Abstract
Dental caries is a biofilm-dependent oral disease, and fermentable dietary carbohydrates are the key environmental factors involved in its initiation and development. However, among the carbohydrates, sucrose is considered the most cariogenic, because, in addition to being fermented by oral bacteria, it is a substrate for the synthesis of extracellular (EPS) and intracellular (IPS) polysaccharides. Therefore, while the low pH environment triggers the shift of the resident plaque microflora to a more cariogenic one, EPS promote changes in the composition of the biofilms’ matrix. Furthermore, it has recently been shown that the biofilm formed in the presence of sucrose presents low concentrations of Ca, Pi, and F, which are critical ions involved in de- and remineralization of enamel and dentin in the oral environment. Thus, the aim of this review is to explore the broad role of sucrose in the cariogenicity of biofilms, and to present a new insight into its influence on the pathogenesis of dental caries.
Keywords: biofilm, sucrose, cariogenic
INTRODUCTION
Dental caries results from the interaction of specific bacteria with constituents of the diet within a biofilm termed ‘dental plaque’ (Bowen, 2002). Sucrose is considered the most cariogenic dietary carbohydrate, because it is fermentable, and also serves as a substrate for the synthesis of extracellular (EPS) and intracellular (IPS) polysaccharides in dental plaque (Newbrun, 1967; Bowen, 2002).
Thus, low pH induced by sucrose fermentation triggers a shift in the balance of resident plaque microflora to a more cariogenic one, according to the ecological plaque hypothesis (Marsh, 1991). This hypothesis has been supported by long-term dietary sugar consumption (De Stoppelaar et al., 1970; Dennis et al., 1975; Staat et al., 1975) and in situ experimental studies (Minah et al., 1981; Pecharki et al., 2005; Ribeiro et al., 2005).
Furthermore, EPS (mainly insoluble glucans) promote bacterial adherence to the tooth surface (Rölla, 1989; Schilling and Bowen, 1992) and contribute to the structural integrity of dental biofilms. They also increase the porosity of biofilm formed, allowing sugar to diffuse into the deepest parts of the biofilm (Dibdin and Shellis, 1988), which would result in low plaque pH values, due to microbial catabolism (Zero et al., 1986b). There is also evidence showing that sucrose exposure and insoluble EPS are associated with the pathogenesis of dental caries (Johnson et al., 1977; Zero et al., 1986b; Cury et al., 1997, 2000; Mattos-Graner et al., 2000; Nobre dos Santos et al., 2002; Paes Leme et al., 2004; Pecharki et al., 2005; Ribeiro et al., 2005; Aires et al., 2006).
Therefore, it is clear that EPS are critical virulence factors in the dental biofilm formed in the presence of sucrose (Bowen, 2002). The relationship between EPS and caries has been supported by in situ and clinical studies, and, simultaneously, it has been found that sucrose reduces the concentrations of calcium (Ca), inorganic phosphorus (Pi), and fluoride (F) in the dental biofilm (Cury et al., 1997, 2000, 2003; Nobre dos Santos et al., 2002; Paes Leme et al., 2004; Pecharki et al., 2005; Ribeiro et al., 2005; Aires et al., 2006).
Ca, Pi, and F are ions that are important in maintaining the mineral equilibrium between the tooth and the oral environment (Margolis et al., 1988; Pearce, 1998). Low pH and the concentrations of these ions are critical factors during the de- and remineralization processes in the saliva/biofilm/teeth milieu (Pearce, 1998), and the reduction of ion availability may increase the cariogenic potential of the biofilm (Margolis and Moreno, 1992; Cury et al., 1997, 2000, 2003; Gao et al., 2001; Ribeiro et al., 2005; Aires et al., 2006). However, the exact mechanisms by which sucrose reduces the inorganic content in the matrix of the cariogenic biofilm remain to be elucidated.
Some hypotheses have been suggested to explain how sucrose changes the inorganic concentrations in biofilms: (1) Constant low-pH values attained in the biofilm matrix, due to persistent sucrose fermentation, would dissolve mineral reservoirs or obviate their storage; (2) enamel could take up these ions from dental biofilm fluid; (3) the low pH values caused by sucrose fermentation in biofilms would release the reservoir of ions bound to bacterial cell walls; (4) low bacterial density due to high insoluble EPS content could result in fewer binding sites for these ions; and (5) low concentrations of specific ion-binding proteins could result in fewer mineral reservoirs in biofilms formed in the presence of sucrose.
Thus, the aim of this review is to consider the broad role of sucrose in the cariogenic properties of the biofilm, and to discuss tenable hypotheses to explain the low inorganic ion concentrations found in the matrix of the biofilms formed in the presence of this carbohydrate.
(1) THE “ECOLOGICAL PLAQUE HYPOTHESIS” AND DENTAL PLAQUE AS A BIOFILM
The ecological plaque hypothesis was proposed in an attempt to unify some of the clinical and laboratory observations (Theilade, 1986; Marsh, 1991) by combining elements of the non-specific (Theilade, 1986) and the specific (Loesche, 1976) theories of the relationship between dental plaque and dental diseases. Thus far, it is the best explanation for the microbial etiology of dental diseases (Theilade, 1996).
With regard to dental caries, and according to this hypothesis, a change in a key environmental factor will trigger a shift in the balance of the resident plaque microflora, which would promote the emergence of more cariogenic bacteria and change the equilibrium toward dental demineralization (Marsh, 1994) (Fig. 1A). Dietary fermentable carbohydrates have been recognized as primary factors responsible for biochemical and physiological changes in dental biofilms. It is well-established that, after the intake of fermentable sugars (glucose, sucrose, or fructose), the pH in plaque falls rapidly, from around neutrality to pH 5.0 or below (Stephan, 1944; Bowen et al., 1966). In addition, frequent long-term carbohydrate consumption increases the proportions of mutans streptococci and lactobacilli, with a concomitant decrease in levels of S. sanguinis and other oral streptococci (De Stoppelaar et al., 1970; Dennis et al., 1975; Staat et al., 1975). However, it was unclear whether the increase in the levels of cariogenic bacteria was due to availability of sugar per se or a response to persistent low pH following constant sugar catabolism (Marsh, 2003). Since these two conditions cannot be distinguished in vivo, Bradshaw et al. (1989) demonstrated in vitro that a decrease of the environmental pH after a glucose pulse increased the percentage of viable counts of S. mutans and L. casei by 19 and 180 times, respectively, as compared with the counts at a constant pH of 7.0. Subsequently, it was shown that mutans streptococci or lactobacilli are competitive at the low pH values attained within biofilms, which inhibits the growth and metabolism of non-cariogenic species (Bradshaw and Marsh, 1998). Collectively, these in vitro studies showed that the breakdown of microbial homeostasis in dental biofilms is caused by low pH generated from carbohydrate metabolism, rather than by carbohydrate availability. The survival of specific bacteria is mainly due to their acid tolerance/adaptation mechanisms within biofilms (Zero, 1993; Burne, 1998; Quivey et al., 2000). In addition, the ecological plaque hypothesis has been supported by in situ studies, which showed a clear relationship between sugar exposure and increase of mutans streptococci and lactobacilli in dental biofilms formed (Pecharki et al., 2005; Ribeiro et al., 2005).
Figure 1.
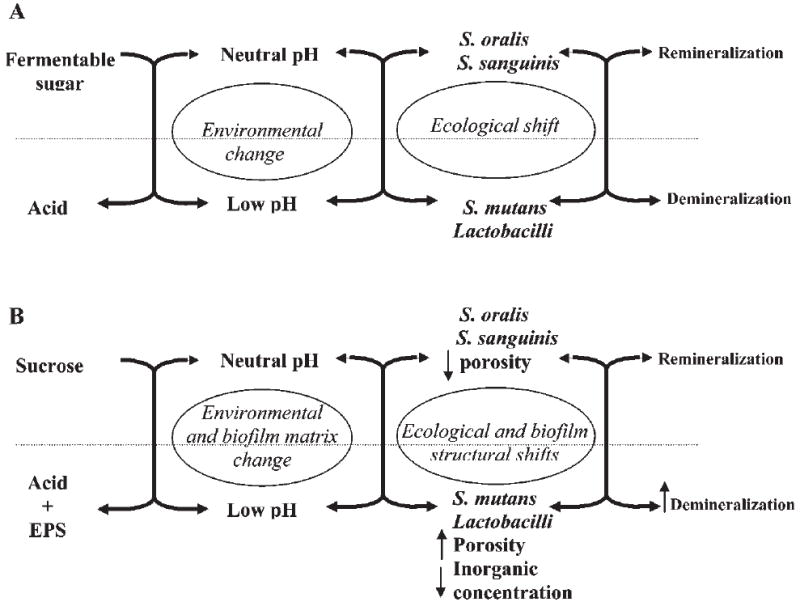
Schematic illustration of a cariogenic biofilm formation in the presence of fermentable sugars (A) or in the presence of sucrose (B); EPS enhances the cariogenic potential of the biofilm formed in the presence of sucrose (adapted from Marsh, 1994).
Nevertheless, the low pH generated by sugar metabolism and the subsequent shifts in microbial composition may not be the only factor involved in the pathogenesis of dental caries. A recent in situ study reported that biofilms formed on enamel by frequent exposure to starch displayed numbers of lactobacilli 200 times higher compared than those formed in the absence of the sugar, but this factor was not enough to induce significant enamel mineral loss (Ribeiro et al., 2005). In contrast, enamel demineralization was detected when the biofilm was formed in the presence of sucrose, which induced similar increases in the levels of aciduric bacteria.
Therefore, factors other than acidogenicity may explain the distinct cariogenic potentials among carbohydrates (Carlsson and Egelberg, 1965; Krasse, 1965; Edwardsson and Krasse, 1967; Carlsson and Sundström, 1968; Birkhed et al., 1980; Lingström et al., 1994; Mattos-Graner et al., 1998; Cury et al., 2000; Ribeiro et al., 2005).
(2) THE ROLE OF SUCROSE IN BIOFILM CARIOGENICITY
There is a clear causal relationship between sucrose and dental caries that has been demonstrated in both epidemiological and experimental studies (Edwardsson and Krasse, 1967; Birkhed et al., 1980; Downer, 1999; Cury et al., 1997, 2000, 2001; Nobre dos Santos et al., 2002; Zero, 2004, and references therein). Sucrose causes major biochemical and physiological changes during the process of biofilm formation, which, in turn, enhance its caries-inducing properties.
Sucrose promotes an increase in the proportions of mutans streptococci and lactobacilli and, simultaneously, a decrease in S. sanguinis levels as a result of the pH fall caused during the fermentation of this carbohydrate (De Stoppelaar et al., 1970; Dennis et al., 1975; Staat et al., 1975; Minah et al., 1981). This observation suggests that acid production from sucrose metabolism disrupts the balance of the microbial community, favoring the growth of cariogenic species (Marsh, 1991). Recent studies have showed that biofilms formed in the presence of sucrose displayed lower fasting and final pH levels, higher mutans streptococci and lactobacilli counts, and enhanced cariogenicity than did those formed in the absence of the sugar (Pecharki et al., 2005; Ribeiro et al., 2005). In addition, the cariogenicity of sucrose has been associated with its frequency of exposure and concentration (König et al., 1968; Hefti and Schmid, 1979; Bowen et al., 1980; Cury et al., 1997; Duggal et al., 2001; Paes Leme et al., 2004; Aires et al., 2006). An increase in the frequency of exposure to carbohydrates results in the plaque being subjected to a prolonged period below the critical pH for enamel demineralization, whereas a greater decrease in pH is observed when sucrose concentration increases. These latter conditions would favor the growth and selection of cariogenic bacteria, thereby converting a healthy biofilm to a diseased one, and consequently enhancing demineralization (Marsh, 1991). This suggests that sucrose may act as a typical fermentable carbohydrate source; however, in comparison with other carbohydrates, sucrose shows enhanced cariogenicity (Bowen et al., 1966; Edwardsson and Krasse, 1967; Birkhed et al., 1980; Horton et al., 1985; Cury et al., 2000; Ribeiro et al., 2005).
Furthermore, two recent in situ studies clearly demonstrated that sucrose has additional properties that enhance its cariogenic potential in comparison with glucose and fructose (Cury et al., 2000) or starch (Ribeiro et al., 2005). For example, sucrose promoted higher enamel mineral loss when compared with a mixture of equimolar concentrations of glucose and fructose (Cury et al., 2000). Sucrose also induced lower pH values, higher mutans streptococci counts in biofilm, and higher mineral loss when compared with starch alone. Indeed, when sucrose and starch were used in combination, the cariogenic potential of starch was enhanced (Ribeiro et al., 2005).
Thus, sucrose is a unique cariogenic carbohydrate, because it is fermentable and also serves as a substrate for extracellular glucan synthesis by glucosyltransferases (GTFs) from mutans streptococci (Newbrun, 1967; Bowen, 2002). Several studies have showed a direct relationship between sucrose exposure and EPS, and caries development (Fig. 1B) (Johnson et al., 1977; Cury et al., 1997, 2000; Mattos-Graner et al., 2000; Nobre dos Santos et al., 2002; Pecharki et al., 2005; Ribeiro et al., 2005).
(3) POLYSACCHARIDE ENHANCES THE CARIOGENICITY OF BIOFILMS
The polysaccharides in biofilms can be divided into two categories: (1) extracellular polysaccharides (EPS), which promote bacterial accumulation to the tooth surface, and influence the physical and biochemical properties of biofilms; and (2) intracellular polysaccharides (IPS), which serve as an endogenous source of carbohydrates that can be metabolized to produce acids during periods of nutrient limitation (Tanzer et al., 1976; Zero et al., 1986a). Both EPS and IPS have important roles in the cariogenicity of biofilms, as discussed below.
Extracellular Polysaccharides (EPS)
Using sucrose primarily as a substrate, the EPS are synthesized mostly by bacterial glucosyltransferases (GTFs) and, to a lesser extent, by fructosyltransferases (FTFs) (Hamada and Slade, 1980; Bowen, 2002). The GTFs from S. mutans synthesize a mixture of α(1→3)-linked insoluble glucans and α(1→6)-linked soluble glucans, whereas FTF produces α(2→6)-linked fructans. The EPS are largely insoluble, have a complex structure (Kopec et al., 1997), and promote selective adherence (Schilling and Bowen, 1992; Vacca-Smith et al., 1996) and accumulation of large numbers of cariogenic streptococci on the teeth of human subjects (Rölla, 1989; Mattos-Graner et al., 2000; Nobre dos Santos et al., 2002) and experimental animals (Krasse, 1965; Frostell et al., 1967; Johnson et al., 1977). Furthermore, EPS increase the bulk and porosity of dental plaque matrix, thereby allowing more substrate to diffuse to the enamel surface (Dibdin and Shellis, 1988). As a result of enhanced substrate diffusibility, deeper layers of dental plaque display lower pH values, due to sugar metabolism by acidogenic micro-organisms (Zero et al., 1992), thereby enhancing the development of dental caries (Cury et al., 1997, 2000; Mattos-Graner et al., 2000; Nobre dos Santos et al., 2002; Ribeiro et al., 2005). A recent study with various mutants constructed by allelic exchange in the regions coding for GTFs and FTFs showed that the wild-type strain produced larger quantities of water-insoluble glucan and allowed faster diffusion of hydrogen ions, compared with mutants (Hata and Mayanagi, 2003).
The relationship among sucrose exposure, EPS, and caries development has been demonstrated in several studies. For example, mutant strains of S. mutans defective in the gtf genes, especially gtfB and gtfC, are significantly less cariogenic than are the parent strains in animals (Johnson et al., 1977; Yamashita et al., 1993). In situ studies have showed that a higher concentration and frequency of sucrose exposure increased EPS concentration in the biofilm matrix, lowered fasting pH values, and enhanced enamel demineralization when compared with biofilms formed in the absence of sucrose (Cury et al., 1997, 2000; Ribeiro et al., 2005; Aires et al., 2006) (Table). Furthermore, clinical studies have also suggested that synthesis of EPS is related to caries activity in children (Mattos-Granner et al., 2000; Nobre dos Santos et al., 2002) (Table). It is evident that sucrose and GTFs are key factors involved in the synthesis of these complex polysaccharides.
Table.
Biofilm Analyses and Enamel Demineralization from Previously Published Studies
| Biofilm Concentrations | Mineral Loss | ||||||
|---|---|---|---|---|---|---|---|
| Paper | Treatment | F, μg/g3 | Ca, mg/g3 | Pi, mg/g3 | EPS, mg/g3 | SMH Change (%)4 | ΔZ5 |
| Cury et al., 19971 | Sucrose 0 | 18.7 ± 3.3c | 12.9 ± 2.5b | 3.7 ± 1.1b | 12.0 ± 2.6b | ||
| Sucrose 2x | 9.6 ± 2.8b | 4.0 ± 1.0a | 0.8 ± 0.2a | 11.5 ± 1.8b | |||
| Sucrose 4x | 2.7 ± 0.8a | 4.0 ± 1.1a | 0.4 ± 0.1a | 14.0 ± 3.3b | -- | -- | |
| Sucrose 8x | 2.7 ± 0.4a | 4.2 ± 1.1a | 0.4 ± 0.04a | 35.8 ± 6.9a | |||
|
| |||||||
| Cury et al., 20001 | Control | 140.6 ± 30.8a | 17.0 ± 2.8a | 11.5 ± 2.1a | 6.5 ± 1.0a | - 3.3 ± 0.7a | χ27,021.7 ± 3951.5a |
| Glucose 10% + Fructose 10% | 27.4 ± 15.9b | 1.9 ± 0.7b | 0.5 ± 0.1b | 11.8 ± 1.9a | -44.2 ± 7.2b | 20,159.5 ± 2213.4b | |
| Sucrose 20% | 5.6 ± 2.2b | 0.6 ± 0.1b | 0.3 ± 0.04b | 35.0 ± 7.8b | -73.4 ± 6.9c | 12,414.4 ± 2419.4c | |
|
| |||||||
| Cury et al., 2001 | Sucrose 0 | χ26,949.2 ± 632.1a | |||||
| Sucrose 2x | 25,527.6 ± 785.0a | ||||||
| Sucrose 4x | -- | -- | -- | -- | 24,000.8 ± 1157.5a | ||
| Sucrose 8x | 15,887.4 ± 2739.2b | ||||||
|
| |||||||
| Nobre dos Santos et al., 20022 | Caries-free | 58.1 ± 21.3a | 10.6 ± 5.4a | 6.0 ± 2.9a | 39.2 ± 7.4a | ||
| Pit/Fissure Caries | 32.5 ± 13.6b | 7.9 ± 4.3a | 4.0 ± 1.9b | 47.4 ± 8.9b | |||
| Nursing Caries | 6.2 ± 2.9c | 3.3 ± 2.6b | 2.6 ± 1.3b | 55.6 ± 17.6b | |||
|
| |||||||
| Cury et al., 20031 | Sucrose Exposure | 1.1 ± 0.3a | 1.2 ± 0.7a | 0.2 ± 0.04a,b | 51.1 ± 13.6a | ||
| Sucrose Interruption: | |||||||
| 24 hrs | 1.6 ± 0.4a | 1.6 ± 0.6a | 0.2 ± 0.1a | 49.7 ± 11.4a | |||
| 48 hrs | 2.7 ± 0.6a | 3.0 ± 1.5a | 0.4 ± 0.1b | 40.6 ± 9.6b | -- | -- | |
| Sucrose Absence | 63.3 ± 23.6a | 12.2 ± 1.9a | 4.3 ± 1.5a | 4.6 ± 0.5a | |||
| Sucrose Exposure: | |||||||
| 24 hrs | 86.1 ± 38.6a | 18.8 ± 4.5a | 4.9 ± 1.3a | 10.0 ± 2.7a,b | |||
| 48 hrs | 67.9 ± 37.5a | 14.8 ± 4.0a | 3.9 ± 1.1a | 11.4 ± 2.3b | |||
|
| |||||||
| Paes Leme et al., 2004b1 | Sucrose 4x | 31.0 ± 41.7a | 0.73 ± 0.52a | 1.0 ± 0.9a | 29.1 ± 17.5a | -19.7 ± 17.5a | δ 655.7 ± 506.2a |
| Sucrose 8x | 17.3 ± 28.6b | 0.53 ± 0.37b | 0.7 ± 0.7a | 44.3 ± 25.5b | -29.5 ± 24.6b | δ 869.8 ± 726.3b | |
|
| |||||||
| Pecharki et al., 20052 | Control | 352.0 ± 271.0a | 45.9 ± 27.0a | 23.5 ± 14.2a | 35.4 ± 7.4a | - 5.1 ± 7.6a | δ 459.4 ± 212.6a |
| 20% Sucrose | 28.2 ± 79.0b | 2.1 ± 1.8b | 3.0 ± 1.5b | 194.0 ± 125.0b | -57.3 ± 32.3b | δ 2,165.5 ± 1890.3b | |
|
| |||||||
| Ribeiro et al., 20052 | Control | 468.4 ± 401.8a | 45.9 ± 50.9a | 27.1 ± 29.5a | 47.5 ± 22.8a | δ 447.9 ± 169.0a | |
| 2% Starch | 239.8 ± 251.3b | 17.4 ± 25.4b | 11.6 ± 14.6b | 49.8 ± 13.5a | δ 420.0 ± 160.1a | ||
| 20% Sucrose | 119.9 ± 179.3c | 5.1 ± 7.9c | 4.1 ± 4.6c | 181.6 ± 115.8b | -- | δ 955.6 ± 543.6b | |
| Starch + Sucrose | 55.7 ± 144.3c | 4.9 ± 10.5c | 3.6 ± 5.0c | 201.6 ± 137.6b | δ 1,421.8 ± 653.8c | ||
|
| |||||||
| Aires et al., 20062 | Control | 55.1 ± 46.1a | 39.4 ± 17.2a | 22.0 ± 10.5a | 28.1 ± 6.5a | - 3.9 ± 5.0a | δ 253.0 ± 129.0a |
| 1% Sucrose | 64.0 ± 82.8a | 25.1 ± 23.1a | 14.9 ± 12.2a | 41.6 ± 13.5b | -10.5 ± 14.5a | 382.0 ± 224.0a | |
| 5% Sucrose | 35.4 ± 53.4ab | 5.7 ± 5.3b | 4.2 ± 3.0b | 109.0 ± 85.9c | -48.8 ± 34.4b | 796.0 ± 600.0b | |
| 10% Sucrose | 11.9 ± 11.9bc | 4.7 ± 5.1b | 3.2 ± 2.2b | 102.0 ± 76.0c | -43.8 ± 31.2b | 1,050.0 ± 1240.0b | |
| 20% Sucrose | 12.3 ± 14.8bc | 5.4 ± 6.1b | 3.7 ± 2.6b | 125.0 ± 85.4c | -58.2 ± 32.6b | 1,580.0 ± 1540.0bc | |
| 40% Sucrose | 5.5 ± 2.4c | 2.6 ± 1.0b | 2.3 ± 0.8b | 268.0 ± 163.0d | -65.7 ± 40.1b | 1,600.0 ± 862.0c | |
Wet biofilm basis.
Dry biofilm basis.
Distinct superscript lower-case letters show statistical difference between treatments (p < 0.05).
Surface microhardness change.
% vol. min × μm.
However, other factors may influence the biochemistry and structural integrity of EPS. A recent in situ study showed that biofilms formed in the presence of sucrose and starch were more cariogenic than those exposed to sucrose alone, despite the fact that the total amounts of EPS in the biofilm matrices were similar in these two conditions (Ribeiro et al., 2005). However, the formation of glucans and the adherence of oral micro-organisms can be modulated by the interaction of amylase and GTF enzymes adsorbed onto the hydroxyapatite surface (Vacca-Smith et al., 1996), which may influence the formation and cariogenicity of dental biofilms.
Furthermore, there is evidence showing that the structure of glucans could be influenced by glucanohydrolases present in the oral cavity. For example, the presence of dextranase and/or mutanase during glucans synthesis by GTFs caused linkage remodeling and branching, which influenced the bacterial binding sites on these glucans (Hayacibara et al., 2004). It is noteworthy that while the synthesis of polysaccharides by plaque bacteria during sucrose-rich diet increases, the levels of dextranase and levanase of plaque bacteria also increase (Gawronski et al, 1975). The presence of glucanohydrolases may have an impact on the development, physical properties, and bacterial binding sites of the polysaccharide matrix in dental biofilms.
Clearly, EPS play a major role in the pathogenesis of dental caries, by promoting biochemical and physiological changes in the matrix of the biofilm, including: (i) enhancing bacterial adherence and further accumulation of organisms, (ii) providing structural integrity and bulk to biofilms, and (iii) increasing the acidogenicity of the biofilm matrix.
Intracellular Polysaccharides (IPS)
IPS are high-molecular-weight metabolizable glycogen-like storage polymers with α(1→4) and α(1→6) linkages, which provide the micro-organisms with an endogenous source of carbohydrate during periods of nutrient limitation in the oral cavity (Hamilton, 1976; Tanzer et al., 1976). Consequently, IPS can promote the formation of dental caries by prolonging the exposure of tooth surfaces to organic acids and maintaining a lower fasting pH in the matrix of the plaque (Tanzer et al., 1976). It is noteworthy that an S. mutans mutant that synthesizes elevated levels of IPS was significantly more cariogenic in animals than was the wild-type (Harris et al., 1992; Spatafora et al., 1995).
The importance of IPS for S. mutans virulence supports previous reports in the literature showing an association of these storage polysaccharides with dental caries in animals and in humans (Gibbons and Socransky, 1962; Loesche and Henry, 1967; van Houte et al., 1969; Tanzer et al., 1976; Ashley and Wilson, 1977; Zero et al., 1986a; Spatafora et al., 1995).
Furthermore, in situ studies have showed that acid production by S. mutans from endogenous substrate caused pronounced and prolonged decreases in pH, and enhanced enamel demineralization (Zero et al., 1986a; van Houte et al., 1989). Recently, it was observed that biofilm formed in situ in the presence of 20% (or higher) sucrose showed a significantly lower fasting pH compared with a negative control (Pecharki et al., 2005; Ribeiro et al., 2005; Aires et al., 2006), which could be related to the metabolism of IPS. In addition, biofilms formed in situ in the presence of glucose+fructose and sucrose displayed higher concentrations of IPS than those formed in the absence of these carbohydrates (Tenuta et al., 2006). Interestingly, the differences in IPS concentration were not only maintained during 3, 7, and 14 days of biofilm formation, but also increased over time (unpublished data). Thus, the metabolism of IPS could explain the fasting low pH observed in biofilms and, consequently, the increased cariogenicity of these biofilms.
Collectively, these observations indicate that the metabolism of endogenous substrate is a significant trait in the pathogenesis of dental caries by influencing the acidogenicity and resting pH of dental biofilm.
Thus, there is clear evidence that EPS and IPS influence the cariogenicity of dental biofilms by at least two pathways: (1) EPS promote bacterial adherence and accumulation on tooth surfaces, and cause biochemical and structural changes in the matrix of the biofilms; and (2) IPS promote lower fasting pH levels during periods of nutrient deprivation, which could result in the selection of cariogenic micro-organisms and caries development. Recently, it was shown that therapeutic agents that diminish EPS and IPS concentrations in biofilms also reduce the development of dental caries in rats (Koo et al., 2003, 2005), confirming the importance of these polysaccharides in S. mutans cariogenicity.
(4) EPS MAY CHANGE THE INORGANIC COMPOSITION OF BIOFILMS
Among the chemical changes that may be associated with the presence of EPS, the low concentrations of Ca, Pi, and F are the most intriguing and a relevant factor in the context of biofilm cariogenicity. The low concentrations of ions are directly related to the saturation levels of biofilm, which determines the driving force of minerals for the demineralization process (Pearce, 1998).
The concentrations of Ca and Pi in dental plaque are critical in terms of caries development, because there is an inverse relationship between concentrations of these ions in the plaque matrix (Ashley and Wilson, 1977) and fluid (Margolis and Moreno, 1992), and caries experience. These ions would be released to the plaque/enamel interface during the fall of pH, thereby maintaining the aqueous phase in a saturated condition.
In situ studies have showed a relationship between sucrose and increased concentrations of EPS and, simultaneously, reduced Ca, Pi, and F content in the matrix of dental plaque, which enhanced tooth enamel mineral loss (Cury et al., 1997, 2000; Paes Leme et al., 2004; Pecharki et al., 2005; Aires et al., 2006) (Table). Furthermore, a recent study showed that biofilms formed in the presence of sucrose, alone or in combination with starch, displayed inorganic concentrations lower than those formed either in the absence of sugar or in the presence of starch only, and which resulted in higher enamel demineralization (Ribeiro et al., 2005). Nobre dos Santos et al. (2002) also found lower concentrations of F, Ca, and Pi in dental plaque samples collected from nursing children with caries, when compared with those from caries-free children. Whether the low concentrations of Ca, Pi, and F are also detected in the fluid of the biofilms needs to be determined in further studies.
Nevertheless, the findings suggest that sucrose, alone or in combination with other carbohydrates, is associated with lower inorganic ion concentrations found in the matrix of dental biofilm, thereby augmenting its cariogenicity (Cury et al., 2000; Ribeiro et al., 2005) (Table). Furthermore, it is likely that the concentrations of inorganic ions are related to EPS content in dental biofilm matrix, but the exact mechanisms of how this phenomenon occurs remain to be elucidated. Therefore, we propose five hypotheses and present some experimental evidence to identify a plausible explanation for the lower inorganic concentrations in cariogenic biofilms.
(5) HOW CAN THE LOW INORGANIC ION CONCENTRATION IN A CARIOGENIC BIOFILM BE EXPLAINED?
Recent studies showing that dental biofilm formed in the presence of sucrose displayed low inorganic ion concentrations in the biofilm matrix (Table) (Cury et al., 1997; 2000; 2003; Paes Leme et al., 2004; Ribeiro et al., 2005; Aires et al., 2006) provide new insight into a better understanding of the pathogenesis of development of cariogenic dental biofilms. Thus, some hypotheses based on the structure, composition, and ion kinetics of biofilms may explain the possible mechanisms for the lower inorganic ion concentrations in the presence of carbohydrates.
(1) Depletion of Mineral Reservoirs
The first hypothesis proposes that the constant low pH, due to sucrose fermentation, would release ions from mineral deposits (Pearce, 1998), which could then diffuse into saliva, resulting in a biofilm with low inorganic concentration. Another alternative explanation is that constant low pH maintained in the biofilm would prevent the precipitation of minerals (Tenuta et al., 2006). However, dental plaque samples in our studies were collected 10-12 hrs after the last sucrose exposure (Cury et al., 1997, 2000, 2003; Paes Leme et al., 2004; Ribeiro et al., 2005; Aires et al., 2006), which would have been enough time for the mineral ions that had been lost to saliva to be replaced by the simple law of mass action. Furthermore, the concentrations of Ca, Pi, and F in dental biofilm formed in the absence or presence of sucrose neither decreased nor increased after sucrose exposure was provided (for biofilms formed in its absence) or interrupted (for biofilms formed in its presence) for a further 48 hrs (Cury et al., 2003). Thus, the inorganic ion concentrations in biofilm may be attributed to changes in the matrix structure, rather than to depletion of inorganic pools by organic acids (Cury et al., 2003) (Fig. 2).
Figure 2.

Schematic representation of the first, second, third, fourth and fifth hypotheses, respectively. First hypothesis: Constant low pH caused by sucrose fermentation would release ions from mineral deposits, which could diffuse to saliva, and promote dental plaque with lower inorganic ion concentrations. However, after 10-12 hrs, there would have been enough time for the mineral ions, which had been lost to saliva, to be replaced by the simple law of mass action. Second hypothesis: Enamel could have taken up ions from biofilm fluid during pH-cycling. After 12 hrs, the biofilms would have been again saturated with these ions. Third hypothesis: Schematic representation adapted from Rose et al. (1996). Binding to bacterial cell wall is another reservoir of minerals. When the pH falls, the minerals are released from biofilm. After the pH is increased, the biofilm is saturated again with the ions from saliva. Fourth hypothesis: Bacterial density. Note that, in the second circle, the density of bacteria is lower, since polysaccharides occupy a large volume of the biofilm. Fifth hypothesis: Low concentrations of specific ion-binding proteins. It has been suggested that biofilm formed in the presence of sucrose shows fewer calcium-binding sites for proteins.
(2) Enamel Uptake of Ions from Biofilm Fluid
The depletion of ions in the biofilm matrix could be a result of their uptake by enamel, since, during falls in pH, the biofilm fluid would be undersaturated relative to hydroxyapatite, but would be still oversaturated relative to fluorapatite, which precipitates on the enamel (Larsen, 1990). This observation could explain the reduction of F concentration in biofilm, but it would not explain the simultaneous low concentrations of Ca and Pi found in our studies (Fig. 2). Furthermore, the mineral ions taken up by enamel could be replaced, since the plaque samples were collected 12 hrs after the last sucrose exposure.
(3) Release of Ions Bound to Bacterial Cells
Another hypothesis is based on the ability of bacterial cell walls to bind ions, which could act as a reservoir of ions in dental plaque (Fig. 2). For example, calcium binding in streptococci is predominantly phosphate-group-based, whereas that in L. casei and A. naeslundii is predominantly carboxylate-group-based (Rose et al., 1997a). This reservoir of ions could explain our findings on not only Ca concentrations in the matrix of the biofilm, but also on fluoride, since Zn2+, Mg2+, and Ca2+ at 5 mmol/L enhance fluoride binding to the cell wall (Rose et al., 1996). These reservoirs are physicochemically sensitive to pH changes, which means that they are depleted during the falls in pH, but would be replenished when the pH rises again. However, this phenomenon would not explain the low concentration of Pi that has been found in dental biofilm formed in the presence of sucrose (Cury et al., 1997, 2000, 2003; Pecharki et al., 2005; Ribeiro et al., 2005; Aires et al., 2006). Furthermore, the low inorganic ion concentrations have been found 12 hrs after the last exposure to sucrose, which would be enough time for this reservoir to be replenished. Therefore, this hypothesis may not be the best explanation for the low concentrations of Ca, Pi, and F found in dental biofilm formed in the presence of sucrose.
(4) Low Density of Bacteria
In contrast, the concept of bacterial binding sites would be extremely important, considering the density of bacteria in biofilm (Carlsson and Sundström, 1968) (Fig. 2), which could be influenced by the amount of insoluble EPS. These polysaccharides occupy a large volume of dental plaque, thereby reducing the number of bacteria and, consequently, ion-binding sites. When the frequency of sucrose exposure was increased, a higher concentration of EPS (Cury et al., 1997; Pearce et al., 2002) and lower cell biomass content in biofilm (Pearce et al., 2002) were observed. Although this hypothesis could explain the low concentrations of Ca (Rose et al. (1997b) and F (Rose et al., 1996), it does not elucidate the simultaneous decrease of Pi.
(5) Low Concentrations of Specific Proteins
The last proposed hypothesis to explain the simultaneous low concentrations of Ca, Pi, and F would be the protein composition of the dental plaque matrix (Fig. 2). Analyses of recent data showed clear differences in the patterns of the matrix proteins extracted from dental plaque formed under three distinct conditions: (1) in the absence of sugar (control), (2) in the presence of glucose and fructose, and (3) in the presence of sucrose (Cury et al., 2000). In terms of the protein profiles and their concentrations in the biofilms, it would be relevant if there were differences in their ability to bind calcium and provide a template for mineral growth.
Recently, it was shown that approximately 33% of the total calcium in dental plaque fluid is free, 17% is bound to phosphate and organic acid anions, and 50% is bound to other species (such as proteins) (Gao et al., 2001). If proteins are responsible for 50% of the calcium concentration of plaque, a change in protein profile could affect calcium-binding sites. This observation may explain the findings that biofilm formed in the presence of sucrose exhibited lower inorganic ion concentrations (Cury et al., 1997, 2000, 2003; Paes Leme et al., 2004; Pecharki et al., 2005; Ribeiro et al., 2005). Whether calcium-binding proteins from saliva or from bacteria can actually serve as a template for mineral growth in dental biofilms awaits further evaluation.
Proline-rich proteins (PRP), statherin, histatins identified in acquired enamel pellicle (Schüpbach et al., 2001), cysteine-containing phosphoproteins in dental plaque (DiPaola et al., 1984), and low-molecular-weight peptides in human parotid saliva (Perinpanayagam et al., 1995) may play significant roles as calcium-binding proteins. A study of the calcium-binding properties of acidic PRP indicated that there is interaction between the calcium-binding N-terminal end and the proline-rich C-terminal (Bennick, 1987). PRP and statherin are also potent inhibitors of calcium phosphate precipitation (Moreno et al., 1979). Moreover, the low-molecular-weight peptides are likely to be in equilibrium with dental plaque fluid, and may therefore help modulate events such as demineralization and remineralization, microbial attachment, and dental plaque metabolism at the tooth-saliva interface (Perinpanayagam et al., 1995). These proteins bind preferentially to hydroxyapatite surfaces, and may bind to calcium. The protein-binding mechanism could be similar to that of casein phosphopeptides (CPP), a protein that stabilizes amorphous calcium phosphate (ACP) to form small clusters, which are able to release calcium to inhibit demineralization and/or enhance remineralization (Rose, 2000). The addition of CPP-ACP to either sorbitol- or xylitol-based sugar-free gum resulted in a dose-dependent increase in enamel subsurface remineralization (Shen et al., 2001). Therefore, the calcium-binding proteins can work as a calcium reservoir and modulate crystal growth, and thus interfere with the de-/remineralization of dental enamel.
Several studies have identified calcium-binding proteins in saliva, acquired pellicle, and gingival crevicular fluid by using two-dimensional gel electrophoresis (2D-PAGE) and peptide mass fingerprinting (Kojima et al., 2000; Ghafouri et al., 2003; Yao et al., 2003; Huang, 2004). Nevertheless, none of them analyzed the protein profile in the matrix of dental biofilms. The protein profiles in biofilms formed in the absence or presence of sucrose were recently evaluated by 2D-PAGE (Paes Leme et al., 2003) and peptide mass fingerprinting (Paes Leme et al., 2004) (Figs. 3A, 3B). Calcium-binding proteins were identified only in biofilm formed in the absence of sucrose (Paes Leme et al., 2004) (Fig. 3A). This finding is the first evidence showing that the absence of calcium-binding proteins in biofilm formed in the presence of sucrose may be associated with the low concentration of calcium in its matrix, which would promote conditions of undersaturation and, consequently, favor the demineralization process.
Figure 3.
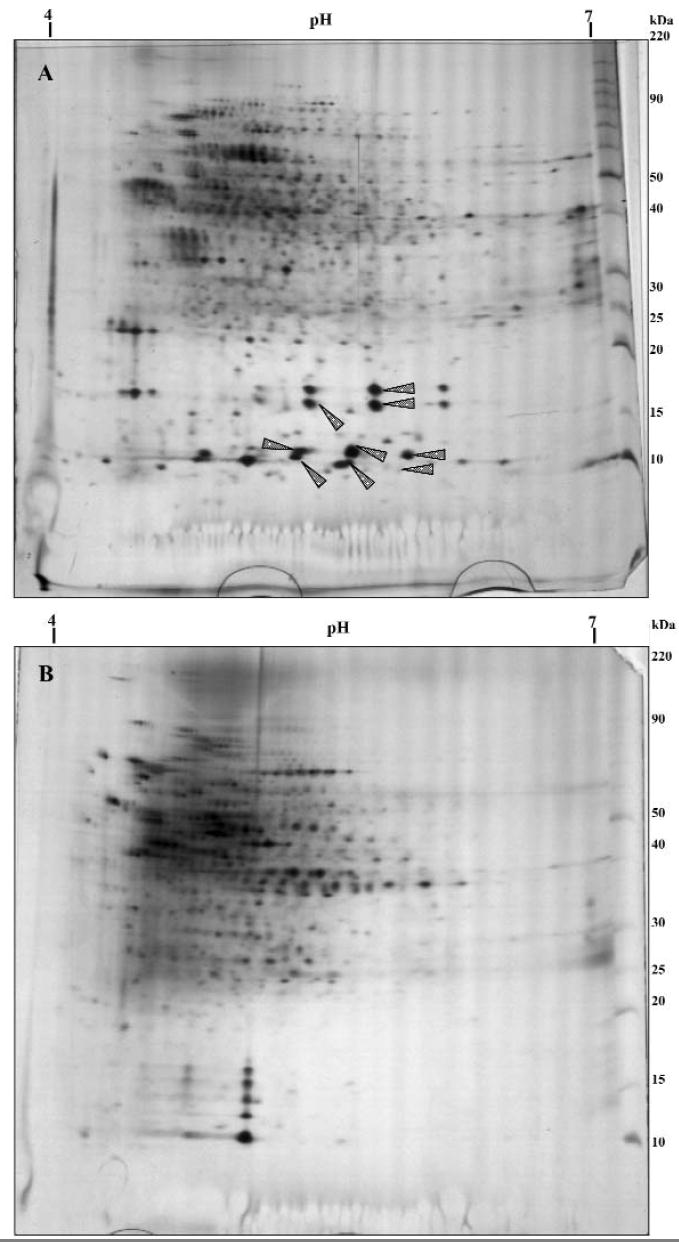
Two-dimensional gel electrophoresis of dental biofilm formed in the absence (A) and presence (B) of sucrose (20 μg of proteins). Isoelectric focusing with pH range 4-7 and PAGE (8-18%). The gels were silver-stained. Spots were excised for in-gel digestion and analyzed by mass spectrometry (MALDITOF). The fingerprints were analyzed in Mascot and Prospector program with the NCBInr database. Arrows show calcium-binding proteins present only in biofilm formed in the absence of sucrose (A) when compared with biofilm formed in the presence of sucrose (B).
The qualitative differences in the protein composition of dental biofilm formed in the presence of sucrose may be related to elevated levels of EPS in the matrix; these polysaccharides occupy a large volume of the biofilm, which decreases the binding sites for proteins. Moreover, it is not known whether the presence of ions, such as calcium, is necessary for protein binding, or if these specific proteins might serve as a template for mineral-binding sites.
The findings of undetectable levels of calcium-binding proteins in biofilm formed in the presence of sucrose offer the most promise among the different hypotheses discussed here, and could be one of the pathways by which this carbohydrate influences the cariogenicity of biofilms.
(6) CONCLUSION
The low concentrations of Ca, Pi, and F in the matrix of the whole dental biofilm formed in the presence of sucrose can be an additional factor contributing to the cariogenicity of this carbohydrate. However, the explanation of these findings remains to be elucidated. It is clear that a better understanding of the unique cariogenic properties of this dietary carbohydrate at physicochemical and molecular levels is worthy of additional exploration.
Acknowledgments
We thank Dr. Mônica Campos Serra, FORP-USP, who encouraged the writing of this article during the discipline "Experimental models for clinical evaluation of dental materials" for the Graduate Program in Dentistry, Cariology Area, Faculty of Dentistry of Piracicaba, UNICAMP. The authors thank Dr. Cínthia P.M. Tabchoury, FOP-UNICAMP, for the English review of the last version of this manuscript. This study was supported by FAPESP (99/07185-7; 02/00293-3; 03/01536-0), CNPq (472392/03-4), and Protein Core Facility grant NIH RR14682.
References
- Aires CP, Tabchoury CP, Del Bel Cury AA, Koo H, Cury JA. Effect of sucrose concentration on dental biofilm formed in situ and on enamel demineralization. Caries Res. 2006;40:28–32. doi: 10.1159/000088902. [DOI] [PubMed] [Google Scholar]
- Ashley FP, Wilson RF. Dental plaque and caries: a 3-year longitudinal study in children. Br Dent J. 1977;142:85–91. doi: 10.1038/sj.bdj.4803870. [DOI] [PubMed] [Google Scholar]
- Bennick A. Structural and genetic aspects of proline-rich proteins. J Dent Res. 1987;66:457–461. doi: 10.1177/00220345870660021201. [DOI] [PubMed] [Google Scholar]
- Birkhed D, Frostell G, Lamm CJ. Cariogenicity of glucose, sucrose and amylopectin in rats and hamsters infected and noninfected with Streptococcus mutans. Caries Res. 1980;14:441–447. [Google Scholar]
- Bowen WH. Do we need to be concerned about dental caries in the coming millennium? Crit Rev Oral Biol Med. 2002;13:126–131. doi: 10.1177/154411130201300203. [DOI] [PubMed] [Google Scholar]
- Bowen WH, Eastoe JE, Cock DJ. The effect of sugar solutions on the pH of plaque in caries-active monkeys (Macaca irus) Arch Oral Biol. 1966;11:833–838. doi: 10.1016/0003-9969(66)90009-4. [DOI] [PubMed] [Google Scholar]
- Bowen WH, Amsbaugh SM, Monell-Torrens S, Brunelle J, Kuzmiak-Jones H, Cole MF. A method to assess cariogenic potential of foodstuffs. J Am Dent Assoc. 1980;100:677–681. doi: 10.14219/jada.archive.1980.0211. [DOI] [PubMed] [Google Scholar]
- Bradshaw DJ, Marsh PD. Analysis of pH-driven disruption of oral microbial communities in vitro. Caries Res. 1998;32:456–462. doi: 10.1159/000016487. [DOI] [PubMed] [Google Scholar]
- Bradshaw DJ, McKee AS, Marsh PD. Effects of carbohydrate pulses and pH on population shifts within oral microbial communities in vitro. J Dent Res. 1989;68:1298–1302. doi: 10.1177/00220345890680090101. [DOI] [PubMed] [Google Scholar]
- Burne RA. Oral streptococci…products of their environment. J Dent Res. 1998;77:445–452. doi: 10.1177/00220345980770030301. [DOI] [PubMed] [Google Scholar]
- Carlsson J, Egelberg J. Effect of diet on early plaque formation in man. Odontol Revy. 1965;16:112–125. [PubMed] [Google Scholar]
- Carlsson J, Sundström B. Variations in composition of early dental plaque following ingestion of sucrose and glucose. Odontol Revy. 1968;19:161–169. [PubMed] [Google Scholar]
- Cury JA, Rebello MA, Del Bel Cury AA. In situ relationship between sucrose exposure and the composition of dental plaque. Caries Res. 1997;31:356–360. doi: 10.1159/000262418. [DOI] [PubMed] [Google Scholar]
- Cury JA, Rebelo MA, Del Bel Cury AA, Derbyshire MT, Tabchoury CP. Biochemical composition and cariogenicity of dental plaque formed in the presence of sucrose or glucose and fructose. Caries Res. 2000;34:491–497. doi: 10.1159/000016629. [DOI] [PubMed] [Google Scholar]
- Cury JA, Francisco SB, Del Bel Cury AA, Tabchoury CP. In situ study of sucrose exposure, mutans streptococci in dental plaque and dental caries. Braz Dent J. 2001;12:101–104. [PubMed] [Google Scholar]
- Cury JA, Marques AS, Tabchoury CP, Del Bel Cury AA. Composition of dental plaque formed in the presence of sucrose and after its interruption. Braz Dent J. 2003;14:147–152. doi: 10.1590/s0103-64402003000300001. [DOI] [PubMed] [Google Scholar]
- Dennis DA, Gawronski TH, Sudo SZ, Harris RS, Folke LE. Variations in microbial and biochemical components of four-day plaque during a four-week controlled diet period. J Dent Res. 1975;54:716–722. doi: 10.1177/00220345750540040401. [DOI] [PubMed] [Google Scholar]
- De Stoppelaar JD, van Houte J, Backer Dirks O. The effect of carbohydrate restriction on the presence of Streptococcus mutans, Streptococcus sanguis and iodophilic polysaccharide-producing bacteria in human dental plaque. Caries Res. 1970;4:114–123. doi: 10.1159/000259633. [DOI] [PubMed] [Google Scholar]
- Dibdin GH, Shellis RP. Physical and biochemical studies of Streptococcus mutans sediments suggest new factors linking the cariogenicity of plaque with its extracellular polysaccharide content. J Dent Res. 1988;67:890–895. doi: 10.1177/00220345880670060101. [DOI] [PubMed] [Google Scholar]
- DiPaola C, Herrera MS, Mandel ID. Immunochemical study of host proteins in human supragingival compared with denture plaque. Arch Oral Biol. 1984;29:161–163. doi: 10.1016/0003-9969(84)90122-5. [DOI] [PubMed] [Google Scholar]
- Downer MC. Caries experience and sucrose availability: an analysis of the relationship in the United Kingdom over fifty years. Community Dent Health. 1999;16:18–21. [PubMed] [Google Scholar]
- Duggal MS, Toumba KJ, Amaechi BT, Kowash MB, Higham SM. Enamel demineralization in situ with various frequencies of carbohydrate consumption with and without fluoride toothpaste. J Dent Res. 2001;80:1721–1724. doi: 10.1177/00220345010800080801. [DOI] [PubMed] [Google Scholar]
- Edwardsson S, Krasse B. Human streptococci and caries in hamsters fed diets with sucrose or glucose. Arch Oral Biol. 1967;12:1015–1016. doi: 10.1016/0003-9969(67)90098-2. [DOI] [PubMed] [Google Scholar]
- Frostell G, Keyes PH, Larson RH. Effect of various sugar and sugar substitutes on dental caries in hamsters and rats. J Nutr. 1967;93:65–76. doi: 10.1093/jn/93.1.65. [DOI] [PubMed] [Google Scholar]
- Gao XJ, Fan Y, Kent RL, Jr, van Houte J, Margolis HC. Association of caries activity with the composition of dental plaque fluid. J Dent Res. 2001;80:1834–1839. doi: 10.1177/00220345010800091201. [DOI] [PubMed] [Google Scholar]
- Gawronski TH, Staat RA, Zaki HA, Harris RS, Folke LE. Effects of dietary sucrose levels on extracellular polysaccharide metabolism of human dental plaque. J Dent Res. 1975;54:881–890. doi: 10.1177/00220345750540042901. [DOI] [PubMed] [Google Scholar]
- Ghafouri B, Tagesson C, Lindahl M. Mapping of proteins in human saliva using two-dimensional gel electrophoresis and peptide mass fingerprinting. Proteomics. 2003;3:1003–1015. doi: 10.1002/pmic.200300426. [DOI] [PubMed] [Google Scholar]
- Gibbons RJ, Socransky SS. Intracellular polysaccharide storage by organisms in dental plaques. Its relation to dental caries and microbial ecology of the oral cavity. Arch Oral Biol. 1962;7:73–80. doi: 10.1016/0003-9969(62)90050-x. [DOI] [PubMed] [Google Scholar]
- Hamada S, Slade HD. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980;44:331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton IR. Intracellular polysaccharide synthesis by cariogenic microorganisms. In: Stiles HM, Loesche WJ, O’Brien TC, editors. Microbial aspects of dental caries. Washington,DC: Information Retrieval; 1976. pp. 680–701. [Google Scholar]
- Harris GS, Michalek SM, Curtiss R., 3rd Cloning of a locus involved in Streptococcus mutans intracellular polysaccharide accumulation and virulence testing of an intracellular polysaccharide-deficient mutant. Infect Immun. 1992;60:3175–3185. doi: 10.1128/iai.60.8.3175-3185.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata S, Mayanagi H. Acid diffusion through extracellular polysaccharides produced by various mutants of Streptococcus mutans. Arch Oral Biol. 2003;48:431–438. doi: 10.1016/s0003-9969(03)00032-3. [DOI] [PubMed] [Google Scholar]
- Hayacibara MF, Koo H, Vacca-Smith AM, Kopec LK, Scott-Anne K, Cury JA, et al. The influence of mutanase and dextranase on the production and structure of glucans synthesized by streptococcal glucosyltransferases. Carbohydr Res. 2004;339:2127–2137. doi: 10.1016/j.carres.2004.05.031. [DOI] [PubMed] [Google Scholar]
- Hefti A, Schmid R. Effect on caries incidence in rats of increasing dietary sucrose levels. Caries Res. 1979;13:298–300. doi: 10.1159/000260414. [DOI] [PubMed] [Google Scholar]
- Horton WA, Jacob EA, Green RM, Hillier VF, Drucker DB. The cariogenicity of sucrose, glucose and maize starch in gnotobiotic rats mono-infected with strains of the bacteria Streptococcus mutans, Streptococcus salivarius and Streptococcus milleri. Arch Oral Biol. 1985;30:777–780. doi: 10.1016/0003-9969(85)90131-1. [DOI] [PubMed] [Google Scholar]
- Huang CM. Comparative proteomic analysis of human whole saliva. Arch Oral Biol. 2004;49:951–962. doi: 10.1016/j.archoralbio.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Johnson MC, Bozzola JJ, Shechmeister IL, Shklair IL. Biochemical study of the relationship of extracellular glucan to adherence and cariogenicity in Streptococcus mutans and an extracellular polysaccharide mutant. J Bacteriol. 1977;129:351–357. doi: 10.1128/jb.129.1.351-357.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima T, Andersen E, Sanchez JC, Wilkins MR, Hochstrasser DF, Pralong WF, et al. Human gingival crevicular fluid contains MRP8 (S100A8) and MRP14 (S100A9), two calcium-binding proteins of the S100 family. J Dent Res. 2000;79:740–747. doi: 10.1177/00220345000790020701. [DOI] [PubMed] [Google Scholar]
- König KG, Schmid P, Schmid R. An apparatus for frequency-controlled feeding of small rodents and its use in dental caries experiments. Arch Oral Biol. 1968;13:13–26. doi: 10.1016/0003-9969(68)90034-4. [DOI] [PubMed] [Google Scholar]
- Koo H, Hayacibara MF, Schobel BD, Cury JA, Rosalen PL, Park YK, et al. Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. J Antimicrob Chemother. 2003;52:782–789. doi: 10.1093/jac/dkg449. [DOI] [PubMed] [Google Scholar]
- Koo H, Schobel B, Scott-Anne K, Watson G, Bowen WH, Cury JA, et al. Apigenin and tt-farnesol with fluoride effects on S. mutans biofilms and dental caries. J Dent Res. 2005;84:1016–1020. doi: 10.1177/154405910508401109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopec LK, Vacca-Smith AM, Bowen WH. Structural aspects of glucans formed in solution and on the surface of hydroxyapatite. Glycobiology. 1997;7:929–934. doi: 10.1093/glycob/7.7.929. [DOI] [PubMed] [Google Scholar]
- Krasse B. The effect of caries-inducing streptococci in hamsters fed diets with sucrose or glucose. Arch Oral Biol. 1965;10:223–226. doi: 10.1016/0003-9969(65)90023-3. [DOI] [PubMed] [Google Scholar]
- Larsen MJ. Chemical events during tooth dissolution. J Dent Res. 1990;69(Spec Iss):575–580. doi: 10.1177/00220345900690S114. [DOI] [PubMed] [Google Scholar]
- Lingström P, Birkhed D, Ruben J, Arends J. Effect of frequent consumption of starchy food items on enamel and dentin demineralization and on plaque pH in situ. J Dent Res. 1994;73:652–660. doi: 10.1177/00220345940730031101. [DOI] [PubMed] [Google Scholar]
- Loesche WJ. Chemotherapy of dental plaque infections. Oral Sci Rev. 1976;9:65–107. [PubMed] [Google Scholar]
- Loesche WJ, Henry CA. Intracellular microbial polysaccharide production and dental caries in a Guatemalan Indian village. Arch Oral Biol. 1967;12:189–194. doi: 10.1016/0003-9969(67)90037-4. [DOI] [PubMed] [Google Scholar]
- Margolis HC, Moreno EC. Composition of pooled plaque fluid from caries-free and caries-positive individuals following sucrose exposure. J Dent Res. 1992;71:1776–1784. doi: 10.1177/00220345920710110301. [DOI] [PubMed] [Google Scholar]
- Margolis HC, Duckworth JH, Moreno EC. Composition of pooled resting plaque fluid from caries-free and caries-susceptible individuals. J Dent Res. 1988;67:1468–1475. doi: 10.1177/00220345880670120601. [DOI] [PubMed] [Google Scholar]
- Marsh PD. Sugar, fluoride, pH and microbial homeostasis in dental plaque. Proc Finn Dent Soc. 1991;87:515–525. [PubMed] [Google Scholar]
- Marsh PD. Microbial ecology of dental plaque and its significance in health and disease. Adv Dent Res. 1994;8:263–271. doi: 10.1177/08959374940080022001. [DOI] [PubMed] [Google Scholar]
- Marsh PD. Are dental diseases examples of ecological catastrophes? Microbiology. 2003;149(Pt 2):279–294. doi: 10.1099/mic.0.26082-0. [DOI] [PubMed] [Google Scholar]
- Mattos-Graner RO, Zelante F, Line RC, Mayer MP. Association between caries prevalence and clinical, microbiological and dietary variables in 1.0 to 2.5-year-old Brazilian children. Caries Res. 1998;32:319–323. doi: 10.1159/000016466. [DOI] [PubMed] [Google Scholar]
- Mattos-Graner RO, Smith DJ, King WF, Mayer MP. Water-insoluble glucan synthesis by mutans streptococcal strains correlates with caries incidence in 12- to 30-month-old children. J Dent Res. 2000;79:1371–1377. doi: 10.1177/00220345000790060401. [DOI] [PubMed] [Google Scholar]
- Minah GE, Lovekin GB, Finney JP. Sucrose-induced ecological response of experimental dental plaques from caries-free and caries-susceptible human volunteers. Infect Immun. 1981;34:662–675. doi: 10.1128/iai.34.3.662-675.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno EC, Varughese K, Hay DI. Effect of human salivary proteins on the precipitation kinetics of calcium phosphate. Calcif Tissue Int. 1979;28:7–16. doi: 10.1007/BF02441212. [DOI] [PubMed] [Google Scholar]
- Newbrun E. Sucrose, the arch criminal of dental caries. Odontol Revy. 1967;18:373–386. [PubMed] [Google Scholar]
- Nobre dos Santos M, Melo dos Santos L, Francisco SB, Cury JA. Relationship among dental plaque composition, daily sugar exposure and caries in the primary dentition. Caries Res. 2002;36:347–352. doi: 10.1159/000065959. [DOI] [PubMed] [Google Scholar]
- Paes Leme AF, Dalcico R, Tabchoury CP, Del Bel Cury AA, Rosalen PL, Cury JA. In situ effect of frequent sucrose exposure on enamel demineralization and on plaque composition after APF application and F dentifrice use. J Dent Res. 2004;83:71–75. doi: 10.1177/154405910408300114. [DOI] [PubMed] [Google Scholar]
- Pearce E. Plaque minerals and dental caries. NZ Dent J. 1998;94:12–15. [PubMed] [Google Scholar]
- Pearce EI, Sissons CH, Coleman M, Wang X, Anderson SA, Wong L. The effect of sucrose application frequency and basal nutrient conditions on the calcium and phosphate content of experimental dental plaque. Caries Res. 2002;36:87–92. doi: 10.1159/000057865. [DOI] [PubMed] [Google Scholar]
- Pecharki GD, Cury JA, Paes Leme AF, Tabchoury CP, Del Bel Cury AA, Rosalen PL, et al. Effect of sucrose containing iron (II) on dental biofilm and enamel demineralization in situ. Caries Res. 2005;39:123–129. doi: 10.1159/000083157. [DOI] [PubMed] [Google Scholar]
- Perinpanayagam HE, VanWuyckhuyse BC, Ji ZS, Tabak LA. Characterization of low-molecular-weight peptides in human parotid saliva. J Dent Res. 1995;74:345–350. doi: 10.1177/00220345950740011001. [DOI] [PubMed] [Google Scholar]
- Quivey RG, Jr, Kuhnert WL, Hahn K. Adaptation of oral streptococci to low pH [review] Adv Microb Physiol. 2000;42:239–274. doi: 10.1016/s0065-2911(00)42004-7. [DOI] [PubMed] [Google Scholar]
- Ribeiro CC, Tabchoury CP, Del Bel Cury AA, Tenuta LM, Rosalen PL, Cury JA. Effect of starch on the cariogenic potential of sucrose. Br J Nutr. 2005;94:44–50. doi: 10.1079/bjn20051452. [DOI] [PubMed] [Google Scholar]
- Rölla G. Why is sucrose so cariogenic? The role of glucosyltransferase and polysaccharides. Scand J Dent Res. 1989;97:115–119. doi: 10.1111/j.1600-0722.1989.tb01439.x. [DOI] [PubMed] [Google Scholar]
- Rose RK. Effects of an anticariogenic casein phosphopeptide on calcium diffusion in streptococcal model dental plaques. Arch Oral Biol. 2000;45:569–575. doi: 10.1016/s0003-9969(00)00017-0. [DOI] [PubMed] [Google Scholar]
- Rose RK, Shellis RP, Lee AR. The role of cation bridging in microbial fluoride binding. Caries Res. 1996;30:458–464. doi: 10.1159/000262360. [DOI] [PubMed] [Google Scholar]
- Rose RK, Matthews SP, Hall RC. Investigation of calcium-binding sites on the surfaces of selected Gram-positive oral organisms. Arch Oral Biol. 1997a;42:595–599. doi: 10.1016/s0003-9969(97)00062-9. [DOI] [PubMed] [Google Scholar]
- Rose RK, Turner SJ, Dibdin GH. Effect of pH and calcium concentration on calcium diffusion in streptococcal model-plaque biofilms. Arch Oral Biol. 1997b;42:795–800. doi: 10.1016/s0003-9969(97)00082-4. [DOI] [PubMed] [Google Scholar]
- Schilling KM, Bowen WH. Glucans synthesized in situ in experimental salivary pellicle function as specific binding sites for Streptococcus mutans. Infect Immun. 1992;60:284–295. doi: 10.1128/iai.60.1.284-295.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüpbach P, Oppenheim FG, Lendenmann U, Lamkin MS, Yao Y, Guggenheim B. Electron-microscopic demonstration of proline-rich proteins, statherin, and histatins in acquired enamel pellicles in vitro. Eur J Oral Sci. 2001;109:60–68. doi: 10.1034/j.1600-0722.2001.00925.x. [DOI] [PubMed] [Google Scholar]
- Shen P, Cai F, Nowicki A, Vincent J, Reynolds EC. Remineralization of enamel subsurface lesions by sugar-free chewing gum containing casein phosphopeptide-amorphous calcium phosphate. J Dent Res. 2001;80:2066–2070. doi: 10.1177/00220345010800120801. [DOI] [PubMed] [Google Scholar]
- Spatafora G, Rohrer K, Barnard D, Michalek S. A Streptococcus mutans mutant that synthesizes elevated levels of intracellular polysaccharide is hypercariogenic in vivo. Infect Immun. 1995;63:2556–2563. doi: 10.1128/iai.63.7.2556-2563.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staat RH, Gawronski TH, Cressey DE, Harris RS, Folke LE. Effects of dietary sucrose levels on the quantity and microbial composition of human dental plaque. J Dent Res. 1975;54:872–880. doi: 10.1177/00220345750540042801. [DOI] [PubMed] [Google Scholar]
- Stephan RM. Intra-oral hydrogen-ion concentrations associated with dental caries activity. J Dent Res. 1944;23:257–266. [Google Scholar]
- Tanzer JM, Freedman ML, Woodiel FN, Eifert RL, Rinehimer LA. Association of Streptococcus mutans virulence with synthesis of intracellular polysaccharide. In: Stiles HM, Loesche WJ, O’Brien TC, editors. Microbial aspects of dental caries. Washington, DC: Information Retrieval; 1976. pp. 597–616. [Google Scholar]
- Tenuta LMA, Del Bel Cury AA, Bortolin MC, Vogel GL, Cury JA. Ca, Pi, and F in the fluid of biofilm formed under sucrose. J Dent Res. 2006;85:834–838. doi: 10.1177/154405910608500911. [DOI] [PubMed] [Google Scholar]
- Theilade E. The non-specific theory in microbial etiology of inflammatory periodontal diseases. J Clin Periodontol. 1986;13:905–911. doi: 10.1111/j.1600-051x.1986.tb01425.x. [DOI] [PubMed] [Google Scholar]
- Theilade E. The experimental gingivitis studies: the microbiological perspective. J Dent Res. 1996;75:1434–1438. doi: 10.1177/00220345960750070201. [DOI] [PubMed] [Google Scholar]
- Vacca-Smith AM, Venkitaraman AR, Quivey RG, Jr, Bowen WH. Interactions of streptococcal glucosyltransferases with alpha-amylase and starch on the surface of saliva-coated hydroxyapatite. Arch Oral Biol. 1996;41:291–298. doi: 10.1016/0003-9969(95)00129-8. [DOI] [PubMed] [Google Scholar]
- van Houte J, Winkler KC, Jansen HM. Iodophilic polysaccharide synthesis, acid production and growth in oral streptococci. Arch Oral Biol. 1969;14:45–61. doi: 10.1016/0003-9969(69)90020-x. [DOI] [PubMed] [Google Scholar]
- van Houte J, Russo J, Prostak KS. Increased pH-lowering ability of Streptococcus mutans cell masses associated with extracellular glucan-rich matrix material and the mechanisms involved. J Dent Res. 1989;68:451–459. doi: 10.1177/00220345890680030301. [DOI] [PubMed] [Google Scholar]
- Yamashita Y, Bowen WH, Burne RA, Kuramitsu HK. Role of the Streptococcus mutans gtf genes in caries induction in the specific-pathogen-free rat model. Infect Immun. 1993;61:3811–3817. doi: 10.1128/iai.61.9.3811-3817.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Berg EA, Costello CE, Troxler RF, Oppenheim FG. Identification of protein components in human acquired enamel pellicle and whole saliva using novel proteomics approaches. J Biol Chem. 2003;278:5300–5308. doi: 10.1074/jbc.M206333200. [DOI] [PubMed] [Google Scholar]
- Zero DT. Adaptations in dental plaque. In: Bowen WH, Tabak LA, editors. Cariology for the nineties. Rochester, NY: University of Rochester Press; 1993. pp. 333–350. [Google Scholar]
- Zero DT. Sugars—the arch criminal? Caries Res. 2004;38:277–285. doi: 10.1159/000077767. [DOI] [PubMed] [Google Scholar]
- Zero DT, van Houte J, Russo J. Enamel demineralization by acid produced from endogenous substrate in oral streptococci. Arch Oral Biol. 1986a;31:229–234. doi: 10.1016/0003-9969(86)90054-3. [DOI] [PubMed] [Google Scholar]
- Zero DT, van Houte J, Russo J. The intra-oral effect on enamel demineralization of extracellular matrix material synthesized from sucrose by Streptococcus mutans. J Dent Res. 1986b;65:918–923. doi: 10.1177/00220345860650061201. [DOI] [PubMed] [Google Scholar]
- Zero DT, Fu J, Anne KM, Cassata S, McCormack SM, Gwinner LM. An improved intra-oral enamel demineralization test model for the study of dental caries. J Dent Res. 1992;71(Spec Iss):871–878. doi: 10.1177/002203459207100S17. [DOI] [PubMed] [Google Scholar]


