Abstract
The signaling pathway initiated by factor Xa on vascular endothelial cells was investigated. Factor Xa stimulated a 5- to 10-fold increased release of nitric oxide (NO) in a dose-dependent reaction (0.1–2.5 μg/ml) unaffected by the thrombin inhibitor hirudin but abolished by active site inhibitors, tick anticoagulant peptide, or Glu-Gly-Arg-chloromethyl ketone. In contrast, the homologous clotting protease factor IXa or another endothelial cell ligand, fibrinogen, was ineffective. A factor Xa inter-epidermal growth factor synthetic peptide L83FTRKL88(G) blocking ligand binding to effector cell protease receptor-1 inhibited NO release by factor Xa in a dose-dependent manner, whereas a control scrambled peptide KFTGRLL was ineffective. Catalytically active factor Xa induced hypotension in rats and vasorelaxation in the isolated rat mesentery, which was blocked by the NO synthase inhibitor l-NG-nitroarginine methyl ester (l-NAME) but not by d-NAME. Factor Xa/NO signaling also produced a dose-dependent endothelial cell release of interleukin 6 (range 0.55–3.1 ng/ml) in a reaction inhibited by l-NAME and by the inter-epidermal growth factor peptide Leu83–Leu88 but unaffected by hirudin. Maximal induction of interleukin 6 mRNA required a brief, 30-min stimulation with factor Xa, unaffected by subsequent addition of tissue factor pathway inhibitor. These data suggest that factor Xa-induced NO release modulates endothelial cell-dependent vasorelaxation and cytokine gene expression. This pathway requiring factor Xa binding to effector cell protease receptor-1 and a secondary step of ligand-dependent proteolysis may preserve an anti-thrombotic phenotype of endothelium but also trigger acute phase responses during activation of coagulation in vivo.
Although primarily recognized for maintaining the hemostatic balance (1), blood proteases of the coagulation and fibrinolytic cascades elicit rapid cellular responses in vascular, mesenchymal, and inflammatory cell types (2, 3). Considerable effort has been devoted to elucidate the molecular interface between protease-dependent signaling and pleiotropic cellular responses. This led to the identification of several membrane protease receptors, initiating intracellular signal transduction and effector functions in vascular cells. In this context, thrombin receptor activation (4) generated second messengers in endothelium (5) and smooth muscle cells (6, 7), released inflammatory cytokines from monocytes (8), fibroblasts (9), and endothelium (10, 11), and increased the expression of leukocyte-endothelial cell adhesion molecules (12). Similarly, binding of factor Xa to effector cell protease receptor-1 (EPR-1) (13) participated in in vivo acute inflammatory responses (14), platelet and brain pericyte prothrombinase activity (15, 16), and endothelial cell and smooth muscle cell signaling and proliferation (17, 18). Despite the potential role of these mechanisms in vascular injury and atherosclerosis (19), the intracellular second messenger(s) mediating protease-dependent cellular effector functions have remained elusive. Here, we report that factor Xa induces the release of endothelial cell NO, thus regulating vasorelaxation in vivo and acute response cytokine gene expression in vitro. This pathway requires a dual step cascade, involving binding of factor Xa to EPR-1 (17, 18) and a secondary as yet unidentified protease-activated mechanism.
MATERIALS AND METHODS
cGMP Accumulation in Human Umbilical Vein Endothelial Cells (HUVEC).
HUVEC were isolated by collagenase treatment and maintained in culture in complete M199 tissue culture medium (GIBCO/BRL) supplemented with 20% fetal bovine serum, endothelial cell growth factor, 100 units/ml penicillin, and 100 μg/ml streptomycin. Before each experiment, confluent HUVEC monolayers were washed with Hanks’ balanced salt solution (GIBCO) and exposed to histamine (10 μM) or increasing concentrations of factor Xa (0.1–5.5 μg/ml) in the presence of isobutylmethylxanthine (1 mM) to inhibit the breakdown of cGMP by phosphodiesterases. After a 15-min incubation at 37°C, the medium was aspirated, and cGMP was extracted in 0.1 M HCl and determined by RIA (Biomedical Technologies, Stoughton, MA). Cell remnants were boiled in 1 M NaOH and used for protein determination (values were calculated as picomoles of cGMP per mg of protein). Specificity for the effect of factor Xa was assessed in the presence of the NO synthase inhibitor l-NG-nitroarginine methyl ester (l-NAME, 100 μM) added for 15 min at 37°C before cGMP determination. Similar experiments were carried out in the presence of factor IXa (2.5 μg/ml), fibrinogen (2.5 μg/ml), or catalytic active site-inhibited Dansyl-Glu-Gly-Arg-chloromethyl ketone (DEGR)-factor Xa (2.5 μg/ml) or factor Xa pretreated with the active site inhibitor, tick anticoagulant peptide (TAP). In parallel experiments, HUVEC cGMP release was determined following stimulation with thrombin (1 unit/ml) or factor Xa (2.5 μg/ml) in the presence or in the absence of hirudin (10 units/ml). For inhibition experiments, HUVEC were preincubated with increasing concentrations of the factor Xa-derived inter-epidermal growth factor (EGF) peptide Leu83-Phe84-Thr85-Arg86-Lys87-Leu88-(Gly) (1–500 μM) inhibiting factor Xa binding to EPR-1 (20) or control scrambled peptide Lys-Phe-Thr-(Gly)-Arg-Leu-Leu (500 μM) (residues in parentheses added to the natural sequence) for 15 min at 37°C, before addition of factor Xa and determination of cGMP production. Alternatively, HUVEC were preincubated with anti-EPR-1 monoclonal antibody 13E5 or control nonbinding antibody 14E11 before factor Xa stimulation and cGMP determination. All coagulation proteins were purchased from Hematologic Technologies, Essex, VT, and characterized in previous studies (20). The interaction of factor Xa with HUVEC and the role of EPR-1 in this recognition have been reported previously (17, 18, 20). All other reagents were from Sigma.
Vasoactive Effects of Factor Xa in Vivo and in the Isolated Rat Mesentery Circulation.
For blood pressure measurements, male Wistar rats were anesthetized with a mixture of ketamine (90 mg/kg intraperitoneally) and xylazine (10 mg/kg intraperitoneally). The right carotid artery and the left jugular vein were cannulated, and a pressure transducer was connected to the artery for measurement of the blood pressure whereas the drugs were administered through the jugular vein. The rats were anticoagulated with heparin (40 units/kg, intravenously). After a 10-min interval, factor Xa (1.1–111 pmol) or DEGR-factor Xa (1111 pmol) was administered as an intravenous bolus. To determine the vasoactive effect of factor Xa on the isolated rat mesentery circulation, rats were anesthetized with urethane (1 g/kg intraperitoneally), the abdomen was opened, and the ileocolic and colic branches of the superior mesentery were tied. The superior mesenteric artery was cannulated and perfused at a flow rate of 2 ml/min with warm (37°C), oxygenated (95% O2/5% CO2) Krebs solution (concentrations in mM: NaCl, 117.5; KCl, 4.7; KH2PO4, 1.2; CaCl2, 2.5; MgSO4, 1.2; NaHCO3, 25; glucose, 5.5) containing heparin (100 units/ml) and indomethacin (10−5 M). Rats were killed by cutting the diaphragm. Perfusion pressures of arterial vasculature were measured with a pressure transducer and recorded on a physiograph. The arterial circulation was preconstricted with methoxamine (10−5 M) until a stable baseline was obtained, and then a bolus injection of either factor Xa (2.2–66 pmol), DEGR-factor Xa (66 pmol), or Ach (10–300 pmol) was administered. l-NAME (100 μM) was infused at a constant rate, and the dose–response curves to the various agonists added were recorded.
Endothelial Cell Cytokine Release.
HUVEC monolayers were incubated with increasing concentrations (0.1–10 μg/ml) of factor Xa, DEGR-factor Xa, or fibrinogen and cultivated in the presence of 0.1% fetal bovine serum for 12 h at 37°C. At the end of the incubation, release of interleukin 6 (IL-6) in the supernatant of stimulated cultures was assessed by ELISA (Immunotech, Marseille, France). To determine the specificity of endothelial cell response to factor Xa, HUVEC were stimulated with thrombin (1 unit/ml), factor IXa (5 μg/ml), transferrin (2.5 μg/ml), tumor necrosis factor α (10 ng/ml), cathepsin G (50 μg/ml), chymotrypsin (100 nM), or urokinase (40 nM) for 12 h at 37°C before IL-6 determination. In peptide inhibition experiments, HUVEC were preincubated with increasing concentrations (10–1000 μM) of the inter-EGF peptide Leu83–Leu88 or control scrambled peptide for 30 min at 37°C, before stimulation with 1 μg/ml factor Xa and determination of IL-6 release. Alternatively, HUVEC were incubated with l-NAME or its inactive analogue d-NAME (5 mM each) for 30 min at 37°C, in the presence or in the absence of increasing concentrations of the NO donor, NOC 15 (Alexis Biochemicals, San Diego, CA; 25–100 μM), before stimulation with factor Xa (5 μg/ml) and determination of IL-6 release. In other experiments, HUVEC were stimulated with 5 μg/ml factor Xa for 1 h at 37°C, mixed with increasing concentrations of recombinant tissue factor pathway inhibitor (TFPI, 0.5–50 μg/ml), and cultivated for an additional 12 h at 37°C, before determination of released IL-6.
Northern Hybridization.
A full-length cDNA encoding human IL-6 was isolated by reverse transcriptase/PCR amplification from cytokine-activated HUVEC. Briefly, total RNA was extracted from confluent HUVEC monolayers after overnight stimulation with 10 ng/ml tumor necrosis factor α by the guanidinium isothiocyanate method. Five μg of total RNA were primed with IL-6-specific oligonucleotide 5′-GTGCCTGCAGCTTCGTCAGCTGG-3′, reverse transcribed with 200 units of Superscript II (GIBCO/BRL) for 50 min at 42°C, and amplified by PCR with 0.5 μg of IL-6 oligonucleotides (forward, 5′-CACACAGACAGCCACTCACCTC-3′), 200 μM dNTPs (New England Biolabs, Beverly, MA), and 2 units of Vent DNA polymerase (New England Biolabs). The amplified IL-6 cDNA of 400 nucleotides was subcloned in pCRII (Invitrogen) and completely sequenced on both strands. For Northern hybridization, HUVEC total RNA was extracted after stimulation with factor Xa (5 μg/ml) for increasing time intervals between 0.5 and 12 h at 37°C. RNA samples (5–10 μg) were separated on agarose–formaldehyde-denaturing gels, transferred overnight to GeneScreen nylon membranes (DuPont), and UV crosslinked. The membrane was hybridized with random-primed [32P]dCTP (Amersham) labeled IL-6 cDNA in 5× SSPE, 10× Denhardt’s solution, 1% SDS, 100 μg/ml denatured salmon sperm DNA for 14 h at 60°C. The membrane was washed in 2× SSC, 1% SDS for 30 min at 60°C twice and 0.2× SSC at 22°C once, and exposed for autoradiography. Control hybridization for densitometric quantitation of IL-6 mRNA induction by factor Xa was carried out with a glyceraldehyde-3-phosphate dehydrogenase 32P-labeled probe under the same experimental conditions.
RESULTS AND DISCUSSION
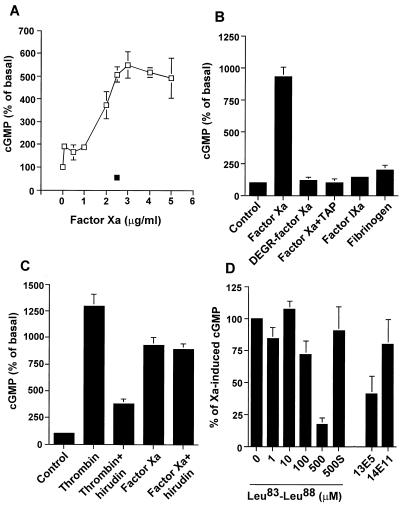
Incubation of HUVEC monolayers with factor Xa resulted in a concentration-dependent release of NO, as determined by cGMP accumulation in these cells, which was inhibited by the nitric oxide synthase (21) antagonist l-NAME (Fig. 1A). Catalytically inactive DEGR-factor Xa or TAP-treated factor Xa failed to stimulate NO release by HUVEC, thus demonstrating the requirement of ligand-dependent proteolysis in this response (Fig. 1B). To determine the specificity of NO release under these conditions, two series of experiments were carried out. First HUVEC stimulation with the homologous clotting protease, factor IXa, or with another endothelial cell ligand, fibrinogen (22), did not stimulate NO release (Fig. 1B). Second, the thrombin-specific antagonist hirudin blocked NO release from thrombin-stimulated HUVEC, in agreement with previous observations (23, 24), but failed to reduce the NO response elicited by factor Xa (Fig. 1C).
Figure 1.
Factor Xa-dependent NO release by HUVEC. (A) HUVEC were exposed to the indicated increasing concentrations of factor Xa in the presence (▪) or in the absence (□) of the NO synthase inhibitor l-NAME (100 μM) for 15 min at 37°C before cGMP determination. (B) HUVEC were treated with factor Xa (2.5 μg/ml), DEGR-factor Xa (2.5 μg/ml), factor IXa (2.5 μg/ml), fibrinogen (2.5 μg/ml), or factor Xa preincubated with TAP (250 μM) for 15 min on ice before determination of cGMP. Histamine (10 μM) stimulation of NO release by HUVEC was not significantly affected by TAP alone (13.2 ± 2.2 versus 10.4 ± 0.6 pmol/mg of protein, n = 4). (C) Thrombin (1 unit/ml) or factor Xa (2.5 μg/ml) were incubated with hirudin (2.5 unit/ml) for 15 min on ice and added to HUVEC for an additional 15 min of incubation before cGMP determination. (D) HUVEC were incubated with the factor Xa-derived inter-EGF peptide Leu83–Leu88, which blocks ligand binding to EPR-1 (20), a control scrambled peptide KFTGRLL(s), anti-EPR-1 monoclonal antibody 13E5 (50 μg/ml), or control nonbinding IgG 14E11 (50 μg/ml). After a 30-min incubation at 37°C, cells were stimulated with factor Xa (2.5 μg/ml) and cGMP was determined. Data are presented as mean ± SEM (n = 3–11; P < 0.05).
The potential role of the endothelial cell factor Xa receptor, EPR-1 (17, 18), in NO release was next investigated. Increasing concentrations of the factor Xa-derived inter-EGF peptide Leu83–Leu88, which prevents ligand binding to EPR-1 (20), blocked NO release from factor Xa-stimulated HUVEC in a dose-dependent manner, whereas a control scrambled peptide KFTGRLL was ineffective (Fig. 1D). A comparable degree of inhibition was obtained with the anti-EPR-1 monoclonal antibody 13E5 but not with control nonbinding antibody 14E11 (Fig. 1D). These data demonstrate that binding of factor Xa to EPR-1 induces a concentration-dependent release of endothelial cell NO through a protease-activated mechanism.
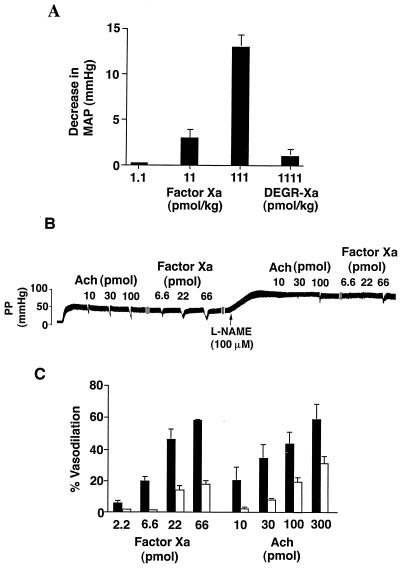
We next investigated a potential role of factor Xa/NO signaling in modulation of vessel wall tone in vivo. Intravenous injection of factor Xa in anticoagulated, anesthetized rats produced an immediate, transient, dose-dependent decrease in mean arterial blood pressure (Fig. 2A). Consistent with the requirement of a protease-activated mechanism in NO release (Fig. 1B), administration of catalytically inactive DEGR-factor Xa did not produce changes in systemic blood pressure under the same experimental conditions (Fig. 2A). To directly investigate the role of factor Xa–NO signaling in modulation of vascular wall tone, experiments in the isolated rat mesentery circulation were carried out next. Increasing concentrations of Ach produced a dose-dependent endothelial cell vasorelaxation in the methoxamine preconstricted isolated mesentery circulation (Fig. 2B), in agreement with previous observations (25). Under these experimental conditions, administration of factor Xa also produced a dose-dependent vasorelaxation in the rat mesenteric circulation, in a response virtually equipotent to that obtained with Ach (Fig. 2B), whereas catalytically inactive DEGR-factor was ineffective (not shown). Perfusion of the mesenteric vascular bed with l-NAME (100 μM) abolished vasodilation induced by 2.2 and 6.6 pmol of factor Xa and partially reduced the response elicited by 22 and 66 pmol of factor Xa (Fig. 2 B and C). Similarly, comparable concentrations of l-NAME completely inhibited vasorelaxation induced by 10 and 30 pmol of Ach and partially reduced the response induced by 100 and 300 pmol of Ach (Fig. 2 B and C).
Figure 2.
Factor Xa causes hypotension in vivo and vasodilation in the isolated rat mesentery. (A) Rats were injected with increasing amounts of factor Xa or catalytically inactive DEGR-factor Xa before determination of changes in mean arterial pressure (MAP). Data are the mean ± SEM for factor Xa (n = 8–10) or DEGR-factor Xa (n = 3). (B) Data depict a representative bioassay trace of Ach- and factor Xa-induced vasodilation in the perfused rat mesentery. Increasing amounts of Ach (10–100 pmol) or factor Xa (6.6–66 pmol) were given as bolus injections into the perfused rat mesentery in the absence or in the presence of l-NAME (100 μM), and perfusion pressures (PP) were monitored. (C) The experimental conditions are the same as in B. Responses in the presence of l-NAME are represented by open bars. Data are the mean ± SEM of the effect of factor Xa and Ach on vasodilation in the perfused rat mesentery expressed as percent relaxation (n = 3–7).
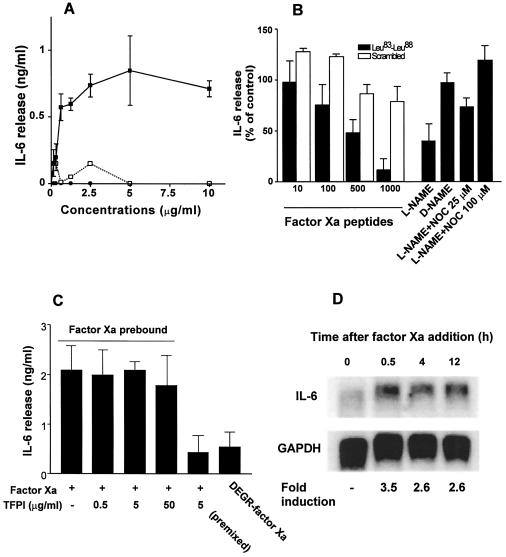
To determine whether factor Xa-induced NO release could also modulate acute phase/inflammatory cytokine gene expression we examined potential changes in IL-6 release following HUVEC stimulation with factor Xa. HUVEC stimulation with factor Xa resulted in a concentration-dependent release of IL-6 (range, 0.55–3.1 ng/ml; Fig. 3A), which was maximal at 0.6 μg/ml added factor Xa. In contrast, catalytically inactive DEGR-factor Xa or fibrinogen did not induce HUVEC release of IL-6 (Fig. 3A) under the same experimental conditions. The specificity of factor Xa-induced cytokine release was next investigated. First, factor Xa-induced IL-6 release from HUVEC was quantitatively indistinguishable from that obtained with tumor necrosis factor α or thrombin stimulation (Table 1). This response was abolished by heat denaturation of factor Xa (Table 1) but was unaffected by the endotoxin inhibitor polymixin B (10 ng/ml, not shown), thus ruling out a potential contribution of contaminating endotoxin in the observed release. Moreover, control protein transferrin or additional proteases urokinase, cathepsin G, or chymotrypsin failed to induce HUVEC release of IL-6 under the same experimental conditions (Table 1). HUVEC preincubation with hirudin completely inhibited IL-6 release induced by thrombin in a dose-dependent manner and in agreement with previous observations (9) but did not reduce IL-6 release stimulated by factor Xa (Table 1).
Figure 3.
Factor Xa/NO induction of IL-6 release in HUVEC. (A) IL-6 release by HUVEC stimulated with increasing concentrations of factor Xa (▪), DEGR-factor Xa (•), or fibrinogen (□) was determined by ELISA after a 12-h culture at 37°C. (B) Effect of the factor Xa-derived inter-EGF peptide Leu83–Leu88 (▪) or control scrambled peptide KFTGRLL (□) on IL-6 release induced by 1 μg/ml factor Xa (peptide concentrations are in μM). Statistical analysis revealed no significant differences in the amount of IL-6 released in the presence of control scrambled peptide KFTGRLL. NO inhibitor l-NAME and control inactive d-NAME were preincubated with HUVEC in the presence or in the absence of the indicated increasing concentrations of the NO donor, NOC 15, before factor Xa stimulation and determination of IL-6 release. (C) Effect of increasing concentrations of TFPI (0.5–50 μg/ml) added 1 h after HUVEC stimulation with factor Xa on IL-6 release. (D) Time course of IL-6 mRNA induction following factor Xa stimulation of HUVEC. Densitometric quantitation of radioactive bands (fold induction) was normalized on control hybridization with glyceraldehyde-3-phosphate dehydrogenase.
Table 1.
Specificity of factor Xa stimulation of IL-6 release by HUVEC
| Additions | IL-6 release ng/ml |
|---|---|
| Factor Xa (5 μg/ml) | 2.29 ± 0.36 |
| Factor Xa denatured | <0.010 |
| Tumor necrosis factor α (10 ng/ml) | 2.3 ± 0.8 |
| Thrombin (1 unit/ml) | 2.26 ± 1.04 |
| Transferrin (2.5 μg/ml) | 0.026 ± 0.026 |
| Cathepsin G (50 μg/ml) | 0.051 ± 0.051 |
| Urokinase (40 nM) | 0.08 ± 0.08 |
| Chymotrypsin (100 nM) | 0.134 ± 0.02 |
| % of control | |
| Thrombin + hirudin (0.08 unit/ml) | 123 ± 6.8 |
| Thrombin + hirudin (10 units/ml) | 3.1 ± 3.1 |
| Factor Xa + hirudin (0.08 unit/ml) | 72 ± 16 |
| Factor Xa + hirudin (10 units/ml) | 92.5 ± 3.5 |
Resting HUVEC monolayers were incubated with the indicated concentrations of the various proteins and cultivated for 12 h at 37°C before determination of IL-6 release by ELISA. Data are the mean ± SD of two independent determinations.
The potential involvement of EPR-1 and NO signaling in factor Xa-induced IL-6 release were next investigated. First, increasing concentrations of the EPR-1 antagonist inter-EGF peptide Leu83–Leu88 (20) inhibited IL-6 release stimulated by factor Xa in a dose-dependent manner, whereas a control scrambled peptide was ineffective (Fig. 3B). Second, HUVEC preincubation with the NO synthase inhibitor l-NAME, but not with the inactive species d-NAME, blocked factor Xa-induced IL-6 response (Fig. 3B). Under these experimental conditions, increasing concentrations of the NO donor NOC 15 reversed the inhibitory effect of l-NAME in a dose-dependent manner (Fig. 3B) and restored the IL-6 response of HUVEC to factor Xa, thus further corroborating the role of NO in cytokine gene induction. Addition of NOC 15 alone did not affect HUVEC IL-6 production (not shown), thus suggesting that binding to EPR-1 and NO release were both necessary for the effect of factor Xa on gene expression. The effect of physiologic anticoagulation pathways on factor Xa/NO cytokine release was next investigated. Consistent with the requirement of a protease-activated mechanism, preincubation of factor Xa with TFPI (26) abrogated IL-6 release by HUVEC (Fig. 3C). However, a short (1 h) exposure of endothelium to factor Xa was sufficient to stimulate maximal release of IL-6, not further inhibited by addition of molar excess TFPI (Fig. 3C). Consistent with these findings, a 30-min stimulation of HUVEC with factor Xa was sufficient to produce a 3.5-fold induction of IL-6 mRNA in these cells, as compared with background levels of unstimulated cells (Fig. 3D). Increased levels of IL-6 mRNA remained 2.5-fold above background of unstimulated cells at 4 and 12 h from addition of factor Xa (Fig. 3D).
In summary, we have identified a signaling pathway centered on the ability of factor Xa to rapidly stimulate endothelial cell NO release (25, 27). This involves a two-step cascade initiated by catalytic active site-independent binding of factor Xa to its receptor, EPR-1 (20), followed by a second step of ligand-dependent proteolysis. Although the substrate of factor Xa activation has not yet been identified, this model is reminiscent of protease-activated signals generated by thrombin on vascular cells (4). Recent studies have demonstrated that activation of coagulation and local protease activity may participate in a panoply of nonhemostatic vascular cell signaling mechanisms (5, 7). Although membrane protease receptors have emerged as modulators of these functions in normal (4, 13, 28) and disease conditions (29), the signaling requirements underlying protease-dependent cellular effector functions have not been conclusively elucidated. Here, we have shown that NO fulfills the criteria of a second messenger for a complex molecular interface between an activated coagulation protease, i.e., factor Xa, and systemic vasorelaxation in vivo and inflammatory cytokine gene induction. This pathway may have important pathophysiologic implications. First, and reminiscent of the anticoagulant properties of the thrombin–thrombomodulin complex (30), the ability of factor Xa to induce NO release may counterbalance a local activation of coagulation by inhibiting platelet activation, reducing leukocyte adhesion, and promoting vasodilation (25, 27). On the other hand, the novel role of NO in regulating inflammatory cytokine gene induction may also participate in acute phase responses in vivo. These are highlighted by the pleiotropic role of IL-6 in inflammation, consumption coagulopathy, and massive organ damage associated with deregulated activation of coagulation in vivo (31, 32). Furthermore, because of the rapidity of NO release and IL-6 gene induction triggered by factor Xa; this mechanism may escape inactivation by physiologic anticoagulants, i.e., TFPI (26, 32), thus further contributing to vascular activation. Elucidation of the novel role of NO (25, 27) at the interface between coagulation and inflammation (3) may offer new therapeutic strategies to interrupt the signaling consequences of deregulated activation of coagulation on vascular cells in vivo.
Acknowledgments
We thank G. Ambrosini for IL-6 cDNA and G. Vlasuk for TAP. This work was supported by National Institutes of Health Grants HL54131 and HL43773 (to D.C.A.) and HL51948 and HL57665 (to W.C.S.). A.P. was supported by a fellowship from the Donaghue Foundation. P.P. was supported by a fellowship from the Regione Abruzzo Fondo Sociale Europeo, Italy. This work was done during the tenure of Established Investigatorship Awards from the American Heart Association (to W.C.S. and D.C.A.).
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: EPR-1, effector cell protease receptor-1; HUVEC, human umbilical vein endothelial cells; l-NAME, l-NG-nitroarginine methyl ester; TAP, tick anticoagulant peptide; EGF, epidermal growth factor; Ach, acetylcholine; IL, interleukin; TFPI, tissue factor pathway inhibitor.
References
- 1.Furie B, Furie B C. N Engl J Med. 1992;326:800–806. doi: 10.1056/NEJM199203193261205. [DOI] [PubMed] [Google Scholar]
- 2.Esmon C T, Taylor F B, Snow T R. Thromb Haemostasis. 1991;66:160–165. [PubMed] [Google Scholar]
- 3.Marcus A J. Semin Hematol. 1994;31:261–269. [PubMed] [Google Scholar]
- 4.Coughlin S R. Proc Natl Acad Sci USA. 1994;91:9200–9202. doi: 10.1073/pnas.91.20.9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gargia J G, Fenton J W, Natarajan V. Blood. 1992;79:2056–2067. [PubMed] [Google Scholar]
- 6.McNamara C A, Sarembock I J, Gimple L W, Fenton J W, Coughlin S R, Owens G K. J Clin Invest. 1993;91:94–98. doi: 10.1172/JCI116206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molloy C J, Pawlowski J E, Taylor D S, Turner C E, Weber H, Peluso M, Seiler S M. J Clin Invest. 1996;97:1173–1183. doi: 10.1172/JCI118531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colotta F, Sciacca F L, Sironi M, Luini W, Rabiet M J, Mantovani A. Am J Pathol. 1994;144:975–985. [PMC free article] [PubMed] [Google Scholar]
- 9.Sower L E, Froelich C J, Carney D H, Fenton J W, II, Klimpel G R. J Immunol. 1995;155:895–901. [PubMed] [Google Scholar]
- 10.Shankar R, de la Motte C A, DiCorleto P E. Am J Physiol. 1992;262:C199–C206. doi: 10.1152/ajpcell.1992.262.1.C199. [DOI] [PubMed] [Google Scholar]
- 11.Ueno A, Murakami K, Yamanouchi K, Watanabe M, Kondo T. Immunology. 1996;88:76–81. doi: 10.1046/j.1365-2567.1996.d01-635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugama Y, Tiruppathi C, Janakidevi K, Andersen T T, Fenton J W, II, Malik A B. J Cell Biol. 1992;119:935–944. doi: 10.1083/jcb.119.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altieri D C. FASEB J. 1995;9:860–865. doi: 10.1096/fasebj.9.10.7615156. [DOI] [PubMed] [Google Scholar]
- 14.Cirino G, Cicala C, Bucci M, Sorrentino L, Ambrosini G, DeDominicis G, Altieri D C. J Clin Invest. 1997;99:2446–2451. doi: 10.1172/JCI119428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouchard B A, Catcher C S, Thrash B R, Adida C, Tracy P B. J Biol Chem. 1997;272:9244–9251. doi: 10.1074/jbc.272.14.9244. [DOI] [PubMed] [Google Scholar]
- 16.Bouchard B A, Shatos M A, Tracy P B. Arterioscler Thromb Vasc Biol. 1997;17:1–9. doi: 10.1161/01.atv.17.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Nicholson A C, Nachman R L, Altieri D C, Summers B D, Ruf W, Edgington T S, Hajjar D P. J Biol Chem. 1996;271:28407–28413. doi: 10.1074/jbc.271.45.28407. [DOI] [PubMed] [Google Scholar]
- 18.Bono F, Herault J-P, Avril P, Lormeau J-C, Herbert J-M. J Cell Physiol. 1997;172:36–43. doi: 10.1002/(SICI)1097-4652(199707)172:1<36::AID-JCP4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 19.Ross R. Nature (London) 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 20.Ambrosini G, Plescia J, Chu K C, High K A, Altieri D C. J Biol Chem. 1997;272:8340–8345. doi: 10.1074/jbc.272.13.8340. [DOI] [PubMed] [Google Scholar]
- 21.Moore P K, al-Swayeh O A, Chong N W S, Evans R A, Gibson A. Br J Pharmacol. 1990;99:408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altieri D C. Semin Cell Biol. 1995;6:269–274. doi: 10.1006/scel.1995.0036. [DOI] [PubMed] [Google Scholar]
- 23.De Meyer E, Van Hove C E, Feng X J, Rampart M, Herman A G. Eur J Pharmacol. 1995;291:67–72. doi: 10.1016/0922-4106(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 24.Grimminger F, Rose F, Sibelius U, Meinhardt M P, Spriestersbach R, Bhakdi S, Suttorp N, Seeger W. J Immunol. 1997;159:1909–1916. [PubMed] [Google Scholar]
- 25.Moncada S, Palmer R M, Higgs E A. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 26.Broze G J, Jr, Girard T J, Novotny W F. Biochemistry. 1990;29:7539–7546. doi: 10.1021/bi00485a001. [DOI] [PubMed] [Google Scholar]
- 27.Sessa W C. J Vasc Res. 1994;31:131–143. doi: 10.1159/000159039. [DOI] [PubMed] [Google Scholar]
- 28.Rao N K, Shi G-P, Chapman H A. J Clin Invest. 1995;96:465–474. doi: 10.1172/JCI118057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Contrino J, Hair G, Kreutzer D L, Rickles F R. Nat Med. 1996;2:209–215. doi: 10.1038/nm0296-209. [DOI] [PubMed] [Google Scholar]
- 30.Rosenberg R D, Rosenberg J S. J Clin Invest. 1984;74:1–6. doi: 10.1172/JCI111389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Poll T, Buller H R, ten Cate H, Wortel C, Bauer K A, van Deventer S J H, Hack E, Sauerwein H P, Rosenberg R D, ten Cate J W. N Engl J Med. 1990;322:1622–1627. doi: 10.1056/NEJM199006073222302. [DOI] [PubMed] [Google Scholar]
- 32.Creasey A A, Chang A C K, Feigen L, Wun T C, Taylor F B, Jr, Hinsaw L B. J Clin Invest. 1993;91:2850–2856. doi: 10.1172/JCI116529. [DOI] [PMC free article] [PubMed] [Google Scholar]