were separated via centrifugation at 2,800 rpm for 20 min. The proliferation assay, described below, was performed.
Euthanasia.
Euthanasia was accomplished by i.v. administration of B Euthanasia-D (1 mg/kg, Shering Plough). Criteria for euthanasia were those used at the California Regional Primate Research Center (CRPRC) for immunodeficient animals and included nonresponse to therapy for opportunistic disease, weight loss >15% in 2 wk or 30% in 2 mo, persistent anorexia with weight loss, or presence of neurological signs. Decisions about euthanasia were made by CRPRC staff veterinarians who were unfamiliar with the social condition assignment of the animals.
Hormonal, Immunological, and Virological Assessments.
Plasma cortisol.Hormone concentrations were assessed using commercially available radioimmunoassay kits (Diagnostic Products, Los Angeles). Inter- and intra-assay coefficients of variability for all plasma cortisol assays reported in this paper were 9.6% and 8.5%, respectively.
Antitetanus IgG.
Microtiter plates were coated with dilute tetanus toxoid and allowed to incubate overnight. Plates were washed twice, and 100 μl of serum (diluted 1:100) was added to each well. A known high titer sample was used as a positive control. Plates were incubated at room temperature for 2 hr and washed, and 100 μl of goat anti-monkey IgG conjugate (Nordic Immunological, Capistrano Beach, CA) was added to each well. Plates were incubated for 1 hr and O-phenylenediamine dihydrochloride (OPD) substrate (Sigma) was added; plates were further incubated for 20 min and then were read on an EL311 plate reader (Bio-Tek, Burlington, VT) with a 405-nm filter. Samples were run in duplicate, and all three samples (days 0, 10, and 24) for a given monkey were run on the same plate. Values are expressed as the proportion of the positive control on each plate (21).
Anti-SIV IgG.
Anti-SIV IgG was assessed using a commercial HIV 1/2 ELISA (Sanofi Diagnostics, Chaska, MN) performed according to manufacturer’s instructions. Samples were assayed in duplicate. Values are expressed as an ELISA ratio, which is defined as the OD for the samples divided by the manufacturer’s suggested cut off (mean OD of the negative control + 0.25) (21). Animals that did not generate a detectable antibody response were assigned a value of one. Intra- and interplate coefficients of variation were 1.2% and 4.8%, respectively.
Lymphocyte proliferation assay.
Lymphocytes were washed twice and resuspended in supplemented RPMI-1640 media at a concentration of 106 cells/ml. Lymphocytes (0.1 ml) were incubated in triplicate in wells of a microtiter plate for 24 hr at 37°C in a 5% humidified CO2 incubator with varying concentrations of the mitogens phytohemagglutinin (6.25, 12.5, 25, and 50 μg/ml) and Con A (5, 10, 20, and 40 μg/ml). Twenty microliters of Alamar Blue (Alamar, Sacramento, CA) was added and the plates were incubated for 18 hr. (22). Plates were read on an EL311 plate reader with filter settings for 570 and 600 nm. Results were expressed as the difference between the mean of stimulated and the mean of unstimulated wells. SIV RNA. SIV RNA was assessed using a branched DNA signal amplification assay developed by Chiron and described in ref. 23. This assay has been optimized to detect SIV RNA by using an SIV-specific probe for the pol gene. Both the between and within run tolerance limits for this assay were less than threefold.
Statistical Analyses.
Survival among SIV-inoculated monkeys was examined using Kaplan–Meier procedures, and the difference in survival between stable and unstable animals was assessed using the log-rank test. For all other measures, ANOVA was used to examine the effects of inoculation condition, social condition, and, where relevant, time. Because of disease-induced mortality, it was necessary to analyze behavior and basal cortisol data in time blocks. Block 1 corresponded to the first 20 wk p.i., during which all of the SIV-inoculated animals were still alive (n = 36). Block 2 comprised the next 14 wk following the deaths of two unstable, inoculated animals (n = 34), and block 3 corresponded to the next 10 wk following the death of a stable, inoculated animal (n = 33). All of the analyses used the full sample (inoculated and control animals) except for analyses involving SIV-specific measures and the lymphocyte proliferation assay, which included only inoculated animals.
RESULTS
Among the SIV-inoculated animals, those in the stable condition survived significantly longer than did animals in the unstable condition (log-rank test, P < 0.05). Median survival for stable animals was 588.5 days with a range of 238–755 days (one animal was still alive >800 days; one animal died from non-SIV-related causes at 302 days.) For the unstable animals, median survival was 420 days with a range of 141–576 days. Four stable animals survived beyond the longest surviving unstable animal. No mortality was observed among control animals in either the stable or unstable conditions.
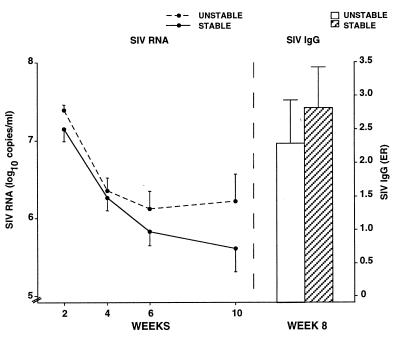
Early measures of infection, such as viral load and the virus-specific IgG response, were related significantly to survival but social condition was not related. We assessed SIV RNA in plasma samples drawn at weeks 2, 4, 6, and 10 p.i. and anti-SIV IgG from plasma collected at week 8 p.i. Measures at most time points were correlated significantly with survival: Log-transformed SIV RNA levels correlated with survival −0.545 (P < .05), −0.221 (not significant), −0.626 (P < .01), and −0.788 (P < 0.001), respectively, and anti-SIV IgG correlated +0.529 (P < 0.05) with survival. A significant decline in SIV RNA levels was found over time [F(3,48) = 41.60, P < 0.001]. Despite stable animals showing consistently lower RNA levels and a higher IgG response, effects of social condition were not statistically significant for either the SIV RNA or anti-SIV IgG measures (Fig. 1).
Figure 1.
Differences between stable and unstable animals in SIV RNA at weeks 2, 4, 6 and 10 p.i., and anti-SIV IgG response at week 8 p.i. For all measures, differences between stable and unstable animals were nonsignificant. Levels of SIV RNA declined significantly for all animals between weeks 2 and 10.
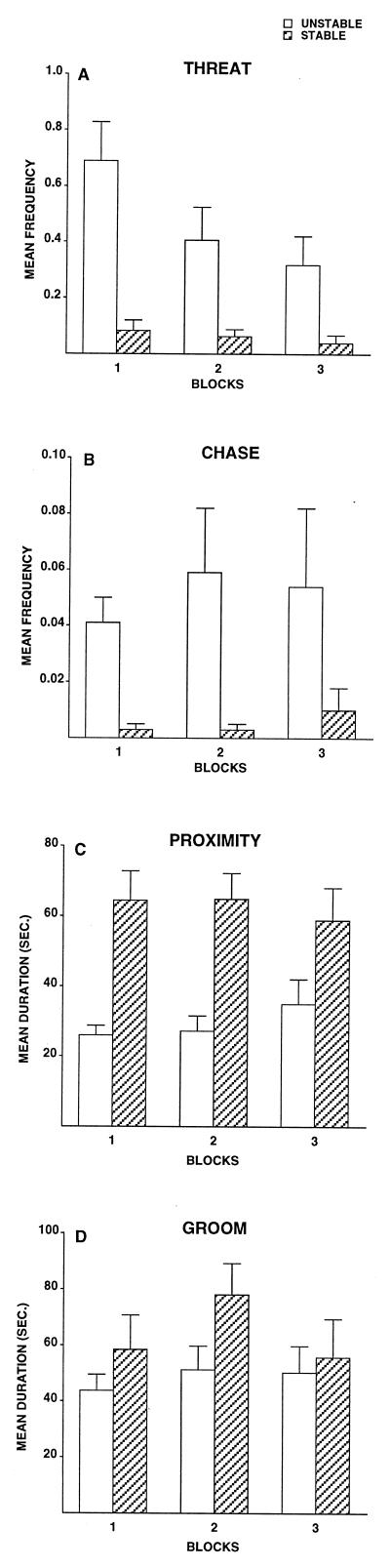
As expected, animals in the unstable condition were more frequently involved in interactions that were conflictual or aggressive than were animals in the stable condition; stable animals, in contrast, engaged in more affiliation than did unstable monkeys. Analyses of variance revealed that unstable animals displayed significantly more facial threats in all three time blocks [see Fig. 2A, block 1: F(1,32) = 18.63, P < 0.001; block 2: F(1,30) = 8.52, P < 0.01; and block 3: F(1,29) = 7.40, P < 0.05], significantly more chases in time blocks 1 and 2; [Fig. 2B, block 1: F(1,32) = 16.21, P < 0.001 and block 2: F(1,30) = 5.90, P < 0.05], and significantly more fear grimaces in time blocks 2 and 3 [block 2: F(1,30) = 6.21, P < 0.05 and block 3: F(1,29) = 5.75, P < 0.05)]. Levels of contact aggression were low overall but were consistently higher in unstable groups. For all three blocks, stable animals spent significantly more time in proximity (within an arm’s reach) [Fig. 2C, block 1: F(1,32) = 16.91, P < 0.001; block 2: F(1,30) = 17.21, P < 0.001; and block 3: F(1,29) = 3.65, P < 0.07] and contact [block 1: F(1,32) = 5.75, P < 0.05; block 2: F(1,30) = 4.55, P < 0.05; and block 3: F(1,29) = 3.28, P < 0.08] than did unstable animals. During blocks 1 and 2, stable animals displayed higher frequencies of groom-solicit [block 1: F(1,32) = 4.64, P < 0.05 and block 2: F(1,30) = 3.67, P < 0.07] and spent less time in nonsocial activity [block 1: F(1,32) = 8.02, P < 0.01 and block 2: F(1,30) = 12.88, P < 0.001]. Finally, duration of social grooming (either initiated or received) was higher among stable animals for all blocks, significantly so for block 2 [Fig. 2D, F(1,30) = 4.36, P < 0.05]. The only result involving inoculation condition was found during block 3: Inoculated animals groomed less than did the control animals [F(1,29) = 8.42, P < 0.01].
Figure 2.
Differences in social behavior between stable and unstable animals. Mean frequency of threats (A) and chases (B) received and mean durations of proximity (C) and groom (D). Significant group differences were found for threats (all time blocks), chases (blocks 1 and 2), proximity (all blocks), and groom (block 2). Block 1 corresponds to weeks 1–20 p.i. (n = 36); block 2 corresponds to weeks 21–34 p.i. (n = 34); and block 3 corresponds to weeks 35–44 p.i. (n = 33).
Regardless of social condition assignment, aggressive and affiliative behaviors displayed by the animals predicted levels of SIV RNA and the anti-SIV IgG response. As an indicator of aggressive interactions, we examined the behavior “receive threat.” Because we were interested in whether affiliative interactions might buffer the effects of social stress (17), we selected the behavior “groom” to reflect affiliation. For the week 2 SIV RNA analysis, animals were classified based on whether they received any threats and initiated or received any grooming in the first 2 wk of the study. Animals that received threats, regardless of social condition, had significantly higher SIV RNA levels at week 2 p.i. than did those that did not [F(1,14) = 17.19, P = 0.001], and animals that were involved in grooming had significantly lower SIV RNA levels [F(1,14) = 13.41, P < 0.01], as indicated by a two-way ANOVA. The interaction was not significant (see Table 1A). For analysis of the week 8 SIV-specific IgG and the week 10 SIV RNA measures, we counted the number of 2-wk periods in which animals received threats and were involved in grooming. (Because of small cell sizes, the number of biweekly periods of receiving threats was recoded into four categories from “0” to “3 or more” for both analyses.) We hypothesized that animals that experienced sustained aggression would show a lower antibody response at week 8 and higher SIV RNA levels at week 10, and animals involved in sustained grooming interactions would show the opposite pattern. Multiple regression analysis supported our hypothesis only for the aggression measure: animals that received threats in more biweekly periods had lower anti-SIV IgG during week 8 p.i. (partial r = −0.556, P < 0.05) and higher SIV RNA levels during week 10 (partial r = +0.703, P < 0.001; see Table 1B). No effect was found for sustained affiliation (partial r = −0.221 and −0.280, respectively).
Table 1.
Social behavior and SIV RNA
| A. Week 2 p.i. | ||||
|---|---|---|---|---|
| Classification | Receive threat
|
Initiate or receive groom
|
||
| no | yes | none | any | |
| Mean log10 copies/ml | 7.048 | 7.439 | 7.431 | 7.132 |
| Standard error | 0.149 | 0.083 | 0.134 | 0.113 |
| Sample size | 8 | 10 | 8 | 10 |
| B. Week 10 p.i. | ||||
| Number of biweekly periods of receiving threats | ||||
| zero | one | two | three or more | |
| Mean log10 copies/ml | 5.120 | 5.585 | 6.385 | 6.753 |
| Standard error | 0.747 | 0.799 | 0.713 | 0.941 |
| Sample size | 5 | 5 | 3 | 5 |
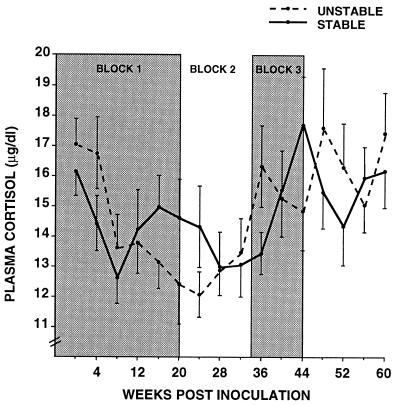
Stable and unstable social conditions resulted in significantly different patterns of basal plasma cortisol secretion. Surprisingly, within 8 wk of group formation, basal cortisol concentrations declined for animals in both the stable and unstable conditions (Fig. 3). Cortisol levels among the stable animals subsequently rose, whereas levels continued to decline through 24 wk p.i. among unstable animals, as indicated by a significant interaction during time block 1 between social condition and time [F(4,128) = 3.89, P < 0.01]. Follow-up analysis for block 1 revealed that, for unstable animals, cortisol concentrations at week 4 were significantly higher than at weeks 8, 12, 16, and 20. For stable animals, cortisol was higher at weeks 4 and 16 compared with week 8. During block 2, cortisol levels for unstable animals increased, and for the remainder of block 2 and all of block 3 stable and unstable animals no longer differed consistently.
Figure 3.
Basal concentrations of plasma cortisol were assessed at 4-wk intervals over a 60-wk period. Week 0 reflects preinoculation levels. See Fig. 2 legend for description of time blocks. Significant interactions of social condition by time were found for blocks 1 and 3.
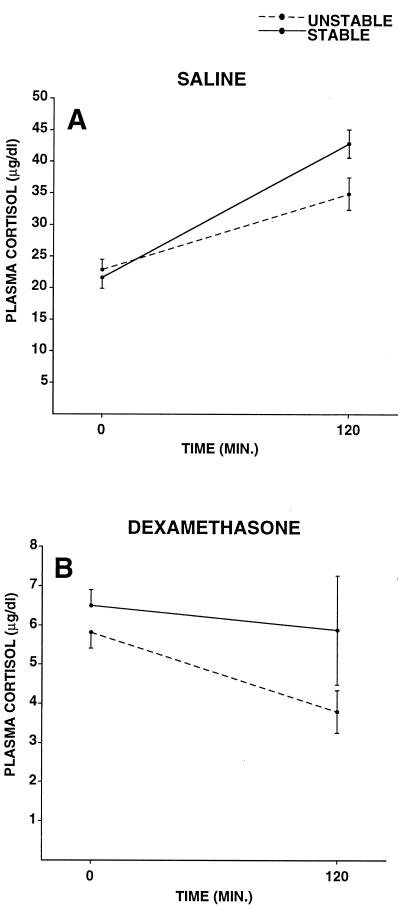
Stable and unstable animals differed in their cortisol responses to an acute stressor and in the ability of dexamethasone to suppress this response between weeks 12 and 18 p.i. Stable animals tended to have higher cortisol concentrations across all of the conditions [F(1,31) = 3.15, P < 0.09] and showed a greater cortisol increase over the 2-hr time period than did unstable animals [social condition by time: F(1,31) = 6.66, P < 0.05]. Pretreatment with dexamethasone attenuated the adrenocortical response to restraint stress [pretreatment by time: F(1,31) = 118.79, P < 0.001], and resulted in a decrease in cortisol concentrations over the 2-hr restraint period for unstable animals [social condition by pretreatment by time: F(1,31) = 5.78, P < 0.05, Fig. 4]. Finally, cortisol concentrations were higher in the saline pretreatment condition overall [F(1,31) = 408.36, P < 0.001], and at the 120-min time point, compared with time 0 [F(1,31) = 58.00, P < 0.001].
Figure 4.
Plasma cortisol responses to an acute stressor in monkeys experiencing stable or unstable social conditions during weeks 12–18 pi. (A) Saline pretreatment condition. (B) Dexamethasone pretreatment condition. The significant three-way interaction of social condition, pretreatment, and time indicated that the stress-induced increase in plasma cortisol from time 0 to time 120 was greater in stable animals; dexamethasone pretreatment resulted in a decline over the 2-hr period for unstable, but not stable, animals.
At the point when group differences in plasma cortisol were maximal (week 21 p.i.), stable and unstable animals showed differences in humoral immune function. The antitetanus IgG response was significantly greater for unstable compared with stable animals [F(1,30) = 7.75, P < 0.01]; values for the proportion of plate positive were 0.75 for stable and 1.03 for unstable animals. Overall, the anti-IgG response increased following boosting [F(2,60) = 93.27, P < 0.001], and inoculated animals showed a lower response than did control animals [F(1,30) = 8.22, P < 0.01], particularly for the days 10 and 24 samples [inoculation condition by time: F(2,60) = 5.92, P < 0.01]. No significant effect for social condition was found for measures of cellular immunity.
DISCUSSION
The data just described provide the most direct evidence that social stress is associated with shorter survival in immunodeficiency disease. Unstable social conditions, which induce a stressful state in adult male rhesus monkeys, were associated with greater agonism, less affiliation, and shorter survival following inoculation with SIV, as well as alterations in plasma cortisol concentrations and humoral immune function. Moreover, important individual differences in behavioral response to the social manipulations were observed early after inoculation that were related strongly to measures of viral load, which themselves predicted survival.
One mechanism whereby social stress might influence progression of immunodeficiency disease is via alteration in pituitary–adrenal hormone regulation. In the present study, social stress produced a transitory decrease in basal glucocorticoid production, and the results of the restraint and dexamethasone testing suggest that this result may be caused by enhanced negative feedback sensitivity. The pattern of cortisol release found in our unstable animals—reduced basal levels and lower levels during an acute stressor—does not conform to the traditional view of “stress” that would predict sustained elevations in cortisol among the stressed animals. Rather, our findings are consistent with data showing a suppression of the HPA axis among individuals suffering from posttraumatic stress disorder (24). The fact that this alteration in pituitary–adrenal regulation had immunological consequences was evident in our finding that humoral immune function differentiated our stable and unstable groups at the time that the basal cortisol difference was maximal. The exact mechanism by which such HPA alterations might affect immunodeficiency disease progression is unknown; inasmuch as our rapid progressors showed a decrease in cortisol but an increase in humoral immune response, however, our data would appear to be inconsistent with hypotheses that disease progression may follow a shift in helper T cell function precipitated by elevations in glucocorticoids (25, 26). Nevertheless, data from a number of sources indicate that socially induced alterations of HPA activity may influence disease progression. These include: posttraumatic stress disorder research that has shown an inverse relationship between cortisol levels and glucocorticoid receptor numbers on lymphocytes (27); the well-known modulatory effects of glucocorticoids on cytokine production (28) and humoral immunity (29); evidence that glucocorticoid response elements have been found in the HIV-1 genome (30, 31); data that glucocorticoids can affect HIV replication and gene expression in vitro (32, 33); and evidence that glucocorticoids exert an antiapoptotic effect on mature, HIV-infected CD4+ T cells in vitro (34).
Altered HPA activity cannot be the only factor linking social condition to shorter survival, however. Even before basal values of cortisol differentiated stable and unstable animals, behaviors observed during the social group formations predicted important virologic and serologic correlates of survival, such as the anti-SIV IgG response and number of copies of SIV RNA in plasma. The individual differences in behavior did not, however, predict basal cortisol concentrations (unpublished data). Together, these data indicate that social stress may affect survival in SIV-inoculated macaques via multiple routes. Inasmuch as the early measures of viral load predicted survival so strongly and were significantly associated with conflictual and affiliative behaviors observed soon after inoculation, our data also suggest that psychosocial events occurring early after initial infection with an immunodeficiency virus might exert a disproportionate effect on disease course (see also ref. 35). Further study of the interaction between social condition and psychobiological characteristics of individuals that might influence their ability to cope with social instability would be useful in identifying individuals who might be particularly at risk for negative health consequences of socially induced stress.
Acknowledgments
We thank P. Allen, J. Bond, D. Chave, P. Dailey, C. Machado, M. Norman, D. Schoen, and the veterinary and animal care staffs of the California Regional Primate Research Center (CRPRC) for technical assistance. M. Marthas and M. McChesney provided helpful comments on an earlier draft of the paper, and M. Marthas kindly provided the virus for inoculation. Supported by Grants MH 49033 from the National Institute of Mental Health (to J.P.C.) and by Grant RR 00169 from the National Center for Research Resources. All procedures were approved by the University of California, Davis, Institutional Animal Care and Use Committee. CRPRC is accredited by the Association and Accreditation of Laboratory Animal Care International.
ABBREVIATIONS
- SIV
simian immunodeficiency virus
- HPA
hypothalamic pituitary adrenal
- p.i.
postinoculation
References
- 1.Rutherford G W, Lifson A R, Hessol N A, Darrow W W, O’Malley P M, Buchbinder S P, Barnhart J L, Bodecker T W, Cannon L, Doll L S, et al. Br Med J. 1990;301:1183–1188. doi: 10.1136/bmj.301.6762.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedland G H, Saltzman B, Vileno J, Freeman K, Schrager L K, Klein R S. J Acquired Immune Defic Syndr. 1991;4:144–153. [PubMed] [Google Scholar]
- 3.Coates T J, Temoshok L, Mandel J. Am Psychol. 1984;39:1309–1314. doi: 10.1037//0003-066x.39.11.1309. [DOI] [PubMed] [Google Scholar]
- 4.Solomon G F. Advances. 1985;2:6–19. [Google Scholar]
- 5.Ader R, Felten D L, Cohen N, editors. Psychoneuroimmunology. 2nd Ed. San Diego: Academic; 1991. [Google Scholar]
- 6.Hendriks A. In: AIDS in the World. Mann J, Tarantola D, Netter T W, editors. Cambridge, MA: Harvard Univ. Press; 1992. pp. 769–774. [Google Scholar]
- 7.Hunter N D, Rubenstein W B, editors. AIDS Agenda: Emerging Issues in Civil Rights. New York: New Press; 1992. [Google Scholar]
- 8.Herek G M, Capitanio J P. Am J Pub Health. 1993;83:574–577. doi: 10.2105/ajph.83.4.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kemeny M E. In: Handbook of Human Stress and Immunity. Glaser R, Kiecolt-Glaser J, editors. San Diego: Academic; 1994. pp. 245–266. [Google Scholar]
- 10.Kemeny M E, Weiner H, Duran R, Taylor S E, Visscher B, Fahey J L. Psychosom Med. 1995;57:547–554. doi: 10.1097/00006842-199511000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Desrosiers R C. Annu Rev Microbiol. 1988;42:607–625. doi: 10.1146/annurev.mi.42.100188.003135. [DOI] [PubMed] [Google Scholar]
- 12.Lindburg D G. Science. 1969;166:1176–1178. doi: 10.1126/science.166.3909.1176. [DOI] [PubMed] [Google Scholar]
- 13.Alberts S C, Sapolsky R M, Altmann J. Horm Behav. 1992;26:167–178. doi: 10.1016/0018-506x(92)90040-3. [DOI] [PubMed] [Google Scholar]
- 14.Mendoza S P, Lyons D M, Saltzman W. Am J Primatol. 1991;23:37–54. doi: 10.1002/ajp.1350230105. [DOI] [PubMed] [Google Scholar]
- 15.Coe C L. Psychosom Med. 1993;55:298–308. doi: 10.1097/00006842-199305000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Laudenslager M L, Boccia M L, Berger C L, Gennaro-Ruggles M M, McFerran B, Reite M L. Dev Psychobiol. 1995;28:199–211. doi: 10.1002/dev.420280402. [DOI] [PubMed] [Google Scholar]
- 17.Cohen S, Kaplan J R, Cunnick J E, Manuck S B, Rabin B S. Psychol Sci. 1992;3:301–304. [Google Scholar]
- 18.Noldus L P J J. Behav Res Methods Instr Comp. 1991;23:415–429. [Google Scholar]
- 19.Capitanio J P. J Comp Psychol. 1985;99:133–144. [PubMed] [Google Scholar]
- 20.Anderson J H, Houghton P. Lab Anim. 1983;12:47–49. [Google Scholar]
- 21.de Savigny D, Voller A. In: Immunoenzymatic Assay Techniques. Malvano R, editor. The Hague, The Netherlands: Martinus Nijhoff; 1980. pp. 116–132. [Google Scholar]
- 22.Ansar Ahmed S, Gogal R M, Walsh J E. J Immunol Methods. 1994;170:211–224. doi: 10.1016/0022-1759(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 23.Pachl C, Todd J A, Kern D G, Sheridan P J, Fong S, Stempien M, Hoo B, Besemer D, Yeghiazarian T, Irvine B, et al. AIDS Res Hum Retroviruses. 1995;8:446–454. doi: 10.1097/00042560-199504120-00003. [DOI] [PubMed] [Google Scholar]
- 24.Yehuda R, Kahana B, Binder-Brynes K, Southwick S M, Mason J W, Giller E L. Am J Psychiatry. 1995;152:982–986. doi: 10.1176/ajp.152.7.982. [DOI] [PubMed] [Google Scholar]
- 25.Rook G A W, Onyebujoh P, Stanford J L. Immunol Today. 1993;14:568–569. doi: 10.1016/0167-5699(93)90190-V. [DOI] [PubMed] [Google Scholar]
- 26.Clerici M, Bevilacqua M, Vago T, Villa M L, Shearer G M, Norbiato G. Lancet. 1994;343:1552–1553. doi: 10.1016/s0140-6736(94)92944-0. [DOI] [PubMed] [Google Scholar]
- 27.Yehuda R, Boisoneau D, Lowy M T, Giller E L. Arch Gen Psychiatry. 1995;52:583–593. doi: 10.1001/archpsyc.1995.03950190065010. [DOI] [PubMed] [Google Scholar]
- 28.Munck A, Guyre P M. In: Psychoneuroimmunology. 2nd Ed. Ader R, Felten D L, Cohen N, editors. San Diego: Academic; 1991. pp. 447–474. [Google Scholar]
- 29.Gross W G, Siegel P B. Avian Dis. 1973;17:807–815. [PubMed] [Google Scholar]
- 30.Ghosh D. J Virol. 1992;66:586–590. doi: 10.1128/jvi.66.1.586-590.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soudeyns H, Geleziunas R, Shyamala G, Hiscott J, Wainberg M A. Virology. 1993;194:758–768. doi: 10.1006/viro.1993.1317. [DOI] [PubMed] [Google Scholar]
- 32.Markham P D, Salahuddin S Z, Veren K, Orndorff S, Gallo R C. Int J Cancer. 1986;37:67–72. doi: 10.1002/ijc.2910370112. [DOI] [PubMed] [Google Scholar]
- 33.Furth P A, Westphal H, Hennighausen L. AIDS Res Hum Retroviruses. 1990;6:553–560. doi: 10.1089/aid.1990.6.553. [DOI] [PubMed] [Google Scholar]
- 34.Lu W, Salerno-Goncalves R, Yuan J, Sylvie D, Han D, Andrieu J. AIDS. 1995;9:35–42. doi: 10.1097/00002030-199501000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Capitanio, J. P. & Lerche, N. W. (in press) Psychosom. Med. [DOI] [PubMed]