Abstract
Substance P, acting via the neurokinin 1 receptor (NK1R), plays an important role in mediating a variety of inflammatory processes. However, its role in acute pancreatitis has not been previously described. We have found that, in normal mice, substance P levels in the pancreas and pancreatic acinar cell expression of NK1R are both increased during secretagogue-induced experimental pancreatitis. To evaluate the role of substance P, pancreatitis was induced in mice that genetically lack NK1R by administration of 12 hourly injections of a supramaximally stimulating dose of the secretagogue caerulein. During pancreatitis, the magnitude of hyperamylasemia, hyperlipasemia, neutrophil sequestration in the pancreas, and pancreatic acinar cell necrosis were significantly reduced in NK1R−/− mice when compared with wild-type NK1R+/+ animals. Similarly, pancreatitis-associated lung injury, as characterized by intrapulmonary sequestration of neutrophils and increased pulmonary microvascular permeability, was reduced in NK1R−/− animals. These effects of NK1R deletion indicate that substance P, acting via NK1R, plays an important proinflammatory role in regulating the severity of acute pancreatitis and pancreatitis-associated lung injury.
The neuropeptide substance P has been shown to play an important role in asthma, inflammatory bowel disease, arthritis, and other inflammatory processes (1, 2). Subsequent to its release from nerve endings, substance P binds to neurokinin 1 receptors (NK1R) on effector cells, increases microvascular permeability, and promotes plasma extravasation from the intravascular to the extravascular space. Although pancreatic acinar cells are known to express NK1R and substance P has been detected within the pancreas (3–5), apparently no studies have been reported that have examined the possibility that this neuropeptide might play a role in the evolution of a pancreatic inflammatory disease such as acute pancreatitis. We have found that pancreatic levels of substance P and the expression of NK1R on pancreatic acinar cells are increased during experimental acute pancreatitis. We have also found that genetic deletion of NK1R reduces the severity of pancreatitis and pancreatitis-associated lung injury. These observations indicate that substance P, acting through NK1R, plays an important proinflammatory role in regulating the severity of acute pancreatitis and associated lung injury.
MATERIALS AND METHODS
All experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee of the Beth Israel Hospital. Breeding pairs of NK1R-deficient mice were generated as described (6), and the identity of their offspring as NK1R-deficient (−/−) homozygotes was confirmed by Southern blotting (6). Animals were bred and housed in standard shoe box cages in a climate-controlled room with an ambient temperature of 23 ± 2°C and a 12-h light/12-h dark cycle. They were fed standard laboratory chow, given water ad libitum, randomly assigned to control or experimental groups, and fasted overnight before each experiment. Caerulein, the decapeptide analog of the potent pancreatic secretagogue cholecystokinin, was obtained from Research Plus (Bayonne, NJ). Other reagents were purchased from sources as reported (7) and were of the highest purity available.
Blood and Tissue Preparation.
Animals were given hourly i.p. injections of saline (control) or saline containing a supramaximally stimulating concentration of caerulein (50 μg/kg) for 12 h. One hour after the last injection, they were sacrificed by an i.p. injection of a lethal dose of pentabarbitone, and blood and samples of the pancreas were rapidly prepared for study. Harvested blood was allowed to clot and centrifuged, and serum was obtained for measurement of amylase and lipase activity. The pancreas was rapidly removed and complete random cross-sections of the head, body, and tail of the pancreas were fixed in 4% neutral phosphate-buffered formalin for histological study. Portions of the pancreas were also prepared for measurement of substance P levels and for immunolocalization of NK1R as described below. In addition, samples of the right lung were fixed in 4% phosphate-buffered formalin for later study. Other samples of pancreas and lung were prepared for measurement of tissue myeloperoxidase (MPO) activity as described below.
Measurement of Substance P Levels.
Pancreatic fragments taken from wild-type animals given either saline or caerulein injections were homogenized in 2 ml of ice-cold 0.1 M HCl for 20 sec. The homogenates were centrifuged (15,000 × g, 15 min, 4°C) and the supernatants were collected. They were adsorbed on C18 cartridge columns (Waters) as described (8). The adsorbed peptides were eluted with 1.5 ml of 5% acetonitrile. The samples were freeze-dried and reconstituted in sample buffer (8). Substance P content was then determined with an ELISA kit (Peninsula Laboratories) according the manufacturer’s instructions and expressed as pg/μg of DNA. DNA assay was performed fluoremetrically by using Hoechst dye 33256 by the method of Labarca and Paigan (9) and calf thymus DNA as standard.
Immunolocalization of NK1R.
An antiserum generated against a peptide representing the last 15 amino acids of the carboxyl terminus of the rat NK1R (10) was prepared by Immuno-Dynamics (La Jolla, CA) according to the m-maleim-idobenzoyl-N-hydroxysuccimide coupling method (11). NK1R antiserum was characterized by ELISA and immunoprecipitation experiments using photoaffinity-labeled NK1Rs expressed in Chinese hamster ovary cells transfected with the rat substance P receptor (10, 11) (data not shown). Immunolocalization of NK1R in pancreas samples taken from wild-type animals given either saline or caerulein injections was accomplished by using confocal microscopy. For these studies, frozen preparations of rat pancreas were fixed in PBS (pH 7.4) containing 4% paraformaldehyde (PFA) for 30 min. Tissues were washed three times in PBS (30-min washes at 4°C) and cryoprotected overnight in PBS containing 30% sucrose before embedded in OCT (Miles). Sections (5 μm) were then cut and mounted on super-frost/plus slides (Fisher Scientific) and then fixed for 3 min in 4% PFA. After washing in Tris-buffered saline (TBS, pH 7.5), sections were first incubated (1 h at room temperature) with blocking solution (TBS containing 50 mM ammonium chloride, 1% normal donkey serum, and 3% BSA). Sections were next incubated (1 h at room temperature) with 1:200 dilution of the rat NK1R antiserum described above. As a negative control, parallel samples were exposed to NK1R antiserum that had been preincubated (1 h, 4°C) with an excess (10 μM) of the carboxyl-terminal 15-amino acid peptide of the NK1R that had been used to generate the NK1R antiserum. This mixture had then been bound to Pansorbin cells (Calbiochem) by following the manufacturer’s protocol. Unbound antibody was used as a negative control. The sections, experimental as well as the negative control, were then washed in TBS and incubated for 1 h at room temperature with 1:100 dilution of fluorescein isothiocyanate (FITC)-conjugated anti-rabbit antibody (Jackson Immunoresearch). All dilutions were made in blocking buffer. After washing in TBS containing 0.2% Triton X-100 (Sigma), sections were mounted in antibleach solution [10% PBS/90% glycerol/n-propyl gallate (1 mg/ml)], examined, and photographed by using a laser confocal microscope (Zeiss).
Morphological Examination.
Paraffin-embedded pancreas and lung samples were sectioned (5 μm), stained with hematoxylin/eosin, and examined by an experienced morphologist who was not aware of the sample identity. Acinar-cell injury/necrosis was quantitated by morphometry as described (7). For these studies, 10 randomly chosen microscopic fields (×125) were examined for each tissue sample and the extent of acinar-cell injury/necrosis was expressed as the percent of the total acinar tissue that was occupied by areas meeting the criteria for injury/necrosis. Those criteria were defined as either (i) the presence of acinar-cell ghosts or (ii) vacuolization and swelling of acinar cells and the destruction of the histoarchitecture of whole or parts of the acini, both of which had to be associated with an inflammatory reaction.
Assays.
Serum amylase activity was measured with 4,6-ethylidene (G7)-p-nitrophenyl (G1)-α1-d-maltoheptoside (Sigma) as the substrate (12). Serum lipase activity was measured colorimetrically as described by Imamura et al. (13). Acini were prepared from mouse pancreas and amylase secretion was studied as a function of caerulein treatment as described (14). Neutrophil sequestration in pancreas and lung was quantitated by measuring tissue MPO activity. For these measurements, tissue samples harvested at the time of sacrifice were stored at −70°C. They were thawed, homogenized in 1 ml of 20 mM phosphate buffer (pH 7.4), centrifuged (10,000 × g, 10 min, 4°C), and the resulting pellet was resuspended in 50 mM phosphate buffer (pH 6.0) containing 0.5% hexadecyltmethylammonium bromide (Sigma). The suspension was subjected to four cycles of freezing and thawing and further disrupted by sonication (40 sec). The sample was then centrifuged (10,000 × g, 5 min, 4°C) and the supernatant was used for MPO assay. The reaction mixture consisted of this extracted enzyme, 1.6 mM tetramethylbenzidine (Sigma), 80 mM sodium phosphate buffer (pH 5.4), and 0.3 mM hydrogen peroxide. This mixture was incubated at 37°C for 110 sec and the absorbance at 655 nm was measured in a CobasBio autoanalyzer. This absorbance was then corrected for the dry weight of the tissue sample used and results were expressed as activity per unit of dry weight (fold increase over control).
Measurement of Pulmonary Microvascular Permeability.
A separate group of animals was used for these studies. Two hours before sacrifice, each animal received an intravenous bolus injection containing FITC-albumin (5 mg/kg, Sigma). Immediately after sacrifice, the trachea was exposed and the lungs were lavaged three times with 1 ml of saline. The lavage fluid was combined and FITC fluorescence was measured in the lavage fluid and serum (excitation = 494 nm; emission = 520 nm). The ratio of fluorescence emission in lavage fluid to blood was calculated and used as a measure of pulmonary microvascular permeability.
Analysis of Data.
The results reported represent the mean ± SEM of values obtained from multiple determinations in three or more experiments. In all figures, vertical bars denote the SEM and the absence of such bars indicates that the SEM is too small to illustrate. The significance of changes was evaluated by using Student’s t test when the data consisted of only two groups or by analysis of variance (ANOVA) when comparing three or more groups. If ANOVA indicated a significant difference, the data were analyzed by using Tukey’s method as a post hoc test for the difference between groups. A P value of ≤0.05 was considered to indicate a significant difference.
RESULTS
Acute Pancreatitis.
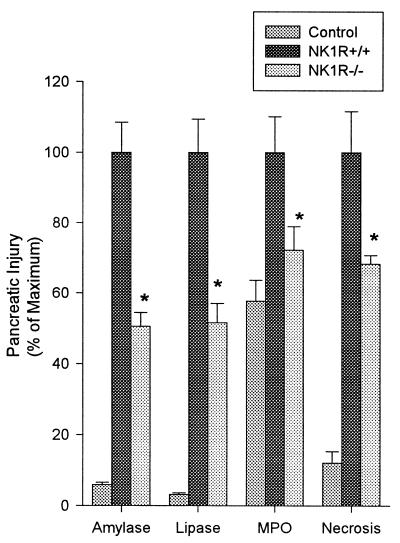
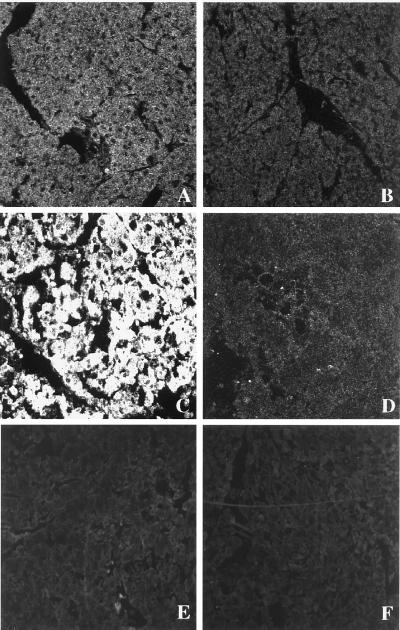
As described (14, 15), wild-type mice given i.p. injections of a supramaximally stimulating dose of the secretagogue caerulein develop acute necrotizing pancreatitis. As shown in Fig. 1, this is manifested by a rise in serum amylase activity, serum lipase activity, pancreatic MPO activity (an indicator of neutrophil sequestration in the pancreas), and morphological evidence of extensive acinar cell necrosis. Substance P levels in pancreas samples taken from control animals were 9.58 ± 3.38 pg/μg of DNA. In samples taken from animals with pancreatitis induced by 4 hourly injections of caerulein, substance P levels were 51.1 ± 13.9 pg/μg of DNA (P < 0.05 vs. control mice). There was a time-dependent increase in pancreatic substance P levels that reached a maximum of 199.65 ± 52.65 pg/μg of DNA after 12 hourly injections of caerulein, a 24-fold increase (P < 0.01 vs. control mice). NK1Rs were found to be present on pancreatic acinar cells in samples prepared form control animals (Fig. 2). Expression of NK1R was markedly increased on acinar cells in samples prepared from animals with pancreatitis (Fig. 2). As expected, no immunoreactivity for NK1 receptor was observed on acinar cells of the NK1R−/− mice, with saline or caerulein treatment (Fig. 2).
Figure 1.
Effects of NK1R deletion on caerulein-induced pancreatitis. Mice were given 12 hourly injections of caerulein (50 μg/kg, i.p.). One hour after the last caerulein injection, mice were sacrificed, and serum amylase activity, serum lipase activity, pancreatic MPO activity, and acinar-cell necrosis were measured. Values are expressed as a percent of the values obtained for wild-type animals given caerulein. These values (100%) were as follows: serum amylase, 32,865 ± 4,064 units/liter; serum lipase, 4,074 + 380 units/liter; pancreas MPO, (1.73 ± 0.18)-fold increase over saline-treated controls; acinar-cell injury/necrosis, 31.3 ± 3.64% of acinar tissue. Results shown are the mean ± SEM for 10 or more animals in each group. Asterisks indicates P < 0.05 when NK1R−/− animals were compared with NK1R+/+ animals.
Figure 2.
Immunohistochemistry of NK1R after caerulein-induced pancreatitis as viewed with confocal microscopy. Mice were given 12 hourly injections of caerulein (50 μg/kg, i.p.). One hour after the last caerulein injection, mice were sacrificed and NK1R expression in the pancreatic cryosections was determined. (A) Section of pancreas from saline-injected NK1R+/+ mouse. (200×.) (B) Section from the same animal with antigen-adsorbed antibody control. (C) Pancreas section from the NK1R+/+ mouse with caerulein-induced pancreatitis. (D) Section from the same animal as C with antigen-adsorbed control. (E) Section of pancreas from saline-injected NK1R−/− mouse. (F) Pancreas section from the NK1R−/− mouse with caerulein-induced pancreatitis.
Effect of NK1R Deletion on Caerulein-Induced Pancreatitis.
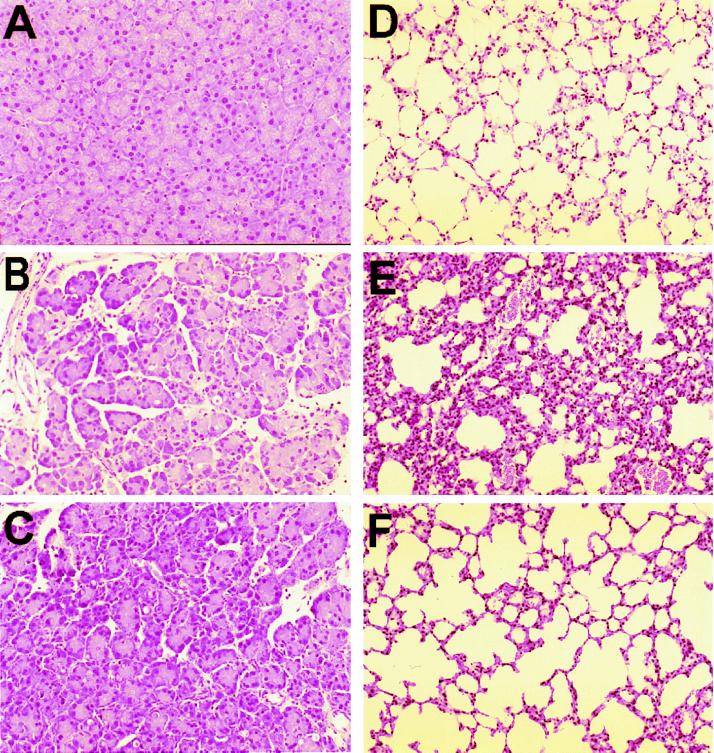
Genetic deletion of NK1R results in marked reduction in the severity of caerulein-induced pancreatitis. As shown in Fig. 1, hyperamylasemia and hyperlipasemia are reduced by 50%, and neutrophil sequestration within the pancreas, as measured by pancreas MPO activity, and acinar cell injury/necrosis (Figs. 1 and 3) are each reduced by 25–30% when NK1R−/− mice are compared with NK1R+/+ wild-type animals with pancreatitis. As can be seen from Fig. 3 C vs. B, NK1R−/− mice had less pancreatic edema than NK1R+/+ mice upon supramaximal stimulation with caerulein.
Figure 3.
Morphologic changes of pancreatitis and pancreatitis-associated lung injury. Representative hematoxylin/eosin-stained sections of pancreas (A–C) and lung (D–F) were examined by light microscopy in normal control animals not given caerulein (A and D), in wild-type NK1R+/+ animals given caerulein (B and E), and in NK1R−/− mice given caerulein (C and F).
Pancreatitis-Associated Lung Injury.
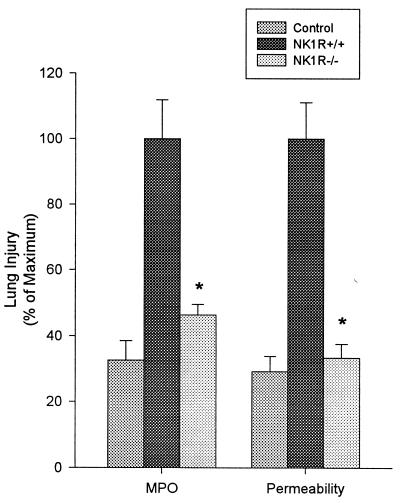
Secretagogue-induced pancreatitis in wild-type mice is associated with lung injury. As shown in Fig. 4, caerulein-induced pancreatitis is associated with a rise in lung MPO activity, indicating the presence of sequestered neutrophils. Furthermore, leakage of intravenously administered FITC-labeled albumin into the alveolar space, a measure of pulmonary microvascular permeability, is increased during secretagogue-induced pancreatitis.
Figure 4.
Effects of NK1R deletion on pancreatitis-associated lung injury. Mice were given 12 hourly injections of caerulein (50 μg/kg, i.p.). One hour after the last caerulein injection, mice were sacrificed and capillary leakage of FITC-albumin and lung MPO activity were measured. Values are expressed as a percent of the value obtained for wild-type animals given caerulein. These values (100%) were as follows: lung MPO activity, 3.06 ± 0.37; FITC-albumin bronchoalveolar lavage fluid to serum ratio 2.13 ± 0.24. Results shown are the mean ± SEM for 10 or more animals in each group. Asterisk indicates P < 0.05 when NK1R−/− animals were compared with NK1R+/+ animals.
Effect of NK1R Deletion on Lung Injury.
Genetic deletion of NK1R results in a marked reduction in the severity of pancreatitis-associated lung injury. Lung MPO activity, lung microvascular permeability, and microscopic evidence of lung injury are reduced in NK1R−/− mice when compared with the NK1R+/+ controls (Figs. 3 and 4).
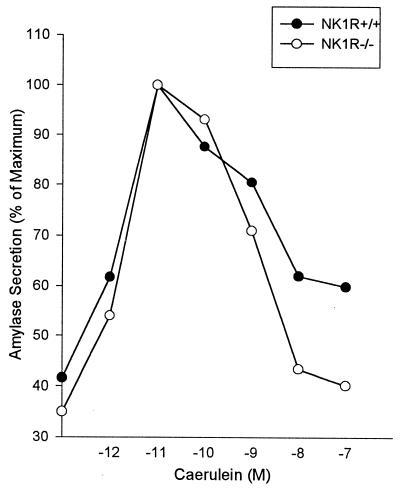
Deletion of NK1R Does Not Alter Caerulein-Stimulated Enzyme Secretion From Pancreatic Acini.
In accord with previous findings (14), biphasic stimulation/inhibition of amylase secretion by increasing concentrations of caerulein was observed when freshly prepared pancreatic acini from wild-type NK1R+/+ mice was evaluated (Fig. 5). Similar changes were also observed when acini obtained from NK1R−/− mice were evaluated. This finding indicates that deletion of NK1R does not alter pancreatic cell responsiveness to the secretagogue caerulein.
Figure 5.
Caerulein-stimulated amylase secretion from pancreatic acini. Acini were prepared from NK1R+/+ and NK1R−/− mice and amylase secretion was determined in response to caerulein treatment as described earlier (14). The results shown are from a representative experiment. The experiment was repeated twice with same results.
DISCUSSION
It is generally believed that the earliest events in the evolution of acute pancreatitis lead to intraacinar cell activation of digestive zymogens and that those enzymes, once activated, cause acinar cell injury (16–18). Recent studies have suggested that the ultimate severity of the resulting pancreatitis may be determined by events that occur subsequent to acinar cell injury including inflammatory cell recruitment and activation and the generation and release of cytokines and other chemical mediators of inflammation (19–21). Although neurogenic factors have been shown to play an important role in a variety of other inflammatory processes (1, 2, 6, 22–26), the potential role of neurogenic factors as regulators of the severity of acute pancreatitis has not been previously evaluated.
Substance P (27) is a neuropeptide that is released from nerve endings in many tissues. Subsequent to its release, substance P binds to NK1Rs on the surface of effector cells and it has been shown to play an important role in many inflammatory states including asthma, immune-complex-mediated lung injury, experimental arthritis, and inflammatory bowel disease. Substance P has been detected within the pancreas and it has been suggested that substance P may act as a neurotransmitter for sensory afferent nerves in the pancreas. Receptors for substance P have also been detected on guinea pig pancreatic acinar cells and the neuropeptide has been shown to act as a secretagogue, stimulating amylase secretion from acinar cells via a G protein-, phospholipase-, inositol phosphate-, and calcium-mediated mechanism in that species (28–30). Rat pancreatic acinar cells apparently do not express receptors for substance P and the neuropeptide does not stimulate enzyme secretion from rat acinar cells (31). Apparently the presence or absence of substance P receptors on mouse pancreatic acinar cells has not been previously examined.
In the current communication, we describe studies that evaluate the role of substance P and the NK1R in regulating the severity of acute pancreatitis. For our studies, we induced pancreatitis by giving mice 12 hourly injections, each containing a supramaximally stimulating dose (50 μg/kg) of the secretagogue caerulein. As reported by us as well as others (7), this secretagogue-induced model of pancreatitis is characterized by hyperamylasemia, hyperlipasemia, sequestration of neutrophils within the pancreas (i.e., increased pancreatic MPO activity), and morphologic evidence of acinar cell necrosis. It is also associated with lung injury that is characterized by sequestration of neutrophils within the lung (i.e., increased lung MPO activity) and increased pulmonary microvascular permeability (i.e., increased leakage of the intravenously administered FITC-labeled albumin into the bronchoalveolar lavage fluid). We now report that this secretagogue-induced model of acute pancreatitis is also associated with a marked increase in the pancreatic content of substance P and that pancreatic acinar cell expression of NK1R is markedly increased during caerulein-induced pancreatitis. These latter observations suggested, to us, that substance P, acting via NK1R, might play a role in mediating acute pancreatitis.
Bozic et al. (6) have recently reported the development of a knockout strain of mice that lack NK1R. They found that immune-complex-mediated lung injury was markedly attenuated in NK1R-deficient animals when compared with wild-type mice that possess NK1R. This report suggested, to us, that the NK1R-deficient mouse strain might be a useful tool with which to examine the potential role of NK1R and substance P as determinants of the severity of acute pancreatitis.
We have found that deletion of NK1R results in a marked decrease in each of the parameters that characterize the severity of secretagogue-induced pancreatitis and pancreatitis-associated lung injury. Specifically, hyperamylasemia and hyperlipasemia are reduced by approximately 50%, pancreatic MPO activity is reduced by roughly 25%, and acinar cell injury/necrosis is reduced by nearly 30% in NK1R-deficient animals. MPO activity in the lung is reduced by 50% and leakage of FITC-albumin is reduced by 65% in the NK1R-deficient knockout animals. The reduction in pancreatitis and lung injury severity that is brought about by NK1R deletion leads us to conclude that, in the NK1R-sufficient state, substance P acting via NK1R has an important proinflammatory role in mediating the severity of pancreatitis and pancreatitis-associated lung injury.
Our results indicate that neurogenic factors, such as substance P, can play an important role in determining the severity of pancreatitis. The mechanisms by which substance P acts to amplify the severity of pancreatitis are not clear. From studies probing the role of substance P in inflammatory processes involving other tissues, one might suspect that the neuropeptide acts primarily on endothelial cells to increase vascular permeability and promote edema formation. Although this explanation could account for the finding that NK1R deletion lessens the increased pulmonary vascular permeability noted in pancreatitis-associated lung injury, it would not account for some of the other effects of NK1R deletion that we have noted including (i) diminished acinar-cell injury/necrosis, (ii) decreased sequestration of neutrophils in the pancreas, and (iii) decreased sequestration of neutrophils in the lung. The observations that acinar-cell expression of NK1R is increased during caerulein-induced pancreatitis and that NK1R deletion does not alter the acinar cell secretory response to the secretagogue caerulein suggests that the proinflammatory effects of substance P may be directly exerted on the acinar cells themselves. It is also tempting to speculate that this phenomenon also explains the role of substance P and NK1R in pancreatitis-associated lung injury, i.e., that substance P acting via NK1R on acinar cells increases the severity of acinar-cell injury and the subsequent increased release of proinflammatory mediators from the pancreas leads to increased lung injury. On the other hand, it remains possible that substance P, acting via NK1R, has a direct injurious effect on the lung during pancreatitis.
Acknowledgments
This work was supported by National Institutes of Health Grants DK-31396 and HL-41587.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: NK1R, neurokinin 1 receptor; MPO, myeloperoxidase; FITC, fluorescein isothiocyanate.
References
- 1.Bowden J J, Garland A M, Baluk P, Lefevre P, Grady E F, Vigna S R, Bunnett N W, McDonald D M. Proc Natl Acad Sci USA. 1994;91:8964–8968. doi: 10.1073/pnas.91.19.8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thurgston G, Baluk P, Hirata A, McDonald D M. Am J Physiol. 1996;271:H2547–H2562. doi: 10.1152/ajpheart.1996.271.6.H2547. [DOI] [PubMed] [Google Scholar]
- 3.Sjodin L, Gylfe E. Biochem Int. 1992;27:145–153. [PubMed] [Google Scholar]
- 4.Jensen R T, Jones S W, Lu Y A, Xu J C, Folkers K, Gardner J D. Biochem Biophys Acta. 1984;804:181–191. doi: 10.1016/0167-4889(84)90148-4. [DOI] [PubMed] [Google Scholar]
- 5.Patto R J, Vinayek R, Jansen R T, Gardner J D. Pancreas. 1992;7:447–452. doi: 10.1097/00006676-199207000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Bozic C R, Lu B, Hopken U E, Gerard C, Gerard N P. Science. 1996;273:1722–1725. doi: 10.1126/science.273.5282.1722. [DOI] [PubMed] [Google Scholar]
- 7.Kaiser A M, Saluja A K, Sengupta A, Saluja M, Steer M L. Am J Physiol. 1995;269:C1295–C1304. doi: 10.1152/ajpcell.1995.269.5.C1295. [DOI] [PubMed] [Google Scholar]
- 8.Castagliuolo I, Keates A C, Qiu B, Kelly C P, Nikulasson S, Leeman S E, Pothoulakis C. Proc Natl Acad Sci USA. 1997;94:4788–4793. doi: 10.1073/pnas.94.9.4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labarca C, Paigan K. Anal Biochem. 1980;102:344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- 10.Li H, Hsu P, Sachais B S, Krause J E, Leeman S E, Boyd N. J Biol Chem. 1996;271:1950–1956. doi: 10.1074/jbc.271.4.1950. [DOI] [PubMed] [Google Scholar]
- 11.MacDonald D,D M, Silberman S C, Lowe J A, III, Drozda S E, Leeman S E, Boyd N D. Mol Pharmacol. 1996;49:808–813. [PubMed] [Google Scholar]
- 12.Pierre K J, Tung K K, Nad H. Clin Chem. 1976;22:1219. [Google Scholar]
- 13.Imamura S, Hirayama T, Arai T, Takao K, Misaka H. Clin Chem. 1989;35:1126. [Google Scholar]
- 14.Saluja A, Hofbauer B, Yamaguchi Y, Yamanaka K, Steer M. Biochem Biophys Res Commun. 1996;220:875–878. doi: 10.1006/bbrc.1996.0498. [DOI] [PubMed] [Google Scholar]
- 15.Ohshio G, Saluja A, Leli U, Sengupta A, Steer M L. Pancreas. 1989;4:739–743. doi: 10.1097/00006676-198912000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Saluja A, Saluja M, Villa A, Leli U, Rutledge P, Meldolesi J, Steer M L. J Clin Invest. 1989;84:1260–1266. doi: 10.1172/JCI114293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grady T, Saluja A, Kaiser A, Steer M. Am J Physiol. 1996;271:G20–G26. doi: 10.1152/ajpgi.1996.271.1.G20. [DOI] [PubMed] [Google Scholar]
- 18.Saluja A K, Saluja M, Printz H, Zavertnik A, Sengupta A, Steer M L. Proc Natl Acad Sci USA. 1989;86:8968–8971. doi: 10.1073/pnas.86.22.8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gross V, Leser H G, Heinisch A, Scholmerich J. Hepatogastroenterology. 1993;40:522–530. [PubMed] [Google Scholar]
- 20.Scholmerich J. Scand J Gastroenterol Suppl. 1996;219:37–42. doi: 10.3109/00365529609104998. [DOI] [PubMed] [Google Scholar]
- 21.Dusseti N J, Ortiz E M, Mallo G V, Dagorn J C, Iovanna J L. J Biol Chem. 1995;270:22417–22421. doi: 10.1074/jbc.270.38.22417. [DOI] [PubMed] [Google Scholar]
- 22.Pothoulakis C, Castagliuolo I, LaMont T, Jaffer A, O’Keene J C, Snider R M, Leeman S E. Proc Natl Acad Sci USA. 1994;91:947–951. doi: 10.1073/pnas.91.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lotz M, Carson D A, Vaughan J H. Science. 1987;235:893–895. doi: 10.1126/science.2433770. [DOI] [PubMed] [Google Scholar]
- 24.Swain M G, Argo A, Blennerhassett P, Stanisz A, Collins S M. Gastroenterology. 1992;102:1913–1919. doi: 10.1016/0016-5085(92)90313-n. [DOI] [PubMed] [Google Scholar]
- 25.Tissot M, Pradelles P, Giroud J P. Inflammation. 1988;12:25–35. doi: 10.1007/BF00915889. [DOI] [PubMed] [Google Scholar]
- 26.Stanisz A M, Befus D, Bienenstock J. J Immunol. 1986;136:152–160. [PubMed] [Google Scholar]
- 27.Chang M M, Leeman S E. J Biol Chem. 1970;245:4784–4790. [PubMed] [Google Scholar]
- 28.Song S Y, Iwashita S, Noguchi K, Konishi S. Neurosci Lett. 1988;95:143–148. doi: 10.1016/0304-3940(88)90647-7. [DOI] [PubMed] [Google Scholar]
- 29.Sjodin L, Dahlen H G, Gylfe E. J Physiol. 1991;444:763–776. doi: 10.1113/jphysiol.1991.sp018905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sjodin L, Viitanen E, Gylfe E. J Physiol. 1994;476:69–77. [PMC free article] [PubMed] [Google Scholar]
- 31.Gardner J D, Jensen R T. In: The Pancreas: Biology, Pathobiology, and Disease. Go V L V, Dimango E P, Gardner J D, Lebenthal E, Reber H A, Scheele G A, editors. New York: Raven; 1993. pp. 151–166. [Google Scholar]