Abstract
Severe malarial anemia (SMA) is a leading cause of mortality among children in sub-Saharan Africa. Although the novel cytokine, interleukin (IL)-23, promotes anemia in chronic inflammatory diseases, the role of IL-23 in SMA remains undefined. Since IL-23 and IL-12 share the IL-12p40 subunit and IL-12Rβ1 receptor, and are down-regulated by IL-10, relationships among these cytokines were explored in Kenyan children with varying severities of malarial anemia. Children with malarial anemia had increased circulating IL-23 and IL-10, and decreased IL-12 relative to healthy controls. Enhanced anemia severity and elevated parasitemia were associated with increased IL-10 relative to IL-23 and IL-12. Further exploration of the relationships among the cytokines using an in vitro model in which peripheral blood mononuclear cells were treated with synthetic hemozoin (sHz, malarial pigment) revealed that IL-12p35 and IL-23p19 transcripts had a sustained induction over 72 hrs, while IL-12p40 and IL-10 message peaked at 24 hrs, and rapidly declined thereafter. Taken together, results here show that IL-23 is elevated in children with malarial anemia, and that IL-10 and IL-12 appear to have important regulatory effects on IL-23 production during childhood malaria.
Keywords: Plasmodium falciparum, malarial anemia, hemozoin, cytokines, IL-23, IL-12, IL-10
INTRODUCTION
Anemia is a common cause of childhood morbidity and mortality in sub-Saharan Africa occurring in 30-60% of children less than 5 years of age [1, 2] and accounting for more than half of all hospital pediatric mortality [3, 4]. One of the leading causes of pediatric anemia in sub-Saharan Africa is Plasmodium falciparum malaria which can culminate in life-threatening severe malarial anemia (SMA) [3, 5, 6]. It is estimated that SMA is responsible for 17-22% of the mortality in African children less than 5 years of age [3, 7].
The etiology of SMA is multi-factorial and results from increased erythrocyte destruction and decreased red blood cell (RBC) production [8-10]. In addition, the relative production of pro- and anti-inflammatory cytokines is important for mediating the development and outcomes of malarial anemia [11-14]. Although not explored in the context of SMA, a recently discovered heterodimeric cytokine, IL-23, is important in regulating the development of anemia in autoimmune diseases [15] and chronic inflammation [16]. IL-23 is composed of p19 and p40 subunits [17] and shares common properties with IL-12, such as the p40 subunit and binding to the IL-12Rβ1 receptor [18]. IL-23 and IL-12 are produced by activated myeloid antigen presenting cells and promote a type 1 immune response [17-21]. In addition, IL-23 and IL-12 are suppressed by both IL-10 [22, 23] and IL-12p40 homodimers [20, 24]. Although IL-23 and IL-12 share common properties, these cytokines also have distinct immunological roles [17, 25]. For example, IL-23 acts on activated memory CD4+ T cells, while IL-12 enhances Th1 differentiation of naïve CD4+T cells [17, 26].
Previous investigations from our laboratory illustrate that phagocytosis of malarial pigment (hemozoin, Hz) by cultured human peripheral blood mononuclear cells (PBMC) generates a profile of cytokine production similar to that observed in children with malarial anemia [27-29]. Hz is a Plasmodium-derived biomineralized product of heme, a prosthetic group of hemoglobin (Hb) liberated during Hb degradation [30]. As part of the host immune response to malaria, monocytes/macrophages and neutrophils acquire Hz through ingestion of parasitized RBC (pRBC) [31], and through phagocytosis of free Hz released into the blood stream following rupture of pRBC [32]. Stimulation of cultured PBMC with Hz, therefore, offers a useful in vitro model for examining the immunological mechanisms that govern host-parasite interactions.
Since no studies to date have reported the role of IL-23, and reciprocal interactions of IL-23 with IL-12 and IL-10 in childhood malarial anemia, absolute cytokine levels and the relative expression of these cytokines were determined in circulating peripheral blood from children with varying severities of malarial anemia. The relationship between absolute cytokine levels, cytokine ratios, Hb, and parasite burden were also examined using multivariate analyses. In addition, since the malaria parasite product(s) that regulate IL-23 are unexplored, the temporal kinetics of de novo IL-23p19 mRNA expression, along with IL-12p35, IL-12p40, and IL-10 transcripts were investigated in cultured PBMC stimulated with synthetic hemozoin (sHz).
MATERIALS AND METHODS
Study area
This study was conducted at Siaya District Hospital (SDH) in Siaya District, Nyanza Province, western Kenya. Siaya District is a holoendemic P. falciparum transmission area where residents receive up to 300 infective bites per annum [33]. SMA is the primary clinical manifestation of severe malaria in children under the age of 5 years in this region, with the peak incidence of malarial anemia occurring in children 7-24 months of age [5, 34]. A detailed description of the study area and the hematological manifestations of pediatric malarial anemia (MA) in the study population can be found in our recent publication [35].
Study population
Children (n=69; age 0-3 years) presenting with acute P. falciparum malaria were recruited from an on-going hospital-based longitudinal study at the SDH, western Kenya [35]. Children with P. falciparum parasitemia (any density) were stratified according to Hb levels into the following categories: mild malarial anemia (MlMA, n=25; Hb 8.0-10.9 g/dL and free from the symptoms of severe malaria such as hypoglycemia); and SMA (n=25; Hb<6.0 g/dL and free from the symptoms of severe malaria such as hypoglycemia). Definitions of MA were based on previous investigations that obtained >10,000 longitudinal Hb measures in children less than 48 months in western Kenya [36]. In addition, healthy, age- and gender-matched aparasitemic controls (AC, n=19; children with malaria-negative smears for P. falciparum parasitemia, Hb≥11.0 g/dL, and free of fever or diarrhea for the last two weeks) were recruited during their visits to SDH for routine immunizations. Since our previous study indicates that HIV-1 promotes enhanced anemia in children with falciparum malaria [37], only HIV-1 negative children were included in the present study. HIV-1 status was determined by rapid serological antibody tests and HIV-1 proviral DNA PCR tests according to our described methods [37]. Also excluded from the study were children with cerebral malaria, a very rare occurrence in this high malaria transmission area [5]. Children with malaria were treated according to the Ministry of Health, Kenya (MOH) guidelines which included the use of Coartem® (artemether and lumefantrin) for uncomplicated malaria and intravenous quinine for severe malaria. Supportive care and blood transfusions were administered according to MOH guidelines. All blood samples were obtained prior to antimalarial and/or any other treatment interventions. Written informed consent was obtained from the parents or legal guardians of all children enrolled in the study. The study was approved by the Institutional Review Board of the University of Pittsburgh and the National Ethical Review Committee of the Kenya Medical Research Institute. The clinical protocol was approved by the National Institutes of Health, USA.
Hemoglobin measurements
Venous blood samples (1-3 mL) were collected into EDTA containing vacutainer tubes at the time of enrollment and a complete blood count was performed using an automated Beckman Coulter® AcT diff2™ (Beckman-Coulter Corporation, Miami, USA).
Parasitemia determination
Thick and thin peripheral blood smears were prepared from venous blood samples and stained with Giemsa reagent for malaria parasite identification and quantification by microscopy. Asexual malaria parasites were counted against 300 leukocytes, and parasite densities were determined by multiplying the parasite count by the absolute leukocyte counts from an automated hematology analyzer (Beckman Coulter® AcT diff2™, Beckman-Coulter Corporation, Miami, USA).
Circulating cytokine measurements
Venous blood samples (<3.0 mL) were centrifuged and plasma was separated immediately, aliquoted, and stored at -70°C until use. Concentrations of IL-10 (Human IL-10; BD PharMingen, San Diego, CA), IL-12 [Human IL-12(p70); BD PharMingen, San Diego, CA], and IL-23 [Human IL-23p19-specific (BMS2023)]; Bender MedSystems, Vienna, Austria] were measured in plasma using sandwich enzyme-linked immunosorbent assays according to the manufacturers’ instructions. Absorbances for each cytokine were measured at 450 nm and cytokine concentrations were calculated using Softmax software (Molecular Devices Corporation, Sunnyvale, CA, USA).
PBMC isolation and culture
Venous blood (70 mL) from healthy, malaria-naïve adult U.S. donors (n=8) was drawn into heparin-containing vials. PBMC were isolated using Ficoll-Hypaque and plated at a density of 1 × 106 cells/mL in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% pooled human sera (heat-inactivated at 56°C for 30 min) according to our previous methods [29, 38]. Cultures were stimulated with media alone (controls) or β-hematin (synthetic Hz, sHz; 10 μg/mL). sHz preparations were sonicated extensively prior to addition to the cultures and were vortexed periodically during addition. Concentrations of sHz used in the present study were comparable to physiological levels of P. falciparum-Hz in children with severe malaria [39]. The study protocol for collection of blood samples from healthy U.S. donors was approved by the Institutional Review Board of the University of Pittsburgh.
sHz preparation
sHz was formed according to the method of Egan et al. [39] by dissolving hemin chloride (bovine, ICN Biomedicals, Aurora, OH) in 0.1 M NaOH, followed by the addition of HCl and acetate stock solution (12.8 M, pH 5) at 60°C. After incubation at 60°C for 150 min in the absence of stirring, the mixture was centrifuged at 10,000 rpm for 10 min, washed with filter-sterilized H2O, and dried on a heat block at 65°C. The resulting pellet was weighed and resuspended at 1.0 mg/mL in filter-sterilized H2O. The re-suspended sHz was sonicated thoroughly before freezing at -20°C. Endotoxin levels were determined to be less than 0.25 EU/mL (0.05 ng/mL) using the Limulus amebocyte lysate test (Cambrex Bio Science, Walkersville, MD).
RNA isolation
Following the removal of culture supernatants, 1.0 mL of Solution D (4 M guanidinium isothiocyanate, 25 mM sodium citrate, 0.5% sarkosyl and β-mercaptoethanol) per 2 × 106 PBMC was added to culture plates, which were stored at -20°C. Total RNA was isolated by the GITC method [40]. Briefly, thawed PBMC in Solution D were transferred to sterile baked glass tubes. Following addition of 100 μL of 2M sodium acetate (pH 4.0), 1 mL phenol, and 200 μL chloroform/isoamyl alcohol (49:1), glass tubes were covered in parafilm and vortexed at high speed for 10 sec. Samples were incubated on ice for 30 min, and then centrifuged at 3750 rpm for 30 min at 4°C. Three quarters (750 μL) of the upper aqueous phase of each centrifuged sample was transferred to a sterile micro-centrifuge tube. An equal volume of cold isopropanol was added to the extracted RNA in each tube, after which the solution was mixed thoroughly and precipitated at -20°C for a minimum of 2 days. Precipitated RNA was pelleted by centrifugation at 10,000 rpm for 30 min at 4°C. Isopropanol was decanted, and tubes were centrifuged repeatedly to remove residual isopropanol with a pipette. Open tubes were placed upside-down under a fume hood for 15-30 min to allow isopropanol to evaporate without complete drying of the RNA pellet. RNA was subsequently dissolved on ice with a minimal (15 μL) volume of DEPC-treated water. RNA samples were then tritriated, held on ice for 15 min, and heated at 65°C for 10 min to solubilize the RNA. Samples were stored at -20°C.
Quantitative real time RT-PCR
Total RNA (1 μg) was reverse-transcribed into cDNA using a PTC-100 Peltier Thermal Cycler (MJ Research, Inc., Waltham, MA). Cytokine gene expression was analyzed by quantitative real time PCR on an Applied Biosystems 7500 Real Time PCR System (Applied Biosystems, Foster City, CA). cDNA was amplified in duplicate with the following primer/probe sets (Applied Biosystems, Foster City, CA): IL-12p35 (Hs00168405m1), IL-12p40 (Hs00233688m1), IL-10 (Hs00174086m1), and IL-23p19 (Hs00372324m1). The endogenous control gene β-actin [Accession Number NM001101 (Applied Biosystems, Foster City, CA )] was used as a reference gene to normalize the expression levels of target genes by correcting differences in the amount of cDNA loaded between samples. Controls without cDNA were included in duplicate for each gene to control for nonspecific background fluorescence. Data were analyzed using the comparative CT method (ΔΔ CT) [28]. The change in CT (ΔCT) for each experimental sample was derived by subtracting the endogenous control gene (β-actin) CT from the experimental gene CT. The ΔCT for each experimental sample was then subtracted from the ΔCT of the control (medium alone) sample. Fold change was expressed as 2-ΔΔCT relative to control conditions.
Statistical analyses
Comparison of the continuous variables between groups was performed using Kruskal-Wallis test. Pair-wise comparisons of cytokine levels and cytokine ratios between groups were performed using Mann-Whitney U test. Differences in the proportional measurements were determined using Pearson’s chi-square test. Relationships between plasma cytokine levels, cytokine ratios, Hb levels, and parasitemia were performed using multivariate linear regression models. Parasitemia, plasma cytokine levels, and cytokine ratios were transformed toward normality prior to correlation analyses. Cytokine transcriptional expression was measured in duplicate and presented as mean ± standard error of the mean.
RESULTS
Characteristics of the study participants
The demographic and clinical characteristics of the study participants are listed in Table 1. Children in the different clinical categories were comparable in age and gender (P=0.514 and P=0.990, respectively). However, there were significant differences in axillary temperature, Hb concentrations, and red blood cell (RBC) counts (P<0.001 for all comparisons) across the groups. Post-hoc testing revealed that relative to the AC group, the MlMA and SMA groups had higher axillary temperatures (P<0.001 and P=0.002, respectively), and decreased Hb levels (P<0.001 for both groups) and RBC counts (P<0.001 for both groups). Mean peripheral parasitemia was not significantly different between the MlMA and SMA groups (P=0.421), although children with SMA had a higher geometric mean parasitemia than children with MlMA.
Table 1.
Demographic and clinical characteristics of the study participants
| Aparasitemic Healthy Controls (AC) | Mild Malarial Anemia (MlMA) | Severe Malarial Anemia (SMA) | P value | |
|---|---|---|---|---|
| Number of individuals | 19 | 25 | 25 | |
| Age (mos) | 6 (4.0 - 11.0) | 8 (6.0 - 12.5) | 8 (6.0 - 11.0) | 0.514a |
| Gender (female, %) | 57.9 | 56.0 | 56.0 | 0.990b |
| Axillary temperature (°C) | 36.50 (36.1 - 36.9) | 37.50 (36.8 - 38.5) | 37.30 (36.5 - 38.3) | <0.001a |
| Hemoglobin (g/dL) | 11.6 (11.4 -12.0) | 9.0 (8.4 - 9.9) | 4.2 (3.6 - 4.2) | <0.001a |
| Red blood cells (× 1012/L) | 5.19 (4.82 - 5.52) | 4.13 (3.67 - 4.57) | 1.75 (1.43 - 1.75) | <0.001a |
| Parasitemia (/μL) | 0 | 38593 (6040 - 130162) | 66130 (12747 - 140622) | 0.421c |
| Geometric mean parasitemia | 0 | 25883 | 37490 |
Data are presented as median (interquartile range) unless stated otherwise.
Differences in the age, temperature, Hb levels, and red blood cell counts were compared using Kruskal-Wallis test.
Differences in the proportion of gender were compared using Pearson’s χ2 test.
Parasitemia between the two malaria groups was compared using Mann-Whitney U test.
Absolute cytokine levels and cytokine ratios in the clinical groups
To investigate the role of IL-23 in the pathogenesis of malarial anemia and to explore the relationship between IL-23, IL-12, and IL-10, plasma cytokine levels and cytokine ratios were determined in children with varying severities of malarial anemia. As shown in Figure 1A, IL-23 levels were elevated in children with MlMA (P<0.05) and SMA groups (P=0.095), relative to AC. IL-12 levels progressively declined with increasing disease severity (P<0.05, MlMA vs. AC and P<0.01, SMA vs. AC, Figure 1B), while IL-10 concentrations were significantly higher in children with malarial anemia (P<0.001 for both the MlMA and SMA vs. AC, Figure 1C). The absolute IL-23, IL-12 and IL-10 plasma cytokine levels did not differ significantly between the MlMA and the SMA groups (P>0.05).
Figure 1. Absolute cytokine levels and cytokine ratios in the clinical groups.
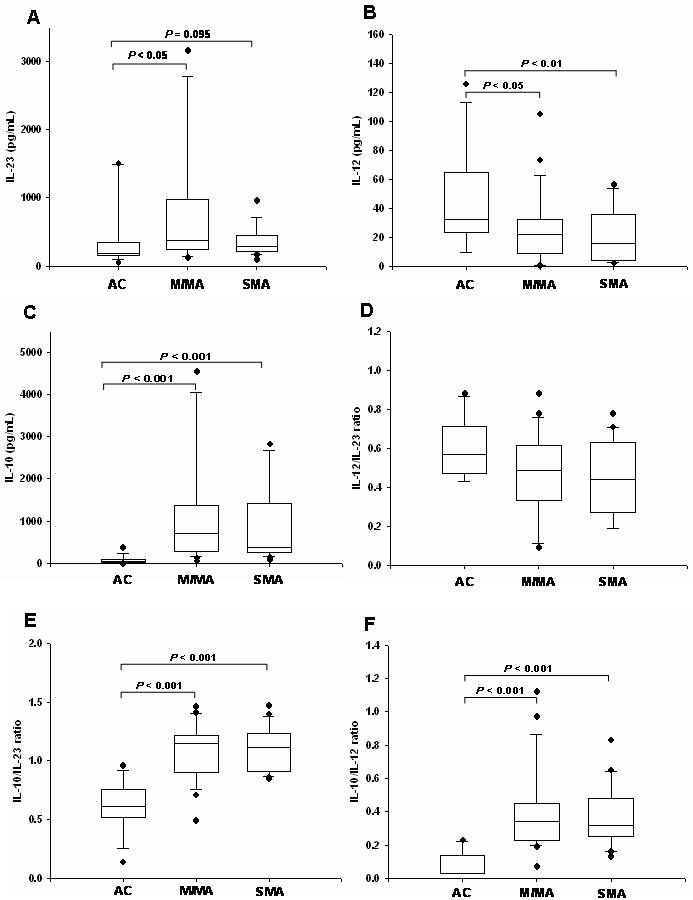
Data are presented as box plots with whiskers and outliers. The box represents the interquartile range, while the whiskers represent the 10th and 90th percentiles, including the outliers (closed circles). The horizontal line in the box indicates the median value. (A) IL-23 levels, (B) IL-12 levels, (C) IL-10 levels, (D) IL-12/IL-23 ratio, (E) IL-10/IL-23 ratio, and (F) IL-10/IL-12 ratio in relation to anemia severity. The group comparisons were done using Kruskal-Wallis test while pair-wise comparisons were done using Mann-Whitney U test.
In addition, since the relative expression of cytokines in the inflammatory milieu is important for mediating malarial anemia pathogenesis [11, 13, 14, 41], and IL-12p70 down-regulates IL-23 production [26], the IL-12 to IL-23 ratio was examined in the different clinical groups. Although there was a decrease in the relative expression of IL-12 to IL-23 in the MlMA and SMA groups relative to AC, the across group comparison was not significant (P=0.215, Figure 1D). Additional relationships were explored between the cytokines since IL-10 suppresses both IL-23 and IL-12 [22, 23, 26]. These analyses revealed that there was a significant difference across the groups for IL-10 production relative to both IL-23 (P<0.001, Figure 1E) and IL-12 (P<0.001, Figure 1F). The IL-10/IL-23 and IL-10/IL-12 ratios showed a similar pattern characterized by an increased IL-10/IL-23 and IL-10/IL-12 ratio in children with MlMA (P<0.001 for both ratios for MlMA vs. AC) and SMA (P<0.001 for both ratios for SMA vs. AC). However, IL-10 production relative to both IL-23 and IL-12 was not significantly different between the MlMA and SMA groups (P>0.05 for both groups). These results suggest that malarial anemia is characterized by elevated levels of IL-10 relative to both IL-23 and IL-12.
Relationship between absolute cytokine levels, cytokine ratios, and parasitemia
To determine the influence of peripheral parasitemia on the patterns of cytokine expression, children were stratified according to parasite density. For these analyses, children were divided into three groups that most appropriately represented the range of parasite burdens among the study participants: aparasitemic, healthy controls (AC, n=19), 1-50,000 parasites/μL (n=24), and >50,000 parasites/μL (n=26). As shown in Figure 2A, IL-23 levels progressively increased with elevated peripheral parasitemia (P=0.063 for 1-50,000 parasites/μL vs. AC and P<0.05 for >50,000 parasites/μL vs. AC). Circulating levels of IL-12 decreased with increasing parasite burden (P=0.069 for 1-50,000 parasites/μL vs. AC and P<0.01 for >50,000 parasites/μL vs. AC, Figure 2B), while plasma IL-10 concentrations progressively increased with elevated peripheral parasitemia (P<0.001 for both 1-50,000 parasites/μL vs. AC and >50,000 parasites/μL vs. AC, Figure 2C).
Figure 2. Plasma cytokine levels/ratios versus parasitemia.

Data are presented as box plots with whiskers and outliers. The box represents the interquartile range while the whiskers represent the 10th and 90th percentiles including the outliers (closed circles). The horizontal line across the box indicates the median value. (A) IL-23 levels, (B) IL-12 levels, (C) IL-10 levels, (D) IL-12/IL-23 ratio, (E) IL-10/IL-23 ratio, and (F) IL-10/IL-12 ratio in relation to parasitemia levels. Differences between groups were tested using Mann-Whitney U test.
Additional analyses were performed to determine the relationship between the relative cytokine production and parasitemia. The expression of IL-12 to IL-23 progressively declined with increasing parasite burden (P=0.330 for 1-50,000 parasites/μL vs. AC and P<0.050 for >50,000 parasites/μL vs. AC, Figure 2D). The relative expression of IL-10 to both IL-23 and IL-12 showed a similar pattern in which the IL-10/IL-23 and IL-10/IL-12 ratios increased in the presence of enhanced parasitemia (P<0.001 for 1-50,000 parasites/μL vs. AC for both ratios and P<0.001 for >50,000 parasites/μL vs. AC for both ratios, Figures 2E and F, respectively).
To further explore the relationship between cytokine patterns and parasitemia, multivariate linear regression analyses were conducted with parasitemia as the dependent variable and age, gender, cytokine levels, and cytokine ratios as independent predictors. For these analyses, a separate model was constructed for absolute cytokine levels and cytokine ratios to maintain a stable regression equation in the sample population (n=69). The multivariate model identified IL-12 (standard partial regression coefficient, β-weight=-0.181, P=0.035) and IL-10 (β-weight=0.724, P<0.001) as significant predictors of parasitemia. In addition, the IL-12/IL-23 (β-weight=-0.616, P=0.004) and IL-10/IL-23 (β-weight=0.876, P<0.001) emerged as predictors of parasitemia. However, in a model containing the significant predictor variables (i.e., IL-10, IL-12, IL-12/IL-23 ratio, and IL-10/IL-23 ratio), only IL-12 (β-weight=-0.881, P=0.036) and IL-10 (β-weight=0.880, P<0.001) remained as significant predictors of parasitemia. Taken together, these results suggest that there is a counter-regulatory mechanism between peripheral parasite density and IL-10 and IL-12 in children with malarial anemia. It is possible that the IL-12 and IL-10 levels also regulate peripheral parasite density, and conversely that peripheral parasite density in turn mediates the production of these cytokines.
Association between absolute cytokine levels, cytokine ratios, and anemia
One of the primary goals of immunological research in children residing in holoendemic P. falciparum transmission areas is to identify the biomarkers associated with SMA. As such, we performed similar multivariate linear regression analyses as those outlined above in which Hb was the dependent variable and age, gender, parasitemia, cytokine levels, and cytokine ratios were the independent predictors. These analyses revealed that none of the cytokines, i.e., IL-10 (β-weight=0.175, P=0.243), IL-12 (β-weight=0.022, P=0.827), or IL-23 (β-weight=0.106, P=0.288), or cytokine ratios i.e., IL-12/IL-23 (β-weight=0.122, P=0.701), IL-10/IL-23 (β-weight=-0.105, P=0.741), or IL-10/IL-12 (β-weight=0.186, P=0.633) were significantly associated with Hb levels.
Effect of sHz on PBMC cytokine gene expression
We have previously shown that parasite-derived products, such as pfHz, cause dysregulation of de novo cytokine transcription in cultured PBMC [27-29, 38]. IL-23 and IL-12 share the IL-12p40 subunit [17] and are both antagonized by IL-10 [22, 23] and secreted forms of IL-12p40 [20, 24]. Aste-Amezaga et al. [22] further reported that IL-10 suppression of IL-12p40 and IL-12p35 occurred at the transcriptional level, with both the IL-12 p40 and IL-12 p35 genes having different requirements for de novo protein synthesis. As such, we examined the effect of sHz on the transcriptional kinetics of IL-23p19, IL-12p35, IL-12p40, and IL-10. PBMC from healthy malaria-naïve adult U.S. donors, (n=8), were stimulated with medium alone (control) or sHz. Cells were harvested at 4, 8, 24, 48, and 72 hrs for determination of transcript levels using real time RT-PCR. Treatment of PBMC with sHz caused a gradual increase in IL-23p19 and IL-12p35 transcripts that were 3.3 and 3.5-fold higher than control conditions at the end of the 72-hr time course experiment (Figures 3A and B). In addition, sHz treatment induced expression of IL-12p40 transcripts that peaked at 24 hrs (∼350-fold vs. control) and rapidly declined to near baseline levels thereafter (Figure 3C). Examination of IL-10 transcripts revealed that IL-10 expression peaked at 24 hrs (3.7-fold vs. controls), but was down-regulated at 72 hrs relative to control conditions (Figure 3D). These results suggest that acquisition of hemozoin by blood mononuclear cells may be, at least in part, responsible for altered production of IL-23, IL-12, and IL-10 in children with malaria. In addition, these results show that sHz causes only marginal increases in IL-23p19, IL-12p35, and IL-10 transcripts, while having a substantial effect on IL-12p40 gene expression.
Figure 3. Effect of sHz on PBMC cytokine gene expression.

PBMC (1 × 106 cells/mL) from healthy, malaria-naïve adult U.S. donors (n=8) were stimulated with medium alone (control; grey line) or with sHz (10μg/mL; black line). Cells were collected at 4, 8, 24, 48, and 72 hrs for determination of IL-23p19 (A), IL-12p35 (B), IL-12p40 (C), and IL-10 (D) transcript levels by real time RT-PCR (determined in duplicate for each time point). Values represent mean ± SEM.
DISCUSSION
Recent studies suggest that IL-23 may be important for promoting anemia in chronic inflammatory diseases [15, 42]. Consistent with these investigations, results presented here illustrate that children with malarial anemia have increased levels of IL-23 in circulation relative to healthy, age-matched controls. Although IL-23 and IL-12 share the p40 subunit [17], levels of IL-23 were elevated in children with malarial anemia, while plasma IL-12 concentrations progressively declined with increasing anemia severity. The decrease in IL-12 with increasing disease severity parallels previous findings in African children residing in areas with intense malaria transmission [12-14, 29, 43-45]. In addition, examination of the IL-12 to IL-23 ratio revealed that SMA is characterized by low levels of IL-12 relative to IL-23. This divergent pattern of IL-23 and IL-12 production in children with malarial anemia may offer important insight into the pathogenesis of SMA since both IL-23 and IL-12 share a common p40 subunit [17] and, as such, may be expected to yield similar directional patterns during active disease.
Although IL-10 can suppress both IL-23 and IL-12 [22, 23], IL-23 can also promote IL-10 production by activated lymphocytes [46]. Results presented here showing that the relative expression of IL-10 to IL-23 progressively increased with increasing anemia severity may suggest that IL-10 could have a suppressive effect on IL-23 production. Our previous investigations illustrate that phagocytosis of malarial pigment by monocytes causes enhanced production of IL-10 that, in turn, suppresses IL-12 [29]. These results have important implications in the pathogenesis of malarial anemia since IL-12 is required for promotion of erythropoiesis through its ability to enhance erythroid burst (BFU-E) and colony forming units (CFU-E) [47, 48]. Results presented here showing high levels of IL-10 relative to IL-12 in children with SMA support previous investigations in children with malarial anemia [12, 14, 29]. Furthermore, findings presented here extend these results by showing that SMA is also characterized by high levels of IL-10 relative to IL-23. However, additional experiments in malaria-infected children that directly examine the effect of IL-10 on IL-23 production are required to confirm the association reported here.
To further explore the relationship between IL-23 and its counter-regulatory cytokines, IL-12 and IL-10, in the context of malaria, children were stratified according to parasite density. These analyses revealed an identical pattern of both absolute cytokine levels and cytokine ratios as those observed when the children were stratified according to anemia severity. However, across group differences in cytokine production and cytokine ratios were more pronounced in children stratified according to parasite density, suggesting that parasitemia, rather than anemia, may be more closely associated with IL-23, IL-12, and IL-10 production. To more quantitatively elucidate the potentially important relationship between parasitemia, absolute cytokine levels, and cytokine ratios, multivariate linear regression analyses were performed. These analyses demonstrated that IL-10, IL-12, IL-12/IL-23 ratio, and IL-10/IL-23 ratio were independent predictors of parasitemia. However, when all of the significant predictor variables were placed in a multivariate model, only IL-10 and IL-12 emerged as significant predictors of parasitemia. The strong positive association between IL-10 and parasitemia and the strong negative association between IL-12 and parasitemia suggest that the overall magnitude of parasitemia may be an important determinant for promoting increased IL-10 and decreased IL-12 production, respectively.
Using a similar modeling approach, we determined the relationship between Hb concentrations, absolute cytokine levels, and cytokine ratios, while controlling for parasitemia. Although IL-23 and IL-10 were increased in children with malarial anemia, while IL-12 levels were suppressed, the multivariate model demonstrated that none of the absolute cytokine levels, or their respective ratios was significant predictors of Hb concentrations after controlling for parasitemia. These results suggest that the pattern of IL-23, IL-12, and IL-10 production observed in children with malarial anemia in the current study were significantly influenced by the strong relationship between parasite density and production of these cytokines. It is also important to point out that in this population of children, we have consistently observed that parasitemia is not strongly associated with malarial anemia severity [29, 35, 38]. This could explain, at least in part, our observation that the absolute levels of these cytokines were comparable between the MlMA and SMA groups. However, there is a need for additional studies to elucidate the precise role of malaria parasites and/or malaria parasite products in conditioning anemia in young children in holoendemic settings.
A number of studies from our laboratory [27-29, 38], as well as others [49-51], have shown that Hz is an important parasitic product that causes dysregulation of de novo cytokine production in human PBMC. As such, Hz was examined as a potential parasitic product that could alter the transcriptional expression of IL-23, IL-12, and IL-10. These results illustrated that IL-23p19 and IL-12p35 have similar transcriptional profiles, characterized by low levels of expression that gradually increased throughout the 72 hr time-course experiment. In contrast, IL-12p40 transcripts were increased over ∼350-fold at 24 hrs prior to rapidly declining to near baseline levels by 48 hrs. Since IL-23 and IL-12p70 share a common p40 subunit [17], these results suggest that IL-12p40 gene products may be the rate-limiting factor for formation of both IL-23 and IL-12p70. Results showing that IL-10 transcriptional expression also peaked at 24 hrs is consistent with the fact that IL-10 is capable of suppressing IL-12p40 [22]. Thus, IL-10 may exert its suppressive effects on IL-23 and IL-12 by down-regulating IL-12p40 gene products. Additional studies in samples collected prospectively in malarial-infected children at different time points throughout the course of infection are required to more definitively examine the inter-relationships among IL-23, IL-12, and IL-10 in vivo. Since IL-23 and IL-12 have distinct roles in the induction of immunity to intracellular pathogens [21, 52-54], as well as different downstream signaling pathways [55], it is important that these pathways be further investigated to delineate the role of IL-23 and IL-12 in malarial anemia pathogenesis. As such, we are currently investigating the role of the IL-23 and IL-12 pathways in prospectively collected samples from children naturally exposed to P. falciparum.
ACKNOWLEDGMENTS
This work was supported by the National Institute of Health grants R01 AI51305-02 (DJP) and D43 TW05884-02 (DJP). This work was also supported by NIH Grant # R01 TW007631 (JMO) from the Fogarty international Center. The content is the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
We are grateful to the parents, guardians, and children from the Siaya District community, western, Kenya for their participation in the study. We also thank all the University of Pittsburgh-KEMRI staff and the Siaya District Hospital staff for their support during this study. We also wish to thank the Director of Kenya Medical Research Institute (KEMRI) for approving this manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Breman JG, Egan A, Keusch GT. The intolerable burden of malaria: a new look at the numbers. Am. J. Trop. Med. Hyg. 2001;64:iv–vii. doi: 10.4269/ajtmh.2001.64.iv. [DOI] [PubMed] [Google Scholar]
- [2].DeMaeyer E, Adiels-Tegman M. The prevalence of anaemia in the world. World Health Stat. Q. 1985;38:302–316. [PubMed] [Google Scholar]
- [3].Lackritz EM, Campbell CC, Ruebush TK, 2nd, Hightower AW, Wakube W, Steketee RW, Were JB. Effect of blood transfusion on survival among children in a Kenyan hospital. Lancet. 1992;340:524–528. doi: 10.1016/0140-6736(92)91719-o. [DOI] [PubMed] [Google Scholar]
- [4].Schellenberg D, Menendez C, Kahigwa E, Font F, Galindo C, Acosta C, Schellenberg JA, Aponte JJ, Kimario J, Urassa H, Mshinda H, Tanner M, Alonso P. African children with malaria in an area of intense Plasmodium falciparum transmission: features on admission to the hospital and risk factors for death. Am. J. Trop. Med. Hyg. 1999;61:431–438. doi: 10.4269/ajtmh.1999.61.431. [DOI] [PubMed] [Google Scholar]
- [5].Bloland PB, Boriga DA, Ruebush TK, McCormick JB, Roberts JM, Oloo AJ, Hawley W, Lal A, Nahlen B, Campbell CC. Longitudinal cohort study of the epidemiology of malaria infections in an area of intense malaria transmission II. Descriptive epidemiology of malaria infection and disease among children. Am. J. Trop. Med. Hyg. 1999;60:641–648. doi: 10.4269/ajtmh.1999.60.641. [DOI] [PubMed] [Google Scholar]
- [6].Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M, Marsh V, Newton C, Winstanley P, Warn P, Peshu N, et al. Indicators of life-threatening malaria in African children. N. Engl. J. Med. 1995;332:1399–1404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- [7].Obonyo CO, Steyerberg EW, Oloo AJ, Habbema JD. Blood transfusions for severe malaria-related anemia in Africa: a decision analysis. Am. J. Trop. Med. Hyg. 1998;59:808–812. doi: 10.4269/ajtmh.1998.59.808. [DOI] [PubMed] [Google Scholar]
- [8].Chang KH, Stevenson MM. Malarial anaemia: mechanisms and implications of insufficient erythropoiesis during blood-stage malaria. Int. J. Parasitol. 2004;34:1501–1516. doi: 10.1016/j.ijpara.2004.10.008. [DOI] [PubMed] [Google Scholar]
- [9].Ekvall H. Malaria and anemia. Curr. Opin. Hematol. 2003;10:108–114. doi: 10.1097/00062752-200303000-00002. [DOI] [PubMed] [Google Scholar]
- [10].Menendez C, Fleming AF, Alonso PL. Malaria-related anaemia. Parasitol. Today. 2000;16:469–476. doi: 10.1016/s0169-4758(00)01774-9. [DOI] [PubMed] [Google Scholar]
- [11].Kurtzhals JA, Akanmori BD, Goka BQ, Adabayeri V, Nkrumah FK, Behr C, Hviid L. The cytokine balance in severe malarial anemia. J. Infect. Dis. 1999;180:1753–1755. doi: 10.1086/315077. [DOI] [PubMed] [Google Scholar]
- [12].Luty AJ, Perkins DJ, Lell B, Schmidt-Ott R, Lehman LG, Luckner D, Greve B, Matousek P, Herbich K, Schmid D, Weinberg JB, Kremsner PG. Low interleukin-12 activity in severe Plasmodium falciparum malaria. Infect. Immun. 2000;68:3909–3915. doi: 10.1128/iai.68.7.3909-3915.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Othoro C, Lal AA, Nahlen B, Koech D, Orago AS, Udhayakumar V. A low interleukin-10 tumor necrosis factor-alpha ratio is associated with malaria anemia in children residing in a holoendemic malaria region in western Kenya. J. Infect. Dis. 1999;179:279–282. doi: 10.1086/314548. [DOI] [PubMed] [Google Scholar]
- [14].Perkins DJ, Weinberg JB, Kremsner PG. Reduced interleukin-12 and transforming growth factor-beta1 in severe childhood malaria: relationship of cytokine balance with disease severity. J. Infect. Dis. 2000;182:988–992. doi: 10.1086/315762. [DOI] [PubMed] [Google Scholar]
- [15].Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- [16].Wiekowski MT, Leach MW, Evans EW, Sullivan L, Chen SC, Vassileva G, Bazan JF, Gorman DM, Kastelein RA, Narula S, Lira SA. Ubiquitous transgenic expression of the IL-23 subunit p19 induces multiorgan inflammation, runting, infertility, and premature death. J. Immunol. 2001;166:7563–7570. doi: 10.4049/jimmunol.166.12.7563. [DOI] [PubMed] [Google Scholar]
- [17].Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- [18].Parham C, Chirica M, Timans J, Vaisberg E, Travis M, Cheung J, Pflanz S, Zhang R, Singh KP, Vega F, To W, Wagner J, O’Farrell AM, McClanahan T, Zurawski S, Hannum C, Gorman D, Rennick DM, Kastelein RA, de Waal Malefyt R, Moore KW. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J. Immunol. 2002;168:5699–5708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- [19].Pirhonen J, Matikainen S, Julkunen I. Regulation of virus-induced IL-12 and IL-23 expression in human macrophages. J. Immunol. 2002;169:5673–5678. doi: 10.4049/jimmunol.169.10.5673. [DOI] [PubMed] [Google Scholar]
- [20].Shimozato O, Ugai S, Chiyo M, Takenobu H, Nagakawa H, Wada A, Kawamura K, Yamamoto H, Tagawa M. The secreted form of the p40 subunit of interleukin (IL)-12 inhibits IL-23 functions and abrogates IL-23-mediated antitumour effects. Immunology. 2006;117:22–28. doi: 10.1111/j.1365-2567.2005.02257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- [22].Aste-Amezaga M, Ma X, Sartori A, Trinchieri G. Molecular mechanisms of the induction of IL-12 and its inhibition by IL-10. J. Immunol. 1998;160:5936–5944. [PubMed] [Google Scholar]
- [23].Schuetze N, Schoeneberger S, Mueller U, Freudenberg MA, Alber G, Straubinger RK. IL-12 family members: differential kinetics of their TLR4-mediated induction by Salmonella enteritidis and the impact of IL-10 in bone marrow-derived macrophages. Int. Immunol. 2005;17:649–659. doi: 10.1093/intimm/dxh247. [DOI] [PubMed] [Google Scholar]
- [24].Gately MK, Carvajal DM, Connaughton SE, Gillessen S, Warrier RR, Kolinsky KD, Wilkinson VL, Dwyer CM, Higgins GF, Jr., Podlaski FJ, Faherty DA, Familletti PC, Stern AS, Presky DH. Interleukin-12 antagonist activity of mouse interleukin-12 p40 homodimer in vitro and in vivo. Ann. N. Y. Acad. Sci. 1996;795:1–12. doi: 10.1111/j.1749-6632.1996.tb52650.x. [DOI] [PubMed] [Google Scholar]
- [25].Belladonna ML, Renauld JC, Bianchi R, Vacca C, Fallarino F, Orabona C, Fioretti MC, Grohmann U, Puccetti P. IL-23 and IL-12 have overlapping, but distinct, effects on murine dendritic cells. J. Immunol. 2002;168:5448–5454. doi: 10.4049/jimmunol.168.11.5448. [DOI] [PubMed] [Google Scholar]
- [26].Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- [27].Keller CC, Davenport GC, Dickman KR, Hittner JB, Kaplan SS, Weinberg JB, Kremsner PG, Perkins DJ. Suppression of prostaglandin E2 by malaria parasite products and antipyretics promotes overproduction of tumor necrosis factor-alpha: association with the pathogenesis of childhood malarial anemia. J. Infect. Dis. 2006;193:1384–1393. doi: 10.1086/503047. [DOI] [PubMed] [Google Scholar]
- [28].Keller CC, Kremsner PG, Hittner JB, Misukonis MA, Weinberg JB, Perkins DJ. Elevated nitric oxide production in children with malarial anemia: hemozoin-induced nitric oxide synthase type 2 transcripts and nitric oxide in blood mononuclear cells. Infect. Immun. 2004;72:4868–4873. doi: 10.1128/IAI.72.8.4868-4873.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Keller CC, Yamo O, Ouma C, Ong’echa JM, Ounah D, Hittner JB, Vulule JM, Perkins DJ. Acquisition of hemozoin by monocytes down-regulates interleukin-12 p40 (IL-12p40) transcripts and circulating IL-12p70 through an IL-10-dependent mechanism: in vivo and in vitro findings in severe malarial anemia. Infect. Immun. 2006;74:5249–5260. doi: 10.1128/IAI.00843-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Slater AF. Malaria pigment. Exp. Parasitol. 1992;74:362–365. doi: 10.1016/0014-4894(92)90162-4. [DOI] [PubMed] [Google Scholar]
- [31].Arese P, Schwarzer E. Malarial pigment (haemozoin): a very active ‘inert’ substance. Ann. Trop. Med. Parasitol. 1997;91:501–516. doi: 10.1080/00034989760879. [DOI] [PubMed] [Google Scholar]
- [32].Schwarzer E, Alessio M, Ulliers D, Arese P. Phagocytosis of the malarial pigment, hemozoin, impairs expression of major histocompatibility complex class II antigen, CD54, and CD11c in human monocytes. Infect. Immun. 1998;66:1601–1606. doi: 10.1128/iai.66.4.1601-1606.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Beier JC, Oster CN, Onyango FK, Bales JD, Sherwood JA, Perkins PV, Chumo DK, Koech DV, Whitmire RE, Roberts CR, et al. Plasmodium falciparum incidence relative to entomologic inoculation rates at a site proposed for testing malaria vaccines in western Kenya. Am. J. Trop. Med. Hyg. 1994;50:529–536. doi: 10.4269/ajtmh.1994.50.529. [DOI] [PubMed] [Google Scholar]
- [34].McElroy PD, ter Kuile FO, Lal AA, Bloland PB, Hawley WA, Oloo AJ, Monto AS, Meshnick SR, Nahlen BL. Effect of Plasmodium falciparum parasitemia density on hemoglobin concentrations among full-term, normal birth weight children in western Kenya, IV. The Asembo Bay Cohort Project. Am. J. Trop. Med. Hyg. 2000;62:504–512. doi: 10.4269/ajtmh.2000.62.504. [DOI] [PubMed] [Google Scholar]
- [35].Ong’echa JM, Keller CC, Were T, Ouma C, Otieno RO, Landis-Lewis Z, Ochiel D, Slingluff JL, Mogere S, Ogonji GA, Orago AS, Vulule JM, Kaplan SS, Day RD, Perkins DJ. Parasitemia, anemia, and malarial anemia in infants and young children in a rural holoendemic Plasmodium falciparum transmission area. Am. J. Trop. Med. Hyg. 2006;74:376–385. [PubMed] [Google Scholar]
- [36].McElroy PD, Lal AA, Hawley WA, Bloland PB, Kuile FO, Oloo AJ, Harlow SD, Lin X, Nahlen BL. Analysis of repeated hemoglobin measures in full-term, normal birth weight Kenyan children between birth and four years of age. III. The Asembo Bay Cohort Project. Am. J. Trop. Med. Hyg. 1999;61:932–940. doi: 10.4269/ajtmh.1999.61.932. [DOI] [PubMed] [Google Scholar]
- [37].Otieno RO, Ouma C, Ong’echa JM, Keller CC, Were T, Waindi EN, Michaels MG, Day RD, Vulule JM, Perkins DJ. Increased severe anemia in HIV-1-exposed and HIV-1-positive infants and children during acute malaria. Aids. 2006;20:275–280. doi: 10.1097/01.aids.0000200533.56490.b7. [DOI] [PubMed] [Google Scholar]
- [38].Awandare GA, Ouma Y, Ouma C, Were T, Otieno R, Keller CC, Davenport GC, Hittner JB, Vulule J, Ferrell R, Ong’echa JM, Perkins DJ. Role of monocyte-acquired hemozoin in suppression of macrophage migration inhibitory factor in children with severe malarial anemia. Infect. Immun. 2007;75:201–210. doi: 10.1128/IAI.01327-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Egan TJ, Mavuso WW, Ncokazi KK. The mechanism of beta-hematin formation in acetate solution. Parallels between hemozoin formation and biomineralization processes. Biochemistry. 2001;40:204–213. doi: 10.1021/bi0013501. [DOI] [PubMed] [Google Scholar]
- [40].Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- [41].Dodoo D, Omer FM, Todd J, Akanmori BD, Koram KA, Riley EM. Absolute levels and ratios of proinflammatory and anti-inflammatory cytokine production in vitro predict clinical immunity to Plasmodium falciparum malaria. J. Infect. Dis. 2002;185:971–979. doi: 10.1086/339408. [DOI] [PubMed] [Google Scholar]
- [42].Means RT, Jr., Krantz SB. Progress in understanding the pathogenesis of the anemia of chronic disease. Blood. 1992;80:1639–1647. [PubMed] [Google Scholar]
- [43].Chaisavaneeyakorn S, Othoro C, Shi YP, Otieno J, Chaiyaroj SC, Lal AA, Udhayakumar V. Relationship between plasma Interleukin-12 (IL-12) and IL-18 levels and severe malarial anemia in an area of holoendemicity in western Kenya. Clin. Diagn. Lab. Immunol. 2003;10:362–366. doi: 10.1128/CDLI.10.3.362-366.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Malaguarnera L, Pignatelli S, Musumeci M, Simpore J, Musumeci S. Plasma levels of interleukin-18 and interleukin-12 in Plasmodium falciparum malaria. Parasite Immunol. 2002;24:489–492. doi: 10.1046/j.1365-3024.2002.00485.x. S. [DOI] [PubMed] [Google Scholar]
- [45].Lyke KE, Burges R, Cissoko Y, Sangare L, Dao M, Diarra I, Kone A, Harley R, Plowe CV, Doumbo OK, Sztein MB. Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1beta), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect. Immun. 2004;72:5630–5637. doi: 10.1128/IAI.72.10.5630-5637.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Vanden Eijnden S, Goriely S, De Wit D, Willems F, Goldman M. IL-23 up-regulates IL-10 and induces IL-17 synthesis by polyclonally activated naive T cells in human. Eur. J. Immunol. 2005;35:469–475. doi: 10.1002/eji.200425677. [DOI] [PubMed] [Google Scholar]
- [47].Mohan K, Stevenson MM. Dyserythropoiesis and severe anaemia associated with malaria correlate with deficient interleukin-12 production. Br. J. Haematol. 1998;103:942–949. doi: 10.1046/j.1365-2141.1998.01126.x. [DOI] [PubMed] [Google Scholar]
- [48].Mohan K, Stevenson MM. Interleukin-12 corrects severe anemia during blood-stage Plasmodium chabaudi AS in susceptible A/J mice. Exp. Hematol. 1998;26:45–52. [PubMed] [Google Scholar]
- [49].Deshpande P, Shastry P. Modulation of cytokine profiles by malaria pigment--hemozoin: role of IL-10 in suppression of proliferative responses of mitogen stimulated human PBMC. Cytokine. 2004;28:205–213. doi: 10.1016/j.cyto.2004.08.002. [DOI] [PubMed] [Google Scholar]
- [50].Mordmuller B, Turrini F, Long H, Kremsner PG, Arese P. Neutrophils and monocytes from subjects with the Mediterranean G6PD variant: effect of Plasmodium falciparum hemozoin on G6PD activity, oxidative burst and cytokine production. Eur. Cytokine Netw. 1998;9:239–246. [PubMed] [Google Scholar]
- [51].Sherry BA, Alava G, Tracey KJ, Martiney J, Cerami A, Slater AF. Malaria-specific metabolite hemozoin mediates the release of several potent endogenous pyrogens (TNF, MIP-1 alpha, and MIP-1 beta) in vitro, and altered thermoregulation in vivo. J. Inflamm. 1995;45:85–96. [PubMed] [Google Scholar]
- [52].Ha SJ, Kim DJ, Baek KH, Yun YD, Sung YC. IL-23 induces stronger sustained CTL and Th1 immune responses than IL-12 in hepatitis C virus envelope protein 2 DNA immunization. J. Immunol. 2004;172:525–531. doi: 10.4049/jimmunol.172.1.525. [DOI] [PubMed] [Google Scholar]
- [53].Khader SA, Pearl JE, Sakamoto K, Gilmartin L, Bell GK, Jelley-Gibbs DM, Ghilardi N, deSauvage F, Cooper AM. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J. Immunol. 2005;175:788–795. doi: 10.4049/jimmunol.175.2.788. [DOI] [PubMed] [Google Scholar]
- [54].Verreck FA, de Boer T, Langenberg DM, Hoeve MA, Kramer M, Vaisberg E, Kastelein R, Kolk A, de Waal-Malefyt R, Ottenhoff TH. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc. Natl. Acad. Sci. U.S.A. 2004;101:4560–4565. doi: 10.1073/pnas.0400983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Watford WT, Hissong BD, Bream JH, Kanno Y, Muul L, O’Shea JJ. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol. Rev. 2004;202:139–156. doi: 10.1111/j.0105-2896.2004.00211.x. [DOI] [PubMed] [Google Scholar]


