Abstract
The posterodorsal aspect of the medial amygdala (MePD) is sexually dimorphic in regional volume, rostrocaudal extent, and neuronal soma size in rats. These dimorphisms are maintained by circulating gonadal hormones, as castration of adult male rats reduces MePD measures, while testosterone treatment of females increases them. We now report that the MePD is also sexually dimorphic in volume, rostrocaudal extent, and somal area in BALB/c mice. Four weeks after castration of adult male mice, MePD regional volume and soma size are reduced, but rostrocaudal extent is not, compared to sham-castrated males. Treatment of adult ovariectomized females with an aromatized metabolite of testosterone, estradiol, for eight weeks increased MePD volume and soma size, but not rostrocaudal extent. To probe the possible role of afferents in the steroid-induced plasticity of the MePD, we examined the effect of removing the olfactory bulbs in gonadally intact males and in estrogen-treated females. Bulbectomy had no effect on MePD morphology with one exception: among gonadally intact males, neuronal soma size was slightly smaller in the right MePD of bulbectomized males compared to males with intact bulbs. These results indicate that the sexual dimorphism and hormone responsiveness of the MePD that has been extensively studied in rats is also present in mice, which offer genetic tools for future research. We detected little or no evidence that olfactory bulb afferents play a role in maintaining MePD morphology in adult mice.
Keywords: olfactory bulbs, testosterone, sexual behavior
1. INTRODUCTION
Sexual dimorphism in the nervous system offers a valuable perspective to learn more about the relationships between neuroanatomy and behavior, as well as the ways in which steroid hormones affect neural function and behavior (Morris, Jordan et al. 2004). In rats, the posterodorsal medial amygdala (MePD) is larger in males than in females in several characteristics, including regional volume and neuronal soma size (Morris et al., 2005). These sexual dimorphisms can be accounted for by circulating hormones in adults, as the measures are reduced in males four weeks after castration and are increased in females after four weeks of testosterone (T) treatment (Cooke, Tabibnia et al. 1999). Treatment of adult gonadectomized rats with two metabolites of T, either dihydrotestosterone, which activates androgen receptors, or estradiol (E), which activates estrogen receptors, indicates that while both receptors contribute to the masculinization of the rat MePD, E is the more effective agent, especially for MePD regional volume (Cooke, Breedlove et al. 2003). Examination of male rats with a genetic defect in the androgen receptor gene, the testicular feminization mutant (Tfm) allele, confirms that both metabolites of T act upon this nucleus (Morris, Jordan et al. 2005). Because the MePD has been implicated in male sexual arousal (Meisel and Sachs 1994; Kondo, Sachs et al. 1997), which waxes and wanes with adult androgen levels, the hormone responsiveness of adult MePD morphology may reflect hormonal activation of sexual arousal.
Aside from Tfm animals, there are very few rat models offering genetic variations that might permit a greater understanding of sexual differentiation of the MePD or other brain regions. Therefore, we asked whether the sexual dimorphism and hormone responsiveness of the MePD also occurs in mice, where many genetic manipulations can be readily accomplished. Because the olfactory bulbs are a major afferent to the medial amygdala (Scalia and Winans 1975), and because removal of the olfactory bulbs causes a profound reduction in male copulatory behavior (Wood and Newman 1995), we also examined whether removal of the olfactory bulbs affects MePD morphology in mice. We found that the MePD of mice is indeed sexually dimorphic and responsive to hormone manipulations in adulthood, but shows virtually no response to the loss of olfactory bulb afferents in adulthood.
2. Results
Sex differences in MePD morphology
We compared males and females subjected to sham gonadectomy using a two-way mixed design ANOVA (sex as an independent variable and hemisphere as a repeated measure) followed by PLSD post-hoc test, and found an overall effect of sex (males larger than females; p < 0.0001) and hemisphere (left larger than right; p < 0.01) on MePD volume (Figure 2A). The interaction was close to significant (p = 0.15), so we further probed with paired T-tests, revealing a significant lateral asymmetry in MePD volume in females (p < 0.01, L>R) but not in males (p = 0.42). This sex difference in asymmetry is opposite to that seen in rats where males have asymmetrical MePD volumes, but females do not (Morris et al., 2005). The direction of asymmetry is reversed as well with female mice having larger left MePD volumes and male rats having larger right MePD volumes.
Figure 2.
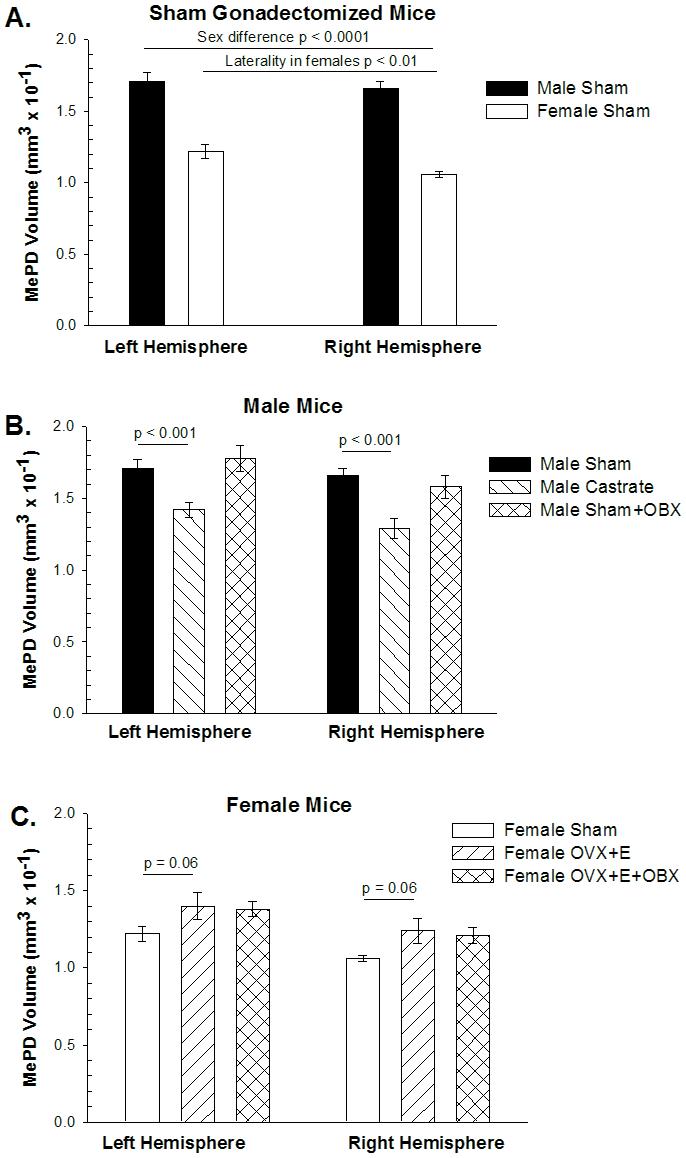
The volume of the posterodorsal medial amygdala (MePD) in BALB/c mice. A. ANOVA of sham-gonadectomized mice revealed that MePD volume is greater in males than in females and larger in the left hemisphere than the right (p < 0.01). Post-hoc repeated-measures t-tests revealed the asymmetry was statistically significant in females, but not in males (p > 0.4). B. Castration of males reduced MePD volume equally in both hemispheres (interaction p > 0.3), but bulbectomy (OBX) had no effect. C. Estradiol (E) treatment of ovariectomized (OvX) females marginally increased MePD volume, while OBX had no discernible effect on MePD volume.
Rostrocaudal extent of the MePD was greater in males than females (main effect of sex; p < 0.03), but there was no lateral asymmetry (main effect of hemisphere; p = 0.49), nor any interaction (p = 0.49) of the two variables among sham-operated males and females (Table 1). Unlike the asymmetry in male rats, where the hemisphere with a greater MePD volume is also longer in extent, in female mice, the larger MePD hemisphere does not have correspondingly longer rostrocaudal extent.
TABLE 1. Rostrocaudal extent of the posterodorsal medial amygdala (MePD) in mice.
Means and standard errors of the mean for number of coronal sections containing the MePD are depicted with N = number of animals in parentheses. MePD rostrocaudal extent was significantly greater in males than in females, but there was no significant effect of hormone manipulations or olfactory bulb removal (OBX) in either sex, nor any interaction of hemisphere with those factors
| LEFT HEMISPHERE | RIGHT | |
|---|---|---|
| HEMISPHERE | ||
| Male Sham | 13.0 ± 0.37 (9) * | 13.0 ± 0.33 (9) * |
| Male Castrate | 13.0 ± 0.26 (6) | 12.2 ± 0.41 (6) |
| Male Sham +OBX | 12.9 ± 0.20 (9) | 12.6 ± 0.38 (9) |
| Female Sham | 12.0 ± 0.44 (9) | 11.7 ± 0.33 (9) |
| Female OvX+E | 12.3 ± 0.62 (8) | 12.2 ± 0.67 (8) |
| Female OvX+E + OBX | 11.7 ± 0.29 (7) | 11.7 ± 0.36 (8) |
sex difference in sham animals main effect p < 0.03.
MePD soma size is larger in male shams than in female shams (main effect of sex: p < 0.0001) but there was no effect of hemisphere (p = 0.36) on soma size, and no interaction (p = 0.87). Therefore, Figure 3 presents soma size averaged across the two hemispheres. These data indicate that soma size is sexually dimorphic but displays no lateral asymmetry in mice. Thus, the asymmetry in female MePD volume cannot be attributed to an asymmetry in neuronal size.
Figure 3.
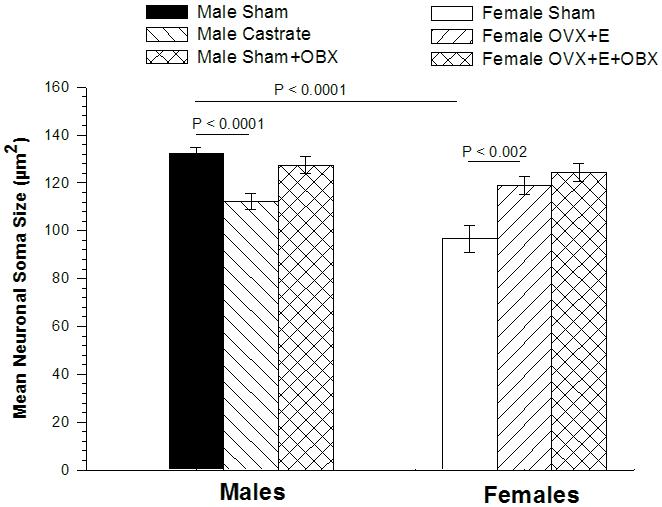
Mean size of neuronal somata in the MePD of BALB/c mice. ANOVA revealed no significant laterality of neuronal soma size in any group of animals, so soma sizes averaged across the two hemispheres are depicted here. Comparison of mice subjected to sham surgery revealed MePD somata to be larger in males than in females. Castration reduced MePD somata in males, while estradiol (E) treatment of ovariectomized (OvX) females enlarged somata size. Olfactory bulbectomy (OBX) had no effect on MePD soma size in OvX females. In males, ANOVA revealed no significant main effect of surgery or hemisphere, but a marginally significant interaction (p < 0.05). Post-hoc tests indicated MePD soma size was slightly smaller in OBX males than sham males (p < 0.04) in the right hemisphere only.
Manipulating steroids affects MePD morphology
Male castrates were compared to sham-operated males using a two-way ANOVA (hormone manipulation as independent measure and hemisphere as repeated measure) followed by PLSD post-hoc test. Castration reduced MePD volume (main effect of castration; p < 0.0003), and there was only a marginal effect of hemisphere (p = 0.08), with no interaction (p = 0.39; Figure 2B). The marginally significant p value for hemisphere led us to conduct repeated-measures t-tests comparing values from the two hemispheres within castrates and sham males, but neither reached statistical significance.
In females, we found OvX + E treated females have larger MePD volumes than intact females (Figure 2C), although the effect was only marginally significant (p = 0.06 two-tailed; p = 0.03 one-tailed if one predicts the direction of effect seen in rats). The overall effect of laterality in females was again present (p < 0.0004), but there was no interaction of laterality with E treatment (p > 0.50).
Rostrocaudal extents of the MePD in male and female shams were not affected by either castration in males or hormone treatment in females (all ps within each sex > 0.30; Table 1). There was no laterality in rostrocaudal extent, nor any statistical interaction between laterality in either sex (all ps > 0.18).
In males, MePD soma size was reduced by castration (p < 0.0001) but there was no difference between hemispheres (p > 0.50), and no interaction (p = 0.41). In females, MePD soma size was greater in E treated females (p < 0.002), but there was no effect of hemisphere, and no interaction (ps > 0.50). Thus, hormone manipulations affect MePD soma size in both sexes, and the two hemispheres respond equivalently (Figure 3).
Bulbectomy has little or no effect on the MePD of adult mice
Male and female bulbectomized mice were compared to their respective same sex controls (bulb-intact sham males and bulb-intact OvX+E females) using ANOVAs within each sex (bulbectomy as an independent variable and hemisphere as a repeated measure) followed by PLSD post-hoc test. In males, MePD volume displayed an overall effect of hemisphere (p < 0.03), but not of bulbectomy (p > 0.50). As the p value for the interaction was non-significant but low (p = 0.17), we used matched pairs t-tests to find a barely significant asymmetry in bulbectomized males (p < 0.05; L>R) that was not seen in bulb-intact males, indicating that bulbectomy may induce an asymmetry in MePD volume in males (Figure 2B).
In females, there was no effect of bulbectomy on MePD volume among OvX + E treated females (p > 0.50). The main effect of laterality was still present (p < 0.002; L>R), but there was no statistical interaction (p > 0.50), indicating bulbectomy has no effect on MePD volume or asymmetry in OvX + E treated females (Figure 2C).
Because there was no effect of bulbectomy on MePD volume in OvX +E females, we collapsed the two groups of females and compared them to gonadally intact females (shams) to determine if the larger sample size would confirm an effect of OvX plus E treatment on MePD volume. Two way ANOVA followed by PLSD post hoc analysis did reveal an overall effect of E treatment on MePD volume (p < 0.02), and the lateral asymmetry (main effect of hemisphere; p < 0.0001; L>R), that did not interact with hormone status (p > 0.50), indicating that hormone manipulation of females does increase MePD volume equally in both hemispheres.
In males, rostrocaudal extent of the MePD displayed no effect of bulbectomy (p = 0.49) or hemisphere, nor interaction (ps > 0.50). Likewise, in females, there was no effect of bulbectomy, or hemisphere, nor an interaction (ps > 0.50) on rostrocaudal extent. Thus, bulbectomy has no effect on the length of the MePD in mice of either sex.
In males, MePD soma size displayed no overall effect of bulbectomy (p = 0.12), or hemisphere (p = 0.24), but a significant interaction (p < 0.05) was found. Post-hoc t-tests revealed that the size of neurons in bulbectomized males are reduced in size compared to bulb intact males in the right MePD (122.4 ± 4.6 vs. 133.6 ± 1.8 um2; p < 0.04), but not the left (p > 0.50).
In females, there was no effect of bulbectomy, or hemisphere, and no interaction (ps > 0.30), on MePD soma size in OvX + E treated females (Figure 3). Thus, of the various MePD measures examined, only soma size in the right hemisphere of male mice was affected by bulbectomy.
3. Discussion
We found the MePD of mice to be sexually dimorphic in many ways. Regional volume, soma size and rostrocaudal extent are all greater in male than in female mice, mirroring the dimorphism in rats (Morris, Jordan et al. 2005). The mouse MePD is also affected by hormone manipulations in adult mice. Castration of males causes regional volume and soma size to shrink, while ovariectomy and E treatment of females causes these same measures to increase. None of our manipulations affected the rostrocaudal extent of the MePD in mice, suggesting that this sexually dimorphic aspect is established early in life and is not susceptible to change in adulthood. The lack of effect on rostrocaudal extent also indicates that the hormone-induced expansion of MePD volume occurs within the dorsoventral and/or lateral planes rather than the rostrocaudal axis.
The sexual dimorphism and hormonal responsiveness of the MePD in adult mice indicate that this may be a fertile model for examining neural sexual differentiation because many tools for manipulating the genome are available in this species. We examined outbred BALB/c mice, but the question remains whether the MePD is sexually dimorphic in other mouse strains. In the hypothalamus, some strains of mice show sexual dimorphisms that other strains lack (Mathieson, Taylor et al. 2000).
There is one sexual dimorphism of the MePD that appears to be reversed in rats versus mice. In rats, MePD regional volume is asymmetric in males, where the MePD is larger on the right than the left, and females display no asymmetry. Furthermore, asymmetrical effects of castration have been seen before in rats (Cooke, Breedlove et al. 2003; Morris, Jordan et al. 2007). But in mice, we found MePD regional volume to be asymmetric in females, with the left MePD larger than the right, while males display no asymmetry. It will be important to see whether the asymmetry in female mice is replicated in other laboratories and/or in other mouse strains.
The olfactory bulbs provide important inputs to the medial amygdala and so the loss of these afferents might be expected to affect regional volume or soma size in this nucleus. Yet olfactory bulbectomy of adult mice had little or no effect on the MePD in mice. The only statistically significant effect we found was that in gonadally intact males: those subjected to OBX had smaller MePD somata in the right hemisphere than bulb-intact males. Because we see no laterality of MePD soma size in bulb-intact mice or rats of either sex, it is difficult to explain why OBX would affect only one hemisphere. Thus, given the number of comparisons made and the modest size of this effect, even this result of bulbectomy should be regarded tentatively until other laboratories have replicated it.
Given the neuroanatomical inputs from the olfactory and pheromonal systems to the medial amygdala (Canteras, Simerly et al. 1995; von Campenhausen and Mori, 2000), the importance of such signaling to sexual behavior in rodents, and the evidence that this nucleus plays a role in sexual behavior in rats (Meisel and Sachs 1994; Kondo, Sachs et al. 1997) and mice (Sipos and Nyby 1998; Kang, Janes et al. 2006), the sexual dimorphism in the MePD likely reflects sex differences in the contribution of this nucleus to sexually differentiated behaviors. The fluctuation in morphology of the male MePD in response to changes in hormone levels, either by castration as in the present study and/or castration and testosterone replacement in studies of rats, presumably contributes to the concomitant changes in male reproductive behavior that accompanies these manipulations. Why should the MePD of rats and mice retain this plasticity in response to adult androgen manipulations? One possibility is that the ancestors of laboratory rats and mice were seasonal breeders and that the MePD, and possibly other brain regions involved in reproduction, waxed and waned with the seasons to support sexual behavior only when it was likely to produce offspring. We have examined the MePD in one seasonally breeding rodent, the Siberian hamster, and find that indeed manipulations of photoperiod that suppress gonadal systems are accompanied by a reduction in MePD regional volume and soma size (Cooke, Hegstrom et al. 2002). The hormone-induced plasticity of the MePD in mice may be a remnant of such plasticity that was regulated by seasons in ancestral mice.
Because a wide variety of transgenic mice and knockout mice are available, the sexual dimorphism and hormone responsiveness of the MePD in this species may be amenable to a fuller understanding of the molecular and cellular processes underlying sex differences in the brain and behavior.
4. Experimental Procedure
Animals
Commercially supplied BALB/c mice (Charles River), approximately 6 months of age, were used. Animals were housed three to a cage (males and females separately) in standard cages with food and water freely available with the exception of the bulbectomized (OBX) females, which were housed singly. Lights were turned off at 1900 hours and on at 0700 hours. The animal care followed standards set by the National Institutes of Health and was approved by the institutional animal care and use committee at Michigan State University.
Bulbectomy
Olfactory bulbectomized (OBX) males and females were purchased from Charles River. Brains were removed from all animals and assessed for complete OBX at sacrifice. Animals with partial bulbectomies were excluded from analysis. Sham bulbectomies were not performed. Because the aim was to determine whether absence of olfactory afferents interfered with steroidal effects on the MePD, bulbectomized males were subjected to sham castration, while bulbectomized females were ovariectomized and treated with E, which has been shown to affect MePD volume and soma size in adult rats.
Hormone manipulation
Ten OBX Females and ten randomly selected bulb-intact females were ovariectomized (OvX) and implanted s.c. with a Silastic capsule (0.058” i.d. and 0.077” o.d.; 5mm effective release length, 15mm total length) containing crystals of 25% free E: 75% cholesterol. Capsules were incubated for 48hrs in phosphate buffered saline (pH 7.4) before implantation and were left in place until sacrifice, approximately 2 months later. Bulb-intact control females (n=10) received sham ovariectomies and blank capsules. Bulb-intact males were either castrated (n=10) or subjected to sham surgery (n=10), while OBX males were subjected to sham surgery (n=10). Animals were sacrificed 28 days after hormonal manipulations.
Histology
On the day of sacrifice, animals were injected IP with an overdose of sodium pentobarbital (210 mg/kg). Deep anesthesia was noted by lack of reflexes to tail and foot pinch as well as lack of a corneal reflex. Animals were then perfused transcardially with 0.9% saline (pH 7.4), followed by 10% neutral buffered formalin (pH 7.4; ∼150 mL/animal). Sex was confirmed at sacrifice by examining external genitalia and noting the presence or absence of gonads. Brains were removed and placed into the same fixative solution.
After at least 1 month fixation, the brains were placed overnight in 20% phosphate-buffered sucrose (pH 7.4) at 4°C prior to slicing. Each brain was scored along the left cortex to mark laterality, blocked at the cerebellum and olfactory tubercle, and coronally sliced on a freezing sliding microtome set to 30 μm. Sections were collected into a phosphate buffer (0.02 M PO4; pH 7.4); every other section was mounted onto gel-subbed glass slides, with a random start from the first series of each brain to ensure that every section had an equal probability of being chosen for sampling. Missing sections due to damage were replaced by the remaining section within that interval, or a space was left on the slide. Mounted tissue was allowed to air-dry, stained with thionin for Nissl substance, and coverslipped with Permount.
Analysis
An investigator, blind to group status, measured the regional volume of the MePD on both sides of the brain. MePD boundaries were determined following nomenclature from a standard mouse atlas (Paxinos and Franklin 2004) using criteria from Hines et al. (Hines, Allen et al. 1992) and Canteras et al. (Canteras, Simerly et al. 1995). The MePD is located in the medial-most aspect of amygdala, and abuts the ventrolateral margin of the optic tract (Figure 1). The MePD contains larger and more darkly stained cells with higher packing density than a surrounding, comparatively cell sparse area. The following landmarks indicated that the caudal-most aspect of the MePD occupied the same rostrocaudal level of the brain in all three groups of animals: size and shape of the lateral ventricle, position and shape of the optic tract with respect to the MePD, and size and shape of the stria terminalis. Moreover, the caudal-most end of the MePD shows a distinct encapsulation by the stria terminalis and therefore was chosen as the anchor point in all measurements.
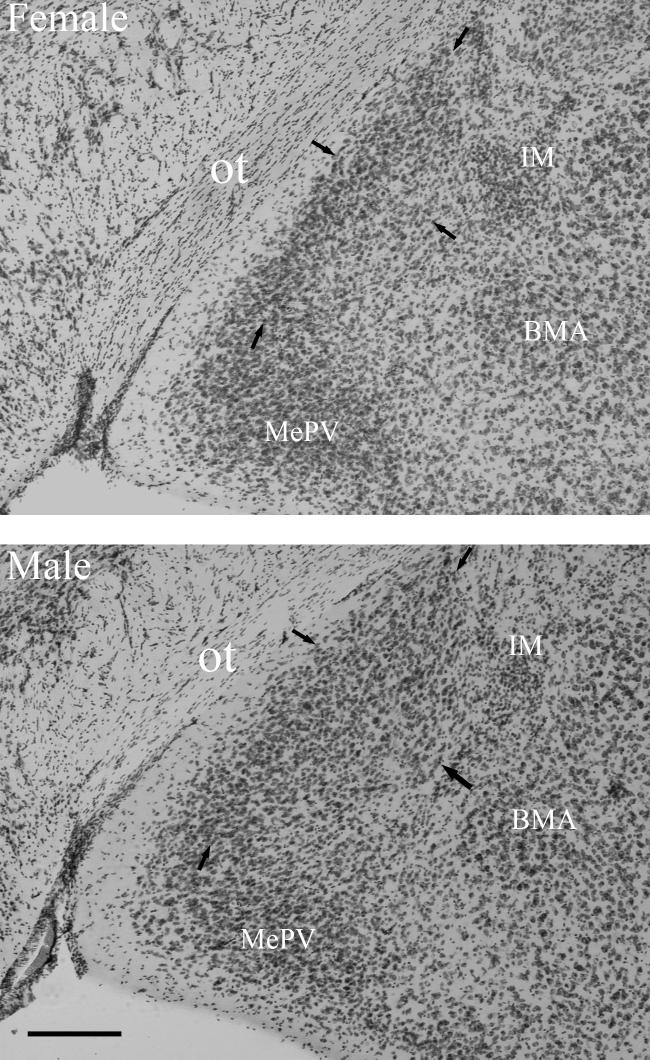
Figure 1.
The posterodorsal medial amygdala (MePD) in BALB/c mice. In these Nissl-stained coronal sections taken approximately in the middle of the rostrocaudal extent of the MePD, the wedge-shaped MePD abuts the ventrolateral margin of the cell-body sparse optic tract (ot; running from lower left corner of photograph to upper right; limits indicated by arrows) and is more slender in female (upper) than male mice (lower), resulting in sexual dimorphism in overall volume of the nucleus. IM = intercalated mass; BMA = basal medial nucleus of the amygdala; MePV = posteroventral medial amygdala. Scale bar = 250 um.
StereoInvestigator (Microbrightfield, Colchester, VT) software was used to estimate the volume and average neuronal soma size of the MePD of both sides of the brain. A digital camera captured at 50X an image containing the area of interest from a Zeiss (Axioplan) compound microscope, and a computer mouse was used to trace the perimeter of the area of interest on a computer monitor in successive sections throughout the rostrocaudal axis. Total volume for each nucleus in each hemisphere was calculated taking into account sampling ratio (every other section) and section thickness (30 μm). Data from animals with damaged or missing tissue in the region of interest were excluded from the analysis. To estimate rostrocaudal extent, the number of sections used for volume estimates were counted. For soma size estimates, neurons were randomly selected by the StereoInvestigator software, which positioned points within the traced MePD of each section without bias for location or appearance so that an investigator could trace the soma of the nearest neuron. An average of 5 neuronal somata from each section was traced (630X) and measured throughout each hemisphere (sampling 25-55 neurons/side). Neuronal somata measures were averaged within each hemisphere yielding a mean soma size per hemisphere for each animal. Neurons were identified by the presence of a distinct nucleolus and Nissl-stained cytoplasm.
Statistical analysis
For each brain measure, groups were compared using separate mixed-design 2 × 2 ANOVAs with hemisphere (left, right) as a repeated measure in every case, and either sex (within sham-operated mice), or gonadal state within males (castrate versus sham), or hormone manipulation within females (OvX plus E versus sham), or olfactory bulb status (OBX or not) within each sex as the independent variable. These were conducted separately for each dependent variable (volume, average perikaryal size, rostrocaudal extent). When there was no significant effect of hemisphere nor a significant interaction between sex and hemisphere, the means for each hemisphere were collapsed, and one-way ANOVAs were conducted to determine whether groups differed from one another. If there was a significant main effect of laterality, or an interaction of laterality and groups, we conducted matched-pairs t-tests within each group of animals to ask which groups displayed a significant hemispheric asymmetry. For each analysis, Fisher’s PLSD post hoc tests were used to determine which groups of animals differed significantly from one another. A probability value of 0.05 was used as the significance criterion, with N representing the number of animals in all analyses. Adobe Photoshop (version 7.0) was used to store and manipulate photographs. No processing of images occurred except for resizing.
ACKNOWLEDGEMENTS
Supported by NIH grant NS28421.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Canteras NS, Simerly RB, et al. Organization of projections from the medial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol. 1995;360(2):213–45. doi: 10.1002/cne.903600203. [DOI] [PubMed] [Google Scholar]
- Cooke B, Tabibnia G, et al. A brain sexual dimorphism controlled by adult circulating androgens. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(13):7538–40. doi: 10.1073/pnas.96.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke BM, Breedlove SM, et al. Both estrogen receptors and androgen receptors contribute to testosterone-induced changes in the morphology of the medial amygdala and sexual arousal in male rats. Horm Behav. 2003;43(2):336–46. doi: 10.1016/s0018-506x(02)00047-8. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Chowanadisai W, et al. Post-weaning social isolation of male rats reduces the volume of the medial amygdala and leads to deficits in adult sexual behavior. Behav Brain Res. 2000;117(12):107–13. doi: 10.1016/s0166-4328(00)00301-6. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Hegstrom CD, et al. Photoperiod-dependent response to androgen in the medial amygdala of the Siberian hamster, Phodopus sungorus. J Biol Rhythms. 2002;17(2):147–54. doi: 10.1177/074873002129002438. [DOI] [PubMed] [Google Scholar]
- Hines M, Allen LS, et al. Sex differences in subregions of the medial nucleus of the amygdala and the bed nucleus of the stria terminalis of the rat. Brain Res. 1992;579(2):321–6. doi: 10.1016/0006-8993(92)90068-k. [DOI] [PubMed] [Google Scholar]
- Kang N, Janes A, et al. Sex difference in Fos induced by male urine in medial amygdala-projecting accessory olfactory bulb mitral cells of mice. Neurosci Lett. 2006;398(12):59–62. doi: 10.1016/j.neulet.2005.12.062. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Sachs BD, et al. Importance of the medial amygdala in rat penile erection evoked by remote stimuli from estrous females. Behavioural brain research. 1997;88:153–160. doi: 10.1016/s0166-4328(97)02287-0. [DOI] [PubMed] [Google Scholar]
- Mathieson WB, Taylor SW, et al. Strain and sex differences in the morphology of the medial preoptic nucleus of mice. J Comp Neurol. 2000;428(2):254–65. doi: 10.1002/1096-9861(20001211)428:2<254::aid-cne5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Sachs BD. The physiology of male sexual behavior. In: Knobil E, Neill JD, editors. The physiology of reproduction. Second edition. 1 and 2. Raven Press; New York, New York, USA: 1994. pp. 3–105. xxv+1878p.(vol. 1); xxv+1372p.(vol. 2) ISBN 0-7817-0086-8(set): [Google Scholar]
- Morris JA, Jordan CL, et al. Sexual differentiation of the vertebrate nervous system. Nat Neurosci. 2004;7(10):1034–9. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, et al. Partial demasculinization of several brain regions in adult male (XY) rats with a dysfunctional androgen receptor gene. J Comp Neurol. 2005;487(2):217–26. doi: 10.1002/cne.20558. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Elsevier Academic Press; Amsterdam ; Boston: 2004. [Google Scholar]
- Scalia F, Winans SS. The differential projections of the olfactory bulb and accessory olfactory bulb in mammals. J Comp Neurol. 1975;161(1):31–55. doi: 10.1002/cne.901610105. [DOI] [PubMed] [Google Scholar]
- Sipos ML, Nyby JG. Intracranial androgenic activation of male-typical behaviours in house mice: concurrent stimulation of the medial preoptic area and medial nucleus of the amygdala. J Neuroendocrinol. 1998;10(8):577–86. doi: 10.1046/j.1365-2826.1998.00215.x. [DOI] [PubMed] [Google Scholar]
- von Campenhausen H, Mori K. Convergence of segregated pheromonal pathways from the accessory olfactory bulb to the cortex in the mouse. Eur J Neurosci. 2000;12:33–46. doi: 10.1046/j.1460-9568.2000.00879.x. [DOI] [PubMed] [Google Scholar]
- Wood RI, Newman SW. The medial amygdaloid nucleus and medial preoptic area mediate steroidal control of sexual behavior in the male Syrian hamster. Horm Behav. 1995;29(3):338–53. doi: 10.1006/hbeh.1995.1024. [DOI] [PubMed] [Google Scholar]