Abstract
Understanding the mechanisms that govern neuronal responses to oxidative and metabolic stress is essential for therapeutic intervention. In vitro modeling is an important approach for these studies, as the metabolic environment influences neuronal responses. Surprisingly, most neuronal culture methods employ conditions that are non-physiological, especially with regards to glucose concentrations, which often exceed 20 mM. This concentration is a significant departure from physiological glucose levels, and even several-fold greater than that seen during severe hyperglycemia. The goal of this study was to establish a physiological neuronal culture system that will facilitate the study of neuronal energy metabolism and responses to metabolic stress. We demonstrate that the metabolic environment during preparation, plating, and maintenance of cultures affects neuronal viability and the response of neuronal pathways to changes in energy balance.
Keywords: Neuronal culture, physiological glucose, AMP-activated protein kinase, ATP, AICAR
INTRODUCTION
Energy homeostasis in the brain is complex, due to high energy demands, different regional energy requirements, heterogeneous cell populations, and the dynamic coupling of neuronal activity and energy consumption (Magistretti, 1999; Mokdad, et al., 2001; Lo, et al., 2003). While some neurons assimilate peripheral and central signals, indexing the overall metabolic status of the organism (Levin, et al., 1999; Routh, et al., 2004), all neurons must monitor their own energy status as well. Although numerous pathways have been identified that relay information concerning overall metabolic status to the brain (Wang, et al., 1998; Magistretti, 1999; Levin, et al., 1999; Routh, 2002), far less is known about mechanisms of cellular energy regulation in neurons. These neuronal mechanisms may serve as targets for intervention in disorders of energy availability, such as stroke and obesity. Therefore, a physiologically relevant in vitro model for cortical neurons may prove an important tool in the study of metabolic pathways.
Studies utilizing neuronal cultures must consider that nutrient concentrations are tightly regulated in the brain (Magistretti, 1999; Bouzier-Sore, et al., 2003), especially regarding glucose, the brain’s preferred fuel. Extracellular glucose concentrations in rodent and human brain are closely coupled to plasma glucose concentrations (Silver, et al., 1994; Abi-Saab, et al., 2002; de Vries, et al., 2003). In euglycemia, plasma glucose can range from 5.5 to 7.8 mM, while brain glucose fluctuates from 0.82 to 2.4 mM, depending on the microdialysis method employed (Silver, et al., 1994; Abi-Saab, et al., 2002; de Vries, et al., 2003). Using glucose microelectrodes to continuously monitor changes in glucose concentrations it has been demonstrated that the extracellular glucose concentration in the brain is altered dramatically during hyper- and hypoglycemia, in which plasma levels may reach 15.2 mM or drop to 2.8 mM, respectively. In these situations, brain glucose can rise to 4.5 mM or drop as low as 0.16 mM (Silver, et al., 1994). Select studies acknowledge these relationships, and emphasize the importance of in vitro culture conditions for CNS research (Wang, et al., 2004; Song, et al., 2005; Lee, et al., 2005; Morgenthaler, et al., 2006; Abe, et al., 2006; Bak, et al., 2006; Kang, et al., 2006; Canabal, et al., 2007a; Canabal, et al., 2007b); however, most studies do not. It is not unusual for culture media to contain 25 mM glucose, a level perhaps seen in the plasma of obese ob/ob mice (Schwartz, et al., 1996), but never seen in the brain. The impact of using media with such high glucose concentrations on the data is still largely unknown.
The effect of nutrient availability on neuronal energy balance requires a marker that reflects cellular energy status. AMP-activated protein kinase (AMPK) is an energy sensor in peripheral tissues, functioning at the cellular level to sense and respond to energy challenge (Hardie, et al., 1997). Stressors that decrease cellular ATP lead to AMPK phosphorylation (pAMPK) and activation [reviewed in (Hardie, et al., 2003; Carling, 2004)] (Hardie, 1999; Winder, 2000; Hardie, 2004; McCullough, et al., 2005). AMPK regulates the activity of metabolic enzymes and alters gene expression to restore energy status by inhibiting ATP-consuming processes and stimulating ATP-producing pathways (Hardie, 1999; Hardie, et al., 2003). Thus, AMPK phosphorylation reflects a cell’s ability to respond to changes in its metabolic state. AMPK is expressed in the brain (Gao, et al., 1996; Stapleton, et al., 1996) where we (Landree, et al., 2004; Kim, et al., 2004a; McCullough, et al., 2005) and others (Turnley, et al., 1999; Culmsee, et al., 2001) have shown its expression predominately in neurons. Importantly, AMPK is now recognized to function as a cellular and organismal energy sensor (Carling, 2004; Andersson, et al., 2004; Minokoshi, et al., 2004; Kim, et al., 2004a; Kola, et al., 2005; McCullough, et al., 2005).
The experiments reported here were designed as an expansion of previous studies that utilized short-term (used by DIV4) dissociated hypothalamic neurons, cultured in media containing 2.5 mM glucose (Wang, et al., 2004; Song, et al., 2005; Canabal, et al., 2007a; Canabal, et al., 2007b). We have expanded this concept to the preparation and maintenance of long-term dissociated cortical neuronal cultures (DIV10-13) in both physiologic and non-physiologic concentrations of glucose. To maintain a consistent extracellular glucose level throughout long-term culture, a novel feeding paradigm was implemented in which cultures were fed fresh media containing a concentration of glucose that at each feeding restored the cultures to the concentration present at plating. Additionally we examined the effect of short-term changes in glucose levels during culture preparation on long-term neuronal viability.
We hypothesize that maintaining neuronal cultures in physiological glucose concentrations is important for neuronal viability, and essential for AMPK to respond to alterations in energy metabolism. Our protocol for preparing and maintaining primary neuronal cultures provides a more consistent glucose environment, resulting in improved neuronal viability and responsiveness to energy challenge as indicated by changes in ATP and pAMPK levels.
MATERIALS AND METHODS
The Johns Hopkins University Institutional Animal Care and Use Committee approved all protocols, and all guidelines for the care and use of laboratory animals from the National Institutes of Health were followed.
Neuronal Culture Paradigms
In order to control media glucose concentrations, primary dissociated cortical neurons were cultured and maintained in a newly available media from Invitrogen (Catalog number: 0050128DJ), based on Neurobasal-A, but devoid of glucose and pyruvate (Wang, et al., 2004; Song, et al., 2005) and referred to here as NB-Af. Physiological and non-physiological media glucose conditions were established by the addition of 3 mM (NB-A3) or 25 mM (NB-A25) glucose to NB-Af, respectively. Neurobasal (NB, Invitrogen), a commonly used neuronal culture media that contains 25 mM glucose and 0.23 mM pyruvate, served as a control in these studies. Three culture paradigms were developed using these medias (Fig. 1A-B). 1) The first paradigm was established to compare neuronal cultures that have been prepared and maintained in either NB-A3 or NB-A25 (Fig. 1A). 2) The second paradigm was designed to alter glucose concentrations by utilizing two feeding strategies, standard and restorative (Fig. 1A). 3) The final paradigm was designed to examine the effect of short-term alterations in glucose concentration during culture preparation on long-term viability. For this purpose, cultures were either plated in media containing glucose concentrations equal to that in which they were prepared, or in media containing a drastically different concentration of glucose from that of preparation (Fig. 1B).
Fig. 1. Culture Paradigms.
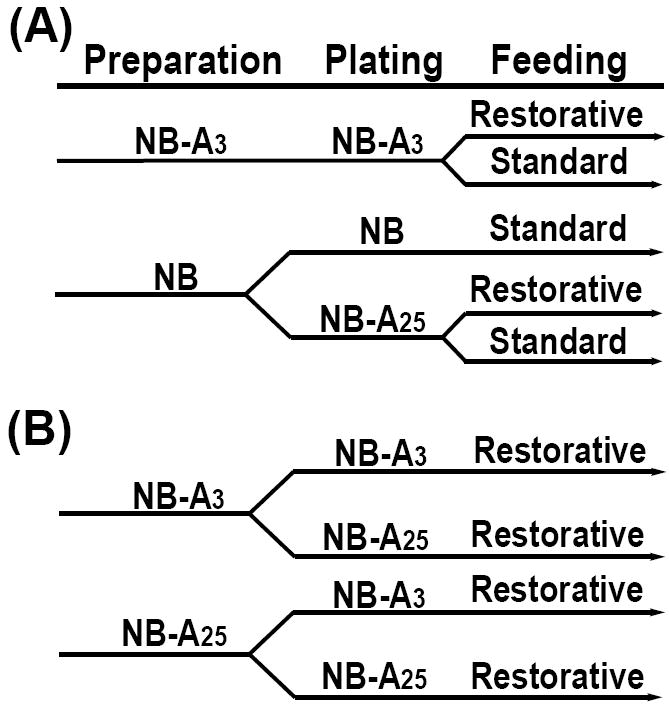
(A) Following dissection and digestion cortical neurons were prepared in either NB or NB-A3 (washes, dissociation and counting). Neurons in NB-A3 remained in this media for plating while neurons in NB media were subsequently divided and placed into either NB or NB-A25 for plating. Neurons plated in NB-A3 or NB-A25 were maintained by either standard or restorative feedings. The NB cultures were maintained by standard feedings only. (B) Following dissection and digestion cortical neurons were divided and prepared in NB-A3 or NB-A25. Following culture preparation each group was further divided into two groups: the first was plated in the same media as the preparation media maintaining a consistent glucose concentration, the second was plated in media supplemented with a different glucose concentration than was present during preparation. All cultures in this paradigm were maintained by the restorative feeding strategy.
Primary Dissociated Cortical Neuron Culture Preparation
For all culture paradigms cortices were dissected from embryonic (E17) Sprague-Dawley rats (Harlan, Indianapolis, Indiana) in Brooks Logan buffer as described previously (Dawson, et al., 1993; Landree, et al., 2004). Tissue was digested in 0.25% (w/v) trypsin (Invitrogen) in HBSS supplemented with 10 μg/ml DNAse (2000 units/mg; Sigma) for 20 min/24-36 cortices in a 37°C water bath following which fetal bovine serum (Hyclone) was used to inactivate the trypsin. Tissue was then placed into NB-A3, NB-A25 or NB media for culture preparation (washes, dissociation via trituration, and cell counting) (Fig. 1A-B). All medias were supplemented with 2% B27, 2 mM glutamine, and 0.5% Penicillin/Streptomycin (Invitrogen). All cells were plated at 5 × 105 cells/ml (~2.5×105 cells/cm2) on poly-D-lysine coated plastic Nunclon culture dishes as required for each type of experiment: 24-well plates for viability and ATP assays; 6-well plates for Western blot analysis.
Culture Maintenance
For the second culture paradigm (Fig. 1A) two feeding strategies (standard and restorative) were utilized. Feedings consisted of a 50% media change on DIV3, 6, 8, and 10. The DIV3 feeding included cytosine arabinoside (AraC) at a final concentration of 1 μM to inhibit cell proliferation. Media glucose concentrations were carefully monitored throughout the culture using the ACCU-CHEK Active blood glucose meter (Roche). The lower detection limit of the meter was 10 mg/dl (0.55 mM) of glucose, in which case a “lo” reading was obtained. For data analysis purposes, “lo” readings were considered 0.5 mM. Glucose measurements were taken immediately prior to and within 30 minutes of each feeding (Fig. 2). For standard feedings, the media was replaced with NB-Af containing either 3 or 25 mM glucose corresponding to the glucose concentration at plating. Cells plated and maintained in NB were fed using the standard feeding strategy as this is the common protocol and glucose concentrations cannot be modulated using this base media. On the other hand, restorative feedings took into account the amount of glucose utilized by the culture between feedings. These feedings involved the addition of NB-Af media that contained enough glucose to reestablish the concentration present at plating. Cultures were used for experimentation before feedings on DIV10 and 13 as specified.
Fig. 2. The effect of feeding strategy on ambient glucose concentrations.
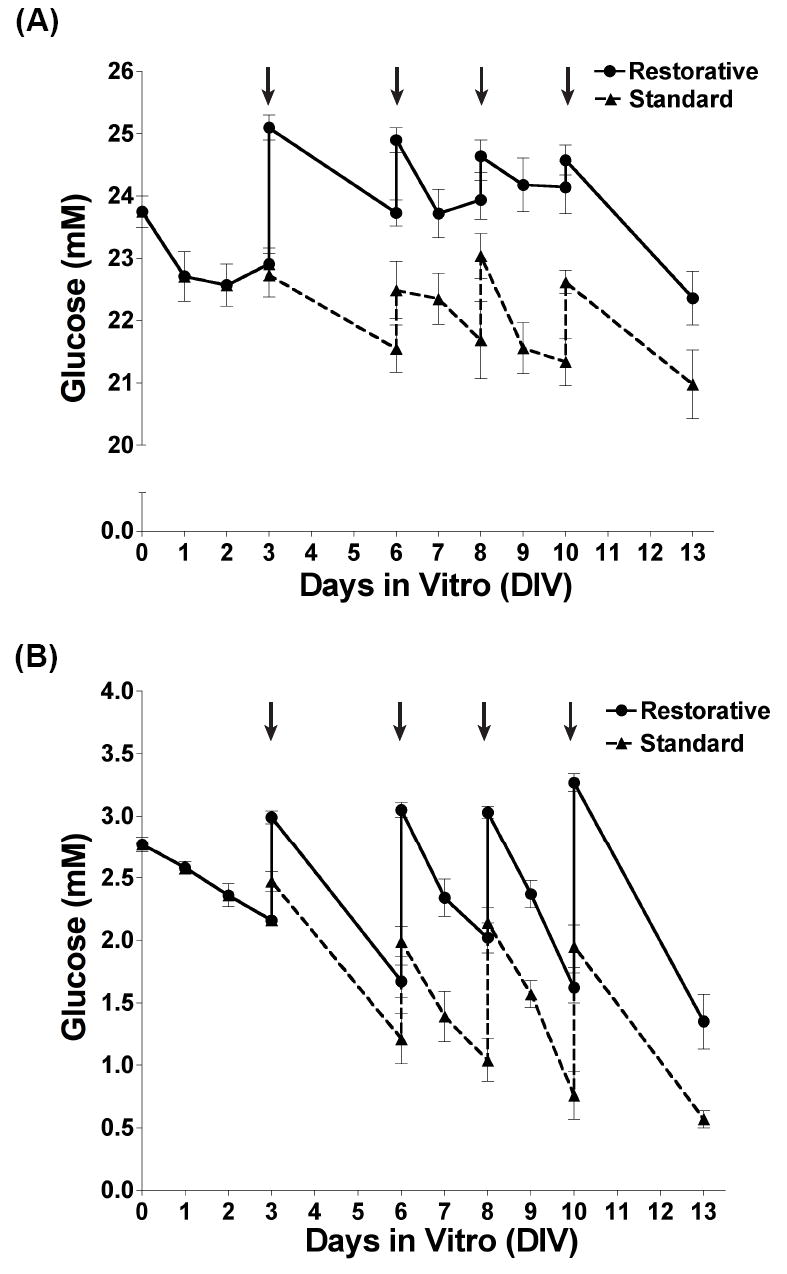
Media glucose concentrations were measured and recorded for cultures prepared and maintained in NB-A3 (B) or NB-A25 (A). Glucose measurements taken from cultures maintained by restorative feedings are represented as solid lines (circles) and those from cultures maintained by standard feedings as dashed lines (triangles).
Pharmacological Agents
C75 (obtained from and characterized by Craig Townsend and Jill McFadden, the Department of Chemistry at the Johns Hopkins University, and from FASgen, Inc.) and 5-Aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR) (Toronto Research Chemicals, Inc.) were solubilized in NB-Af (without B27, glutamine, or glucose) as stock solutions of 6.5 mM and 100 mM, respectively. Cells were treated with 20 μl/well for a 24-well plate or 60 μl/well for a 6-well plate with vehicle (NB-Af) with or without C75, or AICAR for the indicated times and final concentrations.
Measurement of Intracellular ATP Levels
On DIV9, 10 or 13 the media from a 24 well plate was removed via aspiration and cells were washed with 3.5 ml of conditioned culture media (media in which the cells have been maintained, referred to as CM) or NB-Af (without B27 or glutamine) containing the specified glucose concentrations (0.1-25 mM). This wash is extremely important to remove residual glucose and B27, which can affect the experimental outcome. Media was replaced with 500 μl of CM or with NB-Af containing the specified glucose concentrations, as used for the wash. Following equilibration for 30 minutes at 37°C/5% CO2 cells were treated for 1 hour with 20 μl of vehicle (NB-Af) or C75 at a final concentration of 150 μM. Cells were lysed on ice using 500 μl of TE buffer (4 mM Tris, 0.25 mM EDTA at pH 7.4) per well and harvested by trituration into boil-proof tubes (ISC Bioexpress, Kaysville, UT) to prevent sample loss during denaturation at 95°C. ATP levels were measured in the linear range using the ATP Bioluminescence kit CLS II (Roche Applied Science, Indianapolis, IN) according to the manufacturer’s protocol. The plates were analyzed using a Perkin-Elmer Victor2 1420. Data are represented as the percentage of either the vehicle control or the CM control.
Cell Viability Assay
Cortical neurons were incubated in 350 μl of 3 μM Calcein AM (Invitrogen) in 37°C dPBS (PBS supplemented with magnesium and calcium without phenol red; Invitrogen) for 30 minutes at 37°C/5% CO2. The conversion of the cell permeant non-fluorescent Calcein AM dye to the intensely fluorescent calcein dye is catalyzed by intracellular esterase activity and measured at 485 nm/535 nm using a Perkin-Elmer Victor2 1420. Data are represented as the percentage of live cells, using the NB-A3 condition as a control.
Western Blot Analysis
Cultures were treated with 60 μl of vehicle (NB-Af) or AICAR per well at a final concentration of 1.5 mM for 0, 1, or 2 hours at 37°C/5% CO2. Treatments were performed in CM, with the volume adjusted 24 hours before treatment. After incubation, cells were washed with 3 ml of dPBS, lysed in 1X protein sample buffer (62.5 mM Tris-Cl pH 6.8, 10% glycerol, 2% sodium dodecyl sulfate, 1% β-Mercaptoethanol and trace amounts of bromophenol blue), boiled, and stored at -80°C. Samples were run on 10% Tris-HCl SDS-polyacrylamide gels (BioRad) and transferred unto polyvinylidene diflouride (PVDF) membranes (BioRad). Blots were successively probed, stripped, and re-probed with the following antibodies: 1:1000 dilution of either anti-phosphorylated-AMPKα (Thr172) or AMPKα (Cell Signaling) in TBST containing 0.1% tween-20, 5% protease free BSA and 50 mM NaF. Blots were visualized using SuperSignal chemiluminescence kits (Pierce Biotechnology). A 1:10 dilution of the Femto kit was used to detect the pAMPK signal and the undiluted Pico kit was used to detect the AMPKα signal. Band intensity was quantified using Scion Image (NIH) and is reported as the ratio of pAMPKα to AMPKα with the 0 hour time points serving as controls.
Statistical Analysis
Data are represented as means ± S.E. from multiple determinations (n>4). Data were analyzed by one-way ANOVA using either the Dunnett (viability and Western) or the Tukey (ATP) post-tests. Differences from post-tests were considered statistically significant at *, p < 0.05; **, p < 0.01; ***, p < 0.001.
RESULTS
Restorative feedings reduce fluctuations in ambient glucose concentrations
The overall goal of this study was to compare long-term cultures of primary dissociated cortical neurons plated and maintained in media containing non-physiologically high glucose concentrations to those maintained in physiological glucose concentrations. A Neurobasal-A media, devoid of glucose and pyruvate (NB-Af), allowed for the control of glucose concentrations. We supplemented NB-Af with B27 and either a non-physiological high glucose concentration of 25 mM (NB-A25), or 3 mM (NB-A3), a physiological glucose concentration for the brain (Silver, et al., 1994; Abi-Saab, et al., 2002; de Vries, et al., 2003) (Fig. 1A).
One variable examined was the effect of two feeding methods on NB-A3 and NB-A25 cultures. The most common feeding method (here termed standard) for dissociated neuronal cultures involves removing one-half of the media from each well and replacing it with media containing the original plating glucose concentration. This method does not account for the glucose utilized between feedings, and thus the cultures never regain the same glucose levels present at plating. Our restorative feeding method involves removal of one-half of the media and replacement with media containing enough glucose to bring the final concentration back to that of plating. Restorative feedings require glucose readings before each feeding, to calculate the amount of glucose to add to the replacement NB-Af, and after, to confirm that the original glucose concentration is restored (Fig. 2).
Monitoring glucose levels throughout a 13-day culture period allowed comparison of NB-A25 (Fig. 2A, dashed line) and NB-A3 (Fig. 2B, dashed line) cultures. Glucose levels in NB-A25 cultures maintained by standard feedings (standard NB-A25) decreased 2.7 mM from plating to 13 days in vitro (DIV13) but remained well above physiological levels (Fig. 2A, dashed line). Glucose in standard NB-A3 cultures decreased 2.2 mM (>79% decrease) (Fig. 2B, dashed line), causing ambient glucose concentrations to drop below our limit of detection (<0.5 mM) by DIV13. We therefore employed the restorative feeding method, and compared it with the standard approach by continuing to monitor ambient glucose daily.
When maintained by restorative feedings, glucose decreased 1.38 mM from plating to DIV13 in both the NB-A3 and NB-A25 cultures (Fig. 2A-B, solid lines). Thus, restorative feedings maintained ambient glucose within a tighter concentration range than standard feedings. By comparing the rate of glucose decline during feeding intervals (DIV0-3, DIV3-6, DIV10-13) it was noted that glucose consumption increased with age in vitro. Glucose levels in restorative NB-A25 cultures decreased during the DIV0-3, DIV3-6 and DIV10-13 feedings by an average of 0.31 +/- 0.29, 1.36 +/- 0.19, and 2.22 +/- 0.34 mM glucose, respectively (Fig. 2A, solid line). The same trend was observed in restorative NB-A3 cultures, with decreases of 0.54 +/- 0.064 mM from DIV0-DIV3, 1.34 +/- 0.089 mM from DIV3 to DIV6, and greater than 1.96 +/- 0.15 mM between the DIV10 feeding and DIV13 (Fig. 2B, solid line). Thus, when maintaining long-term neuronal cultures, particularly in physiological glucose concentrations, restorative feedings are essential in sustaining desired glucose levels and preventing large fluctuations in ambient glucose concentrations.
Restorative feedings improve long-term neuronal viability
Due to fluctuations in glucose concentrations observed during long-term culture (Fig. 2A-B), we sought to determine whether the more consistent glucose environment afforded by restorative feedings improved long-term neuronal viability. NB-A3 and NB-A25 cultures were maintained with either standard or restorative feedings, while cells maintained in the commonly used media, Neurobasal (NB), supplemented with B27 and containing 25 mM glucose and 0.23 mM pyruvate were fed using the standard protocol. Neuronal viability was assessed before feedings on DIV10 and DIV13 using the fluorogenic esterase substrate, Calcein AM (Fig. 3). We emphasize here our overall hypothesis that the cumulative glucose environment, and not necessarily the glucose at the time of the experiment, has a significant impact on neuronal viability. We do note; however, that the cultures used on DIV10 and DIV13 had very similar glucose levels at the time of the experiment within each feeding paradigm.
Fig. 3. Changes in glucose concentration during culture preparation, plating and maintenance affect neuronal viability.
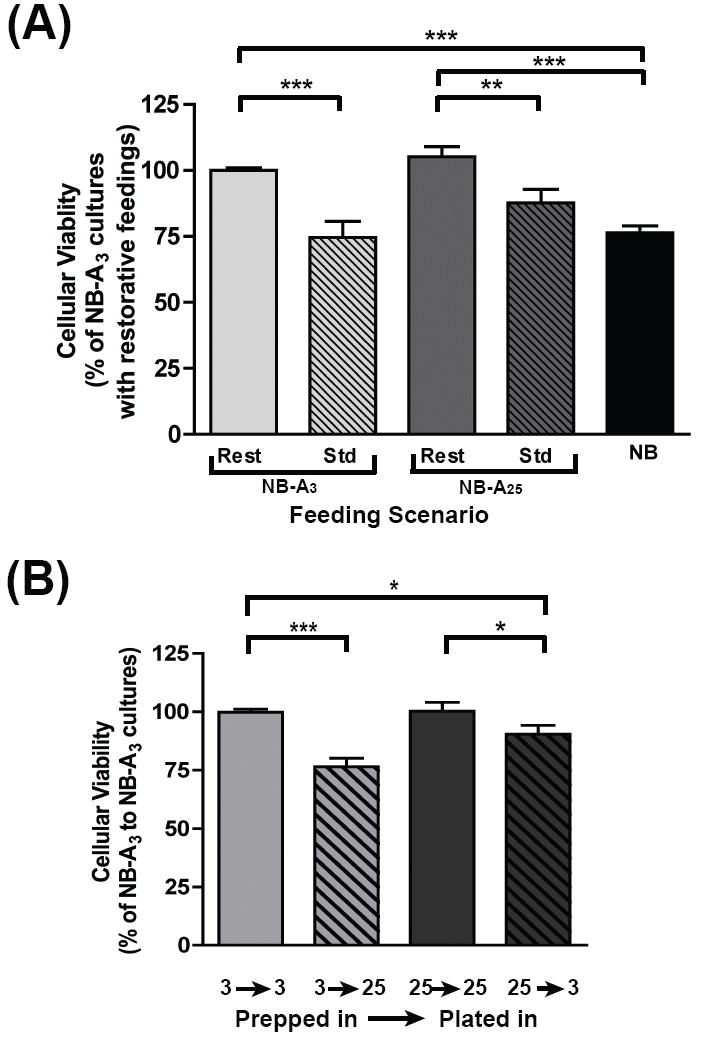
(A) Dissociated primary cortical neurons were cultured in NB, NB-A3 or NB-A25 and maintained by restorative (‘rest’) or standard (‘std’) feedings. Calcein AM was used to evaluate neuronal viability before feedings on DIV10 and 13 and represented as a percentage of restorative NB-A3 cultures. (B) Neurons were prepared in NB-A3 or NB-A25 then further divided into two groups for plating. Neurons were either plated in the same media that they were prepared in (3→3 and 25→25) or switched into a media containing a different concentration of glucose from what they had been prepared in (3→25 and 25→3). Cell viability was determined before feeding on DIV10 using Calcein AM and represented as a percentage of NB-A3 → NB-A3 cultures *, p<0.05; **, p<0.01; ***, p<0.001.
All cultures maintained by standard feedings displayed similar levels of fluorescence, whether plated in NB, NB-A3 or NB-A25. However, cultures maintained by restorative feedings were significantly more viable than those given standard feedings, whether prepared in NB-A3 or NB-A25 (Fig. 3A). Standard NB-A3 cultures were 25.5% less viable than restorative NB-A3 cultures. Likewise, standard NB-A25 cultures were 20.4% less viable than those maintained by restorative feedings. Strikingly, cultures maintained using the generally accepted NB media were 23.7% and 28.8% less viable than restorative NB-A3 and NB-A25 cultures, respectively. Also important to note is that there are no significant differences in viability between NB-A3 and NB-A25 cultures when both are maintained via restorative feedings. These data suggest that fluctuations in ambient glucose concentrations during long-term culture are detrimental to neuronal viability.
Significant fluctuations in glucose concentration during neuronal culture preparation and plating are detrimental to long-term neuronal viability
Given that tightly maintaining glucose levels during culture maintenance had a significant effect on neuronal viability, we hypothesized that changes in glucose concentration during culture preparation and plating might also be detrimental to neuronal viability. Neuronal cultures were prepared (washed, triturated, and counted) in NB-A3 or NB-A25, and then plated in either the same media as during preparation, or in media containing a different glucose concentration (Fig. 1B). Given the improved viability due to restorative feedings, all neurons for these experiments were maintained by this protocol. Neuronal viability was assessed before feeding on DIV10 using Calcein AM.
Neuronal viability was decreased when glucose levels had been changed drastically between culture preparation and plating (Fig. 3B). Neurons prepared in NB-A3 but plated in NB-A25 were 23.3% less viable than those that remained in NB-A3 throughout preparation and plating. Similarly, neurons prepared in NB-A25 but plated in NB-A3 were 9.8% less viable than those prepared and maintained in NB-A25. Notably, neurons prepared and plated in the same glucose, whether 3 or 25 mM, showed no significant differences in viability. These results demonstrate that any dramatic change in glucose concentration during preparation and plating is detrimental to long-term neuronal survival. Based on these results, cultures in subsequent experiments were prepared and plated in the same media, and maintained by restorative feedings. This difference in viability could be due to changes in osmolarity during culture preparation and plating. To be consistent with the majority of published studies that employ media “switches” into higher or lower media glucose concentrations prior to the experiment we did not adjust the osmolarity of our medias (which include glutamine and B27): NB-A3 = 258.2 +/- 3.9 mOsm, NB-A25 = 278.6 +/- 4.2 mOsm and NB = 235.8 +/- 2.6 mOsm.
Neurons are responsive to metabolic changes when maintained in physiological glucose concentrations
Our method was designed with the ultimate goal of developing neuronal cultures that could respond to changes in energy balance in a manner that more closely approximates in vivo responses. We hypothesized that maintaining neuronal cultures in physiological glucose concentrations would be important not only for neuronal viability, but also to provide a more accurate reproduction of the changes in ATP and AMPK seen in vivo in response to modulators of energy metabolism. We (Kim, et al., 2004a; McCullough, et al., 2005) and others (Clough-Helfman, et al., 1990; Culmsee, et al., 2001; Andersson, et al., 2004; Minokoshi, et al., 2004; Kim, et al., 2004b; Kim, et al., 2005; Carling, 2005) have demonstrated that neuronal AMPK is activated in vitro following metabolic stress, as well as in vivo during energy-poor states such as CNS ischemia. We have previously shown that C75, a synthetic inhibitor of fatty acid synthase and stimulator of carnitinepalmitoyl-transferase-1, increases ATP levels in cortical and hypothalamic neurons (Landree, et al., 2004; Kim, et al., 2004a). We therefore used C75 to alter ATP levels in order to investigate how NB-A3 and NB-A25 cultures respond to changes in extracellular glucose availability.
Neurons were cultured in NB-A3 or NB-A25 and maintained by restorative feedings. On DIV9, 10 and 13 the media of the conditioned media (CM) controls was removed, used to wash the cells, and replaced. For all other treatment groups the media was removed, the cells were washed with and their media replaced by NB-Af (without B27 and glutamine) supplemented with varying glucose concentrations (0, 0.1, 0.5, 1, 1.5, 3, 5, 10, and 25 mM). After 30 minutes, neurons were treated with C75 (150 μM) or vehicle (NB-Af) for 1 hour. Intracellular ATP levels were analyzed following the treatment by measuring bioluminescence and represented as percent of each vehicle control (Fig. 4A-B) or as percent of the CM control (Fig. 4C-D).
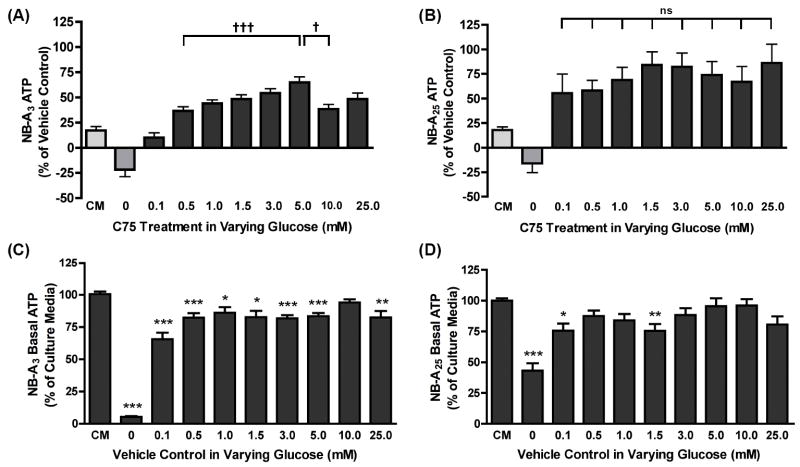
Fig. 4. Neurons maintained in non-physiological glucose concentrations have a blunted response to metabolic changes.
Dissociated primary cortical neurons were cultured in NB-A3 (A and C) or NB-A25 (B and D) and maintained by restorative feedings. On DIV9-13 culture media was removed, cells washed, and media replaced with NB-Af supplemented with varying glucose concentrations (0.1-25 mM). Following a 30 minute equilibration in the new media, cells were treated with 150 μM C75 or vehicle (NB-Af) for 1 hour. Changes in ATP levels were evaluated by luminescence and represented as the percent change over each vehicle control (A and B). The vehicle control data from (A) and (B) are re-represented as a percent of the culture media control (CM) for the neurons cultured in NB-A3 (C) or NB-A25 (D). ATP levels from the NB-A3 (A) and NB-A25 (B) cultures treated with C75 in the presence of 0.1-25 mM glucose were considered statistically significant at †, p<0.05; †††, p<0.001. As indicated on the graph, ATP levels from the NB-A3 (A) cultures treated in 5.0 mM glucose are significantly increased from those in 0.5 mM glucose †††, p<0.001). Decreases in ATP are observed above 5.0 mM in the 10 and 25 mM treatment groups, where 10 mM is significantly reduced relative to 5.0 mM (†, p<0.05). As indicated by the ‘ns’, there is no significant difference between any of the glucose conditions (0.1-25 mM) in the NB-A25 cultures (B). The basal ATP values from the NB-A3 cultures (C) are all significantly lower than the CM control with the exception of the 10 mM condition as illustrated on the graph, *, p<0.05; **, p<0.01; ***, p<0.001. Additionally, the only differences observed in this culture between glucose conditions was between the 0.1 mM condition and the following: 0.5 mM (p<0.05), 1.0 mM (p<0.01), 5.0 mM (p<0.05), and 10 mM. The basal ATP values from the NB-A25 cultures (D) that were significantly lower than the CM control are indicated. No significant difference was observed between any of the glucose conditions (0.1-10 mM). The 0 mM glucose conditions on all graphs (A, B, C & D) were statistically different from all other conditions (p≤0.05).
As reported previously (Landree, et al., 2004; Kim, et al., 2004a), C75 treatment produced a moderate but significant increase in ATP over vehicle control in CM for both the NB-A3 (17.2%) (Fig. 4A) and NB-A25 cultures (17.9%) (Fig. 4B). C75-induced increases in ATP were observed only when glucose was included in the test media. In test media devoid of glucose, C75 decreased ATP 21.7% (Fig. 4A) and 16.1% (Fig. 4B) below the vehicle controls in NB-A3 or NB-A25 cultures, respectively. These results obtained in the absence of glucose support the previous hypothesis that the C75-induced increase in neuronal ATP requires glycolysis (Landree, et al., 2004).
If ambient glucose determines the rate at which C75 increases neuronal ATP, then increases in ATP should positively correlate with increased media glucose concentration. This was indeed seen in cultures maintained in physiological glucose concentrations (Fig. 4A). Notably, the most robust response in NB-A3 cultures occurred in the presence of physiological glucose concentrations (0.5 to 5 mM), and decreased significantly in the presence of non-physiological glucose concentrations (above 5 mM). A 36.6% increase in ATP was detected in 0.5 mM glucose and a 64.8% increase was seen in 5 mM glucose (†††, p<0.001) (Fig. 4A), whereas the C75-induced increase in ATP was significantly attenuated by 10 mM glucose compared with 5 mM glucose (Fig.4A).
In contrast to NB-A3 cultures (Fig. 4A), NB-A25 cultures did not show a statistically discernible glucose concentration-response curve (Fig 4B). In these non-physiological cultures, the magnitude of the C75-induced increase in ATP was not affected by the amount of glucose present in the test media, as random increases of 55.5% to 86.1% were observed regardless of the glucose concentration present (Fig 4B). Thus, a relationship between ambient glucose concentrations and the effect of C75 on ATP (as seen in vivo) is only discernible when the cultures have been maintained under physiological conditions.
To examine the effect of media washes and changes on basal ATP levels the vehicle treated cultures presented in Fig. 4A-B are represented as percent of the conditioned culture media control (CM) in Fig. 4C-D. When glucose was withdrawn during the treatment period, neurons cultured in NB-A3 or NB-A25 displayed a reduction in ATP (Fig. 4C-D). Despite including a wash before providing 0 mM test media, the ATP in NB-A25 cultures only dropped to 43.1% of the CM control (Fig. 4D), whereas ATP in NB-A3 cultures decreased to 5.3% of the CM control (Fig. 4C). All cultures with supplemented glucose (0.1 to 25 mM) had basal ATP levels that were decreased from CM control, but not significantly different from each other. We believe that this observation indicates that neurons may require more than 1.5 hours to re-equilibrate following the stress of a complete media change, which involves exposure to air. However, the amount of glucose present in the test media does not further affect basal levels of ATP. Interestingly, even when the same glucose concentration was added back after the wash (i.e 3 mM → 3 mM and 25 mM → 25 mM) the basal ATP levels were still decreased, also suggesting that perhaps B27 and/or glutamine, play a role in this response as they are not present in the test medias. In all, these results demonstrate that neurons are most responsive to alterations in energy balance when both the culture and the test medias contain physiological glucose concentrations.
Non-physiological glucose concentrations reduce the ability to pharmacologically activate AMPK
Given previous results demonstrating that the effect of AICAR is inhibited when hypothalamic neurons are cultured in high glucose (5 mM) as opposed to physiologic glucose (2.5 mM) (Canabal, et al., 2007b) and our results that glucose levels present during neuronal culture maintenance altered C75-induced increases in ATP, we then analyzed the effect of ambient glucose on the ability of the AMP-mimetic, AICAR (5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside), to increase AMPK phosphorylation. The response of AMPK to changes in neuronal energy status and metabolic stress makes the pAMPK/AMPK ratio a reasonable readout of neuronal responsiveness to changes in energy status.
Cortical neurons were prepared and maintained in NB-A3 or NB-A25 via restorative feedings. Neurons were treated in conditioned media with 1.5 mM AICAR for 0, 1 or 2 hours and harvested for Western blots to detect levels of both AMPK and pAMPK. The volume of conditioned media for these experiments was adjusted the night before the experiment to minimize effects of media changes on basal levels of pAMPK as observed in the previous ATP experiment (Fig. 4C-D). A time-dependent increase in phosphorylation was detected over 2 hours in NB-A3 cultures with AICAR treatment (Fig. 5A,C). After 2 hours, the pAMPK/AMPK ratio was significantly greater than the 0 hour control (192.7%). In contrast, identical AICAR treatment of NB-A25 cultures produced no significant changes in the pAMPK/AMPK ratio (Fig. 5B-C). These results demonstrate that a pharmacological agent can be ineffective in neuronal cultures grown in non-physiologically high concentrations of glucose, further emphasizing the importance of the metabolic environment in the study of neuronal energy balance.
Fig. 5. Neuronal AMPK is most responsive to AICAR when cultures are maintained at physiological glucose concentrations.
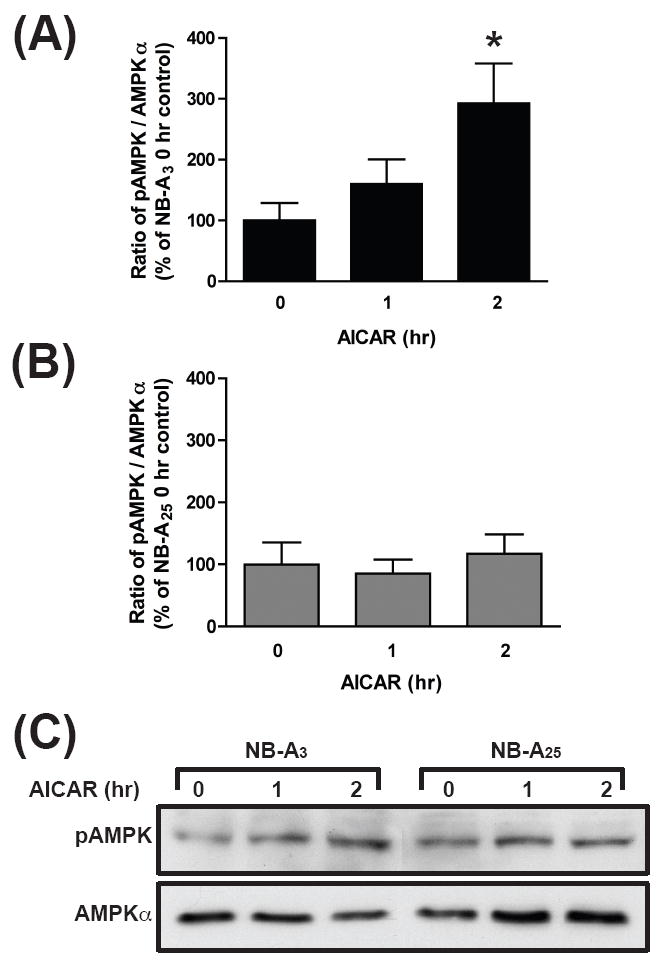
Western blot analysis of cortical lysates from cultures maintained in either NB-A3 or NB-A25 and treated on DIV9-10 with 1.5 mM AICAR for 0, 1 and 2 hrs. Antibodies detecting both phosphorylated AMPK catalytic isoforms, α1 and α2, and unphosphorylated AMPKα were utilized (A-C). Band intensity was quantified using Scion Image (NIH) and reported as the ratio of pAMPKα to AMPKα with the 0 hour time points serving as a control for the NB-A3 (A) and NB-A25 (B). *, p<0.05.
DISCUSSION
In the present study, we demonstrate that the metabolic environment alters long-term neuronal viability as well as the responsiveness of neurons to alterations in energy balance. For these studies, a previously established primary dissociated cortical neuronal culture (Dawson, et al., 1993; Landree, et al., 2004) was maintained in a glucose-free media supplemented with glutamine and B27 and either a physiological concentration of 3 mM glucose (NB-A3), or a non-physiological high concentration of 25 mM glucose (NB-A25). The present study was designed to further develop previous studies that considered the role of media glucose concentrations present during short-term culture (used by DIV4) and/or experimental medias (Wang, et al., 2004; Song, et al., 2005; Lee, et al., 2005; Morgenthaler, et al., 2006; Abe, et al., 2006; Bak, et al., 2006; Kang, et al., 2006; Canabal, et al., 2007a; Canabal, et al., 2007b).
Our culture system permitted examination of the effects of different glucose concentrations on long-term (DIV10-13) neuronal viability and energy metabolism and led to several conclusions: 1) restorative feedings reduce fluctuations in ambient glucose concentrations, improving long-term neuronal viability; 2) brief, but significant changes in glucose levels during culture preparation and plating decrease long-term neuronal viability; 3) only when maintained in a physiological glucose environment do neuronal ATP levels appear to respond to changes in the metabolic environment; and 4) the ability to pharmacologically modulate AMPK activity is blunted by culturing neurons in non-physiologically high glucose levels as seen by Canabal, et al (Canabal, et al., 2007b). The findings presented here may explain some of the discordance in studies examining the role of AMPK in neuroprotection (Culmsee, et al., 2001; McCullough, 2004). Currently, many in vitro systems used to study neuronal responses to nutrient challenge, oxidative stress, and oxygen deprivation are maintained in non-physiological concentrations of glucose, such as those seen in disease states. The implementation of a neuronal culture system in which glucose can be strictly controlled throughout may prove necessary to further investigate mechanisms that regulate neuronal energy metabolism during both normal and disease states.
There is mounting evidence that changes in pathways that regulate energy metabolism are incurred from exposure to dysregulated metabolic environments such as during diabetes, obesity or hypoglycemia (Sango, et al., 1991; Sango, et al., 1994; Cryer, 2001; Chen, et al., 2005; Song, et al., 2006; Martin, et al., 2006; Somoza, et al., 2007). In states of disrupted energy balance animals respond differently to known modulators of neuronal metabolism. For instance, rats fed a carbohydrate-free diet and considered to be ketotic do not experience the hypophagia usually associated with i.c.v. administration of C75, possibly due to an inability to oxidize glucose (Wortman, et al., 2003). Previous studies in primary cortical neurons demonstrated increases in glucose oxidation upon addition of C75 (Landree, et al., 2004). The results from C75 treatment of NB-A3 and NB-A25 cultures in 0 mM glucose are consistent with those seen in the previously mentioned in vivo and in vitro studies. Therefore, these neurons provide a basic system for the study of natural responses to treatments under both physiologic and non-physiologic conditions.
Our results are consistent with others in the literature, in which extracellular glucose levels are shown to be critical for eliciting relevant physiological responses from glucose sensing neurons in the hypothalamus (Wang, et al., 2004; Song, et al., 2005; Kang, et al., 2006; Canabal, et al., 2007b), as well as for determining the oxidative capacity of astroglia in vitro (Abe, et al., 2006). However, it is common practice in many studies to maintain cultures in high glucose and then transfer them into media containing lower glucose concentrations prior to experimentation (Ioudina, et al., 2004; Abe, et al., 2006; Bak, et al., 2006; Morgenthaler, et al., 2006). As mentioned in the results, the osmolarity of our NB-A3 and NB-A25 medias differ by approximately 20 mOsm and to be consistent with the majority of published “switching” paradigms we did not adjust the osmolarity in this study. However, the effect of osmolarity is an important issue to explore in future studies.
We also demonstrated that baseline ATP levels are reduced 1.5 hours following a media change; therefore, transferring cells into test media prior to experimentation does not allow them time to equilibrate to a new environment nor does it account for any long-term changes that may have been incurred due to exposure to non-physiological levels of glucose.
The mechanisms whereby alterations in ambient glucose concentrations cause profound changes in neuronal survival and responsiveness are unclear. The entry of glucose into the brain and neurons occurs via facilitative diffusion. As a high-affinity neuronal glucose transporter, GLUT3 would be expected to be saturated or near saturation in our cultures. Altered transporter expression and/or translocation to the cellular membrane has been shown during hyperglycemia (Boileau, et al., 1995; Merriman-Smith, et al., 2003), hypoglycemia (Nagamatsu, et al., 1994), and following ischemia [reviewed in (Maher, et al., 1994)]. Additionally, AMPK is recognized as a regulator of glucose transport [for review see (Fujii, et al., 2006)]. It is therefore quite plausible that dramatic changes in extracellular glucose concentrations could alter intracellular glucose levels, glucose metabolism and thus ATP levels.
We hypothesize that the manner in which neurons are cultured and maintained permits the acclimation to a physiological environment, subsequently allowing cells to respond to energy challenges in a more physiological manner. There are a small number of studies involving neuronal cultures and AMPK modulation with AICAR (Lee, et al., 2005; Chau-Van, et al., 2007; Dasgupta, et al., 2007; Canabal, et al., 2007a; Canabal, et al., 2007b; Mountjoy, et al., 2007), but few which utilize physiological glucose culture conditions (Lee, et al., 2005; Canabal, et al., 2007a; Canabal, et al., 2007b). A previous study using neuroblastoma cell lines cultured in 25 mM glucose demonstrated that basal levels of pAMPK are increased following incubation (16 hour) in media containing glucose below physiological concentrations (0 and 1 mM) relative to those switched to 2.5 mM glucose, and decreased in media containing glucose above physiological levels (5, 10, and 25 mM) (Lee, et al., 2005). Hyperglycemia during short-term culture (1-3 days) or acute treatment has been shown to inhibit AMPK signaling in hypothalamic neurons. This inhibition in was reversed with the treatment of AICAR (Canabal, et al., 2007b). Together, these studies demonstrate that the glucose environments during culture and at the time of experimentation, have a significant effect on AMPK signaling. This further suggests that neuronal cultures exposed to varying glucose environments may experience long-term metabolic differences, consistent with our hypothesis that culture conditions must be tightly regulated within physiological parameters in order to study neuronal energy balance.
In addition to glucose, other fuels such as lactate, pyruvate, fatty acids, and ketone bodies have been shown to play roles in neuronal energy metabolism both in vivo and in vitro [reviewed in (Pierre, et al., 2005)] (Morgan, et al., 2004). Although the system presented in this paper is simplified, as we chose to focus on only glucose to limit the variables, we recognize the importance of other nutrients to neuronal metabolism. This culture system now allows the systematic evaluation of other nutrients and metabolites. Additionally, this culture paradigm may prove useful in the future study of metabolic stress using different culture systems including explants and organotypic slices.
Acknowledgments
This work was funded by NIH grants from the NINDS, NIDCD, and NIDDK to G.V.R. We would like to thank FASgen, Inc. for providing C75 and V.H. Routh for helpful discussion at the onset of this project.
Footnotes
COMPETING INTERESTS STATEMENT Under a license agreement between FASgen, Inc. and the Johns Hopkins University, L.E.L. and G.V.R. are entitled to a share of royalties received by the University on sales of products related to reagents described in this article. G.V.R. has an interest in FASgen, Inc. stock, which is subject to certain restrictions under University policy. The Johns Hopkins University, in accordance with its conflict of interest policies, is managing the terms of this arrangement in accordance with its policies on conflict-of-interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe T, Takahashi S, Suzuki N. Oxidative metabolism in cultured rat astroglia: Effects of reducing the glucose concentration in the culture medium and of d-aspartate or potassium stimulation. J Cereb Blood Flow Metab. 2006;26:153–60. doi: 10.1038/sj.jcbfm.9600175. [DOI] [PubMed] [Google Scholar]
- Abi-Saab WM, Maggs DG, Jones T, Jacob R, Srihari V, Thompson J, Kerr D, Leone P, Krystal JH, Spencer DD, During MJ, Sherwin RS. Striking differences in glucose and lactate levels between brain extracellular fluid and plasma in conscious human subjects: Effects of hyperglycemia and hypoglycemia. J Cereb Blood Flow Metab. 2002;22:271–9. doi: 10.1097/00004647-200203000-00004. [DOI] [PubMed] [Google Scholar]
- Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ. Amp-activated protein kinase plays a role in the control of food intake. J Biol Chem. 2004;279:12005–8. doi: 10.1074/jbc.C300557200. [DOI] [PubMed] [Google Scholar]
- Bak LK, Schousboe A, Sonnewald U, Waagepetersen HS. Glucose is necessary to maintain neurotransmitter homeostasis during synaptic activity in cultured glutamatergic neurons. J Cereb Blood Flow Metab. 2006;26:1285–97. doi: 10.1038/sj.jcbfm.9600281. [DOI] [PubMed] [Google Scholar]
- Boileau P, Mrejen C, Girard J, Hauguel-de Mouzon S. Overexpression of glut3 placental glucose transporter in diabetic rats. J Clin Invest. 1995;96:309–17. doi: 10.1172/JCI118036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzier-Sore AK, Voisin P, Canioni P, Magistretti PJ, Pellerin L. Lactate is a preferential oxidative energy substrate over glucose for neurons in culture. J Cereb Blood Flow Metab. 2003;23:1298–306. doi: 10.1097/01.WCB.0000091761.61714.25. [DOI] [PubMed] [Google Scholar]
- Canabal DD, Song Z, Potian JG, Beuve A, McArdle JJ, Routh VH. Glucose, insulin, and leptin signaling pathways modulate nitric oxide synthesis in glucose-inhibited neurons in the ventromedial hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2007a;292:R1418–28. doi: 10.1152/ajpregu.00216.2006. [DOI] [PubMed] [Google Scholar]
- Canabal DD, Potian JG, Duran RG, McArdle JJ, Routh VH. Hyperglycemia impairs glucose and insulin regulation of nitric oxide production in glucose-inhibited neurons in the ventromedial hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2007b;293:R592–600. doi: 10.1152/ajpregu.00207.2007. [DOI] [PubMed] [Google Scholar]
- Carling D. The amp-activated protein kinase cascade - a unifying system for energy control. Trends Biochem Sci. 2004;29:18–24. doi: 10.1016/j.tibs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Carling D. Amp-activated protein kinase: Balancing the scales. Biochimie. 2005;87:87–91. doi: 10.1016/j.biochi.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Chau-Van C, Gamba M, Salvi R, Gaillard RC, Pralong FP. Metformin inhibits adenosine 5’-monophosphate-activated kinase activation and prevents increases in neuropeptide y expression in cultured hypothalamic neurons. Endocrinology. 2007;148:507–11. doi: 10.1210/en.2006-1237. [DOI] [PubMed] [Google Scholar]
- Chen MB, McAinch AJ, Macaulay SL, Castelli LA, O’Brien PE, Dixon JB, Cameron-Smith D, Kemp BE, Steinberg GR. Impaired activation of amp-kinase and fatty acid oxidation by globular adiponectin in cultured human skeletal muscle of obese type 2 diabetics. J Clin Endocrinol Metab. 2005;90:3665–72. doi: 10.1210/jc.2004-1980. [DOI] [PubMed] [Google Scholar]
- Clough-Helfman C, Phillis JW. 5-aminoimidazole-4-carboxamide riboside (aicar) administration reduces cerebral ischemic damage in the mongolian gerbil. Brain Res Bull. 1990;25:203–6. doi: 10.1016/0361-9230(90)90277-7. [DOI] [PubMed] [Google Scholar]
- Cryer PE. Hypoglycemia-associated autonomic failure in diabetes. Am J Physiol Endocrinol Metab. 2001;281:E1115–21. doi: 10.1152/ajpendo.2001.281.6.E1115. [DOI] [PubMed] [Google Scholar]
- Culmsee C, Monnig J, Kemp BE, Mattson MP. Amp-activated protein kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival following glucose deprivation. J Mol Neurosci. 2001;17:45–58. doi: 10.1385/JMN:17:1:45. [DOI] [PubMed] [Google Scholar]
- Dasgupta B, Milbrandt J. Resveratrol stimulates amp kinase activity in neurons. Proc Natl Acad Sci U S A. 2007;104:7217–22. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson TM, Arriza JL, Lefkowitz RJ, Jaworsky DE, Ronnett GV. Beta-adrenergic receptor kinase-2 and beta-arrestin-2: Mediators of odorant-induced desensitization. Science. 1993;259:825–29. doi: 10.1126/science.8381559. [DOI] [PubMed] [Google Scholar]
- de Vries MG, Arseneau LM, Lawson ME, Beverly JL. Extracellular glucose in rat ventromedial hypothalamus during acute and recurrent hypoglycemia. Diabetes. 2003;52:2767–73. doi: 10.2337/diabetes.52.11.2767. [DOI] [PubMed] [Google Scholar]
- Fujii N, Jessen N, Goodyear LJ. Amp-activated protein kinase and the regulation of glucose transport. Am J Physiol Endocrinol Metab. 2006;291:E867–77. doi: 10.1152/ajpendo.00207.2006. [DOI] [PubMed] [Google Scholar]
- Gao G, Fernandez CS, Stapleton D, Auster AS, Widmer J, Dyck JR, Kemp BE, Witters LA. Non-catalytic beta-and gamma-subunit isoforms of the 5’-amp-activated protein kinase. J Biol Chem. 1996;271:8675–81. doi: 10.1074/jbc.271.15.8675. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Carling D. The amp-activated protein kinase--fuel gauge of the mammalian cell? Eur J Biochem. 1997;246:259–73. doi: 10.1111/j.1432-1033.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the amp-activated protein kinase system. FEBS Lett. 2003;546:113–20. doi: 10.1016/s0014-5793(03)00560-x. [DOI] [PubMed] [Google Scholar]
- Hardie DG. Roles of the amp-activated/snf1 protein kinase family in the response to cellular stress. Biochem Soc Symp. 1999;64:13–27. [PubMed] [Google Scholar]
- Hardie DG. The amp-activated protein kinase pathway--new players upstream and downstream. J Cell Sci. 2004;117:5479–87. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- Ioudina M, Uemura E, Greenlee HW. Glucose insufficiency alters neuronal viability and increases susceptibility to glutamate toxicity. Brain Res. 2004;1004:188–92. doi: 10.1016/j.brainres.2003.12.046. [DOI] [PubMed] [Google Scholar]
- Kang L, Dunn-Meynell AA, Routh VH, Gaspers LD, Nagata Y, Nishimura T, Eiki J, Zhang BB, Levin BE. Glucokinase is a critical regulator of ventromedial hypothalamic neuronal glucosensing. Diabetes. 2006;55:412–20. doi: 10.2337/diabetes.55.02.06.db05-1229. [DOI] [PubMed] [Google Scholar]
- Kim EK, Miller I, Aja S, Landree LE, Pinn M, McFadden J, Kuhajda FP, Moran TH, Ronnett GV. C75, a fatty acid synthase inhibitor, reduces food intake via hypothalamic amp-activated protein kinase. J Biol Chem. 2004a;279:19970–6. doi: 10.1074/jbc.M402165200. [DOI] [PubMed] [Google Scholar]
- Kim MS, Lee KU. Role of hypothalamic 5’-amp-activated protein kinase in the regulation of food intake and energy homeostasis. J Mol Med. 2005;83:514–20. doi: 10.1007/s00109-005-0659-z. [DOI] [PubMed] [Google Scholar]
- Kim MS, Park JY, Namkoong C, Jang PG, Ryu JW, Song HS, Yun JY, Namgoong IS, Ha J, Park IS, Lee IK, Viollet B, Youn JH, Lee HK, Lee KU. Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic amp-activated protein kinase. Nat Med. 2004b;10:727–33. doi: 10.1038/nm1061. [DOI] [PubMed] [Google Scholar]
- Kola B, Hubina E, Tucci SA, Kirkham TC, Garcia EA, Mitchell SE, Williams LM, Hawley SA, Hardie DG, Grossman AB, Korbonits M. Cannabinoids and ghrelin have both central and peripheral metabolic and cardiac effects via amp-activated protein kinase. J Biol Chem. 2005;280:25196–201. doi: 10.1074/jbc.C500175200. [DOI] [PubMed] [Google Scholar]
- Landree LE, Hanlon AL, Strong DW, Rumbaugh G, Miller IM, Thupari JN, Connolly EC, Huganir RL, Richardson C, Witters LA, Kuhajda FP, Ronnett GV. C75, a fatty acid synthase inhibitor, modulates amp-activated protein kinase to alter neuronal energy metabolism. J Biol Chem. 2004;279:3817–27. doi: 10.1074/jbc.M310991200. [DOI] [PubMed] [Google Scholar]
- Lee K, Li B, Xi X, Suh Y, Martin RJ. Role of neuronal energy status in the regulation of adenosine 5’-monophosphate-activated protein kinase, orexigenic neuropeptides expression, and feeding behavior. Endocrinology. 2005;146:3–10. doi: 10.1210/en.2004-0968. [DOI] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA, Routh VH. Brain glucose sensing and body energy homeostasis: Role in obesity and diabetes. Am J Physiol. 1999;276:R1223–31. doi: 10.1152/ajpregu.1999.276.5.R1223. [DOI] [PubMed] [Google Scholar]
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L. Astrocytes couple synaptic activity to glucose utilization in the brain. News Physiol Sci. 1999;14:177–82. doi: 10.1152/physiologyonline.1999.14.5.177. [DOI] [PubMed] [Google Scholar]
- Maher F, Vannucci SJ, Simpson IA. Glucose transporter proteins in brain. Faseb J. 1994;8:1003–11. doi: 10.1096/fasebj.8.13.7926364. [DOI] [PubMed] [Google Scholar]
- Martin TL, Alquier T, Asakura K, Furukawa N, Preitner F, Kahn BB. Diet-induced obesity alters amp kinase activity in hypothalamus and skeletal muscle. J Biol Chem. 2006;281:18933–41. doi: 10.1074/jbc.M512831200. [DOI] [PubMed] [Google Scholar]
- McCullough L. C75 is neuroprotective in and ischemia-reperfusion model and inhibits amp-activated kinase. 2004 [Google Scholar]
- McCullough LD, Zeng Z, Li H, Landree LE, McFadden J, Ronnett GV. Pharmacological inhibition of amp-activated protein kinase provides neuroprotection in stroke. J Biol Chem. 2005;280:20493–502. doi: 10.1074/jbc.M409985200. [DOI] [PubMed] [Google Scholar]
- Merriman-Smith BR, Krushinsky A, Kistler J, Donaldson PJ. Expression patterns for glucose transporters glut1 and glut3 in the normal rat lens and in models of diabetic cataract. Invest Ophthalmol Vis Sci. 2003;44:3458–66. doi: 10.1167/iovs.02-1235. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. Amp-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–74. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the united states. Jama. 2001;286:1195–200. doi: 10.1001/jama.286.10.1195. [DOI] [PubMed] [Google Scholar]
- Morgan K, Obici S, Rossetti L. Hypothalamic responses to long-chain fatty acids are nutritionally regulated. J Biol Chem. 2004;279:31139–48. doi: 10.1074/jbc.M400458200. [DOI] [PubMed] [Google Scholar]
- Morgenthaler FD, Kraftsik R, Catsicas S, Magistretti PJ, Chatton JY. Glucose and lactate are equally effective in energizing activity-dependent synaptic vesicle turnover in purified cortical neurons. Neuroscience. 2006;141:157–65. doi: 10.1016/j.neuroscience.2006.03.065. [DOI] [PubMed] [Google Scholar]
- Mountjoy PD, Bailey SJ, Rutter GA. Inhibition by glucose or leptin of hypothalamic neurons expressing neuropeptide y requires changes in amp-activated protein kinase activity. Diabetologia. 2007;50:168–77. doi: 10.1007/s00125-006-0473-3. [DOI] [PubMed] [Google Scholar]
- Nagamatsu S, Sawa H, Inoue N, Nakamichi Y, Takeshima H, Hoshino T. Gene expression of glut3 glucose transporter regulated by glucose in vivo in mouse brain and in vitro in neuronal cell cultures from rat embryos. Biochem J. 1994;300(Pt 1):125–31. doi: 10.1042/bj3000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre K, Pellerin L. Monocarboxylate transporters in the central nervous system: Distribution, regulation and function. J Neurochem. 2005;94:1–14. doi: 10.1111/j.1471-4159.2005.03168.x. [DOI] [PubMed] [Google Scholar]
- Routh VH. Glucose-sensing neurons: Are they physiologically relevant? Physiol Behav. 2002;76:403–13. doi: 10.1016/s0031-9384(02)00761-8. [DOI] [PubMed] [Google Scholar]
- Routh VH, Song Z, Liu X. The role of glucosensing neurons in the detection of hypoglycemia. Diabetes Technol Ther. 2004;6:413–21. doi: 10.1089/152091504774198133. [DOI] [PubMed] [Google Scholar]
- Sango K, Horie H, Sotelo JR, Takenaka T. A high glucose environment improves survival of diabetic neurons in culture. Neurosci Lett. 1991;129:277–80. doi: 10.1016/0304-3940(91)90480-h. [DOI] [PubMed] [Google Scholar]
- Sango K, Horie H, Takano M, Inoue S, Takenaka T. Diabetes-induced reduction of neuronal survival in hypotonic environments in culture. Brain Res Bull. 1994;34:365–8. doi: 10.1016/0361-9230(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Baskin DG, Bukowski TR, Kuijper JL, Foster D, Lasser G, Prunkard DE, Porte D, Jr, Woods SC, Seeley RJ, Weigle DS. Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide y gene expression in ob/ob mice. Diabetes. 1996;45:531–5. doi: 10.2337/diab.45.4.531. [DOI] [PubMed] [Google Scholar]
- Silver IA, Erecinska M. Extracellular glucose concentration in mammalian brain: Continuous monitoring of changes during increased neuronal activity and upon limitation in oxygen supply in normo-, hypo-, and hyperglycemic animals. J Neurosci. 1994;14:5068–76. doi: 10.1523/JNEUROSCI.14-08-05068.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somoza B, Guzman R, Cano V, Merino B, Ramos P, Diez-Fernandez C, Fernandez-Alfonso MS, Ruiz-Gayo M. Induction of cardiac uncoupling protein-2 expression and adenosine 5’-monophosphate-activated protein kinase phosphorylation during early states of diet-induced obesity in mice. Endocrinology. 2007;148:924–31. doi: 10.1210/en.2006-0914. [DOI] [PubMed] [Google Scholar]
- Song Z, Routh VH. Differential effects of glucose and lactate on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes. 2005;54:15–22. doi: 10.2337/diabetes.54.1.15. [DOI] [PubMed] [Google Scholar]
- Song Z, Routh VH. Recurrent hypoglycemia reduces the glucose sensitivity of glucose-inhibited neurons in the ventromedial hypothalamus nucleus. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1283–7. doi: 10.1152/ajpregu.00148.2006. [DOI] [PubMed] [Google Scholar]
- Stapleton D, Mitchelhill KI, Gao G, Widmer J, Michell BJ, Teh T, House CM, Fernandez CS, Cox T, Witters LA, Kemp BE. Mammalian amp-activated protein kinase subfamily. J Biol Chem. 1996;271:611–4. doi: 10.1074/jbc.271.2.611. [DOI] [PubMed] [Google Scholar]
- Turnley AM, Stapleton D, Mann RJ, Witters LA, Kemp BE, Bartlett PF. Cellular distribution and developmental expression of amp-activated protein kinase isoforms in mouse central nervous system. J Neurochem. 1999;72:1707–16. doi: 10.1046/j.1471-4159.1999.721707.x. [DOI] [PubMed] [Google Scholar]
- Wang D, Sul H. Insulin stimulation of the fatty acid synthase promoter is mediated by the phosphatidylinositol 3-kinase pathway. J Biol Chem. 1998;273:25420–26. doi: 10.1074/jbc.273.39.25420. [DOI] [PubMed] [Google Scholar]
- Wang R, Liu X, Hentges ST, Dunn-Meynell AA, Levin BE, Wang W, Routh VH. The regulation of glucose-excited neurons in the hypothalamic arcuate nucleus by glucose and feeding-relevant peptides. Diabetes. 2004;53:1959–65. doi: 10.2337/diabetes.53.8.1959. [DOI] [PubMed] [Google Scholar]
- Winder WW. Amp-activated protein kinase: Possible target for treatment of type 2 diabetes. Diabetes Technol Ther. 2000;2:441–8. doi: 10.1089/15209150050194305. [DOI] [PubMed] [Google Scholar]
- Wortman MD, Clegg DJ, D’Alessio D, Woods SC, Seeley RJ. C75 inhibits food intake by increasing cns glucose metabolism. Nat Med. 2003;9:483–5. doi: 10.1038/nm0503-483. [DOI] [PubMed] [Google Scholar]