Abstract
The clinical need for improved blood vessel substitutes, especially in small-diameter applications, drives the field of vascular tissue engineering. The blood vessel has a well-characterized structure and function, but it is a complex tissue, and it has proven difficult to create engineered tissues that are suitable for widespread clinical use. This review is focused on approaches to vascular tissue engineering that use proteins as the primary matrix or “scaffold” material for creating fully biological blood vessel replacements. In particular, this review covers four main approaches to vascular tissue engineering: 1) cell-populated protein hydrogels, 2) cross-linked protein scaffolds, 3) decellularized native tissues, and 4) self-assembled scaffolds. Recent advances in each of these areas are discussed, along with advantages of and drawbacks to these approaches. The first fully biological engineered blood vessels have entered clinical trials, but important challenges remain before engineered vascular tissues will have a wide clinical effect. Cell sourcing and recapitulating the biological and mechanical function of the native blood vessel continue to be important outstanding hurdles. In addition, the path to commercialization for such tissues must be better defined. Continued progress in several complementary approaches to vascular tissue engineering is necessary before blood vessel substitutes can achieve their full potential in improving patient care.
Introduction
The driving force behind the many efforts in vascular tissue engineering is the clear need for better replacements for blood conduits, especially for small-diameter (< 6 mm) applications, in which synthetic materials are prone to clotting and failure. For larger-diameter applications, polymers such as polytetrafluoroethylene and polyethylene terephthalate have been used to create vascular grafts for peripheral applications. However, for indications such as coronary bypass or below-the-knee bypass grafting, synthetic materials do not function well in the longer term. This clinical need has fueled the search for better, more biologically compatible blood vessel replacements.
Many approaches have been used to recreate the structure and function of blood vessels. The archetypal tissue engineering approach is to use a degradable synthetic scaffold that is seeded with cells. The general concept is that the polymer provides initial mechanical support to the engineered tissue and that, as it degrades, it is replaced by a protein matrix secreted by the cells. This method has been used widely for many tissues, including blood vessels, and the first engineered tissues based on this technology have entered the clinic.1,2 However, it is not clear that this approach will be the most suitable for all applications, and the broader field of tissue engineering includes many complementary paths. In this review, we will focus on approaches to vascular tissue engineering that aim to create fully biological engineered tissues without the use of synthetic materials for support.
Blood Vessel Structure
Tissues consist of cells embedded in an extracellular matrix (ECM) composed largely of proteins, glycoproteins, glycosaminoglycans, and proteoglycans. The protein components are often fibrillar and provide strength in tension and shear, whereas the proteoglycan “ground substance” sequesters water and ions and provides compressive resistance. The most abundant proteins in most tissue types are forms of collagen and elastin, with lesser amount of fibronectin, laminin, vitronectin, and other ECM proteins. Tissues contain specific cell types, which can produce and secrete proteins, as well as carry out mechanical functions such as cell contraction and migration. Tissues also have characteristic ECM composition and arrangement. It is the interplay between tissue-specific cells and the structure of the ECM that gives different tissues their specialized functions.
Blood vessels have a concentric layered structure, with each layer being distinct in its cell and protein composition. Figure 1 shows a schematic of the structure of a medium-sized artery, with the main features, components, and environmental influences listed. The innermost layer consists of a monolayer of specialized endothelial cells (ECs) called the intima, which forms a tight nonthrombogenic barrier between the lumen of the vessel and the rest of the vessel wall. This layer is critical not only in preventing unwanted clot formation, but also in preventing infection and inflammation of the underlying tissue, as well as in signaling to the muscular component of the vessel wall. Beneath the intimal layer is a layer of basement membrane enriched in collagen IV and laminin, followed by a fenestrated but acellular layer of elastin called the internal elastic lamina.
FIG. 1.
Schematic of structure of a medium-sized blood vessels, showing the main cellular, extracellular matrix, and environmental components (not to scale). Color images available online at www.liebertpub.com/ten.
The muscular layer of the artery, called the media, is composed largely of collagens Type I and III, as well as lesser amounts of other proteins and proteoglycans. Smooth muscle cells (SMCs) that have a specialized contractile function populate this layer. The collagen matrix and the SMCs are generally aligned circumferentially or in a spiral pattern along the axis of the vessel. In larger vessels, elastic laminae further subdivide the media. Stimulation of SMCs by signals from the ECs of the lumen or directly by cytokines can cause the cells to contract or dilate in a coordinated fashion, which in turn leads to vessel contraction or dilation. In larger arteries, the medial layer is innervated, allowing the muscular layer to receive signals directly from the nervous system, and in some cases, microvessels, which supply the tissue with nutrients and oxygen, also infiltrate thick medial layers. Another elastin layer, called the external elastic lamina, which separates the medial layer from the adventitia, the outermost layer of the vessel wall, surrounds the medial layer. The adventitia consists mainly of a loose collagen matrix with embedded fibroblasts. This layer provides a substrate for a vascular supply to the artery wall and serves to anchor the blood vessel to the surrounding tissue, as well as to provide additional structural support.
Blood vessels can fail in a variety of ways. Injury, disease, or inflammation can weaken the vascular wall, which can lead to aneurysms and dissections, but the most common reason for vessel failure is occlusion due to atherosclerosis, an inflammatory disease that causes plaque build-up beneath the intimal layer of the vessel wall. The infiltration of monocytes into the intima, and a resulting increase in the migration, proliferation, and secretory activity of the vascular SMCs, forms this plaque. As the plaque grows and calcifies, it can cause a narrowing of the blood vessel lumen and can thereby decrease blood flow to the downstream tissues. Plaque rupture and subsequent clot formation also are potentially serious complications that can lead to infarction of the downstream tissue. The wide prevalence of atherosclerotic disease in western society has resulted in a strong need for better treatments for blocked vessels, including better options for blood vessel replacement. (For recent statistics on cardiovascular disease, see, e.g.,3.)
Scope of Review
This article is intended to be a focused review of the approaches to vascular tissue engineering that use proteins as the primary matrix or “scaffold” material for creating fully biological blood vessel replacements. There is a large body of work in this area, which dates back 20- to 30 years. In this review, we have concentrated on advances that have been made in the last decade. Our intent is not to neglect the important contributions of the pioneers in this field but rather to provide a concise update of recent work in this area.
To limit the scope of material, we have concentrated on methods to produce small- and medium-sized arteries and have not included the engineering of capillary networks or the microvasculature. Our emphasis is on the creation of the vascular wall (media and adventitia), rather than EC seeding, which is a complex field on its own. Again, this is not meant to play down the importance of the endothelium, because it is a critical component of any functional blood vessel. Instead, we recognize that EC-seeding will be a key enabling technology if engineered small- and medium-sized vessels are to be successful, and we refer the interested reader to recent reviews on this important topic.4,5 In addition, we have excluded approaches that use synthetic polymers (e.g., polyglycolic acid, polylactic acid, polycaprolactone, polyurethane) for creating tissue engineering constructs to focus on fully biological approaches. Several comprehensive reviews in the broader field of vascular tissue engineering have been published in recent years (see e.g.,6–8), and the reader interested in other approaches is referred to these sources.
Finally, this review is not intended to give a detailed analysis of the many different materials and techniques that have been used to engineering fully biological, protein-based blood vessels. Rather, it is intended as an overview of the main approaches that have developed over the last 2 decades, with some commentary on the advantages and disadvantages of each method. It is hoped that this review will be a starting resource for those interested in this topic, and the reader is encouraged to delve into the cited primary literature for details. For readers who already are in the field of protein-based vascular tissue engineering, we hope this review will provide a concise compilation of recent advances and the context in which they were made.
Approaches to Vascular Tissue Engineering Using Protein-Based Biomaterials
Although their general structure is straightforward and well understood, native arteries are complex tissues with multiple cell types and ECM components. To understand these tissues, and therefore to engineer fully functional arterial replacements, requires expertise in cell biology, hemodynamics, cell—matrix interactions, biomechanics, and protein chemistry. At the stage of development at which most engineered vascular tissues are currently, the main functional parameters of interest are mechanical strength and differentiated cell function. Mechanical properties are usually expressed in terms of stiffness (or tensile modulus), as well as ultimate tensile strength and burst pressure. Biological function typically is expressed in terms of cell viability, morphology, and gene and protein expression, often with an emphasis on ECM deposition. Because of the critical importance of the EC layer in maintaining patency of vascular grafts, this is usually one of the most important endpoints of in vivo studies of such constructs. In the following subsections, we have outlined several approaches to producing blood vessel substitutes based on protein matrices and have commented on the key findings in terms of biological and mechanical function of these constructs.
Cell-populated protein hydrogels
Because of the abundance and structural importance of fibrillar proteins in the blood vessel, one of the earliest approaches to vascular tissue engineering was to recreate protein matrices seeded with cells. The idea is to provide the appropriate ECM and cell type to create a tissue. Collagen type I is often used in this context because it is abundant in many tissues and can be isolated, solubilized, and reconstituted in a variety of ways. Use of a solubilized protein matrix allows living cells to be suspended in the protein solution, and this suspension then can be poured into a mold of the desired shape (e.g., an annular mold is used to create tubes for vascular tissue engineering). The protein matrix is then reconstituted by changing the environmental conditions (pH, temperature) such that, once the protein hydrogel has been formed, the cells are uniformly dispersed and immobilized in the matrix. This method is distinct from the common tissue-engineering approach of seeding cells onto a preformed scaffold, because the cells are directly embedded in the protein matrix at the time of gelation. Figure 2 shows an SMC-seeded collagen type 1 hydrogel that was molded into a tubular shape and cultured for 7 days.
FIG. 2.
Image of a collagen type I hydrogel construct containing vascular smooth muscle cells after 7 days in static culture. Color images available online at www.liebertpub.com/ten.
In theory, protein hydrogel matrices have the advantage that a defined ECM surrounds the embedded cells in the same way that matrices surround many cells (e.g., SMCs) in native tissues. In addition, cells have receptors for the proteins used in such hydrogels and therefore can recognize and bind to them, as well as migrate through them and remodel or secrete them as needed. Indeed, the vigorous compaction that occurs over the first few days after preparation of a cell-seeded collagen gel is a hallmark of cell-mediated remodeling,9 and this process leads to the formation of a rudimentary tissue.10 In native tissues, ECM proteins are known to sequester and release growth factors and other biochemicals, and therefore there is potential that this mechanism could also be used in engineered tissues.
In practice, it has proven difficult to recapitulate the structure and function of even relatively simple tissues using only cell-seeded protein hydrogels. In the case of blood vessels, the seminal work in this area used cells embedded in tubular collagen type I hydrogels.11 A multilayer structure was created with the intent of mimicking the structure of the native vessel, using SMCs and collagen as the medial layer and fibroblasts in collagen as the adventitial layer. A similar early model added dermatan sulfate proteoglycan to the hydrogel matrix.12 In both these studies, a polyethylene terephthalate mesh was incorporated into the construct to provide mechanical reinforcement to the hydrogel matrices. These important early studies demonstrated the feasibility of using protein hydrogels in vascular applications but also pointed out the challenges of creating fully biological graft materials.
The lack of appropriate mechanical properties for use in the vascular system is a problem that has consistently hampered the development of protein hydrogel-based blood vessels. In the past decade, a variety of methods have been used to strengthen such constructs without the need for a synthetic reinforcement, based on the belief that a fully biological blood vessel substitute would provide the best solution over the long term. These approaches have targeted the organization and composition of the matrix proteins, as well as function of the cells, in an effort to enhance mechanical properties.
Supplementation of the culture medium used to grow the cells and constructs used for vascular tissue engineering is one approach to enhancing the properties of the engineered tissue. Ascorbic acid and retinoic acid (forms of vitamin C and vitamin A, respectively) have been shown to alter SMC metabolism and in particular the expression of ECM proteins.13 Stimulation of cells with growth factors also can alter their ability to compact and remodel protein lattices, which can result in altered mechanical properties. For example, stimulation of SMCs in collagen type 1 gels with exogenous platelet-derived growth factor or -transforming growth factor-beta increases gel compaction, whereas heparin decreases the degree of compaction.14 Although a wide panel of small molecules, growth factors, cytokines, and other biologically active factors are available for modulating the function of SMCs, the effects and mechanisms of action of these supplements are not well understood. In particular, the use of these factors in 3-dimensional protein matrices presents challenges and opportunities in terms of dosing and delivery, and these issues need to be clarified to achieve consistent biological control of cell function in vascular tissue engineering.
Crosslinking of the protein matrix is another potential avenue to increasing the mechanical strength of engineered vascular tissues. The main challenge in crosslinking cell-seeded protein hydrogels is the need to avoid damage to the cellular component, because many crosslinking agents and methods are cytotoxic. One way to circumvent this problem has been to exploit the process of nonenzymatic glycation of proteins by reducing sugars to stiffen and strengthen collagen-based vascular constructs.15 Another approach has been to transfect SMCs to overexpress lysyl oxidase, the enzyme responsible for collagen crosslinking in most tissues, which produces stronger and stiffer collagen matrices.16 Although these crosslinking methods alone do not result in protein hydrogels of sufficient strength for implantation into the arterial system, they demonstrate the ingenuity that has been applied to increasing hydrogel mechanical properties, and it is likely that such techniques in combination with others described here will lead to mechanically suitable blood vessel substitutes.
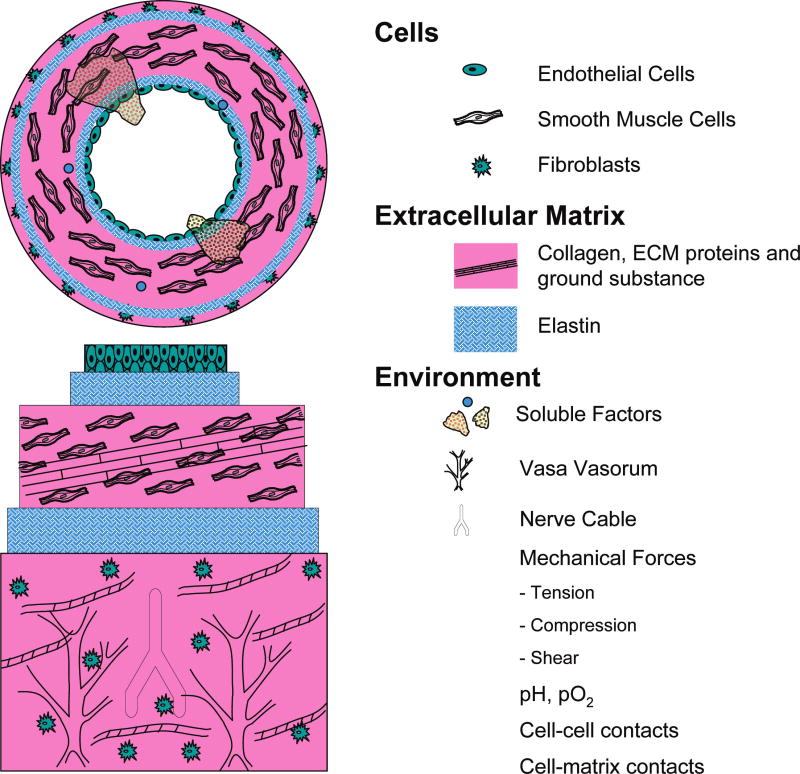
The blood clotting protein fibrin also has been used to create 3-dimensional protein hydrogel matrices for vascular tissue engineering. This protein is of interest in tissue engineering because it is a main wound-healing protein, and it is known to stimulate regenerative and remodeling responses in a variety of cell types. Fibrinogen, which is the precursor to fibrin, is abundant in blood and can be isolated, purified, and solubilized in aqueous solution. Addition of the enzyme thrombin cleaves fibrinogen to yield insoluble fibrin, which aggregates to form protein fibers. This process has been harnessed to mold engineered vascular constructs that also undergo cell-mediated compaction in a manner similar to collagen, and fibrin has been used to create vascular conduits seeded with SMCs.17,18 As with collagen, a variety of parameters, including the concentration of fibrinogen and calcium19 and thrombin20 and the presence of growth factors and medium supplements, influence the properties of these constructs.21 Collagen and fibrin also have been used in combination to form composite materials that have mechanical properties that are superior to those of the pure components alone.22 Varying the ratio of these proteins, as well as the total protein content, has been shown to change the mechanical properties of such constructs.23 Although fibrin is not an ECM protein that is normally found in the blood vessel wall, it has the potential to augment the biochemical and mechanical properties of scaffolds for vascular tissue engineering. Figure 3 shows scanning electron micrographs of collagen, fibrin, and collagen—fibrin composite matrices.
FIG. 3.
Scanning electron microscope images of protein hydrogel matrices: (A) collagen type I, (B) fibrin, (C) collagen–fibrin composite (scale bar 300 nm).
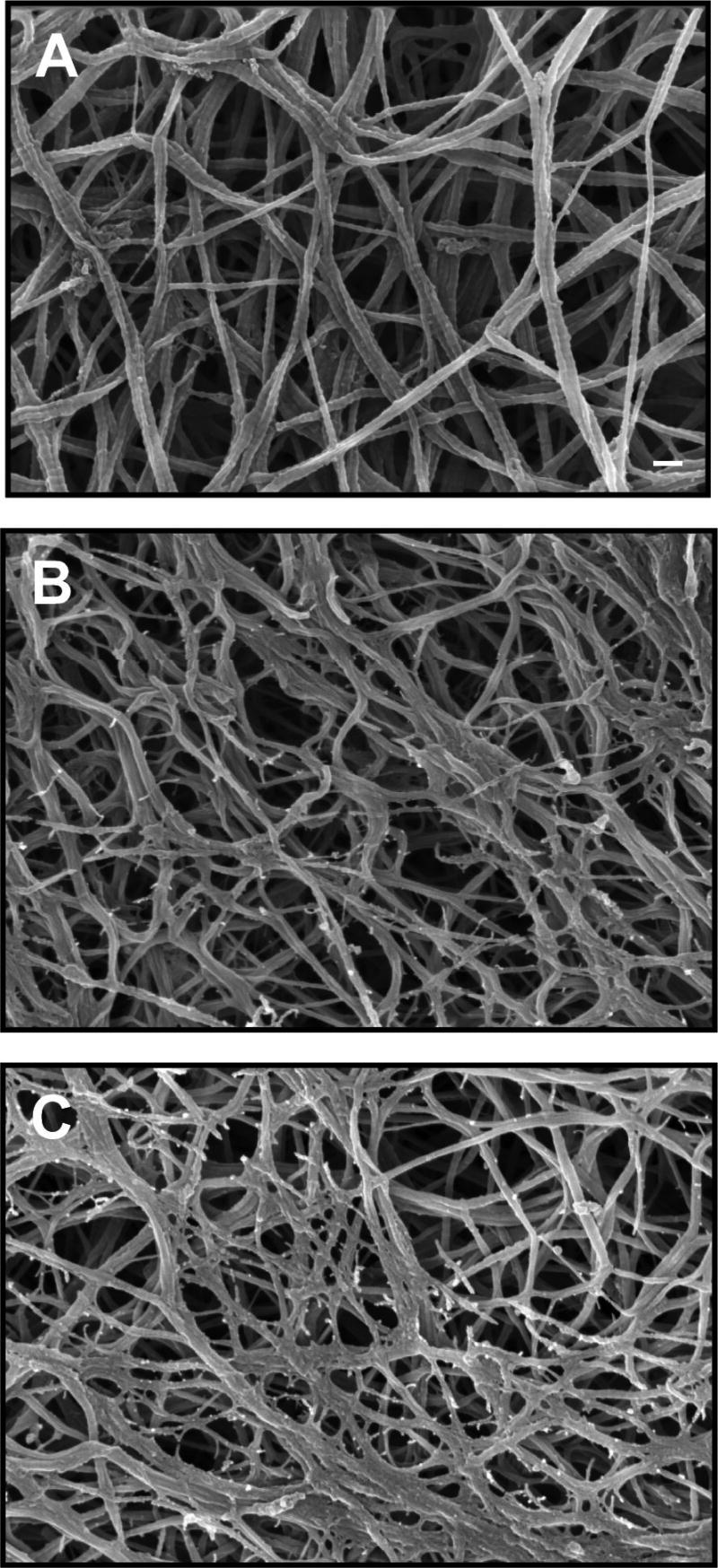
Mechanical stimulation of protein hydrogel constructs has been used as a means of changing the structure of the matrix, as well as directing cell function. It has long been known that mechanical forces, in particular fluid shear, strongly affect ECs (for a recent review see, e.g.,24,25). It also is now clear that a variety of mechanical forces, including tension26 and shear,27,28 affect vascular SMC function. Bioreactors designed to mimic these forces have been developed and used to examine the role of these forces in tissue remodeling. Figure 4 shows an example of a modular bioreactor system that provides flow through the lumen of the construct, as well as crossflow across the external surface. Cyclic circumferential strain often is applied in such systems in an effort to mimic the pulsatile stresses in the cardiovascular system. Such stimulation has been shown to induce remodeling of SMC-seeded collagen gels,29 as well as to affect SMC phenotype30 and induce elastin synthesis.31 The mechanisms of SMC-induced remodeling are not completely understood, although the embedded cells are presumably able to degrade the matrix around them, as well as synthesize new matrix in response to mechanical cues.32 In general, bioreactor culture has been shown to increase the mechanical properties of protein hydrogel constructs, and newer bioreactor systems have begun to combine circumferential tensile strain with perfusion and shear,33,34 longitudinal strain,35 and even electrical stimulation.36 (For a review on this topic, see37.)
FIG. 4.
Example of a bioreactor system used to mechanically stimulate engineered vascular tissues in vitro: (A) three-module bioreactor showing lumen and external flow direction (arrows), (B) schematic of bioreactor flow circuit during construct culture. (Used by permission from Williams, C., and Wick, T.M. Perfusion bioreactor for small diameter tissue-engineered arteries. Tissue Eng 10, 930, 2004.)
The technology of creating and controlling ever more-complex protein-based hydrogels has advanced steadily over the past 2 decades. An attractive feature of using such reconstituted protein hydrogel matrices is that one has excellent control over the composition of the starting scaffold material. Living cells can be incorporated directly at the time of scaffold fabrication, and these cells will recognize and interact with the provided matrix. By controlling the composition of the protein matrix, it is potentially possible to direct cell differentiation, function, and remodeling in desired ways. However, the major challenge to this approach remains the difficulty in achieving appropriate mechanical properties for use in the arterial system. Longer-term culture (weeks to months) of such hydrogels in the presence of the appropriate medium supplements has been shown to increase their mechanical properties, and the first in vivo experiments using fully biological fibrin hydrogel scaffolds have recently been initiated.18
Cross-linked protein scaffolds
Efforts to increase the mechanical function of pure protein vascular constructs have focused mainly on crosslinking the protein matrix to increase its resistance to tension. As mentioned previously, most crosslinking methods are toxic to cells, and therefore the approach taken has been to create separate protein scaffolds that subsequently are seeded with cells. This method is more analogous to the traditional tissue-engineering approach of combining cells with a pre-formed scaffold, with the important distinction that the scaffold is made of protein instead of the more typically used synthetic polymers.
Proteins that are present in the vascular wall have been a main focus in attempting to make fully biological scaffolds. Reconstituted collagen type I has been used in this application. To obtain a mechanically robust protein scaffold from this material, collagen solutions typically are molded into a tube or sheet and are then dehydrated or lyophilized to remove the water and create a dense matrix.38,39 The resulting material can be further crosslinked to increase its mechanical properties before cells are added to the scaffold. Common agents used for this purpose include aldehydes and carbodiimides,40,41 although other methods also have been used.42,43 After the scaffold has been prepared, SMCs or other cell types can be seeded onto the scaffold, and because cells have receptors for these proteins, they will recognize the matrix and attach to it. Because native proteins are used, the cellular component also can remodel these materials during in vitro culture and after implantation, although it is not clear how the decellularization procedure and subsequent crosslinking affects this process.
Other proteins and blends of proteins also have been used in this approach. Because of elastin's importance in the recoil properties of blood vessels, elastin materials,44 as well as collagen—elastin matrices,45 in some cases also containing chondroitin sulfate (a glycosaminoglycan),46 also have been examined for use as protein scaffolds for engineered blood vessels. In general, these materials also are cross-linked to increase their mechanical integrity before being seeded with cells. Hyaluronan, a glycosaminoglycan found in many tissues, including the blood vessel wall, has been used as a scaffold for vascular tissue engineering, but because it is not a protein matrix, it generally needs to be surface-modified to promote cell attachment.47,48 Other materials that have been investigated for use in creating protein scaffolds for blood vessels include fibronectin49 and chitosan,50 a polysaccharide with amine groups, which gives it some properties of proteins.
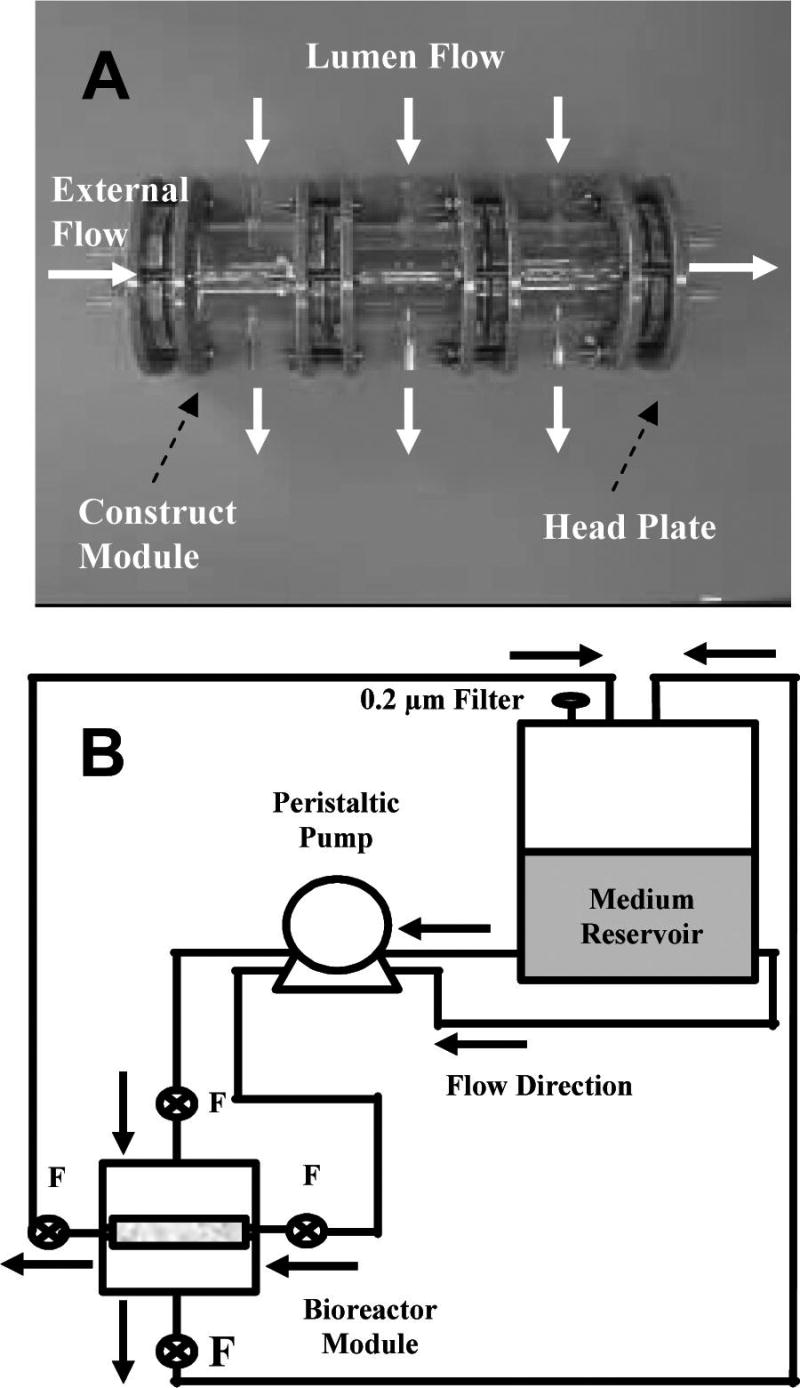
Other techniques aimed at creating pure protein scaffolds for vascular tissue engineering have emerged. The process of electrospinning involves drawing a jet of charged polymer solution between a spinneret needle and a grounded target. The solvent evaporates as the polymer solution travels through the air, leaving a solid polymer fiber that gradually builds up into a non-woven fibrous scaffold. By controlling the flow rate of polymer through the needle, as well as the voltage and geometry of the spinning rig, one can vary the fiber size and density, as well as the overall shape of the scaffold that is created. For vascular tissue-engineering applications, a rotating mandrel is often used as the grounded target, and deposition of the fibers therefore creates a tubular mesh scaffold. A variety of synthetic polymers have been used, and the technology is advancing to create multi-component materials, as well as more-complex geometries. (For a recent review on the broader topic, see e.g., 51,52). Electrospinning of protein fibers also has been demonstrated, including for vascular applications. As with other approaches, collagen and elastin are the main proteins of interest in these tissues, and tubular scaffolds have been created using electrospun versions of these proteins.53,54 Figure 5 shows scanning electron micrographs of electrospun collagen and elastin scaffolds. Electrospun fibrinogen also has been used in vascular applications,55 and electrospun blends of proteins and synthetic polymers are being developed.56,57 The application of electrospinning to tissue engineering has focused mainly on synthetic polymers, and these systems are much better characterized than electrospun proteins. The process of electrospinning proteins remains less well understood and controlled, but there is considerable effort being applied to developing electrospinning for biomedical applications, and it is likely that protein spinning will be similarly developed in the coming years.
FIG. 5.
Scanning electron microscope images of (A) electrospun type I collagen, (B) electrospun elastin (scale bar = 1 micron in both panels). (Used by permission from Boland, E.D., Matthews, J.A., Pawlowski, K.J., Simpson, D.G., Wnek, G.E., and Bowlin, G.L. Electrospinning collagen and elastin: preliminary vascular tissue engineering. Front Biosci 9, 1422, 2004.)
Decellularized native tissues
Another response to the poor mechanical properties of pure protein hydrogel constructs has been the use of decellularized tissues from a variety of sources as mechanically robust scaffold materials. It is the cellular component of transplanted allogeneic or xenogeneic tissues that is the most potent initiator of the immune response. For this reason, it has been reasoned that removal of foreign cells from a blood vessel will leave a suitable ECM from which to recreate a new tissue. In more recent efforts, the concept is to recellularize the “empty” tissue matrix with autologous cells to create an immune-acceptable engineered tissue before implantation. The potential advantage of this approach is that animal vessels, which are in relatively abundant supply, could be used as the protein scaffold and autologous cells could be expanded in culture to overcome cell supply limitations.
The idea of using decellularized vessels in this manner has been around for at least 2 decades,58 but attempts at recellularizing this material are more recent and are now one of the more common tissue-engineering technologies. Decellularization is usually achieved using solvent extraction of the lipid component and partial digestion using proteases to remove the cells,59 although there are a number of protocols for achieving this end. The goal is to retain the protein scaffold structure of the tissue while removing all potentially immunogenic cells. (For a recent review on this topic, see, e.g.,60.) In some cases, the isolated scaffolds are subsequently crosslinked before being re-seeded with the desired cells. Because of the variability in tissue across different donors, more-standardized and -automated processing techniques are being developed.61 Modification of the extraction protocol can be used to preferentially remove some components of the matrix, such that the remaining scaffold is enriched in a desired protein, (e.g., collagen or elastin).62 Figure 6 shows a scanning electron micrograph of a decellularized and purified elastin matrix. Such purified elastin scaffolds have been used as support sleeves for collagen-based hydrogel constructs. Figure 7 shows histological samples of a native artery, a collagen hydrogel construct, and a collagen hybrid construct that is reinforced with an elastin sleeve.
FIG. 6.

Scanning electron microscope image of decellularized elastin scaffold (scale bar 100 nm). (Used by permission from Lu, Q., Ganesan, K., Simionescu, D.T., and Vyavahare, N.R. Novel porous aortic elastin and collagen scaffolds for tissue engineering. Biomaterials 25, 5227, 2004.)
FIG. 7.
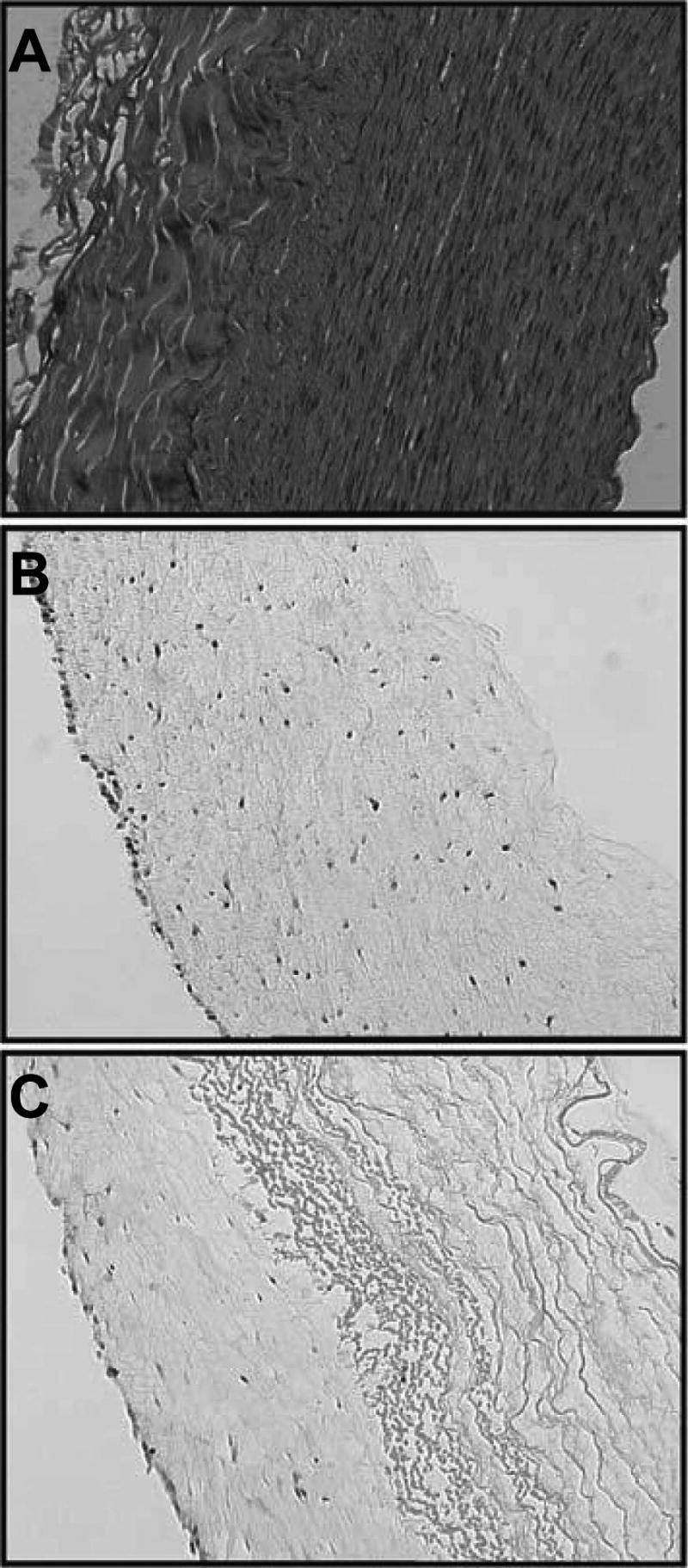
Hematoxylin and eosin staining of (A) native porcine carotid artery, (B) collagen hydrogel construct, (C) hybrid construct with elastin sleeve and collagen hydrogel. (Used by permission from Berglund, J.D., Nerem, R.M., and Sambanis, A. Viscoelastic testing methodologies for tissue engineered blood vessels. Tissue Eng 10, 1526, 2004.)
For vascular applications, arteries (carotid, aorta) and veins (jugular) of porcine and canine origin are the most widely used, 63–65 presumably because of their similarity in size to important small-diameter human vessels (e.g., the main coronary vessels). Depending on the method used to process and decellularize the matrix, the mechanical and biochemical properties can vary. In general, the decellularization process produces weaker tissues, but subsequent crosslinking makes the materials stronger and stiffer. In some cases, the decellularized vessels are similar in mechanical behavior to native vessels after processing. Creating engineered vessels using allogeneic or xenogeneic cells and then decellularizing these to create a scaffold for autologous cells has also been proposed.66 The potential advantage of this approach is that custom scaffolds could be created before they are needed using foreign cells and then could be seeded and implanted when needed using host cells.
Another material that has been used in this context for more than 2 decades is small intestinal submucosa (SIS).67,68 This matrix is usually of porcine origin and consists of the fibrous protein-rich layer immediately below the mucosal layer of the small intestine. Its main components are collagens, as well as some remaining proteoglycans, and it has been suggested that SIS also retains various growth factors that are important in its regenerative function.69 Already successful as a clinical biomaterial in a variety of soft tissue applications, SIS continues to be investigated for vascular applications (see, e.g.,70–72). As with other protein-based matrices, it has been shown that SIS matrix can be remodeled using cellular action after transplantation.73,74 The initial material tends to be stiffer than native vascular tissue,75 and attempts have been made to combine SIS with elastin sleeves to more closely mimic native compliance.76
Self-assembled scaffolds
The idea of combining cells with a scaffold is central to most tissue-engineering approaches, and in most cases, the scaffold is “exogenously” supplied. Indeed, the concept behind the approaches described above is that supplying a suitable protein scaffold will provide the mechanical robustness and biochemical cues required for engineered constructs to become functional blood vessels. An alternate approach is to allow the cellular component to synthesize and assemble its own scaffold. Culturing monolayers of fibroblasts and SMCs in medium enriched in ascorbic acid causes greater production of collagen type I, which over time in culture leads to the creation of collagen-rich cell “sheets” that can be lifted off the culture surface intact. Such sheets have been used to create vascular constructs by wrapping them around a mandrel to produce a tubular structure, and multilayered constructs that mimic the cellular composition of the artery can be produced in this way.77 These fully biological engineered vessels have displayed burst pressures similar to those of native vessels, and there is evidence of biological functions such as response to vascular agonists that control vasodilation and constriction.78,79 Animal studies have shown that these constructs can be lined with an endothelium and remain patent over weeks to months in an autologous nude rat model, as well as in immunosuppressed canine and primate models using xenogeneic (human) cells.80 Initial clinical trials to test the safety of these constructs as vascular access grafts recently have begun,81 and this model is the first fully biological engineered blood vessel to be implanted in the arterial system of humans.
In vivo self-assembly of vascular conduits also has been developed recently using a modification of the Sparks mandril method for creating fibrous collagen conduits. In this model, the body's fibrotic response to foreign materials is harnessed. Silicone tubing is implanted in the peritoneal cavity of the intended graft recipient through a minimally invasive procedure. Over several weeks of implantation, the silicone material becomes encapsulated in granulation tissue consisting largely of collagen type I and fibroblasts and eventually is covered by a mesothelial monolayer.82 The silicone tubing and encasing tissue then can be harvested via a small incision. Removal of the inner silicone material and eversion (i.e., turning inside-out) of the granulation tissue sleeve creates a fully biological tube with mesothelial cells in the lumen and a collagen-fibroblast wall. Figure 8 shows a histological section of the wall of such a tube created in the rat. Subsequent transplantation of these constructs into the vascular system of the host animal has shown that they can remain patent for periods of months and that extensive tissue remodeling and cell differentiation takes place such that the construct changes to resemble a native vessel over time. The initial studies on this approach to vascular tissue engineering were performed in the rat and rabbit, although canine large-animal studies have also been completed.83
FIG. 8.

Hematoxylin and eosin–stained longitudinal section of tube of granulation tissue after 2 weeks of growth in a rat. Arrow indicates side from which silicone tubing was removed (magnification ×300). (Used by permission from Campbell, J.H., Efendy, J.L., and Campbell, G.R. Novel vascular graft grown within recipient's own peritoneal cavity. Circ Res 85, 1173, 1999.) Color images available online at www.liebertpub.com/ten.
Current and Future Challenges
The science and technology needed to produce fully biological blood vessel substitutes has progressed steadily over the last 25 years. The field has benefited from incremental advances in our understanding of vascular biology and tissue engineering and from important and innovative solutions to key challenges. It is encouraging that the first clinical trials using fully biological blood vessel substitutes have been initiated. The first trial has targeted the vascular access indication, rather than the more complex and risky by-pass application, but this is an important first step in establishing tissue engineering as a therapy in the vasculature. In addition, most of the other complementary approaches described in the preceding sections are progressing into more-advanced animal studies.
In spite of these advances, there are serious challenges that prevent the widespread clinical application of fully biological vascular grafts in small-diameter applications. Overcoming the immune response in any vascular application is particularly difficult because of the direct exposure of the material to the bloodstream. In this respect, the endothelial lining of any graft will be crucial to its success, and at this stage, it appears likely that only living, autologous ECs will be able to meet the joint challenges of being nonthrombogenic and immune-tolerated. This topic has not been covered in detail in this review, but there is an abundance of research being conducted in the area of EC sourcing and functional reconstruction of the endothelium (reviewed in e.g.,4,5). An advantage of protein-based biomaterials in this arena is that it is likely that EC function will be easier to achieve and maintain on such a matrix than on a synthetic biomaterial. In addition, the protein matrix itself is likely to be better tolerated and more easily remodeled and replaced than hydrocarbon polymer scaffolds.
Sourcing of ECs, SMCs, and other cell types needed for construction of a blood vessel substitute remains a challenge. Availability of cells for creating autologous replacement tissues is a primary concern, especially because the patients who require bypass surgeries are often elderly, with systemic cardiovascular disease. Stem and progenitor cells offer a potential solution to this sourcing problem. Embryonic stem cells have been shown to differentiate toward EC84,85 and SMC86,87 lineages, but the science of control of embryonic stem cell differentiation is still in its infancy, and the problems of immune tolerance are not solved with this cell type. Bone marrow–-derived stem cells also have been shown to differentiate toward vascular phenotypes88–90 and represent an autologous cell source. The broader topic of stem cells in vascular tissue engineering is addressed in several recent reviews,91–93 and this is a topic that is of importance across the field of tissue engineering.
As fully biological vascular constructs have been developed, functional concerns beyond simple burst strength and cell viability have become more of a focus. More physiological cell function in terms of vasoactivity has been achieved,94,95 which may lead to better graft performance in vivo. Using such biologically functional blood vessel substitutes as models to study vascular biology in vitro, as well as testbeds for drug screening or evaluation, is another potential application of vascular tissue engineering. Similarly, the mechanical characterization of protein-based vascular grafts has progressed. In recognition of the complex behavior of native tissues, more emphasis is being placed on characterizing the viscoelastic nature of the constructs that are produced.96 In particular, the incorporation of elastin or elastin-mimetic peptides into protein matrices has become a main goal,97,98 in order to enhance construct recoil and prevent aneurysm formation. The movement of the field toward mimicking more-specialized features of vascular tissue is a testament to the progress that has been made, although the recapitulation of native tissue mechanics and contractility is an ambitious endpoint. It is not clear how close to native properties a blood vessel construct must be to be used as a therapy.
Beyond the scientific and technical challenges lie a range of other hurdles that engineered blood vessel substitutes must overcome to have an important clinical impact. For such therapies to be adopted and used widely, they must also be commercially viable. A recent review on the topic of translating vascular tissue-engineering technologies to the clinic does an excellent job of summarizing some of the key obstacles that need to be addressed.81 These include not only the scientific and technical challenges described above, but also such things as manufacturing ease, regulatory affairs, and product reimbursement. A key point is that the most important parameter in evaluating a new therapy should be clinical efficacy, and over the longer term, tissue-engineering approaches have the potential to improve efficacy dramatically, although perhaps at a higher initial cost. These points are critical to the field of tissue engineering as a whole, and as these technologies near the clinic, there has been an increased focus on them (see, e.g.,99–101).
The protein-based biomaterials described in this review offer some clear advantages as scaffolds for vascular tissue engineering over alternate approaches based on synthetic polymers. They also bring their own distinct challenges, and several decades of focused research in the field has recently resulted in only limited clinical testing. We would like to reiterate the fact that this review has focused on one branch of the field of vascular tissue engineering and that information and insight from all the complementary approaches will be needed to bring about the clinical effect that this field promises. With continued progress, we are confident that the approaches described above will lead to new ways to treat cardiovascular disease and to improve patient care.
Acknowledgments
The authors are grateful to their colleagues in the field of vascular tissue engineering for their valuable insight, discussions, and support. We apologize to those whose work could not be included because of space limitations. The authors are supported in part by the American Heart Association through Grant SDG-043511N and the National Institute of Biomedical Imaging and Bioengineering through Grant R21-EB003978.
References
- 1.Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 2.Shin'oka T, Matsumura G, Hibino N, Naito Y, Watanabe M, Konuma T, Sakamoto T, Nagatsu M, Kurosawa H. Midterm clinical result of tissue-engineering vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg. 2005;129:1330. doi: 10.1016/j.jtcvs.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 3.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 4.Williams SK. Endothelial cell transplantation. Cell Transplant. 1995;4:401. doi: 10.1177/096368979500400411. [DOI] [PubMed] [Google Scholar]
- 5.Zilla P, Deutsch M, Meinhart J. Endothelial cell transplantation. Semin Vasc Surg. 1999;12:52. [PubMed] [Google Scholar]
- 6.Stegemann JP, Rowe SL, Nerem RM. Engineered blood vessel substitutes. In: Elisseeff J, Ma PX, editors. Scaffolding in Tissue Engineering. New York: Marcel Dekker; 2005. [Google Scholar]
- 7.Hoenig MR, Campbell GR, Rolfe BE, Campbell JH. Tissue-engineered blood vessels: alternative to autologous grafts? Arterioscler Thromb Vasc Biol. 2005;25:1128. doi: 10.1161/01.ATV.0000158996.03867.72. [DOI] [PubMed] [Google Scholar]
- 8.Isenberg BC, Williams C, Tranquillo RT. Small diameter artificial arteries engineered in vitro. Circ Res. 2006;98:25. doi: 10.1161/01.RES.0000196867.12470.84. [DOI] [PubMed] [Google Scholar]
- 9.Hong H, McCullough CM, Stegemann JP. The role of ERK signaling in protein hydrogel remodeling by vascular smooth muscle cells. Biomaterials. 2007;28:3824. doi: 10.1016/j.biomaterials.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogle BM, Mooradian DL. The role of vascular smooth muscle cell integrins in the compaction and mechanical strengthening of a tissue-engineered blood vessel. Tissue Eng. 1999;5:387. doi: 10.1089/ten.1999.5.387. [DOI] [PubMed] [Google Scholar]
- 11.Weinberg CB, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231:397. doi: 10.1126/science.2934816. [DOI] [PubMed] [Google Scholar]
- 12.Miwa H, Matsuda T, Iida F. Development of a hierarchically structured hybrid vascular graft biomimicking natural arteries. ASAIO J. 1993;39:M273. [PubMed] [Google Scholar]
- 13.Ogle BM, Mooradian DL. Manipulation of remodeling pathways to enhance the properties of a tissue engineered blood vessel. J Biomech Eng. 2002;124:724. doi: 10.1115/1.1519278. [DOI] [PubMed] [Google Scholar]
- 14.Stegemann JP, Nerem RM. Altered response of vascular smooth muscle cells to exogenous biochemical stimulation in two- and three-dimensional culture. Exp Cell Res. 2003;283:146. doi: 10.1016/s0014-4827(02)00041-1. [DOI] [PubMed] [Google Scholar]
- 15.Girton TS, Oegema TR, Grassl ED, Isenberg BC, Tranquillo RT. Mechanisms of stiffening and strengthening in media-equivalents fabricated using glycation. J Biomech Eng. 2000;122:216. doi: 10.1115/1.429652. [DOI] [PubMed] [Google Scholar]
- 16.Elbjeirami WM, Yonter EO, Starcher BC, West JL. Enhancing mechanical properties of tissue-engineered constructs via lysyl oxidase crosslinking activity. J Biomed Mater Res A. 2003;66:513. doi: 10.1002/jbm.a.10021. [DOI] [PubMed] [Google Scholar]
- 17.Grassl ED, Oegema TR, Tranquillo RT. Fibrin as an alternative biopolymer to type-I collagen for the fabrication of a media equivalent. J Biomed Mater Res. 2002;60:607. doi: 10.1002/jbm.10107. [DOI] [PubMed] [Google Scholar]
- 18.Swartz DD, Russell JA, Andreadis ST. Engineering of fibrin-based functional and implantable small-diameter blood vessels. Am J Physiol Heart Circ Physiol. 2005;288:H1451. doi: 10.1152/ajpheart.00479.2004. [DOI] [PubMed] [Google Scholar]
- 19.Yao L, Swartz DD, Gugino SF, Russell JA, Andreadis ST. Fibrin-based tissue-engineered blood vessels: differential effects of biomaterial and culture parameters on mechanical strength and vascular reactivity. Tissue Eng. 2005;11:991. doi: 10.1089/ten.2005.11.991. [DOI] [PubMed] [Google Scholar]
- 20.Rowe SL, Stegemann JP. Influence of thrombin concentration on the mechanical and morphological properties of cell-seeded fibrin hydrogels. Acta Biomaterialia. 2007;3:59. doi: 10.1016/j.actbio.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neidert MR, Lee ES, Oegema TR, Tranquillo RT. Enhanced fibrin remodeling in vitro with TGF-beta1, insulin and plasmin for improved tissue-equivalents. Biomaterials. 2002;23:3717. doi: 10.1016/s0142-9612(02)00106-0. [DOI] [PubMed] [Google Scholar]
- 22.Cummings CL, Gawlitta D, Nerem RM, Stegemann JP. Properties of engineered vascular constructs made from collagen, fibrin, and collagen-fibrin mixtures. Biomaterials. 2004;25:3699. doi: 10.1016/j.biomaterials.2003.10.073. [DOI] [PubMed] [Google Scholar]
- 23.Rowe SL, Stegemann JP. Interpenetrating collagen-fibrin composite matrices with varying protein contents and ratios. Biomacromolecules. 2006;7:2942. doi: 10.1021/bm0602233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol. 2007;292:H1209. doi: 10.1152/ajpheart.01047.2006. [DOI] [PubMed] [Google Scholar]
- 25.Davies PF, Spaan JA, Krams R. Shear stress biology of the endothelium. Ann Biomed Eng. 2005;33:1714. doi: 10.1007/s10439-005-8774-0. [DOI] [PubMed] [Google Scholar]
- 26.Grenier G, Rémy-Zolghadri M, Bergeron F, Guignard R, Baker K, Labbé R, Auger FA, Germain L. Mechanical loading modulates the differentiation state of vascular smooth muscle cells. Tissue Eng. 2006;12:3159. doi: 10.1089/ten.2006.12.3159. [DOI] [PubMed] [Google Scholar]
- 27.Wang S, Tarbell JM. Effect of fluid flow on smooth muscle cells in a 3-dimensional collagen gel model. Arterioscler Thromb Vasc Biol. 2000;20:2220. doi: 10.1161/01.atv.20.10.2220. [DOI] [PubMed] [Google Scholar]
- 28.Lee AA, Graham DA, Dela Cruz S, Ratcliffe A, Karlon WJ. Fluid shear stress-induced alignment of cultured vascular smooth muscle cells. J Biomech Eng. 2002;124:37. doi: 10.1115/1.1427697. [DOI] [PubMed] [Google Scholar]
- 29.Seliktar D, Black RA, Vito RP, Nerem RM. Dynamic mechanical conditioning of collagen-gel blood vessel constructs induces remodeling in vitro. Ann Biomed Eng. 2000;28:351. doi: 10.1114/1.275. [DOI] [PubMed] [Google Scholar]
- 30.Stegemann JP, Nerem RM. Phenotype modulation in vascular tissue engineering using biochemical and mechanical stimulation. Ann Biomed Eng. 2003;31:391. doi: 10.1114/1.1558031. [DOI] [PubMed] [Google Scholar]
- 31.Isenberg BC, Tranquillo RT. Long-term cyclic distention enhances the mechanical properties of collagen-based media-equivalents. Ann Biomed Eng. 2003;31:937. doi: 10.1114/1.1590662. [DOI] [PubMed] [Google Scholar]
- 32.Seliktar D, Nerem RM, Galis ZS. Mechanical strain-stimulated remodeling of tissue engineered blood vessel constructs. Tissue Eng. 2003;9:657. doi: 10.1089/107632703768247359. [DOI] [PubMed] [Google Scholar]
- 33.Williams C, Wick TM. Perfusion bioreactor for small diameter tissue-engineered arteries. Tissue Eng. 2004;10:930. doi: 10.1089/1076327041348536. [DOI] [PubMed] [Google Scholar]
- 34.Sodian R, Lemke T, Fritsche C, Hoerstrup SP, Fu P, Potapov EV, Hausmann H, Hetzer R. Tissue-engineering bioreactors: a new combined cell-seeding and perfusion system for vascular tissue engineering. Tissue Eng. 2002;8:863. doi: 10.1089/10763270260424222. [DOI] [PubMed] [Google Scholar]
- 35.Mironov V, Kasyanov V, McAllister K, Oliver S, Sistino J, Markwald R. Perfusion bioreactor for vascular tissue engineering with capacities for longitudinal stretch. J Craniofac Surg. 2003;14:340. doi: 10.1097/00001665-200305000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Abilez O, Benharash P, Miyamoto E, Gale A, Xu C, Zarins CK. P19 progenitor cells progress to organized contracting myocytes after chemical and electrical stimulation: implications for vascular tissue engineering. J Endovasc Ther. 2006;13:377. doi: 10.1583/06-1844.1. [DOI] [PubMed] [Google Scholar]
- 37.Barron V, Lyons E, Stenson-Cox C, McHugh PE, Pandit A. Bioreactors for cardiovascular cell and tissue growth: a review. Ann Biomed Eng. 2003;31:1017. doi: 10.1114/1.1603260. [DOI] [PubMed] [Google Scholar]
- 38.Berglund JD, Mohseni MM, Nerem RM, Sambanis A. A biological hybrid model for collagen-based tissue engineered vascular constructs. Biomaterials. 2003;24:1241. doi: 10.1016/s0142-9612(02)00506-9. [DOI] [PubMed] [Google Scholar]
- 39.Wu HC, Wang TW, Kang PL, Tsuang YH, Sun JS, Lin FH. Coculture of endothelial and smooth muscle cells on a collagen membrane in the development of a small-diameter vascular graft. Biomaterials. 2007;28:1385. doi: 10.1016/j.biomaterials.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 40.Gratzer PF, Pereira CA, Lee JM. Solvent environment modulates effects of glutaraldehyde crosslinking on tissue-derived biomaterials. J Biomed Mater Res. 1996;31:533. doi: 10.1002/(SICI)1097-4636(199608)31:4<533::AID-JBM14>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 41.Wissink MJ, van Luyn MJ, Beernink R, Dijk F, Poot AA, Engbers GH, Beugeling T, van Aken WG, Feijen J. Endothelial cell seeding on crosslinked collagen: effects of crosslinking on endothelial cell proliferation and functional parameters. Thromb Haemost. 2000;84:325. [PubMed] [Google Scholar]
- 42.Sung HW, Chang WH, Ma CY, Lee MH. Crosslinking of biological tissues using genipin and/or carbodiimide. J Biomed Mater Res A. 2003;64:427. doi: 10.1002/jbm.a.10346. [DOI] [PubMed] [Google Scholar]
- 43.Chan BP, Hui TY, Chan OC, So KF, Lu W, Cheung KM, Salomatina E, Yaroslavsky A. Photochemical cross-linking for collagen-based scaffolds: a study on optical properties, mechanical properties, stability, and hematocompatibility. Tissue Eng. 2007;13:73. doi: 10.1089/ten.2006.0004. [DOI] [PubMed] [Google Scholar]
- 44.Leach JB, Wolinsky JB, Stone PJ, Wong JY. Crosslinked alpha-elastin biomaterials: towards a processable elastin mimetic scaffold. Acta Biomater. 2005;1:155. doi: 10.1016/j.actbio.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 45.Buttafoco L, Engbers-Buijtenhuijs P, Poot AA, Dijkstra PJ, Daamen WF, van Kuppevelt TH, Vermes I, Feijen J. First steps towards tissue engineering of small-diameter blood vessels: preparation of flat scaffolds of collagen and elastin by means of freeze drying. J Biomed Mater Res B Appl Biomater. 2006;77:357. doi: 10.1002/jbm.b.30444. [DOI] [PubMed] [Google Scholar]
- 46.Daamen WF, van Moerkerk HT, Hafmans T, Buttafoco L, Poot AA, Veerkamp JH, van Kuppevelt TH. Preparation and evaluation of molecularly-defined collagen-elastin-glycosaminoglycan scaffolds for tissue engineering. Biomaterials. 2003;24:4001. doi: 10.1016/s0142-9612(03)00273-4. [DOI] [PubMed] [Google Scholar]
- 47.Remuzzi A, Mantero S, Colombo M, Morigi M, Binda E, Camozzi D, Imberti B. Vascular smooth muscle cells on hyaluronic acid: culture and mechanical characterization of an engineered vascular construct. Tissue Eng. 2004;10:699. doi: 10.1089/1076327041348347. [DOI] [PubMed] [Google Scholar]
- 48.Amarnath LP, Srinivas A, Ramamurthi A. In vitro hemocompatibility testing of UV-modified hyaluronan hydrogels. Biomaterials. 2006;27:1416. doi: 10.1016/j.biomaterials.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 49.Harding SI, Afoke A, Brown RA, MacLeod A, Shamlou PA, Dunnill P. Engineering and cell attachment properties of human fibronectin-fibrinogen scaffolds for use in tissue engineered blood vessels. Bioprocess Biosyst Eng. 2002;25:53. doi: 10.1007/s004490100268. [DOI] [PubMed] [Google Scholar]
- 50.Zhang L, Ao Q, Wang A, Lu G, Kong L, Gong Y, Zhao N, Zhang X. A sandwich tubular scaffold derived from chitosan for blood vessel tissue engineering. J Biomed Mater Res A. 2006;77:277. doi: 10.1002/jbm.a.30614. [DOI] [PubMed] [Google Scholar]
- 51.Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 2006;12:1197. doi: 10.1089/ten.2006.12.1197. [DOI] [PubMed] [Google Scholar]
- 52.Murugan R, Ramakrishna S. Nano-featured scaffolds for tissue engineering: a review of spinning methodologies. Tissue Eng. 2006;12:435. doi: 10.1089/ten.2006.12.435. [DOI] [PubMed] [Google Scholar]
- 53.Boland ED, Matthews JA, Pawlowski KJ, Simpson DG, Wnek GE, Bowlin GL. Electrospinning collagen and elastin: preliminary vascular tissue engineering. Front Biosci. 2004;9:1422. doi: 10.2741/1313. [DOI] [PubMed] [Google Scholar]
- 54.Buttafoco L, Kolkman NG, Engbers-Buijtenhuijs P, Poot AA, Dijkstra PJ, Vermes I, Feijen J. Electrospinning of collagen and elastin for tissue engineering applications. Biomaterials. 2006;27:724. doi: 10.1016/j.biomaterials.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 55.McManus MC, Boland ED, Koo HP, Barnes CP, Pawlowski KJ, Wnek GE, Simpson DG, Bowlin GL. Mechanical properties of electrospun fibrinogen structures. Acta Biomater. 2006;2:19. doi: 10.1016/j.actbio.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 56.Jin HJ, Fridrikh SV, Rutledge GC, Kaplan DL. Electrospinning Bombyx mori silk with poly(ethylene oxide) Biomacromolecules. 2002;3:1233. doi: 10.1021/bm025581u. [DOI] [PubMed] [Google Scholar]
- 57.Lee SJ, Yoo JJ, Lim GJ, Atala A, Stitzel J. In vitro evaluation of electrospun nanofiber scaffolds for vascular graft application. J Biomed Mater Res A. 2007 doi: 10.1002/jbm.a.31287. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 58.Malone JM, Brendel K, Duhamel RC, Reinert RL. Detergent-extracted small-diameter vascular prostheses. J Vasc Surg. 1984;1:181. doi: 10.1067/mva.1984.avs0010181. [DOI] [PubMed] [Google Scholar]
- 59.McFetridge PS, Daniel JW, Bodamyali T, Horrocks M, Chaudhuri JB. Preparation of porcine carotid arteries for vascular tissue engineering applications. J Biomed Mater Res A. 2004;70:224. doi: 10.1002/jbm.a.30060. [DOI] [PubMed] [Google Scholar]
- 60.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 61.Lu Q, Ganesan K, Simionescu DT, Vyavahare NR. Novel porous aortic elastin and collagen scaffolds for tissue engineering. Biomaterials. 2004;25:5227. doi: 10.1016/j.biomaterials.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 62.Berglund JD, Nerem RM, Sambanis A. Incorporation of intact elastin scaffolds in tissue-engineered collagen-based vascular grafts. Tissue Eng. 2004;10:1526. doi: 10.1089/ten.2004.10.1526. [DOI] [PubMed] [Google Scholar]
- 63.Schaner PJ, Martin ND, Tulenko TN, Shapiro IM, Tarola NA, Leichter RF, Carabasi RA, Dimuzio PJ. Decellularized vein as a potential scaffold for vascular tissue engineering. J Vasc Surg. 2004;40:146. doi: 10.1016/j.jvs.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 64.Roy S, Silacci P, Stergiopulos N. Biomechanical properties of decellularized porcine common carotid arteries. Am J Physiol Heart Circ Physiol. 2005;289:H1567. doi: 10.1152/ajpheart.00564.2004. [DOI] [PubMed] [Google Scholar]
- 65.Amiel GE, Komura M, Shapira O, Yoo JJ, Yazdani S, Berry J, Kaushal S, Bischoff J, Atala A, Soker S. Engineering of blood vessels from acellular collagen matrices coated with human endothelial cells. Tissue Eng. 2006;12:2355. doi: 10.1089/ten.2006.12.2355. [DOI] [PubMed] [Google Scholar]
- 66.Dahl SL, Koh J, Prabhakar V, Niklason LE. Decellularized native and engineered arterial scaffolds for transplantation. Cell Transplant. 2003;12:659. [PubMed] [Google Scholar]
- 67.Lantz GC, Badylak SF, Coffey AC, Geddes LA, Blevins WE. Small intestinal submucosa as a small-diameter arterial graft in the dog. J Invest Surg. 1990;3:217. doi: 10.3109/08941939009140351. [DOI] [PubMed] [Google Scholar]
- 68.Badylak SF, Lantz GC, Coffey A, Geddes LA. Small intestinal submucosa as a large diameter vascular graft in the dog. J Surg Res. 1989;47:74. doi: 10.1016/0022-4804(89)90050-4. [DOI] [PubMed] [Google Scholar]
- 69.Hodde JP, Record RD, Liang HA, Badylak SF. Vascular endothelial growth factor in porcine-derived extra-cellular matrix. Endothelium. 2001;8:11. doi: 10.3109/10623320109063154. [DOI] [PubMed] [Google Scholar]
- 70.Yavuz K, Gevik S, Pavcnik D, Uchida BT, Corless CL, Hartley DE, Goktav A, Correa LO, Timmermans H, Hodde JP, Kaufman JA, Keller FS, Rosch J. Comparison of the endothelialization of small intestinal submucosa, Dacron, and expanded polytetrafluoroethylene suspended in the thoracoabdominal aorta in sleep. J Vasc Interv Radiol. 2006;17:873. doi: 10.1097/01.RVI.0000217938.20787.BB. [DOI] [PubMed] [Google Scholar]
- 71.Kasvanov V, Isenburg J, Draughn RA, Hazard S, Hodde J, Ozolanta I, Murovska M, Halkes SB, Vrasidas I, Liskamp RM, Pieters RJ, Simionescu D, Markwald RR, Mironov V. Tannic acid mimicking dendrimers as small intestine submucosa stabilizing nanomordants. Biomaterials. 2006;27:745. doi: 10.1016/j.biomaterials.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 72.Nemcova S, Noel AA, Jost CJ, Gloviczki P, Miller VM, Brockbank KG. Evaluation of a xenogeneic acellular collagen matrix as a small-diameter vascular graft in dogs—preliminary observations. J Invest Surg. 2001;14:321. doi: 10.1080/089419301753435693. [DOI] [PubMed] [Google Scholar]
- 73.Huynh T, Abraham G, Murray J, Brockbank K, Hagen PO, Sullivan S. Remodeling of an acellular collagen graft into a physiologically responsive neovessel. Nat Biotechnol. 1999;17:1083. doi: 10.1038/15062. [DOI] [PubMed] [Google Scholar]
- 74.Roeder RA, Lantz GC, Geddes LA. Mechanical remodeling of small-intestine submucosa small-diameter vascular grafts—a preliminary report. Biomed Instrum Technol. 2001;35:110. [PubMed] [Google Scholar]
- 75.Hiles MC, Badylak SF, Lantz GC, Kokini K, Geddes LA, Morff RJ. Mechanical properties of xenogeneic small-intestinal submucosa when used as an aortic graft in the dog. J Biomed Mater Res. 1995;29:883. doi: 10.1002/jbm.820290714. [DOI] [PubMed] [Google Scholar]
- 76.Hinds MT, Rowe RC, Ren Z, Teach J, Wu PC, Kirkpatrick SJ, Breneman KD, Gregory KW, Courtman DW. Development of a reinforced porcine elastin composite vascular scaffold. J Biomed Mater Res A. 2006;77:458. doi: 10.1002/jbm.a.30571. [DOI] [PubMed] [Google Scholar]
- 77.L'Heureux N, Paquet S, Labbe R, Germain L, Auger FA. A completely biological tissue engineered human blood vessel. FASEB J. 1998;12:47. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- 78.Laflamme K, Roberge CJ, Labonte J, Pouliot S, Dorleans-Juste P, Auger FA, Germain L. Tissue-engineered human vascular media with a functional endothelin system. Circulation. 2005;111:459. doi: 10.1161/01.CIR.0000153850.53419.50. [DOI] [PubMed] [Google Scholar]
- 79.Laflamme K, Roberge CJ, Pouliot S, D'Orleans-Juste P, Auger FA, Germain L. Tissue-engineered human vascular media produced in vitro by the self-assembly approach present functional properties similar to those of their native blood vessels. Tissue Eng. 2006;12:2275. doi: 10.1089/ten.2006.12.2275. [DOI] [PubMed] [Google Scholar]
- 80.L'Heureux N, Dusserre N, Konig G, Victor B, Keire P, Wight TN, Chronos NA, Kyles AE, Gregory CR, Hoyt G, Robbins RC, McAllister TN. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med. 2006;12:361. doi: 10.1038/nm1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.L'Heureux N, Dusserre N, Marini A, Garrido S, de la Fuente L, McAllister T. Technology insight: the evolution of tissue-engineered vascular grafts—from research to clinical practice. Nat Clin Pract Cardiovasc Med. 2007;4:389. doi: 10.1038/ncpcardio0930. [DOI] [PubMed] [Google Scholar]
- 82.Campbell JH, Efendy JL, Campbell GR. Novel vascular graft grown within recipient's own peritoneal cavity. Circ Res. 1999;85:1173. doi: 10.1161/01.res.85.12.1173. [DOI] [PubMed] [Google Scholar]
- 83.Chue WL, Campbell GR, Caplice N, Muhammed A, Berry CL, Thomas AC, Bennett MB, Campbell JH. Dog peritoneal and pleural cavities as bioreactors to grow autologous vascular graft. J Vasc Surg. 2004;39:859. doi: 10.1016/j.jvs.2003.03.003. [DOI] [PubMed] [Google Scholar]
- 84.Hirashima M, Kataoka H, Nishikawa S, Matsuyoshi N, Nishikawa S. Maturation of embryonic stem cells into endothelial cells in an in vitro model of vasculogenesis. Blood. 1999;93:1253. [PubMed] [Google Scholar]
- 85.Kaufman DS, Lewis RL, Hanson ET, Auerbach R, Plendl J, Thomson JA. Functional endothelial cells derived from rhesus monkey embryonic stem cells. Blood. 2004;103:1325. doi: 10.1182/blood-2003-03-0799. [DOI] [PubMed] [Google Scholar]
- 86.Drab M, Haller H, Bychkov R, Erdmann B, Lindschau C, Haase H, Morano I, Luft FC, Wobus AM. From totipotent embryonic stem cells to spontaneously contracting smooth muscle cells: a retinoic acid and db-cAMP in vitro differentiation model. FASEB J. 1997;11:905. doi: 10.1096/fasebj.11.11.9285489. [DOI] [PubMed] [Google Scholar]
- 87.Sinha S, Hoofnagle MH, Kingston PA, McCanna ME, Owens GK. Transforming growth factor-beta1 signaling contributes to development of smooth muscle cells from embryonic stem cells. Am J Physiol Cell Physiol. 2004;287:C1560. doi: 10.1152/ajpcell.00221.2004. [DOI] [PubMed] [Google Scholar]
- 88.Cho SW, Lim SH, Kim IK, Hong YS, Kim SS, Yoo KJ, Park HY, Jang Y, Chang BC, Choi CY, Hwang KC, Kim BS. Small-diameter blood vessels engineered with bone marrow derived cells. Ann Surg. 2005;241:506. doi: 10.1097/01.sla.0000154268.12239.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hamilton DW, Maul TM, Vorp DA. Characterization of the response of bone marrow-derived progenitor cell to cyclic strain: implication for vascular tissue-engineering applications. Tissue Eng. 2004;10:361. doi: 10.1089/107632704323061726. [DOI] [PubMed] [Google Scholar]
- 90.Park JS, Chu JS, Cheng C, Chen F, Chen D, Li S. Differential effects of equiaxial and uniaxial strain on mesenchymal stem cells. Biotechnol Bioeng. 2004;88:359. doi: 10.1002/bit.20250. [DOI] [PubMed] [Google Scholar]
- 91.Sales KM, Salacinski HJ, Alobaid N, Mikhail M, Balakrishnan V, Seifalian AM. Advancing vascular tissue engineering: the role of stem cell technology. Trends Biotechnol. 2005;23:461. doi: 10.1016/j.tibtech.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 92.Riha GM, Lin PH, Lumsden AB, Yao Q, Chen C. Application of stem cells for vascular tissue engineering. Tissue Eng. 2005;11:1535. doi: 10.1089/ten.2005.11.1535. [DOI] [PubMed] [Google Scholar]
- 93.Huang NF, Lee RJ, Li S. Chemical and physical regulation of stem cells and progenitor cells: potential for cardiovascular tissue engineering. Tissue Eng. 2007;13:1809. doi: 10.1089/ten.2006.0096. [DOI] [PubMed] [Google Scholar]
- 94.L'Heureux N, Stoclet JC, Auger FA, Lagaud GJ, Germain L, Andrianstitohaina R. A human tissue-engineered vascular media: a new model for pharmacological studies of contractile responses. FASEB J. 2001;15:515. doi: 10.1096/fj.00-0283com. [DOI] [PubMed] [Google Scholar]
- 95.Liu JY, Swartz DD, Peng HF, Gugino SF, Russell JA, Andreadis ST. Functional tissue-engineered blood vessels from bone marrow progenitor cells. Cardiovasc Res. 2007;75:618. doi: 10.1016/j.cardiores.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 96.Berglund JD, Nerem RM, Sambanis A. Viscoelastic testing methodologies for tissue engineered blood vessels. Tissue Eng. 2005;10:1526. doi: 10.1115/1.2073487. [DOI] [PubMed] [Google Scholar]
- 97.Gobin AS, West JL. Val-ala-pro-gly, an elastin-derived non-integrin ligand: smooth muscle cell adhesion and specificity. J Biomed Mater Res A. 2003;67:255. doi: 10.1002/jbm.a.10110. [DOI] [PubMed] [Google Scholar]
- 98.Patel A, Fine B, Sandig M, Mequanint K. Elastin biosynthesis: the missing link in tissue-engineered blood vessels. Cardiovasc Res. 2006;71:40. doi: 10.1016/j.cardiores.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 99.Lysaght MJ, Hazlehurst AL. Tissue engineering: the end of the beginning. Tissue Eng. 2004;10:309. doi: 10.1089/107632704322791943. [DOI] [PubMed] [Google Scholar]
- 100.Hunziker E, Spector M, Libera J, Gertzman A, Woo SL, Ratcliffe A, Lysaght M, Coury A, Kaplan D, Vunjak-Novakovic G. Translation from research to applications. Tissue Eng. 2006;12:3341. doi: 10.1089/ten.2006.12.3341. [DOI] [PubMed] [Google Scholar]
- 101.Aper T, Schmidt A, Duchrow M, Bruch HP. Autologous blood vessels engineered from peripheral blood sample. Eur J Vasc Endovasc Surg. 2007;33:33. doi: 10.1016/j.ejvs.2006.08.008. [DOI] [PubMed] [Google Scholar]