Abstract
When olfactory receptor neurons respond to odors, a depolarizing Cl− efflux is a substantial part of the response. This requires that the resting neuron accumulate Cl− against an electrochemical gradient. In isolated olfactory receptor neurons, the Na+-K+-2Cl− cotransporter NKCC1 is essential for Cl− accumulation. However, in intact epithelium, a robust electrical olfactory response persists in mice lacking NKCC1. To determine whether NKCC1 is required for normal olfactory sensitivity, olfactory sensitivity was compared between knockout (KO) mice carrying a null mutation for NKCC1 and wild-type (WT) littermates. Using operant behavioral techniques, olfactory sensitivity was measured using a commercial liquid-dilution olfactometer. Detection thresholds for the simple odorants cineole, 1-heptanol, and 1-propanol were compared in KO and WT animals. Regardless of the stimulus conditions employed, no systematic differences in behavioral thresholds were evident between KO and WT animals. We conclude that NKCC1 is not required for normal olfactory sensitivity.
1. Introduction
In vertebrates, odor stimuli are initially transduced into an electrical signal by primary sensory neurons in the olfactory epithelium. In the majority of these neurons, a G-protein-coupled cascade in the neuronal cilia underlies odor transduction (for review see ref. 1). An odor molecule binds to a receptor protein in the ciliary membrane. This activates a G-protein (Golf) that in turn activates a type III adenylate cyclase. The resulting cAMP activates cyclic-nucleotide-gated (CNG) channels, which allow a depolarizing influx of Na+ and Ca2+. The Ca2+ then activates Cl− channels, which generate a further inward current via an efflux of Cl−. In rat [2] and mouse [3,4], the Cl− efflux accounts for at least 80% of the receptor current. Such an efflux cannot occur unless the neuron accumulates Cl− against an electrochemical gradient at rest.
By measuring [Cl−] in the neuronal dendrites, Kaneko et al. [5] discovered that the Na+-K+-2Cl− cotransporter NKCC1 contributes to Cl− accumulation. Subsequently, Reisert et al. [6] found that isolated olfactory receptor neurons cannot accumulate Cl− if NKCC1 activity is eliminated by genetic or pharmacological means. This suggested that NKCC1 is required for Cl− accumulation. However, in intact epithelium, odors induce a robust neuronal Cl− efflux even in mice lacking NKCC1 [3]. It is not yet understood why loss of NKCC1 has a more profound effect in isolated neurons.
Behavioral methods offer an independent way to assess the importance of molecules thought to underlie olfactory transduction. Mice lacking the type III adenylate cyclase [7,8] and the channel subunits CNGA2 [9,10; but see also 11] and CNGB1b [12] have been found to have severely reduced olfactory behaviors. We now report that mice lacking NKCC1 show no systematic deficits in olfactory behavioral thresholds. It is concluded that NKCC1 is not required for normal olfactory sensitivity.
2. Materials and methods
Olfactory sensitivity was compared between knockout (KO) mice carrying a null allele for NKCC1 and wild-type (WT) littermates. Sensitivity was determined by measuring odor detection thresholds to cineole, 1-heptanol, and 1-propanol.
2.1. Animals
The behavioral experiments were performed with NKCC1+/+ and NKCC1−/− mice that were in an inbred FVBN background [13]. Eight KO animals (5 male, 3 female) and seven WT littermates (4 male, 3 female) were tested. The KO mice contain a null allele for the gene NKCC1; no NKCC1 mRNA is made [13]. NKCC1 homozygous mutant and WT mice were obtained by breeding gene-targeted NKCC1 heterozygous mutant mice. The genotype of each mouse was determined by polymerase chain reaction of DNA from tail biopsies as previously described [13]. NKCC1−/− mice exhibit defects in hearing, balance, salivation, blood pressure, and spermatogenesis (reviewed in ref. 14). FVBN mice also reportedly exhibit retinal degeneration [15].
Mice were 24 to 26 weeks old at the start of training. Their ad-libitum weights, before water control, ranged from 23 to 33.5 g. Mice were individually housed during testing periods; this allowed monitoring of water intake. Water was restricted to maintain weight at about 85% of free access weight. Additional water was supplied if an animal’s weight dropped below 80%. Mice were fed Purina mouse chow ad libitum. They were maintained on a ~23-hr restricted access to water. During each session, the animals were allowed to remain in the testing chamber and earn as much nutritional liquid food (Ensure, Abbott Laboratories, Columbus, OH) as they cared to, and were removed when they stopped working. The amount of Ensure was monitored during each session and, depending on its weight (i.e., how close it was to 80%), each animal was given an additional amount of water immediately after leaving the olfactometer, up to a total of 3 ml/day (USDA recommended daily allotment). If the animal was being tested the following day, no additional fluids were provided.
2.2. Olfactometer
A commercial liquid-dilution rodent olfactometer (Knosys Olfactometers, Lutz, FL; ref. 16) was employed in this study to assess olfactory sensitivity in a group of behaviorally trained mice. To facilitate use of the nutritional liquid food (Ensure) as a reinforcer, the animals were maintained on a water-restriction schedule. Animals were tested once daily, five days per week. All behavioral methods in this study were approved by the University of Florida Institutional Animal Care and Use Committee.
The instrumentation and methods employed in this study are identical to those used in previous works with this olfactometer, and detailed discussions of the training and testing techniques can be found in those presentations [16,17,18]. The mouse olfactometer consists of a 15-cm deep, 20-cm wide, and 13-cm tall ventilated Plexiglas operant chamber. The chamber is fitted with a conductive metal floor and a glass sniffing port containing a metal licking tube. The ventilation provides a steady stream of fresh room air in the chamber, maintaining positive pressure and ensuring that the odorant remains within the sniffing port air stream.
When the animal inserted its nose into the carrier stream within the sampling port, this broke a photo beam and initiated a trial sequence. The mouse was required to keep its nose within the air stream and sample the air for a minimum of 0.2 s, at which time a stimulus, either the S+ or S− (as defined below), was introduced into the carrier stream through the bottom of the glass port. The air stream and stimulus were drawn through the sampling port, across the animal’s nose, and exhausted out of the top of the tube by an in-line exhaust fan and fed into a central room-evacuation system.
A primary use of the Knosys olfactometer has been to estimate sensitivity via acquisition of a two-sample discrimination task [16,17,18]. In this study, as well as in the previous works, animals were trained to discriminate dilutions of the target odorant in a diluent (S+) from the diluent alone (S−). The diluent was light mineral oil (Sigma-Aldrich, St. Louis, MO) or water, depending on the particular odorant. Reinforcement was contingent upon the animal reporting detection of the S+ odorant by licking on the metal water tube (correct detection), which completed an electrical circuit with the metal floor and registered the response with the computer-based olfactometer control program. A correct detection was followed by presentation of approximately 5 μl of Ensure through the lick tube. Failure to report the presence of the S+ (a miss), and licking the response tube during presentation of an S− stimulus (false alarm), were recorded as incorrect responses and required the animal to withdraw its nose from the sampling port for 2 s before re-inserting its nose in the sampling port to initiate a subsequent trial sequence.
Trials were presented in blocks of 20 (10 S+ and 10 S−). Within each block, the sequence of the 20 trials was quasi-random. The percent correct was calculated (for both correct detection and correct rejection) individually for each block. When the percent correct reached 85% on three successive blocks, the concentration of the S+ stimulus was decreased tenfold for the following block. During a given session, animals were allowed to remain in the testing chamber for as long as they continued to initiate trials. That day’s performance was recorded as the average percent correct on the final five blocks (100 trials). When an animal failed to reach 85% correct on three consecutive blocks during a given session, the same concentration was presented the following day. This was repeated for two days. If the animal failed to reach the 85% criterion for the next serial dilution, then the final value recorded (for that dilution) was the average of the last five blocks. “Threshold” was the lowest concentration at which the animal achieved 85% or higher on three consecutive blocks.
Simultaneous testing was carried out in three olfactometers and the animals were switched randomly on a daily basis between chambers. Control tests were conducted to determine whether or not inadvertent odorant or non-odorant cues were available to the animals as discriminative cues. Control tests were conducted by replacing the S+ odorant bottle with the diluent alone. In this case, both the S+ and S− saturator bottles contained the same (S−) stimulus. A second, quick control check was also made by simply pinching off the S+ saturator bottle tubes during an S+ trial. Under both control conditions, trained animals performed at chance levels, indicating a lack of reliable discrimination cues.
2.3. Odorants
Cineole, 1-heptanol, and 1-propanol were purchased from Sigma-Aldrich (St. Louis, MO). Serial dilutions of the odorants in odorless mineral oil or water were made fresh daily from the stock solutions, and 5 ml was placed in a saturation bottle for testing. The pure odorants were stored under inert gas (nitrogen) to prevent oxidation. The actual concentration of the odorant at the animal’s nose was unknown. However, for many odorants, the concentration in the vapor above a solution is proportional to the concentration in the solution [19]. The concentrations given here refer to the fraction by volume of the odorant in the liquid phase in the saturation bottle.
In the nose, the odorants may reach not only the olfactory receptor neurons but also neurons of the vomeronasal and trigeminal systems. Some volatile odorants appear to stimulate the vomeronasal system [20]. However, we are aware of no evidence that the particular odorants we used stimulate the vomeronasal system. At high concentrations, these odorants may activate trigeminal receptors, but perithreshold concentrations were well below those of the trigeminal system. For 1-propanol and 1-heptanol, for example, the olfactory thresholds in humans are lower than the trigeminal thresholds by factors of 500 and 2000, respectively [see Fig. 2 in ref.21]. Thus the thresholds we report in WT mice represent normal olfactory sensitivity.
3. Results
Knockout mice exhibited significant motor deficits associated with deletion of the NKCC1 gene and were immediately distinguishable from their wild-type littermates by the stereotypical “shaker/waltzer” phenotype. The motor dysfunctions were observed primarily as rapid, bidirectional rotational behaviors thought to result from loss of normal vestibular function [22]. The absence of normal labyrinthine function extends to the inner ear and, as a consequence, the KO mice are also deaf [23,24]. Remarkably, these motor and sensory deficits notwithstanding, the KO mice were readily trained to perform the necessary behavioral responses for testing in the olfactometer, and there were no apparent differences across genotype in the time required to reach criterion for the initial phase of odorant discrimination training. On average, KO animals passed the initial behavioral training stage in 2.5 d, compared with 3.2 d for the WT littermates. These times were not significantly different (paired t-test, P = 0.87). Furthermore, when compared, there was also no difference in the number of trial blocks that it took WT and KO animals to initially reach the 85% correct response criterion for the first odorant discrimination concentration (undiluted odorant; Fig. 1).
Fig. 1.
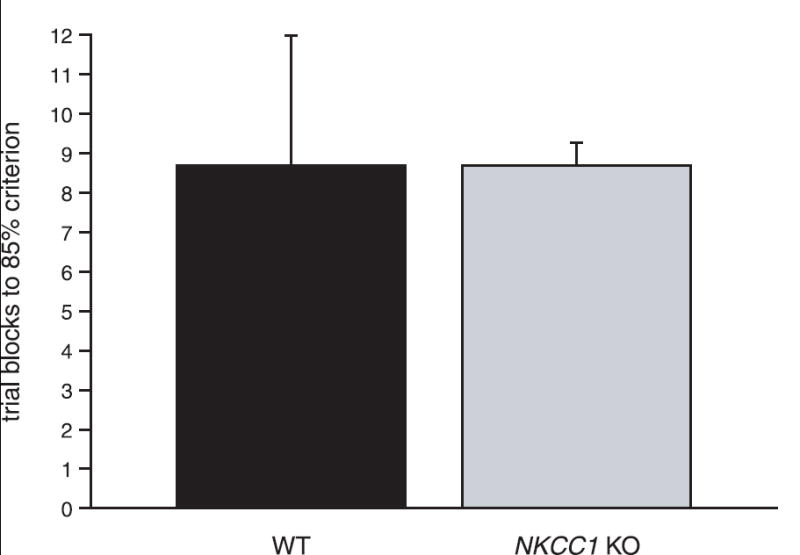
Number of trial blocks to criterion for the odorant discrimination task. Number of trial blocks required to reach an initial discrimination criterion of 85% for a group of WT (n = 3) and KO (n = 6) mice for two different odorants (cineole and 1-propanol).
To determine whether deletion of NKCC1 produces measurable decreases in odor sensitivity, we compared estimates of odor detection threshold in KO mice with WT littermates for the odorant 1-propanol. The animals were required to discriminate 1-propanol, diluted in mineral oil, from mineral oil alone. Fig. 2 plots the average percent correct as a function of 1-propanol concentration for groups of WT and KO animals. Threshold for each animal was defined as the lowest concentration of 1-propanol at which an animal achieved 85% correct for three consecutive blocks. Threshold for each genotype was defined as the lowest concentration of 1-propanol at which the averaged group response attained an 85% correct level. On average, this threshold concentration was 10−10 for the WT mice and 10−11 for the KO mice. The thresholds were not significantly different (paired t-test, P = 0.91).
Fig. 2.
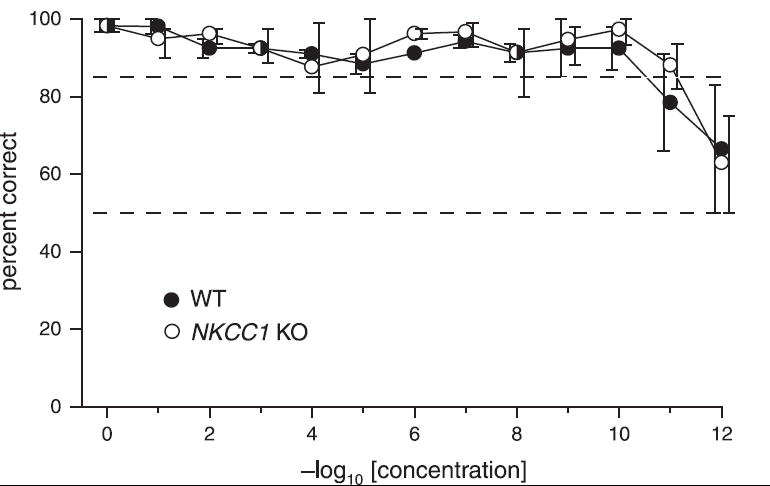
Response accuracy of WT and KO mice in detection of serial dilutions of 1-propanol. Means are shown for 2 WT mice and 3 KO mice. For each animal, each data point is the average of three successive blocks of 20 trials (10 S+ and 10 S−, presented randomly) once the criterion of 85% on three successive blocks was attained. Testing was continued for a maximum of five sessions at a given dilution. Concentrations represent the fraction of 1-propanol by volume in the liquid phase within the saturation bottle. Threshold was the last dilution at which an animal reached the 85% criterion. Bars represent the full range of values for each point. Bars on the left side of each point are from WT mice; bars to the right are from KO mice. Dashed lines are shown at 50% (expected chance performance) and 85% (criterion for stimulus detection).
The 1-propanol thresholds described above represent an average of three trial blocks once the animals reached the 85% criterion level. In that paradigm, each animal was given up to five sessions to reach threshold, providing extensive practice to detect the stimulus at low odorant concentrations. To determine whether differential reinforcement might influence threshold estimates, thresholds were determined using the same odor discrimination paradigm, but the animals were only given two sessions to meet the 85% correct response criterion to pass to a successive, lower odorant concentration. Thresholds were determined for 1-heptanol and cineole (Figs. 3A and B, respectively). For each odorant, thresholds were virtually identical for the two genotypes.
Fig. 3.

Response accuracy of a WT mouse and a KO mouse in detection of serial dilutions of 1-heptanol (A) or cineole (B). Each data point is the average of three successive blocks of 20 trials (10 S+ and 10 S−, presented randomly) once the criterion of 85% on three successive blocks was attained. Testing was continued for a maximum of two sessions at a given dilution. Concentrations represent the fraction of odorant by volume in the liquid phase within the saturation bottle. Threshold concentrations for the WT mice were 10−2 (1-heptanol, A) and 5 × 10−5 (cineole, B). Threshold concentrations for the KO mice were 10−2 (1-heptanol, A) and 10−3 (cineole, B). Dashed lines are shown at 50% (expected chance performance) and 85% (criterion for stimulus detection).
4. Discussion
Studies in isolated olfactory epithelia [3,5] and in olfactory receptor neurons [6] indicate that elimination of NKCC1 impairs odor transduction. To learn whether NKCC1 is required for normal olfactory sensitivity, we assessed the odor sensitivities of WT and NKCC1 KO mice. Two approaches were taken. In the first, mice were given extensive operant training in repeated sessions to learn to discriminate the S+ and S− stimuli. Using this approach, WT and KO mice yielded similar discrimination threshold estimates approximating a concentration of 10−11 to 10−10 for the simple odorant 1-propanol (Fig. 2). This threshold concentration is comparable to 1-propanol thresholds measured in CB57 mice using the same instrumentation (D.W. Smith and B.W. Ache, unpublished observations) but is somewhat lower that that previously reported for the mouse using air-dilution olfactometry [25], where more precise control of odorant concentration is possible. The precise concentration of 1-propanol presented to the animal within the sampling port is unknown. However, as both the WT and KO animals were tested under identical conditions, any relative differences in sensitivity resulting from genotypic differences would have been readily evident.
The second technique, a variant of the first approach, differed in that only two sessions were given for the animal to reach an 85% threshold. This approach was tested with the odorants cineole and 1-heptanol. Again, there were no systematic differences in sensitivity between the WT and KO mice (Fig. 3).
The rationale for the use of two different threshold criteria to estimate odor sensitivity comes from previous work showing that use of differential reinforcement might reveal different aspects of neural processing compared with methods based on nonreinforced or “spontaneous” behaviors [26]. In that study, rats had difficulty spontaneously discriminating enantiomers of limonene and terpinen-4-ol yet could readily discriminate those same odorants when reinforced in an olfactometer [26]. While these comparisons were not possible with the KO mice in this study, we sought to characterize the effects of threshold criterion (i.e. the opportunity for reinforcement and experience) on threshold estimates. To do this, we limited the number of experimental sessions allowed for the animal to reach the 85% correct criterion. While this manipulation had the expected effect of increasing threshold estimates for cineole and 1-heptanol, there was still no systematic difference in sensitivity between WT and KO animals.
In the present study, the physical disabilities present in the KO animals resulting from deletion of the NKCC1 gene precluded meaningful application of habituation methods to assess changes in sensitivity. The KO animals, however, had no trouble with the operant olfactometer because each was physically supported by the wall of the operant chamber while its head was inserted into the sampling port.
The majority of vertebrate olfactory receptor neurons transduce odor stimuli via a G-protein-coupled cascade and the second messenger cAMP [1]. The initial elements of this cascade include the odor receptor proteins, the G-protein Golf, a type III adenylate cyclase, and the three different subunits of the cyclic-nucleotide-gated (CNG) channels. The importance of each of these transduction components has been demonstrated by electrophysiological means. Expression of the odor receptors I7 and MOR23 confer sensitivity to octanal and lyral, respectively [27,28]. Genetic ablation of Golf [29], type III adenylate cyclase [7,8], or CNG channel subunit A2 [10,30; but see also 11] greatly reduces or eliminates the epithelial field potential measured during odor stimulation. Mice lacking the A4 [10,31,32] or B1b [12,32] subunit of the CNG channel show an electrophysiological response to odor stimuli but are defective in stimulus adaptation.
More recent studies have demonstrated that the initial proteins of the cAMP transduction cascade are also required for behaviors mediated by olfaction. Mice lacking type III adenylate cyclase are severely defective in odor detection, pheromone detection, and male-male aggression [7,8]. Mice lacking the CNGA2 channel subunit fail to mate or fight [9] or to show the normal preference for disparate peptides of the major histocompatibility complex [10]. Finally, genetic ablation of the CNGB1b channel subunit results in mice that are three times slower than WT littermates when searching for buried food [12].
Beyond the cAMP-activated current, there is a secondary transduction current carried by Cl−. Although it accounts for at least 80% of the total receptor current [2,3,4], it has never been clear whether the Cl− current is required for normal olfactory function. As Ca2+ enters the neuronal cilia, it gates Cl− channels [1]. Because the neurons accumulate Cl− at rest, opening the Cl− channels causes an efflux of Cl− that also depolarizes the neuron. The Na+-K+-2Cl− cotransporter NKCC1 contributes to neuronal Cl− accumulation, so olfactory receptor neurons of mice lacking NKCC1 show a substantially reduced Cl− current during odor stimulation [3,6,33]. In isolated olfactory neurons, absence of NKCC1 eliminates the odor-induced Cl− current [6].
In the present study, we found that deletion of NKCC1 has no effect on the threshold for detection of any of the three odors tested. Two explanations are possible. First, there may be other mechanisms for neuronal Cl− uptake in the absence of NKCC1 [3,33]. Second, it may be that the secondary Cl− current is not required at all for normal olfactory sensitivity. Perhaps the initial elements of the transduction cascade, including the small current through the CNG channels, are sufficient. Mice lacking the Cl− channels would allow a more definitive test of this but are not available. However, a candidate gene for the Cl− channel has recently been identified [34]. This may ultimately allow a more direct test of the importance of the Cl− channel in olfactory perception.
Acknowledgments
We are grateful to Gary Shull, Lara Gawenis, and Emily Bradford for KO mice and many helpful discussions; to Nancy Kleene for maintaining the mouse colonies; to Randall Sakai and Alan Spector for critical reviews of the manuscript; to Dr. Burton Slotnick for assistance with the Knosys olfactometer; and to UF honors students Erica Rodriguez, Benjamin Burns, Eric Przybylinski and Swati Pradeep for assistance in conducting the day-to-day behavioral test sessions with the mice. This work was supported in part by research grant R01 DC00926 from the National Institute on Deafness and Other Communicative Disorders, National Institutes of Health (SJK); by the College of Medicine Dean’s Bridge Funding Program and the University Research Council of the University of Cincinnati (SJK); and by startup funding from the Department of Psychology, University of Florida (DWS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schild D, Restrepo D. Transduction mechanisms in vertebrate olfactory receptor cells. Physiol Rev. 1998;78:429–66. doi: 10.1152/physrev.1998.78.2.429. [DOI] [PubMed] [Google Scholar]
- 2.Reisert J, Bauer PJ, Yau K-W, Frings S. The Ca-activated Cl channel and its control in rat olfactory receptor neurons. J Gen Physiol. 2003;122:349–63. doi: 10.1085/jgp.200308888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nickell WT, Kleene NK, Gesteland RC, Kleene SJ. Neuronal chloride accumulation in olfactory epithelium of mice lacking NKCC1. J Neurophysiol. 2006;95:2003–6. doi: 10.1152/jn.00962.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boccaccio A, Menini A. Temporal development of CNG and Ca2+-activated Cl− currents in isolated mouse olfactory sensory neurons. J Neurophysiol. 2007;98:153–60. doi: 10.1152/jn.00270.2007. [DOI] [PubMed] [Google Scholar]
- 5.Kaneko H, Putzier I, Frings S, Kaupp UB, Gensch T. Chloride accumulation in mammalian olfactory sensory neurons. J Neurosci. 2004;24:7931–8. doi: 10.1523/JNEUROSCI.2115-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reisert J, Lai J, Yau K-W, Bradley J. Mechanism of the excitatory Cl− response in mouse olfactory receptor neurons. Neuron. 2005;45:553–61. doi: 10.1016/j.neuron.2005.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong ST, Trinh K, Hacker B, Chan GCK, Lowe G, Gaggar A, Xia Z, Gold GH, Storm DR. Disruption of the type III adenylyl cyclase gene leads to peripheral and behavioral anosmia in transgenic mice. Neuron. 2000;27:487–97. doi: 10.1016/s0896-6273(00)00060-x. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z, Sindreu CB, Li V, Nudelman A, Chan GC-K, Storm DR. Pheromone detection in male mice depends on signaling through the type 3 adenylyl cyclase in the main olfactory epithelium. J Neurosci. 2006;26:7375–9. doi: 10.1523/JNEUROSCI.1967-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandiyan VS, Coats JK, Shah NM. Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nature Neurosci. 2005;8:1660–2. doi: 10.1038/nn1589. [DOI] [PubMed] [Google Scholar]
- 10.Spehr M, Kelliher KR, Li X-H, Boehm T, Leinders-Zufall T, Zufall F. Essential role of the main olfactory system in social recognition of major histocompatibility complex peptide ligands. J Neurosci. 2006;26:1961–70. doi: 10.1523/JNEUROSCI.4939-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin W, Arellano J, Slotnick B, Restrepo D. Odors detected by mice deficient in cyclic nucleotide-gated channel subunit A2 stimulate the main olfactory system. J Neurosci. 2004;24:3703–10. doi: 10.1523/JNEUROSCI.0188-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michalakis S, Reisert J, Geiger H, Wetzel C, Zong X, Bradley J, Spehr M, Hüttl S, Gerstner A, Pfeifer A, Hatt H, Yau K-W, Biel M. Loss of CNGB1 protein leads to olfactory dysfunction and subciliary cyclic nucleotide-gated channel trapping. J Biol Chem. 2006;46:35156–66. doi: 10.1074/jbc.M606409200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flagella M, Clarke LL, Miller ML, Erway LC, Giannella RA, Andringa A, Gawenis LR, Kramer J, Duffy JJ, Doetschman T, Lorenz JN, Yamoah EN, Cardell EL, Shull GE. Mice lacking the basolateral Na-K-2Cl cotransporter have impaired epithelial chloride secretion and are profoundly deaf. J Biol Chem. 1999;274:26946–55. doi: 10.1074/jbc.274.38.26946. [DOI] [PubMed] [Google Scholar]
- 14.Delpire E, Mount DB. Human and murine phenotypes associated with defects in cation-chloride cotransport. Annu Rev Physiol. 2002;64:803–43. doi: 10.1146/annurev.physiol.64.081501.155847. [DOI] [PubMed] [Google Scholar]
- 15.Wong AA, Brown RE. Visual detection, pattern discrimination and visual acuity in 14 strains of mice. Genes Brain Behav. 2006;5:389–403. doi: 10.1111/j.1601-183X.2005.00173.x. [DOI] [PubMed] [Google Scholar]
- 16.Bodyak N, Slotnick B. Performance of mice in an automated olfactometer: odor detection, discrimination and odor memory. Chem Senses. 1999;24:637–45. doi: 10.1093/chemse/24.6.637. [DOI] [PubMed] [Google Scholar]
- 17.Kelliher KR, Ziesmann J, Munger SD, Reed RR, Zufall F. Importance of the CNGA4 channel gene for odor discrimination and adaptation in behaving mice. Proc Natl Acad Sci USA. 2003;100:4299–304. doi: 10.1073/pnas.0736071100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laska M, Shepherd GM. Olfactory discrimination ability of CD-1 mice for a large array of enantiomers. Neuroscience. 2007;144:205–301. doi: 10.1016/j.neuroscience.2006.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cometto-Muñiz JE, Cain WS, Abraham MH. Quantification of chemical vapors in chemosensory research. Chem Senses. 2003;6:467–77. doi: 10.1093/chemse/28.6.467. [DOI] [PubMed] [Google Scholar]
- 20.Brennan PA, Zufall F. Pheromonal communication in vertebrates. Nature. 2006;444:308–15. doi: 10.1038/nature05404. [DOI] [PubMed] [Google Scholar]
- 21.Cometto-Muñiz JE, Cain WS. Thresholds for odor and nasal pungency. Physiol Behav. 1990;48:719–25. doi: 10.1016/0031-9384(90)90217-r. [DOI] [PubMed] [Google Scholar]
- 22.Delpire E, Lu J, England R, Dull C, Thorne T. Deafness and imbalance associated with inactivation of the secretory Na-K-2Cl co-transporter. Nat Genet. 1999;22:192–5. doi: 10.1038/9713. [DOI] [PubMed] [Google Scholar]
- 23.Dixon MJ, Gazzard J, Chaudhry SS, Sampson N, Schulte BA, Steel KP. Mutation of the Na-K-Cl co-transporter gene Slc12a2 results in deafness in mice. Hum Mol Genet. 1999;8:1579–84. doi: 10.1093/hmg/8.8.1579. [DOI] [PubMed] [Google Scholar]
- 24.Pace AJ, Madden VJ, Henson OW, Jr, Koller BH, Henson MM. Ultrastructure of the inner ear of NKCC1-deficient mice. Hear Res. 2001;156:17–30. doi: 10.1016/s0378-5955(01)00263-5. [DOI] [PubMed] [Google Scholar]
- 25.Youngentob SL, Margolis FL. OMP gene deletion causes an elevation in behavioral threshold sensitivity. Neuroreport. 1999;18:15–9. doi: 10.1097/00001756-199901180-00003. [DOI] [PubMed] [Google Scholar]
- 26.Linster C, Johnson BA, Morse A, Yue E, Leon M. Spontaneous versus reinforced olfactory discriminations. J Neurosci. 2002;22:6842–5. doi: 10.1523/JNEUROSCI.22-16-06842.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao H, Ivic L, Otaki JM, Hashimoto M, Mikoshiba K, Firestein S. Functional expression of a mammalian odorant receptor. Science. 1998;279:237–42. doi: 10.1126/science.279.5348.237. [DOI] [PubMed] [Google Scholar]
- 28.Grosmaitre X, Vasalli A, Mombaerts P, Shepherd GM, Ma M. Odorant responses of olfactory sensory neurons expressing the odorant receptor MOR23: A patch clamp analysis in gene-targeted mice. Proc Natl Acad Sci USA. 2006;103:1970–5. doi: 10.1073/pnas.0508491103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belluscio L, Gold GH, Nemes A, Axel R. Mice deficient in Golf are anosmic. Neuron. 1998;20:69–81. doi: 10.1016/s0896-6273(00)80435-3. [DOI] [PubMed] [Google Scholar]
- 30.Brunet LJ, Gold GH, Ngai J. General anosmia caused by a targeted disruption of the mouse olfactory cyclic nucleotide-gated cation channel. Neuron. 1996;17:681–93. doi: 10.1016/s0896-6273(00)80200-7. [DOI] [PubMed] [Google Scholar]
- 31.Munger SD, Lane AP, Zhong H, Leinders-Zufall T, Yau K-W, Zufall F, Reed RR. Central role of the CNGA4 channel subunit in Ca2+-calmodulin-dependent odor adaptation. Science. 2001;294:2172–6. doi: 10.1126/science.1063224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bradley J, Reuter D, Frings S. Facilitation of calmodulin-mediated odor adaptation by cAMP-gated channel subunits. Science. 2001;294:2176–8. doi: 10.1126/science.1063415. [DOI] [PubMed] [Google Scholar]
- 33.Nickell WT, Kleene NK, Kleene SJ. Mechanisms of neuronal chloride accumulation in intact mouse olfactory epithelium. J Physiol. 2007;583:1005–1020. doi: 10.1113/jphysiol.2007.129601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pifferi S, Pascarella G, Boccaccio A, Mazzatenta A, Gustincich S, Menini A, Zucchelli S. Bestrophin-2 is a candidate calcium-activated chloride channel involved in olfactory transduction. Proc Natl Acad Sci USA. 2006;103:12929–34. doi: 10.1073/pnas.0604505103. [DOI] [PMC free article] [PubMed] [Google Scholar]


