Abstract
Convection-enhanced delivery (CED) permits the homogeneous distribution of therapeutic agents throughout localized regions of the brain parenchyma without causing tissue damage as occurs with bolus injection. Here, we examined whether CED infusion of the N-type calcium channel antagonists ω-conotoxin GVIA (ω-CTX-G) and ω-conotoxin MVIIA (ω-CTX-M) can attenuate kindling measures in fully amygdala-kindled rats. Rats were implanted with a combination infusion cannula-stimulating electrode assembly into the right basolateral amygdala. Fully kindled animals received infusions of vehicle, ω-CTX-G (0.005, 0.05, and 0.5 nmol), ω-CTX-M (0.05, 0.15, and 0.5 nmol), proteolytically inactivated ω-CTX-M (0.5 nmol), or carbamazepine (500 nmol) into the stimulation site. CED of ω-CTX-G and ω-CTX-M over a 20-min period resulted in a dose-dependent increase in the afterdischarge threshold and a decrease in the afterdischarge duration and behavioral seizure score and duration during a period of 20 min to 1 week after the infusion, indicating an inhibitory effect on the triggering and expression of kindled seizures. The protective effects of ω-conotoxins reached a maximum at 48 h postinfusion, and then they gradually resolved over the next 5 days. In contrast, carbamazepine was active at 20 min but not at 24 h after the infusion, whereas CED of vehicle or inactivated ω-CTX-M had no effect. Except for transient tremor in some rats receiving the highest toxin doses, no adverse effects were observed. These results indicate that local CED of high-molecular-weight presynaptic N-type calcium channel blockers can produce long-lasting inhibition of brain excitability and that they may provide prolonged seizure protection in focal seizure disorders.
Mesial temporal lobe epilepsy (TLE), the most common type of focal epilepsy, remains a significant therapeutic challenge (Nadkarni et al., 2005). It is estimated that 30% of TLE patients are resistant to currently available antiepileptic drugs (Berg et al., 2003). Although resective surgery can lead to seizure control in some of these refractory patients, not all such individuals are candidates for surgery, and even among those considered to be candidates, a substantial portion (17%) will refuse to accept the risks of a major surgical procedure (Berg et al., 2003). The ultimate limiting factor in conventional systemic antiepileptic drug therapy is the unwanted side effects of the therapeutic agent on brain systems not involved in the epileptic process. In addition, systemic toxicities and teratogenicity may be a concern.
In focal epilepsies such as TLE, it may be possible to minimize or avoid side effects and systemic toxicities by restricting delivery of the therapeutic agent to a limited region of brain (Nilsen and Cock, 2004). The success of resective surgery indicates that local targeting has the potential to provide therapeutic benefit. Bolus injection of solutions into the brain parenchyma causes local tissue deformation and damage and provides poor control over the extent of tissue exposure. In addition, drug concentrations in the region exposed are highly inhomogeneous. Convection-enhanced delivery (CED) is an alternative means of delivering therapeutic substances to a localized brain region (Haroun and Brem, 2000). In this technique, the therapeutic agent is distributed by slow infusion of a solution into the interstitial space under positive pressure through a fine cannula (Bobo et al., 1994; Chen et al., 1999). Unlike intraparenchymal injection, CED does not cause structural or functional damage to the infused tissue, and it provides greater control over the distribution of the therapeutic substance. In CED, solutes are distributed homogeneously throughout a distribution volume that is proportional to the infusion volume regardless of the solute’s molecular weight (Bobo et al., 1994; Chen et al., 1999; Groothuis et al., 1999; Occhiogrosso et al., 2003). CED is particularly applicable to slowly diffusing substances of high molecular weight because the distribution of such large solutes is effectively restricted to the region of the infusion.
In the present study, we explored the potential utility of CED for the treatment of focal epilepsy through the local delivery of the highly selective peptide N-type calcium channel antagonists ω-conotoxin GVIA (ω-CTX-G) and ω-conotoxin MVIIA (ω-CTX-M; ziconotide). In brain slice preparations, N-type calcium channel antagonists inhibit epileptiform activity (Boulton and O’Shaughnessy, 1991) by suppressing synaptic transmission (Kamiya et al., 1988; Dutar et al., 1989; Wu and Saggau, 1994). However, the toxins have limited bioavailability, and they distribute poorly in the brain so that they are not active systemically (Olivera et al., 1985; Miljanich and Ramachandran, 1995; Newcomb et al., 2000). They do have anticonvulsant activity when administered at high doses intraventricularly, but effective doses are invariably associated with profound generalized tremor (Jackson and Scheideler, 1996). Here, we sought to determine whether local CED delivery of the toxins would attenuate kindling measures in amygdala-kindled rats, a model of TLE.
Materials and Methods
Animals
Experimentally naive, male Sprague-Dawley rats, weighing 225 to 250 g at the beginning of the study, were obtained from Taconic Farms (Germantown, NY). Rats were housed individually under a controlled environment (24 ± 2°C; 45 ± 5% humidity; 12-h light/dark cycle with lights on between 6:00 AM and 6:00 PM). Each rat had free access to tap water and a nutritionally balanced rodent diet supplemented regularly with fresh fruit and sweetened gelatin dessert. Experiments were conducted between 9:00 AM and 4:00 PM.
All experiments were conducted under a protocol approved by the Institutional Animal Care and Use Committee of the National Institute of Neurological Disorders and Stroke in strict compliance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996; http://www.nap.edu/readingroom/books/labrats/). The animal facilities were fully accredited by the American Association for the Accreditation of Laboratory Animal Care.
Test Substances
Synthetic ω-CTX-G (27 amino acids; mol. wt. 3037) and ω-CTX-M (25 amino acids; mol. wt. 2639) were from Sigma-Aldrich (St. Louis, MO). Each toxin was dissolved in a 0.01% (w/v) solution of bovine serum albumin in 0.1 M phosphate-buffered saline (PBS). Inactivated ω-CTX-M was obtained by mild treatment with chymotrypsin as described by Chung et al. (1995). Briefly, 4 μg of sequencing grade modified bovine chymotrypsin (Princeton Separations, Adelphia, NJ) was added to a solution of 100 μg of ω-CTX-M in 150 μl of 0.1 M PBS. The solution was then incubated at 30°C overnight. At the end of the incubation, the solution was boiled in a water bath for 60 min to inactivate the chymotrypsin, and then diluted with 0.1 M PBS to the final concentration of 0.5 nmol/5 μl. Such an enzymatic digestion of ω-CTX-M results in the selective cleavage of the peptide link between tyrosine-13 and aspartic acid-14, and it results in a 99% decrease in the binding affinity of the toxin for neuronal N-type calcium channels (Nadasdi et al., 1995). Carbamazepine (Sigma-Aldrich) was dissolved in 30% (w/v) polyethylene glycol 400 in 0.1 M PBS.
Kindling
Each rat was chronically implanted with a custom-made guide cannula-bipolar stimulating electrode assembly (Plastics One, Roanoke, VA) (Fig. 1) during an aseptic surgical procedure under general anesthesia induced by a mixture of ketamine (60–75 mg/kg) and medetomidine (0.25–0.5 mg/kg). The cannula-electrode assembly consisted of a 26-gauge, 6.5-mm-long stainless steel guide cannula with two 0.23-mm-diameter stainless steel electrode wires attached diametrically opposed at the periphery. The electrode wires were polyamide-insulated except for the 0.5 mm most distal extent; the tips of the wires were separated by 0.5 mm, and they projected 3.5 mm past the end of the guide cannula. The cannula-electrode was fixed to a threaded central plastic pedestal. The electrode wires passed to a second threaded pedestal with pin connectors to allow a connection with the kindling stimulator. The bipolar electrodes were used for recording and stimulating. Between infusions, the guide cannula was plugged with a dummy cannula assembly consisting of a solid wire of the same length (6.5 mm) as the guide cannula and a threaded plastic cap (Plastics One). The electrode tip was implanted into the basolateral nucleus of the right amygdala at stereotaxic coordinates, measured from bregma (AP, −2.8 mm; ML, 5.0 mm; DV, −8.7 mm) (Paxinos and Watson, 1998). Dental acrylic cement (Lang Dental, Wheeling, IL) and stabilizing stainless steel screws (Plastics One) were used to secure the cannula-electrode assembly to the skull. Ketoprofen was given subcutaneously after surgery, followed by atipamezole (1 mg/kg) to reverse anesthesia. At least 10 days were allowed for recovery after the surgery. The position of the cannula-electrode assembly tip was histologically verified in randomly selected rats at the end of the study.
Fig. 1.
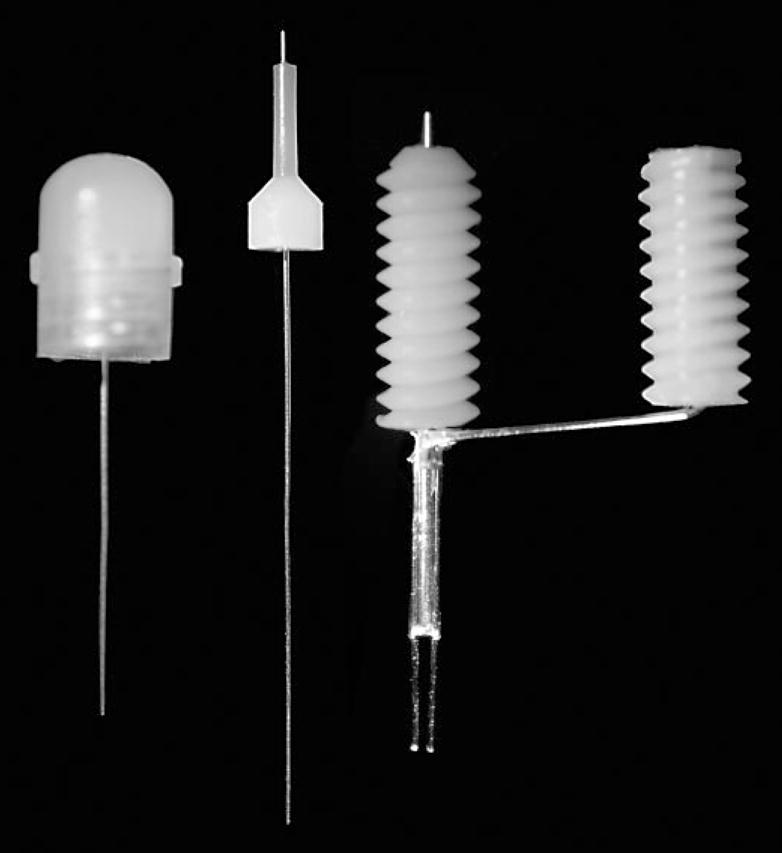
Components of the cannula-bipolar stimulating electrode assembly system used for kindling stimulation and recording, and for CED delivery of solutions into the basolateral amygdala. The combination guide cannula and bipolar stimulating assembly is shown to the right. The guide cannula passes through the threaded left-most pedestal; stimulating electrodes are affixed diametrically opposed to the exterior of the guide cannula below the pedestal. The two wire electrodes pass to the right-most pedestal containing pin connectors to allow electrical connection to the kindling stimulator. The dummy cannula wire-cap assembly is shown to the left. The plastic cap is internally threaded to match the treads of the pedestal of the cannula-electrode assembly. The CED cannula is shown in the center. The plastic stop maintains the tip of the CED cannula 0.5 mm above the tips of the stimulating electrode wires.
Rats were stimulated individually within a 29-cm-diameter Plexiglas cylinder. Each rat was connected to a custom-made stimulator (National Institutes of Health Research Services Branch, Bethesda, MD) via a swivel attachment to allow free movement within the chamber. The stimulator was set to deliver 1-ms duration, bipolar, square current pulses at 60 Hz for 1 s at variable current intensities. Depth electroencephalogram (EEG) signals were recorded via the stimulating electrode (except during the 1-s stimulation interval) with a Grass CP511 AC EEG preamplifier (Astro-Med, West Warwick, RI), and they were stored in digital form using Axotape 9 (Axon Instruments, Foster City, CA).
Kindled seizure activity was assessed using four dependent measures: afterdischarge (AD) threshold, AD duration, severity of behavioral seizures, and behavioral seizure duration. AD threshold refers to the lowest stimulating current intensity (in microamperes) that induces an AD consisting of a train of EEG spikes at 1 Hz or more lasting for at least 5-s duration, with amplitude at least twice the baseline amplitude. AD duration is the total duration of the AD (in seconds). The severity of behavioral seizures was scored according to Racine (1972b), with the following designations: stage 0, no apparent change in behavior; stage 1, facial twitching; stage 2, head nodding associated with more severe facial twitching; stage 3, unilateral forelimb clonus; stage 4, rearing; and stage 5, rearing and loss of balance. Behavioral seizure duration is the duration (in seconds) of limbic seizures (stages 1–2) or motor seizures (stages 3–5). Behavioral changes such as immobility with occasional facial twitches that often occurred after the end of motor seizures were not considered in the duration determination.
After the rats recovered from the surgery, kindling began and it consisted of three phases: 1) prekindling determination of the AD threshold, 2) kindling development, and 3) postkindling redetermination of the AD threshold (Pinel et al., 1976; Freeman and Jarvis, 1981). On day 1, AD threshold was determined by delivering a series of stimulations of increasing intensities (starting at 50 μA and increasing in 25% increments every 3–5 min) until AD was triggered. Rats were excluded from the study if a current of 466-μA intensity failed to produce AD on the first day of kindling. (Rats excluded for this and other reasons, including headmount loss and guide cannula blockage, did not exceed 15% of animals.) During the second phase of kindling, each rat was stimulated daily at a current intensity of 125% of its individual AD threshold value determined on day 1. Daily stimulations continued until the rat exhibited stage 5 seizures during five consecutive days or on 8 days out of the last 10 stimulation days. Rats meeting this criterion were considered “kindled”. Rats that failed to meet the kindling criterion within 30 stimulations were excluded from the study. During the third phase of kindling, AD threshold was redetermined in the same way as during the first phase of kindling. Threshold was redetermined on several consecutive days until AD threshold and the behavioral seizure score produced by stimulation at the AD threshold were stable and reproducible. Rats that did not show stable responses to the electrical stimulation at their respective AD thresholds for several consecutive days were excluded from further testing.
Convection-Enhanced Delivery
The CED system consisted of a programmable infusion pump (model KDS200; KD Scientific, Holliston, MA), a gastight 50-μl Hamilton syringe with a 22-gauge needle (Hamilton Co., Reno, NV), a counter-weighted swivel to allow free movement of the rat (Instech Laboratories Inc., Plymouth Meeting, PA), and a 33-gauge infusion cannula (Plastics One). All components were connected with thick-wall polyethylene 50 tubing (Plastics One). After gently restraining the rat, the infusion cannula (Fig. 1) was slowly inserted into the brain through the guide cannula. The tip of the infusion cannula extended to a depth 0.5 mm above the tips of the stimulating electrode wires, and it was maintained at the appropriate depth by a plastic stop at the top of the cannula. The rat was released and placed in a plastic cylinder for the entire infusion. All infusions were performed in conscious and unrestrained animals. After infusion cannula insertion, the brain tissue was allowed to seal around the cannula for a few minutes before initiation of the infusion. Infusions were delivered at a constant rate of 0.25 μ l/min, which has previously been determined to be optimal for CED of large molecules into the rat brain (Chen et al., 1999). The total infusion volume was 5 μ l. At the end of the infusion, the cannula was left in place for a few minutes to minimize infusate backflow and to ensure better distribution of infusate. Experiments to assess the effects of bolus injection were carried out in an identical manner except that the infusion rate was 2.5 μ l/min.
Evaluation of the Effects of Test Substances on Kindling Measures and for Neurological Toxicity
Effects of test substances on seizure sensitivity in fully kindled rats were assessed by establishing the AD threshold as described previously and by measuring the AD duration, seizure stage, and behavioral seizure duration. After CED infusion of the test substances, animals were stimulated, and kindling measures were determined 20 min postinfusion and on the subsequent days at 24, 48, 72, and 96 h and 1 week postinfusion. Each rat was observed for the occurrence of tremor (rhythmic oscillatory movements of the limbs, head, and trunk) or other neurological signs during the test substance infusion, for at least 1 h after the infusion, and before each subsequent stimulation session. In some animals, stimulation and determination of kindling measures was carried out only during the baseline period and at 7 and 8 days postinfusion.
The effects produced by the test substances on kindling measures were fully reversible within 1 week after infusion so that rats could be reinfused and retested, allowing the total number of animals used in the study to be minimized. Rats were reused for testing different toxin doses. In addition, randomly selected rats from groups previously tested with toxins were reused for testing inactivated ω-CTX-M and carbamazepine. All infusions were separated by at least 14 days. To ensure that there were no interactions between the treatments, all four kindling measures were reevaluated for several days before reuse, and they were compared with the initial baseline values for that animal. Animals were reused only if the AD threshold value and other kindling measures determined upon reevaluation were close to the baseline values for that animal.
Locomotor Activity
At the end of the studies characterizing toxin effects on kindling measures, 18 fully kindled rats were randomly selected for locomotor activity testing with a VersaMax Animal Activity Monitoring System (AccuScan Instruments, Inc., Columbus, OH). The locomotor activity chamber consisted of a Plexiglas arena (40 × 40 cm) surrounded by two sets of infrared light beam sensors detecting horizontal movement of 1.8 cm (at a level of 2.5 cm above the floor) and vertical movement 17.5 cm above the floor. The number of horizontal and vertical beam interruptions (reflecting ambulatory activity and rearing, respectively) were measured during daily sessions lasting 60 min each.
Each rat was exposed to the locomotor activity chamber for 60 min on five successive days to allow habituation. Horizontal and vertical activity trended toward a stable baseline over the 5-day period; the means of the activity counts during the test session on the final 2 days of the habituation period were taken as the baseline for the infusion studies. On the day after the completion of the 5-day habituation period, each rat received an infusion of a test substance. The parameters of the infusion and other factors, including animal handling and external cues were identical to those in the kindled seizure experiments. Horizontal and vertical beam interruptions were determined in 60-min periods beginning 20 min postinfusion and on subsequent days at 24, 48, 72, and 96 h postinfusion.
Intraventricular Infusion
Nonkindled rats for intraventricular infusion studies had a guide cannula assembly (without stimulating electrodes) implanted at stereotaxic coordinates with respect to bregma of AP, −0.80 mm; ML, 1.4 mm; and DV, − 2.6 mm. At the time of infusion, an infusion cannula was inserted through the guide cannula so that the tip extended 1 mm beyond the end of the guide cannula to a DV depth of − 3.6 mm within the right lateral ventricle. The toxin infusion proceeded as for CED at a rate of 0.25 μl/min over 20 min.
Histological Analysis
After the completion of testing, selected animals were perfused transcardially with 4% paraformaldehyde, and the brains were removed for sectioning and cresyl violet and silver staining to assess cannula placement and evidence of neuronal damage.
Presentation of Experimental Data and Statistical Analyses
Kindling measures and locomotor activity test values are presented as group means ± S.E.M. For kindling measures, baseline values (used to calculate percentage of change values) are the averages of values collected during at least three sessions before CED infusion. Averaged relative values with respect to the corresponding average baseline value are used to present the results in the CED infusion experiments. Specifically, effects of each drug treatment are expressed as a change (in percentage) from baseline calculated using the following formula: 100 × [(value before treatment) − (value after treatment)]/(value before treatment). Treatment effects with respect to baseline for each rat were calculated separately and then averaged for a group. Statistical analyses of the data from the kindling and locomotor activity testing were performed by one-way (within a group) and two-way (between groups) repeated measures analysis of variance (ANOVA) after transformation of the percentage change data using arcsine-root transformation. When appropriate, post hoc analysis was performed using Dunnett’s test or Tukey’s test. Tremor data are expressed as frequencies and were analyzed by the Fisher’s exact probability test.
All calculations were made using SigmaStat (SPSS Inc., Chicago, IL) and GraphPad Prism (GraphPad Software Inc., San Diego, CA). Group differences were considered statistically significant at p < 0.05.
Results
Kindling
The data presented represent the results of experiments with 28 fully amygdala-kindled rats. In these animals, daily stimulation at a current intensity of 125% of the AD threshold value resulted in the attainment of the criterion for full kindling (stage 5 seizures) in 15 ± 1 (mean ± S.E.M.) days. The mean AD threshold value in these animals was 197 ± 24 μA on the first day of kindling. All animals in the test group exhibited stable thresholds to elicit stage 5 seizures during the redetermination period. The mean redetermination AD threshold was 96 ± 8 μA, and the mean AD duration was 95 ± 4 s. Stimulations at the individual threshold value resulted in behavioral seizures of stage 4.8 ± 0.1 that lasted for 89 ± 3.5 s.
Effects of CED of ω-CTX-G and ω-CTX-M on Kindling Measures
Animals receiving CED infusions of vehicle exhibited stable kindling measures (AD threshold, AD duration, seizure stage, and behavioral seizure duration) when tested 20 min after the infusion and at intervals up to 1 week (Figs. 2 and 3). In contrast, a single 20 min CED infusion of ω-CTX-G (0.005–0.5 nmol) and ω-CTX-M (0.05–0.5 nmol) was associated with an elevation in AD threshold at time points from 20 min to 96 h postinfusion. In addition, there were effects of both toxins on AD duration, seizure stage, and behavioral seizure duration. Two-way ANOVA indicated statistically significant treatment effects of ω-CTX-G on AD threshold (F3,101 = 6.286; p = 0.002), with accompanying decreases in AD duration (F3,101 = 5.611; p = 0.003) and behavioral seizure duration (F3,101 = 3.049; p = 0.041). ω-CTX-M was also associated with significant treatment effects on AD threshold (F3,124 = 4.157; p = 0.015), AD duration (F3,124 = 3.498; p = 0.028) and behavioral seizure duration (F3,124 = 5.693; p = 0.004). Post hoc analyses indicated statistically significant effects of ω-CTX-G at doses of 0.05 and 0.5 nmol and of ω-CTX-M at doses of 0.15 and 0.5 nmol. The lowest doses tested of ω-CTX-G (0.005 nmol) and ω-CTX-M (0.05 nmol) were not associated with statistically significant effects on any of these measures. Finally, none of the doses tested of either toxin were associated with effects on seizure stage. Table 1 summarizes the results of the statistical analyses.
Fig. 2.
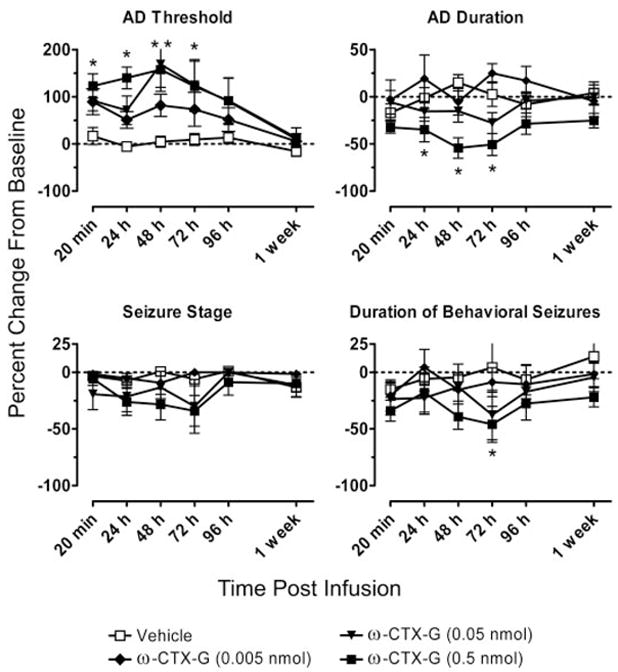
Mean relative AD threshold, AD duration, seizure stage, and duration of behavioral seizures values at intervals from 20 min to 1 week after CED infusion of ω-CTX-G (0.005, 0.05, and 0.5 nmol/infusion) or vehicle. The protocol for AD threshold determination was as described under Materials and Methods. Data are presented as a percentage of change from baseline values calculated as an average of the values obtained in response to at least two stimulation sessions before the infusion. Each data point represents a mean ± S.E.M. of values from 10 rats. *, significantly different from vehicle value at that time point (Tukey’s test) following the detection of a statistically significant main effect by a two-way repeated measures ANOVA. Results of ANOVA and post hoc analysis are presented in Table 1.
Fig. 3.
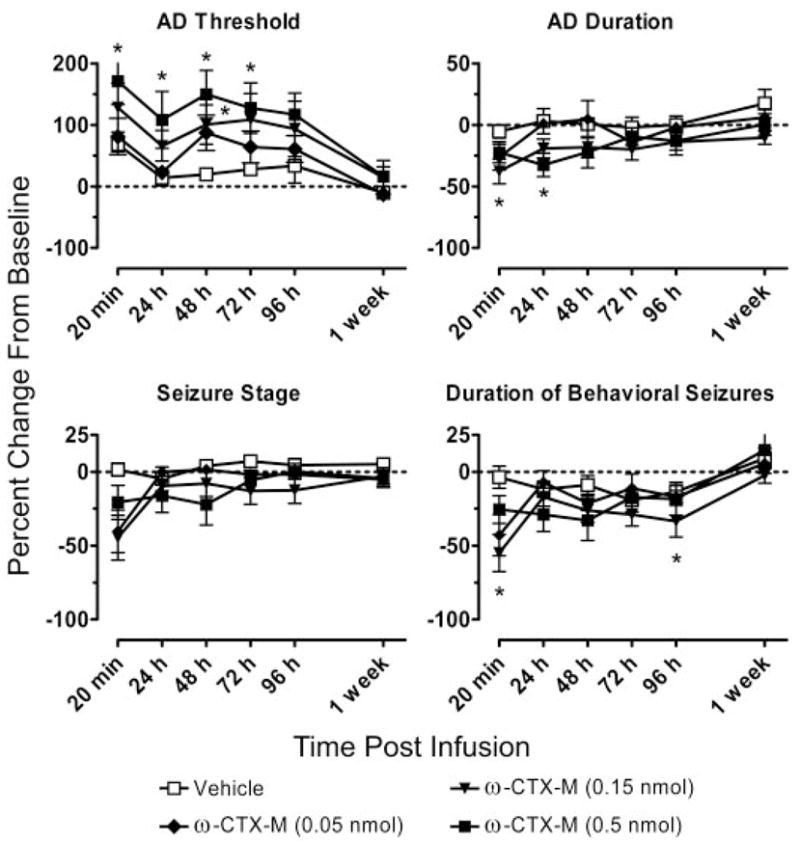
Relative AD threshold, AD duration, seizure stage, and duration of behavioral seizures at intervals from 20 min to 1 week after CED infusion of ω-CTX-M (0.05, 0.15, and 0.5 nmol/infusion) or vehicle. Data are presented as a percentage of change from baseline values calculated as an average of the values obtained in response to at least two stimulation sessions before the infusion. Each data point represents a mean ± S.E.M. of values from 10 to 12 rats. *, significantly different from vehicle value at that time point (Dunnett’s test) following the detection of a statistically significant main effect by a one-way repeated measures ANOVA. Results of ANOVA and post hoc analysis are presented in Table 1.
TABLE 1.
Summary of the outcomes of two-way repeated measures ANOVA and post hoc statistical analyses (Tukey’s test) for ω-CTX-G and ω-CTX-M effects on kindling measures in the experiments of Figs. 2 and 3
Numerical values represent p values.
|
ω-CTX-G
|
ω-CTX-M
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Treatment Effect | Post Hoc Analysis at Dose
|
Treatment Effect | Post Hoc Analysis at Dose
|
|||||
| 0.005 nmol | 0.05 nmol | 0.5 nmol | 0.05 nmol | 0.15 nmol | 0.5 nmol | |||
| AD threshold | 0.002 | N.S. | <0.05 | <0.05 | 0.015 | N.S. | <0.05 | <0.05 |
| AD duration | 0.003 | N.S. | NS | <0.05 | 0.028 | N.S. | <0.05 | N.S. |
| Seizure stage | N.S. | N.S. | ||||||
| Duration of behavioral seizure | 0.041 | N.S. | N.S. | <0.05 | 0.004 | N.S. | <0.05 | N.S. |
N.S., statistically insignificant (p > 0.05).
The effects on kindling measures in the experiments with ω-CTX-G and ω-CTX-M were time-dependent (Figs. 2 and 3). The AD threshold was elevated at all time points from 20 min to 72 h after CED infusion of the highest does tested (0.5 nmol) of ω-CTX-G and ω-CTX-M. At 96 h, the threshold elevations were smaller and not significantly different from the corresponding control values. The threshold values had nearly returned to the baseline pre-CED infusion values at 1 week. The two toxins at the highest doses produced effects of similar magnitude, with elevations in the AD threshold ranging from 106 to 153% with ω-CTX-G and from 95 to 131% with ω-CTX-M at postinfusion intervals between 20 min and 72 h. Mean increases in the AD threshold during the same time period after CED infusion of the intermediate doses (0.05 and 0.15 nmol, respectively) of ω-CTX-G and ω-CTX-M ranged from 75 to 164% and from 52 to 81%, with the maximum increase at 48 h following the CED infusion. The changes produced by the lowest toxin dose (0.005 nmol of ω-CTX-G and 0.05 nmol of ω-CTX-M) were not statistically significant.
Analyses of the results of the results presented in Figs. 2 and 3 by an area-under-the-curve method are presented in Fig. 4. ω-CTX-G exhibited statistically significant, dose-dependent effects on all kindling measures (AD threshold: F3,39 = 5.05, p = 0.007; AD duration: F3,39 = 6.565, p = 0.002; seizure stage: F3,39 = 3.866, p = 0.020; and behavioral seizure duration: F3,39 = 4.466, p = 0.011). Similarly, ω-CTX-M showed statistically significant treatment effects with respect to all kindling measures (AD threshold: F3,45 = 3.305, p = 0.033; AD duration: F3,45 = 3.824, p = 0.019; seizure stage: F3,45 = 3.125, p = 0.040; and behavioral seizure duration: F3,45 = 3.651, p = 0.023). Although the effect on AD threshold was dose-dependent for ω-CTX-M, dose dependence for the 0.15- and 0.5-nmol doses was not obtained for the other kindling measures.
Fig. 4.
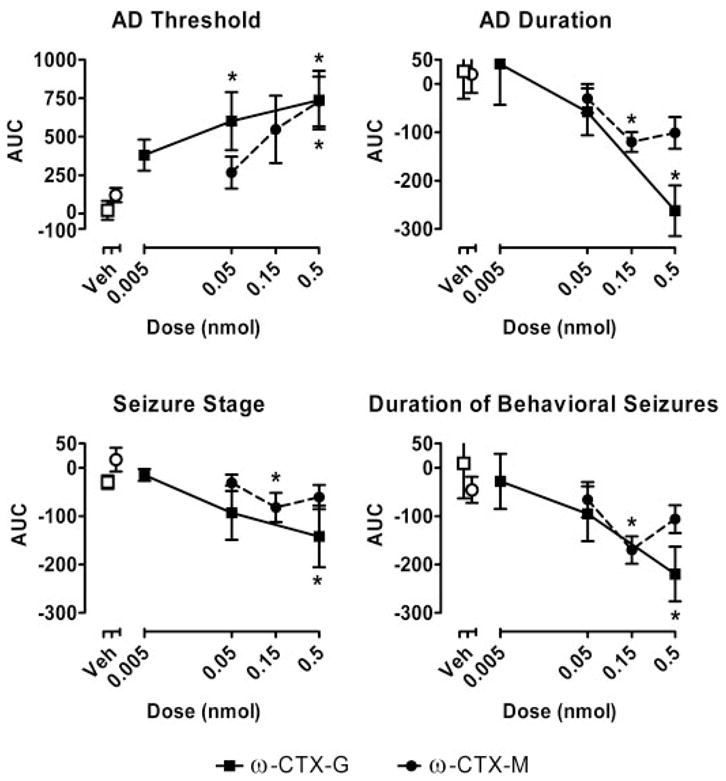
Area-under-the-curve analysis of the data from Figs. 2 and 3. The area under the curve of the percentage of change values for the period 20 min to 1 week following infusion for every time course data set in each animal was determined using the trapezoidal method. The data points represent the mean ± S.E.M. of the values (in percent-days) for all animals receiving a specific toxin and dose. Open squares (□) and circles (○) indicate control values for the group of animals tested with ω-CTX-G (■) and ω-CTX-M (●), respectively. *, significantly different from corresponding vehicle value at that dose (Tukey’s test) following the detection of a statistically significant main effect by a two-way repeated measures ANOVA.
The highest doses of ω-CTX-G and ω-CTX-M were associated with transient whole-body tremor. In rats treated with ω-CTX-G (0.5 nmol), tremor was evident in 3 of 10 rats within 20 min following the CED infusion and in 9 of 10 rats 1 day after the infusion. In each of the animals that exhibited tremor, the tremor resolved by the third day after the infusion. The 0.5-nmol dose of ω-CTX-M was associated with tremor in 4 of 10 rats immediately after the infusion, but no tremor was apparent on subsequent days in any of the animals. No amygdala depth EEG abnormalities were detected in animals exhibiting tremor, indicating that the motor behavior probably did not represent seizure activity. No tremor was observed after infusions of lower doses of the toxins or vehicle. Other than tremor, the CED infusions were not associated with any observable effects on neurological function or behavior.
To minimize the use of animals, kindled rats were reused for the testing of different toxin doses and vehicle. Thus, each animal received at least four CED infusions (three doses plus vehicle). During this sequence, the animals received multiple kindling stimulations. To verify that the baseline was stable throughout, mean baseline kindling measures for the complete group of 22 rats determined before each of the four sequential CED infusions were compared. As shown in Fig. 5, the baseline values in the kindling experiments were stable during the course of repeated infusion and testing (AD threshold: F3,63 = 1.533, p = 0.215; AD duration: F3,63 = 1.078, p = 0.365; seizure stage: F3,63 = 1.501, p = 0.233; and behavioral seizure duration: F3,63 = 0.620, p = 0.604). Thus, it is unlikely that a history of previous testing had an influence on the response to subsequent testing.
Fig. 5.
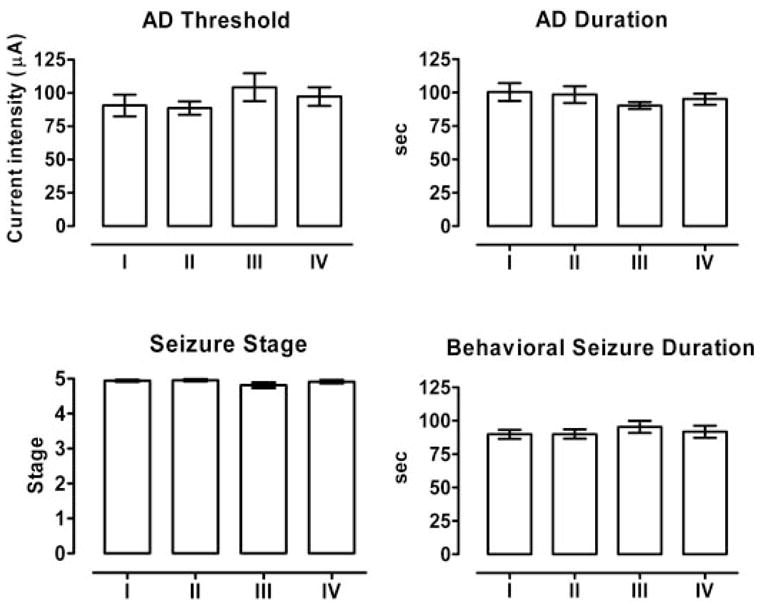
Baseline values for AD threshold, AD duration, seizure stage, and duration of behavioral seizures before each of four sequential CED infusions (test sessions I–IV) of ω-CTX-G and ω-CTX-M at various doses and vehicle in the experiments presented in Figs. 2 and 3. The values for test session I represent the kindling measures for 22 animals before any infusion (true baseline). The treatments in later test session (II–IV) were presented in random order. The baseline values were calculated by averaging seizure measures in response to at least three electrical stimulations before an infusion. Each bar represents the mean ± S.E.M. There were no significant differences between the mean values of any of the seizure measures among the four test sessions (two-way repeated measures ANOVA), indicating that the baseline before CED infusion was stable during repeated CED infusion and testing.
Histological examination of the brains from five animals tested with full sequences of toxin and vehicle demonstrated that multiple CED episodes were only associated with minimal damage to the amygdala and surrounding brain structures at the site of the infusion cannula-stimulating electrode assembly (Fig. 6A). An additional eight rats received bolus infusions of 0.05 nmol of ω-CTX-G in the same volume (5 μl) as for CED but at a 10-fold higher infusion rate (2.5 μl/min) using identical implanted infusion cannula-stimulating electrode assemblies. As in the example in Fig. 6B, seven of these animals exhibited cavitation at the infusion site. The brain of the remaining animal was damaged during removal of the electrode assembly, and it was not be processed for histology
Fig. 6.
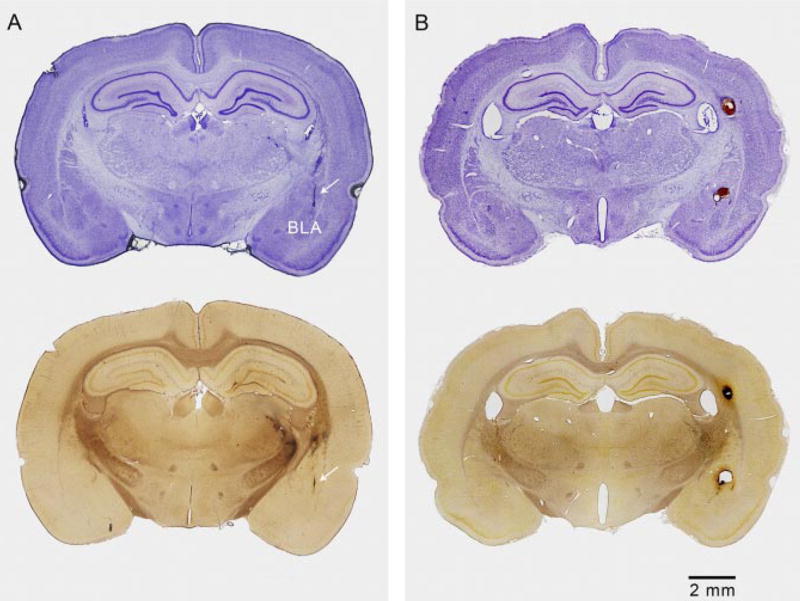
CED causes minimal damage in the amygdala and surrounding brain structures in contrast to bolus infusion, which causes cavitation. A, brain obtained from an animal that had been fully kindled and that had received three CED infusions of ω-CTX-G and an additional vehicle infusion. The animal had experienced multiple stimulation-induced kindled seizures. Top, cresyl violet-stained coronal section through the track of the cannula-electrode assembly and right amygdala. Arrow indicates location of track. There is minimal tissue disruption along the track and no damage apparent in the amygdala. Bottom, the adjacent silver-stained section shows some staining in the right striatum, thalamus, and internal capsule, indicating neuronal disintegration. There is no damage apparent in the amygdala. B, brain obtained from a rat infused with 0.05 nmol of ω-CTX-G at a rate of 2.5 μl/min (10-fold greater than for CED). Tracks of the stimulating electrode wires are seen to extend into the right basolateral amygdala and a cavity is present above the nucleus. A second cavity is present in the alveus due to damage from the guide cannula and tracking of the infused solution up the cannula. Cavitation was observed in seven of eight rats treated in a similar manner.
Does Continued Kindling Account for the Apparent Recovery from the Toxin Effects?
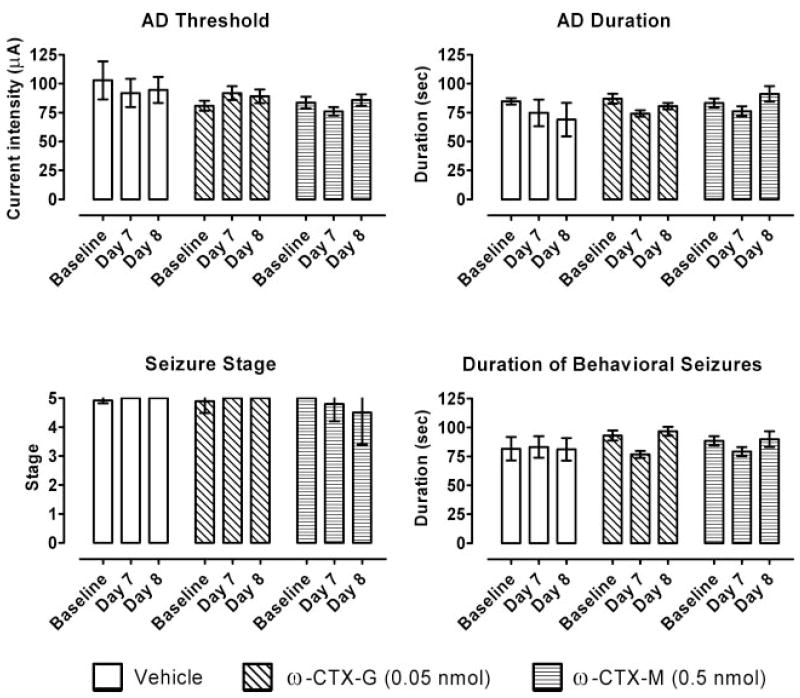
It is possible that the apparent recovery from the effects of toxin exposure over the 1-week period following toxin infusion in the experiments of Figs. 2 and 3 was due to continued kindling resulting from repeated test stimulations and not to a decrease of the toxin effects. In this case, toxin effects on the kindling measures would be expected to be observed at time points beyond the apparent recovery period, if kindling stimulation is not imposed during the intervening period. To determine whether such persistent effects of the toxin do occur, a subset of animals received CED infusion of ω-CTX-G (0.05 nmol) and ω-CTX-M (0.5 nmol), but they did not receive kindling test stimulation until day 7 after the infusion. As shown in Fig. 7, in these animals the kindling measures to test stimulations at days 7 and 8 were no different from the baseline values (p > 0.05). Thus, the apparent recovery of the toxin effects in the experiments of Figs. 2 and 3 was not likely to be due to repeated electrical stimulation.
Fig. 7.
Assessment of the influence of repeated kindling stimulation on recovery from the toxin effects. AD threshold, AD duration, seizure stage, and duration of behavioral seizures before the infusion (baseline control values) and 7 and 8 days after CED infusion of vehicle, ω-CTX-G (0.05 nmol), and ω-CTX-M (0.5 nmol). After the infusions, the animals were not electrically stimulated until 7 days after the infusion. Bars represent the mean ± S.E.M. of the values from six rats. Baseline values for each animal are averages from at least three electrical stimulations before the infusion.
Lack of Effect of Proteolyzed ω-CTX-M on Kindling Measures
To determine whether the observed effects of the toxins were due to their activity as calcium channel antagonists, we conducted control experiments with proteolyzed ω-CTX-M, which is devoid of effects on calcium channels. As shown in Fig. 8, CED infusion of proteolyzed ω-CTX-M (0.5 nmol) failed to cause a significant alteration in any of the kindling measures (AD threshold: F1,21 = 0.0882, p = 0.379; AD duration: F1,21 = 1.115, p = 0.318; seizure stage: F1,21 = 0.486, p = 0.508; and behavioral seizure duration: F1,21 = 0.0007, p = 0.981).
Fig. 8.
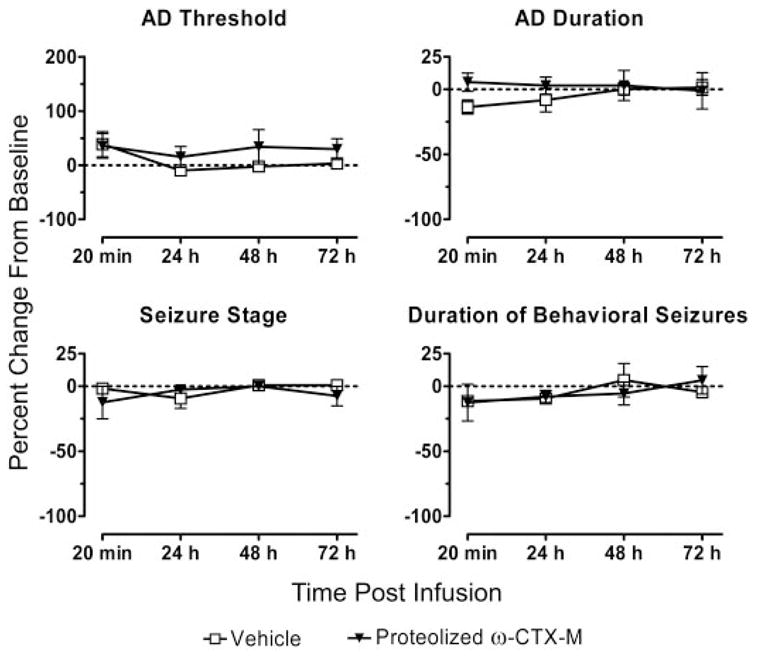
Lack of effect of CED infusion of proteolyzed ω-CTX-M (0.5 nmol) on kindling measures. Mean relative AD, AD duration, seizure stage, and duration of behavioral seizures values at intervals from 20 min to 72 h after CED infusion of proteolyzed ω-CTX-M (eight rats) or vehicle (eight rats). Each data point represents a mean ± S.E.M. There were no significant differences between the values for the proteolyzed toxin and vehicle by a two-way repeated measures ANOVA.
Effects of Carbamazepine on Kindling Measures
To determine whether CED administration of a conventional nonpeptide antiepileptic drug would produce long-lasting effects on seizure measures like the peptide toxins, we conducted a series of experiments with the widely used antiepileptic drug carbamazepine. CED infusion of carbamazepine (500 nmol) resulted in a significant increase in the mean AD threshold and decreases in mean seizure stage and mean behavioral seizure duration at the 20-min time point only (p < 0.05) (Fig. 9). There were no differences in kindling measures at any of the later time points (from 24 h to 1 week). Thus, by comparison, CED administration of the peptide toxins is associated with a distinctively long duration of action. Carbamazepine infusion was not associated with any observable adverse neurological or behavioral effects.
Fig. 9.
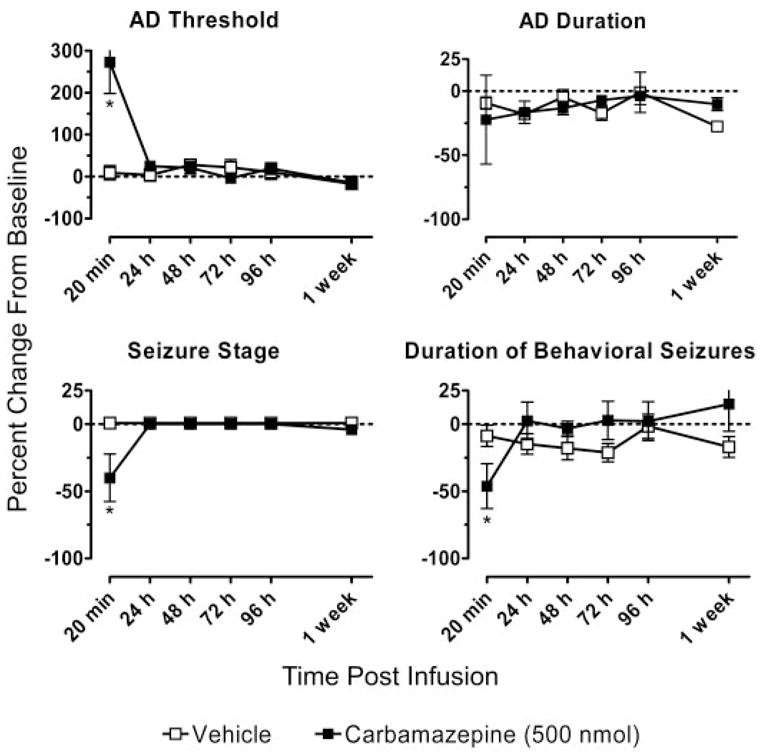
Mean relative AD threshold, AD duration, seizure stage, and duration of behavioral seizures values at intervals from 20 min to 1 week after CED infusion of 500 nmol of carbamazepine (eight rats) or vehicle (six rats). *, significantly different from vehicle value (Tukey’s test) following the detection of a statistically significant main effect by a two-way repeated measures ANOVA.
Effects of CED Infusion of ω-CTX-G and ω-CTX-M on Locomotor Activity
Animals selected for testing were habituated for five consecutive days to the locomotor activity chamber while recording horizontal and vertical activity. As Fig. 10 shows, the rats quickly habituated to the new environment as reflected by stable, low-level locomotor activity after several 60-min habituation sessions. Habituated animals received CED infusions of ω-CTX-G (0.05 nmol), ω-CTX-M (0.5 nmol), or vehicle. The toxin doses chosen were the highest doses that produced significant effects on kindling measures but that did not cause more than transient tremor. Locomotor activity was recorded in five 60-min sessions at intervals following the infusion. Nether toxin was associated with a change in horizontal activity (F2,60 = 0.0413, p = 0.960) or vertical activity (F2,60 = 0.0021, p = 0.998) in comparison with vehicle.
Fig. 10.
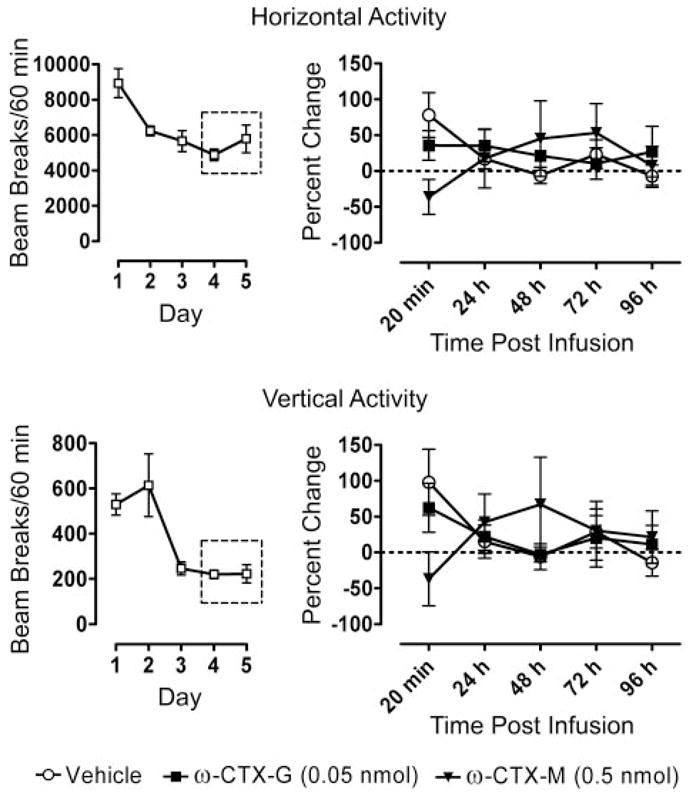
Effects of CED infusion of ω-CTX-G (0.05 nmol), ω-CTX-M (0.5 nmol), or vehicle on locomotor activity at intervals from 20 min to 96 h after the infusion presented as the percentage of change from baseline (right). Each data point represents the mean ± S.E.M. of experiments with six rats. The dotted lines represent no change from the baseline values. The 18 animals were habituated to the locomotor-activity chamber in five daily sessions before the infusion (left). Baseline values were the averages of the activity counts during the last two habituation sessions.
Behavioral Effects of Intraventricular ω-CTX-G
To determine whether CED infusion provided reduced toxicity compared with conventional intraventricular delivery, we administered ω-CTX-G intraventricularly and we observed the animals for gross behavioral disturbances. All six rats injected intraventricularly with 0.05 nmol of ω-CTX-G exhibited locomotor activity arrest [beginning 5.0 ± 0.4 min (mean ± S.E.M.) following the infusion] followed by rhythmic whole-body tremors (beginning 10.8 ± 0.9 min following the infusion). Calculated from the infusion rate and time from infusion onset, the cumulative doses corresponding to the thresholds for locomotor arrest and whole-body tremors were 0.013 ± 0.001 and 0.027 ± 0.002 nmol, respectively. The full dose of toxin was associated with rapid, rhythmic shaking of the entire body. The forelimbs and hindlimbs were abducted so that the animal was unable to stand, causing it to rest on its abdomen. Rats experiencing tremor did not engage in normal behaviors, such as cage exploration, ambulation, eating, or drinking. Consequently, there was significant body weight loss [10.3 ± 0.9% (mean ± S.E.M.)] at 24 h compared with the animals that had received the same dose of toxin by CED (body weight increase at 24 h, 0.1 ± 2.1%; p < 0.001 by t test). Tremors persisted for 24 h, when the animals were euthanized for animal welfare concerns. The overall behavioral syndrome associated with intraventricular infusion of 0.05 nmol of ω-CTX-G was much more severe than that observed after CED infusion of 0.5 nmol of ω-CTX-G. In the latter instance, tremor was more modest in intensity, usually only evident during handling, and it was not associated with an interruption of normal behaviors such as exploration or feeding.
Three additional rats were injected intraventricularly with a 0.005-nmol dose of ω-CTX-G, which is below the previously determined threshold for intraventricular toxicity. Accordingly, these animals exhibited no or only mild behavioral effects of the toxin infusion. One of these animals did not exhibit any locomotor disruption or tremor. The second animal showed transient locomotor disruption at the end of the 20-min infusion, and no tremor during the entire 24-h observation period. The third rat did exhibit transient locomotor activity disruption and mild, transient tremor during the infusion; both signs had resolved at 24 h. On average, these three rats showed a body weight gain of 3.0 ± 1.8% at 24 h.
Discussion
ω-CTX-G and ω-CTX-M are peptide toxins originally isolated from the venoms of fish-hunting cone snails (Olivera et al., 1985, 1987). The toxins selectively inhibit N-type voltage-activated calcium channels (Cav2.2; Dubel et al., 1992). At central synapses, multiple calcium channel types, including N-type calcium channels, mediate the influx of calcium into presynaptic nerve terminals that is required for neurotransmitter release (Wu and Saggau, 1997). By depressing N-type calcium currents in presynaptic terminals, ω-CTX-G and ω-CTX-M inhibit both excitatory and inhibitory synaptic transmission (Kamiya et al., 1988; Dutar et al., 1989; Horne and Kemp, 1991; Burke et al., 1993; Luebke et al., 1993). As a result of the effects on calcium channels, the toxins can inhibit epileptiform activity in some in vitro preparations (Boulton and O’Shaughnessy, 1991). In the present study, we sought to determine whether ω-CTX-G and ω-CTX-M infused using the CED method can interfere with the electrical activation of kindled seizures and their subsequent expression electrographically and behaviorally. Our results demonstrate that local CED administration of both toxins at the site of kindling stimulation can produce a prolonged increase in the intensity of electrical stimulation required to activate an electrographic seizure and that it can depress the seizures elicited.
A key result obtained in the present study is that the toxins can produce long-lasting effects on electrical excitability that persist for up to 1 week. The persistent action of the toxins contrasts with the duration of the response to carbamazepine, whose effect on stimulation threshold and on stimulation-induced seizures was apparent at 20 min but not at 24 h. The persistent action of ω-conotoxins has not been described previously. In a study of the analgesic effects of 7-day infusion of ω-CTX-M, Malmberg and Yaksh (1995) found that the action of the toxin had resolved when tested after stopping the infusion. The ability of CED-administered μ-conotoxins to cause long-lasting changes in excitability indicates that the toxin could have diverse applications as research tools. They may also have potential clinical applications. Two factors could account for the prolonged duration of action of the toxins in these experiments. The binding of ω-CTX-G and ω-CTX-M to N-type calcium channels under ordinary condition is extremely stable, and it is generally considered to be irreversible (Cruz and Olivera, 1986; Grantham et al., 1994; Fox, 1995). If the turnover of N-type calcium channels is low (or if toxin binding inhibits turnover), then the irreversible nature of toxin binding could account for the persistent blocking action of the toxin. In this case, recovery would result from resynthesis of new channel protein. Little is known about the stability of N-type calcium channels at central nervous system synapses. Our results are compatible with the possibility that channel turnover is slow and that it occurs over the course of days. We note that under this scenario the channel turnover rate may not necessarily be the dominant rate accounting for recovery from the toxin effects inasmuch as dissociation of toxin from N-type calcium channels, although slow, could nevertheless occur over the time course of days. The second factor that could contribute to prolonged toxin action is the inability of toxin molecules to leave the site of deposition in the extracellular space following CED infusion. Carbamazepine is a strongly hydrophobic molecule (log P of 2.69 by XLog P; Wang et al., 1997), which readily diffuses across biological membranes. Thus, it can easily leave the site of deposition. Accordingly, even though a relatively large amount of carbamazepine was delivered in the CED experiments, its effects dissipated rapidly. By contrast, ω-CTX-G and ω-CTX-M are hydrophilic (grand average of hydropathicity values, −0.893 and −0.464, respectively; Kyte and Doolittle, 1982), and they would be expected to remain in the extracellular space at the site of deposition. Although the toxins are susceptible to cleavage by endopeptidases and exopeptidases at multiple peptide bonds, cerebrospinal fluid exhibits minimal hydrolytic activity for the peptides, so that local degradation is not expected to limit the duration of the functional activity. That we observed a prolonged duration of action of the toxins when they were administered by CED, but the duration was not prolonged with instillation into the cerebrospinal fluid in the study of Malmberg and Yaksh (1995), suggests that slow toxin clearance may be of greater importance than slow dissociation from the target site as a determinant of the long duration of action of the toxins.
In binding studies, ω-CTX-G and ω-CTX-M have been shown to compete for the same target in brain, which represents the N-type calcium channel α1B subunit (Cav2.2) (Feng et al., 2003). The kinetics of binding and affinity of the two toxins for expressed recombinant rat Cav2.2 calcium channel subunits is similar (KD ~ 100 nM). Mutagenesis studies have indicated that the two toxins bind to overlapping domains on the channel, but that the recognition sites do not share identical structural determinants (Nielsen et al., 2000; Feng et al., 2003). We found that the efficacy and time course of action of the toxins were similar, except that ω-CTX-G produced a significant effect on AD threshold at the 0.05-nmol dose, whereas the effects of ω-CTX-M at this dose did not reach significance (Table 1). In a study with audiogenic seizure susceptible mice, intraventricularly administered ω-CTX-G protected against seizures, whereas ω-CTX-M did not (Jackson and Scheideler, 1996), indicating that as in our results ω-CTX-G may have greater anticonvulsant potency than ω-CTX-M. However, upon intraventricular administration, both toxins induced intense shaking at very low doses. We confirmed that intraventricular infusion of 0.05 nmol of ω-CTX-G, a dose that was devoid of any gross behavioral effects when delivered by CED, caused profound behavioral disruption, including persistent whole-body tremor. Thus, untargeted administration of the conotoxins by instillation into the cerebrospinal fluid is not likely to be a useful therapeutic approach. In contrast, our results indicate that local CED delivery of the toxins can protect against seizures with reduced or no untoward neurological side effects, highlighting the potential of local CED delivery for epilepsy therapy.
To confirm that the effects of toxin infusion were due to functional blockade of N-type calcium channels, we demonstrated that CED infusion of proteolyzed ω-CTX-M, which has less than 1% of the binding affinity of the native toxin and is inactive physiologically (Nadasdi et al., 1995), had no effect on kindling measures. In an additional series of control experiments, we excluded superkindling as an explanation for the apparent recovery from the toxin effects over the 1-week course of the CED studies. Superkindling is a phenomenon whereby continued daily kindling stimulations cause a progressive decrease in the AD threshold, presumably due to continuing kindling (Racine, 1972a). Superkindling could lead to an apparent diminution in activity of the toxin. However, in experiments where we did not apply kindling stimulations until the 7th day after the toxin infusion, we found no change in any kindling measures on days 7 and 8, indicating that the toxin effect decreases even when kindling stimulations are not applied.
Although toxin infusion produced a remarkably long-lasting effect on kindling measures, there was no evidence that the repeated CED infusions or episodes of toxin exposure caused functionally significant tissue damage. Histological examination after several cycles of toxin infusion and multiple kindling stimulations failed to detect any evidence of tissue damage at sites beyond the tract caused by the physical placement of the infusion cannula-stimulating electrode assembly. In addition, as shown in Fig. 4, baseline kindling measures were stable over multiple CED infusions of vehicle and toxins at different doses. Thus, repeated CED infusion of the toxins did not produce permanent alterations in the electrical excitability properties of the brain at the infusion site. Moreover, there was no indication that the toxins had anti-epileptogenic properties to reverse the epileptic state generated by the initial kindling stimulations.
Carbamazepine was highly protective when administered by CED infusion, consistent with its well recognized activity against amygdala-kindled seizures in rats when administered systemically (Albertson et al., 1984). The peak action of carbamazepine occurred earlier than the toxins and its duration was dramatically shorter. The more rapid onset and recovery from carbamazepine are likely due to its ability to diffuse to the target within the infusion zone and then leave the infusion zone, as discussed above. Indeed, carbamazepine has a short duration of action when it is administered systemically or by the intracerebroventricular route in amygdala-kindled rats (Albertson et al., 1984; Barcia et al., 1999). Although the minimum effective dose of carbamazepine was not determined, carbamazepine seems to be markedly less potent than the toxins. The high potency of the ω-conotoxins might be advantageous in a clinical application in which an implanted pump of limited capacity is used to deliver the therapeutic agents.
ω-CTX-G and ω-CTX-M are well recognized to cause “shaking” when injected intracerebroventricularly in mice and, in fact, were initially isolated from cone snail venom on the basis of this activity (Olivera et al., 1985; Miljanich and Ramachandran, 1995). In our CED experiments, the highest doses of ω-CTX-G and ω-CTX-M were associated with tremor in some animals, but lower doses, even those that had long-lasting effects on kindling measures, did not cause tremor. Thus, in contrast to the situation when the toxins are instilled into the cerebrospinal fluid, when administered by CED tremor can be dissociated from therapeutic activity. There were no other observable neurobehavioral effects of the CED toxin infusions. Specifically, the toxins did not cause any significant changes in locomotor activity during the 96-h period after CED infusion. ω-CTX-G was associated with more prolonged tremor than ω-CTX-M, further supporting the view that it is modestly more potent. Because toxin effects on kindling measures could be obtained in the absence of tremor, we presume that the tremor obtained with high toxin doses is due to leakage of the toxins from the amygdala infusion site. Such leakage is likely to be a particular problem in animals with small brains inasmuch as the distribution of substances administered by CED can be more effectively controlled in larger brain volumes (Lieberman et al., 1995; Lonser et al., 2002).
In conclusion, our study provides the first experimental evidence that brief (20-min), localized CED infusions of ω-CTX-G and ω-CTX-M can produce prolonged alterations in brain excitability at the site of the infusion that persist for up to 1 week. Moreover, our results raise the possibility that the CED technique could have utility in suppressing focal seizures. Although we used ω-conotoxins to demonstrate the potential of the CED method, CED is equally applicable to other large blood-brain barrier-impermeable anticonvulsant substances or biological agents, including other anticonvulsant peptides, gene vectors, or cell grafts. In our experimental paradigm, seizures were elicited by electrical stimulation. Further studies are required to demonstrate that CED is similarly effective in suppressing spontaneous seizures emanating from an endogenous seizure focus. If this can be confirmed, CED infusion could eventually be used as a pre-surgical diagnostic tool to determine the functional activity of a localized epileptic brain region and the consequences of its later removal. In addition, the method might be used therapeutically as an alternative to resective surgery in intractable focal epilepsies or where systemic toxicities or the teratogenicity of systemically administered antiepileptic drugs must be avoided.
Acknowledgments
We are grateful to Rebecca Tang for technical assistance, Howard Jaffe for assistance preparing inactivated toxin, Stuart Walbridge for guidance with the CED methodology, and Brita Fritsch for advice on the stereotaxic surgery.
ABBREVIATIONS
- TLE
mesial temporal lobe epilepsy
- CED
convection-enhanced delivery
- ω-CTX-G
ω-conotoxin GVIA
- ω-CTX-M
ω-conotoxin MVIIA
- PBS
phosphate-buffered saline
- EEG
electroencephalogram
- AD
afterdischarge
- ANOVA
analysis of variance
Footnotes
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
References
- Albertson TE, Joy RM, Stark LG. A pharmacological study in the kindling model of epilepsy. Neuropharmacology. 1984;23:1117–1123. doi: 10.1016/0028-3908(84)90227-2. [DOI] [PubMed] [Google Scholar]
- Barcia JA, Rubio P, Alos M, Serralta A, Belda V. Anticonvulsant and neurotoxic effects of intracerebroventricular injection of phenytoin, phenobarbital and carbamazepine in an amygdala-kindling model of epilepsy in the rat. Epilepsy Res. 1999;33:159–167. doi: 10.1016/s0920-1211(98)00085-0. [DOI] [PubMed] [Google Scholar]
- Berg AT, Vickrey BG, Langfitt JT, Sperling MR, Walczak TS, Shinnar S, Bazil CW, Pacia SV, Spencer SS, Multicenter Study of Epilepsy Surgery The multicenter study of epilepsy surgery: recruitment and selection for surgery. Epilepsia. 2003;44:1425–1433. doi: 10.1046/j.1528-1157.2003.24203.x. [DOI] [PubMed] [Google Scholar]
- Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A. 1994;91:2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton CL, O’Shaughnessy CT. The effect of calcium channel antagonists on spontaneous and evoked epileptiform activity in the rat neocortex in vitro. Eur J Neurosci. 1991;3:992–1000. doi: 10.1111/j.1460-9568.1991.tb00035.x. [DOI] [PubMed] [Google Scholar]
- Burke SP, Adams ME, Taylor CP. Inhibition of endogenous glutamate release from hippocampal tissue by Ca2+ channel toxins. Eur J Pharmacol. 1993;238:383–386. doi: 10.1016/0014-2999(93)90870-n. [DOI] [PubMed] [Google Scholar]
- Chen MY, Lonser RR, Morrison PF, Governale LS, Oldfield EH. Variables affecting convection-enhanced delivery to the striatum: a systematic examination of rate of infusion, cannula size, infusate concentration, and tissue-cannula sealing time. J Neurosurg. 1999;90:315–320. doi: 10.3171/jns.1999.90.2.0315. [DOI] [PubMed] [Google Scholar]
- Chung D, Gaur S, Bell JR, Ramachandran J, Nadasdi L. Determination of disulfide bridge pattern in ω-conopeptides. Int J Pept Protein Res. 1995;46:320–325. doi: 10.1111/j.1399-3011.1995.tb00604.x. [DOI] [PubMed] [Google Scholar]
- Cruz LJ, Olivera BM. Calcium channel antagonists. ω-conotoxin defines a new high affinity site. J Biol Chem. 1986;261:6230–6233. [PubMed] [Google Scholar]
- Dubel SJ, Starr TV, Hell J, Ahlijanian MK, Enyeart JJ, Catterall WA, Snutch TP. Molecular cloning of the α1 subunit of an ω-conotoxin-sensitive calcium channel. Proc Natl Acad Sci U S A. 1992;89:5058–5062. doi: 10.1073/pnas.89.11.5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutar P, Rascol O, Lamour Y. ω-Conotoxin GVIA blocks synaptic transmission in the CA1 field of the hippocampus. Eur J Pharmacol. 1989;174:261–266. doi: 10.1016/0014-2999(89)90318-x. [DOI] [PubMed] [Google Scholar]
- Feng ZP, Doering CJ, Winkfein RJ, Beedle AM, Spafford JD, Zamponi GW. Determinants of inhibition of transiently expressed voltage-gated calcium channels by ω-conotoxins GVIA and MVIIA. J Biol Chem. 2003;278:20171–20178. doi: 10.1074/jbc.M300581200. [DOI] [PubMed] [Google Scholar]
- Fox JA. Irreversible and reversible blockade of IMR32 calcium channel currents by synthetic MVIIA and iodinated MVIIC ω-conopeptides. Pflugers Arch. 1995;429:873–875. doi: 10.1007/BF00374813. [DOI] [PubMed] [Google Scholar]
- Freeman FG, Jarvis MF. The effect of interstimulation interval on the assessment and stability of kindled seizure thresholds. Brain Res Bull. 1981;7:629–633. doi: 10.1016/0361-9230(81)90109-x. [DOI] [PubMed] [Google Scholar]
- Grantham CJ, Bowman D, Bath CP, Bell DC, Bleakman D. ω-Conotoxin MVIIC reversibly inhibits a human N-type calcium channel and calcium influx into chick synaptosomes. Neuropharmacology. 1994;33:255–258. doi: 10.1016/0028-3908(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Groothuis DR, Ward S, Itskovich AC, Dobrescu C, Allen CV, Dills C, Levy RM. Comparison of 14C-sucrose delivery to the brain by intravenous, intraventricular, and convection-enhanced intracerebral infusion. J Neurosurg. 1999;90:321–331. doi: 10.3171/jns.1999.90.2.0321. [DOI] [PubMed] [Google Scholar]
- Haroun RI, Brem H. Local drug delivery. Curr Opin Oncol. 2000;12:187–193. doi: 10.1097/00001622-200005000-00001. [DOI] [PubMed] [Google Scholar]
- Horne AL, Kemp JA. The effect of ω-conotoxin GVIA on synaptic transmission within the nucleus accumbens and hippocampus of the rat in vitro. Br J Pharmacol. 1991;103:1733–1739. doi: 10.1111/j.1476-5381.1991.tb09855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources. Institute of Laboratory Animal Resources, Commission on Life Sciences. 7. National Research Council; Washington DC: 1996. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- Jackson HC, Scheideler MA. Behavioural and anticonvulsant effects of Ca2+ channel toxins in DBA/2 mice. Psychopharmacology (Berl) 1996;126:85–90. doi: 10.1007/BF02246415. [DOI] [PubMed] [Google Scholar]
- Kamiya H, Sawada S, Yamamoto C. Synthetic ω-conotoxin blocks synaptic transmission in the hippocampus in vitro. Neurosci Lett. 1988;91:84–88. doi: 10.1016/0304-3940(88)90253-4. [DOI] [PubMed] [Google Scholar]
- Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lieberman DM, Laske DW, Morrison PF, Bankiewicz KS, Oldfield EH. Convection-enhanced distribution of large molecules in gray matter during interstitial drug infusion. J Neurosurg. 1995;82:1021–1029. doi: 10.3171/jns.1995.82.6.1021. [DOI] [PubMed] [Google Scholar]
- Lonser RR, Walbridge S, Garmestani K, Butman JA, Walters HA, Vortmeyer AO, Morrison PF, Brechbiel MW, Oldfield EH. Successful and safe perfusion of the primate brainstem: in vivo magnetic resonance imaging of macromolecular distribution during infusion. J Neurosurg. 2002;97:905–913. doi: 10.3171/jns.2002.97.4.0905. [DOI] [PubMed] [Google Scholar]
- Luebke JI, Dunlap K, Turner TJ. Multiple calcium channel types control glutamatergic synaptic transmission in the hippocampus. Neuron. 1993;11:895–902. doi: 10.1016/0896-6273(93)90119-c. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Yaksh TL. Effect of continuous intrathecal infusion of ω-conopeptides, N-type calcium-channel blockers, on behavior and antinociception in the formalin and hot-plate tests in rats. Pain. 1995;60:83–90. doi: 10.1016/0304-3959(94)00094-U. [DOI] [PubMed] [Google Scholar]
- Miljanich GP, Ramachandran J. Antagonists of neuronal calcium channels: structure, function, and therapeutic implications. Annu Rev Pharmacol Toxicol. 1995;35:707–734. doi: 10.1146/annurev.pa.35.040195.003423. [DOI] [PubMed] [Google Scholar]
- Nadasdi L, Yamashiro D, Chung D, Tarczy-Hornoch K, Adriaenssens P, Ramachandran J. Structure-activity analysis of a Conus peptide blocker of N-type neuronal calcium channels. Biochemistry. 1995;34:8076–8081. doi: 10.1021/bi00025a013. [DOI] [PubMed] [Google Scholar]
- Nadkarni S, LaJoie J, Devinsky O. Current treatments of epilepsy. Neurology. 2005;64:S2–S11. doi: 10.1212/wnl.64.12_suppl_3.s2. [DOI] [PubMed] [Google Scholar]
- Newcomb R, Abbruscato TJ, Singh T, Nadasdi L, Davis TP, Miljanich G. Bioavailability of Ziconotide in brain: influx from blood, stability, and diffusion. Peptides. 2000;21:491–501. doi: 10.1016/s0196-9781(00)00175-3. [DOI] [PubMed] [Google Scholar]
- Nielsen KJ, Schroeder T, Lewis R. Structure-activity relationships of ω-conotoxins at N-type voltage-sensitive calcium channels. J Mol Recognit. 2000;13:55–70. doi: 10.1002/(SICI)1099-1352(200003/04)13:2<55::AID-JMR488>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Nilsen KE, Cock HR. Focal treatment for refractory epilepsy: hope for the future? Brain Res Brain Res Rev. 2004;44:141–153. doi: 10.1016/j.brainresrev.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Occhiogrosso G, Edgar MA, Sandberg DI, Souweidane MM. Prolonged convection-enhanced delivery into the rat brainstem. Neurosurgery. 2003;52:388–393. doi: 10.1227/01.neu.0000043696.83722.8d. [DOI] [PubMed] [Google Scholar]
- Olivera BM, Cruz LJ, de Santos V, LeCheminant GW, Griffin D, Zeikus R, McIntosh JM, Galyean R, Varga J, Gray WR, et al. Neuronal calcium channel antagonists. Discrimination between calcium channel subtypes using ω-conotoxin from Conus magus venom. Biochemistry. 1987;26:2086–2090. doi: 10.1021/bi00382a004. [DOI] [PubMed] [Google Scholar]
- Olivera BM, Gray WR, Zeikus R, McIntosh JM, Varga J, Rivier J, de Santos V, Cruz LJ. Peptide neurotoxins from fish-hunting cone snails. Science. 1985;230:1338–1343. doi: 10.1126/science.4071055. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; Sydney, Australia: 1998. [Google Scholar]
- Pinel JP, Skelton R, Mucha RF. Kindling-related changes in afterdischarge “thresholds. Epilepsia. 1976;17:197–206. doi: 10.1111/j.1528-1157.1976.tb03397.x. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. I. Afterdischarge threshold. Electroencephalogr Clin Neurophysiol. 1972a;32:269–279. doi: 10.1016/0013-4694(72)90176-9. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972b;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Wang R, Fu F, Lai L. A new atom-additive method for calculating partition coefficients. J Chem Inf Comput Sci. 1997;37:615–621. [Google Scholar]
- Wu LG, Saggau P. Pharmacological identification of two types of presynaptic voltage-dependent calcium channels at CA3-CA1 synapses of the hippocampus. J Neurosci. 1994;14:5613–5622. doi: 10.1523/JNEUROSCI.14-09-05613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LG, Saggau P. Presynaptic inhibition of elicited neurotransmitter release. Trends Neurosci. 1997;20:204–212. doi: 10.1016/s0166-2236(96)01015-6. [DOI] [PubMed] [Google Scholar]