Abstract
The molecular mechanisms underlying long-term potentiation in the hippocampus have received much attention because of the likely functional importance of synaptic plasticity for information storage and the development of neuronal connectivity. Surprisingly, it remains unclear whether activity modifies the strength of individual synapses in a digital (all-or-none) or analog (graded) manner. Here we characterize step-like all-or-none transitions from baseline synaptic transmission to potentiated states following protocols for inducing potentiation at putative single CA3-CA1 synaptic connections. Individual synapses appear to have all-or-none potentiation indicative of highly cooperative processes but different thresholds for undergoing potentiation. These results raise the possibility that some forms of synaptic memory may be stored in a digital manner in the brain.
There is increasing evidence that activity-dependent synaptic plasticity plays an important role in certain forms of learning and memory as well as in the development of neural circuitry. In part because of its experimental accessibility and reliability, the most intensively studied form of synaptic plasticity has been long-term potentiation (LTP) in the CA1 region of the hippocampus. A major research effort by many investigators has addressed the involvement of specific intracellular signaling cascades in this phenomenon as well as the pre- and postsynaptic modifications that may be responsible for it (1–4). Despite this intense effort, an important question that has not been addressed is whether the LTP that occurs at individual synapses is graded or all-or-none. That is, is synaptic strength at individual synapses controlled by activity in an analog or digital manner? The answer to this question has important mechanistic implications for the types of molecular changes that should be considered when examining the mechanisms responsible for LTP. Of equal importance are the theoretical implications of digital vs. analog control of synaptic strength for the mechanisms by which information is stored in the pattern of synaptic weights in a distributed neural network.
To address this issue, we have attempted to determine whether the N-methyl-d-aspartate (NMDA) receptor-dependent potentiation of synaptic efficacy at putative single synapses onto CA1 pyramidal neurons occurs in a graded or all-or-none manner. Our approach to investigating this question was first to define protocols that would induce apparently graded amounts of potentiation in cells upon which many synapses were simultaneously stimulated. Analysis of the potentiation evoked by the same protocols in cells where putative single fiber inputs were stimulated could then be used to determine if the apparently graded nature of the potentiation was due to graded potentiation at single synapses or alternatively whether single synapses exhibit all-or-none potentiation with individual synapses showing different thresholds for potentiation.
METHODS
Transverse hippocampal slices (500 μm) were prepared from 10- to 14-day-old Sprague–Dawley rats. Slices were incubated at room temperature in a medium containing 119 mM NaCl, 26 mM NaHCO3, 10 mM d-glucose, 2.5 mM KCl, 2.5 mM CaCl2, 1.3 mM MgCl2, and 1 mM NaH2PO4 equilibrated with 95% O2 and 5% CO2. Slices were then transferred to an immersion type recording chamber superfused with a solution containing 119 mM NaCl, 26 mM NaHCO3, 10 mM d-glucose, 2.5 mM KCl, 2 mM CaCl2, 1 mM NaH2PO4, 1 mM MgCl2, and 0.1 mM picrotoxin (0.04% dimethyl sulfoxide) equilibrated with 95% O2 and 5% CO2. Experiments were done at room temperature. Whole-cell patch-clamp recordings were made from CA1 neurons with the “blind” recording technique (5) after making a cut between the CA3 and CA1 regions. Patch electrodes were filled with 120 mM Cs-gluconate, 15 mM CsCl, 10 mM HEPES, 5 mM NaCl, 2 mM Mg3ATP2, 0.3 mM Na3GTP, and 0.2 mM Cs-EGTA (pH 7.2). Stimulating electrodes were placed in stratum radiatum close to the cell body layer and Schaffer collateral/commissural fibers were stimulated at a frequency of 1 Hz. To maximize the stability of evoked excitatory postsynaptic current (EPSCs), stimulation began several minutes before entering the whole-cell configuration. For experiments involving activation of a population of fibers bipolar stainless steel electrodes were used and for minimal stimulation experiments patch pipettes filled with the standard extracellular solution were used. To identify putative single synaptic connections using minimal stimulation, the stimulus strength was gradually reduced until no EPSCs were detected and then increased until at a sharp threshold synaptic responses were reliably evoked with a low probability (around 0.5) of transmission. Data were collected with an Axopatch-1D amplifier, filtered at 1 kHz, sampled at 5 kHz, and analyzed on-line as described (6). EPSC amplitudes were measured by using a 2-ms window at the peak of the event relative to the baseline taken immediately before the stimulus artifact. The magnitude of the potentiation was calculated by comparing the average size of the EPSCs for the 50 events immediately prior to pairing to the average size of the EPSCs for the 50 events beginning 50 sec after the pairing. All results are expressed as mean ± SEM.
RESULTS
EPSCs were recorded with the whole-cell patch-clamp technique from CA1 pyramidal cells voltage-clamped at −80 mV and synapses were stimulated at 1 Hz. To induce potentiation, the postsynaptic cell was depolarized to ≈0 mV and held at this potential while maintaining the stimulation frequency constant, a procedure referred to as “pairing.” We initially used relatively large synaptic inputs to find a pairing protocol that would reliably induce a small, nonsaturating amount of potentiation. An example of one of these experiments is shown in Fig. 1A. At 200 s, the cell was depolarized to 0 mV for 10 stimuli, a procedure that resulted in a small amount of potentiation. To ensure that this protocol did not induce a saturating level of potentiation, a more prolonged pairing procedure (100 stimuli at 0 mV) was performed starting at 400 s and this resulted in substantially more potentiation. Potentiation was not observed in separate interleaved experiments carried out in the presence of the NMDA receptor antagonist d-2-amino-5-phosphoonovaleric acid (D-APV) (Fig. 1B). These interleaved experiments were averaged together and are shown in Fig. 1C (n = 10 in the absence and n = 10 in the presence of D-APV). Fig. 1D (n = 18) plots the individual results of all the experiments (those from the interleaved experiments in the absence of D-APV and an additional eight cells). The results in Fig. 1 demonstrate that 10 pairings induce a small, subsaturating level of potentiation that is dependent on the activation of NMDA receptors.
Figure 1.
Potentiation of EPSCs evoked by activation of multiple synapses is graded and dependent on NMDA receptor activation. (A) The amplitude of EPSCs evoked by stimulation of many synapses can be potentiated in a graded manner. Thus pairing 10 stimuli with postsynaptic depolarization evoked a small amount of potentiation and subsequent pairing of 100 stimuli evoked further potentiation. Averages of 50 consecutive current traces from the baseline period, following 10 pairings and following 100 pairings are superimposed (Right). The response to a −2 mV hyperpolarization is shown prior to the synaptic response. (B) EPSCs are not potentiated by pairing 10 stimuli and 100 stimuli in the presence of 100 μM D-APV. Averages of 50 stimuli from the baseline period, following 10 pairings and following 100 pairings are superimposed (Right). (C) Summary of experiments showing 35 ± 2% (n = 10) potentiation with 10 pairings and a further 43 ± 2% (n = 10) potentiation with 100 pairings (•). Potentiation is completely blocked in paired experiments carried out in the presence of 100 μM D-APV (n = 10, □). (D) The potentiation observed following pairing of 10 stimuli and the additional potentiation evoked by pairing 100 stimuli are plotted for each individual experiment (n = 18).
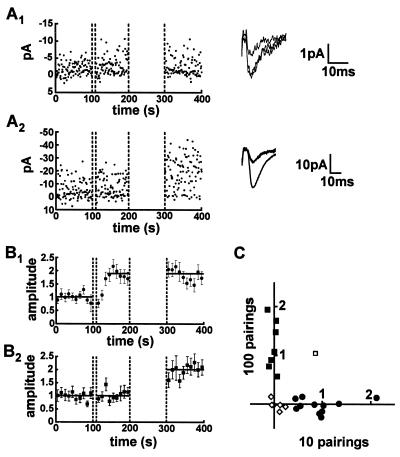
Having defined parameters for inducing small amounts of potentiation, we next repeated the above experiments by using minimal stimulation techniques in an attempt to activate single fibers. To maximize the occurrence of activating single synaptic sites we used young animals (10–14 days) because the number of synapses made by a single axon onto a single postsynaptic cell is considerably less than in older animals (7). The results fell clearly into three categories: (i) Potentiation (defined as >25% increase in EPSC amplitude) was observed following 10 pairings, in which case we were unable to generate further potentiation with 100 pairings (Fig. 2 A1 and B1). (ii) No potentiation was observed after 10 pairings, but could be observed after 100 pairings (Fig. 2 A2 and B2). (iii) No potentiation could be generated with either 10 or 100 pairings and thus the synapse appeared to be incapable of undergoing potentiation. The data from each cell in this series of experiments is plotted in Fig. 2C. Except for one cell, all cells capable of expressing potentiation either exhibited potentiation with 10 pairings and no further potentiation with 100 pairings (•), or exhibited no potentiation with 10 pairings but did express potentiation with 100 pairings (▪). Finally, six cells (◊) failed to show any significant potentiation with either the short or long pairing protocol. Importantly, the potentiation observed with 100 pairings (98 ± 7%, n = 7) (Fig. 2B2) was no different from the potentiation observed with 10 pairings (88 ± 7%, n = 11) (Fig. 2B1). These results are consistent with the hypothesis that potentiation occurs in an all-or-none manner, because once a synapse had undergone potentiation it was unable to exhibit further potentiation. Additionally the experiments demonstrate that individual synapses appear to have different “thresholds” for potentiation, because only a subset of synapses became potentiated following 10 pairings. This finding provides an explanation for the apparently graded nature of the potentiation observed when multiple synapses were monitored (Fig. 1).
Figure 2.
Potentiation is all-or-none at presumed single synaptic connections. (A) The amplitude of EPSCs evoked by minimal stimulation was monitored in a baseline period, following 10 pairings and 100 pairings. (A1) In some experiments potentiation of EPSC amplitude was evoked with 10 pairings but no further potentiation was induced with a subsequent pairing of 100 stimuli. Averages of 50 consecutive EPSCs in baseline, following 10 pairings and following 100 pairings are superimposed (Right). (A2) In other experiments no potentiation of EPSC amplitude was observed following 10 pairings, but potentiation could be evoked by subsequent pairing of 100 stimuli. Averages of 50 consecutive EPSCs from the baseline period, following 10 pairings and following 100 pairings are superimposed (Right). (B) The experiments using minimal stimulation were separated into two groups. (B1) One group contained the experiments showing potentiation following 10 pairings (88 ± 7%, n = 11) but did not show further potentiation when 100 stimuli were paired (−2 ± 4%, n = 11). It thus appears that once a synapse becomes potentiated it is no longer capable of further potentiation, suggesting that potentiation is an all-or-none event. (B2) The other group of experiments showed no potentiation with 10 pairings (4 ± 6%, n = 7) but did show potentiation when 100 stimuli were paired (98 ± 7%, n = 7). (C) The potentiation observed following pairing of 10 stimuli and additional potentiation evoked by 100 pairings are plotted for each individual experiment (n = 25). Eleven experiments showed potentiation only in response to the first 10 pairings (•, equivalent to the experiments grouped together in B1). Seven experiments showed no potentiation with 10 pairings but did show potentiation with 100 pairings (▪, equivalent to the experiments grouped together in B2). In six experiments (◊) no potentiation was observed in response to 10 pairings or 100 pairings and only in one experiment (□) was potentiation observed in response to both 10 pairings and 100 pairings.
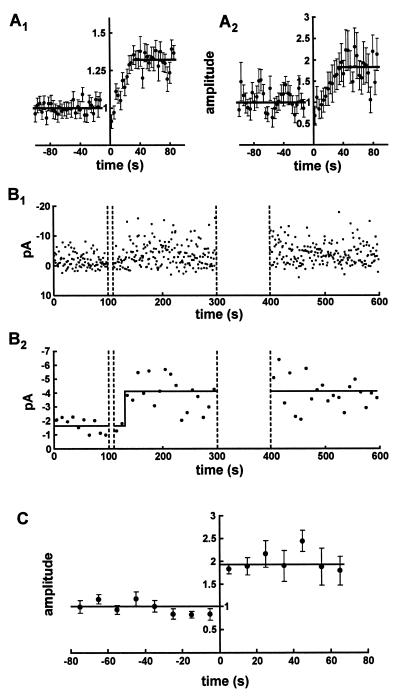
We next examined the time course for the development of potentiation. With large inputs there was a gradual growth of the EPSCs following 10 pairings and the potentiation reached half maximal after about 20 s (Fig. 3A1) in agreement with previously published data (8). When all of the minimal stimulation experiments showing potentiation following 10 pairings were averaged together a similar time course was seen (Fig. 3A2). Does this time course averaged over many synapses reflect the time it takes for potentiation to occur at an individual synapse? In the minimal stimulation experiment illustrated in Fig. 3B1 there appears to be a delay after the 10 pairings before potentiation occurs. When every 10 responses are averaged together (Fig. 3B2) the delay is more obvious and, in addition, there appears to be a relatively abrupt transition to the potentiated state. To examine this conclusion further 10-response average EPSC amplitudes were plotted for all the minimal stimulation experiments in which potentiation occurred following the pairing of 10 stimuli. This data was then aligned by setting time zero as the time when potentiation first occurred. Time zero was operationally defined as the time at which 4 of 5 consecutive data points following the pairing had values greater than half of the potentiated state. Importantly, this never occurred during the baseline itself. It is clear (Fig. 3C) that the potentiation at putative single sites is considerably more abrupt than seen with the simple population average (Fig. 3A2). These results suggest that there is a variable delay to the onset of potentiation following the pairing protocol, but that when potentiation occurs it is complete within 10 s.
Figure 3.
Although potentiation develops gradually when examined with multiple synapses, it develops in a step-like fashion with a variable latency when examined at presumed single synapses. (A) Time course of the development of potentiation following the pairing of 10 stimuli in experiments with a large synaptic input (A1, n = 28) and in experiments with minimal stimulation (A2, n = 11). In both experimental protocols the time to half maximum potentiation following the pairing is ≈20 s. (B) Amplitude of individual EPSCs evoked by minimal stimulation (B1) and 10-s averages of EPSC amplitudes for the same experiment (B2). After pairing 10 stimuli with postsynaptic depolarization the EPSC amplitude initially remains unchanged, but after a latent period of around 20 s larger amplitude EPSCs are suddenly observed. Subsequent pairing of 100 stimuli did not evoke further potentiation. (C) The zero time of experiments similar to that shown in the lower part of B was recalculated such that time zero indicates the time where the transition from unpotentiated to potentiated states was thought to occur (22 ± 3 s after end of pairing). The aligned data traces were then averaged (n = 11) and show that a step-like transition occurs from the unpotentiated to the potentiated state. (For details of alignment procedure see text.)
A limitation with the above experiments is that the pairing protocol consisted of depolarizing the cell for 10 consecutive stimuli, making it impossible to monitor synaptic transmission during this period. Thus it is conceivable that the potentiation was graded during the induction of the potentiation. To examine more closely the change in synaptic strength during the induction protocol, we performed another series of experiments during which we paired every tenth stimulus with depolarization. This permitted us to monitor synaptic strength following each pairing event. Fig. 4A shows that when a large input is used and multiple synapses are monitored, this pairing protocol resulted in the gradual development of potentiation that reached a value of 53 ± 2% (n = 16). When the identical procedure was carried out in the presence of the NMDA receptor antagonist D-APV (100 μM), no potentiation was observed (3 ± 2%, n = 7). When this protocol was performed by using minimal stimulation, in 8 of 20 experiments, a potentiation occurred with some delay following the start of the pairing procedure and once it occurred rapidly reached a stable level (Fig. 4B). When the time of the potentiation in each experiment was set at time zero, using the same criteria as described for the experiments in Fig. 3, the potentiation again appeared to be abrupt and step-like (Fig. 4C). These observations support the previous set of findings and suggest that, depending on the state of the synapse, an abrupt (within 10 s) and stable potentiation can be induced following a few pairings. Individual synapses appear to have different “thresholds” for potentiation because the number of pairings before potentiation was observed ranged from 3 to 15 (i.e., from ≈30–150 s after the first pairing).
Figure 4.
Potentiation is all-or-none at presumed single synapses when induced by pairing every tenth stimuli. (A) EPSCs evoked by stimulation of many synapses can be potentiated (53 ± 2%, n = 16) by pairing of every tenth stimuli with postsynaptic depolarization. This allows the simultaneous monitoring of synaptic transmission while attempting to induce potentiation. The mean time to reach a half-maximal potentiation measured from the first paired stimulus was 101 ± 14 s. (B) The amplitude of EPSCs evoked by minimal stimulation could also be potentiated by pairing every tenth stimuli with postsynaptic depolarization. B1 graphs the amplitude of each response. The average of nine EPSCs immediately before and after the transition from unpotentiated to potentiated state is shown to the right. B2 shows 9-s averages of EPSC amplitude between depolarizations from the same experiment. Following a delay of around 50 s from the beginning of the pairing procedure larger amplitude EPSCs are suddenly observed. (C) Nine second averages of EPSC amplitudes from eight experiments (similar to the example shown in B2) were aligned at a point where the transition from unpotentiated to potentiated states was thought to occur (mean time from the first paired stimulus to the transition point was 78 ± 16 s). This alignment suggests that potentiation occurs in a step-like all-or-none transition. (For details of alignment procedure see text.)
Because there is a delay on the order of 20 s between induction and expression of potentiation (Fig. 3), the results suggest that a single pairing may be sufficient to cause potentiation at a small subset of synapses. To determine if, in fact, a single release can induce potentiation, we used large inputs and examined the consequence of a single pairing. Consistent with previous data (9), a significant potentiation occurred after a single pairing (not shown) and the time course of the onset of this potentiation was similar to that observed in the previous experiments.
DISCUSSION
Implications for LTP.
The present experiments were designed to allow us to focus on the events that immediately surround the induction of potentiation at excitatory synapses on CA1 pyramidal cells. The data we obtained are consistent with the hypothesis that at individual synapses, activation of NMDA receptors during postsynaptic depolarization, the conditions known to be required for inducing LTP (1–3), results in a relatively abrupt, all-or-none “digital” increase in synaptic strength. For these experiments we used minimal stimulation in which it is presumed that a single or a few synapses are monitored. While our results are most easily explained if we were monitoring a single synapse, the results do not depend on this assumption. The ability to observe rapid, all-or-none transitions in synaptic strength suggests that if we were stimulating more than a single synapse, only one synapse showed potentiation or the potentiation occurred abruptly at the same time at all of the synapses. It might be argued that with the cell depolarized to zero millivolt, the release of a single vesicle during pairing will produce a saturating level of synapse change. In such an explanation, synapse change is “all-or-none” according to whether a single vesicle has been released during the pairing. Thus the ability to generate potentiation during the first 10 pairings would depend on whether transmitter release occurred and not to differences in the threshold for potentiation among synapses. However, because the release probability (calculated from the failure rate) was about 0.5 for all the synapses studied, including those that did not potentiate during the first 10 pairings, it can safely be assumed that transmitter release did occur during the 10 pairings. An interesting question remains as to whether potentiation would occur in an all-or-none manner under all induction conditions and in particular whether it occurs physiologically in vivo. An alternative approach to addressing the issue of all-or-none potentiation would be to vary the membrane potential and therefore the level of Ca2+ in the postsynaptic spine. In practice, however, this approach is complicated by the lack of quantitative data on the dependence of spine Ca2+ levels on the holding potential and the difficulty of reliably controlling the membrane potential in the range of the negative slope conductance of the NMDA receptors.
A limitation of our study was that synaptic strength was monitored for a relatively short period of time after the induction of potentiation. Thus we did not confirm that we actually induced LTP rather than an NMDA receptor-dependent short-term potentiation (see ref. 10 for review). However the fact that a 100 stimuli pairing protocol, which is a strong LTP induction protocol that routinely elicits LTP, did not cause an increase in synaptic strength when it followed a potentiation that was first elicited by a 10 stimuli pairing protocol (Figs. 2B1 and 3B), strongly argues that the all-or-none potentiation does in fact occur during LTP. It is possible that additional events, which would not show up as a change in synaptic strength, are required to stabilize the initial potentiation.
The number of pairings required to induce an essentially all-or-none potentiation at individual synapses varied as did the interval between the activation of NMDA receptors and the onset of the potentiation. The apparent difference in the “threshold” for inducing potentiation may reflect differences in the probability of release at individual synapses. During a fixed number of afferent stimuli, synapses with low probability of release would undergo fewer actual pairings than synapses with higher probability of release. However, no correlation between probability of release (estimated from the failure rate) and the induction of potentiation was observed. Alternatively, the state of the synapse in terms of its detailed molecular properties could strongly influence the degree of NMDA receptor activation that is required to induce potentiation as well as the time at which it occurs (11). Finally, because we have not potentiated (and depotentiated) a given synapse multiple times, we cannot tell whether an individual synapse has a fixed, definite threshold and these are distributed in value or whether all synapses are essentially identical and there is a pronounced stochastic element to the induction of the potentiation event. These physically different models could yield essentially identical results and analysis for the present experiments.
The NMDA receptor-dependent rise of calcium concentration in the dendritic spine appears to be sufficient to trigger potentiation (12, 13). Considerable evidence suggests that one important function of this rise in spine calcium is to activate calcium/calmodulin-dependent protein kinase II (CaMKII) (14–17). The Ca2+ signal evoked by activation of NMDA receptors and required for potentiation appears to last only a few seconds (18, 19). Because there was a delay of many sec after the pairing to the onset of the potentiation, it is clear that the actual potentiation occurs considerably after Ca2+ has returned to normal levels. Although the kinetics of the activation of CaMKII in the postsynaptic density has not been determined, it seems reasonable to assume that it is considerably faster than the onset of potentiation. This would suggest that the delay between induction and expression of potentiation resides in the events that occur well after the activation of CaMKII. Whether these events entail the all-or-none up-regulation of clusters of α-amino-3-hydroxy-5-methyl-4-isoazolepropionate receptors (20–22) and/or the enhancement of glutamate release (23, 24) remains to be firmly established. One intriguing possibility is that the step-like all-or-none transition from baseline synaptic transmission to a potentiated state is mediated by a postsynaptic membrane fusion event (25), perhaps involving the insertion of α-amino-3-hydroxy-5-methyl-4-isoazolepropionate receptors into the postsynaptic density.
Implications for Neural Network Models.
Whereas mathematical algorithms have most often invoked graded changes in “synaptic” strengths, it appears from our data that synaptic memories at hippocampal CA3-CA1 are encoded in a digital manner. What are the possible advantages for the biological system in such all-or-none synaptic modifications? The major problems that real biological synapses face in terms of long-term information storage are generic to any perishable storage medium in a noisy world. Two specific potential difficulties may be resolved through the digital encoding of information at synapses.
First, if long-term memory is described as residing in the pattern of strengths of the synapses, how can a memory remain fixed whilst the synaptic molecules reflecting the stored information are subject to changes over time? All memory elements that have continuous adjustability have a noise problem that can best be described by analogy. Suppose we wish to remember a particular analog value between 0 and 1, say 0.7. We might in concept do so by etching a line 1-μm long that can confine a large molecule, and “writing” the memory by placing a large molecule at 0.7 μm from the left-hand end. When we want to recall the stored information at a later time, we measure the distance from the left-hand end to the large molecule. However, because of noise, the molecule will now not be exactly at 0.7 μm, but perhaps at 0.65 μm. We therefore will obtain an answer that is in error because there is no way to know that 0.65 is not the correct answer. The root-mean-square error increases with time such that eventually the molecule is equally likely to be found anywhere between 0 and 1. At this point, there is no remaining information about what was written. If information is to be stored as graded synaptic strengths then from the above analogy we can see that there is no way of knowing what error noise has introduced over time and hence it becomes impossible to store information reliably for long periods of time.
If on the other hand, the information is written in a digital (e.g., binary) form, the same system can be used for reliable storage of information. Suppose we agree to put the molecule only at 0 μm or 1 μm initially, that is either at the left or the right hand end of the line. We are thus storing one bit of information, not an analog number. If the molecule is initially placed at the right hand end of the line, then after a short time noise may have moved the molecule to a new position 0.05 μm from the right hand end. However, for short intervals of time we can safely conclude that it is extremely unlikely that the molecule was initially at the left end. We can thus restore the molecule by pushing it fully back to the right hand end. In so doing, we eliminate the effect of the noise. Thus, sufficiently frequent repeated restoration can preserve digital information for arbitrarily long times in a noisy environment.
The restoration process must be carried out correctly, or it itself becomes the source of inaccuracy. For example, if a very fine scale of digital synaptic strengths was envisioned, based perhaps on the number of receptor molecules in the membrane, restoration events would need to replace old individual molecules with new ones on an exact 1:1 basis. The difficulty in biology of having exact stochiometry for such a molecular event, independent of the total number of molecules, is exemplified by the frequent insertion and deletion errors that occur when a normally accurate DNA polymerase is faced with a long monotone string like GGGGGGGGGGGGGGGGGG to copy.
Restoration can be either active (as in typical computer RAM electronics) or passive (as in magnetic memories). In the context of synaptic memories, our data would suggest that restoration is achieved from the existence of only two stable states, “potentiated” and “not potentiated.” The potentiated state could then simply be maintained by pushing the synapse into the state of highest efficacy. At a molecular level the long-lasting activity of the Thr-286-autophosphorylated state of αCaMKII, which has recently been shown to be required for potentiation (26), could achieve this task of restoration.
The second difficulty is the problem of not storing information under the wrong circumstances. This involves developing an appropriate threshold for deciding whether to store the information or not. When memory is to be written by physical signals, but also not written by somewhat similar signals of the same modality, a sharp threshold for storage is important. For example, in EEPROM (electrically erasable programmable ROM) readout signals or thermal processes must not erase or over-write a memory. High voltages are used to write or erase information, lower voltages are used to read it, and a sharp writing threshold prevents a confusion between these events.
The present data suggest that there is a true all-or-none threshold for potentiation at individual synapses. This threshold presumably can only be reached with appropriate temporally correlated pre- and postsynaptic activity. Because there is likely to be high levels of background neural activity, a sharp threshold for potentiation is necessary if synapse modification by irrelevant events is not to occur. The sharpness of a threshold is determined by the degree of cooperativity, seldom high at the single enzyme level. However, a system containing a positive feedback loop involving many molecules in a cooperative fashion, has effectively infinite cooperativity, step-like thresholds, and an all-or-none response. One system in the postsynaptic density that could play such a role involves CaMKII (27) for which the activation occurs in a “cooperative, positive feedback loop” (28) with a sharp threshold.
Many descriptions of learning and development in models of neurobiology rely on synapses that can make continuous or infinitesimal adjustments. If the synapses involved, like those studied here, can be modified only in a binary fashion, these descriptions will need to be rethought, although some models may be reinterpretable on a population basis. Interestingly, there is one model of associative memory in which binary change for excitatory synapses was invoked because it improved behavior compared with continuously adjustable synapses (29). It will be interesting to determine if other models of neurobiological processes also improve with binary, rather than analog, synaptic modifications and sharp modification thresholds.
Acknowledgments
We thank M. Frerking, G.O. Hjelmstad, and K. Vogt for useful discussions, and Helen Czerwonka for secretarial assistance. This study was supported by grants from the Wellcome Trust (C.C.H.P.), the National Institutes of Health (R.A.N. and R.C.M.), the Human Frontier Science Program (R.C.M.), and the McKnight Endowment Fund for Neuroscience (R.C.M.). R.A.N. is a member of the Keck Center for Integrative Neuroscience Research and R.C.M. is a member of the Center for Neurobiology and Psychiatry and the Center for the Neurobiology of Addiction. J.J.H. is supported in part by the National Science Foundation Engineering Research Center for Neuromorphic Systems at the California Institute of Technology.
ABBREVIATIONS
- CaMKII
calcium/calmodulin-dependent protein kinase II
- EPSC
excitatory postsynaptic current
- LTP
long-term potentiation
- NMDA
N-methyl-d-aspartate
- D-APV
d-2-amino-5-phosphonovaleric acid
Footnotes
A commentary on this article begins on page 4086.
References
- 1.Bliss T V P, Collingridge G L. Nature (London) 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 2.Nicoll R A, Malenka R C. Nature (London) 1995;377:115–118. doi: 10.1038/377115a0. [DOI] [PubMed] [Google Scholar]
- 3.Larkman A U, Jack J J B. Curr Opin Neurobiol. 1995;5:324–334. doi: 10.1016/0959-4388(95)80045-x. [DOI] [PubMed] [Google Scholar]
- 4.Kullmann D M, Siegelbaum S A. Neuron. 1995;15:997–1002. doi: 10.1016/0896-6273(95)90089-6. [DOI] [PubMed] [Google Scholar]
- 5.Blanton M G, Lo Turco J J, Kriegstein A R. J Neurosci Methods. 1989;30:203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- 6.Mulkey R M, Malenka R C. Neuron. 1992;9:967–975. doi: 10.1016/0896-6273(92)90248-c. [DOI] [PubMed] [Google Scholar]
- 7.Hsia, A., Malenka, R. C. & Nicoll, R. A. (1998) J. Neurophysiol. , in press. [DOI] [PubMed]
- 8.Gustafsson B, Asztely F, Hanse E, Wigström H. Eur J Neurosci. 1989;1:382–394. doi: 10.1111/j.1460-9568.1989.tb00803.x. [DOI] [PubMed] [Google Scholar]
- 9.Colino A, Huang Y-Y, Malenka R C. J Neurosci. 1992;12:180–187. doi: 10.1523/JNEUROSCI.12-01-00180.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malenka R C, Nicoll R A. Trends Neurosci. 1993;16:521–527. doi: 10.1016/0166-2236(93)90197-t. [DOI] [PubMed] [Google Scholar]
- 11.Abraham W C, Bear M F. Trends Neurosci. 1996;19:126–30. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- 12.Malenka R C, Kauer J A, Zucker R J, Nicoll R A. Science. 1988;242:81–84. doi: 10.1126/science.2845577. [DOI] [PubMed] [Google Scholar]
- 13.Neveu D, Zucker R S. Neuron. 1996;16:619–29. doi: 10.1016/s0896-6273(00)80081-1. [DOI] [PubMed] [Google Scholar]
- 14.Silva A J, Stevens C F, Tonegawa S, Wang Y. Science. 1992;257:201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- 15.Lledo P-M, Hjelmstad G O, Mukherji S, Soderling T R, Malenka R C, Nicoll R A. Proc Natl Acad Sci USA. 1995;92:11175–11179. doi: 10.1073/pnas.92.24.11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pettit D L, Perlman S, Malinow R. Science. 1994;266:1881–1885. doi: 10.1126/science.7997883. [DOI] [PubMed] [Google Scholar]
- 17.Barria A, Muller D, Derkach V, Griffith L C, Soderling T R. Science. 1997;276:2042–5. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- 18.Regehr W G, Connor J A, Tank D W. Nature (London) 1989;341:533–536. doi: 10.1038/341533a0. [DOI] [PubMed] [Google Scholar]
- 19.Malenka R, Lancaster B, Zucker R S. Neuron. 1992;9:121–128. doi: 10.1016/0896-6273(92)90227-5. [DOI] [PubMed] [Google Scholar]
- 20.Liao D, Hessler N A, Malinow R. Nature (London) 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- 21.Isaac J T R, Nicoll R A, Malenka R C. Neuron. 1995;15:427–434. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- 22.Durand G M, Kovalchuk Y, Konnerth A. Nature (London) 1996;381:71–5. doi: 10.1038/381071a0. [DOI] [PubMed] [Google Scholar]
- 23.Stevens C F, Wang Y. Nature (London) 1994;371:704–707. doi: 10.1038/371704a0. [DOI] [PubMed] [Google Scholar]
- 24.Bolshakov V Y, Siegelbaum S A. Science. 1995;269:1730–1734. doi: 10.1126/science.7569903. [DOI] [PubMed] [Google Scholar]
- 25.Lledo P-M, Zhang X, Südhof T C, Malenka R C, Nicoll R A. Science. 1998;279:399–403. doi: 10.1126/science.279.5349.399. [DOI] [PubMed] [Google Scholar]
- 26.Giese K P, Fedorov N B, Filipkowski R K, Silva A J. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- 27.Lisman J E. Proc Natl Acad Sci USA. 1985;82:3055–3057. doi: 10.1073/pnas.82.9.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanson P I, Meyer T, Stryer L, Schulman H. Neuron. 1994;12:943–56. doi: 10.1016/0896-6273(94)90306-9. [DOI] [PubMed] [Google Scholar]
- 29.Gelperin A, Hopfield J J, Tank D W. In: Model Neural Networks and Behavior. Selverston A I, editor. New York: Plenum; 1985. pp. 237–261. [Google Scholar]