Abstract
Objective
Caffeine increases cortisol secretion in people at rest or undergoing mental stress. It is not known whether tolerance develops in this response with daily intake of caffeine in the diet. We therefore tested the cortisol response to caffeine challenge after controlled levels of caffeine intake.
Methods
Men (N = 48) and women (N = 48) completed a double-blind, crossover trial conducted over 4 weeks. On each week, subjects abstained for 5 days from dietary caffeine and instead took capsules totaling 0 mg, 300 mg, and 600 mg/day in 3 divided doses. On day 6, they took capsules with either 0 mg or 250 mg at 9:00 AM, 1:00 PM, and 6:00 PM, and cortisol was sampled from saliva collected at 8 times from 7:30 AM to 7:00 PM.
Results
After 5 days of caffeine abstinence, caffeine challenge doses caused a robust increase in cortisol across the test day (p < .0001). In contrast, 5 days of caffeine intake at 300 mg/day and 600 mg/day abolished the cortisol response to the initial 9:00 AM caffeine dose, although cortisol levels were again elevated between 1:00 PM and 7:00 PM (p = .02 to .002) after the second caffeine dose taken at 1:00 PM. Cortisol levels declined to control levels during the evening sampling period.
Conclusion
Cortisol responses to caffeine are reduced, but not eliminated, in healthy young men and women who consume caffeine on a daily basis.
Keywords: caffeine, cortisol, men, women, tolerance
Introduction
Caffeine is a widely consumed pharmacologic agent found in coffee, tea, and soft drinks (1). Its popularity is attributable to its effects in the nervous system (2), including its ability to increase rates of dopamine release in the anterior cingulate gyrus (3). Caffeine also activates the stress axis, elevating glucocorticoid and catecholamine, output along with increases in blood pressure (4). As such, caffeine intake during times of stress may contribute to the duration and magnitude of blood pressure and stress endocrine responses (5,6). However, in assessing caffeine's contribution to stress reactions during daily life, we have asked whether caffeine's effects are reduced by pharmacologic tolerance in proportion to a person's level of daily consumption (7,8). This paper addresses the effect of tolerance formation on cortisol responses to repeated caffeine doses in people consuming varying amounts of caffeine over several days before testing.
Cortisol is secreted in a diurnal pattern, with a peak around the time of awakening, a declining course across the waking hours, and a nadir seen during the early phases of sleep (9,10). This diurnal cycle is important in maintaining optimal bodily function: the nadir contributes to memory consolidation during sleep (11). Cortisol helps regulate energy balance (12). It maintains normal autonomic function by regulating adrenoreceptor synthesis and maintains receptor sensitivity (13). Perturbations in cortisol's diurnal secretion pattern therefore have deleterious consequences if sustained for prolonged periods (14). Caffeine in dietary doses increases both adrenocorticotropin (ACTH) and cortisol secretion in humans (15). Caffeine's effect on glucocorticoid regulation therefore has the potential to alter circadian rhythms and to interact with stress reactions. By extension, these cortisol alterations may have implications for health if maintained in the face of daily caffeine intake.
It is generally assumed that caffeine consumed in the diet leads to development of tolerance to its effects, with a consequent elimination of any potential untoward consequences (16). However, others and we have shown that daily caffeine intake does not abolish the blood pressure response to caffeine on test days (8,17). We accordingly conducted a randomized trial of the effect of caffeine intake on acute physiological responses to caffeine (8). We report here the effect of 3 levels of caffeine intake on cortisol responses to caffeine challenges. Daily maintenance doses were chosen to mimic a range of consumption commonly found in the US diet, from none (0 mg/day), to moderate (300 mg/day, approximately 3 cups of brewed coffee), to high (600 mg/day, about 6 cups per day) levels of intake (18). Weekly testing then determined the resulting changes in cortisol response to caffeine across the day.
Methods
Subjects
Subjects were 98 healthy adults recruited through advertisement from the general populations of Buffalo, NY, and Oklahoma City, OK, as described in Table 1. All volunteers were nonobese and in good health by self-report and routine physical examination. They had screening blood pressure <135/85 mm Hg, consumed 50 to 700 mg/day of caffeine by structured report, were nonsmokers, and used no medications having cardiovascular or metabolic effects. Women were free from oral contraceptives and were not pregnant, as determined by urine pregnancy test (One Step Pregnancy Test, Inverness Medical Ltd., Beachwood Park, North Inverness, Scotland). Half of the volunteers were tested in Buffalo NY, and half in Oklahoma City, OK. All participants signed a consent form approved by the institutional review board of the University of Oklahoma Health Sciences Center and the Veterans Affairs Medical Center in Oklahoma City and SUNY Buffalo, Buffalo, NY, and were paid for participating.
TABLE 1. Subject Characteristics.
| Total Group (n = 96) | Males (n = 48) | Females (n = 48) | t (p) | |
|---|---|---|---|---|
| Age (yr) | 28 (0.6) | 27 (0.8) | 30 (0.9) | 2.3 (0.02) |
| Weight (lb) | 159 (2.7) | 176 (3.7) | 143 (2.3) | 7.6 (0.001) |
| Height (in) | 68 (0.4) | 71 (0.4) | 65 (0.3) | 11.9 (0.001) |
| Body fat (%) | 20 (0.7) | 16 (0.8) | 23 (0.8) | 7.1 (0.001) |
| BMI (kg/m2) | 24 (0.3) | 24 (0.4) | 24 (0.4) | NS |
| Caffeine intake (mg/d) | 458 (47.9) | 451 (71.4) | 464 (64.9) | NS |
| Screening BP (mmHg) | ||||
| Systolic | 112 (1.0) | 118 (1.1) | 107 (1.1) | 6.8 (0.001) |
| Diastolic | 66 (0.6) | 66 (0.9) | 66 (0.9) | NS |
| Heart rate | 69 (1.1) | 68 (1.4) | 71 (1.6) | NS |
Entries show (mean, standard error). Males and females were compared using Student's t test. Caffeine intake reflects usual consumption from all sources based on structured interview. Screening blood pressure is average of three readings over 5 min after 5 min seated.
BMI = body mass index; BP = blood pressure; NS = not significant.
Study Design, Caffeine Dosing, and Compliance
The study design was a randomized, placebo-controlled, double-blind, 4-week crossover trial of acute caffeine effects in relation to differing levels of daily intake. Each study week included 5 days of home self-administration of placebo (P = 0 mg/day) or caffeine (C = 300 mg/day or 600 mg/day), followed by 1 test day (3 × 250 mg = 750 mg), and 1 crossover day to buffer sudden changes in intake between study weeks (C = 100 mg, 0 mg, and 0 mg). Weekly maintenance and lab dose combinations are shown in Table 2. The development of tolerance was indexed by a decreasing response to the repeated challenge doses as a function of prior daily intake level.
TABLE 2. Placebo and Caffeine Doses on Study Weeks.
| Study Week | Maintenance Days | Protocol Days |
|---|---|---|
| P-P | 0 mg (3 × 0 mg) | 0 mg (3 × 0 mg) |
| P-C | 0 mg (3 × 0 mg) | 750 mg (3 × 250 mg) |
| C300-C | 300 mg (3 × 100 mg) | 750 mg (3 × 250 mg) |
| C600-C | 600 mg (3 × 200 mg) | 750 mg (3 × 250 mg) |
Week order was randomized across subjects.
P = placebo, C = caffeine; C300 = 300 mg/day maintenance; C600 = 600 mg/day maintenance.
Home maintenance doses were supplied in bottles of identical gelatin capsules (College of Pharmacy, University of Oklahoma, Oklahoma City, OK) containing either lactose or lactose mixed with 100 mg or 200 mg of USP caffeine (Gallipot, St. Paul, MN). Subjects were instructed to take one capsule at 8:00 AM, 1:00 PM, and 6:00 PM each day. Test-day challenge doses were supplied in capsules containing either lactose or lactose mixed with 250 mg of caffeine, administered at 9:00 AM, 1:00 PM, and 6:00 PM.
Compliance was assessed by capsule counts in bottles returned on laboratory days, by caffeine assay of saliva specimens collected at home each day at 7:00 PM (19), and from saliva specimens collected each morning on entering the laboratory. Subjects found to be noncompliant by any of these criteria were dropped and replaced. To avoid significant withdrawal effects when subjects stopped their usual caffeine intake, all subjects started the 4-week protocol with a 3-day tapering and washout period with stepped-down caffeine doses (200 mg on day 1, 100 mg on day 2, and 0 mg on day 3). Subjects completed a daily self-report instrument designed to assess withdrawal symptoms and mood states. None of the subjects complained of severe withdrawal, although there were some reports of headache. We controlled for reported habitual daily intake in preliminary analyses and found no effect on the results.
Laboratory and Ambulatory Protocol
The protocol included arrival at the laboratory at 7:30 AM, breakfast and instrumentation, predrug baseline 1, P or C capsule, postdrug response, stress challenge, stress recovery, lunch break, predrug baseline 2, P or C capsule, postdrug response, followed by the ambulatory phase with a final P or C capsule. The stress testing and ambulatory periods involved cardiovascular measurements, as reported elsewhere, and collection of saliva for measurement of cortisol as reported here. The stressors varied by site, with mental testing done in Oklahoma City and exercise in Buffalo. Although the specific pattern of acute cortisol response differed to the 2 types of stressors, preliminary analyses indicated that the effect of caffeine on the daily secretion of cortisol was comparable across sites. The acute stress effects will be reported elsewhere. On the test days, caffeine challenge doses of 250 mg or placebo were taken 9:00 AM, 1:00 PM, and 6:00 PM, for total daily doses of 750 mg versus 0 mg.
Saliva was collected using a commercial device (Salivette, Sarstedt, Newton, NC) at the following 8 times: 7:30 AM on arrival at the laboratory, 9:00 AM after the predrug baseline, 10:30 AM at the end of stress testing, 11:10 AM after stress recovery, 1:00 PM after predrug baseline 2, 2:00 PM after postdrug response 2, at home at 6:00 PM before the third capsule, and again at 7:00 PM. Specimens were stored at −70°F until assayed. Caffeine concentrations were measured after precipitation of proteins by high-performance liquid chromatography using a methanol and water mobile phase and ultraviolet detection (Waters Corp., Milford, MA). Cortisol concentrations were quantified using a commercial kit (Orion Diagnostica, Espoo, Finland) adapted to the low concentrations of free cortisol found in saliva. Specimens were mixed with a fixed amount of 125I-labeled cortisol derivative and cortisol antiserum. Competitive binding of labeled cortisol was then assessed using liquid scintillation and comparison to a standard curve. The respective intra- and interassay coefficients of variation were 6% and 12%.
The caffeine values in saliva were tested in an analysis of variance (ANOVA) model including 4 weeks (PP, PC, C300 and C600) × 8 periods (7:30 AM, 9:00 AM, 10:30 AM, 11:10 AM, 1:00 PM, 2:00 PM, 6:00 PM, 7:00 PM) and the interaction. The analysis of cortisol evaluated whether increasing levels of daily caffeine consumption (0 mg/day, 300 mg/day, or 600 mg/day) in the preceding 5 days would reduce or eliminate the cortisol responses to the 3 × 250-mg challenge doses on test days. Results were analyzed by Student's t test, χ2 test, and ANOVA using SAS (SAS System for Windows, ver. 8.2, SAS Institute Inc., Cary, NC) and SPSS (SPSS for Windows, rel. 10.1.0, SPSS, Inc., Chicago, IL). Potential violation of the sphericity assumption in univariate repeated-measures ANOVA was avoided using a multivariate solution for repeated measures factors. For pair wise comparisons, family-wise error rate was controlled across tests at the 0.05 level using Holm's sequential Bonferroni procedures.
Men and women did not differ in their patterns of tolerance formation, and so data are presented only for the combined sample. In preliminary tests on the model, covarying for reported habitual caffeine intake had no effect on the results of the ANOVA, and so this variable was not considered further. A small number of saliva specimens were missing, constituting 2.2% (69 of 3104) of the total. These were replaced by the mean value of all other samples at that time point. This procedure introduces minimum bias while retaining subjects for the full analysis.
Results
The subject demographics and comparison of male and female participants are shown in Table 1. The females were significantly older than the males. The males were heavier and taller than the females, lower in body fat, and they had higher systolic blood pressure at screening. Correlations were computed of acute cortisol response to the morning caffeine dose with these variables. There was no relationship between resting systolic blood pressure and cortisol response (r = 0.045, p = .67). However, there were significant relationships between cortisol response and age (r = 0.212, p = .038) and percent body fat (r = 0.274, p = .006). Further analysis indicated that the greater percent body fat in older subjects explained the relationship of cortisol response with age. The primary cortisol analysis was redone using body fat as a covariate, and there were no changes from the unadjusted results, which are presented here.
Figure 1 shows the caffeine concentrations in saliva on each of the 4 test days. On the PP week, caffeine levels were at or near 0 μg/ml, indicating good compliance with dietary restrictions and accuracy of dosing on that week. These values were higher on caffeine weeks than on the PP week (Week × Periods, F(21,74) = 46, p < .0001), with the highest values on the C600 week and lower values on C300 and PC weeks, respectively (main effect of Week, F (3,92) = 206, p < .0001). These levels were greater in the afternoon and evening, after the 1:00 PM and 6:00 PM doses, as reflected in a significant Periods effect (F(7,88) = 133, p < .0001). The higher caffeine levels on the C600 week are consistent with the higher daily doses in the preceding 5 days and small residual levels on entering the laboratory for testing. The analysis was rerun taking these residual caffeine levels into account with no change in results.
Figure 1.
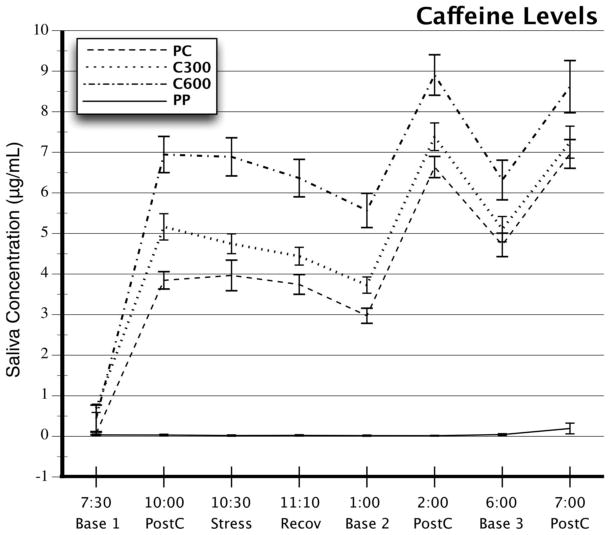
Caffeine concentrations in saliva on 4 test days. Entries show means, and error bars represent standard errors. Samples from 7:30 AM to 2:00 PM were collected in the laboratory, and the samples at 6:00 and 7:00 PM were collected at home. PC = placebo maintenance followed by 3 × 250-mg caffeine challenges on the test day. C300 = 300 mg/day of caffeine at home followed by caffeine challenge on the test day. C600 = 600 mg/day at home followed by caffeine challenge on the test day. PP = placebo at home and placebo on test day. Base 1, Base 2, Base 3 = saliva samples taken immediately before taking a caffeine or placebo capsule. PostC = samples taken 1 hour postdrug. Stress and Recov = samples taken at the end of a 30-minute behavioral stress period or after 30 minutes of recovery.
Cortisol values varied significantly as a function of caffeine administration (Week, F(3,93) = 19.4, p < .0001), the time of day (Period, F(7,88) = 56.4, p < .0001), and the level of daily caffeine intake before testing (Week × Period, F(21,74) = 2.39, p = .003). Morning cortisol values at 7:30 AM varied slightly with the level of prior daily intake, but the difference at that time point was not significant, and the primary results did not change when the ANOVA was rerun using 7:30 AM cortisol as a covariate. Contrasts of each caffeine week against the mean values on the PP week showed that cortisol values were higher on the PC (p < .001) and C300 weeks (p = .002) but not on the C600 week (p < .113).
Based on our planned comparisons of the effects of caffeine intake on cortisol responses, we tested contrasts of caffeine weeks against the PP week control value at each time point, using multivariate correction for multiple tests. Figure 2 shows p values at each time point. From top to bottom, these reflect the respective contrasts of PC, C300, and C600 versus the PP cortisol value. On the PC week, caffeine challenge after prior abstinence resulted in significantly higher cortisol values at all times during the day. In contrast, the cortisol values on the C300 and C600 days were not significantly higher than the PP week values until the afternoon hours at 1:00 and 2:00 PM. At 6:00 PM, only the C300 week values were still significantly above the PP level, and by 7:00 PM, this comparison was also nonsignificant. This pattern is consistent with an incomplete tolerance formation after 5 days of moderate caffeine intake on the C300 week and a more complete tolerance after high-level intake on the C600 week.
Figure 2.
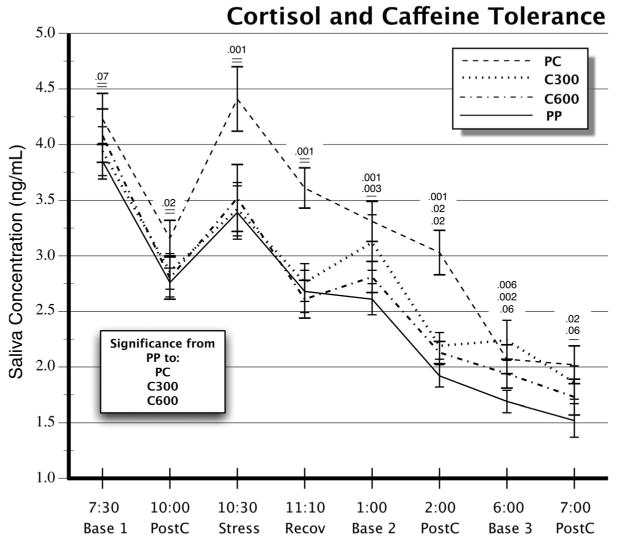
Cortisol concentrations in saliva on 4 test days. Entries show means and standard errors. Abbreviations, sampling times, and designations as in Figure 1. Numbers above error bars refer from top to bottom to p values of PC, C300, and C600 week contrasts against the PP week control value.
Discussion
The data presented here were developed as part of a larger project to establish the extent to which daily consumption of caffeine would diminish its physiological effects. Other findings from this study have been published elsewhere (8,20,21). The present study examined the effect of moderate to high levels of short-term daily caffeine intake on the cortisol response to repeated challenge doses on test days. We predicted that as tolerance to caffeine developed with regular daily intake, the cortisol values on test days would approximate those on the PP control week. In contrast, if tolerance were absent, the cortisol values on the C300 and C600 maintenance weeks would remain closer to the levels seen on the PC week. The results depict an intermediate outcome. Five days of moderate caffeine intake, at 300 mg/day, resulted in incomplete tolerance formation. Challenge doses given in the morning and afternoon caused a significant elevation of cortisol at 1:00 PM, lasting for approximately 6 hours. In contrast, a high daily dose of 600 mg produced a more complete tolerance to caffeine effects on the hypothalamic-pituitary-adrenocortical axis (HPAC), with cortisol rising above the PP week control level only for the hours immediately after the 1:00 PM caffeine dose.
Most investigators agree that in dietary amounts (<10–15 mg/kg/day), caffeine exerts its physiological effects via partial blockade of the A1 and A2a members of the adenosine receptor family (22). There is evidence that caffeine counteracts soporific effects of adenosine in the central nervous system to increase alertness, elevate mood, and perhaps to increase HPAC activation (23). Caffeine acts to increase cortisol secretion by elevating production of ACTH at the pituitary (15), although the precise mechanisms remain to be characterized.
Cortisol secretion is regulated by the HPAC, such that neurosecretory cells in the hypothalamic paraventricular nucleus release corticotropin releasing factor into the pituitary stalk, causing ACTH release by the anterior pituitary, resulting in increased rates of adrenal cortisol production. This action is restrained in turn by negative feedback of cortisol at the pituitary, hypothalamus, and hippocampus (24). Chronic elevations of cortisol secretion may have implications for long-term health (25). Cortisol can alter immune-system responses (26). Its long-term elevation is associated with depression (27) and altered central nervous system responsiveness (28), including sensitization of the limbic system (29), alterations in declarative memory (30), and alterations in frontal lobe function (31).
In practical terms, our results suggest that tolerance to caffeine's physiological effects is incomplete at the levels of intake seen in the US population, where reported consumption ranges from 250 to 300 mg/day. We have also reported that caffeine's ability to elevate blood pressure is not abolished in these same subjects even after consuming 600 mg/day at home (8). Taken together with the present findings, it appears that caffeine responses of the cardiovascular and pituitary adrenal systems are not abolished in people consuming caffeine on a daily basis. It is likely that an overnight abstinence, as used in this study, is partially sufficient to overcome tolerance formation in central nervous system adenosine receptors systems (32,33). This interpretation is consistent with the persistent mood and alertness effects of daily caffeine intake in most people. Generalizing from the present findings, statistically significant increases in cortisol secretion may occur during the afternoon hours in healthy consumers taking repeated doses of caffeine during the day. Repeated dosing mimics a common pattern of consumption for many consumers, and the doses are within the range of intake reported by most regular adult consumers. However, despite the maintenance of physiological responses to caffeine after overnight abstinence, the existence of caffeine dependence and withdrawal symptoms shows that there is also a partial tolerance formation that can vary substantially from person to person (7,34).
Other evidence, in light of the present results, indicates that the pattern of cortisol tolerance formation may vary as a function of hypertension risk (35,36). Hypertension is accompanied by enhanced responsivity of the HPAC (36,37), consistent with reports of greater numbers of hypothalamic corticotropin-releasing factor neurons in spontaneously hypertensive rats and in brain tissue taken from deceased hypertensive patients (38). The greater HPAC responses seen in hypertension risk groups are accompanied by greater cortisol responses to caffeine. Borderline hypertensives and those with a positive family history have more rapid and prolonged cortisol responses to caffeine than do low-risk persons (36).
Tolerance is defined in 2 ways, as the need for larger doses of a drug to accomplish the same effect or as a diminishing response to a fixed dose. The second approach to evaluating tolerance was used here. James (39) tested this same form of tolerance in a blood pressure study. Although James did not examine cortisol responses, it is noteworthy that the 2 studies are in agreement that the blood pressure response to caffeine is reduced but not eliminated by daily consumption.
The generality of the present results is limited by several factors. We tested only younger adults and did not examine tolerance formation in older adults. Also we used only a relatively brief, 5-day period of steady daily intake to establish tolerance levels. Although the 5-day run-in periods adequately manipulated the responsiveness of the HPAC and cardiovascular responses, longer periods of stable intake should be tested to further generalize findings to truly chronic levels of caffeine intake. We attempted to address the question of longer periods of caffeine intake on the results by preliminary analyses that included each subject's habitual caffeine intake level as a covariate in the analysis. Including this variable in the analysis had no effect on the pattern of cortisol response to acute challenge doses across the study weeks. The use of saliva obtained by the subjects to measure cortisol has the inherent drawback that sampling is limited to the waking hours and is absent during sleep. Therefore, this study does not allow us to examine the HPAC effects of caffeine tolerance during sleep. The well-known effects of caffeine on alertness and sleepiness suggest that this is a potentially important area for further study, given the possibility that caffeine intake could counteract the depth of slow-wave sleep in the early part of the sleep cycle, a time of minimal cortisol secretion now thought to be important for memory consolidation. The potentially larger and more prolonged effect of caffeine on cortisol secretion in borderline hypertensives suggests that caffeine's HPAC effects should be tested in persons at high risk of hypertension in order to fully explore the range of response variability and tolerance variation in the general population.
The present study suggests that daily caffeine intake causes a partial but not complete tolerance to caffeine's effects on cortisol secretion. The responsiveness of the HPAC was particularly evident in persons consuming moderate doses of 300 mg per day at home but was effectively abolished in those consuming higher doses. The potential for a persistent response to caffeine in consumers of moderate dietary doses suggests that an elevation of cortisol may occur in the afternoon hours in those consuming repeated doses throughout the day. This finding may have implications for health status in persons who are especially reactive to caffeine, such as persons developing hypertension.
Acknowledgments
Supported by NHLBI grant HL32050, NIRR grant M01-RR14467, and the Department of Veterans Affairs Medical Research Service.
Plasma cortisol assays were performed by M. L'Hermite-Baleriaux, Universite Libre de Bruxelles, Belgium, whom we thank for her dedicated efforts. We also thank our recently retired colleague, Richard M. Passey, PhD, who performed the caffeine assays. His collegial manner and selfless service are sorely missed.
- ANOVA
analysis of variance
- C
caffeine
- HPAC
hypothalamic-pituitary-adrenocortical axis
- P
placebo
- ACTH
adrenocorticotropin
References
- 1.Dews PB. Caffeine. Annu Rev Nutr. 1982;2:323–41. doi: 10.1146/annurev.nu.02.070182.001543. [DOI] [PubMed] [Google Scholar]
- 2.Nehlig A, Daval JL, Debry G. Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res Brain Res Rev. 1992;17:139–70. doi: 10.1016/0165-0173(92)90012-b. [DOI] [PubMed] [Google Scholar]
- 3.Daly JW, Fredholm BB. Caffeine: an atypical drug of dependence. Drug Alcohol Depend. 1998;5:199–206. doi: 10.1016/s0376-8716(98)00077-5. [DOI] [PubMed] [Google Scholar]
- 4.al'Absi M, Lovallo WR. Caffeine effects on the human stress axis. In: Nehlig A, editor. Coffee, Tea, Chocolate and the Brain. Boca Raton, FL: CRC Press LLC; 2004. pp. 113–31. [Google Scholar]
- 5.Pincomb GA, Lovallo WR, McKey BS, Sung BH, Passey RB, Everson SA, Wilson MF. Acute blood pressure elevations with caffeine in men with borderline systemic hypertension. Am J Cardiol. 1996;77:270–4. doi: 10.1016/s0002-9149(97)89392-7. [DOI] [PubMed] [Google Scholar]
- 6.Lovallo WR, al'Absi M, Pincomb GA. Caffeine raises blood pressure during extended mental stress in borderline hypertensive men. Int J Behav Med. 2000;7:183–8. doi: 10.1207/s15327558ijbm0203_5. [DOI] [PubMed] [Google Scholar]
- 7.Griffiths RR, Chausmer AL. Caffeine as a model drug of dependence: recent developments in understanding caffeine withdrawal, the caffeine dependence syndrome, and caffeine negative reinforcement. Nihon Shinkei Seishin Yakurigaku Zasshi. 2000;20:223–31. [PubMed] [Google Scholar]
- 8.Lovallo WR, Wilson MF, Vincent AS, Sung BH, McKey BS, Whitsett TL. Blood pressure response to caffeine shows incomplete tolerance after short-term regular consumption. Hypertension. 2004;43:760–5. doi: 10.1161/01.HYP.0000120965.63962.93. [DOI] [PubMed] [Google Scholar]
- 9.Czeisler CA, Klerman EB. Circadian and sleep-dependent regulation of hormone release in humans. Recent Prog Horm Res. 1999;54:97–130. [PubMed] [Google Scholar]
- 10.Weitzman ED, Zimmerman JC, Czeisler CA, Ronda J. Cortisol secretion is inhibited during sleep in normal man. J Clin Endocrinol Metab. 1983;56:352–8. doi: 10.1210/jcem-56-2-352. [DOI] [PubMed] [Google Scholar]
- 11.Plihal W, Born J. Memory consolidation in human sleep depends on inhibition of glucocorticoid release. Neuroreport. 1999;10:2741–7. doi: 10.1097/00001756-199909090-00009. [DOI] [PubMed] [Google Scholar]
- 12.DeBold CR, Orth DN, DeCherney GS, Jackson RV, Sheldon WR, Jr, Nicholson WE, Island DP. Corticotropin-releasing hormone: stimulation of ACTH secretion in normal man. Horm Metab Res Suppl. 1987;16:8–16. [PubMed] [Google Scholar]
- 13.Davies AO, Lefkowitz RJ. Regulation of beta-adrenergic receptors by steroid hormones. Annu Rev Physiol. 1984;46:119–30. doi: 10.1146/annurev.ph.46.030184.001003. [DOI] [PubMed] [Google Scholar]
- 14.Starkman MN, Schteingart DE, Schork MA. Cushing's syndrome after treatment: changes in cortisol and ACTH levels, and amelioration of the depressive syndrome. Psychiatry Res. 1986;19:177–88. doi: 10.1016/0165-1781(86)90096-x. [DOI] [PubMed] [Google Scholar]
- 15.Lovallo WR, al'Absi M, Blick K, Whitsett TL, Wilson MF. Stress-like adrenocorticotropin responses to caffeine in young healthy men. Pharmacol Biochem Behav. 1996;55:365–9. doi: 10.1016/s0091-3057(96)00105-0. [DOI] [PubMed] [Google Scholar]
- 16.Robertson D, Frolich JC, Carr RK, Watson JT, Hollifield JW, Shand DG, Oates JA. Effects of caffeine on plasma renin activity, catecholamines and blood pressure. N Engl J Med. 1978;298:181–6. doi: 10.1056/NEJM197801262980403. [DOI] [PubMed] [Google Scholar]
- 17.James JE. Effects of habitual caffeine consumption on ambulatory blood pressure. Am J Cardiol. 1996;78:129. doi: 10.1016/s0002-9149(96)90323-9. [DOI] [PubMed] [Google Scholar]
- 18.Barone JJ, Roberts HR. Caffeine consumption. Food Chem Toxicol. 1996;34:119–29. doi: 10.1016/0278-6915(95)00093-3. [DOI] [PubMed] [Google Scholar]
- 19.Lelo A, Miners JO, Robson R, Birkett DJ. Assessment of caffeine exposure: caffeine content of beverages, caffeine intake, and plasma concentrations of methylxanthines. Clin Pharmacol Ther. 1986;39:54–9. doi: 10.1038/clpt.1986.10. [DOI] [PubMed] [Google Scholar]
- 20.Farag NH, Vincent AS, McKey BS, Whitsett TL, Lovallo WR. Hemodynamic mechanisms underlying the incomplete tolerance to caffeine's pressor effects. Am J Cardiol. doi: 10.1016/j.amjcard.2005.01.093. In press. [DOI] [PubMed] [Google Scholar]
- 21.Farag NH, Vincent AS, Sung BH, Whitsett TL, Wilson MF, Lovallo WR. Tolerance to the pressor effects of caffeine is incomplete after daily intake: an analysis of blood pressure across the day. Am J Hypertens. In press. [Google Scholar]
- 22.Ammon HP. Biochemical mechanism of caffeine tolerance. Arch Pharm (Weinheim) 1991;324:261–7. doi: 10.1002/ardp.19913240502. [DOI] [PubMed] [Google Scholar]
- 23.Karcz-Kubicha M, Antoniou K, Terasmaa A, Quarta D, Solinas M, Justinova Z, Pezzola A, Reggio R, Muller CE, Fuxe K, Goldberg SR, Popoli P, Ferre S. Involvement of adenosine A1 and A2a receptors in the motor effects of caffeine after its acute and chronic administration. Neuropsychopharmacology. 2003;28:1281–91. doi: 10.1038/sj.npp.1300167. [DOI] [PubMed] [Google Scholar]
- 24.Dallman MF, Akana SF, Jacobson L, Levin N, Cascio CS, Shinsako J. Characterization of corticosterone feedback regulation of ACTH secretion. Ann N Y Acad Sci. 1987;512:402–14. doi: 10.1111/j.1749-6632.1987.tb24976.x. [DOI] [PubMed] [Google Scholar]
- 25.McEwen BS, Biron CA, Brunson KW, Bulloch K, Chambers WH, Dhabhar FS, Goldfarb RH, Kitson RP, Miller AH, Spencer RL, Weiss JM. The role of adrenocorticoids as modulators of immune function in health and disease: neural, endocrine and immune interactions. Brain Res Brain Res Rev. 1997;23:79–133. doi: 10.1016/s0165-0173(96)00012-4. [DOI] [PubMed] [Google Scholar]
- 26.Laudenslager ML, Rasmussen KL, Berman CM, Suomi SJ, Berger CB. Specific antibody levels in free-ranging rhesus monkeys: relationships to plasma hormones, cardiac parameters, and early behavior. Dev Psychobiol. 1993;26:407–20. doi: 10.1002/dev.420260704. [DOI] [PubMed] [Google Scholar]
- 27.Starkman MN, Giordani B, Gebarski SS, Berent S, Schork MA, Schteingart DE. Decrease in cortisol reverses human hippocampal atrophy following treatment of Cushing's disease. Biol Psychiatry. 1999;46:1595–602. doi: 10.1016/s0006-3223(99)00203-6. [DOI] [PubMed] [Google Scholar]
- 28.Buchanan TW, Brechtel A, Sollers JJ, Lovallo WR. Exogenous cortisol exerts effects on the startle reflex independent of emotional modulation. Pharmacol Biochem Behav. 2001;68:203–10. doi: 10.1016/s0091-3057(00)00450-0. [DOI] [PubMed] [Google Scholar]
- 29.Shepard JD, Barron KW, Myers DA. Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Res. 2000;861:288–95. doi: 10.1016/s0006-8993(00)02019-9. [DOI] [PubMed] [Google Scholar]
- 30.Buchanan TW, Lovallo WR. Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology. 2001;26:307–17. doi: 10.1016/s0306-4530(00)00058-5. [DOI] [PubMed] [Google Scholar]
- 31.Lupien SJ, Gillin CJ, Hauger RL. Working memory is more sensitive than declarative memory to the acute effects of corticosteroids: a dose-response study in humans. Behav Neurosci. 1999;113:420–30. doi: 10.1037//0735-7044.113.3.420. [DOI] [PubMed] [Google Scholar]
- 32.Boulenger JP, Patel J, Post RM, Parma AM, Marangos PJ. Chronic caffeine consumption increases the number of brain adenosine receptors. Life Sci. 1983;32:1135–42. doi: 10.1016/0024-3205(83)90119-4. [DOI] [PubMed] [Google Scholar]
- 33.Watson J, Deary I, Kerr D. Central and peripheral effects of sustained caffeine use: tolerance is incomplete. Br J Clin Pharmacol. 2002;54:400–6. doi: 10.1046/j.1365-2125.2002.01681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Evans SM, Griffiths RR. Caffeine withdrawal: a parametric analysis of caffeine dosing conditions. J Pharmacol Exp Ther. 1999;289:285–94. [PubMed] [Google Scholar]
- 35.Lovallo WR, Pincomb GA, Sung BH, Passey RB, Sausen KP, Wilson MF. Caffeine may potentiate adrenocortical stress responses in hypertension-prone men. Hypertension. 1989;14:170–6. doi: 10.1161/01.hyp.14.2.170. [DOI] [PubMed] [Google Scholar]
- 36.al'Absi M, Lovallo WR, McKey B, Sung BH, Whitsett TL, Wilson MF. Hypothalamic-pituitary-adrenocortical responses to psychological stress and caffeine in men at high and low risk for hypertension. Psychosom Med. 1998;60:521–7. doi: 10.1097/00006842-199807000-00021. [DOI] [PubMed] [Google Scholar]
- 37.al'Absi M, Lovallo WR, McKey BS, Pincomb GA. Borderline hypertensives produce exaggerated adrenocortical responses to mental stress. Psychosom Med. 1994;56:245–50. doi: 10.1097/00006842-199405000-00011. [DOI] [PubMed] [Google Scholar]
- 38.Goncharuk VD, Van Heerikhuize J, Swaab DF, Buijs RM. Paraventricular nucleus of the human hypothalamus in primary hypertension: activation of corticotropin-releasing hormone neurons. J Comp Neurol. 2002;443:321–31. doi: 10.1002/cne.10124. [DOI] [PubMed] [Google Scholar]
- 39.James JE. Chronic effects of habitual caffeine consumption on laboratory and ambulatory blood pressure levels. J Cardiovasc Risk. 1994;1:159–64. doi: 10.1177/174182679400100210. [DOI] [PubMed] [Google Scholar]


