Abstract
Objectives
To analyze the trend of dextromethorphan abuse in California and to compare these findings with national trends.
Design
A 6-year retrospective review.
Setting
California Poison Control System (CPCS), American Association of Poison Control Centers (AAPCC), and Drug Abuse Warning Network (DAWN) databases from January 1, 1999, to December 31, 2004.
Participants
All dextromethorphan abuse cases reported to the CPCS, AAPCC, and DAWN. The main exposures of dextromethorphan abuse cases included date of exposure, age, acute vs long-term use, coingestants, product formulation, and clinical outcome.
Main Outcome Measure
The annual proportion of dextromethorphan abuse cases among all exposures reported to the CPCS, AAPCC, and DAWN databases.
Results
A total of 1382 CPCS cases were included in the study. A 10-fold increase in CPCS dextromethorphan abuse cases from 1999 (0.23 cases per 1000 calls) to 2004 (2.15 cases per 1000 calls) (odds ratio, 1.48; 95% confidence interval, 1.43–1.54) was identified. Of all CPCS dextromethorphan abuse cases, 74.5% were aged 9 to 17 years; the frequency of cases among this age group increased more than 15-fold during the study (from 0.11 to 1.68 cases per 1000 calls). Similar trends were seen in the AAPCC and DAWN databases. The highest frequency of dextromethorphan abuse occurred among adolescents aged 15 and 16 years. The most commonly abused product was Coricidin HBP Cough & Cold Tablets.
Conclusions
Our study revealed an increasing trend of dextromethorphan abuse cases reported to the CPCS that is paralleled nationally as reported to the AAPCC and DAWN. This increase was most evident in the adolescent population.
Dextromethorphan has been used safely for years as a cough suppressant and is available in many over-the-counter (OTC) cough and cold preparations. Whereas therapeutic doses of dextromethorphan act at the sigma receptor to produce its anti-tussive effects, high doses are metabolized to dextrorphan, an active metabolite that antagonizes N-methyl-D-aspartate receptors.1 Because of its similar pharmacology to phencyclidine and ketamine, dextrorphan and, to a lesser degree, dextromethorphan may produce dissociative hallucinations at high doses.2,3 In addition, tachycardia, hypertension, agitation, ataxia, and psychosis also have been reported following high-dose dextromethorphan.4–7
Dextromethorphan abuse was recognized as early as the 1960s when it was marketed as the sole active ingredient in Romilar, an OTC product that was voluntarily removed from the market because of abuse.8 Since the late 1990s, adolescents have been increasingly abusing OTC dextromethorphan products because of their easy accessibility and false perception of safety. Several states, such as California, North Dakota, Texas, and New York, have even proposed legislation to control the sale of dextromethorphan-containing products to minors. There are numerous slang names for dextromethorphan, such as CCC, Triple C, DXM, Dex, Poor Man’s PCP, Skittles, and Robo.9 Many of these slang terms refer to particular brand name products containing dextromethorphan. Two previously reported studies, one in California6 and another in Texas,10 identified Coricidin products (Schering-Plough HealthCare Products Inc, Kenilworth, NJ) as agents of abuse with a high prevalence in the adolescent population. The Texas Poison Center study identified a possible increasing trend of Coricidin abuse from 1998 to 1999.
The goals of this retrospective review were to analyze the trend of dextromethorphan abuse in California and to compare the findings with national trends. We also hoped to gain insight into the particular products involved and the most common age groups abusing dextromethorphan, and the effect on health care facilities. Evaluating patterns of dextromethorphan abuse may help health care practitioners target interventions, such as prevention and treatment.
METHODS
We conducted a retrospective case record review analyzing all suspected dextromethorphan abuse cases reported to the California Poison Control System (CPCS) from January 1, 1999, through December 31, 2004. The CPCS is a 24-hour emergency telephone consultation service that offers treatment advice to the public and health care professionals regarding poisonings and overdoses. The CPCS database consists of case records documenting all consultations. All data in the case records are collected by the CPCS staff in accordance with American Association of Poison Control Centers (AAPCC) guidelines.11 This study was approved by the Committee on Human Research, University of California, San Francisco. Subject consent was not required because the study involved a retrospective review of case records, in which all patient identifiers were removed before evaluation.
Case records were identified for evaluation by electronically searching the CPCS database for all cases coded as abuse and containing any of the AAPCC dextromethorphan-containing generic or product codes. Cases were excluded if they involved any of the following: (1) drug information, (2) unintentional misuse, (3) duplicate cases, (4) product miscoded as dextromethorphan abuse, and (5) symptoms unrelated to dextromethorphan ingestion. Cases were reviewed by 1 of 4 data reviewers (J.K.B., U.K.W., J.W.H., or M.B.), with 183 (13.2%) of the 1382 cases undergoing a second review to ensure consistency. All major outcome cases underwent a second review. Data collected included age, sex, caller site, disposition, product ingested, dose form, coingestants, clinical symptoms, short- vs long-term use, ethanol level, and acetaminophen level. Outcomes were coded according to the AAPCC definitions.11 Examples of each category include the following: (1) no effect; (2) minor effect (self-limiting minimal symptoms that generally resolve rapidly, including drowsiness, gastrointestinal symptoms, and/or sinus tachycardia); (3) moderate effect (more pronounced, prolonged, or systemic symptoms that are not life threatening, including agitation, disorientation, hallucinations, brief hypotension, and/or a single brief seizure); and (4) major effect (life-threatening symptoms or symptoms that resulted in significant residual disability, including cardiovascular and respiratory compromise and/or prolonged or multiple seizures).
The primary outcome measured was the proportion of dextromethorphan abuse cases among all cases reported to the CPCS yearly from 1999 to 2004. Secondary outcomes analyzed included the proportion of dextromethorphan abuse cases in patients aged 9 to 17 years, the proportion of users classified as long-term abusers, the proportion of solid vs other dose forms, the frequency of individual product exposures, and the frequency of clinical outcomes (classified as no effect, minor, moderate, or major). Changes in primary and secondary outcomes over the study years were analyzed using logistic regression. A multivariate logistic regression model was constructed to determine the effect of age, sex, year, presence of coingestants, and product on clinical outcome.
Data were analyzed from 2 additional sources reporting nationwide drug abuse. The first was composed of dextromethorphan abuse cases reported to the AAPCC from January 1, 1999, through December 31, 2004. The AAPCC data were derived from a written report generated from the Toxic Exposure Surveillance System involving human exposures to dextromethorphan reported as intentional abuse to US Poison Control Centers from 1999 to 2004 (AAPCC, Washington, DC, Report 27, unpublished data, December 16, 2005).11 The second was composed of dextromethorphan abuse cases reported to the Substance Abuse and Mental Health Services Administration program, Drug Abuse Warning Network (DAWN), from January 1, 1999, through December 31, 2002.12 Dextromethorphan-related exposures reported to DAWN are not currently available after 2002. The following data from CPCS were compared with data from the DAWN and AAPCC databases: (1) age distribution (for AAPCC only, not available through DAWN) and (2) total number of dextromethorphan abuse cases reported annually. Only the AAPCC cases for which the exact age was known and the age was at least 9 years were included in the analysis. The AAPCC and DAWN databases were analyzed for temporal trends in dextromethorphan reporting by logistic regression, and the proportion of dextromethorphan cases reported in adolescents was investigated in the AAPCC database. The general approach we used in this study of combining detailed analysis of CPCS data juxtaposed with general surveillance data from other sources parallels the methods previously used to study γ-hydroxybutyrate, another drug of abuse.13
RESULTS
During the 6-year study period, the CPCS had 1411 reports coded as dextromethorphan abuse and all underwent manual case record review. Ultimately, 1382 dextromethorphan abuse cases were included in the final analysis. On review, 29 cases were excluded because of the following reasons: drug information cases (n=10), duplicate cases (n=2), product reported did not contain dextromethorphan (n=1), misclassified as abuse (n=14), and reported symptoms were unrelated to dextromethorphan ingestion (n=2). During the study, the CPCS received 1 336 475 human exposure calls.
From 1999 through 2004, the frequency of all dextromethorphan abuse cases reported to the CPCS increased 10-fold (Table). The average effect was an almost 50% increase in the frequency of reported cases each year compared with the previous year (odds ratio, 1.48; 95% confidence interval, 1.43–1.54). This increase in the number of dextromethorphan abuse cases (Figure 1) and the proportion of dextromethorphan cases among all exposures reported (Figure 2) was also seen in the data collected by the AAPCC (odds ratio, 1.31; 95% confidence interval, 1.29–1.33) and DAWN (through 2002) (odds ratio, 1.15; 95% confidence interval, 1.13–1.18). The CPCS dextromethorphan abuse reports are included in the cases reported to the AAPCC, but these alone would not account for the national AAPCC trend.
Table.
Dextromethorphan Abuse Cases Reported to the CPCS From 1999 Through 2004*
| Case Characteristics | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | All Years |
|---|---|---|---|---|---|---|---|
| CPCS data | |||||||
| Dextromethorphan cases | |||||||
| Total | 48 | 125 | 149 | 226 | 356 | 478 | 1382 |
| Those aged 9–17 y† | 23 (47.9) | 79 (63.2) | 114 (76.5) | 152 (67.3) | 286 (80.3) | 375 (78.5) | 1029 (74.5) |
| Total CPCS human exposure cases | 209 991 | 220 418 | 230 819 | 231 716 | 220 867 | 222 664 | 1 336 475 |
| Dextromethorphan cases/1000 calls | |||||||
| All | 0.23 | 0.57 | 0.65 | 0.97 | 1.61 | 2.15 | 1.03 |
| Those aged 9–17 y | 0.11 | 0.36 | 0.49 | 0.66 | 1.30 | 1.68 | 0.77 |
| Case characteristics of CPCS data | |||||||
| Males† | 30 (62.5) | 75 (60.0) | 105 (70.5) | 145 (64.2) | 184 (51.7) | 289 (60.5) | 828 (59.9) |
| Mean age, y | 21.5 | 18.8 | 17.9 | 17.4 | 16.6 | 16.6 | 18.1 |
| Median age, y | 18.0 | 16.0 | 16.0 | 16.0 | 15.0 | 16.0 | 16.0 |
| Disposition† | |||||||
| HCF | 31 (64.6) | 78 (62.4) | 102 (68.5) | 132 (58.4) | 235 (66.0) | 303 (63.4) | 881 (63.7) |
| NHCF | 17 (35.4) | 47 (37.6) | 47 (31.5) | 94 (41.6) | 121 (34.0) | 175 (36.6) | 501 (36.3) |
| Long-term abusers† | 7 (14.6) | 8 (6.4) | 4 (2.7) | 8 (3.5) | 3 (0.8) | 2 (0.4) | 32 (2.3) |
| Outcome† | |||||||
| Minor or no effect | 26 (54.2) | 80 (64.0) | 75 (50.3) | 137 (60.6) | 185 (52.0) | 227 (47.5) | 730 (52.8) |
| Moderate effect | 19 (39.6) | 42 (33.6) | 73 (49.0) | 83 (36.7) | 142 (39.9) | 219 (45.8) | 578 (41.8) |
| Major effect | 1 (2.1) | 0 | 1 (0.7) | 1 (0.4) | 2 (0.6) | 2 (0.4) | 7 (0.5) |
| Unknown | 2 (4.2) | 3 (2.4) | 0 | 5 (2.2) | 27 (7.6) | 30 (6.3) | 67 (4.8) |
| Most common products abused†‡ | |||||||
| Coricidin HBP Cough & Cold Tablets | 9 (18.8) | 72 (57.6) | 106 (71.1) | 144 (63.7) | 248 (69.7) | 330 (69.0) | 909 (65.8) |
| Robitussin formulations with dextromethorphan | 11 (22.9) | 24 (19.2) | 27 (18.1) | 41 (18.1) | 38 (10.7) | 50 (10.5) | 191 (13.8) |
| Coricidin, other dextromethorphan-containing formulations | 3 (6.2) | 3 (2.4) | 0 | 7 (3.1) | 23 (6.5) | 26 (5.4) | 62 (4.5) |
| Nyquil formulations with dextromethorphan | 10 (20.8) | 9 (7.2) | 4 (2.7) | 5 (2.2) | 10 (2.8) | 10 (2.1) | 48 (3.5) |
| Slang term used to identify dextromethorphan | 0 | 3 (2.4) | 2 (1.3) | 14 (6.2) | 8 (2.2) | 17 (3.6) | 44 (3.2) |
| Tussin DM liquid | 1 (2.1) | 0 | 0 | 5 (2.2) | 3 (0.8) | 6 (1.3) | 15 (1.1) |
| Vicks formulations with dextromethorphan | 2 (4.2) | 3 (2.4) | 2 (1.3) | 1 (0.4) | 4 (1.1) | 2 (0.4) | 14 (1.0) |
| Other§ | 0 | 5 (4.0) | 1 (0.7) | 1 (0.4) | 8 (2.2) | 16 (3.3) | 31 (2.2) |
| Unknown dextromethorphan-containing product | 12 (25.0) | 6 (4.8) | 7 (4.7) | 8 (3.5) | 14 (3.9) | 21 (4.4) | 68 (4.9) |
Abbreviations: CPCS, California Poison Control System; HCF, health care facility; NHCF, non-HCF (home or other site).
The age was unknown for 22 subjects; the sex was known for all subjects. The exact dates of the study were from January 1, 1999, through December 31, 2004.
Data are given as number (percentage) of total for each year. Percentages may not total 100 because of rounding.
Coricidin HBP Cough & Cold Tablets are a combination of chlorpheniramine maleate and dextromethorphan hydrobromide and are manufactured by Schering-Plough HealthCare Products Inc, Kenilworth, NJ; Robitussin formulations are manufactured by Wyeth, Madison, NJ; Nyquil formulations are manufactured by Proctor & Gamble, Cincinnati, Ohio; Tussin DM is a combination of guaifenesin and dextromethorphan hydrobromide and is manufactured by various manufacturers; and Vicks formulations are manufactured by Proctor & Gamble.
Individual brand name products each had a frequency of 10 or fewer.
Figure 1.
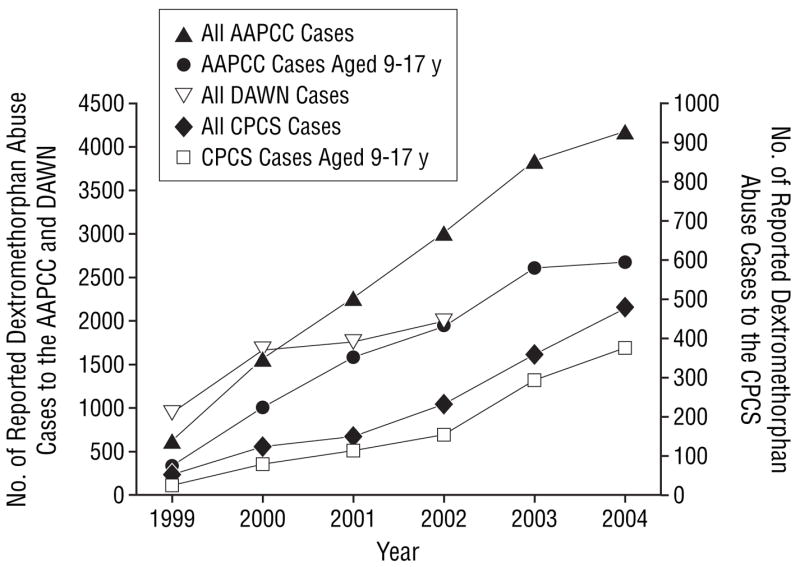
A comparison of California Poison Control System (CPCS), American Association of Poison Control Centers (AAPCC), and Drug Abuse Warning Network (DAWN)12 dextromethorphan abuse cases. Dextromethorphan DAWN data were unavailable for 2003 and 2004. Dextromethorphan abuse reported to the AAPCC represents 15 543 cases; and to DAWN, 6447 cases.
Figure 2.
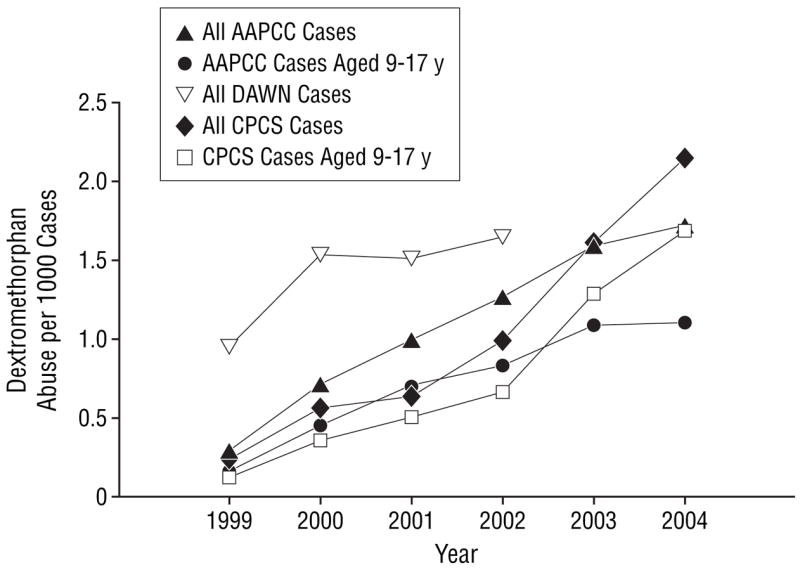
The proportion of California Poison Control System (CPCS), American Association of Poison Control Centers (AAPCC), and Drug Abuse Warning Network (DAWN)12 dextromethorphan abuse cases compared with the total exposures reported to each database. Dextromethorphan DAWN data are unavailable for 2003 and 2004.
The overall increase in the frequency of dextromethorphan abuse cases was paralleled by an increase in the frequency of dextromethorphan abuse cases reported in adolescents (defined as those aged 9–17 years). During the 6-year study period, 74.5% of all reported CPCS dextromethorphan abuse cases involved adolescents. The overall median age was 16 years. The proportion of all dextromethorphan abuse cases that occurred in adolescents increased over the duration of the study according to the CPCS (odds ratio, 1.25; 95% confidence interval, 1.15–1.36) and AAPCC (odds ratio, 1.03; 95% confidence interval, 1.01–1.05) databases. The largest proportional increase in adolescent abuse was seen from 1999 to 2000 (Table). The highest frequency of dextromethorphan abuse in the CPCS and AAPCC databases was among adolescents aged 15 and 16 years (Figure 3). The younger adolescent age distribution is further underscored by the observation that in CPCS reporting the combined frequency among 12- to 13-year-old subjects exceeded that of 18-year-old subjects.
Figure 3.
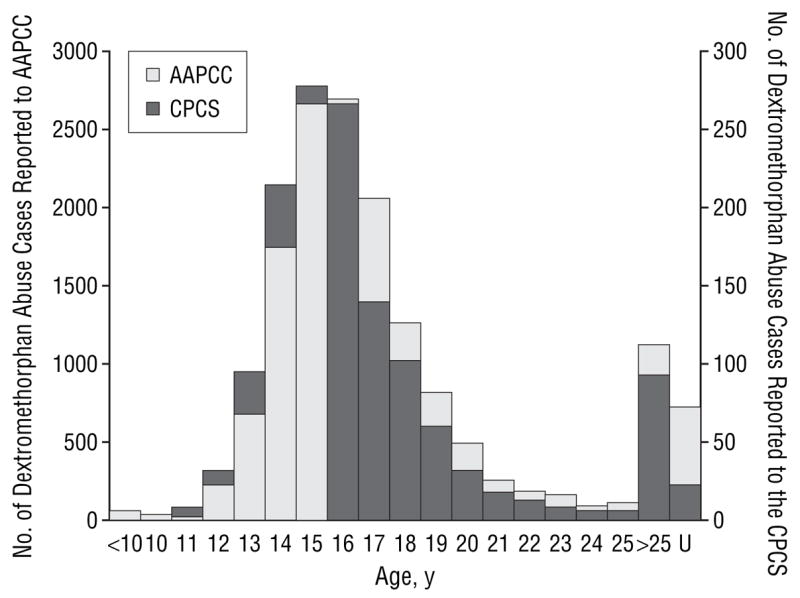
A comparison of the trends in abuse according to age for cases reported to the California Poison Control System (CPCS) and the American Association of Poison Control Centers (AAPCC) during the study. The age was not reported in 22 of the CPCS cases and in 733 of the AAPCC cases. U indicates unknown.
Of the 1382 cases of dextromethorphan abuse included in our study, the most commonly abused product was Coricidin HBP Cough & Cold Tablets. This was followed by dextromethorphan-containing Robitussin products (Wyeth, Madison, NJ) (Table). The proportion of abused dextromethorphan products represented by Coricidin HBP Cough & Cold Tablets increased over the study period (odds ratio, 1.23; 95% confidence interval, 1.13–1.26). This increasing trend in abuse of Coricidin HBP Cough & Cold Tablets corresponded to an increase in the number of exposures that involved a solid dose form of dextromethorphan (odds ratio, 1.43; 95% confidence interval, 1.31–1.56). In contrast to the increase in acute cases of dextromethorphan abuse, long-term abuse reports to the CPCS were uncommon (n=32) and declined over the study period (odds ratio, 0.51; 95% confidence interval, 0.40–0.64).
Most dextromethorphan cases resulted in minor (636 cases [46.0%]) or moderate (578 cases [41.8%]) outcomes. Seven cases (0.5%) had major outcomes. For the remaining cases, either no symptoms were reported (94 cases [6.8%]) or the outcome was unknown (67 cases [4.8%]) (Table). There were no fatalities reported. All 7 major outcome cases involved serious pulmonary complications, such as respiratory depression or aspiration requiring intubation. Five of the major outcome cases involved coingestants, 3 of which involved ethanol. Among the major outcome cases, only 2 involved minors. There was an increase in combined moderate and major outcomes during the study (odds ratio, 1.09; 95% confidence interval, 1.01–1.17). A multivariate model incorporating study year, age, sex, presence of coingestants, and exposure to Coricidin HBP Cough & Cold Tablets showed an increased incidence of moderate and major outcomes among patients in later years (odds ratio, 1.09; 95% confidence interval, 1.01–1.18) and among male adolescents (odds ratio, 1.22; 95% confidence interval, 1.09–1.37).
Polysubstance use was reported by history in 278 (20.1%) of all subjects. The most common agents reported were ethanol (n=93), marijuana (n=40), opiates (n=23), acetaminophen (n=17), amphetamines (n=14), selective serotonin reuptake inhibitors (n=10), pseudoephedrine (n=10), and benzodiazepines (n=9). Self-reported ethanol coingestion was documented among 93 (6.7%) subjects; however, only 23 cases had laboratory confirmation. Three cases were coded as having major outcomes (levels ranged from 230–373 mg/dL), and 11 were coded as having moderate outcomes. Only 17 patients reported ingesting acetaminophen by history; however, there were 26 patients with confirmed laboratory acetaminophen levels (range, 9.1–111.0 μg/mL). More important, 8 patients had a delayed presentation to the hospital (range, 8 hours–4 days), and in 4 additional patients the time of ingestion was unknown. Sixteen patients received N-acetylcysteine therapy, of whom 7 experienced elevated transaminase levels. Of these 7 patients, 1 was a long-term alcohol user. Of the 26 subjects with acetaminophen involvement, 14 were coded as having a moderate outcome and 0 were coded as having a major outcome. There was no significant difference in the occurrence of the combined major and moderate outcomes between patients who did or did not have any coingestant (odds ratio, 0.96; 95% confidence interval, 0.83–1.14).
The most common adverse effects reported were tachycardia (n=593), lethargy (n=481), hypertension (n=289), confusion or altered mental status (n=277), mydriasis (n=251), agitation (n=171), gastrointestinal effects (n=159), dizziness (n=94), ataxia (n=83), hallucinations (n=80), slurred speech (n=77), nystagmus (n=75), fever (n=54), loss of consciousness (n=26), tachypnea (n=20), seizure (n=12), and hypotension (n=12).
COMMENT
Our study reveals a 10-fold increase in all dextromethorphan abuse cases reported to the CPCS from 1999 through 2004. This increasing trend of dextromethorphan abuse is consistent with the national trend documented by the AAPCC, whose data revealed a 7-fold increase during the same time frame. In addition, DAWN also reported an overall increase in dextromethorphan abuse from 1999 to 2002, although the increase was not as pronounced. The highest frequency of abuse was in adolescents aged 15 and 16 years, as reported to the CPCS and the AAPCC. Abuse of solid dose forms increased more dramatically during the study, compared with the liquid formulations. According to CPCS reports, Coricidin HBP Cough & Cold Tablets was the most commonly reported dextromethorphan-containing product abused, followed by dextromethorphan-containing Robitussin formulations (Table). There may be multiple explanations for the apparent preference of Coricidin HBP Cough & Cold Tablets abuse, including widespread OTC availability, high dextromethorphan content, palatability of the tablet formulation, and, most important, its frequent promotion as a product of abuse on many Internet Web sites. An increase in combined moderate and major outcomes was also identified during the study. The explanation for this trend is likely multifactorial; however, it may be because of the increased abuse of Coricidin HBP Cough & Cold Tablets and the contribution of chlorpheniramine in the formulation following high doses. However, a detailed examination of the actual cause of this particular trend is beyond the scope of this study.
The increasing trend of teenage dextromethorphan abuse is multifactorial. Dextromethorphan is available in multiple OTC cough and cold products legally sold at pharmacies and most grocery stores. As little as 1 package may contain enough dextromethorphan to produce euphoric and hallucinatory effects.14 Adolescents are increasingly abusing OTC medications because they are easily available and relatively inexpensive, and many have a false perception that even high doses of dextromethorphan are not dangerous. In contrast, other drugs of abuse, such as γ-hydroxybutyrate, methylenedioxymeth-amphetamine (Ecstasy), and lysergic acid diethylamide, which are not as easily accessible to minors, have shown an annual decline in use among adolescents and all age groups over the past few years.13,15 Considering ease of availability as a reason for teenage abuse, it is not surprising that abuse of a readily available OTC medication like dextromethorphan, with its euphoric effects, is increasing. Another factor is that it is easy to fool parents because these OTC products are commonly kept in the household. Because dextromethorphan is an OTC medication, it lacks the stigma of a drug of abuse and there are numerous Web sites promoting dextromethorphan abuse and even providing instructions on how to abuse and manufacture the drug.16,17 More and more children younger than 18 years have unsupervised access to the World Wide Web, including dextromethorphan abuse Web sites. The peer pressure that commonly plagues adolescents could certainly play an important role in the increasing abuse.
Another serious concern with taking large amounts of OTC cough and cold products for the dextromethorphan content involves toxicity from the hidden ingredients, such as pseudoephedrine, acetaminophen, and antihistamines. For example, high doses of acetaminophen may result in delayed liver failure.18 Typically, the intoxication from the dextromethorphan resolves within 6 to 8 hours; however, acetaminophen may not even start to show signs of toxicity for 10 hours and, even then, the first signs may be gastrointestinal upset only. Unless these patients seek medical care, acetaminophen-induced hepatotoxicity may ensue. Our study revealed that of the 26 patients who had elevated serum acetaminophen levels, only 17 reported acetaminophen use by history. It is highly likely that some of these patients were unaware of their acetaminophen exposure because it is a common “hidden” ingredient in many OTC cough and cold products. Sixteen patients received the antidote, N-acetylcysteine, and 7 had elevated transaminase levels.
The major limitation of our study was the retrospective design, primarily because of incomplete documentation of clinical data on some of the poison center charts. Because the poison center reporting is a passive reporting system, calls to the poison center regarding drugs of abuse are usually secondary to an adverse reaction. To our knowledge, there is no study that correlates the true incidence of abuse in the general population to the incidence of poison center reports. Therefore, dextromethorphan abuse may be far higher than the cases reported in our study. Another limitation of our study is that laboratory testing of dextromethorphan is not routinely available at hospital laboratories. Consequently, our patients’ dextromethorphan exposure was based on history rather than urine or blood test confirmation. In addition, dextromethorphan may trigger a false positive on the phencyclidine laboratory drug screen, resulting in some patients being falsely diagnosed as having phencyclidine intoxication when in actuality dextromethorphan was the agent responsible for the intoxication, further lowering the dextromethorphan incidence reported.6 Moreover, dextromethorphan abuse is likely underreported to DAWN for 2 reasons. First, dextromethorphan was not a drug that was specifically listed on the DAWN data collection form during the early years of this study, but rather would only be documented in the “other” category. Second, dextromethorphan is a hidden ingredient in OTC cough and cold preparations and, therefore, may have been missed by the DAWN hospital medical record evaluator during the medical record review process. Last, another limitation of our study is our inability to attribute case-specific symptoms to dextromethorphan as opposed to other ingredients found in combination products. For example, in some cases, tachycardia, a common finding, could have been due, at least in part, to the antihistamine (eg, chlorpheniramine) or the decongestant (eg, pseudoephedrine) components in the OTC cough and cold products.
In conclusion, this study showed an increasing trend of dextromethorphan abuse over a 6-year period, particularly in adolescents younger than 18 years. The data from the CPCS showed a 10-fold increase in dextromethorphan abuse in all ages and a 15-fold increase in adolescents from 1999 through 2004. This increase in dextromethorphan abuse in adolescents is most likely due to the hallucinogenic effects of these easily accessible inexpensive OTC products and the false perception that high-dose dextromethorphan is safe. It is important for health care practitioners, manufacturers, and retail establishments selling dextromethorphan-containing products to be aware of increasing dextromethorphan abuse to educate and hopefully prevent dextromethorphan abuse and the ensuing toxicity from occurring. Preventive measures, such as placing dextromethorphan-containing products behind pharmacy counters, may be an effective action to limit this increasing trend of abuse in adolescents.
Acknowledgments
We thank Terry S. Carlson, PharmD, at the CPCS, for his invaluable assistance as a computer systems analyst in extracting the poison center case records.
Funding/Support: This study was supported in part by grant R01 DA 14935 from the National Institute on Drug Abuse.
Footnotes
Financial Disclosure: None reported.
Additional Information: This study was conducted as a partial requirement to earn a doctor of pharmacy degree from the School of Pharmacy, University of California, San Francisco, for the following authors: Bryner, Wang, Hui, and Bedodo.
Author Contributions: Dr Anderson had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Bryner, Wang, Hui, Bedodo, and Anderson. Acquisition of data: Bryner, Wang, Hui, Bedodo, and Anderson. Analysis and interpretation of data: Bryner, Wang, Hui, Bedodo, MacDougall, and Anderson. Drafting of the manuscript: Bryner, Wang, Hui, Bedodo, and MacDougall. Critical revision of the manuscript for important intellectual content: Bryner, Wang, Hui, MacDougall, and Anderson. Statistical analysis: MacDougall. Obtained funding: Anderson. Administrative, technical, and material support: Bryner, Hui, and Bedodo. Study supervision: Anderson. Background data search: Bedodo.
Role of the Sponsor: The funding body had no role in data extraction and analyses, in the writing of the manuscript, or in the decision to submit the manuscript for publication.
References
- 1.Ramachander G, Williams FD, Emele JF. Determination of dextrorphan in plasma and evaluation of bioavailability of dextromethorphan hydrobromide in humans. J Pharm Sci. 1977;66:1047–1048. doi: 10.1002/jps.2600660740. [DOI] [PubMed] [Google Scholar]
- 2.Ginski MJ, Witkin JM. Sensitive and rapid behavioral differentiation of N-methyl-D-aspartate receptor antagonists. Psychopharmacology (Berl) 1994;114:573–582. doi: 10.1007/BF02244987. [DOI] [PubMed] [Google Scholar]
- 3.Bobo WV, Fulton RB. Commentary on: severe manifestations of Coricidin intoxication. Am J Emerg Med. 2004;22:624–625. doi: 10.1016/j.ajem.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Price LH, Lebel J. Dextromethorphan-induced psychosis [letter] Am J Psychiatry. 2000;157:304. doi: 10.1176/appi.ajp.157.2.304. [DOI] [PubMed] [Google Scholar]
- 5.Sharma A, Dewan V, Petty F. Acute psychosis with Coricidin cold medicine. Ann Pharmacother. 2005;39:1577–1578. doi: 10.1345/aph.1G193. [DOI] [PubMed] [Google Scholar]
- 6.Banerji S, Anderson IB. Abuse of Coricidin HBP Cough & Cold Tablets: episodes recorded by a poison center. Am J Health Syst Pharm. 2001;58:1811–1814. doi: 10.1093/ajhp/58.19.1811. [DOI] [PubMed] [Google Scholar]
- 7.Kirages TJ, Sule HP, Mycyk MB. Severe manifestations of Coricidin intoxication. Am J Emerg Med. 2003;21:473–475. doi: 10.1016/s0735-6757(03)00168-2. [DOI] [PubMed] [Google Scholar]
- 8.Wolfe TR, Caravati EM. Massive dextromethorphan ingestion and abuse. Am J Emerg Med. 1995;13:174–176. doi: 10.1016/0735-6757(95)90088-8. [DOI] [PubMed] [Google Scholar]
- 9.Dextromethorphan (DXM) [Accessed March 18, 2006]; [ www.streetdrugs.org Web site.] http://www.streetdrugs.org/dxm.htm.
- 10.Baker SD, Borys DJ. A possible trend suggesting increased abuse of Coricidin exposures reported to the Texas Poison Network: comparing 1998 to 1999. Vet Hum Toxicol. 2002;44:169–171. [PubMed] [Google Scholar]
- 11.Watson WA, Litovitz TL, Rodgers GC, Jr, et al. 2004 Annual report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerg Med. 2005;23:589–666. doi: 10.1016/j.ajem.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Drug Abuse Warning Network. ED mentions for respiratory agents by drug category: estimates for the coterminous US by half year: trends from the Drug Abuse Warning Network, final estimates 1995–2002–2003. [Accessed January 10, 2006]; [report 2.9.0 and 2.10.0]. http://dawninfo.samhsa.gov/old_dawn/pubs_94_02/pickatable/drugtable1.asp.
- 13.Anderson IB, Kim SY, Dyer JE, et al. Trends in γ-hydroxybutyrate (GHB) and related drug intoxication: 1999 to 2003. Ann Emerg Med. 2006;47:177–183. doi: 10.1016/j.annemergmed.2005.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The vaults of Erowid. [The Vaults of Erowid Web site.] [Accessed March 18, 2006]; http://www.erowid.org/chemicals/dxm/
- 15.National Institute on Drug Abuse. Monitoring the Future Survey: overview of key findings. [Accessed March 22, 2006]; 2005. http://www.monitoringthefuture.org/
- 16.The third plateau: beginner’s guide to DXM. [The Third Plateau: Beginner’s Guide to DXM Web site.] [Accessed April 24, 2006]; http://www.third-plateau.org/knowledgebase/beginners.shtml.
- 17.Dextroverse. [Dextroverse Web site.] [Accessed February 19, 2006]; http://www.dextroverse.org.
- 18.Rumack BH, Peterson RC, Koch GG, Amara IA. Acetaminophen overdose: 662 cases with evaluation of oral acetylcysteine treatment. Arch Intern Med. 1981;141:380–385. doi: 10.1001/archinte.141.3.380. [DOI] [PubMed] [Google Scholar]


