Abstract
Introduction
There is widespread belief that caffeine consumption is linked to atrial arrhythmias; however, there is a relative lack of systematic evidence to support the assertion. The purpose of this study was to investigate whether caffeine, in doses equivalent to daily use in the general population, alter the propensity for atrial fibrillation (AF) in an experimental model comparing normal and simulated predisposition to AF.
Methods and Material
Caffeine (caffeine Na benzoate, 50:50 mixture) was administered intravenously at 1, 3, and 5 mg/kg doses in dogs producing serum levels of 2 to 4, 5 to 7, and 8 to 10 μg/mL. To simulate focal AF, premature stimulation from the right superior pulmonary vein was delivered at 2×, 4×, and 10× threshold at a rate of 180/min (S1-S2 = 330 milliseconds) without and then with low-level stimulation of ganglionated plexi (GP) at the entrance of the right superior pulmonary vein. The window of vulnerability (WOV), a measure of the propensity for AF inducibility, was determined by the longest coupling interval of the premature beat (S1-S2) minus the shortest S1-S2, which induced AF. The cumulative WOV is the sum of the individually determined WOV.
Results
At each serum level of caffeine, the cumulative WOV was lower without rather than with GP stimulation compared with control. The cumulative WOV for both the stimulated, that is, predisposed to AF, and nonstimulated, that is, normal groups, exhibited a significantly lower average as compared with that exhibited by the control group (P ≤ .003-.02).
Conclusion
These findings suggest that the presence of caffeine may result in an unexpected reduction in the propensity for AF in healthy individuals and in those with a predisposition for AF (enhanced AF inducibility caused by the stimulation of the GP).
Keywords: Caffeine, Atrial fibrillation, Predisposition
Introduction
Coffee, tea, and other caffeine-containing beverages have been among the most popular for hundreds of years. Ninety percent of the adult population acknowledges drinking caffeinated beverages. Americans alone consume more than 109 kg of caffeine per year.1 The use of caffeine for its various therapeutic qualities (atopic dermatitis, anorexic affects, treatment of neonatal apnea, arousal, and others) has made caffeine the most widely used drug in the Western hemisphere. Although caffeine toxicity has been clinically designated to occur at serum levels of 25 mg/L, various studies over the past 90 years have linked caffeine to an array of insidious effects at much lower serum levels.2 Various studies have associated caffeine use with anxiety disorders, fibrocystic breast disease, birth defects, gastrointestinal maladies, various forms of cancer, and cardiac arrhythmias, as well as numerous other ailments.3 Despite widespread belief among physicians and the general public alike that caffeine consumption leads to the induction of cardiac arrhythmias, until relatively recently, there was a deficiency of scientific evidence supporting such perceptions.
The purpose of our study was to determine the association between daily caffeine consumed and the risk for atrial fibrillation (AF) in an animal model, which, under controlled circumstances, shows a low propensity for the AF but, with local cardiac autonomic nerve stimulation, demonstrates a significantly greater disposition for AF inducibility.4 Thus, the former state can be considered as the normal response and the latter represents a surrogate for the response of an individual predisposed for paroxysmal AF.5
Materials and methods
Seven adult mongrel dogs were used as experimental subjects for this study. All dogs weighed between 20 and 24 kg. The dogs were initially anesthetized using 30 mg/kg of sodium pentobarbital delivered intravenously. Additional anesthesia was administered as needed throughout the experimental study. Animals were ventilated with room air (Harvard Apparatus Co, Holliston, Mass) and maintained at a steady temperature (36.5°C ± 1.5°C) by placing a heating pad under the dog's chest and abdomen. Femoral arteries and veins were exposed in both legs and were used as portals for various recording catheters (His bundle and right atrial electrogram), a cannula-connected pressure transducer, intravenous saline drip, and a thermistor catheter to monitor core temperature. Electrocardiographic (ECG) leads II and aVR and blood pressure were continuously monitored.
A right-sided thoracotomy was performed at the fourth intercostal space to expose the right lung. The right lung was reflected, and moist gauze pads measuring 4 × 4 were placed over the lung to prevent excessive drying. A small perforation was made in the pleura surrounding the right superior pulmonary vein (RSPV) to properly mount an octapolar electrode catheter against the vein using 3-0 sutures. This catheter was connected to a Medtronic 5328 programmable stimulator (Medtronic, Minneapolis, Minn) to provide pacing and induction of programmed electric stimulation. A pericardiotomy was performed to expose the right atrium and base of the right ventricle. An acrylic plaque electrode with bipolar pairs of electrodes was sutured onto the fat pad (at the base of RSPV) to provide a reproducible means of stimulating the ganglionated plexi (GP) that lay within the fat pad.6
Electric stimulation (ranging between 0.6 and 3.2 V) was delivered to the GP using a Grass stimulator (stimulation frequency, 20 Hz; pulse duration, 0.1 milliseconds; voltage, 0.6-3.2 V; Grass stimulator S-88; Astro Med Inc, West Warwick, RI) to ensure that incremental electric stimulation progressively slowed the heart rate without atrial excitation. The ECG, blood pressure, heart rate, His bundle electrogram, and right atrial electrograms were registered throughout the study using a computerized recording system (CR Bard Inc, Billerica, Mass).
Initially, a blood sample was obtained to determine baseline levels of caffeine. Programmed electrical stimulation was applied at the distal electrode pair at the RSPV, which consisted of 8 atrial paced beats at a cycle length of 330 milliseconds (S1-S2 = 330 milliseconds) followed by a premature stimulus (S1-S2 = 150 milliseconds). S1-S2 was progressively decremented to determine the refractory period. In addition, this pacing algorithm was repeated at 2×, 4×, and 10× the diastolic threshold. The window of vulnerability (WOV) for AF was determined at each threshold level by the subtracting the shortest S1-S2 that induced AF from the longest S1-S2 that induced AF. The cumulative WOV was measured as the sum of the individual WOVs determined at threshold level.
This process of determining the WOV was repeated without stimulation of the GP at the different threshold intensities. After this process, we repeated the procedure but with stimulation of the GP at the level that slowed the heart to lower than 100 beats per minute (normal heart rate under pentobarbital anesthesia ranged from 130 to 150/min). For the first experimental trial in each dog, 1 mg/kg of caffeine (caffeine Na benzoate, 50:50 mixture) was administered intravenously. After 2 minutes, an arterial blood sample was taken. The same procedures that were used in the control trials were used for each experimental trial. In some of the 7 animals, multiples of a given dose were administered. After completing 1 experimental trial, the next dose of caffeine was administered until each of the experimental trials was completed. Blood samples were taken within 2 minutes after each dose of caffeine was given. Upon completion of the control and experimental procedures, an additional maintenance dose of sodium pentobarbital was administered. The animals were then euthanized by inducing ventricular fibrillation with a 9-V battery applied to the right ventricle. Fibrillation was monitored for 10 minutes to ensure a terminal state.
Guidelines for animal use
Studies were performed according to the regulations for humane care and treatment for animals established by the National Institutes of Health and were locally reviewed and approved by the Animal Studies Subcommittee and the Research and Development Committee of the Department of Veterans Affairs Medical Center, Oklahoma City, Okla, as well as the Institutional Animal Care and Use Committee of the University of Oklahoma Health Sciences Center.
Statistical analysis
The collected electrophysiologic data were analyzed using the Bard LabSystem software (Bard, Inc). Statistical analysis was performed using a standard Student t test for comparison of paired and unpaired data. A P value of .05 was considered significant.
Results
The data were organized and analyzed based on various parameters. The cumulative WOV was calculated and used to compare the experimental data obtained by electric stimulation of the ganglia with the nonstimulated or baseline state (Table 1, Fig. 1). The average WOV in the control state without GP stimulation was 162 ± 79 milliseconds. The average WOV in the control state with stimulation of the GP was significantly wider 271 ± 85 milliseconds (P < .05). The average WOV with serum caffeine levels between 2 and 4 μg/mL without stimulation was 104 ± 91 and 154 ± 114 milliseconds with ganglionated plexus stimulation compared with control values. Atrial fibrillation inducibility (with GP stimulation) was significantly reduced by caffeine at this serum concentration (P = .02). With each of the subsequent serum caffeine concentrations, there was a significant reduction in WOV values compared with control, both without and with GP stimulation (P < .05) (Table 1). In each of the experiments, with the exception of the trial in which the serum caffeine level was 11 to 20 μg/ml, electric stimulation of the GP located at the RSPV resulted in a significant decrease in the WOV (P ≤ .02, compared with control levels).
Table 1.
The effect of various concentrations of caffeine inducibility of AF
| Experiment | Control WOV
|
CAF, 2-4 μg/mL
|
CAF, 5-7 μg/mL
|
CAF, 8-10 μg/mL
|
CAF, 11-20 μg/mL
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| No STM | STM-GP | No STM | STM-GP | No STM | STM-GP | No STM | STM-GP | No STM | STM-GP | |
| 1 | 52 | 198 | 3 | 10 | 202 | 226 | 54 | 88 | 48 | 18 |
| 2 | 245 | 330 | 63 | 115 | 29 | 128 | 120 | 108 | 165 | 167 |
| 3 | 70 | 136 | 140 | 320 | 46 | 178 | 5 | 116 | 256 | 200 |
| 4 | 220 | 310 | 165 | 150 | 5 | 125 | 78 | 112 | 20 | 75 |
| 5 | 210 | 350 | 230 | 298 | 88 | 260 | 70 | 165 | ||
| 6 | 215 | 351 | 234 | 296 | 148 | 160 | 160 | 164 | ||
| 7 | 125 | 223 | 148 | 160 | 74 | 165 | 10 | |||
| 8 | 20 | 105 | 18 | 90 | ||||||
| 9 | 18 | 45 | ||||||||
| 10 | 15 | 45 | ||||||||
| AV ± SD | 162 ± 79 | 271 ± 85 | 104 ± 91 | 154 ± 114 | 76 ± 68 | 167 ± 55 | 71 ± 56 | 126 ± 32 | 122 ± 109 | 115 ± 84 |
| P | <.05 | .02 | .02 | .05 | .8 | |||||
| P | .2 | .03 | .04 | .02 | .03 | .003 | .5 | .02 | ||
CAF indicates caffeine; no STM, no stimulation; STM-GP, stimulation of ganglionic plexus; AV, average.
Fig. 1.
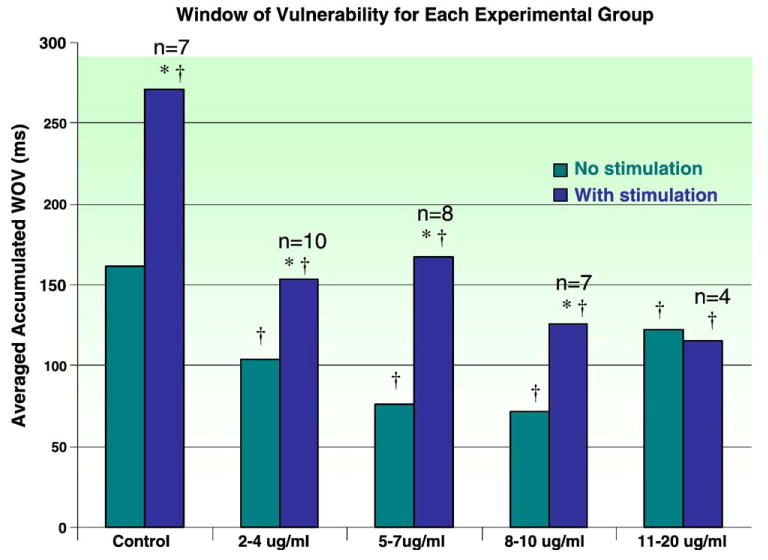
Bar graph presentation of the average cumulative WOV for each experimental group with different serum levels of caffeine compared with the control group. The first bar in each group represents the average WOV determined without GP stimulation; the second bar depicts the average WOV due to premature depolarization from the PV with concomitant stimulation of the GP. Statistical comparison were made within groups (*P < .05, stimulation compared with no stimulation) as well as between each experimental group compared with the control group (†P < .05, compared with control).
Discussion
We hypothesized that caffeine, in doses equivalent to those seen with human daily consumption, would increase the prevalence of AF in this animal model. Scherlag et al4 developed an experimental model that simulates patients predisposed to AF. By electrically stimulating the autonomic GP located within a fat pad at the base of RSPV, the experimental model consistently induced a greater propensity for AF compared with that determined without GP stimulation. In this context, the effects of caffeine were tested in the control state, that is, simulating healthy patients and during the GP stimulation, that is, simulating the patients predisposed to AF. This propensity for AF was quantitated by comparing the average cumulative WOV when premature stimuli were delivered at the RSPV, without and with concomitant GP stimulation. The latter was consistently and significantly greater than the WOV when the GP were not activated electrically. Thus, this model simulates the clinical situation of focal pulmonary vein ectopy, which has been shown to be responsible for paroxysmal AF in most patients.6,7 The GP at the RSPV is 1 of 7 GP, 3 in the ventricles and 4 in the atria, found within the canine intrinsic cardiac nervous system.8 These ganglia, located within fat pads on the epicardium, function as an integrative system for parasympathetic efferent, sympathetic efferent, interneurons, mechanosensitve, and chemosensitive afferent neuron function.9 With synapses from extracardiac neurons, the intrinsic cardiac ganglia function together to mediate various cardiac properties. By incremental electric stimulation of these ganglia (specifically the GP at the base of the RSPV), the response to premature beats is altered; this results in the aforementioned predisposition for AF.
Stimulation of the GP before caffeine administration induced AF with a significantly larger WOV showing that the heart was more susceptible to AF, which confirms previous findings by Scherlag et al.4
The WOVs for each of the stimulated and nonstimulated conditions of the experimental groups subject to caffeine (2-4, 5-7, 8-10, and 11-20 μg/mL) exhibited a significantly lower averaged WOV as compared with that exhibited by the control group (P < .003-.02). The decrease in WOV is present regardless of whether the ganglionic plexus was stimulated or not, suggesting that the antiarrhythmic effect may occur in both healthy individuals and those with a marked predisposition for AF. The presence of an antiarrhythmic effect refutes the hypothesis that caffeine would increase the propensity for induction of AF, for the focal form of AF, that is, arising from the pulmonary veins and using autonomic mechanisms as a substrate. Although the hypothesis appeared to be a logical conjecture based on past studies, the method of determining the effects of caffeine on the arrhythmogenicity and analysis techniques may be responsible for the unexpected results. This study provides a unique approach to investigating the effect of caffeine on cardiac arrhythmias by refining the method of detecting the arrhythmogenic potential. The antiarrhythmic effect of caffeine seen in this study appears to contradict some of the preexisting data. Most previous studies investigating the arrhythmogenicity of caffeine, examiners simply calculated the prevalence or frequency of various arrhythmias in response to different doses of caffeine. Mehta et al1 described administering caffeine to normal dogs at 3 dose levels and recording the arrhythmogenic effect by analyzing a 20-minute ECG recording and tallying any abnormal rhythms. These studies did not use the electrophysiologic method of determining the propensity for AF or other arrhythmias. The study by the Mehta et al1 showed that only the highest dose of caffeine induced atrial and ventricular tachycardia. These doses were not based on the average or maximal consumption of caffeine within the general population.
The mechanism behind the potential antiarrhythmic effect is unknown. Caffeine has been shown to increase intracellular calcium levels, increase renin activity, increase plasma catecholamines, increase adenylate cyclase and cyclic adenosine monophosphate levels, and inhibit adenosine A1 and A2 receptors. The A1 and A2 adenosine receptors are the most likely mediators of the protective effect exhibited upon administration of caffeine. A report by Grant10 postulates that reentrant AF may be impeded by prolongation of the atrial refractory period induced by the selective A1 receptors at the atrial myocardium and atrioventricular node. In a study by Brandts et al,11 selective blockade of the adenosine A1 receptor was shown to result in a decrease in AF propensity. The authors attribute this to the inhibition of the negative chronotropic and reduced atrial refractory period that is seen with adenosine A1 activation. These effects of adenosine A1 receptor blockade suggest that caffeine cannot only inhibit the onset of AF but can also impede its propagation throughout the atria via increased refractoriness.
A recently published article by Frost and Vestergaard12 showed that the consumption of caffeine is not associated with increase incidence of AF. Study population consisted of 50 000 middle-aged people with follow-up for about 6 years. During this time, only 555 people developed AF or flutter, but analysis of risk by fifth of the distribution of caffeine intake did not revealed any hint of a trend.
Limitations
The present model for AF inducibility is one, which simulates the focal form of AF related to a distinct dysautonomia, that is, hyperactivity of intrinsic autonomic ganglia on the heart.4 Another form of AF is based on multiple or macroentrant circuit of electrical activation, which can initiate and maintain AF13-15 rather than a focal mechanism.6 The response of the macroreentrant form of AF to caffeine has not been tested in the present study.
This study did not examine common stress–related effects that could interact with caffeine in arrhythmia prone persons. During periods of emotional distress, vagal outflow to the heart decreases, whereas sympathetic output may increase. In addition, distress is often associated with increasing output of epinephrine. These stress-related physiological changes are potentiated in the presence of caffeine16 and may be destabilizing to an arrhythmia-prone myocardium. At present, it is unknown whether the doses of caffeine tested here would exert proarrhythmic or antiarrhythmic effects under these circumstances. Future studies may effectively explore these conditions to increase our base of knowledge about caffeine's effects in patients subject to arrhythmias who are undergoing acute episodes of severe emotional disturbance.
Conclusions
Contrary to the prevailing views, this study suggests that intravenously administered caffeine results in a reduced propensity for AF in normal hearts and those artificially predisposed to focal forms of this arrhythmia. Blockade of the adenosine A1 receptors is a likely mechanism for producing the observed affect; however, more testing must be completed to elucidate the precise nature of this association.
Acknowledgments
This study was supported by the Medical Research Service of the Department of Veterans Affairs, Washington, DC, and by Grant HL 32050 from the National Heart, Lung and Blood Institute, Bethesda, Md.
We thank Andrea Moseley, Cameron Hogan, and Dr Gopireddy for their assistance and expertise throughout the project tenure.
References
- 1.Mehta A, Jain AC, Mehta MC, et al. Caffeine and cardiac arrhythmias: an experimental study in dogs with review of literature. Acta Cardiol. 1997;52:273. [PubMed] [Google Scholar]
- 2.Cannon ME, Cooke CT, McCarthy JS. Caffeine induced cardiac arrhythmia: an unrecognized danger of health food products. Med J. 2001;174:520. doi: 10.5694/j.1326-5377.2001.tb143404.x. [DOI] [PubMed] [Google Scholar]
- 3.Curatolo PW, Robertson D. The health consequences of caffeine. An Intern Med. 1983;98:641. doi: 10.7326/0003-4819-98-5-641. [DOI] [PubMed] [Google Scholar]
- 4.Scherlag BJ, Yamanashi W, Patel U, et al. Autonomically induced conversion of pulmonary vein focal firing into atrial fibrillation. J Am Coll Cardiol. 2005;45:1878. doi: 10.1016/j.jacc.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 5.Scherlag BJ, Nakagawa H, Jackman WM, et al. Electrical stimulation to identify neural elements in the heart: their role in atrial fibrillation. J Interv Card Electrophysiol. 2005;13:37. doi: 10.1007/s10840-005-2492-2. [DOI] [PubMed] [Google Scholar]
- 6.Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;95:7. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 7.Pappone C, Santinelli V, Manguso F, et al. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation. 2004;109:327. doi: 10.1161/01.CIR.0000112641.16340.C7. [DOI] [PubMed] [Google Scholar]
- 8.Armour JA. Intrinsic cardiac neurons. J Cardiovasc Electrophysiol. 1991;2:331. [Google Scholar]
- 9.Ardell JL. Neurocardiology. Oxford Press; 1994. Structure and function of mammalian cardiac neurons; p. 95. [Google Scholar]
- 10.Grant AO. Mechanisms of atrial fibrillation and action of drugs in its management. Am J Cardiol. 1998;82:43N. doi: 10.1016/s0002-9149(98)00585-2. [DOI] [PubMed] [Google Scholar]
- 11.Brandts B, Borchard R, Dirkmann D, et al. Diadenosine-5-phosphate exerts A1-receptor–mediated proarrhythmic effects in rabbit atrial myocardium. Br J Pharmacol. 2003;139:1265. doi: 10.1038/sj.bjp.0705361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frost L, Vestergaard P. Caffeine and risk of atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Clin Nutr. 2005;81:578. doi: 10.1093/ajcn/81.3.578. [DOI] [PubMed] [Google Scholar]
- 13.Moe GK, Rheinboldt WC, Abildskov JA. A computer model of atrial fibrillation. Am Heart J. 1964;67:200. doi: 10.1016/0002-8703(64)90371-0. [DOI] [PubMed] [Google Scholar]
- 14.Allessie MA, Lammers WJEP, Bonke FIM, et al. Experimental evaluation of Moe's multiple wavelet hypothesis of atrial fibrillation. chap 30 Orlando: Grune & Stratton; 1985. [Google Scholar]
- 15.Li D, Benardeau A, Nattel S. Contrasting efficacy of dofetilide in differing experimental model of atrial fibrillation. Circulation. 2000;102:104. doi: 10.1161/01.cir.102.1.104. [DOI] [PubMed] [Google Scholar]
- 16.Lovallo WR, Wilson MF, Vincent AS, et al. Blood pressure response to caffeine shows incomplete tolerance after short-term regular consumption. Hypertension. 2004;43:760. doi: 10.1161/01.HYP.0000120965.63962.93. [DOI] [PubMed] [Google Scholar]


