Abstract
Injury or loss of the knee meniscus is associated with altered joint stresses that lead to progressive joint degeneration. The goal of this study was to determine if dynamic mechanical compression influences the production of inflammatory mediators by meniscal cells. Dynamic compression increased prostaglandin E2 (PGE2) and nitric oxide (NO) production over a range of stress magnitudes (0.0125-0.5 MPa) in a manner that depended on stress magnitude and zone of tissue origin. Inner zone explants showed greater increases in PGE2 and NO production as compared to outer zone explants. Meniscal tissue expressed NOS2 and NOS3 protein, but not NOS1. Mechanically-induced NO production was blocked by NOS inhibitors, and the non-selective NOS inhibitor L-NMMA augmented PGE2 production in the outer zone only. These findings suggest that the meniscus may serve as an intra-articular source of pro-inflammatory mediators, and that alterations in the magnitude or distribution of joint loading could significantly influence the production of these mediators in vivo.
Keywords: osteoarthritis, proteoglycan, collagen, prostaglandin, mechanobiology, knee
Introduction
The meniscus is an intra-articular fibrocartilaginous structure that is essential for load transmission, shock absorption, stability, and lubrication of the knee joint [1]. Injury or loss of the meniscus significantly changes the magnitude and distribution of stress in the knee and is associated with inflammatory and degenerative changes of the joint [1-5]. However, the underlying mechanisms by which meniscal injury leads to osteoarthritis are not fully understood and may involve interrelated biochemical and biomechanical responses to an altered joint environment.
Osteoarthritis is characterized with the increased production of pro-inflammatory mediators such as prostaglandin E2 (PGE2) and nitric oxide (NO) in joint tissues [6], including the meniscus [7,8]. Similar to synovium or articular cartilage, meniscal cells can produce significant levels of NO [7-9] that can be significantly enhanced by cytokines or mechanical stress [10-15]. However, the relationship between stress magnitude and NO production is not fully understood. Meniscal cells also increase the production of PGE2 in response to cytokines such as tumor necrosis factor alpha (TNF-α), interleukin 1 (IL-1), or IL-17 [9,11,13]. Furthermore, cytokines may alter the response of meniscal cells to mechanical stimulation [14,16,17].
The meniscus is a highly inhomogeneous tissue, exhibiting regional differences in composition, structure, and cell phenotype [18]. Cells of the inner radial two-thirds of the meniscus (inner zone) express high mRNA levels for chondrocytic markers including type II collagen and aggrecan [18-20]. In contrast, cells of the outer one-third of the meniscus (outer zone) express high collagen type I mRNA and protein, characteristics of a more fibroblastic phenotype [20-22]. The mechanical properties of the meniscus also differ with site and are associated with a spatially heterogeneous mechanical environment in the tissue, including differing magnitudes of pressure, compression, and tension [23].
The goal of this study was to examine the hypothesis that dynamic mechanical compression induces a pro-inflammatory response in the meniscus in a manner that depends on the magnitude of stress as well as the site of origin of the tissue (inner versus outer zone). Explants from the inner or outer zone of porcine medial meniscus were subjected to defined loading regimens, and the production of PGE2 and NO was determined. We also examined interactions between the NOS2/NO and COX2/PGE2 systems to determine whether the biomechanical stimulation of one pathway has significant influences on the other, as has been shown in other system [13,24-27].
Materials and Methods
Meniscus samples
Cylindrical explants (5 mm diameter, 2 mm thick) were harvested from the outer one-third of the femoral surface (outer zone) or the inner two-thirds of the medial meniscus (inner zone) from the knee joints of 2-3 year-old female pigs, as described previously [12,14]. Test (compressed) and control (uncompressed) explants were paired from adjacent sites. Only menisci without visible degenerative changes or tears were used. Samples were incubated in Dulbecco's Modified Eagle Medium (DMEM) (Gibco, Grand Island, NY) with 10% heat-inactivated fetal bovine serum (HyClone, Logan, UT), 0.1 mM non-essential amino acids (Gibco), 10 mM HEPES Buffer solution (Gibco), and 100 U/ml penicillin-streptomycin (Gibco) at 37°C, 5% CO2 for 72 hours, at which time the rates of metabolism in culture had equilibrated [12].
Meniscal explant compression
Dynamic compression was applied to the explants (Biopress™, Flexcell International, Hillsborough, NC), as described previously [12,28] at a frequency of 0.5 Hz at stresses (σ) of 0.0125, 0.025, 0.05, 0.1, 0.2 or 0.5 MPa for 24 hours in an unconfined state. In separate experiments, meniscal explants were tested under the same conditions using a materials testing system (ELF 3200, Enduratec, Minnetonka MN) and the peak strains during the final compression cycles (εmax) was measured to range from 0.06 to 0.41, described by the equation: εmax=0.23 log (σ) + 0.47 (R2=0.98). Cell viability was examined using a fluorescent live/dead assay (Invitrogen, Eugene OR) in specimens compressed for 24 hours at 0.5 MPa, and no change in cell viability was observed under any of the loading conditions.
In a second set of experiments, explants from either the inner or outer zones were compressed at 0.1 MPa, 0.5 Hz for 24 hrs and the effects of a selective COX2 inhibitor (100 μM NS398), a non-selective NOS inhibitor (2 mM L-NMMA), and a selective NOS2 inhibitor (2 mM 1400W) (Cayman Chemical, Ann Arbor, MI, USA) were tested.
Quantification of PGE2 and NO synthesis
PGE2 concentrations were measured in the media by ELISA (PGE2 Immunoassay, R&D Systems) and normalized to the wet weight of each explant. Nitric oxide production was measured as the total concentration of nitrate and nitrite (termed “NOx”) in the media [29] and normalized to the wet weight of each explant.
Immunoblots
Immunoblots were performed using highly isoform-specific monoclonal antibodies: anti-COX2 (1:250 dilution, BD Biosciences, San Jose, CA); or anti-NOS1 (1:500 dilution, BD Biosciences and 1:200 Santa Cruz Biotechnology, CA); anti-NOS2 (1:50 dilution, BD Biosciences); or anti-NOS3 (1:2000, R & D Systems). Immunoblots were detected using the Supersignal West Femto Maximum sensitivity substrate (Pierce). Positive controls for COX2 and NOS2 were mouse macrophages treated with LPS and interferon, or pig meniscus treated with 10 ng/ml IL-1 for 24 hrs. Positive control for NOS1 was pig brain lysate, and for NOS3 was pig lung lysate. Since NOS2 and NOS3 are similar sizes, a further negative control was run to rule out cross-reaction of the anti-NOS3 antibody with NOS2 by testing the anti-NOS3 antibody on protein extract from pig articular cartilage incubated with IL-1 for 24 hrs, since this tissue only expresses NOS2 [6].
Statistical analysis
A two-factor ANOVA with repeated measures and Newman-Keuls post hoc test was used to compare the effects of compression (control versus pair compressed specimens at 0.0125-0.5 MPa) and zone of origin (inner and outer zones). In the second set of experiments, two-factor ANOVA with repeated measures and Newman-Keuls post hoc test was used to compare the effects of compression (control versus 0.1 MPa) and the various COX/NOS inhibitors.
Results
Effect of compression on PGE2 synthesis
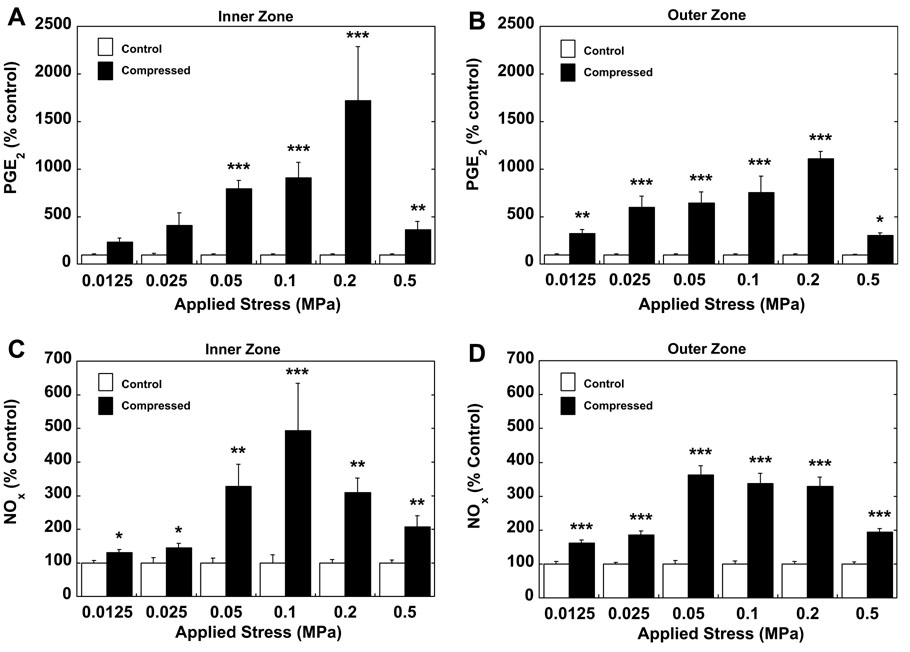
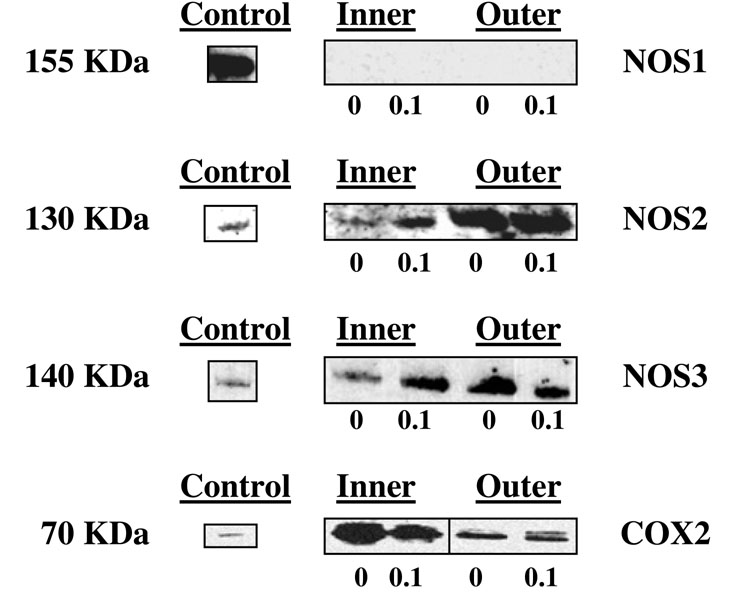
Dynamic compression significantly increased PGE2 production with increasing compression at most magnitudes of stress for the inner zone (Figure 1a) and all magnitudes of stress for the outer zone (Figure 1b). Significantly greater PGE2 production was observed in the inner as compared to the outer zone at 0.2 MPa only (p<0.001). There was no difference in baseline PGE2 production between the zones. Mechanical compression induced expression of COX2 protein (Figure 2).
Figure 1.
PGE2 production from (a) inner and (b) outer zone meniscal explants and NO production from (c) inner and (d) outer zone meniscal explants subjected to 24 hours of dynamic compression at magnitudes from 0.0125–0.5 MPa and a frequency of 0.5 Hz (mean±SEM, expressed as percent of control from the same zone, *p<0.05, **p<0.01, ***p<0.001 vs. control samples, n=16 for inner zone, n=24 for outer zone per level of compression).
Figure 2.
Immunoblot analysis for meniscal explants from the inner and outer zones following 24 hours of dynamic compression at 0.5 Hz and 0.1 MPa. (0=uncompressed control, 0.1=compressed at 0.1 MPa). Antibodies to NOS1, NOS2, NOS3 and COX2 were used. A positive control is also shown for each antibody used.
Effect of compression on NOx synthesis
In both zones of the meniscus, NOx production increased significantly in a stress-dependent manner. Inner zone explants showed maximum NOx production at 0.1 MPa (Figure 1c). Outer zone explants demonstrated a slightly greater increase in NOx synthesis, although there were similar levels of significance at all magnitudes of stress (p<0.001) with maximum stimulation at 0.05 MPa (Figure 1d). A significant difference (p<0.05) in NOx production was observed between inner and outer zone at 0.05 and 0.1 MPa. There was no difference in baseline production of NOx in between zones.
NOS1 protein was not detected in control or compressed samples from either zone (Figure 2). NOS2 and NOS3 were constitutively expressed in both zones, but were increased by mechanical compression in the inner zone only.
Effect of NOS and COX inhibitors on mechanically induced PGE2 production
The inhibition of NOS2 by 1400W resulted in a trend towards a further increase in PGE2 production, particularly in the inner zone (Figure 3a), but had no effect on PGE2 production in the outer zone (Figure 3b). Compression in the presence of the non-selective NOS inhibitor L-NMMA inhibited PGE2 production in the inner zone (Figure 3c) but induced a significant further increase in mechanically induced PGE2 production in the outer zone (Figure 3d). In both zones, inhibition of COX2 by NS398 abolished the stimulatory effect of compression on PGE2 synthesis (Figures 3e, 3f).
Figure 3.
PGE2 production from the inner (a,c,e) and outer (b,d,f) zone meniscal explants subjected to 24 hours of dynamic compression at 0.1 MPa and a frequency of 0.5 Hz in the absence or presence of (a,b) 2 mM 1400W, a NOS2 selective inhibitor, (c,d) 2mM L-NMMA a non-selective NOS inhibitor, and (e,f) 100 μM NS398, a COX2 selective inhibitor (mean±SEM, expressed as percent of control, *p<0.05, ** p<0.01 and ***p<0.001, n=12 per group).
Effect of NOS and COX inhibitors on mechanically induced NO production
In the explants from zones, inhibition of NOS2 with 1400W significantly decreased NO production compared to the explants compressed without 1400W, although NO production was still greater than uncompressed controls, which was statistically significant only for the outer zone (Figures 4a and 4b). In the presence of L-NMMA, compression did not increase NO production in explants from either the outer or inner zones (Figures 4c and 4d). Inhibition of COX2 with NS398 had no effects on mechanically-induced NO synthesis in either zone (Figure 4e and 4f).
Figure 4.
NO production from the inner (a,c,e) and outer (b,d,f) zone meniscal explants subjected to 24 hours of dynamic compression at 0.1 MPa and a frequency of 0.5 Hz in the absence or presence of (a,b) a NOS2 selective inhibitor 2 mM 1400W, (c,d) a non-selective NOS inhibitor 2 mM L-NMMA, or (e,f) a selective COX2 inhibitor 100 μM NS398 (mean±SEM, expressed as percent of control, *p<0.05, **p<0.01 and ***p<0.001, n=12 per group).
Discussion
The findings of this study show that dynamic compression significantly increases the production of PGE2 and NO by meniscal cells. This response was related to the magnitude of applied stress as well as the zone of origin of the tissue. These findings confirm the ability of meniscal cells to respond biologically to different mechanical stimuli [12,14,15,19,30], and further suggest that the meniscus could serve as a significant source of pro-inflammatory mediators in the joint and synovial fluid. In this respect, alterations in the magnitude or distribution of stress on the meniscus (e.g., due to injury) may influence the production of these mediators in vivo.
The meniscus experiences compression during normal activity due to contact with the tibia and femoral articular surfaces, although joint loading also induces spatially and time-varying stresses at tissue level, including tension, compression, shear, and hydrostatic pressure [31,32] that engender a complex mechanical environment at the cellular level [23,33]. Nominal compressive strains (ε) in situ have been estimated to range from ε=0.1−0.15 under physiologic conditions [32], suggesting that the stresses applied in this study induced a range of physiologic and hyperphysiologic conditions predicted to exist within the meniscus. While significant production of NO and PGE2 was stimulated at strains in the “physiologic” range (ε=0.1-0.15), the production of pro-inflammatory mediators was maximal at stress magnitudes (i.e., 0.2 MPa) that induced “hyperphysiologic” strains of 0.25-0.3. Both NO and PGE2 may have varied effects on matrix biosynthesis and degradation, depending on concentration and duration of exposure, and thus further studies are necessary to determine the overall influence of these mediators on various joint tissues.
Our study showed significant quantitative differences in the magnitude of mechanically induced PGE2 and NO production between the inner and outer zones of the meniscus, although similar trends were observed qualitatively. Inner zone explants produced greater relative increases in PGE2 and NO in response to compression than those from the outer zone. These differences likely reflect intrinsic phenotypic differences in the cells populating these different regions, with evidence for fibroblastic phenotype of the outer zone cells compared with the more chondrocytic phenotype of the inner zone cells [15,20,34], which are responsive to dynamic compression [27].
Previous studies have shown that mechanical compression of meniscus tissue increased NO production via NOS2 induction [12,14], although these studies did not distinguish between the inner and outer zones. In the present study, selective NOS2 inhibition did not completely block the mechanical induction of NO in outer zone explants, suggesting the activity of other NOS enzymes. Similarly, other studies have shown similar findings in response to biaxial stretch of isolated meniscal cells [11]. Although there are no selective NOS3 inhibitors available at this time, the non-selective NOS inhibitor L-NMMA inhibited mechanically-induced NO in the outer zone, and NOS3 protein was more abundant in the outer zone. Together, these results provide new evidence for the presence and activity of both NOS2 and NOS3 in the outer zone of the meniscus, and their regulation by mechanical stress. Previous studies have shown constitutive NOS2 mRNA expression in the inner, but not the outer zone of the meniscus [15], consistent with the overall phenotypic differences of these cells. Furthermore, in experimental osteoarthritis induced in rabbits, increased nitrites have been found in the central meniscus compared with the peripheral zone of the meniscus [8]. In contrast, similar studies on articular cartilage explants showed no difference in the effects of 1400W and L-NMMA on mechanically induced NO, as well as the absence of any NOS3 expression [28]. These differences in inflammatory mediator production between zones may partially contribute to the differences in the repair capacity of tears located in the inner zone of the meniscus [35], due to the potential inhibitory effects of NO on meniscus matrix synthesis [9].
In a number of cell types such as articular cartilage, meniscus, or macrophages, NO appears to suppress PGE2 production, and inhibition of NO can lead to further increases in PGE2 production [13,24-26]. In the present study, the inhibition of NO with L-NMMA enhanced PGE2 production in response to mechanical stress in the outer zone. These findings are consistent with studies showing similar effects of interleukin-1-stimulated osteoarthritic human meniscus, which showed significant further increases in PGE2 production with L-NMMA or 1400W [13]. These results have potential implications with respect to the potential clinical use of NOS inhibitors for the treatment of joint disease [36], and suggest that the inhibition of NOS could potentially influence the inflammatory response of different joint tissues due to a “superinduction” of PGE2 [25]. It is hypothesized that the inhibition of PGE2 by NO is due to the nitration of the tyrosine residue (Tyr385) leading to decreased COX2 activity [37].
COX2 inhibition by NS398 abolished the mechanical induction of PGE2, but did not affect NO production. These findings are in contrast to the effects of the COX2 inhibitor on mechanically induced NO production from articular cartilage explants [27], in which NS398 partially inhibited NO production. In other studies, NOS inhibitors have also been shown to decrease PGE2 production [38], or to have no effect on the COX pathway [39] in cartilaginous tissues. Differences in concentration, timing and source (endogenous versus exogenous) of NO can also lead to different results in vitro. Our findings indicate that dynamic loading of the meniscus significantly increases PGE2 and NO production in a stress-dependent manner. A better understanding of such interactions between the NOS and COX pathways may provide important insights into the potential therapeutic use of NOS inhibitors in arthritis.
Acknowledgments
We thank Maureen Upton and Drs. Lori Setton and Christian Fink for valuable discussions, and Robert Nielsen and Stephen Johnson for excellent technical assistance. Supported by the Department of Veterans Affairs, NIH AR50245, AR49790, AG15768 and AR48182. Dr. Alfred Hennerbichler was the recipient of an Erwin-Schroedinger-Fellowship of the Austrian Science Fund (project no. J2294) at Duke University Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aagaard H, Verdonk R. Function of the normal meniscus and consequences of meniscal resection. Scand J Med Sci Sports. 1999;9:134–40. doi: 10.1111/j.1600-0838.1999.tb00443.x. [DOI] [PubMed] [Google Scholar]
- 2.LeRoux MA, Arokoski J, Vail TP, Guilak F, Hyttinen MM, Kiviranta I, Setton LA. Simultaneous changes in the mechanical properties, quantitative collagen organization, and proteoglycan concentration of articular cartilage following canine meniscectomy. Journal of Orthopaedic Research. 2000;18:383–92. doi: 10.1002/jor.1100180309. [DOI] [PubMed] [Google Scholar]
- 3.Carlson CS, Guilak F, Vail TP, Gardin JF, Kraus VB. Synovial fluid biomarker levels predict articular cartilage damage following complete medial meniscectomy in the canine knee. Journal of Orthopaedic Research. 2002;20:92–100. doi: 10.1016/S0736-0266(01)00066-3. [DOI] [PubMed] [Google Scholar]
- 4.Fairbank TJ. Knee joint changes after meniscectomy. Journal of Bone & Joint Surgery. 1948;30-B:664–670. [PubMed] [Google Scholar]
- 5.Roos H, Lauren M, Adalberth T, Roos EM, Jonsson K, Lohmander LS. Knee osteoarthritis after meniscectomy: prevalence of radiographic changes after twenty-one years, compared with matched controls. Arthritis & Rheumatism. 1998;41:687–693. doi: 10.1002/1529-0131(199804)41:4<687::AID-ART16>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 6.Amin AR, Attur M, Abramson SB. Nitric oxide synthase and cyclooxygenases: distribution, regulation, and intervention in arthritis. Current Opinion in Rheumatology. 1999;11:202–9. doi: 10.1097/00002281-199905000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto S, Takahashi K, Ochs RL, Coutts RD, Amiel D, Lotz M. Nitric oxide production and apoptosis in cells of the meniscus during experimental osteoarthritis. Arthritis & Rheumatism. 1999;42:2123–2131. doi: 10.1002/1529-0131(199910)42:10<2123::AID-ANR12>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi K, Mishima H, Hashimoto S, Goomer RS, Harwood FL, Lotz M, Moriya H, Amiel D. Chondrocyte apoptosis and regional differential expression of nitric oxide in the medial meniscus following partial meniscectomy. Journal of Orthopaedic Research. 2001;19:802–808. doi: 10.1016/S0736-0266(01)00023-7. [DOI] [PubMed] [Google Scholar]
- 9.Cao M, Stefanovic-Racic M, Georgescu HI, Miller LA, Evans CH. Generation of nitric oxide by lapine meniscal cells and its effect on matrix metabolism: stimulation of collagen production by arginine. Journal of Orthopaedic Research. 1998;16:104–111. doi: 10.1002/jor.1100160118. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal S, Long P, Gassner R, Piesco NP, Buckley MJ. Cyclic tensile strain suppresses catabolic effects of interleukin-1beta in fibrochondrocytes from the temporomandibular joint. Arthritis & Rheumatism. 2001;44:608–17. doi: 10.1002/1529-0131(200103)44:3<608::AID-ANR109>3.0.CO;2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fermor B, Jeffcoat D, Hennerbichler A, Pisetsky DS, Weinberg JB, Guilak F. The effects of cyclic mechanical strain and tumor necrosis factor alpha on the response of cells of the meniscus. Osteoarthritis and Cartilage. 2004;12:956–962. doi: 10.1016/j.joca.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Fink C, Fermor B, Weinberg JB, Pisetsky DS, Misukonis MA, Guilak F. The effect of dynamic mechanical compression on nitric oxide production in the meniscus. Osteoarthritis and Cartilage. 2001;9:481–487. doi: 10.1053/joca.2001.0415. [DOI] [PubMed] [Google Scholar]
- 13.LeGrand A, Fermor B, Fink C, Pisetsky DS, Weinberg JB, Vail TP, Guilak F. Interleukin-1, tumor necrosis factor alpha, and interleukin-17 synergistically up-regulate nitric oxide and prostaglandin E2 production in explants of human osteoarthritic knee menisci. Arthritis & Rheumatism. 2001;44:2078–2083. doi: 10.1002/1529-0131(200109)44:9<2078::AID-ART358>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 14.Shin SJ, Fermor B, Weinberg JB, Pisetsky DS, Guilak F. Regulation of matrix turnover in meniscal explants: role of mechanical stress, interleukin-1, and nitric oxide. Journal of Applied Physiology. 2003;95:308–313. doi: 10.1152/japplphysiol.00131.2003. [DOI] [PubMed] [Google Scholar]
- 15.Upton ML, Hennerbichler A, Fermor B, Guilak F, Weinberg JB, Setton LA. Biaxial strain effects on cells from the inner and outer regions of the meniscus. Connect Tissue Res. 2006;47:207–14. doi: 10.1080/03008200600846663. [DOI] [PubMed] [Google Scholar]
- 16.Ferretti M, Madhavan S, Deschner J, Rath-Deschner B, Wypasek E, Agarwal S. Dynamic Biophysical Strain Modulates Proinflammatory Gene Induction In Meniscal Fibrochondrocytes. American Journal of Physiology - Cell Physiology. 2006 doi: 10.1152/ajpcell.00529.2005. epub. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deschner J, Wypasek E, Ferretti M, Rath B, Anghelina M, Agarwal S. Regulation of RANKL by biomechanical loading in fibrochondrocytes of meniscus. Journal of Biomechanics. 2006 doi: 10.1016/j.jbiomech.2005.05.034. epub. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hellio Le Graverand MP, Ou Y, Schield-Yee T, Barclay L, Hart D, Natsume T, Rattner JB. The cells of the rabbit meniscus: their arrangement, interrelationship, morphological variations and cytoarchitecture. Journal of Anatomy. 2001;198:525–35. doi: 10.1046/j.1469-7580.2000.19850525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Upton ML, Chen J, Guilak F, Setton LA. Differential effects of static and dynamic compression on meniscal cell gene expression. Journal of Orthopaedic Research. 2003;21:963–969. doi: 10.1016/S0736-0266(03)00063-9. [DOI] [PubMed] [Google Scholar]
- 20.Upton ML, Chen J, Setton LA. Region-specific constitutive gene expression in the adult porcine meniscus. J Orthop Res. 2006;24:1562–70. doi: 10.1002/jor.20146. [DOI] [PubMed] [Google Scholar]
- 21.Spindler KP, Miller RR, Andrish JT, McDevitt CA. Comparison of collagen synthesis in the peripheral and central region of the canine meniscus. Clinical Orthopaedics & Related Research. 1994;303:256–263. [PubMed] [Google Scholar]
- 22.Spindler KP, Mayes CE, Miller RR, Imro AK, Davidson JM. Regional mitogenic response of the meniscus to platelet-derived growth factor (PDGF-AB) Journal of Orthopaedic Research. 1995;13:201–207. doi: 10.1002/jor.1100130208. [DOI] [PubMed] [Google Scholar]
- 23.Upton ML, Guilak F, Laursen TA, Setton LA. Finite element modeling predictions of region-specific cell-matrix mechanics in the meniscus. Biomech Model Mechanobiol. 2006;5:140–9. doi: 10.1007/s10237-006-0031-4. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh DK, Misukonis MA, Reich C, Pisetsky DS, Weinberg JB. Host response to infection: the role of CpG DNA in induction of cyclooxygenase 2 and nitric oxide synthase 2 in murine macrophages. Infection and Immunity. 2001;69:7703–10. doi: 10.1128/IAI.69.12.7703-7710.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amin AR, Attur M, Patel RN, Thakker GD, Marshall PJ, Rediske J, Stuchin SA, Patel IR, Abramson SB. Superinduction of cyclooxygenase-2 activity in human osteoarthritis-affected cartilage. Influence of nitric oxide. Journal of Clinical Investigation. 1997;99:1231–7. doi: 10.1172/JCI119280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henrotin YE, Zheng SX, Deby GP, Labasse AH, Crielaard JM, Reginster JY. Nitric oxide downregulates interleukin 1beta (IL-1beta) stimulated IL-6, IL-8, and prostaglandin E2 production by human chondrocytes. Journal of Rheumatology. 1998;25:1595–601. [PubMed] [Google Scholar]
- 27.Fermor B, Weinberg JB, Pisetsky DS, Misukonis MA, Fink C, Guilak F. Induction of cyclooxygenase-2 by mechanical stress through a nitric oxide-regulated pathway. Osteoarthritis and Cartilage. 2002;10:792–798. doi: 10.1053/joca.2002.0832. [DOI] [PubMed] [Google Scholar]
- 28.Fermor B, Weinberg JB, Pisetsky DS, Misukonis MA, Banes AJ, Guilak F. The effects of static and intermittent compression on nitric oxide production in articular cartilage explants. Journal of Orthopaedic Research. 2001;19:729–737. doi: 10.1016/S0736-0266(00)00049-8. [DOI] [PubMed] [Google Scholar]
- 29.Weinberg JB, Misukonis MA, Shami PJ, Mason SN, Sauls DL, Dittman WA, Wood ER, Smith GK, McDonald B, Bachus KE. Human mononuclear phagocyte inducible nitric oxide synthase (iNOS): analysis of iNOS mRNA, iNOS protein, biopterin, and nitric oxide production by blood monocytes and peritoneal macrophages. Blood. 1995;86:1184–1195. [PubMed] [Google Scholar]
- 30.Imler SM, Doshi AN, Levenston ME. Combined effects of growth factors and static mechanical compression on meniscus explant biosynthesis. Osteoarthritis and Cartilage. 2004;12:736–744. doi: 10.1016/j.joca.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Setton LA, Guilak F, Hsu EW, Vail TP. Biomechanical factors in tissue engineered meniscal repair. Clinical Orthopaedics & Related Research. 1999;367:S254–72. doi: 10.1097/00003086-199910001-00025. [DOI] [PubMed] [Google Scholar]
- 32.Spilker RL, Donzelli PS, Mow VC. A transversely isotropic biphasic finite element model of the meniscus. Journal of Biomechanics. 1992;25:1027–45. doi: 10.1016/0021-9290(92)90038-3. [DOI] [PubMed] [Google Scholar]
- 33.Guilak F, Mow VC. The mechanical environment of the chondrocyte: a biphasic finite element model of cell-matrix interactions in articular cartilage. Journal of Biomechanics. 2000;33:1663–73. [PubMed] [Google Scholar]
- 34.Collier S, Ghosh P. Effects of transforming growth factor beta on proteoglycan synthesis by cell and explant cultures derived from the knee joint meniscus. Osteoarthritis and Cartilage. 1995;3:127–138. doi: 10.1016/s1063-4584(05)80045-7. [DOI] [PubMed] [Google Scholar]
- 35.Arnoczky SP, Warren RF. The microvasculature of the meniscus and its response to injury. An experimental study in the dog. American Journal of Sports Medicine. 1983;11:131–141. doi: 10.1177/036354658301100305. [DOI] [PubMed] [Google Scholar]
- 36.Pelletier JP, Jovanovic D, Fernandes JC, Manning P, Connor JR, Currie MG, Di Battista JA, Martel-Pelletier J. Reduced progression of experimental osteoarthritis in vivo by selective inhibition of inducible nitric oxide synthase. Arthritis & Rheumatism. 1998;41:1275–86. doi: 10.1002/1529-0131(199807)41:7<1275::AID-ART19>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 37.Clancy R, Varenika B, Huang W, Ballou L, Attur M, Amin AR, Abramson SB. Nitric oxide synthase/COX cross-talk: nitric oxide activates COX-1 but inhibits COX-2-derived prostaglandin production. Journal of Immunology. 2000;165:1582–1587. doi: 10.4049/jimmunol.165.3.1582. [DOI] [PubMed] [Google Scholar]
- 38.Manfield L, Jang D, Murrell GA. Nitric oxide enhances cyclooxygenase activity in articular cartilage. Inflammation Research. 1996;45:254–8. doi: 10.1007/BF02259612. [DOI] [PubMed] [Google Scholar]
- 39.Jarvinen TA, Moilanen T, Jarvinen TL, Moilanen E. Endogenous nitric oxide and prostaglandin E2 do not regulate the synthesis of each other in interleukin-1 beta-stimulated rat articular cartilage. Inflammation. 1996;20:683–92. doi: 10.1007/BF01488804. [DOI] [PubMed] [Google Scholar]