Abstract
Background
Alcohol misuse is more common in persons with a family history of alcoholism (FH+) than in those with no such history (FH−). Among FH+, behavioral disinhibition and male sex seem to signal the presence of an increased risk.
Methods
This study examined cognitive and behavioral characteristics of 175 nonabusing 18- to 30-year-olds, 87 FH+ and 88 FH−, who were further characterized by their degree of behavioral disinhibition using the Sociability scale of the California Personality Inventory. Working memory and decision making were tested using the Stroop Color-Word Test and the Iowa Gambling Task, a simulated card game.
Results
Persons with a family history of alcoholism who were behaviorally disinhibited displayed significantly greater interference on the Stroop task than the other subgroups. On the Iowa Gambling Task, FH+ males, but not the females, were significantly more attentive to financial gains than other subgroups, and they had greater consistency in their choice behaviors.
Conclusions
Persons with a family history of alcoholism, in combination with behavioral disinhibition, appears to signal working memory deficits and in combination with male sex indicates an attraction to the rewarding aspects of a risk-taking challenge. These findings are not secondary to heavy exposure to alcohol or other drugs, but instead reflect intrinsic risk-related familial and personal characteristics of the subjects.
Keywords: Alcoholism, Family History, Antisocial Behavior, Working Memory, Gambling, Male, Female
Persons with a family history of alcoholism (FH+) have an increased lifetime risk of 4.5 or greater of developing alcoholism relative to the general population (Finn et al., 1990; Lieb et al., 2002; Merikangas et al., 1998). The behavioral and psychological characteristics that distinguish FH+ persons from those with no such history (FH−) are not well understood. The Oklahoma Family Health Patterns Project is a study of nonabusing FH+, who are free of detrimental effects of heavy alcohol intake. Our premise is that familial risk for alcoholism should be reflected in altered functioning of brain motivational systems and that these alterations can be detectable in the behavioral and psychological characteristics of the FH+ group.
We were guided in this formulation by the influential studies of Robert Cloninger (1987) who postulated the presence of disorders of appetitive mechanisms in persons at high risk for alcoholism. Other guidance came from the work of Kenneth Sher, who identified in FH+ young adults a pattern of behavioral disinhibition, or undercontrol, including a tendency toward sensation seeking, impulsivity and aggressiveness, and antisocial behaviors (Sher, 1991; Sher and Trull, 1994). In a similar fashion, Peter Finn has shown that FH+ are high in excitement seeking and display antisocial behaviors, with the latter including poor norm adherence, risk taking, and low harm avoidance (Finn et al., 1990). These formulations all converge on departures from normative regulation of affect in the FH+ person, with an attraction for hedonically pleasurable experiences and a willingness to violate norms of conduct. As Cloninger observed, these alterations map onto regulation of behavior by dopaminergic and perhaps serotonergic projections to the limbic system and prefrontal cortex. This article presents tests of working memory and risky decision making that depend on these same brain regions. We compare performance of the FH groups and discuss implications for brain mechanisms that underlie risk for alcoholism.
Working memory encompasses short-term storage of information and the executive processes that govern conscious manipulation of that information (focusing attention, managing tasks, and updating contents of the temporary store) (Smith and Jonides, 1999). Lesion, neuroimaging, and cellular recording studies of primates and humans have indicated a central role for the dorsolateral regions of the prefrontal cortex in performance of working memory tasks (Andres, 2003; Levy and Goldman-Rakic, 2000; Owen, 1997; Smith and Jonides, 1998). Working memory deficiencies have been implicated in studies of alcoholism and its associated risk factors (Finn et al., 2002; Tapert et al., 2001, 2002). Alcoholics and non-alcoholic FH+ have impairments of set formation and response shifting on the Wisconsin Card Sorting Test (Alterman et al., 1987; Corral et al., 2003), a task that taps frontal executive processes. The Stroop Color-Word Test is an ideal challenge to the executive processes of working memory because it requires attention to the correct characteristic of the stimulus and response conflict resolution at each item (Demakis, 2004; Kane and Engle, 2003). Neuroimaging during the Stroop and other response conflict tasks shows activation in the anterior cingulate gyrus (Barch et al., 2001; Soeda et al., 2005). Stroop performance may depend in part on dopaminergic circuits, as the interference effect is diminished by a dopamine agonist (Roesch-Ely et al., 2005). Stroop performance is impaired in persons with prefrontal cortex damage (Wildgruber et al., 2000), attention deficit-hyperactivity disorder (Rapport et al., 2001), alcoholism in remission (Tedstone and Coyle, 2004), and a FH+ of alcoholism (Silveri et al., 2004). More generally, FH+ adolescents display attentional and neurocognitive deficits, and the degree of attention deficit is predictive of substance use disorders over 8 years of follow-up (Tapert et al., 2002).
We also tested the subjects on a gambling simulation developed to study decision-making biases in patients with damage to the ventromedial prefrontal cortex (Bechara et al., 1997). The player chooses from 4 decks of cards that yield different levels of payoff and loss, making the task sensitive to a player's attraction to monetary gains or aversion to losses. In contrast to the Stroop task, which is a relatively pure challenge to attention and executive processes, the gambling task engages affective biases that develop as the subject gains experience with the card decks. Play is impaired in persons that have a breakdown in a postulated emotion-based learning system (Bechara et al., 1994; Damasio et al., 1990). Patients with bilateral damage to the medial prefrontal cortex are particularly poor at avoiding losses in this game (Bechara et al., 1994), even when they can describe the relative payoffs and losses among the 4 decks. Patients with bilateral amygdala damage are even more impaired (Bechara et al., 1999).
Some workers have implicated faulty decision-making processes as targets of study in risk for addiction, on the theory that risk-prone decision making may dispose the person to make poor choices about alcohol consumption in social situations (Adinoff et al., 2003; Bechara, 2003). Reports show poor performance on the Iowa Gambling Task by abstinent heroin addicts (Bechara and Damasio, 2002; Bechara et al., 2002) and young adults with early-onset alcoholism and antisocial personality disorder (Mazas et al., 2000). Some of these substance abusers were unimpaired, others were overly sensitive to rewards, and still others devalued future gains and losses (Bechara et al., 2002). Because this is a complex task with 4 card decks having payoffs that vary or may vary over time, overall wins and losses may not be the most sensitive index of the behavioral components characterizing a subject's play (Busemeyer and Stout, 2002; Yechiam et al., 2005).
In evaluating the gambling behavior of FH groups, the present article uses a cognitive model, called the Expectancy Valence model (Busemeyer and Stout, 2002), to examine the player's attention to losses and sensitivity to gains. Studies using this model have shown that chronic cocaine (Stout et al., 2005) and marijuana abusers (Yechiam et al., 2005) display high attention to gains on the task. However, college-age alcohol abusers (Finn et al., submitted for publication) and polydrug abusers (Yechiam et al., 2005) showed no significant increases in attention to gains.
The cited studies indicate altered motivation and risky decision making in persons with a significant history of exposure to alcohol and illicit drugs, but they are not clear on whether such results reflect a predisposition to abuse or if they are a consequence of exposure. Given the high comorbidity of substance use disorders and antisocial personality disorder (Langbehn et al., 2003), we presumed that FH+ who have antisocial tendencies would be at especially high risk for future drinking and drug use problems. Accordingly, we examined the data from both tasks including FH grouping alone and in relation to scores on the Sociability (So) scale of the California Personality Inventory (Gough, 1969). We predicted that a family history of alcoholism and antisocial behavior would predict poorer performance on both tasks.
METHODS
Subjects
This sample included 175 healthy young adults, averaging 23.5 years of age, recruited by advertisement and personal referral from the Oklahoma City community and local colleges and technical schools (Table 1). To achieve a diverse sample, ads were placed in a variety of community newspapers appealing to a range of demographic groups, as well as on the exteriors of city buses. We also used a television news spot, personal referrals, and posters in public places. Subjects and parents signed a consent form approved by the Institutional Review Board of the University of Oklahoma Health Sciences Center and the VA Medical Center and were paid for their participation.
Table 1.
Sample Characteristics
| Family history
|
||||||
|---|---|---|---|---|---|---|
| Negative
|
Positive
|
p Values
|
||||
| Sociability group | High | Low | High | Low | All | Neg/high vs pos/low |
| N (M/F) | 68 (28/40) | 20 (15/5) | 43 (11/32) | 44 (20/24) | ||
| Age (year) | 23 (4) | 23 (0.8) | 24 (0.5) | 24 (0.5) | ||
| Education (year) | 16 (0.3) | 16 (0.5) | 16 (0.3) | 14 (0.3) | 0.006 | 0.005 |
| SES | 49 (1.6) | 49 (2.8) | 43 (2.1) | 43 (2.0) | 0.054 | 0.16 |
| Shipley vocabulary | 30 (0.5) | 30 (0.9) | 30 (0.6) | 28 (0.6) | 0.098 | 0.11 |
| BDI | 3.5 (0.5) | 5.6 (0.1) | 4.7 (0.7) | 7.3 (0.7) | 0.0003 | 0.0001 |
| EPI—Neuroticism | 5.3 (0.5) | 5.4 (1.0) | 6.5 (0.6) | 7.3 (0.6) | 0.09 | 0.09 |
| AUDIT | 2.8 (0.3) | 2.8 (0.6) | 3.5 (0.4) | 3.3 (0.4) | ||
| Cahalan (oz/mo) | 40 (4) | 35 (8) | 48 (5) | 46 (5) | ||
| Caffeine (mg/d) | 126 (19) | 110 (31) | 102 (16) | 160 (28) | ||
| Tobacco (% using weekly) | 9 | 19 | 17 | 21 | ||
| Drug use (n) | 0 | 1 | 1 | 8 | ||
| Failed drug screen n (%) | 0 (0%) | 2 (10%) | 1 (2%) | 7 (16%) | ||
Entries (M, SE) unless given otherwise. SES, Hollingshead & Redlich Socioeconomic Status score. Higher scores reflect higher SES. All scores shown are considered “Middle Class.” Shipley, Shipley Institute of Living vocabulary score. BDI, Beck Depression Inventory. EPI, Eysenck Personality Inventory. AUDIT, Alcohol Use Disorders Identification Test. Caffeine, structured interview, all sources. Drug use, number of subjects in a group who reported the most frequent use of one or more of 11 categories of abused or psychoactive drugs other than alcohol. Drug Screen entries reflect the number of subjects (% of the respective group) who failed a urine drug screen given on days of testing.
Recruitment and Screening
Persons calling to inquire about the project were asked if they were aware of their parents' and grandparents' drinking patterns and potential drinking problems. If they answered in the affirmative and were 18 to 30 years of age and indicated no history of severe abuse or dependence, they were told about the study and invited to the lab for a full screening. Among subjects undergoing screening in the lab, 62% were excluded for 3 primary reasons: (1) insufficient parent or family information or the family did not meet FH criteria (e.g. alcoholism in a grandparent but not in a parent); (2) the subject met criteria for an alcohol or substance use disorder; or (3) the subject had a current depression or anxiety disorder or required psychotropic medication.
Family History of Alcoholism and Other Drug Problems
Family history of alcoholism status was established by the Family History Research Diagnostic Criteria (FH-RDC) (Andreasen et al., 1977), a structured interview with an interrater reliability of .95 for reports of substance use disorders (Andreasen et al., 1977; Mann et al., 1985; Zimmerman et al., 1988). Persons were considered FH+ if either biological parent met at least 2 of a possible 6 criteria for alcohol or substance abuse. Subjects were excluded if they or the parent reported possible fetal exposure to alcohol or other drugs. Persons without a family history of alcoholism were those reporting an absence of alcohol or substance use disorders in their biological parents and grandparents. Parent interviews were successfully conducted for 80% of the subjects reported here, and the parent confirmed the subjects' FH reports in 88% of these interviews. Of the 12% of interviews yielding conflicting parent reports, one-third of the subjects could be retained in the sample by reassigning their FH status, while the remaining two-thirds had to be dropped because of the interviewer's judgment of an unreliable parent report, lack of information about the grandparents, or other sources of unreliability. Therefore, 92% of the interviewed subsample could be considered accurately classified, the remainder having been discarded. Among the 20% of the sample without parent interviews, we infer that 88% are accurately classified. Together with the retained group with parent interviews, this yields an estimated correct classification rate of 91% of all subjects included in this report, a figure consistent with other reliability estimates (Schuckit et al., 1995). Among the 87 FH+ subjects, 39 families (44.5%) had a history of only alcohol use disorders, another 39 families (44.5%) had both alcohol and other substance use disorders, and the remaining 9 (10%) had a history only of other substance use disorders.
Physical and Mental Health, Alcohol and Drug Use, and Personality Assessments
Physical health was assessed through a structured medical history and by self-report of current good health. Socioeconomic status (SES) was measured using updated occupational categories on the Hollingshead and Redlich scale (Hollingshead, 1975) and was based on the primary occupation of the main breadwinner in the household in which the subject grew up. Intelligence and lack of cerebral impairment were estimated from the vocabulary score on the Shipley Institute of Living Scale (John and Rattan, 1992).
Alcoholism-related personality characteristics were assessed using the Tridimensional Personality Questionnaire (TPQ) (Cloninger, 1987). Behavioral undercontrol was assessed as in earlier studies of healthy, college-age FH+ (Adinoff and Risher-Flowers, 1991; Finn et al., 1990). Socialization tendencies were measured by the Sociability scale of the California Personality Inventory (CPI-So) (Gough, 1994), which is reliable for assessing antisocial behavior in alcoholic patients (Cooney et al., 1990). Psychiatric history was obtained by the Diagnostic Interview Schedule-IV (DIS-IV) conducted by a certified research assistant and through the Beck Depression Inventory II (Beck et al., 1996). Mood regulation was assessed using the Neuroticism scale from the Eysenck Personality Inventory (EPI) (Eysenck and Eysenck, 1964), and current depression was assessed by the Beck Depression Inventory (BDI) (Beck et al., 1996).
Alcohol and drug use were assessed through the Cahalan Drinking Habits Questionnaire (Cahalan et al., 1969), the Alcohol Use Disorders Identification Test (AUDIT) (Babor et al., 1992), and a Drug Use Questionnaire modeled on the Cahalan instrument (Cognitive Studies Laboratory, 1994). Caffeine intake and smoking were assessed by questionnaires.
TESTING PROTOCOL
The main study involved 2 days of laboratory testing including psychophysiological testing and behavioral measures (Collins et al., submitted for publication; Sorocco et al., 2006). Both sessions were held at the same time of day for a given subject. The tasks reported here were always given on Day 2.
Tasks
The Stroop Color-Word Test used here was developed by Dodrill (Salinsky et al., 2002). It consists of 176 repetitions of the color words “red, orange, green, and blue,” each one printed in a discrepant ink color (e.g., the word “red” printed in blue ink) and laid out in 16 lines of 11 words each. The subject reads the list aloud 2 times. On the first reading, he or she reads the printed words while the time is recorded to the nearest second. On the second reading, the subject recites the ink colors, and that time is recorded. The interference score is the difference between the time to read the ink colors and the time to read the words. The interference arises from a prepotent tendency to read the words rather than the ink colors, making performance dependent on the subject's ability to resolve the response conflict on each recitation of the ink colors during the second reading (Stroop, 1935).
The Iowa Gambling Task (Bechara et al., 1997, 2001) is a simulated card game presented on a computer display. The subject sees 4 decks of cards face down on the screen, and on each of the 100 plays he or she turns face up the top card on any of the deck. After each play the subject is informed of the amount of money won on that trial along with the amount of loss. The net difference determines the winnings for that play. In this version of the game, the decks were stacked to provide a balance of wins to losses that changed as play progressed. Decks A and B, initially yield large gains ($100 per play) with few losses, although after 20 plays from each of those decks, the losses become larger, so that consistent play from these “bad” decks results in a net loss for the game. In the “good” decks, C and D, the initial losses are larger than the gains, but on later plays, the wins increase relative to the losses, so that consistent play from these decks results in net winnings for the game. The player cannot predict when a given deck will yield a gain or a penalty and does not know how many plays he or she will have. The only adequate strategy is to respond according to the long-run payoffs from each deck. To enhance the realism and motivation value of the game, the subjects are told they will be paid 1 cent US for each dollar shown on the screen at the end.
Scoring and Analysis
Performance on the Iowa Gambling Task was scored in terms of the final dollar value of the subject's bank, and this value was analyzed using the same ANOVA model as for the Stroop interference scores. In addition, trial-by-trial performance was examined to estimate parameters characterizing the subject's decision-making style using the Expectancy Valence model (Busemeyer and Stout, 2002; Yechiam et al., 2005). The model produces 3 measures: (1) Relative attention to gains and losses (W), which denotes the weighting of gains compared with losses at each decision point; (2) relative weighting to recent and past outcomes (φ), which denotes the weighting given to the recent trials compared with past trials; and (3) choice consistency (c), which measures the degree of random guessing.
The 3 measures are based on the following rationales:
(a) Relative weighting to gains and losses: After seeing the outcome of a play, the subject is presumed to experience an affective reaction, called a valence, to the consequences produced by the deck chosen on that trial. The valence of the payoffs experienced on trial t is denoted v(t), and it is calculated as a weighted average of the gains (Win) and losses (Loss) experienced in trial t. An attention weight parameter W determines the weight of gains and losses. The parameter denotes the motivational difference in attention distribution. For example, drug abusers may persist in choosing from disadvantageous decks because they are overly sensitive to the large gains produced by these decks. Such sensitivity to gains is indicated by high attention gain weight. Formally:
| (1) |
where if W is larger than 0.5, then the weight to gains is larger than losses, and if W is smaller than 0.5, then the weight of losses is larger than gains. Finally, a parameter value of .5 indicates that gains and losses impact the decision equally.
(b) Relative weighting to recent and past outcomes: Performers are assumed to form expectancies for each deck that represent the anticipated consequences of choosing a card from that deck. When a deck is chosen, the expectancy Ej for deck j is updated as a function of past experience, as well as on the basis of newly experienced payoffs. The second parameter of the model φ denotes the relative weight of recent payoffs compared with payoffs from the more distant past, as follows:
| (2) |
The parameter φ is limited from 0 to 1. Small parameter values produce more persistent influences across longer lags and less discounting of past outcomes. Large values of φ produce rapid discounting of past outcomes. For example, some drugs such as cannabis impair working memory (see Bolla et al., 2002) and produce a tendency to rapidly discount past outcomes, including past losses from disadvantageous decks (Yechiam et al., 2005). This is a second potential contributor to the tendency to choose disadvantageously in the task.
(c) Choice consistency: According to the model, the probability of choosing decks 1 to k is determined by strength of that deck relative to the sum of the strengths of all decks:
| (3) |
The variable θ(t) controls the consistency between choices and the expectancies, and it is assumed to change with experience. Consistency is assumed to increase with task repetition, reflecting greater reliance on one's expectancies for making successive choices. This is formalized by a power function for the sensitivity change over trials, θ(t) = (t/10)c, where c is the response sensitivity parameter. When the value of c is high, choices converge toward the deck with the maximum expectancy. For example, some subjects may fail to select a high proportion of advantageous cards because they respond unreliably to their own expectations, and their choices include more random guesses, an effect that would lead to lower values on the consistency parameter. This erratic choice pattern is a third reason for performers not to learn to choose from advantageous decks.
The parameters of the model were optimized separately for each individual performer by maximizing the likelihood of the observed sequence of 100 choices produced by an individual. Optimization is a process wherein the fit of the model (in log likelihood) is compared with the fit of a baseline model. The baseline model prediction is based on the optimized choice proportion of the different decks. Accordingly, the baseline model has 3 parameters denoting the average choice proportions of decks A, B, and C (deck D's is calculated accordingly). The improvement in the fit of the learning model over the baseline model is measured by the G2 index ( = 2×log likelihood difference between the models), a model fit statistic analogous to the chi-square (see Busemeyer and Wang, 2000). Positive values of the G2 statistic indicate that a cognitive model performs better than the baseline model, while negative values indicate the reverse.
It is therefore possible to examine trial-by-trial Iowa Gambling Task data to calculate the extent to which a subject was attentive to amounts lost or if they were relatively sensitive to gains (Busemeyer and Stout, 2002). Preliminary exploration of the present data set indicated that none of the parameters derived from the Expectancy Valence model varied as a function of Sociability, and so the final analysis of variance model included only sex and FH.
DATA ANALYSIS
All summary statistics are given as mean ± standard error of the mean. The FH groups were compared for demographic variables, psychological status, and drug and alcohol use by 2-tailed Student's t tests and χ2, and performance data were analyzed by analyses of variance (ANOVA) all performed using SAS (SAS System for Windows, version 8.2, SAS Institute, Cary, NC).
In preliminary analyses, we noted that the FH+ were markedly lower than FH− on the CPI-So scale (28.5 ± 0.59 vs. 32.8 ± 0.49, t = 5.67, p<0.0001). Based on our formulation that some FH+ would be at high risk because of behavioral disinhibition, we used an a priori cutpoint of 30 to divide each FH group into high and low So groups, as shown in Table 1. The cutoff score of 30 has strong empirical validation. Scores ≥ 30 characterize groups that are more norm-abiding, such as research scientists (32.0) and nursing students (31.5), whereas scores <30 often are seen in more deviant groups, such as infrequent and frequent marijuana smokers (26.3 and 28.7), shoplifters (27.9), and children of less—and more—severe alcoholics (27.4 and 25.1). Still lower scores are seen in alcoholics (22.8 and 23.8) and pathological gamblers (21.3) (Gough, 1994). Accordingly, So group classification was included as a categorical variable in subsequent analyses.
The Stroop interference data and final winnings on the Iowa Gambling Task were subjected to 2 Sex×2 FH×2 So group ANOVAs that included main effects and their interaction terms. Results were examined as univariate and multivariate ANOVAs.
The trial-by-trial data from the Iowa Gambling Task data were analyzed to calculate the extent to which a subject was attentive to amounts lost and how sensitive they were to gains along with their choice consistency (Busemeyer and Stout, 2002). Preliminary exploration of the data indicated that none of the parameters varied as a function of sociability scores, and so the final analysis of variance model included only sex and FH.
RESULTS
The characteristics of the FH and So groups are shown in Table 1. Subjects in both FH groups were comparable in age, socioeconomic status, and estimated intelligence (all p's>0.05). At the time of testing, the FH+ had achieved about 1 less year of education than the FH− (15 ± 0.3 vs 16 ± 0.4 years, p<0.01), despite being about a year older (24 ± 0.5 vs 23 ± 0.5 years, NS). The FH+ had higher scores on the Beck Depression Inventory (6.0 ± 0.7 vs 4.0 ± 0.45, p<0.001) although both groups scored within the normal range. The FH groups did not differ in ounces per month of alcohol intake, in signs of alcohol abuse as reflected in AUDIT scores, or in recreational drug use (data not shown). The high-risk subgroup appears more willing to use drugs other than alcohol, as indicated in the frequency of reports of having experimented with 1 or more of 11 classes of illicit or prescribed psychoactive drugs. The high-risk group also was more likely to fail the urine drug screen on test days. In FH+ persons, behavioral undercontrol, as represented in low scores on the CPI So scale, carries over into lower educational achievement, poorer mood regulation, and risky substance use patterns.
The Stroop Color Word Test
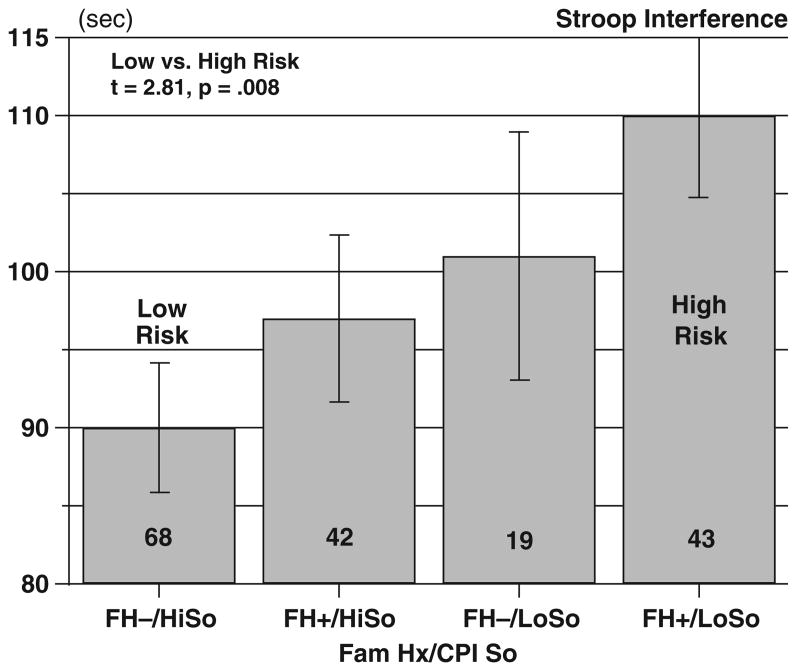
The Stroop interference scores are shown in Fig. 1. Three male subjects were colorblind and did not complete the test, and so the ANOVA included 172 subjects. As the figure suggests, the FH groups and the So groups differed in Stroop interference effects to approximately equal degrees, with FH− having less interference than the FH+ (96 ± 4.5 and 103 ± 3.8 seconds, respectively, F(1, 164) = 3.99, p<0.05) and high So subjects having less interference than low So subjects (94 ± 3.4 and 105 ± 4.8 seconds, respectively F(1, 164) = 3.96, p<0.05). There was no main effect of sex, and there were no interactions. Because the FH groups differed in their So scores, we considered it desirable to test the model using multivariate sums of squares. In this case, the FH− were not significantly different from the FH+(F(1, 164) = 0.62, NS). However, the high So group again showed lower interference scores than the low So group (F(1, 164) = 4.50, p = 0.035). There were no interactions involving any of the factors. The apparent difference in Stroop interference between the FH groups appears to be accounted for by the low So scores in the FH+ group.
Fig. 1.
Interference scores from the Stroop Color-Word Test. Bars show multivariate corrected means ± standard errors. Group sizes are shown in the bars.
Based on our hypothesis that risk for alcoholism depends on behavioral disinhibition in relation to family history, we considered FH−, high So subjects to form a low-risk reference group, and we compared them with each of the other 3 groups using 1-tailed Student's t tests with Dunnet's correction for multiple comparisons. The high-risk group (FH+, low So) had a significantly higher Stroop interference score than the low-risk group (109 ± 5.3 vs 90 ± 4.2 seconds, t = 2.81, p = .008). The other comparisons were not significant (p's ≥ 28).
Iowa Gambling Task
We used the Sex × FH × So ANOVA model to analyze the percentage of choices each subject made from the disadvantageous decks (A and B) as well as their final bank totals. The results showed no significant main effects or interactions. These groups of healthy subjects all achieved similar final outcomes regardless of FH or So score.
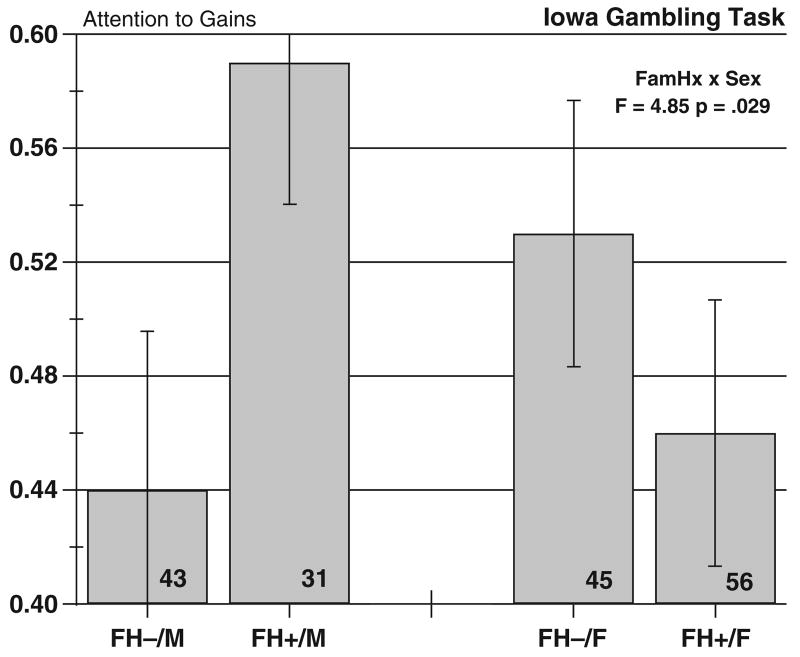
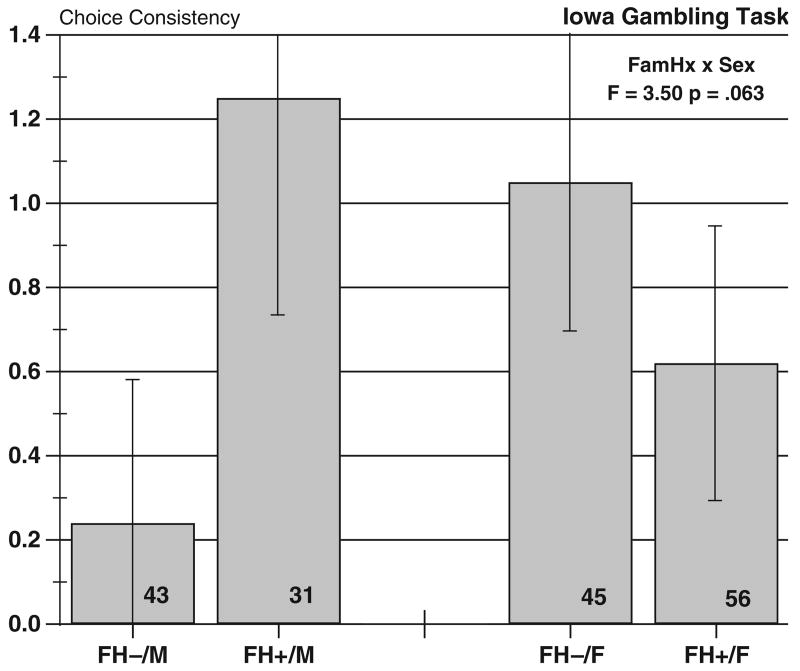
We then determined whether modeling could usefully identify group differences in trial-to-trial behavior. The fit of the total Expectancy-Valence model was relatively high, with a mean of 10.65, significantly above value of 0 specified by the null hypothesis (t = 6.92, p<.0001). We next examined the parameter estimates for attention to gains and choice consistency, shown respectively in Figs. 2 and 3. The ANOVA on attention to gains yielded a significant Sex×FH interaction (F(1, 171) = 4.85, p<.029). Inspection of Fig. 2 indicates that the FH+ men had a high parameter value, indicating greater attention to monetary gains in making their choices, whereas the FH− men were less attentive to gains, taking losses into account in a more balanced fashion. The women in both FH groups were more similar in attention to gains, although in this case, FH+ women were slightly but not significantly less attentive to gains then the FH− women. This result for FH+ men suggests that monetary gains provide a dominant focus for their performance. The choice consistency parameter showed a similar interaction that just missed the criterion of statistical significance (F(1, 171) = 3.50, p = 0.063). The pattern of results (Fig. 3) shows the FH+males were the most choice-consistent in their play and the FH− men the least. Again, women showed an intermediate and opposing pattern.
Fig. 2.
Gambling task parameter estimates for attention to gains. Bars show means ± standard errors. Group sizes are shown in the bars.
Fig. 3.
Gambling task parameter estimates for choice consistency. Bars show means ± standard errors. Group sizes are shown in the bars.
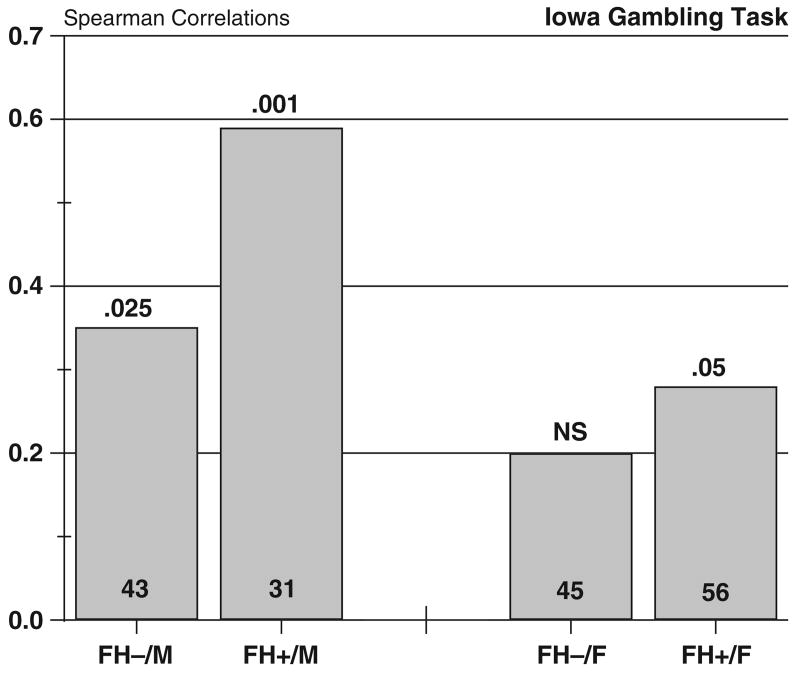
We questioned whether the FH+ males' tendency to make consistent choices might have offset potentially detrimental effects associated with a high attention to gains, resulting in their having winnings similar to the FH− men. We therefore asked if the subjects who were high in choice consistency tended to make advantageous choices (plays from safe decks C and D vs. risky decks A and B). We calculated Spearman's correlation coefficients between these values for each group, as shown in Fig. 4. The FH+ males had the largest association between choice consistency and plays from the safer decks. We then addressed this question more thoroughly by carrying out a multiple regression analysis using the 3 model parameters to predict disadvantageous choices in the sample as a whole. The recency parameter did not contribute to the prediction and was dropped from the analysis. The final model was highly significant. The percentage of choices made from disadvantageous decks = 0.469 − .017*Choice Consistency + .086*Attention to Gains, F(1, 173) = 13.1, p<0.001, r = 0.37. The partial rs for the individual parameters were: Choice Consistency partial r = −0.28, t = 4.83, p<0.01; and Attention to Gains: partial r = 0.24, t = 3.32, p<.05. The direction of these associations indicates that attention to gains was associated with a tendency toward disadvantageous plays while choice consistency was negatively associated with this behavior. The FH+ males in this sample therefore were attentive to monetary gains, but they had other behavioral tendencies that moderated that influence. The result is that their net winnings were equivalent to the other groups', although they showed a distinctive pattern of influences in their choice behavior.
Fig. 4.
Spearman's correlations between the numerical value of the choice consistency parameter and the number of plays from the advantageous decks in the gambling task, calculated for each group. Entries above the bars are p values of the respective Spearman's correlation. Group sizes are shown in the bars.
The TPQ assesses personality characteristics that might also vary in relation to risk taking, attraction to gains, and stability of performance on the Gambling task. We examined Pearson correlations between the Expectancy Valence Model and the main scales and subscales on the TPQ and found some corroboration for the relationship between personality and choice behavior. In the FH+ group, low fatigability (high vigor) was associated with high choice consistency on the Gambling Task, r(87) = −0.267, p<0.05. It appears that FH+ who are easily fatigued are more likely to choose erratically on the task, while the FH− did not appear susceptible to this effect of fatigability. Among females, regardless of FH status, disorderliness (low regimentation) was associated with high attention to gains, r(100) = −0.274, p<0.01. Those women who were high in the trait of persistence were also likely to be high in recency, r(100) = 0.244, p<0.05, suggesting sustained responsiveness to recent events even with the accumulation of payoff information. These correlations indicate a degree of correspondence between self-reported behavior tendencies on the TPQ and the subjects' behavioral characteristics observed during the Gambling task.
We then used Pearson's r to address whether the tendency to have a high Stroop interference score might also be reflected in performance parameters on the Gambling Task. The results showed no significant correlations between Stroop interference scores and any of the 3 model parameters. Moreover, the model parameters were not associated with So scores and they did not interact with So in their association with FH. The So scores and their relationship to working memory appear to be independent of the relationship between family history and gambling choice behavior.
DISCUSSION
The present results are of interest in characterizing cognitive processes and choice behaviors in FH+ young adults who are at presumed high risk for alcoholism. These persons were selected exclusively on their family history status and were screened to be free of psychiatric disorders and current abuse of alcohol or other substances. It is therefore noteworthy that the FH+ were then found to be low on behavioral undercontrol, having low scores on the CPI-So scale. In dividing the FH groups at the cutpoint of 30, we then found the suspected high-risk group (FH+, low So) to be lower in educational achievement in light of their slightly greater age. This group also had a modestly lower SES, indicating lower achievement in the heads of their childhood households. The high-risk subjects had poorer regulation of affect, indicated by their higher BDI and EPI scores, and they were more likely to experiment with psychoactive substances other than alcohol. This pattern of findings substantiates the potential influence of behavioral undercontrol in FH+ and sets a background for understanding the higher Stroop interference scores seen in the high-risk subjects.
The incongruent response condition of the Stroop test challenges the person to maintain attention to the relevant stimulus cue, to resolve response conflict, and to select responses based on the low-dominance cue on each trial (Kane and Engle, 2003). Performance on the Stroop task is impaired in persons with alcoholism, attention-deficit hyperactivity disorder, and neurological conditions associated with altered prefrontal function (Dao-Castellana et al., 1998; Duka et al., 2003; Rapport et al., 2001; Tedstone and Coyle, 2004). Poor functioning on tests of attention and executive function predicts future substance abuse (Tapert et al., 2002). Our results suggest that both a FH in combination with antisocial tendencies is related to a reduced ability to maintain attention and resolve response conflict to perform well on the Stroop test. The multivariate results showed that the apparent difference in Stroop performance between FH groups was attributable to differences in sociability scores. Offspring of alcoholics show a similar pattern of disinhibitory tendencies that account for much of the difference between FH groups. Twin-adoption data indicate that antisocial personality disorder tends to be inherited together with a tendency toward alcohol and drug abuse (Langbehn et al., 1998). Chassin and colleagues show that over 50% of the variance between FH groups is mediated by measures of behavioral undercontrol, which in turn predicts alcohol and drug use (King and Chassin, 2004). It is noteworthy that only about half of the present FH+group scored in the antisocial range of the So scale (<30). This is consistent with the idea that not all FH+will have inherited equally great levels of risk. The relative scarcity of persons with low-So scores in the FH− group (20%) suggests that the underlying tendencies toward behavioral undercontrol are less likely to be manifest in persons lacking a family history of alcoholism. Behavioral undercontrol therefore appears to predict lower levels of functioning in several domains including mood regulation, working memory, educational achievement, and experimentation with drugs.
Data from the Iowa Gambling Task offer a complimentary perspective on how FH groups differ in processes governing behavioral choice. We observed a high attention to gains during the Gambling task among the FH+ males, but there was no effect of antisocial tendencies on this behavior. While the gender difference was not predicted, it is consistent with findings that suggest males have a greater liability for alcoholism than do females. Twin adoption studies show that sons of alcoholic parents are more likely to become alcoholics then are their daughters (Cloninger et al., 1981), as supported by a recent meta-analysis, although the sex differences in risk were not large (Walters, 2002). We are unaware of other studies on FH+ that tested subjects on both the Gambling task and the Stroop, and so it is not possible to directly compare the present data with other work. Theories of choice behavior in drug abusers indicate that signals of immediate reward may carry larger motivational weight than signals of potential punishments, suggesting stronger appetitive processes and weaker inhibitory mechanisms (see reviews in Finn et al., 2002; Gorenstein and Newman, 1980). Moreover, recent findings indicate that a high attention to rewards is more characteristic of male drug abusers than female abusers. First, male smokers report using tobacco in relation to stronger social and enhancement motives, whereas adolescent females report stronger expectations of weight and anxiety reduction (Chassin et al., 2004). Secondly, male, college-age drug abusers performed worse than male controls on the Gambling task, while female abusers performed significantly better than the control women, while tests of the Expectancy Valence model showed an increased sensitivity to rewards for the male drug abusers but not for females (Stout et al., 2005). The present behavioral effects were seen in healthy young adults who were free of prolonged, heavy use of alcohol or drugs. The groups were also equivalent in estimated intelligence. These considerations suggest that sensitivity to rewards may represent inherited differences in brain function underlying a tendency to value positive and rewarding cues and that this may be part of a behavioral spectrum of risk for alcohol abuse in sons, but not daughters, of alcoholic parents.
While a family history of alcoholism played a role in accounting for performance differences on both of the present tasks, somewhat different variables predicted performance on each one. This may be consistent with the fact that the tasks call on different cognitive abilities that are used differently in each case. This raises the question of the tasks having dissociable brain mechanisms serving their cognitive requirements. A consideration of brain regions shows a pattern of overlapping, but distinguishable, functional specializations. Work on the Stroop task is accompanied by activation in the dorsal anterior cingulate gyrus (Brodmann's area 32) (Taylor et al., 1994), an area active when a subject is forced to choose between competing response alternatives (Barch et al., 2001). The Gambling task was designed to tap decision-making biases and the attraction of early gains from the disadvantageous decks in patients with damage to the ventromedial prefrontal cortex. These patients typically perform poorly on the Gambling task, even in the absence of working memory deficits (Bechara et al., 1998).
The ventromedial prefrontal cortex is continuous with the ventral extent of the anterior cingulate gyrus, and it also receives inputs from the amygdala. Some writers have therefore described the ventromedial prefrontal cortex, along with the orbitofrontal cortex, as a brain region where the reward value of stimulus inputs may be processed as part of a cognitive stream of information arriving from more dorsal and parietal regions of the cerebral cortex (Damasio, 1994; LaBar et al., 2003; Rolls, 2000). A meta-analysis of the human functional neuroimaging literature suggests that cognitive functions that include monitoring of unfavorable outcomes, detecting errors, resolving response conflict, and overcoming decision uncertainty all elicit overlapping regions of activation in the anterior cingulate gyrus (Brodmann's area 32) (Ridderinkhof et al., 2004). The Stroop task and the Gambling task both call on the processes of decision making, monitoring outcomes, and subsequent behavioral adjustments. The functional imaging data and lesion data relating to the medial prefrontal cortex would suggest that anterior cingulate areas are more heavily involved in Stroop conflict while this same region would also be involved in the Gambling task, but also engaging the ventromedial prefrontal cortex in the affective weighting of the decks, a process that is absent in the Stroop task. This functional differentiation within a broad network of medial prefrontal areas presumes that the 2 tasks have overlapping, but distinguishable task requirements served by related brain regions.
Neuromaging studies of healthy FH+ are called for to examine functional activation in the amygdala, ventromedial prefrontal cortex, and anterior cingulate gyrus in relation to decision making and performance under motivational demands. The importance of the anterior cingulate cortex during work on the Stroop test, and the poorer performance of our high-risk group, implicates suboptimal functioning of this brain region in relation to risk for future alcohol problems. This line of reasoning suggests that the behavioral disinhibition in this high-risk group may have a basis in deficient integration of functions dependent on the prefrontal cortex. Support comes from a finding that low working memory capacity predicts problem drinking in young adults with signs of behavioral disinhibition (Finn et al., 2002). Studies of recently abstinent alcohol-dependent women also show reduced frontal activation during tests of attention and working memory (Tapert et al., 2001). Studies of this sort on nonabusing FH+ would aid in distinguishing risk factors from consequences of abuse.
These findings may have implications for substance abuse treatment. First these findings highlight certain risk factors, in particular behavioral undercontrol, that appear to predict lower levels of functioning in several domains including mood regulation, working memory, educational achievement, and experimentation with drugs. In addition, male gender is a risk factor among individuals with a positive family history to have a higher attention in gains in situations that might not warrant this attention. Therefore, treatments could focus on improving these areas of functioning for individuals meeting these risk criteria. These risk factors might also be used to predict treatment response and relapse potential, allowing clinicians to design their treatment plans accordingly. Second, although the tasks used here differ in many respects, they both depend on decision-making processes. To the extent that alterations in decision making are characteristic of persons at risk for alcoholism, prevention and treatment strategies could focus on improving decision-making skills in affected individuals. Third, these data highlight the fact that FH+ individuals comprise a heterogeneous group in which severity of risk will vary as a function of such characteristics as sociability. This suggests that persons with a family history of alcoholism may possess more or less severe individual patterns of risk factors, suggesting that risk for future substance use disorders is not uniformly high across this group. The data presented here indicate that behavioral undercontrol tendencies captured in low scores on the Sociability scale are predictive of altered choice behaviors. The subject sample was found to have very little history of childhood conduct disorder and few present symptoms of antisocial personality disorder. Accordingly, the findings presented here have little to say about core antisocial characteristics relating to these diagnostic categories.
Young adults who have a family history of alcoholism demonstrated a pattern of poor working memory performance, on the Stroop test, and the males displayed high affective and attentional responses to monetary rewards in a gambling simulation. The Stroop deficits in the alcoholic offspring are present only in those displaying the disinhibitory trait of low sociability. The attention to gains in the gambling task was seen only in male offspring and not in females, with no contribution by differences in sociability. In this sample, a family history of alcoholism in conjunction with behavioral disinhibition is associated with moderate impairments in mood regulation, lower educational achievement, and potentially risky experimentation with psychoactive substances. The differences between risk groups do not appear to be secondary to prolonged heavy drinking or drug abuse. These findings point to impairments in response conflict resolution and biases in decision making as potentially important cognitive-affective alterations that distinguish persons at high risk for alcoholism.
Acknowledgments
Supported by the Department of Veterans Affairs Medical Research Service, the National Institutes of Health, NHLBI Grant HL32050, NIAAA Grant AA12207, and NIRR Grant M01 RR14467.
References
- Adinoff B, Devous MD, Sr, Cooper DB, Best SE, Chandler P, Harris T, Cervin CA, Cullum CM. Resting regional cerebral blood flow and gambling task performance in cocaine-dependent subjects and healthy comparison subjects. Am J Psychiatry. 2003;160:1892–1894. doi: 10.1176/appi.ajp.160.10.1892. [DOI] [PubMed] [Google Scholar]
- Adinoff B, Risher-Flowers D. Disturbances of hypothalamic-pituitary-adrenal axis functioning during ethanol withdrawal in six men. Am J Psychiatry. 1991;148:1023–1025. doi: 10.1176/ajp.148.8.1023. [DOI] [PubMed] [Google Scholar]
- Alterman AI, Gerstley LJ, Goldstein G, Tarter RE. Comparisons of the cognitive functioning of familial and nonfamilial alcoholics. J Stud Alcohol. 1987;48:425–429. doi: 10.15288/jsa.1987.48.425. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria. Arch Gen Psychiatry. 1977;34:1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- Andres P. Frontal cortex as the central executive of working memory: time to revise our view. Cortex. 2003;39:871–895. doi: 10.1016/s0010-9452(08)70868-2. [DOI] [PubMed] [Google Scholar]
- Babor TF, de la Fuente JR, Saunders J, Grant M. AUDIT: The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary health care. World Health Organization; Geneva, Switzerland: 1992. [Google Scholar]
- Barch DM, Braver TS, Akbudak E, Conturo T, Ollinger J, Snyder A. Anterior cingulate cortex and response conflict: effects of response modality and processing domain. Cereb Cortex. 2001;11:837–848. doi: 10.1093/cercor/11.9.837. [DOI] [PubMed] [Google Scholar]
- Bechara A. Risky business: emotion, decision-making, and addiction. J Gambl Stud. 2003;19:23–51. doi: 10.1023/a:1021223113233. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H. Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40:1675–1689. doi: 10.1016/s0028-3932(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Anderson SW. Dissociation of working memory from decision making within the human prefrontal cortex. J Neurosci. 1998;18:428–437. doi: 10.1523/JNEUROSCI.18-01-00428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275:1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Hindes A. Decision-making and addiction (part II): myopia for the future or hypersensitivity to reward? Neuropsychologia. 2002;40:1690–1705. doi: 10.1016/s0028-3932(02)00016-7. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Busemeyer JR, Stout JC. A contribution of cognitive decision models to clinical assessment: decomposing performance on the Bechara gambling task. Psychol Assess. 2002;14:253–262. doi: 10.1037//1040-3590.14.3.253. [DOI] [PubMed] [Google Scholar]
- Busemeyer JR, Wang YM. Model comparisons and model selections based on generalization criterion methodology. J Math Psychol. 2000;44:171–189. doi: 10.1006/jmps.1999.1282. [DOI] [PubMed] [Google Scholar]
- Cahalan U, Cisin I, Crossely HM. American Drinking Practices. Rutgers Center for Alcohol Studies; Newark, NJ: 1969. [Google Scholar]
- Chassin L, Hussong A, Molina B, Barrera M, Trim R, Ritter J. Adolescent substance use and abuse. In: Steinberg L, Lerner R, editors. Handbook of Adolescent Research. Wiley; New York: 2004. pp. 665–696. [Google Scholar]
- Cloninger CR. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236:410–416. doi: 10.1126/science.2882604. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse: cross fostering analysis of adopted men. Arch Gen Psychiatry. 1981;38:861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- Cognitive Studies Laboratory. The Drug Use Inventory. Center for Alcohol and Drug Related Studies, University of Oklahoma Health Sciences Center; Oklahoma City, OK: 1994. [Google Scholar]
- Cooney NL, Kadden RM, Litt MD. A comparison of methods for assessing sociopathy in male and female alcoholics. J Stud Alcohol. 1990;51:42–48. doi: 10.15288/jsa.1990.51.42. [DOI] [PubMed] [Google Scholar]
- Corral M, Holguin SR, Cadaveira F. Neuropsychological characteristics of young children from high-density alcoholism families: a three-year follow-up. J Stud Alcohol. 2003;64:195–199. doi: 10.15288/jsa.2003.64.195. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descartes' Error: Emotion, Reason, and the Human Brain. G.P. Putnam's Sons; New York, NY: 1994. [Google Scholar]
- Damasio AR, Tranel D, Damasio H. Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behav Brain Res. 1990;41:81–94. doi: 10.1016/0166-4328(90)90144-4. [DOI] [PubMed] [Google Scholar]
- Dao-Castellana MH, Samson Y, Legault F, Martinot JL, Aubin HJ, Crouzel C, Feldman L, Barrucand D, Rancurel G, Feline A, Syrota A. Frontal dysfunction in neurologically normal chronic alcoholic subjects: metabolic and neuropsychological findings. Psychol Med. 1998;28:1039–1048. doi: 10.1017/s0033291798006849. [DOI] [PubMed] [Google Scholar]
- Demakis GJ. Frontal lobe damage and tests of executive processing: a meta-analysis of the category test, Stroop test, and trail-making test. J Clin Exp Neuropsychol. 2004;26:441–450. doi: 10.1080/13803390490510149. [DOI] [PubMed] [Google Scholar]
- Duka T, Townshend JM, Collier K, Stephens DN. Impairment in cognitive functions after multiple detoxifications in alcoholic inpatients. Alcohol Clin Exp Res. 2003;27:1563–1572. doi: 10.1097/01.ALC.0000090142.11260.D7. [DOI] [PubMed] [Google Scholar]
- Eysenck SB, Eysenck HJ. An improved short questionnaire for the measurement of extraversion and neuroticism. Life Sci. 1964;305:1103–1109. doi: 10.1016/0024-3205(64)90125-0. [DOI] [PubMed] [Google Scholar]
- Finn PR, Kleinman I, Pihl RO. The lifetime prevalence of psychopathology in men with multigenerational family histories of alcoholism. J Nerv Ment Dis. 1990;178:500–504. [PubMed] [Google Scholar]
- Finn PR, Mazas CA, Justus AN, Steinmetz J. Early-onset alcoholism with conduct disorder: go/no go learning deficits, working memory capacity, and personality. Alcohol Clin Exp Res. 2002;26:186–206. [PubMed] [Google Scholar]
- Gorenstein EE, Newman JP. Disinhibitory psychopathology: a new perspective and a model for research. Psychol Rev. 1980;8:301–315. [PubMed] [Google Scholar]
- Gough H. Manual for the California Psychological Inventory. Consulting Psychologists Press; Palo Alto, CA: 1969. [Google Scholar]
- Gough H. Theory, development, and interpretation of the CPI socialization scale. Psychol Rep. 1994;75:651–700. doi: 10.2466/pr0.1994.75.1.651. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. Yale University; New Haven, CT: 1975. p. 22. [Google Scholar]
- John KR, Rattan G. In: Shipley Institute of Living Scale-Revised. Keyser DJ, Sweetland RC, editors. IX. Pro-Ed Inc; Austin, TX: 1992. pp. 490–495. [Google Scholar]
- Kane MJ, Engle RW. Working-memory capacity and the control of attention: the contributions of goal neglect, response competition, and task set to Stroop interference. J Exp Psychol Gen. 2003;132:47–70. doi: 10.1037/0096-3445.132.1.47. [DOI] [PubMed] [Google Scholar]
- King KM, Chassin L. Mediating and moderated effects of adolescent behavioral undercontrol and parenting in the prediction of drug use disorders in emerging adulthood. Psychol Addict Behav. 2004;18:239–249. doi: 10.1037/0893-164X.18.3.239. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Crupain MJ, Voyvodic JT, McCarthy G. Dynamic perception of facial affect and identity in the human brain. Cereb Cortex. 2003;13:1023–1033. doi: 10.1093/cercor/13.10.1023. [DOI] [PubMed] [Google Scholar]
- Langbehn DR, Cadoret RJ, Caspers K, Troughton EP, Yucuis R. Genetic and environmental risk factors for the onset of drug use and problems in adoptees. Drug Alcohol Depend. 2003;69:151–167. doi: 10.1016/s0376-8716(02)00310-1. [DOI] [PubMed] [Google Scholar]
- Langbehn DR, Cadoret RJ, Yates WR, Troughton EP, Stewart MA. Distinct contributions of conduct and oppositional defiant symptoms to adult antisocial behavior: evidence from an adoption study. Arch Gen Psychiatry. 1998;55:821–829. doi: 10.1001/archpsyc.55.9.821. [DOI] [PubMed] [Google Scholar]
- Levy R, Goldman-Rakic PS. Segregation of working memory functions within the dorsolateral prefrontal cortex. Exp Brain Res. 2000;133:23–32. doi: 10.1007/s002210000397. [DOI] [PubMed] [Google Scholar]
- Lieb R, Merikangas KR, Hofler M, Pfister H, Isensee B, Wittchen HU. Parental alcohol use disorders and alcohol use and disorders in offspring: a community study. Psychol Med. 2002;32:63–78. doi: 10.1017/s0033291701004883. [DOI] [PubMed] [Google Scholar]
- Mann RE, Sobell LC, Sobell MB, Pavan D. Reliability of a family tree questionnaire for assessing family history of alcohol problems. Drug Alcohol Depend. 1985;15:61–67. doi: 10.1016/0376-8716(85)90030-4. [DOI] [PubMed] [Google Scholar]
- Mazas CA, Finn PR, Steinmetz JE. Decision-making biases, antisocial personality, and early-onset alcoholism. Alcohol Clin Exp Res. 2000;24:1036–1040. [PubMed] [Google Scholar]
- Merikangas KR, Stolar M, Stevens DE, Goulet J, Preisig MA, Fenton B, Zhang H, O'Malley SS, Rounsaville BJ. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998;55:973–979. doi: 10.1001/archpsyc.55.11.973. [DOI] [PubMed] [Google Scholar]
- Owen AM. The functional organization of working memory processes within human lateral frontal cortex: the contribution of functional neuroimaging. Eur J Neurosci. 1997;9:1329–1339. doi: 10.1111/j.1460-9568.1997.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Rapport LJ, Van Voorhis A, Tzelepis A, Friedman SR. Executive functioning in adult attention-deficit hyperactivity disorder. Clin Neuropsychol. 2001;15:479–491. doi: 10.1076/clin.15.4.479.1878. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Roesch-Ely D, Scheffel H, Weiland S, Schwaninger M, Hundemer HP, Kolter T, Weisbrod M. Differential dopaminergic modulation of executive control in healthy subjects. Psychopharmacology (Berlin) 2005;178:420–430. doi: 10.1007/s00213-004-2027-z. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex and reward. Cereb Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Salinsky MC, Binder LM, Oken BS, Storzbach D, Aron CR, Dodrill CB. Effects of gabapentin and carbamazepine on the EEG and cognition in healthy volunteers. Epilepsia. 2002;43:482–490. doi: 10.1046/j.1528-1157.2002.22501.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Klein JL, Twitchell GR. The misclassification of family history status in studies of children of alcoholics. J Stud Alcohol. 1995;56:47–50. doi: 10.15288/jsa.1995.56.47. [DOI] [PubMed] [Google Scholar]
- Sher KJ. Psychological characteristics of children of alcoholics. Overview of research methods and findings. Recent Dev Alcohol. 1991;9:301–326. [PubMed] [Google Scholar]
- Sher KJ, Trull TJ. Personality and disinhibitory psychopathology: alcoholism and antisocial personality disorder. J Abnorm Psychol. 1994;103:92–102. doi: 10.1037//0021-843x.103.1.92. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Tzilos GK, Pimentel PJ, Yurgelun-Todda DA. Trajectories of adolescent emotional and cognitive development: effects of sex and risk for drug use. Ann N Y Acad Sci. 2004;1021:363–370. doi: 10.1196/annals.1308.046. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Neuroimaging analyses of human working memory. Proc Natl Acad Sci USA. 1998;95:12061–12068. doi: 10.1073/pnas.95.20.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Soeda A, Nakashima T, Okumura A, Kuwata K, Shinoda J, Iwama T. Cognitive impairment after traumatic brain injury: a functional magnetic resonance imaging study using the Stroop task. Neuroradiology. 2005;47:501–506. doi: 10.1007/s00234-005-1372-x. [DOI] [PubMed] [Google Scholar]
- Sorocco KH, Lovallo WR, Vincent AS, Collins FL. Blunted hypothalamic-pituitary-adrenocortical axis responsivity to stress in persons with a family history of alcoholism. Int J Psychophysiol. 2006;59:210–217. doi: 10.1016/j.ijpsycho.2005.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout JC, Busemeyer JR, Lin A, Grant SR, Bonson KR. Cognitive modeling analysis of the decision-making processes used by cocaine abusers. Psychonom Bull Rev. 2005;11:742–747. doi: 10.3758/bf03196629. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- Tapert SF, Baratta MV, Abrantes AM, Brown SA. Attention dysfunction predicts substance involvement in community youths. J Am Acad Child Adolesc Psychiatr. 2002;41:680–686. doi: 10.1097/00004583-200206000-00007. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown GG, Kindermann SS, Cheung EH, Frank LR, Brown SA. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcohol Clin Exp Res. 2001;25:236–245. [PubMed] [Google Scholar]
- Taylor SF, Kornblum S, Minoshima S, Oliver LM, Koeppe RA. Changes in medial cortical blood flow with a stimulus-response compatibility task. Neuropsychologia. 1994;32:249–255. doi: 10.1016/0028-3932(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Tedstone D, Coyle K. Cognitive impairments in sober alcoholics: performance on selective and divided attention tasks. Drug Alcohol Depend. 2004;75:277–286. doi: 10.1016/j.drugalcdep.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Walters GD. The heritability of alcohol abuse and dependence: a meta-analysis of behavior genetic research. Am J Drug Alcohol Abuse. 2002;28:557–584. doi: 10.1081/ada-120006742. [DOI] [PubMed] [Google Scholar]
- Wildgruber D, Kischka U, Fassbender K, Ettlin TM. The frontal lobe score: part II: evaluation of its clinical validity. Clin Rehabil. 2000;14:272–278. doi: 10.1191/026921500676524726. [DOI] [PubMed] [Google Scholar]
- Yechiam E, Busemeyer JR, Stout JC, Bechara A. Using cognitive models to map relations between neuropsychological disorders and human decision making deficits. Psychol Sci. 2005;16:973–978. doi: 10.1111/j.1467-9280.2005.01646.x. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Coryell W, Pfohl B, Stangl D. The reliability of the family history method for psychiatric diagnoses. Arch Gen Psychiatr. 1988;45:320–322. doi: 10.1001/archpsyc.1988.01800280030004. [DOI] [PubMed] [Google Scholar]