Abstract
Background
Caffeine in dietary doses is a well-established pressor agent. Tolerance to this pressor effect occurs in only about half of regular consumers in acute laboratory tests. The clinical significance of this incomplete tolerance depends on whether the pressor effect is maintained throughout the day with repeated intake. Therefore, we examined the ability of a standard dose of caffeine (250 mg × 3) to maintain a blood pressure (BP) elevation during 18 hours of ambulatory BP monitoring (ABPM) after 5 days of regular daily intake of varying background doses.
Methods
Eighty-five men and women completed a four-week double blind, crossover trial. During each week, subjects consumed capsules totaling 0, 300, or 600 mg/day of caffeine in 3 divided doses. On day 6, they consumed capsules with either 0 or 250 mg at 9:00 am and 1:00 pm, in the laboratory, and again at 6:00 pm during ABPM. Tolerance was defined as a reduction in the diastolic BP response to two challenge doses given in the lab in response to increasing daily intake. Data were analyzed using multivariate repeated measures analysis of variance.
Results
BP responses to caffeine above those found on placebo-placebo (P-P) week were found for both tolerance groups when caffeine was consumed after a week of receiving a placebo. However, only the low tolerance group showed increases, above those found on P-P week, after 300 mg/day in systolic/diastolic BP during the waking hours (mean ± standard error of the mean = 2.8 ± 1.1, P = .01/2.2 ± 0.9, P = .02) and in systolic BP during sleep (2.3 ± 1, P = .03).
Conclusions
Persistent elevations in BP occurring on a daily basis in some habitual caffeine consumers may hold clinical significance.
Keywords: caffeine, tolerance, blood pressure, ambulatory
Caffeine is by far the most widely used, socially accepted psychoactive substance worldwide. Among US adults, 54% drank coffee daily and 25% drank coffee occasionally in a year 2000 survey (National Coffee Association). The popularity of caffeine stems from its stimulant and positive mood effects.1 Caffeine's stimulatory action extends to the cardiovascular and neuroendocrine systems.2 Blood pressure (BP) increases in the range of 5–10 mm Hg following caffeine consumption are well-documented.3–7 Caffeine exerts its effect on BP through its blockade of vascular adenosine receptors.8,9
Epidemiological studies document linear relationships between BP levels and risk of cardiovascular disease (CVD). A 5-mm Hg difference in diastolic BP is associated with an increase of 34% in the incidence of stroke and a 21% increase in the incidence of coronary heart disease. Such relationships persist even for BP within the normal range.10 Thus, caffeine's pressor effect should be considered in relation to BP elevations in regular consumers.
Concern regarding caffeine's effect on BP is dampened by the belief that tolerance occurs with daily ingestion.11 Systematic study of tolerance to caffeine with regular consumption suggests that tolerance may not be complete. In a prior report we examined caffeine's acute BP effect in the laboratory to repeated morning and midday doses after different amounts of daily intake.12 This study and others showed that daily caffeine intake produces only a partial tolerance leaving a persistent BP response to repeated daily dosing.12–14 In addition, it has been suggested that caffeine's acute BP effect in the lab is not readily demonstrable across the day and during a range of activities.13
The extent of tolerance formation tested by acute response to caffeine in the lab has been published previously.12 In this paper, we examine tolerance effects on the BP response to caffeine across the day in the ambulatory setting. Tolerance formation was tested in relation to daily maintenance doses chosen to mimic the range of consumption commonly found in the U.S. diet,15 ranging from none (0 mg/day) to moderate (300 mg/day) to high (600 mg/day) levels in a within-subject, crossover design. Testing then determined the effect of these background intake levels on 18-hour ambulatory BP. Tolerance was defined as a reduction in the acute BP response to a fixed challenge dose (250 mg × 3/day) occurring with increasing dietary intake (0, 300, and 600 mg/day), all referenced to BP levels during a no-caffeine control week (0 mg/day dietary intake and a 0 mg × 3/day challenge doses).
Methods
Study Population
A total of 132 subjects ages 21-35 years were recruited from the community and enrolled in the study. Twenty-one subjects withdrew voluntarily at various points in the protocol, 11 were found to be noncompliant and dropped, 1 was excluded due to abnormal lab values, 2 reported side effects and were excused, and 12 had missing ambulatory data on one or more of the 4 testing weeks reducing the sample size to 85 (men = 47 and women = 38) (Table 1). All were nonobese (body mass index [BMI] < 30) and in good health by self-report and routine physical exam. They had BPs in the normotensive range (BPs < 135/85 mmHg) at screening, and used no medications having cardiovascular or metabolic effects. They regularly consumed 50-700 mg/day of caffeine by structured interview. Approximately 64% reported consumption greater than 200 mg/day (equivalent to 2 or more cups of coffee) based on a structured interview administered by a research nurse. Subjects were asked about a typical week's volume and frequency of consumption of the following dietary caffeine sources: coffee, tea, soft drinks, chocolates, and caffeine-containing medications. They were also asked about the brewing method in the case of coffee and tea. Caffeine content per ounce was calculated using International Food Information Council Foundation standards.16 Women were free from oral contraceptives and were not pregnant, as determined by urine pregnancy test (One Step Pregnancy Test, Inverness Medical Ltd., Beachwood Park, North Inverness, Scotland). Half of the volunteers were tested in Buffalo, NY, and half in Oklahoma City, OK. All participants signed a consent form approved by the Institutional Review Board of the University of Oklahoma Health Sciences Center and the Veterans Affairs Medical Center in Oklahoma City and SUNY Buffalo, Buffalo, NY, and were paid for participating.
Table 1.
Demographics by tolerance group
| Total Group
(N = 85) |
High Tolerance Group
(N = 42) |
Low Tolerance Group
(N = 43) |
|
|---|---|---|---|
| Sex (M/F) | 47/38 | 20/22 | 27/16 |
| Age (yr) | 28 (0.6) | 28 (0.9) | 28 (0.8) |
| BMI (kg/m2) | 23.7 (0.3) | 24.2 (0.4) | 23.2 (0.4) |
| Body Fat (%) | 19.4 (0.7) | 20.2 (1.1) | 18.5 (0.98) |
| Caffeine intake (mg/day)a | 394 (43) | 353 (57) | 435 (63) |
| Morning saliva caffeine levels on each week (μmol/L) | |||
| Placebo-placebo (0 mg/day) | 0.03 (0.02) | 0.06 (0.04) | 0 (0) |
| Placebo-caffeine (0 mg/day) | 0.05 (0.03) | 0.09 (0.06) | 0.01 (0.01) |
| Caffeine low (300 mg/day) | 0.71 (0.11) | 0.83 (0.20) | 0.59 (0.11) |
| Caffeine high (600 mg/day) | 2.24 (0.25) | 2.37 (0.38) | 2.12 (0.34) |
| Screening BP (mmHg) | |||
| Systolic | 113 (1.0) | 111 (1.3) | 114 (1.5) |
| Diastolic | 66 (0.7) | 65 (1.0) | 68 (0.9) |
ANOVA = analysis of variance; BMI = body mass index; BP = blood pressure; SEM = standard error of the mean. Note: Entries show (mean ± SEM). Caffeine intake reflects usual consumption from all sources based on structured interview. Screening BP is an average of three readings over 5 min after 5 min of seated rest. High and low tolerance groups were not different in numbers of males and females (χ2 = 1.98, P = .16), or in any other listed variable (ts < 1.73, ps > .09) with the exception of screening diastolic BP (P = .03). Caffeine levels from saliva specimens collected at 8:00 am on each test day after 5 days on the indicated daily maintenance dose of caffeine. The ANOVA on Tolerance Group × Week for C300 (300 mg/day) and C600 (600 mg/day) weeks showed that the indicated groups did not differ in residual caffeine levels, F1,81 = .001; P = .97.
Level of regular daily caffeine consumption was not related to the degree of tolerance formation.
Study Design, Caffeine Dosing, and Compliance
The design was a four-week, placebo-controlled, double-blind, randomized crossover trial of caffeine tolerance effects on BP responses to fixed challenge doses. Subjects were instructed to eliminate all sources of caffeine from their diet (coffee, tea, soft drinks, and chocolates) during the four weeks of the study. Each study week consisted of 5 maintenance days with placebo (P) or caffeine (C) self-administration at home. Day 6 consisted of a 6-hour lab protocol and 18 hours of ambulatory BP monitoring accompanied by placebo or caffeine challenges. Day 7 was a crossover day, with successive caffeine doses of 100 mg, 0 mg, and 0 mg, to buffer sudden changes in intake at weekly crossovers. Weekly dose schedules are shown in Table 2.
Table 2.
Placebo and caffeine doses on study weeks
| Study Week | Maintenance Days | Protocol Days |
|---|---|---|
| P-P | 0 mg (3 × 0 mg) | 0 mg (3 × 0 mg) |
| P-C | 0 mg (3 × 0 mg) | 750 mg (3 × 250 mg) |
| C300-C | 300 mg (3 × 100 mg) | 750 mg (3 × 250 mg) |
| C600-C | 600 mg (3 × 200 mg) | 750 mg (3 × 250 mg) |
Note: P = placebo, C = caffeine, C500-500 mg/day maintenance. C600-600 mg/day maintenance. Week order was randomized across subjects. P-P week is the control week with placebo for 5 days and placebo on the testing day. P-C provides the maximum response to caffeine (C) doses following 5 days of C withdrawal. The C300 and C600 weeks provide moderate and high daily levels of intake to examine a decrease in response to the C challenge doses.
Home maintenance doses were supplied in bottles of identical gelatin capsules (College of Pharmacy, University of Oklahoma, Oklahoma City, OK) containing either lactose or lactose mixed with 100 mg or 200 mg of USP caffeine (Gallipot, St. Paul, MN). Subjects were instructed to take a capsule at 08.00, 13.00, and 18.00 hours each day. Test-day challenge doses were supplied in capsules containing either lactose or lactose mixed with 250 mg of caffeine administered at 09.00, 13.00, and 18.00 hours.
Compliance was assessed by capsule counts, by caffeine assay of saliva specimens collected at home each day at 13.00 hours (Salivette®, Starstedt, Germany), and from a saliva specimen collected each morning upon entering the lab. Subjects found to be noncompliant by any of these criteria were dropped and replaced.
Lab Protocol
Laboratory testing took place at hospitals on the respective campuses (Oklahoma University Health Sciences Center and SUNY Buffalo). Details of the lab protocol have been published previously.12 There were no significant differences in the demographic characteristics and BPs between the two population samples. Acute BP responses to caffeine intake were reported separately.12
Ambulatory Blood Pressure Monitoring (ABPM)
The laboratory protocol ended at 14.00 hours. Before dismissal from the lab, subjects were outfitted with a Spacelabs Model 90205 ABPM unit (Spacelabs, Redmond, WA). The apparatus was programmed to obtain a BP measurement every 15 minutes until 22.00 hours and every 30 minutes from 22.00 to 07.00 hours. Subjects returned the monitor in the morning. The data were edited using standard artifact detection software provided by Spacelabs. Acceptable records included at least two recordings per hour during daytime and one per hour during sleep.17 Sleep was defined by diary entries indicating “bedtime” and “morning awakening” times.
Caffeine concentrations in saliva were measured by high performance liquid chromatography (Waters Corp., Milford, MA), following precipitation of proteins, using a methanol and water mobile phase and ultraviolet detection.18
Statistical Analysis
The high and low tolerance groups were formed previously using a median split of the averaged diastolic BP responses to the acute 9:00 am and 1:00 pm laboratory caffeine doses during the C600 week, when tolerance would be expected to be at its greatest, as previously described.12 Diastolic BP was used to define tolerance groups because caffeine exerts its pressor effect by raising peripheral resistance,9 and diastolic BP is more reflective of peripheral resistance than is systolic BP. The ABPM data were analyzed using multivariate, repeated measures analyses of variance (MANOVAs) that included 4 test weeks (placebo-placebo [P-P], placebo-caffeine [P-C], caffeine low [C300], and caffeine high [C600]) × 2 tolerance groups (high vs low). We first tested the effect of caffeine tolerance on the averaged afternoon and evening systolic and diastolic BPs (awake BP). The same analysis was conducted for BP averaged during sleep. For each set of ANOVAs, we tested specific contrasts between the P-P control week versus the P-C, C300, and C600 weeks. Significance was set at α < .05. Preliminary analyses found no BP differences by gender or site (Oklahoma City vs. Buffalo), thus subsequent analyses did not include these factors.
Data were analyzed using SAS (SAS System for Windows, version 8.2, SAS Institute Inc., Cary, NC) and SPSS (SPSS for Windows, rel. 10.1.0, SPSS Chicago, IL). Potential violation of the sphericity assumption in univariate repeated measures ANOVA was avoided using a multivariate solution for repeated measures factors.
Results
The tolerance groups did not differ by age, weight, height, BMI, percent body fat, daily caffeine consumption, or BPs at screening, as shown in Table 1 (columns 2 and 3).
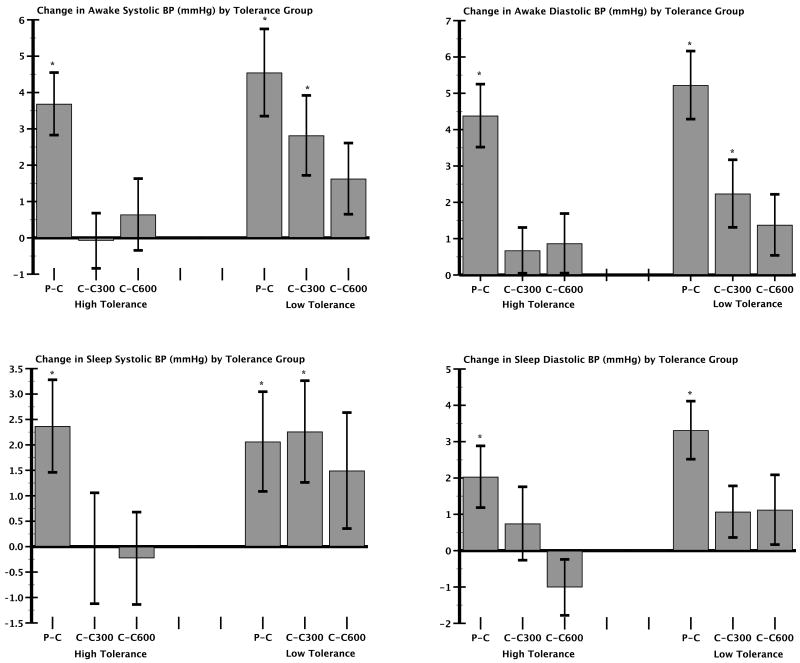
During the waking period of the ABPM, MANOVAs revealed significant BP differences across weeks (systolic BP: F1,84 = 12.9, P < .0001, partial η2 = 0.32; diastolic BP: F1,84 = 23.8, P < .0001, partial η2 = 0.47) and between tolerance groups (systolic BP: F1,84 = 8.4, P = .005, partial η2 = 0 .09; diastolic BP: F1,84 = 10.2, P = .002, partial η2 = 0.11). There were no significant week × tolerance group interactions (systolic BP: F1,84 = 1.7, P = .1, partial η2 = 0.06; diastolic BP: F1,84 = 0.7, P = .6, partial η2 = 0.03). However, examination of Figures 1A and 1B shows distinct differences between tolerance groups in awake BPs. Ambulatory BP was highest in both tolerance groups after 5 days of caffeine abstinence (P-C week). The BP response to caffeine was reduced in both groups after daily intake on the C300 and C600 weeks. Nevertheless, the low-tolerance group maintained significant BP elevations relative to the P-P week on the C300 week (systolic BP: 2.8 ± 1.1, t = 2.6, P = .01; diastolic BP: 2.2 ± 0.9, t = 2.4, P = .02) as well as the PC week (systolic BP: 4.6 ± 1.2, t = 3.8, P < .0001; diastolic BP: 5.2 ± .9, t = 5.6, P < .0001). In contrast, the high-tolerance group showed significant BP elevations only on the PC week (systolic BP: 3.7 ± 0.9, t = 4.3, P < .0001; diastolic BP: 4.4 ± 0.9, t = 5.1, P < .0001), suggesting that relatively complete caffeine tolerance appears to have developed for these subjects. The awake BP levels on C600 week did not reach statistical significance (systolic BP: 1.6 ± 1.1, t = 1.7, P = .1; diastolic BP: 1.4 ± 0.8, t = 1.6, P = .1) in either tolerance group.
FIG. 1.
Systolic and diastolic BP responses to caffeine averaged during the awake period (A) and (B) and during sleep (C) and (D) in subgroups that show high vs low tolerance to caffeine challenge (250 mg × 3) in the laboratory as a function of 5 days of consuming 0 mg/day (P-C), 300 mg/day (C300) or 600 mg/day (C600) of caffeine at home. For visual clarity, bars reflect differences between PP week and each of the other 3 weeks. Pairwise contrasts of each week against P-P week are designated as *P < .05. (BP = blood pressure; C = caffeine; P-C = placebo-caffeine; P-P = placebo-placebo.)
Figure 1C and D show caffeine effects on BP during sleep in the two tolerance groups. The sleep BPs differed across weeks (systolic BP: F1,84 = 3.8, P = .01, partial η2 = 0.12; diastolic BP: F1,84 = 8.7, P < .0001, partial η2 = 0.24), and the difference between tolerance groups approached significance for diastolic BP (F184 = 3.4, P = .07, partial η2 = 0.04). There were no significant week × tolerance group interactions for sleep systolic BP (F1,84f = 1.24, P = .3, partial η2 = 0.04) or diastolic BP (F1,84 = 1.18, P = .3, partial η2 = 0.04). Sleep systolic BP was significantly elevated above P-P week values on P-C (2.1 ± 0.9, t = 2.1, P = .04) and C300 (2.3 ± 1, t = 2.3, P = .03) weeks in the low-tolerance group (Fig. 1C). In the high-tolerance group, systolic (diastolic) BP during sleep was significantly elevated above P-P week values on P-C week only (2.4 ± 0.9, t = 2.6, P = .01/2 ± 0.9, t = 2.4, P = .02).
Discussion
The acute effects of caffeine on BP are well documented. However, it is commonly held that tolerance develops with habitual use, thus negating concerns about long-term effects of caffeine on cardiovascular health. However, a thorough review of the evidence from experimental and epidemiologic studies confirms that tolerance to caffeine's pressor effects is not well documented.19 Larger BP effects have been reported for caffeine administered in the lab as compared with the ambulatory setting.2,20 Consequently, it has been suggested that in laboratory studies, caffeine's effects on BP are measured during peak blood concentrations (45-90 minutes after ingestion), accounting for larger BP responses in the lab as opposed to the ambulatory setting. In this study, we examined the BP responses to caffeine across a 24-hour period including the lab and ambulatory settings. We have recently demonstrated that daily caffeine consumption for 5 days reduced, but did not abolish, the acute BP response to caffeine challenge doses in the lab.12 In this report we extend these findings by reporting the effects of caffeine on BP in the ambulatory setting. We found that caffeine's pressor effect was maintained across the day into the ambulatory setting despite daily consumption.
The largest BP response occurred to caffeine challenge doses given after 5 days of placebo maintenance (P-C week), and these BP effects persisted across the ambulatory period. The change in BP decreased after 5 days of both low (300 mg) and high (600 mg) caffeine intake. However, these responses were still greater than the BP levels seen on P-P week suggesting partial, but not complete, tolerance to the effects of caffeine on ambulatory BP. There was also significant interindividual variability in the degree of tolerance. Half of the subjects became completely tolerant to caffeine after consuming either 300 or 600 mg/day for 5 days. The other half had little or no reduction in the ambulatory BP response, particularly to the 300 mg/day dose. To the extent that these findings are generalizable in approximately half of the population, caffeine produces similar effects every day that it is ingested. The findings of this study support those of prior research confirming an incomplete tolerance to caffeine's effects on BP,13,14,21 We have extended such findings by demonstrating that such BP effects are not transient, but rather maintained throughout the day with repeated caffeine intake.
The magnitude of BP elevations to caffeine during sleep were smaller than those during the waking hours. However, Figures 1C and 1D show that BP responses to repeated challenge doses of caffeine in the low tolerance group did not return to baseline levels on the C300 week, when daily caffeine intake was equivalent to three cups of coffee per day. If indeed, caffeine's pressor effects are transient and limited to the waking hours as previously hypothesized,13 one would have expected nighttime BP to fall to P-P week levels for all subjects. It is of note that BP levels were sustained during sleep given that the average elimination half life of caffeine in humans is 4 to 5 hours,22 and the last dose was administered at 18.00 hours, approximately 4 to 6 hours before bedtime. The normal fall in BP during sleep is a healthy phenomenon and a restorative physiologic process.23 Individuals with diminished nocturnal BP fall are at a higher risk of target organ damage and cardiovascular complications.24,25
It is noteworthy that BP elevations above P-P week were significant when subjects consumed 300 mg/day of caffeine which is equivalent to 2–3 cups of coffee. Consumption of 600 mg/day (4–6 cups) did not produce a significant elevation in ambulatory BP. Nevertheless, we have previously demonstrated a persistent acute BP elevation at the 600 mg dose in the laboratory setting.12 This suggests that, in the ambulatory setting, moderate doses of caffeine are more likely to lead to only a partial tolerance than higher doses. Given that the 300 mg dose is the level of caffeine most commonly consumed by U.S. adults,15 this confirms the persistent BP effects of caffeine in a considerable portion of regular consumers.
Although the mechanism of tolerance development was beyond the scope of this study, some points are worth consideration in evaluating these findings. Tolerance to caffeine may be due to an altered metabolism26 or adaptive changes in the number of adenosine receptors.27 In the present study, differences in metabolism do not appear to be the cause for the observed differences. The tolerance groups had similar residual morning caffeine concentrations after overnight abstinence and comparable caffeine levels in saliva after dosing in the lab. Adenosine receptor density or dynamics may account for the observed differences; however, we did not evaluate such effects in the current study. However, given that even in low tolerance individuals, a higher dose of caffeine lead to an attenuation of the pressor effect of caffeine suggests the involvement of adenosine receptor mechanisms. Caffeine tolerance formation should be tested after longer periods of exposure than used here. It should be pointed out, however, that although the subjects were kept for only 5 days at each maintenance dose, they were all regular consumers of caffeine, suggesting that this short-term maintenance may generalize to longer periods of intake.
The pressor response to caffeine is greater in hypertensive or hypertension-prone subjects than in normotensive subjects.28 Unmedicated hypertensive patients have greater rises in diastolic BP to caffeine during exercise than do normotensive controls.29 Additionally, the pressor effects of caffeine are not diminished by the administration of antihypertensive medications.30 The present results show that BP responses to repeated caffeine intake during the day and night are sustained in persons who are resistant to forming a complete pharmacologic tolerance to its effects. If low-tolerance forming persons are also hypertensive, caffeine consumption may reduce the effectiveness of hypertension control efforts. The small BP rises seen in this study may be of clinical significance in hypertensives. The Hypertension Detection and Follow-up Program reported that a 5-mmHg reduction in BP was associated with a 20% reduction in 5-year mortality.31 The present results indicate that clinicians may suggest reductions in caffeine consumption as a viable step in hypertension control efforts. This is particularly so for those patients with uncontrolled hypertension where pharmacological treatment and conventional lifestyle modifications have failed to produce the desired results. Future research should directly assess whether caffeine plays a role in BP regulation of uncontrolled hypertension and if lowering or eliminating caffeine intake would result in lowering of BP in such patients.
Acknowledgments
This study was supported by the Medical Research Service of the Department of Veterans Affairs and by Grants HL 32050, HL 32050-S2, and HL 07640 from the National Heart, Lung, and Blood Institute and M01-RR14467 from the National Center for Research Resources, Bethesda, MD.
References
- 1.Lieberman HR. The effects of ginseng, ephedrine and caffeine on cognitive performance, mood and energy. Nutr Rev. 2001;59:91–102. doi: 10.1111/j.1753-4887.2001.tb06995.x. [DOI] [PubMed] [Google Scholar]
- 2.James JE. Caffeine and health. Academic Press; New York, NY: 1991. [Google Scholar]
- 3.Pincomb GA, Lovallo WR, Passey RB, Whitsett TL, Silverstein SM, Wilson MF. Effects of caffeine on vascular resistance, cardiac output and myocardial contractility in young men. Am J Cardiol. 1985;56:119–122. doi: 10.1016/0002-9149(85)90578-8. [DOI] [PubMed] [Google Scholar]
- 4.Lovallo WR, Pincomb GA, Sung BH, Passey RB, Sausen KP, Wilson MF. Caffeine may potentiate adrenocortical stress response in hypertension-prone men. Hypertension. 1989;14:170–176. doi: 10.1161/01.hyp.14.2.170. [DOI] [PubMed] [Google Scholar]
- 5.Robertson R, Frolich JC, Carr RK, Watson JT, Hollifield JW, Shand DG, Oates JA. Effects of caffeine on plasma renin activity, catecholamines and blood pressure. N Engl J Med. 1978;298:181–186. doi: 10.1056/NEJM197801262980403. [DOI] [PubMed] [Google Scholar]
- 6.Lane JD. Caffeine and cardiovascular responses to stress. Psychosom Med. 1983;45:447–451. doi: 10.1097/00006842-198310000-00008. [DOI] [PubMed] [Google Scholar]
- 7.France C, Ditto B. Caffeine effects on several indices of cardiovascular activity at rest and during stress. J Behav Med. 1988;11:47–82. doi: 10.1007/BF00844840. [DOI] [PubMed] [Google Scholar]
- 8.Smits P, Boekema P, De Abreu R, Thien T, van't Laar A. Evidence for an antagonism between caffeine and adenosine in the human cardiovascular system. J Cardiovasc Pharmacol. 1987;10:136–143. doi: 10.1097/00005344-198708000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Pincomb GA, Lovallo WR, Passey RB, Whitsett TL, Silverstein SM, Wilson MF. Effects of caffeine on vascular resistance, cardiac output and myocardial contractility in young men. Am J Cardiol. 1985;56:119–122. doi: 10.1016/0002-9149(85)90578-8. [DOI] [PubMed] [Google Scholar]
- 10.MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, Abbott R, Godwin J, Dyer A, Stamler J. Blood pressure, stroke, and coronary heart disease: Part 1. Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335:765–774. doi: 10.1016/0140-6736(90)90878-9. [DOI] [PubMed] [Google Scholar]
- 11.Robertson D, Wade D, Workman R, Woosley RI, Oates JA. Tolerance to the humoral and hemodynamic effects of caffeine in man. J Clin Invest. 1981;67:1111–1117. doi: 10.1172/JCI110124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lovallo WR, Wilson MF, Vincent AS, Sung BH, McKey BS, Whitsett TL. Blood pressure response to caffeine shows incomplete tolerance after short-term regular consumption. Hypertension. 2004;43:760–765. doi: 10.1161/01.HYP.0000120965.63962.93. [DOI] [PubMed] [Google Scholar]
- 13.James JE. Chronic effects of habitual caffeine consumption on laboratory and ambulatory blood pressure levels. J Cardiovasc Risk. 1994;1:159–164. doi: 10.1177/174182679400100210. [DOI] [PubMed] [Google Scholar]
- 14.Lane JD, Peiper CF, Phillips-Bute B, Bryant JE, Kuhn CM. Caffeine affects cardiovascular and neuroendocrine activation at work and home. Psychosom Med. 2002;64:595–603. doi: 10.1097/01.psy.0000021946.90613.db. [DOI] [PubMed] [Google Scholar]
- 15.Barone JJ, Roberts HR. Caffeine consumption. Food Chem Toxic. 1996;34:119–129. doi: 10.1016/0278-6915(95)00093-3. [DOI] [PubMed] [Google Scholar]
- 16.International Food Information Council Foundation 2004 http://ific.org.
- 17.Di Rienzo M, Grassi G, Pedotti A, Mancia G. Continuous vs intermittent blood pressure measurements in estimating 24 hours average blood pressure. Hypertension. 1983;5:264–269. doi: 10.1161/01.hyp.5.2.264. [DOI] [PubMed] [Google Scholar]
- 18.Christensen HD, Whitsett TL. Measurements of xanthines and their metabolites by means of high pressure liquid chromatography. In: Hawk GL, editor. Biological/biomedical applications of liquid chromatography science. New York, NY: Marcel Dekker; 1979. pp. 507–538. [Google Scholar]
- 19.James JE. Critical review of dietary caffeine and blood pressure: A relationship that should be taken more seriously. Psychosom Med. 2004;66:63–71. doi: 10.1097/10.psy.0000107884.78247.f9. [DOI] [PubMed] [Google Scholar]
- 20.Eggertsen R, Andreasson A, Hedner R, Karlberg BE, Hansson L. Effect of coffee on ambulatory blood pressure in patients with treated hypertension. J Intern Med. 1993;233:351–355. doi: 10.1111/j.1365-2796.1993.tb00683.x. [DOI] [PubMed] [Google Scholar]
- 21.Watson J, Deary I, Kerr D. Central and peripheral effects of sustained caffeine use: tolerance is incomplete. Br J Clin Pharmacol. 2002;54:400–406. doi: 10.1046/j.1365-2125.2002.01681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smits P, Thien T, van't Laar A. Circulatory effects of coffee in relation to the pharmacokinetics of caffeine. Am J Cardiol. 1985;56:958–963. doi: 10.1016/0002-9149(85)90412-6. [DOI] [PubMed] [Google Scholar]
- 23.Rosansky SJ, Menachery SJ, Whittman D, Rosenberg JC. The relationship between sleep deprivation and tehnocturnal decline of blood pressure. Am J Hypertens. 1996;9:1136–1138. doi: 10.1016/0895-7061(96)00300-7. [DOI] [PubMed] [Google Scholar]
- 24.Verdechia P, Schillaciv G, Bldrini F, Guerrieri M, Porcellati C. Sex, cardiac hypertrophy and diurnal blood pressure variations in essential hypertension. J Hypertens. 1992;10:683–692. [PubMed] [Google Scholar]
- 25.Timio M, Vnanzi S, Lolli S, Lippi G, Verdura C, Monarca C, Guerrini E. Non-dipper hypertensive patients and progressive renal insufficiency: a 3-year longitudinal study. Clin Nephrol. 1995;43:382–387. [PubMed] [Google Scholar]
- 26.Daly JW. Mechanisms of action of caffeine. In: Garattini S, editor. Caffeine, coffee and health. New York, NY: Raven; 1993. pp. 97–150. [Google Scholar]
- 27.Ramkumar V, Bumgarner JR, Jacobson KA, Stiles GL. Multiple components of the A1 adenosine receptor-adenylate cyclase system are regulated in rat cerebral cortex by chronic caffeine ingestion. J Clin Invest. 1988;82:242–247. doi: 10.1172/JCI113577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lovallo WR, al'Absi M, Pincomb GA, Everson SA, Sung BH, Passey RB, Wilson MF. Caffeine and behavioral stress effects on blood pressure in borderline hypertensive Caucasian men. Health Psychol 15. 1996;15:11–17. doi: 10.1037//0278-6133.15.1.11. [DOI] [PubMed] [Google Scholar]
- 29.Sung BH, Lovallo WR, Whitsett T, Wilson MF. Caffeine elevates blood pressure response to exercise in mild hypertensive men. Am J Hypertens. 1995;8(Pt 1):1184–1188. doi: 10.1016/0895-7061(95)00331-2. [DOI] [PubMed] [Google Scholar]
- 30.Smits P, Hoffmann H, Thien T, Huben H, Van't Laar A. Hemodynamic and humoral effects of coffee after β1-selective and nonselective β-blockade. Clin Pharmacol Ther. 1983;34:153–158. doi: 10.1038/clpt.1983.145. [DOI] [PubMed] [Google Scholar]
- 31.Hypertension Detection and Follow-up Program Cooperative Group. Five-year findings of the hypertension detection and follow-up program: I. Reduction in mortality of persons with high blood pressure, including mild hypertension. JAMA. 1979;242:2562–2571. [PubMed] [Google Scholar]