Abstract
The capacity for neuromodulation and biophysical plasticity is a defining feature of most mature neuronal cell types. In several cases, modulation at the level of the individual neuron has been causally linked to changes in the functional output of a neuronal circuit and subsequent adaptive changes in the organism’s behavioral responses. Understanding how such capacity for neuromodulation develops therefore may provide insights into the mechanisms both of neuronal development and learning and memory. We have examined the development of multiple forms of neuromodulation triggered by a common neurotransmitter, serotonin, in the pleural sensory neurons of Aplysia californica. We have found that multiple signaling cascades within a single neuron develop sequentially, with some being expressed only very late in development. In addition, our data suggest a model in which, within a single neuromodulatory pathway, the elements of the signaling cascade are developmentally expressed in a “retrograde” manner with the ionic channel that is modulated appearing early in development, functional elements in the second messenger cascade appearing later, and finally, coupling of the second messenger cascade to the serotonin receptor appearing quite late. These studies provide the characterization of the development of neuromodulation at the level of an identified cell type and offer insights into the potential roles of neuromodulatory processes in development and adult plasticity.
Over the past two decades, significant progress has been made in elucidating the cellular and molecular mechanisms underlying behavioral plasticity and learning in a wide variety of experimental systems. One of the general principles that has emerged from this work is that the modulation of ionic conductances and the resultant impact on synaptic efficacy plays an integral role in determining the functional output of a neuronal circuit (1). Analyzing the developmental emergence of such biophysical plasticity has the potential to provide insights into both the basic principles of neuronal development and the functional significance of plasticity in the adult nervous system.
We have combined the advantages of a “simple systems” approach with the power of a developmental analysis to examine the ontogenetic emergence of modulation in the sensory neurons that mediate the defensive withdrawal reflexes of the marine mollusc, Aplysia californica. Specifically, we have analyzed the development of serotonin (5HT)-induced increased excitability and spike broadening in identified tail sensory neurons in the pleural ganglia. The facilitatory neurotransmitter 5HT is known to have multiple effects on the biophysical properties of mature tail sensory neurons (see Fig. 4C): it produces an increase in input resistance, a depolarization of membrane potential, an increase in somatic excitability, and an increase in action potential duration (2, 3). The effects of 5HT on action potential duration are mediated primarily through decreases in the magnitude of the delayed rectifier potassium conductance (IKV; refs. 4 and 6) produced by modulation of its activation and inactivation rates (4, 5). The kinetic properties of IKV, including its relatively rapid activation (peak current amplitude achieved within 20–30 msec) and high voltage dependency, are consistent with it playing a major role in repolarization of the action potential (5, 7–10). Decreasing the magnitude of IKV therefore results in a significant increase in the duration of the action potential. However, because IKV is not active at rest, it does not contribute to the resting input conductance or membrane potential. At a biochemical level, the effect of 5HT on IKV has been shown to be mediated primarily by activation of protein kinase C (PKC), which, through phosphorylation of ion channels or closely associated proteins, reduces the macroscopic IKV current (6, 11, 12).
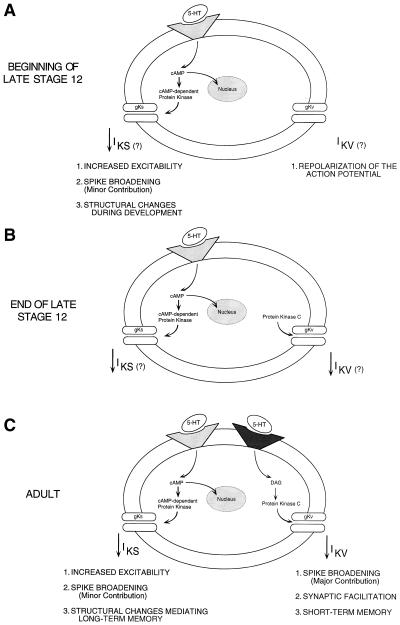
Figure 4.
A model for the sequential development of the 5HT-activated signaling cascades in the tail sensory neurons. The subcellular pathway that links 5HT to modulation of excitability is fully expressed at the youngest stage of development that has been examined. Conversely, maturation of the subcellular pathway that links 5HT to spike broadening occurs through the final stage of juvenile development. For the effects of 5HT on excitability and spike duration in adult sensory neurons (C), the ionic currents that mediate these two forms of neuromodulation have been well characterized (IKS and IKV, respectively). Moreover, the second messenger systems that link the 5HT receptor to modulation of the ionic conductances have been characterized (PKA and PKC, respectively). Although we have not directly assayed identified ionic currents in juvenile sensory neurons, we hypothesize (indicated by question marks in A and B) that the modulation of ionic conductances underlying spike broadening and increased excitability are similar to those characterized in the adult. The posited developmental sequence of the pathway responsible for major spike broadening is illustrated in the three steps of the model. In the first step, the ionic conductance is expressed but cannot yet be modulated (A). In the second step, the conductance can be modulated by the second messenger but the second messenger cannot be activated by the neurotransmitter (B). In the final step, the neurotransmitter is now able to activate the second messenger and thereby complete the pathway (C).
The effects of 5HT on input resistance, membrane potential, and excitability are mediated by a reduction in the 5HT-sensitive potassium current, IKS (13, 14). The properties of IKS have been characterized in detail at the macroscopic level: it is a slowly activating (peak current amplitude occurring at 100–120 msec), relatively voltage-independent K+ current that does not inactivate (9, 10). The time and voltage dependence of IKS are thus consistent with its effects on input resistance and excitability; because it is active at rest and slowly activating, it contributes to both the resting potential and the accommodation of firing rate. However, because of the kinetic properties of IKS it provides a relatively minor contribution to spike broadening compared with IKV¶ (4, 6, 11, 12). Computer simulations of the relative contributions of IKS and IKV modulation to the increase in action potential duration have demonstrated that in mature sensory neurons 5HT-induced decreases in IKS contribute approximately 25% to the overall increase in action potential duration produced by 5HT whereas reduction in IKV contributes the remaining 75% (15). Biochemically, the effects of 5HT on IKS are mediated by elevations in cAMP and the resultant activation of protein kinase A (PKA) (4, 13). Thus, there are parallel signaling cascades mediating the effects of 5HT on spike duration and increased excitability with one acting predominantly through PKC and IKV and the other through PKA and IKS (see Fig. 4C).
MATERIALS AND METHODS
Animals.
All staged juvenile Aplysia were obtained from the University of Miami Aplysia facility. The life cycle of Aplysia is divided into five phases: embryonic, planktonic, metamorphic, juvenile, and adult (16). The embryonic phase lasts 10 days, at the end of which the animals hatch out of the egg case as free-swimming veliger larvae. The larval or planktonic phase lasts 34 days, at the end of which the larvae settle on a specific species of algae and metamorphose. After a brief metamorphic phase (2–3 days), the animal enters the juvenile phase, which lasts 90 days and ends with the onset of sexual maturity that marks the beginning of the adult phase. The juvenile phase has been subdivided into four stages (stages 9–12), which can be distinguished on the basis of changes in the external morphology of the animal (16). Stage 12, the last stage of juvenile development, lasts about 60 days and is characterized by the emergence of a genital groove. Because of the length of stage 12, this stage has been further subdivided into early, mid, and late based on the weight of the animal (17).
Surgery.
Animals were anesthetized with an injection of isotonic MgCl2 into the hemocoel and then were pinned ventral side up in a Sylgard-coated Petri dish. An incision was made along the midline of the foot to expose the central nervous system (CNS). The pedal-pedal and pleural-pleural connectives were transected, and the cerebral ganglion was cut in half to allow the right and left pleural ganglia to be placed in separate, small Sylgard-coated Petri dishes in isotonic artificial seawater (460 mM NaCl/10.4 mM KCl/11 mM CaCl2/55 mM MgCl2/10 mM Trizma, pH 7.6). Each pleural ganglion remained attached to the ipsilateral cerebral and pedal ganglia via the connectives; these connections were maintained to allow for appropriate stabilization for recording. The ganglia were pinned (dorsal side up) with minutien pins through the pedal and cerebral ganglia, the cerebral-pleural connective, and the pleuro-abdominal connective. The ganglia then were treated with a nonspecific pronase (Dispase, Boehringer Manheim) for 1 hr (2 mg/ml) and washed for 1 hr. This treatment was designed to chemically soften the sheath. Fine pulled micropipettes then were used to mechanically remove the sheath overlying the region of the pleural sensory neurons.
Intracellular Recording.
Standard intracellular recording techniques were used. Sensory neurons were impaled with borosilicate capillary glass microelectrodes (20–45 MΩ) filled with 3 M KCl. Voltage records were amplified by an Axoclamp (model 2A) preamplifier and then simultaneously displayed on a dual-beam oscilloscope (Tektronix model 511A) and a computer (Leading Edge) connected to a data analysis system (Spike, Hilal Associates, Englewood Cliffs, NJ). The data also were recorded on an FM tape recorder (Hewlett Packard model 3964A) for subsequent analysis. Sensory neurons were identified on the basis of their position, lack of synaptic input, silent intrinsic firing pattern, characteristic action potential waveform, and accommodation of firing rate to prolonged current injection. Neuronal excitability was quantified by measuring the number of spikes elicited by a 200-msec depolarizing constant-current pulse. The magnitude of the current pulse was determined empirically so as to elicit 1–3 spikes in the baseline condition; the current then remained constant throughout each experiment. In each experiment a stable baseline consisting of three sequential measurements was established before the delivery of any pharmacological agents. Current pulses were delivered at a rate of 1/min or 1/30 sec. Modulation of excitability was measured by comparing the average spike number elicited by three sequential current injections in the presence of the pharmacological agent with the average obtained in the baseline.
Action potentials were triggered at a frequency of 1 per min with a suprathreshold 2-msec current injection; the frequency and duration of the current injection was controlled by a Grass stimulator (S88). Spike duration was measured on the repolarization phase of the spike as the time from the peak to one-third of peak voltage by the automated data acquisition system. Experiments examining the effect of 5HT on spike duration were carried out in a continuously perfused chamber. After the establishment of a stable baseline recording, which included three consecutive stable action potential durations, 50 μM of 5HT was applied through the perfusion system. The perfusion rate was approximately 5 ml/min. The three consecutive maximum spike durations in 5HT were averaged and compared with the average of the three baseline durations obtained in artificial seawater. The concentration of 5HT was chosen based on the known effective concentration for adult pleural sensory neurons (4, 5). Experiments examining the effect of direct activation of PKC were carried out in a static bath recording chamber to minimize drug solution volumes. After the establishment of a stable baseline spike duration, 100 μl of a 100 μM solution of 12–13 phorbol diactetate (Sigma) was added to a standing 3 ml bath for a final phorbol diacetate (PDAc) concentration of 3 μM. The concentration of PDAc was selected based on the known effective concentrations for adult pleural sensory neurons (11). Spike duration in the presence of PDAc was measured 1/min for approximately 20 min.
Biochemical Assays.
Activation of adenylate cyclase was measured by using a protocol adapted from Salomon (19) and modified by Abrams et al. (20) and was based on the detection of the incorporation of labeled 32P into cAMP. The CNS of animals from stages 11, early 12, late 12, and adult were mechanically homogenized in 50 mM Tris buffer. For each juvenile stage, several animals were dissected and the CNSs were pooled in the homogenization procedure. For stage 11, 20–25 CNSs were pooled; for early stage 12, 10–15; for late stage 12, 3–5; adult CNSs were assayed individually. The homogenates were centrifuged at 2,000 rpm on an Eppendorf 5415 for 5 min at 4°C to remove large particulate matter. Each assay was carried out in triplicate. The reaction was carried out in a solution consisting of 10 μl of assay mixture [25 mM creatine phosphate/250 units/ml of creatine phosphokinase/125 mM Tris acetate/25 mM MgCl2/0.167 mM ATP/0.25 mM cAMP/5 mM DTT/0.5 mg/ml BSA/0.05 mM GTP/32P-labeled ATP (2 × 107 cpm)], 10 μl of Tris buffer, and 5 μl of 5HT or small cardioactive peptide (SCP). The concentration of 5HT or SCP was varied to provide an estimate of the linearity of the cyclase stimulation; for each homogenate, parallel reactions also were carried out in the absence of transmitter to assess basal cyclase activity. Homogenate samples were added to the reaction mixture, and the reaction was allowed to proceed for 15 min in 30°C water bath. The reaction then was arrested with the addition of 100 μl of stopping solution (2% sodium lauryl sulfate/45 mM ATP/1.3 mM cAMP). Tritiated cAMP (50 μl) was added to each reaction tube to allow for an assessment of the efficiency of the column purification. The reaction tubes were boiled for 3 min and allowed to cool. The samples then were purified over a Dowex affinity column to remove unincorporated labeled ATP. The cAMP-containing fraction then was eluted off the Dowex column onto the alumina column with 0.1 M imidazole. Finally, the cAMP was eluted off the alumina column into scintillation vials with imidazole. The vials were counted simultaneously on 3H and 32P channels. The efficiency of the purification process was determined by comparing the 3H-cAMP counts with the known amount of 3H-cAMP added to the reaction solution just before the purification. The 32P-labeled cAMP counts then were adjusted according to the efficiency of each column to provide a measure of the amount of labeled cAMP produced in the reaction. Preliminary results of these experiments appeared in abstract form (21).
Statistical Analyses.
Group data were analyzed by using a standard Student’s t test for independent or pairwise measures. All P values are two-tailed.
RESULTS
Properties of Juvenile Sensory Neurons.
To examine the developmental emergence of biophysical plasticity in the tail sensory neurons, we have characterized the effects of 5HT on excitability and spike duration in juvenile Aplysia. As a first step in this analysis we compared the fundamental electrical properties of adult and juvenile sensory neurons. Juvenile sensory neurons had a mean resting potential of −37 mV +/−1.8, which was not significantly different from that observed in adults (mean = 38.4 mV +/− 1.9). Likewise, juvenile sensory neurons had a mean action potential duration (1.48 msec +/− 0.1), which was not significantly different from the duration of the adult action potential (mean = 1.44 +/− 0.2). Although juvenile and adult sensory neurons had comparable resting potentials and action potential durations, they did differ significantly in their input resistance: juvenile sensory neurons mean = 94.7 mΩ +/− 8.9; adult sensory neurons mean = 39.7 +/− 4.9, P < 0.001. This difference presumably reflects the smaller size of sensory neurons in juvenile animals.
5HT-Induced Excitability Is Present in Juvenile Sensory Neurons.
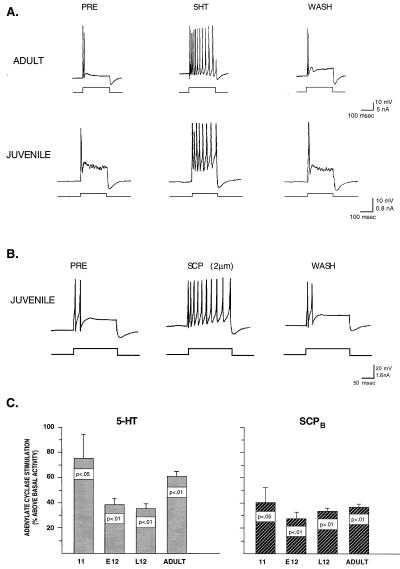
In our first series of experiments, we examined the ability of 5HT to produce increases in excitability in the tail sensory neurons of late stage 12 juveniles. In the baseline condition, the firing rate of both adult and juvenile sensory neurons accommodates within the first 50 msec of a prolonged depolarizing pulse (Fig. 1A). This accommodation reflects at least in part the delayed activation of IKS, which develops across the initial phase of the depolarization and acts to inhibit prolonged spike activity. In the presence of 50 μM 5HT, the excitability of the sensory neurons dramatically increases in both adult and juvenile sensory neurons, and both fire throughout the duration of the injected current pulse. A statistical analysis of juvenile sensory neurons revealed a significant increase in excitability in the presence of 5HT (mean increase = 133%, P < 0.004). This increase is comparable in magnitude to that observed in adult sensory neurons with 50 μM 5HT. For example, Baxter and Byrne (4) have characterized in detail the effect of this same concentration of 5HT on excitability in adult sensory neurons and report an 80–130% increase. Thus, by late stage 12 5HT is capable of producing an increase in excitability equivalent in magnitude to that observed in adults. These data are consistent with the hypothesis that by the end of juvenile development the signaling cascade that links 5HT to modulation of IKS (see Fig. 4C) is mature, including expression of the 5HT receptor, the second messenger PKA, and the ionic conductance itself. As a further test of this hypothesis, we examined the effects of another modulatory neurotransmitter, SCP, which is known to modulate excitability in mature tail sensory neurons by the same signaling pathway as 5HT (18). There is no evidence in adult Aplysia that SCP modulates IKV or stimulates PKC; therefore its modulatory effects on sensory neurons are limited to a subset of 5HT’s effects. We found that SCP applied to sensory neurons of late stage 12 juveniles also produced dramatic increases in excitability (Fig. 1B). Thus it appears that SCP, like 5HT, is capable of activating PKA and modulating IKS in late stage 12. Finally, as a direct test of the ability of 5HT and SCP to activate PKA in juvenile Aplysia, we performed biochemical analyses to measure stimulated cAMP activity in whole CNS homogenates (16) and found that both transmitters are able to produce significant increases in cAMP activity over basal levels as early as stage 11 (Fig. 1C). Taken collectively, these data demonstrate that at least one of the neuromodulatory pathways activated by 5HT in mature Aplysia sensory neurons is present and functional by the end of the juvenile phase of development, and may in fact be expressed even earlier.
Figure 1.
In sensory neurons from juvenile Aplysia, 5HT-induced activation of PKA and modulation of IKS appears to be fully mature as indicated by the significant increase in excitability that is identical in magnitude to that observed in mature sensory neurons (A, see text). In addition, as in the adult CNS, 5HT is capable of producing significant increases above basal levels in adenylate cyclase activity in the pleural ganglia as early as stage 11. SCP (2 μm), a neuromodulatory peptide, which is known in adult Aplysia to activate PKA and modulate IKS, is also capable of producing increases in excitability and increases in adenylate cyclase activity in juvenile neurons.
5HT-Induced Spike Broadening Emerges at the End of Juvenile Development.
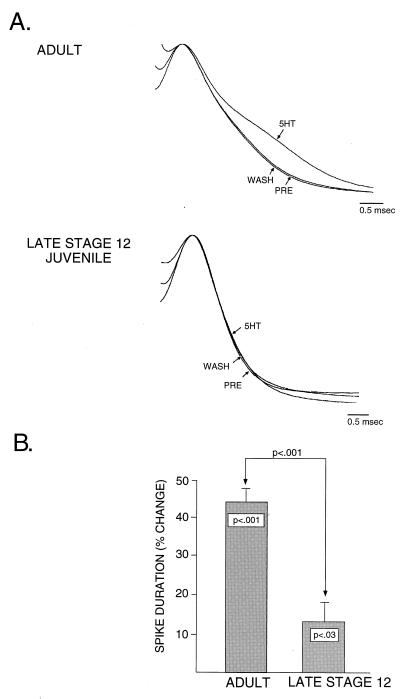
Having established that 5HT is capable of producing an increase in excitability in late stage 12 juveniles that is comparable in magnitude to that observed in adults, we next examined the ability of 5HT to produce changes in action potential duration at this same developmental stage. Fig. 2 shows the effects of 5HT on spike duration in mature and juvenile sensory neurons. Typically, the magnitude of broadening observed in response to 50 μM 5HT in adults is 45%. Approximately 25% of this increase (which in the case of 50 μM 5HT amounts to a 10–12% increase in spike duration) is estimated to be caused by a PKA-mediated reduction in IKS; the remaining 75% of the increase is estimated to be caused by a PKC-dependent reduction in IKV (15). In the example shown in Fig. 2A, in late stage 12, the same concentration of 5HT has little effect on spike duration, suggesting the hypothesis that 5HT is incapable of modulating one or more of the currents responsible for repolarizing the action potential (e.g., IKV) at this stage. A pooled comparison of adult pleural sensory neurons and late stage 12 sensory neurons (Fig. 2B) demonstrates two important points. First, in late stage 12, 5HT produces a modest, but significant, increase in action potential duration (approximately 12%). This degree of spike broadening is consistent with the magnitude of spike broadening attributable to 5HT-induced decreases in IKS (see Fig. 4C). This observation is again consistent with our earlier results examining increases in excitability (Fig. 1), which demonstrated that by late stage 12 the ability of 5HT to modulate IKS appears relatively mature. Second, the amount of spike broadening in late stage 12 is significantly less than that observed in adults, demonstrating that some aspect of the signaling cascade linking 5HT to modulation of the outward currents responsible for repolarization (e.g., IKV) is not yet functionally expressed at this stage of development. Thus, at the end of the juvenile phase, well after the tail sensory neurons have acquired many of their adult characteristics, they still do not possess the full mature complement of neuromodulatory pathways.
Figure 2.
In juvenile sensory neurons, 5HT is not able to produce the full adult complement of spike broadening, suggesting that 5HT-induced activation of PKC and modulation of repolarizing currents such as IKV are not yet developmentally expressed (A). In comparing group data (B) from adult sensory neurons (n = 5) and late stage 12 juvenile sensory neurons (n = 13), there is a small but significant average increase in action potential duration in juvenile neurons of 12%. The magnitude of this increase corresponds to the increase typically caused by modulation of IKS. In the adult sensory neurons, there is approximately a 42% increase in action potential duration in response to the same regimen of 5HT application, consistent with 5HT producing modulation of both IKS and IKV. Notably, there is a significant difference between the amount of spike broadening produced in juvenile and adult sensory neurons (B).
PKC-Induced Spike Broadening Emerges Before 5HT-Induced Spike Broadening.
The absence of 5HT-induced spike broadening in late stage 12 juveniles could be caused by the delayed developmental expression of one or more steps in the transduction of a neuromodulatory signal. First, it is possible that the plasma membrane receptors necessary to transduce the 5HT signal are not yet expressed. Although it is clear that late stage 12 sensory neurons are capable of responding to 5HT based on the mature 5HT-induced increases in excitability that we observe, the possibility exists that a second subtype of 5HT receptor is linked selectively to PKC activation and is expressed developmentally only after late stage 12. This possibility is particularly interesting in light of data from adult tail sensory neurons demonstrating two pharmacologically distinct types of 5HT receptor, one that appears to be coupled to PKA activation and one that is coupled to PKC activation (22). Second, it is possible that one or more of the delayed rectifier potassium conductances is not yet expressed at this stage. This does not appear to be the case because, as described above, the action potential duration and wave form at this stage is not significantly different from that observed in mature sensory neurons, suggesting that the major repolarizing currents are fully expressed. In other systems in which the development of IKV has been directly examined, changes in the expression of IKV have been associated with marked changes in action potential duration (23–25). In addition, preliminary experiments using whole-cell patch clamp to examine the macroscopic currents present in dissociated tail sensory neurons of late stage 12 juveniles suggest that there is an outwardly rectifying potassium current with similar voltage dependence and activation kinetics to the well-characterized adult IKV (data not shown). This leaves open a third possibility, that some aspect of the signal transduction cascade that links activation of the 5HT receptor to modulation of the repolarizing currents (e.g., the delayed rectifier potassium channel) is missing at this stage. Specifically, drawing on what is known about 5HT-induced modulation of IKV in mature sensory neurons, 5HT may be incapable of activating PKC or alternatively, PKC may be incapable of modulating IKV.
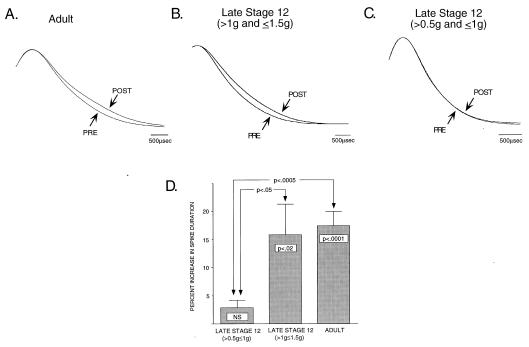
To test whether the lack of 5HT-induced spike broadening in late stage 12 is caused by an inability of 5HT to activate PKC or an inability of PKC to modulate repolarizing currents, we have used direct pharmacological activation of PKC with phorbol esters at a stage when 5HT does not produce the major PKC-dependent component of spike broadening, to determine whether it is possible to bypass the requirement for 5HT activation of PKC and produce spike broadening at this stage. Fig. 3 illustrates the effects of bath application of a phorbol ester (PDAc) to sensory neurons of adult and late stage 12 juvenile Aplysia. Consistent with earlier reports (11) in mature sensory neurons, 3 μM PDAc produces a significant increase in action potential duration (Fig. 3A, P < 0.001) while having no effect on excitability (data not shown). Previous work in adult sensory neurons has shown that PDAc-induced increases in action potential duration are blocked by the PKC inhibitor staurosporine, indicating that the phorbol ester effect in the sensory neurons is mediated by activation of PKC (11). PDAc-induced spike broadening of identical magnitude is produced in sensory neurons of juveniles at the end of late stage 12 (Fig. 3B). These data suggest that the inability of 5HT to produce spike broadening at this stage is because of an inability of 5HT to activate PKC. To further explore the developmental expression of PKC-mediated spike broadening, we also examined sensory neurons from younger juvenile animals (as determined by body weight). As shown in Fig. 3C, it appears that the ability of PKC to modulate repolarizing currents emerges during late stage 12, because in the first half of late stage 12, direct activation of PKC with PDAc does not produce spike broadening. A summary comparison of the effects of PDAc at all three stages examined is presented in Fig. 3D. At the earliest stage, the beginning of late stage 12, PDAc produces no significant increase in action potential duration. By the end of late stage 12, PDAc produces significant spike broadening (P < 0.02) that is statistically indistinguishable from that observed in adults.
Figure 3.
In adult sensory neurons, direct activation of PKC by application of the phorbol ester PDAc produces a significant increase in action potential duration consistent with a resultant modulation of IKV (A and D). By the end of late stage 12, at a time when 5HT is not able to produce the adult complement of spike broadening, PDAc produces significant broadening that is identical in magnitude to that observed in mature neurons (B and D). These data suggest that the inability of 5HT to modulate repolarizing currents such as IKV is because of an inability of 5HT to activate PKC. At the beginning of late stage 12, even direct activation of PKC is unable to produce significant spike broadening (C and D), demonstrating that PKC is not able to modulate the ion channel protein(s) responsible for repolarization at this earlier developmental stage.
DISCUSSION
Our data provide two insights into the development of neuromodulatory pathways. First, by comparing the developmental expression of 5HT-induced increases in excitability and 5HT-induced increases in spike duration, we have shown that multiple signaling cascades activated in the same neuron by a single neurotransmitter can develop according to different timetables. Our data suggest the hypothesis that as early as we have looked, the 5HT–cAMP–IKS cascade is in place and appears mature whereas the 5HT–PKC–IKV cascade does not emerge until much later. A similar temporal dissociation in the development of 5HT activation of PKA- and PKC-dependent pathways has been demonstrated for the development of 5HT responsiveness in the Retzius cell of the leech (26), suggesting that there may be a conserved function for the sequence of expression of PKA- and PKC-mediated pathways in development, perhaps in the regulation of outgrowth and neuronal morphogenesis (27).
A second important principle that can be inferred from our results is that, within a single neuromodulatory pathway, the elements comprising the signaling cascade may be developmentally assembled in a “retrograde” manner, counter to the normal flow of information in the pathway, that is, from ion channel back to receptor (Fig. 4). From this perspective, the complete pathway would be assembled in a series of three sequential steps. The first step is expression of the channels mediating the ionic conductance, which we posit are expressed early in development to serve the function of repolarizing the action potential. The second step is the development of the ability to modulate the ionic conductance with a second messenger (in this case PKC). In the youngest late stage 12 animals examined, phorbol esters, which directly activate PKC, do not produce spike broadening. This result suggests that in at the beginning of late stage 12, repolarizing currents, although functionally expressed, are not modulated by the second messenger. Slightly later in development, at the end of late stage 12, phorbol esters are capable of producing the full adult amount of spike broadening. Based on these observations, we postulate that there is a developmental switch in the properties of the channels carrying the repolarizing currents that endows them with the capacity for modulation. Precedents for such developmental regulation has been demonstrated for ligand-gated conductances such as the nicotinic acetylcholine and glutamate receptors (28). The third and final step of the model is the development of the ability to activate the second messenger with a neurotransmitter (in this case 5HT). Surprisingly, even as late as the end of juvenile development, 5HT is unable to produce the full adult amount of spike broadening. Because direct activation of PKC by phorbol esters does produce adult-like spike broadening at this stage, we postulate that the second messenger PKC is now capable of modulating the ionic conductance, although the neurotransmitter is not yet capable of activating the second messenger. Thus the last step in the assembly of the subcellular cascade would be the developmental expression of either a specific 5HT receptor subtype that activates PKC or a specific PKC subtype that is capable of being activated by 5HT.
In addition to clear implications for developmental issues, our analysis of the differential expression of 5HT-induced spike broadening and excitability also has potentially important implications for the cellular and molecular mechanisms of learning and memory in adult Aplysia. In light of the known role of the 5HT–PKA–IKS pathway in mediating long-term structural and functional changes in adult sensory neurons (29–33), our observation that this pathway is expressed early in development suggests that the mechanisms of long-term memory storage in the mature CNS represent a maintained subset of mechanisms involved in neuronal development. This possibility is consistent with the intriguing view, first articulated by Ramon y Cajal (34) and subsequently proposed for a wide range of systems (35–41), that development and learning may be mechanistically related. Finally, given that 5HT-induced activation of the PKC pathway and spike broadening emerges quite late in development, it will be of considerable interest to determine the functional significance of this major form of neuromodulation, including its possible contributions to late-emerging forms of learning and memory.
Acknowledgments
We thank TeNing Chang and Yadin Dudai for invaluable help in performing the biochemical assays of cAMP activity. This work was supported by National Institutes of Health Grant R01-MH-14-1083 and National Science Foundation Grant BNS 8614961 to T.J.C. and a National Institutes of Health predoctoral fellowship to E.A.M.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: 5HT, serotonin; IKV, delayed rectifier potassium current; IKS, 5HT-sensitive potassium current; PKC, protein kinase C; PKA, protein kinase A; CNS, central nervous system; SCP, small cardioactive peptide; PDAc, phorbol diacetate.
It is important to point out that under the appropriate conditions, spike broadening can be mediated by a modulation of IKS. Specifically, in experiments in which IKV is blocked pharmacologically with tetraethylammonium (TEA) the relative contribution of IKS to repolarization is enhanced; thus changes in the duration of TEA-broadened spikes reflect IKS modulation. (For general discussion see ref. 6.)
References
- 1.Kaczmarek L K, Levitan I B. Neuromodulation: The Biochemical Control of Neuronal Excitability. Oxford: Oxford Univ. Press; 1987. [Google Scholar]
- 2.Klein M, Hochner B, Kandel E R. Proc Natl Acad Sci USA. 1986;83:7994–7998. doi: 10.1073/pnas.83.20.7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stark L L, Mercer A R, Emptage N J, Carew T J. J Neurophys. 1996;75:855–866. doi: 10.1152/jn.1996.75.2.855. [DOI] [PubMed] [Google Scholar]
- 4.Baxter D A, Byrne J H. J Neurophysiol. 1990;64:978–990. doi: 10.1152/jn.1990.64.3.978. [DOI] [PubMed] [Google Scholar]
- 5.Baxter D A, Byrne J H. J Neurophysiol. 1989;62:665–679. doi: 10.1152/jn.1989.62.3.665. [DOI] [PubMed] [Google Scholar]
- 6.Byrne J H, Kandel E R. J Neurosci. 1996;16:425–435. doi: 10.1523/JNEUROSCI.16-02-00425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams D J, Gage P W. J Physiol (London) 1980;304:297–313. doi: 10.1113/jphysiol.1980.sp013325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams D J, Smith S J, Thompson S H. Annu Rev Neurosci. 1980;3:141–167. doi: 10.1146/annurev.ne.03.030180.001041. [DOI] [PubMed] [Google Scholar]
- 9.Klein M, Camardo J S, Kandel E R. Proc Natl Acad Sci USA. 1982;79:5713–5717. doi: 10.1073/pnas.79.18.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pollock J D, Bernier L, Camardo J S. J Neurosci. 1985;5:1862–1871. doi: 10.1523/JNEUROSCI.05-07-01862.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugita S, Goldsmith J R, Baxter D A, Byrne J H. J Neurophysiol. 1992;68:643–651. doi: 10.1152/jn.1992.68.2.643. [DOI] [PubMed] [Google Scholar]
- 12.Goldsmith B A, Abrams T W. Proc Natl Acad Sci USA. 1992;89:11481–11485. doi: 10.1073/pnas.89.23.11481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siegelbaum M J, Camardo J S, Kandel E R. Nature (London) 1982;299:413–417. doi: 10.1038/299413a0. [DOI] [PubMed] [Google Scholar]
- 14.Pollock J D, Camardo J S. Brain Res. 1987;410:367–370. doi: 10.1016/0006-8993(87)90340-4. [DOI] [PubMed] [Google Scholar]
- 15.White J A, Baxter D A, Byrne J H. Biophys J. 1994;66:710–718. doi: 10.1016/s0006-3495(94)80845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kriegstein A R. J Exp Zool. 1977;199:275–288. doi: 10.1002/jez.1401990212. [DOI] [PubMed] [Google Scholar]
- 17.Nolen T G, Carew T J. J Neurosci. 1988;8:212–222. doi: 10.1523/JNEUROSCI.08-01-00212.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abrams T W, Castellucci V F, Camardo J S, Kandel E R, Lloyd P E. Proc Natl Acad Sci USA. 1984;81:7956–7960. doi: 10.1073/pnas.81.24.7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salomon Y. Adv Cyclic Nucleotide Res. 1979;10:35–55. [PubMed] [Google Scholar]
- 20.Abrams T, W, Karl K A, Kandel E R. J Neurosci. 1991;11:2655–2665. doi: 10.1523/JNEUROSCI.11-09-02655.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang T N, Marcus E A, Dudai Y, Carew T J. Soc Neurosci Abstr. 1989;15:1019. [Google Scholar]
- 22.Mercer A R, Emptage N J, Carew T J. Science. 1991;254:1811–1813. doi: 10.1126/science.1662413. [DOI] [PubMed] [Google Scholar]
- 23.Barish M E. J Physiol. 1986;375:229–250. doi: 10.1113/jphysiol.1986.sp016114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Dowd D K, Ribera A B, Spitzer N C. J Neurosci. 1988;8:792–805. doi: 10.1523/JNEUROSCI.08-03-00792.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spitzer N C. J Neurobiol. 1991;22:659–673. doi: 10.1002/neu.480220702. [DOI] [PubMed] [Google Scholar]
- 26.Lessmann V, Dietzel I D. J Neurosci. 1991;11:800–809. doi: 10.1523/JNEUROSCI.11-03-00800.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mattson M P, Taylor-Hunter A, Kater S B. J Neurosci. 1988;8:1704–1711. doi: 10.1523/JNEUROSCI.08-05-01704.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuetze S M, Role L W. Annu Rev Neurosci. 1987;10:403–457. doi: 10.1146/annurev.ne.10.030187.002155. [DOI] [PubMed] [Google Scholar]
- 29.Bailey C H, Chen M. Science. 1983;220:91–93. doi: 10.1126/science.6828885. [DOI] [PubMed] [Google Scholar]
- 30.Bailey C H, Chen M. Proc Natl Acad Sci USA. 1988;85:2373–2377. doi: 10.1073/pnas.85.7.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dash P K, Hochner B, Kandel E R. Nature (London) 1990;345:718–721. doi: 10.1038/345718a0. [DOI] [PubMed] [Google Scholar]
- 32.Schacher S, Castellucci V F, Kandel E R. Science. 1988;240:1667–1669. doi: 10.1126/science.2454509. [DOI] [PubMed] [Google Scholar]
- 33.Nazif F, Byrne J H, Cleary L J. Brain Res. 1991;539:324–327. doi: 10.1016/0006-8993(91)91638-h. [DOI] [PubMed] [Google Scholar]
- 34.Cajal S R. Histologie du système nerveux de l’homme et des vertébrés. Paris: Maloine; 1911. [Google Scholar]
- 35.Goelet P, Castellucci V F, Schacher S, Kandel E R. Nature (London) 1986;322:419–422. doi: 10.1038/322419a0. [DOI] [PubMed] [Google Scholar]
- 36.Greenough W T, Bailey C H. Trends Neurosci. 1988;11:142–147. [Google Scholar]
- 37.Kandel E R, O’Dell T J. Science. 1992;258:243–245. doi: 10.1126/science.1411522. [DOI] [PubMed] [Google Scholar]
- 38.Marcus E A, Emptage N J, Marois R, Carew T J. Prog Brain Res. 1994;100:179–188. doi: 10.1016/s0079-6123(08)60784-0. [DOI] [PubMed] [Google Scholar]
- 39.Pfenninger K H. Trends Neurosci. 1986;9:562–565. [Google Scholar]
- 40.Rose S P R. Trends Neurosci. 1991;14:390–397. doi: 10.1016/0166-2236(91)90027-r. [DOI] [PubMed] [Google Scholar]
- 41.Wolpaw J R, Schmidt J T, Vaughn T M. Ann NY Acad Sci. 1991;627:1–398. [Google Scholar]