Abstract
Voltage-dependent Ca2+ currents evoke synaptic transmitter release. Of six types of Ca2+ channels, L-, N-, P-, Q-, R-, and T-type, only N- and P/Q-type channels have been pharmacologically identified to mediate action-potential-evoked transmitter release in the mammalian central nervous system. We tested whether Ca2+ channels other than N- and P/Q-type control transmitter release in a calyx-type synapse of the rat medial nucleus of the trapezoid body. Simultaneous recordings of presynaptic Ca2+ influx and the excitatory postsynaptic current evoked by a single action potential were made at single synapses. The R-type channel, a high-voltage-activated Ca2+ channel resistant to L-, N-, and P/Q-type channel blockers, contributed 26% of the total Ca2+ influx during a presynaptic action potential. This Ca2+ current evoked transmitter release sufficiently large to initiate an action potential in the postsynaptic neuron. The R-type current controlled release with a lower efficacy than other types of Ca2+ currents. Activation of metabotropic glutamate receptors and γ-aminobutyric acid type B receptors inhibited the R-type current. Because a significant fraction of presynaptic Ca2+ channels remains unidentified in many other central synapses, the R-type current also could contribute to evoked transmitter release in these synapses.
Biophysical and pharmacological analysis has led to the description of six classes of Ca2+ channels, usually referred to as L-, N-, P-, Q-, R-, and T-type (1, 2). Among them, N- and P/Q-type Ca2+ channels have been found to control transmitter release evoked by action potentials in most synapses examined so far (1, 2). In the presence of high concentrations of L-, N-, and P/Q-type channel blockers, a significant fraction (≈25%) of presynaptic Ca2+ channels remained unidentified at hippocampal (3) and cerebellar (4) synapses and in rat brain synaptosomes (5). These unidentified channels could be of R- or T-type. However, it has not been shown unequivocally that action potentials evoke transmitter release in the presence of saturating concentrations of L-, N-, and P/Q-type channel blockers (3, 4), even when release was enhanced by a train of stimulations (6) or by the K+ channel blocker 4-aminopyridine (7). In several other studies, combined application of the N-type channel blocker ω-conotoxin-GVIA and the P/Q-type blocker ω-agatoxin-IVA did not completely block synaptic transmission (8–10). In these studies, however, the concentration of ω-agatoxin-IVA was at most 100–200 nM, which is not sufficient to completely block Q-type channels (11, 12). In brief, whether Ca2+ channels other than N- and P/Q-type participate in controlling action-potential-evoked transmitter release is not known.
Recently, simultaneous presynaptic and postsynaptic recordings were made at single calyx-type synapses in rat medial nucleus of the trapezoid body (MNTB) (13, 14). At this axosomatic synapse, voltage control can be obtained for both the presynaptic Ca2+ current and the excitatory postsynaptic current (EPSC) (13, 15, 16). The large amplitude of the EPSC makes it very suitable for testing the effect of Ca2+ channel blockers on synaptic transmission. We used this synapse to test whether Ca2+ channels other than N- and P/Q-type are involved in action-potential-evoked synaptic transmission at the level of a single synapse. We found that about one-quarter of the presynaptic Ca2+ current is contributed by the R-type Ca2+ channel. This channel controls transmitter release with a lower efficacy than other types of Ca2+ channels.
METHODS
Electrophysiology.
Slices were cut from the brainstem of 8- to 10-day-old Wistar rats, transferred to a recording chamber, and perfused at room temperature (23–24°C) with a solution containing: 125 mM NaCl, 2.5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 25 mM dextrose, 1.25 mM NaH2PO4, 0.4 mM ascorbic acid, 3 mM myo-inositol, 2 mM sodium pyruvate, 25 mM NaHCO3, pH 7.4 when bubbled with 95% O2, 5% CO2 (13). Whole-cell current clamp recordings from terminals were made with an AxoClamp-2B amplifier (Axon Instruments) and glass pipettes (8–12 MΩ) containing: 115 mM potassium gluconate, 20 mM KCl, 4 mM MgATP, 10 mM Na2-phosphocreatine, 0.3 mM GTP, 10 mM Hepes, 0.05 mM fura-2 (Molecular Probes), pH 7.2, adjusted with KOH. Whole-cell voltage clamp recordings from postsynaptic cells were made with an Axopatch-200A amplifier. Pipettes had a resistance of 1.5–2 MΩ and were filled with the same solution as the pipettes used for presynaptic recordings, except they contained 0.5 mM EGTA instead of 50 μM fura-2. Series resistance in postsynaptic recordings (<15 MΩ) was always compensated to 98% (lag 10 μs).
Whole-cell Ca2+ current recordings from terminals were made with an Axopatch-200A amplifier and pipettes with a resistance of 4–6 MΩ that contained the same intracellular solution as used for current clamp recordings except that potassium was replaced by cesium. In addition, 1 μM tetrodotoxin and 0.1 mM 3,4-diaminopyridine (Sigma) were added to the extracellular solution and 20 mM NaCl was replaced with 20 mM tetraethylammonium chloride (Sigma) to block sodium and potassium channels (13). Unless mentioned otherwise, 2 mM Ca2+ was used as the charge carrier. Series resistance (<35 MΩ) compensation was set at 90%, with a lag of 10 μs, prediction was set at 60%. Subtraction of the passive response was by the P/-5 method. Terminals with a capacitance of less than 35 pF were selected to avoid long axons (16). The fura-2 fluorescence image was used to confirm the presynaptic origin of the recording at the end of the experiment. For the waveform command, we used the presynaptic action potential displayed in figure 2A of ref. 13, interpolated to 20 μs per point with a cubic spline. Potentials were corrected for a liquid junction potential of −11 mV between the extracellular and the pipette solution. Holding potential for voltage-clamp experiments was −80 mV, unless noted otherwise. Potentials or currents were low-pass filtered at 2–5 kHz and digitized at 20–50 kHz with a 16-bit analogue-to-digital converter (Instrutech, Greatneck, NY).
Optical Recordings.
The optical recording system was comprised of an upright epifluorescence microscope (Axioskop, Achroplan 40×, numerical aperture 0.75, Zeiss), equipped with a polychromatic illumination system (T.I.L.L. Photonics, Munich, Germany), a dichroic mirror (410 nm), a long pass (415 nm) emission filter, and two photodiodes on the image plane for signal and background subtraction, respectively (17). Excitation light was coupled to the microscope via a light guide. Fura-2 measurements of Ca2+ concentration were made by forming ratios between recordings (100–500 ms) of fluorescence (after background subtraction) at excitation wavelengths of 357 nm (isosbestic) and 380 nm (18). Rmax and Rmin, the parameters used for calculating Ca2+ concentrations, were obtained from in situ calibrations as described in ref. 19. A value of 273 nM for the Kd of fura-2 measured in situ was taken from ref. 19. Fluorescence signals recorded by a photodiode (Hamamatsu, Hamamatsu City, Japan) were filtered at 30 Hz (8-pole Bessel filter). Because the Ca2+ transient evoked by an action potential decays with a time constant of more than 400 ms in the presence of 50 μM fura-2 (19), filtering this transient at 30 Hz did not affect the measurement of its amplitude. Fura-2 was far from saturation during single action potentials, because (i) the peak Ca2+ concentrations evoked by single action potentials were usually less than the Kd of fura-2 measured in situ (19), and (ii) paired-pulse-action-potential stimulation (interval: 50 ms) caused approximately the same Ca2+ influx for each action potential (not shown).
For simultaneous presynaptic and postsynaptic recordings, only synapses in which the postsynaptic cells discharged an action potential in response to afferent stimulation were selected for recording (13). Single afferent stimuli were given via a bipolar electrode (3–30 V, 100 μs) placed at the midline of the trapezoid body. Stimulation interval was 20–30 sec.
Pharmacology.
The effect of a toxin was evaluated after it reached equilibrium (e.g., Fig. 1A). Data are expressed as mean ± SEM. If not mentioned in the text, the concentrations of ω-conotoxin-MVIIC (MVIIC) (Research Biochemicals, Natick, MA), ω-conotoxin-GVIA (Bachem), ω-agatoxin-IVA (a gift from N.A. Saccomano, Pfizer) and NiCl2 were 8 μM, 1 μM, 100 nM, and 100 μM, respectively. The metabotropic glutamate receptor agonist L-AP4 was purchased from Tocris Neuramin (Bristol, U.K.). The γ-aminobutyric acid type B (GABAB) receptor agonist (R)-(−)-baclofen was a gift from CIBA–Geigy. The toxins did not significantly affect the resting membrane potential, the action potential, and the resting Ca2+ concentration of the terminal (e.g., Fig. 1B). The toxins were applied in the presence of 0.1 mg/ml cytochrome c to block nonspecific binding sites. Cytochrome c did not significantly affect the EPSC (n = 16, P > 0.5), or presynaptic Ca2+ influx (n = 3, P > 0.5). Statistical significance was tested with a paired t test, unless noted otherwise.
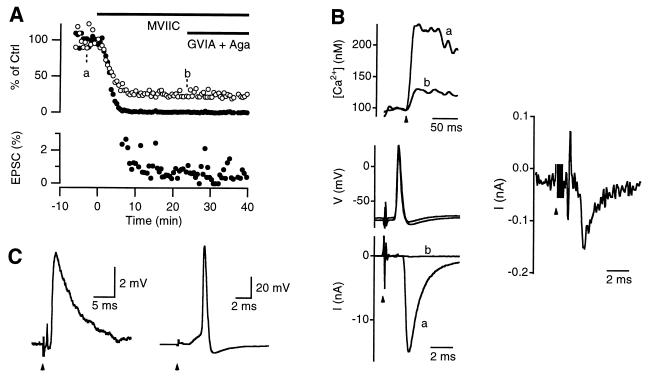
Figure 1.
The MVIIC-insensitive Ca2+ channel mediates action-potential-evoked transmitter release. (A) MVIIC (8 μM) reduced both the Δ[Ca2+] (○) and the EPSC (•) evoked by a single action potential. Additional application of ω-conotoxin-GVIA (GVIA, 1 μM) and ω-agatoxin-IVA (Aga, 100 nM) did not further reduce either the Δ[Ca2+] or the EPSC. The EPSC was clearly not abolished, as shown on a different amplitude scale (Lower). (B) Sample recordings of the presynaptic Ca2+ influx (Upper), presynaptic action potentials and the EPSCs (Lower) before (a) and after (b) MVIIC application, as indicated in A. The EPSC trace b was plotted on a different scale in the right. Arrow heads point to the time at which the stimulus was given (same for C). Stimulation artifacts were truncated (same for C). Note that each sample recording was taken from a single sweep (the same as in all the following figures). (C) Sample recordings of sub-threshold (Left) and supra-threshold (Right) excitatory postsynaptic potentials in the presence of MVIIC from a different principal cell. The resting membrane potential was −74 mV.
RESULTS AND DISCUSSION
Ca2+ Channels Other Than L-, N-, and P/Q-Type Contribute to Synaptic Transmission.
We studied the effect of different Ca2+ channel blockers on synaptic transmission in rat MNTB synapses in acute brainstem slices. Presynaptic action potentials were evoked by electrical stimulation of the afferent axon via a bipolar electrode positioned at the midline of the trapezoid body. Presynaptic Ca2+ influx (Δ[Ca2+]) was quantified with the Ca2+ indicator fura-2. Transmitter release was monitored by measuring the amplitude of the EPSC. Application of 8 μM MVIIC, a blocker of N- and P/Q-type Ca2+ channels (20, 21), reduced the Δ[Ca2+] to 26 ± 1% (n = 9) and the EPSC to 0.8 ± 0.1% (n = 7, Fig. 1A). This effect was reached about 10 min after the toxin application (Fig. 1A) and was largely irreversible during a washout period of up to 45 min (not shown). Because of the large size of the EPSC (>2 nA) in control solution, the EPSC that remained after MVIIC application was well resolved (Fig. 1 A, Lower, and B). This MVIIC-resistant synaptic current generated excitatory postsynaptic potentials, which often reached the threshold for initiation of an action potential in the postsynaptic neuron (Fig. 1C). These results show that the MVIIC-insensitive Ca2+ current evoked by a presynaptic action potential contributes to synaptic transmission.
The blocking effect of MVIIC was saturating at 8 μM, because application of high concentrations of the N-type channel blocker ω-conotoxin-GVIA (1 μM) and the P/Q-type blocker ω-Agatoxin-IVA (100 nM) did not further reduce either the Δ[Ca2+] or the EPSC (Fig. 1A). Application of MVIIC alone, MVIIC+Aga+GVIA, or Aga+GVIA reduced Δ[Ca2+] to 26 ± 1% (n = 9), 26 ± 2% (n = 8) or 27 ± 1% (n = 6), respectively. These blockers reduced the EPSC to 0.8 ± 0.1% (n = 7), 0.9 ± 0.2% (n = 4), or 0.7 ± 0.1% (n = 4), respectively. These effects were not significantly different (P > 0.5, ANOVA). In the presence of 8 μM MVIIC, addition of another 8 μM MVIIC did not further reduce the Δ[Ca2+] significantly (n = 3, P > 0.5, data not shown). Application of high concentrations of the L-type channel blockers nimodipine (10 μM) or nifedipine (10 μM) did not significantly change either the Δ[Ca2+] (n = 3, P > 0.5) or the EPSC (n = 3, P > 0.5, data not shown), suggesting that L-type Ca2+ channels are not present in the terminal. We conclude that presynaptic Ca2+ currents resistant to L-, N-, and P/Q-type blockers can be isolated by application of 8 μM MVIIC.
Recently, we found that the MVIIC-sensitive channels at the terminal of the MNTB synapse are subdivided into ω-conotoxin-GVIA sensitive N-type and ω-agatoxin-IVA sensitive P/Q-type channels (36). These two types of channels colocalize with MVIIC-insensitive channels at the same terminal (Fig. 1). These results are different from the results of Takahashi et al. (14), who found that on average 96% of the presynaptic Ca2+ current at the MNTB synapse is P/Q-type. The reason for this discrepancy is unknown.
About one-quarter of the presynaptic Ca2+ influx evoked by single action potentials is resistant to saturating concentrations of L-, N-, and P/Q-type channel blockers at hippocampal (3) and cerebellar (4) synapses. This unidentified Ca2+ influx does not evoke a clearly detectable synaptic response (3, 4, 6, 7). However, the signal-to-noise ratio of synaptic responses at these synapses was relatively low.
The Ca2+ Current Resistant to L-, N-, and P/Q-Type Blockers Is of R-Type.
To study the gating, conductance, and pharmacological properties of MVIIC-insensitive Ca2+ channels, terminals were voltage-clamped and presynaptic Ca2+ currents were pharmacologically isolated (13). In control conditions, the threshold for current activation was at around −40 mV and the maximum current was reached at between −10 and 0 mV (Fig. 2A). At −30 to −20 mV, the current activation had a fast and a slow component (e.g., Fig. 2A, Right), the latter of which became faster at more depolarized potentials (16). MVIIC reduced the Ca2+ current at all potentials (Fig. 2A). The slow activation component at −30 to −20 mV was abolished (Fig. 2A, n = 9), indicating that the gating of MVIIC-sensitive and -insensitive currents is different. At a test potential of −10 mV, MVIIC reduced the Ca2+ current to 26 ± 3% (n = 9) of control, similar to its effect on the Δ[Ca2+] during an action potential. Both activation (τ < 1 ms at −10 mV) and deactivation (τ < 1 ms at −80 mV) of the MVIIC-insensitive current were fast (Fig. 2A, see also Fig. 2C and Fig. 4).
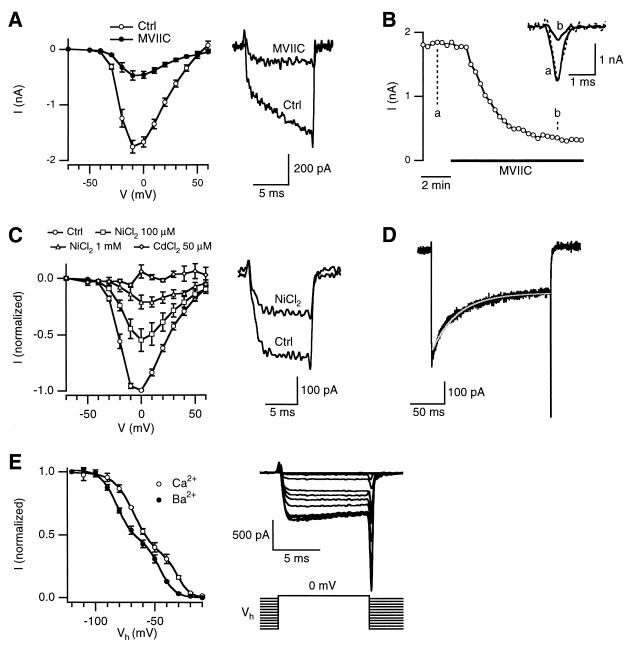
Figure 2.
The biophysical and pharmacological properties of the MVIIC-insensitive current. (A) Peak-current-voltage (I–V) relationship before (Ctrl) and after MVIIC (8 μM) application (Left, n = 9). Sample Ca2+ current recordings elicited at −30 mV before and after MVIIC application (Right). The holding potential was −80 mV with Ca2+ as the charge carrier (also in B and C). (B) Time course of the MVIIC block monitored by Ca2+ current evoked by an action potential waveform command. (Inset) Sample Ca2+ currents before (a) and after (b) MVIIC application. The dashed line is trace b scaled to trace a. (C) I–V relationship of the MVIIC-insensitive Ca2+ current before (ctrl) and after application of NiCl2 (100 μM, n = 4; 1 mM, n = 3) or CdCl2 (50 μM, n = 4). (Right) Sample currents elicited at −20 mV before (ctrl) and after application of 100 μM NiCl2. The experiments were performed in the presence of MVIIC (same for D and E). (D) Time course of inactivation of the MVIIC-insensitive Ba2+ current. The current was elicited by a 200-ms pulse to +10 mV with 2 mM Ba2+ as the charge carrier (black trace). The inactivation time course was fit with a single exponential curve with a time constant of 43 ms (grayish white trace). (E) Voltage-dependence of inactivation of the MVIIC-insensitive Ba2+ (n = 6) and Ca2+ (n = 4) currents (Left). The current (I) was elicited from various holding potentials (Vh = −120 to −10 mV) to 0 mV, and was normalized with respect to the peak current elicited from Vh = −120 mV. (Right) An example with Ba2+ as the charge carrier. The interval between each test pulse was 30 sec. The grouped data (Left) was fit with a sum of two Boltzmann expressions: I = C1/{1 + exp[(Vh − Vhalf1)/k1]} + (1 − C1)/{1 + exp[(Vh − Vhalf2)/k2]}. When Ba2+ was the charge carrier, C1 = 0.56, Vhalf1 = −82 mV, k1 = 7 mV, Vhalf2 = −46 mV and k2 =6 mV. For Ca2+ as the charge carrier, C1 = 0.66, Vhalf1 = −67 mV, k1 = 9 mV, Vhalf2 = −31 mV and k2 = 5 mV.
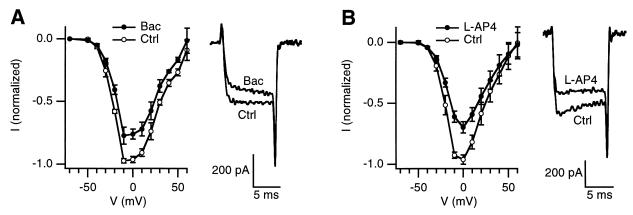
Figure 4.
Modulation of the R-type current by activation of GABAB or metabotropic glutamate receptors. I–V curves of the R-type current before (Ctrl) and after application of 20 μM baclofen (n = 4, A), or 100 μM L-AP4 (n = 4, B). Sample currents elicited at +10 mV before (Ctrl) and after drug application (Right, A and B). Experiments were performed in the presence of MVIIC.
During application of MVIIC, the effect of MVIIC was monitored by recording Ca2+ currents evoked by an action potential waveform command (ICa(AP), Fig. 2B) (15). At equilibrium, MVIIC reduced the amplitude of the ICa(AP) to 27 ± 4% (n = 3) without significantly affecting the waveform (measured as the half-width, P > 0.3, n = 3) of the ICa(AP) (Fig. 2B). This effect is very similar to the effect of MVIIC on Δ[Ca2+] (26 ± 1, n = 9), confirming that the fluorescence recording of Δ[Ca2+] is proportional to the peak ICa(AP) (17, 19).
Next, we studied the block of the MVIIC-insensitive current by NiCl2 and CdCl2. In the presence of MVIIC, 100 μM NiCl2 reduced the MVIIC-insensitive current at all test potentials, but with a stronger block at the more hyperpolarizing test potentials (Fig. 2C). At −10 mV, the MVIIC-insensitive current was reduced to 46 ± 8% (n = 4) of control (Fig. 2C). Interestingly, even at 1 mM, NiCl2 did not completely block the MVIIC-insensitive current (n = 3, Fig. 2C), suggesting that this current consists of two components, with one more sensitive to NiCl2 than the other (see also ref. 22). The MVIIC-insensitive current was abolished by 50 μM CdCl2 (n = 4, Fig. 2C).
The time course of inactivation of the MVIIC-insensitive current was studied with 2 mM Ba2+ rather than Ca2+ as the charge carrier to avoid possible Ca2+-dependent channel inactivation. The holding potential was at −100 mV instead of at −80 mV in these experiments to minimize inactivation (see below). The Ba2+ current inactivated with a time constant of 38–51 ms (mean = 44 ± 3 ms, 8 terminals) to 53 ± 1% of the peak current at the end of 200 ms pulses ranging from −30 to 30 mV (Fig. 2D). The time constant and the percentage of the current remaining at the end of the voltage pulse were not significantly different between these test potentials (P > 0.5, ANOVA). Voltage-dependence of steady-state inactivation of the MVIIC-insensitive current showed two components with the midpoints of inactivation at −82 and −46 mV (n = 6), respectively, when 2 mM Ba2+ was the charge carrier (Fig. 2E). When Ca2+ was the charge carrier, the midpoints of inactivation of both components shifted 15 mV toward more positive potentials (n = 4, Fig. 2E).
In summary, the Ca2+ current resistant to L-, N-, and P/Q-type channel toxins activated at around −40 mV, peaked at around −10 to 0 mV, deactivated fast, inactivated fast with significant inactivation at negative potentials, and was relatively sensitive to NiCl2 and CdCl2. These properties are similar to those of the R-type current in granule neurons of the rat cerebellum (11, 12), and the currents of the class E recombinant Ca2+ channel (11, 23, 24), which presumably mediates the R-type current (11, 12). In addition, these properties show that the MVIIC-insensitive current is not a T-type current, because the latter activates at more negative potentials, deactivates slowly, and is relatively resistant to cadmium block (25–27). Therefore, the MVIIC-insensitive current in terminals of MNTB can be classified as an R-type current. The steady-state inactivation curve (Fig. 2E) and the incomplete block by nickel (Fig. 2C) suggest two components of the R-type current, but the results are also consistent with the different rates of inactivation and sensitivities to the block by nickel reported for R-type currents at different preparations (12, 22, 28) and for the class E recombinant Ca2+ channels coexpressed with different β subunits (23, 29, 30).
R-Type Ca2+ Channels Control Transmitter Release with a Lower Efficacy.
Because the R-type current is sensitive to block by NiCl2 (Fig. 2C), we examined whether NiCl2 can be used as a selective blocker for the R-type current evoked by action potentials. The presynaptic Ca2+ influx evoked by single action potentials was recorded fluorometrically, and the effect of 100 μM NiCl2 on Δ[Ca2+] was compared in the absence and presence of MVIIC (Fig. 3A). The Δ[Ca2+] was reduced by 21 ± 1% (n = 13) and 17 ± 1% (n = 7), respectively (Fig. 3B, percentages are all relative to the total Δ[Ca2+] measured before addition of toxins). Thus, NiCl2 preferentially blocked R-type current with only 19% (= (21 − 17)/21) of its effect being on the MVIIC-sensitive currents.
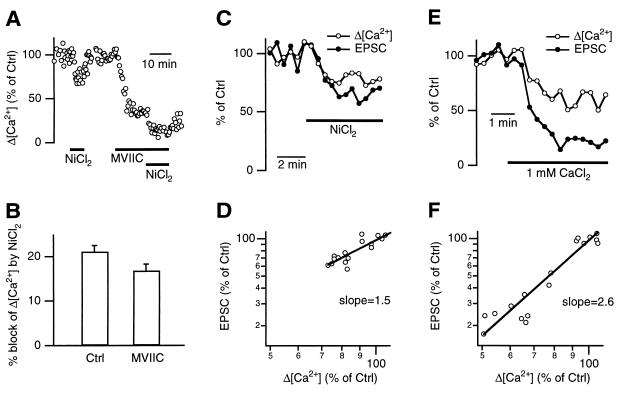
Figure 3.
R-type Ca2+ current mediates action-potential-evoked transmitter release with a lower efficacy. (A) NiCl2 (100 μM) reversibly reduced Δ[Ca2+] in control and in the presence of MVIIC in the same terminal. (B) Block of the Δ[Ca2+] by 100 μM NiCl2 in control (n = 13) and in the presence of MVIIC (n = 7). Percentages were normalized to values before drug application. (C) Application of 100 μM NiCl2 simultaneously reduced the EPSC and the Δ[Ca2+]. (D) EPSCs plotted against the Δ[Ca2+] from C on double logarithmic scales. The slope of the linear regression line was 1.5. (E and F) Similar plots as C and D, respectively, but with a decrease of the extracellular CaCl2 concentration from 2 to 1 mM by substitution with MgCl2. The slope of the regression line in F was 2.6.
Using NiCl2 as a selective blocker, we examined the efficacy of the R-type current in triggering release. The presynaptic Δ[Ca2+] and the EPSC from single synapses were recorded simultaneously. NiCl2 (100 μM) reduced both the Δ[Ca2+] and the EPSC (Fig. 3C). The relation between the EPSC and the Δ[Ca2+] (both in logarithmic scales) was fit with a linear regression line with a slope of 1.4 ± 0.1 (n = 4 pairs, Fig. 3D). In contrast, reducing the [Ca2+]o from 2 to 1 mM (substituted with Mg2+) resulted in a slope of 2.7 ± 0.2 (n = 5 synapses, Fig. 3 E and F), suggesting a cooperative mechanism for Ca2+ to trigger release (for review see refs. 1 and 31). The slope of 2.7 estimated here with paired recordings is lower than that determined by separate presynaptic and postsynaptic current recordings, which was about 4 (15). This difference is probably caused by some rundown of the EPSCs during recordings in which the EPSC amplitude was determined a long time (15–20 min) after the [Ca2+]o was changed (15). On average, NiCl2 reduced the EPSC by 36 ± 4% (n = 4). To estimate how much of this reduction is caused by a block of the MVIIC-sensitive current by NiCl2, the efficacy of the MVIIC-sensitive current needs to be measured. A slope of 3.3 ± 0.3 was observed in three simultaneous recordings (e.g., Fig. 1A, slope = 3.6) in which MVIIC was applied (not shown). The high efficacy of the MVIIC-sensitive channels in triggering release is consistent with measurements in many other synapses (see ref. 31 for review). If NiCl2 would reduce the EPSC only by inhibiting the MVIIC-sensitive current, it would reduce, on average, the EPSC by only 13% (100% − [100% − (21% − 17%)]3.3). This is much smaller than the reduction of the EPSC by NiCl2 that we observed (36%). Thus, R-type currents mediate action-potential-evoked transmitter release to a significant amount, but with a lower efficacy than other Ca2+ currents.
The slope measured with application of NiCl2 (1.4 ± 0.1) was significantly (P < 0.01, t test) smaller than that obtained with lowering [Ca2+]o (2.7 ± 0.2) or with application of MVIIC (3.3 ± 0.3), suggesting a lower efficacy of R-type channels in controlling release. The mechanism underlying this observation is unclear. It is not caused by a difference in the Ca2+ current time course, because the kinetics of the R-type current evoked by action potential waveform commands was not significantly different from the total current (Fig. 2B). The location of the R-type Ca2+ channels at terminals might be important. For example, R-type channels could be located further away from the release sites than MVIIC-sensitive channels.
R-Type Current Is Inhibited by Activation of Presynaptic Metabotropic Glutamate Receptors and GABAB Receptors.
Neurotransmitters and neuromodulators have been shown to cause presynaptic inhibition of evoked transmitter release primarily by inhibition of Ca2+ channels (see ref. 31 for review). Presynaptic inhibition may be a mechanism causing synaptic depression (31). To explore whether the R-type current is a target for presynaptic inhibition, we studied the effect of the GABAB receptor agonist baclofen (20 μM, Fig. 4A, n = 4) and the metabotropic glutamate receptor agonist L-AP4 (100 μM, Fig. 4B, n = 4). Both substances partially inhibited the R-type current (Fig. 4). Baclofen induced a slow phase in the activation of the R-type current (sample traces in Fig. 4A, n = 4), similar to G-protein-mediated modulation (32) of the α1E current (33). Our results demonstrate direct modulation of presynaptic Ca2+ currents by activation of GABAB receptors.
In summary, we found that about one-quarter of the Ca2+ current evoked by a presynaptic action potential was carried by R-type current in MNTB synapses (Figs. 1 and 2). This current evokes transmitter release with a low efficacy (Fig. 3) and is modulated by neurotransmitters (Fig. 4), suggesting that it is a target for presynaptic inhibition of transmitter release. Because R-type channels control fast transmitter release with a lower efficacy than MVIIC-sensitive channels, they may contribute preferentially to mechanisms involving slower cytoplasmic calcium signaling, such as paired-pulse facilitation (34), posttetanic potentiation (34), and mobilization of synaptic vesicles (35). These findings may be of general significance because a significant fraction of presynaptic Ca2+ channels remained unidentified in a number of other synapses (3–5).
Acknowledgments
We thank Drs. J. M. Bekkers and A. Reyes for helpful comments and Dr. W. Jarolimek for providing baclofen. This work was supported by the Alexander von Humboldt Foundation and Human Frontier Science Program to L.G.W. and a Training and Mobility of Researchers fellowship to J.G.G.B.
ABBREVIATIONS
- MNTB
medial nucleus of the trapezoid body
- EPSC
excitatory postsynaptic current
- MVIIC
ω-conotoxin-MVIIC
- ICa(AP)
Ca2+ currents evoked by an action potential waveform command
- GABAB
γ-aminobutyric acid type B
References
- 1.Dunlap K, Luebke J I, Turner T J. Trends Neurosci. 1995;18:89–98. [PubMed] [Google Scholar]
- 2.Reuter H. Curr Opin Neurobiol. 1996;6:331–337. doi: 10.1016/s0959-4388(96)80116-4. [DOI] [PubMed] [Google Scholar]
- 3.Wu L G, Saggau P. J Neurophysiol. 1995;73:1965–1972. doi: 10.1152/jn.1995.73.5.1965. [DOI] [PubMed] [Google Scholar]
- 4.Mintz I, Sabatini B L, Regehr W G. Neuron. 1995;15:675–688. doi: 10.1016/0896-6273(95)90155-8. [DOI] [PubMed] [Google Scholar]
- 5.Turner T J, Lampe R A, Dunlap K. Mol Pharmacol. 1995;47:348–353. [PubMed] [Google Scholar]
- 6.Castillo P E, Weisskopf M G, Nicoll R A. Neuron. 1994;12:261–269. doi: 10.1016/0896-6273(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 7.Wheeler D B, Randall A, Tsien R W. J Neurosci. 1996;16:2226–2237. doi: 10.1523/JNEUROSCI.16-07-02226.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luebke J, Dunlap K, Turner T J. Neuron. 1993;11:895–902. doi: 10.1016/0896-6273(93)90119-c. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi T, Momiyama A. Nature (London) 1993;366:156–158. doi: 10.1038/366156a0. [DOI] [PubMed] [Google Scholar]
- 10.Regehr W G, Mintz I. Neuron. 1994;12:605–613. doi: 10.1016/0896-6273(94)90216-x. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J-F, Randall A D, Ellinor P T, Horne W A, Sather W A, Tanabe T, Schwarz T L, Tsien R W. Neuropharmacology. 1993;32:1075–1088. doi: 10.1016/0028-3908(93)90003-l. [DOI] [PubMed] [Google Scholar]
- 12.Randall A, Tsien R W. J Neurosci. 1995;15:2995–3012. doi: 10.1523/JNEUROSCI.15-04-02995.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borst J G G, Helmchen F, Sakmann B. J Physiol. 1995;489:825–840. doi: 10.1113/jphysiol.1995.sp021095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi T, Forsythe I D, Tsujimoto T, Barnes-Davies M, Onodera K. Science. 1996;274:594–597. doi: 10.1126/science.274.5287.594. [DOI] [PubMed] [Google Scholar]
- 15.Borst J G G, Sakmann B. Nature (London) 1996;383:431–434. doi: 10.1038/383431a0. [DOI] [PubMed] [Google Scholar]
- 16.Borst J G G, Sakmann B. J Physiol. 1998;506:143–157. doi: 10.1111/j.1469-7793.1998.143bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borst J G G, Helmchen F. In: Methods in Enzymology. Conn P M, editor. Vol. 295. San Diego: Academic; 1998. , in press. [DOI] [PubMed] [Google Scholar]
- 18.Grynkiewicz G, Poenie M, Tsien R Y. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 19.Helmchen F, Borst J G G, Sakmann B. Biophys J. 1997;72:1458–1471. doi: 10.1016/S0006-3495(97)78792-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hillyard D R, Monje V D, Mintz I, Bean B P, Nadasdi L, Ramachandran J, Miljanich G, Azimi-Zoonooz A, Mcintosh J M, Cruz L J, et al. Neuron. 1992;9:69–77. doi: 10.1016/0896-6273(92)90221-x. [DOI] [PubMed] [Google Scholar]
- 21.McDonough S I, Swartz K J, Mintz I M, Boland L M, Bean B P. J Neurosci. 1996;16:2612–2623. doi: 10.1523/JNEUROSCI.16-08-02612.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tottene A, Moretti A, Pietrobon D. J Neurosci. 1996;16:6353–6363. doi: 10.1523/JNEUROSCI.16-20-06353.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soong T W, Stea A, Hodson C D, Dubel S J, Vincent S R, Snutch T P. Science. 1993;260:1133–1136. doi: 10.1126/science.8388125. [DOI] [PubMed] [Google Scholar]
- 24.Williams M E, Marubio L M, Deal C R, Hans M, Brust P F, Philipson L H, Miller R J, Johnson E C, Harpold M M, Ellis S B. J Biol Chem. 1994;269:22347–22357. [PubMed] [Google Scholar]
- 25.Fox A P, Nowicky M C, Tsien R W. J Physiol. 1987;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armstrong C M, Matteson D R. Science. 1985;227:65–67. doi: 10.1126/science.2578071. [DOI] [PubMed] [Google Scholar]
- 27.Kaneda M, Akaike N. Brain Res. 1989;497:187–190. doi: 10.1016/0006-8993(89)90987-6. [DOI] [PubMed] [Google Scholar]
- 28.Yu B, Shinnick-Gallagher P. J Neurophysiol. 1997;77:690–701. doi: 10.1152/jn.1997.77.2.690. [DOI] [PubMed] [Google Scholar]
- 29.Olcese R, Qin N, Schneider T, Neely A, Wei X, Stefani E, Birnbaumer L. Neuron. 1994;13:1433–1438. doi: 10.1016/0896-6273(94)90428-6. [DOI] [PubMed] [Google Scholar]
- 30.Stephens G J, Page K M, Burley J R, Berrow N S, Dolphin A C. Pflügers Arch. 1997;433:523–532. doi: 10.1007/s004240050308. [DOI] [PubMed] [Google Scholar]
- 31.Wu L G, Saggau P. Trends Neurosci. 1997;20:204–212. doi: 10.1016/s0166-2236(96)01015-6. [DOI] [PubMed] [Google Scholar]
- 32.Hille B. Trends Neurosci. 1994;17:531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 33.Mehrke G, Pereverzev A, Grabsch H, Hescheler J, Schneider T. FEBS Lett. 1997;408:261–270. doi: 10.1016/s0014-5793(97)00437-7. [DOI] [PubMed] [Google Scholar]
- 34.Kamiya H, Zucker R S. Nature (London) 1994;371:603–606. doi: 10.1038/371603a0. [DOI] [PubMed] [Google Scholar]
- 35.Heinemann C, von Rüden L, Chow R H, Neher E. Pflügers Arch. 1993;424:105–112. doi: 10.1007/BF00374600. [DOI] [PubMed] [Google Scholar]
- 36.Wu L-G, Borst J G G, Sakmann B. Soc Neurosci Abstr. 1997;23:365. [Google Scholar]