Abstract
This cross-sectional study evaluated the association between radiographic evidence of alveolar bone loss and the concentration of host-derived bone resorptive factors (interleukin-1 beta, tumor necrosis factor-alpha, interleukin-6, prostaglandin-E2), and markers of bone turnover [pyridinoline cross-linked carboxyterminal telopeptide of type I collagen (ICTP), osteocalcin, osteonectin] in stimulated human whole saliva collected from 110 untreated dental patients. Alveolar bone loss scores for each patient were derived from radiographic examination. Variables positively associated with increased bone loss score were: age, current smoking, use of bisphosphonate drugs, and salivary interleukin-1beta levels above the median. Salivary osteonectin levels above the median were associated with a decreased bone loss score. Additional in vitro studies were carried out to determine the fate of interleukin-1beta, interleukin-6 and tumor necrosis factor-alpha added to whole and parotid saliva. All cytokines added to saliva were detected in significantly lower concentrations than when added to buffer alone. Protease inhibitors added to saliva did not prevent the reduction in detection of biomarkers. Variation in time of incubation, repeated cycles of freezing and thawing, or exposure to dimethylsulfoxide did not appreciably affect the measurement of cytokines in saliva. These results suggest that detection of biomarkers by conventional immunoassays may underestimate the actual quantity of molecules in saliva.
Keywords: periodontal disease, salivary biomarkers, pro-inflammatory cytokines, bone turnover
Introduction
It is well known that bacterial biofilms initiate and advance the pathogenesis of chronic periodontitis. An outcome of this process is alveolar bone destruction and eventual tooth loss. Oral bacteria induce inflammation by activating host cells to produce pro-inflammatory mediators, which in turn promote connective tissue and alveolar bone destruction (Offenbacher, 1996). Components of microbial plaque, especially lipopolysaccharide and other soluble products, stimulate lymphocytes, macrophages and neutrophils to secrete a wide range of proinflammatory components, for example interleukin-1 beta (IL-1β), tumor necrosis factor-alpha (TNF-α), prostaglandin E2 (PGE2), and IL-6. These molecules have been previously reported to be elevated in gingival crevicular fluid (GCF) of subjects with active periodontal disease (Giannopoulou et al., 1990; Rossomando et al., 1990; Heasman et al., 1993; Reinhardt et al., 1993).
Osteoblasts, osteocytes and osteoclasts are three cell types involved in the development, growth and remodeling of bone. Several proteins found in bone such as osteonectin (Bowers et al., 1989) and osteocalcin (Giannobile et al., 1995; Nakashima et al., 1996) have also been shown to be elevated in GCF of subjects with periodontal disease. Osteocalcin has also been utilized as a biomarker in serum for bone formation (Delmas, 1993). It has been found to be elevated in GCF in the dog ligature model of periodontal bone loss. This finding may reflect an increase in alveolar bone turnover in subjects with periodontal disease.
The majority of research reports of biomarkers and periodontitis have used GCF as a sample fluid (Taba et al., 2005). However, sampling of GCF is time consuming and only reflects gingival inflammation at each specific site sampled. The use of saliva to measure biomarkers offers several advantages over GCF. As collection of saliva requires no specialized equipment or techniques, it is faster and more convenient for the patient and the practitioner to collect. In addition, as whole saliva represents a pooled sample with contributions from all periodontal sites, analysis of biomarkers in saliva may provide an overall assessment of disease status as opposed to site-specific GCF analysis (Miller et al., 2006).
Although saliva has the potential to be used as a diagnostic fluid for oral disease, its use may be limited by a number of factors. Inhibitors or enzymes in saliva may obscure or destroy immunodeterminants necessary for immunoassay. Proteases are well known to be elevated in the saliva of periodontitis patients, which may reduce the levels of protein biomarkers therein (Nakamura & Slots, 1983; Zambon et al., 1985; Ingman et al., 1993). Also, variations in flow rate, oral hygiene status, and xerostomia may further complicate measurement of analytes in saliva (Mandel & Wotman, 1976). There have been no reports to date of immunomodulatory inhibitors in saliva, although inhibitors of cysteine proteinases have been described (Balbin et al., 1994; Baron et al., 1999). The detection of both cytokines and antibodies has been found to be inhibited by cervical mucus glycoproteins, a common constituent of cervico-vaginal secretions (Ginsburg et al., 1997). Saliva contains similar mucin glycoproteins (Tabak, 1995).
The goals of the present study were thus (1) to identify salivary biomarkers that alone or in combination with other well known risk factors (e.g. age, tobacco use and diabetes) are associated with alveolar bone loss, and (2) to examine if saliva might have inhibitory effects on the detection of the several biomarkers targeted in the present study.
Materials and methods
Study subjects
Subjects for the cross-sectional study were recruited from untreated subjects initially presenting to the University at Buffalo School of Dental Medicine Screening Clinic. Following receipt of informed consent, about 3–5 mL of paraffin-stimulated whole saliva was collected into sterile tubes on ice, centrifuged at 6000 g for 10 min to remove cells and debris, and supernatants were frozen at −70 °C.
Subjects demonstrating the following conditions were not included in the study: heart murmur, artificial heart valve(s) or a history of rheumatic heart disease having excess risk for subacute bacteria endocarditis; artificial joints; history of untreated cancer or recent cancer therapy; history of recent oral surgery; history of periodontal therapy within the previous 3 months; and current use of medications such as corticosteroids, nonsteroidal anti-inflammatory drugs such as ibuprofen or antibiotic therapy within the previous 3 months.
Due to the organization of this clinic and time constraints, it was not possible to perform a rigorous oral examination of each subject at the time of saliva collection. Necessary radiographs were, however, obtained at the time of sample collection.
To study its effect on detection of biomarkers, whole saliva was also collected from several periodontal healthy volunteers (age range 24–65 years), without a history of systemic disease or recent medication use. Subjects were asked to expectorate about 15 mL of paraffin-stimulated whole saliva into a 50-mL plastic centrifuge tube. Parotid saliva was stimulated with 2% citric acid and collected directly from Stenson’s duct using a sterile modified Carlson–Crittenden apparatus (Curby, 1953). Saliva was clarified by centrifugation for 20 min at 3000 g at 4 °C. The supernatant solutions were aliquoted into 1-mL volumes and frozen at −70 °C until needed.
Radiographic examination
Alveolar bone loss scores for each patient were derived from bitewing and/or panoramic radiographs that were examined and scored by two periodontists (EH and FAS). Third molars were not included in the analysis. A score of 0 was assigned to the mesial and distal surfaces of all remaining teeth showing alveolar bone within 2 mm of the cementoenamel junction (CEJ). The distance of 2 mm was chosen because it had been previously demonstrated to represent the outer limit of normal (Hausmann et al., 1991). A score of 1 was assigned to each tooth surface when crestal bone was observed > 2 mm from the CEJ but when ≤ one half of the root length (from CEJ to apex) was found to show bone loss. A score of 2 was assigned to each tooth surface when the alveolar crest height was lost > one half of the root length.
The degree of furcation involvement was also scored for each molar tooth. A score of 0 was assigned to molar teeth having no bone loss in the furcation. A score of 1 was assigned to each furcation showing detectable but incomplete bone loss. A score of 2 was assigned when the radiographic lucency was consistent with complete furcation patency. A ‘bone loss score’ was then calculated as the sum of the scores from all nonmissing teeth (excluding third molars).
In vitro studies of biomarker detection in whole saliva
Various amounts of purified IL-1β, IL-6 and TNF-α (R & D Systems, Minneapolis, MN) were added directly into whole and parotid saliva samples, or to whole saliva samples with and without a protease inhibitor cocktail (Sigma Chemical Co.) containing 4-(2-aminoethyl) benzenesulfonyl fluoride (AEBSF), aprotinin, leupeptin, bestatin, pepstatin A, E-64, supplied in dimethylsulfoxide. In addition, whole saliva samples were incubated; at room temperature for different times (0 min, 30 min, 60 min); with and without 1% dimethylsulfoxide; and held at −70 °C and then thawed to room temperature multiple times. Controls included the addition of IL-1β, IL-6 and TNF-α to PBS-TBN instead of to saliva. The following concentrations of reagents were supplied from stocks: antibody coated beads: 8×106 mL (Luminex Corp., Oosterhout, the Netherlands), biotinylated detection antibody: 50 μg mL−1 (R&D Systems), Phycoprobe R-Phycoerythrin (PE)-avidin: 300 μg mL−1 Caltage Laboratories (Burlingame, CA).
Immunoassay
The concentration of IL-1β, TNF-α and IL-6 in saliva samples were determined by Luminex100 immunoassay (Vignali, 2000). The concentration of cytokine present was determined by reference to a standard curve constructed for each assay.
Osteonectin, osteocalcin and PGE2 were measured by conventional ELISA (United States Biological, Swampscott, MA). The concentration of ICTP was measured by conventional ELISA (Nordic Bioscience, Denmark).
Statistical analysis
Statistical analysis for the cross-sectional study was performed using Poisson regression analysis. All univariate and multivariate analyses were adjusted for the variable ‘number of missing teeth’. Subjects who had no teeth were not included in the analysis. The primary outcome was the sum of radiographic bone loss scores across nonmissing teeth (excluding the third molars). Biomarker levels were dichotomized above and below the median. Bone loss scores, adjusted for the number of missing teeth, were modeled as a function of salivary biomarker levels, gender, age, smoking history (never smoked, still smoking, former smoker), diabetes (yes/no) and the use of bisphosphonate drugs (a surrogate for osteoporosis). Statistical significance was determined at the 0.05 level. All analyses were performed using the SAS version 9.0 statistical software package.
Results
Demographic and clinical characteristics of participants in the cross-sectional study
Characteristics of participants recruited for this study are summarized in Table 1. After eliminating four subjects who were edentulous, and eight subjects because of missing data, 56 men and 42 women who consented to provide whole saliva samples were included in the analysis. The mean age for men was 49 years and for women 44 years. The unadjusted mean total bone loss score for men was 20.4 (SD 22.9) and for women 15.8 (SD 21.3). The average number of teeth showing bone loss for men was 10 and for women seven. The smoking status for men was 33% never smokers, 41% former smokers and 22% current smokers. The smoking status for women was 57% never smokers, 18% former smokers and 25% current smokers. It was found that 7% of the men and 2% of the women reported a history of diabetes. Men reporting use of bisphosphonate drugs (used as a surrogate indicator for osteoporosis) was 1%; for women 5%. Most of the subjects in this population had fewer than nine missing teeth, 74% of the subjects had four or fewer missing teeth, and 35% of the subjects had no missing teeth.
Table 1.
Summary of the characteristics of study subjects (mean±SD)
| Men (n = 56) | Women (n = 42) | |
|---|---|---|
| Age | 48.7±19.1 | 43.9±18.9 |
| Total bone loss score | 20.4±22.9 | 15.8±1.3 |
| Number of teeth with bone loss | 10.1±9.0 | 7.3±8.7 |
| Smoking | 33% never smoked | 57% never smoked |
| 44% former smoker | 18% former smoker | |
| 22% current smoker | 25% current smoker | |
| Diabetes | 7% | 2% |
| Use of bisphosphonate drugs | 1% | 5% |
Concentration of biomarkers found in saliva
Table 2 summarizes data on the concentration of each biomarker detected in saliva. Of the target biomarkers examined, IL-1β, osteonectin and IL-6 were found in greatest abundance in the saliva samples. Osteocalcin, PGE2 and TNF-α were found in very low levels in saliva.
Table 2.
Biomarkers concentration found in saliva (mean±SD)
| Men (n = 56) | Women (n = 42) | Combined population (n = 98) | |
|---|---|---|---|
| Osteonectin (ng mL−1) | 62.2±41.9 | 56.3±322.2 | 59±34.4 |
| Osteocalcin (ng mL−1) | 0.9±0.6 | 0.7±0.2 | 0.7±0.5 |
| PGE2 (ng mL−1) | 0.6±0.8 | 0.7±0.9 | 0.7±0.9 |
| IL-1β (pg mL−1) | 126.6±145.6 | 131.7±149.1 | 123.5±144.5 |
| IL-6 (pg mL−1) | 37.6±46.3 | 107.4±202.9 | 15.1±47.8 |
| TNF-α (pg mL−1) | 1.9±0.2 | 1.9±0.2 | 1.9±0.2 |
Although an attempt was made to detect ICTP in saliva, useful results were not obtained due to what appeared to be inhibition of the signal by saliva to levels below baseline.
Statistical analysis
From the univariate Poisson regression analysis (Table 3), males were found to have higher bone loss scores (P<0.0001) than women. Age was found to be directly proportional to bone loss (P<0.0001). Subjects reporting use of bisphosphonate drugs (presumably for treatment of osteoporosis) showed lower bone loss scores than did subjects not taking these medications (P<0.0001). A higher bone loss score (P<0.0001) was found among subjects reporting a history of diabetes than among subjects who did not. Subjects who reported having never smoked (P<0.0001), or having quit smoking (P<0.0001) showed lower bone loss scores compared to subjects that were current smokers.
Table 3.
Univariate Poisson regression estimates for demographic and health history variables adjusted, for number of missing teeth related to alveolar bone score
| Parameter n = 98 | Regression estimate for β2* | P value |
|---|---|---|
| Female | −0.2872 | <0.0001 |
| Male | Reference category | |
| Age | 0.03 | < 0.0001 |
| Bisphosphonate drugs “Yes” | −2.39 | <0.0001 |
| Bisphosphonate drugs “No” | Reference category | |
| Diabetes | 0.53 | < 0.0001 |
| None | Reference category | |
| Smoking status | ||
| Never smoked | −0.31 | < 0.0001 |
| Quit | −0.46 | < 0.0001 |
| Still smoking | Reference category |
Sign of regression estimate corresponds to the direction of the effect: +, increased bone loss score; −, decreased bone loss score.
Biomarkers that were significantly correlated with bone loss score in the univariate Poisson regression analysis were IL-1β (P<0.0001), IL-6 (P<0.001), PGE2 (P<0.0001), osteocalcin (P<0.0001) and osteonectin (P<0.0001). According to the regression estimates, the concentrations of IL-1β and IL-6 were directly proportional to bone loss score, whereas PGE2, osteocalcin and osteonectin were inversely proportional to bone loss score (Table 4).
Table 4.
Univariate Poisson regression estimates for individual biomarkers (dichotomized) adjusted for number of missing teeth related to alveolar bone score
| Parameter n = 98 | Median cut point | Regression estimate* | P value |
|---|---|---|---|
| IL-1β (pg mL−1) | 60.7 | 0.55 | Significant <0.0001 |
| TNF-α (pg mL−1) | 1.9 | −0.11 | Not significant = 0.3623 |
| IL-6 (pg mL−1) | 7.9 | 0.06 | Significant <0.001 |
| PGE2 (pg mL−1) | 0.4 | −0.21 | Significant <0.0001 |
| Osteocalcin | 0.7 | −0.23 | Significant <0.0001 |
| Osteonectin | 48 | −0.51 | Significant <0.0001 |
Sign of regression estimate corresponds to the direction of the effect: +, increased bone loss score; −, decreased bone loss score.
A multivariate Poisson regression model was fit and adjusted for the number of missing teeth (Table 5). The significant variables related to alveolar bone loss in order of absolute relative effect sizes were: age, current smoker, IL-1β (above the median), use of bisphosphonate drugs, former smoker and osteonectin (below the median). No other markers were significant in the full model. These results suggest that salivary concentrations of IL-1 β (above the median) and osteonectin (below the median) were related to the alveolar bone loss score (results were summarized in Table 4).
Table 5.
Multivariable Poisson regression comparing bone score to variables
| Parameter | Estimate* | P value |
|---|---|---|
| Intercept | 1.09 | <0.0001 |
| Number of missing teeth | −0.039 | <0.0001 |
| Age | 0.034 | <0.0001 |
| IL-1β (pg mL−1) | 0.4178 | <0.0001 |
| Current smoker | 0.7024 | |
| Former smoker | −0.2248 | <0.0001 |
| Never smoker | Reference category | |
| Osteonectin (ng mL−1) | −0.1717 | =0.0008 |
| Use of bisphosphonate drugs (no = reference) | −2.352 | <0.0001 |
All significant variables in univariate analysis were combined in the full model, adjusted for the number of missing teeth.
In vitro studies
The results summarized in Fig. 1 show that, when compared to the concentration of biomarkers following addition to PBS-TBN, IL-1β was detected at a significantly lower concentration following addition to parotid saliva (P = 0.0130) and to whole saliva (P<0.0001). IL-6 (P<0.0001) and TNF-α (P<0.0001) were also found in significantly lower concentrations in whole saliva samples than when added to PBS-TBN. A similar reduction in detection was seen for cytokines added to saliva at 5 and 50 pg mL−1 (data not shown).
Fig. 1.
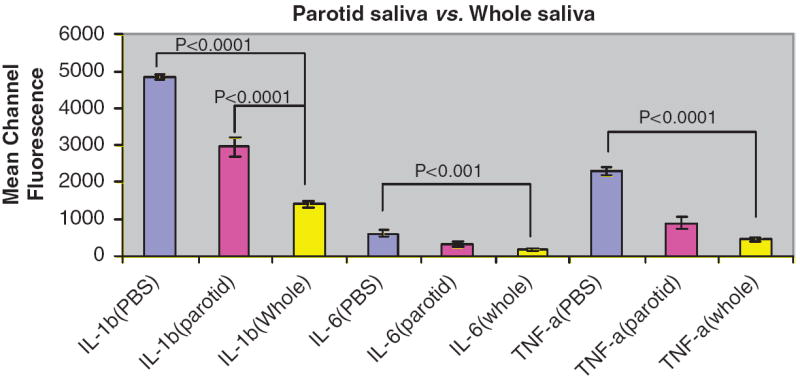
Effect of parotid saliva and whole saliva on detection of biomarkers. Results are expressed as mean channel fluorescence detected of each biomarker added at 500 pg mL−1 in PBS-TBN, whole saliva or parotid saliva (n = 3). Statistically significant reductions are indicated compared to PBS-TBN control.
Figure 2 shows that addition of protease inhibitors to saliva samples followed by incubation at room temperature for 1 h did not prevent the reduction in detection of biomarkers subsequently added to whole saliva. Results were the same for those samples to which protease inhibitors were added and incubated at room temperature at 0 time and after 30 min (data not shown).
Fig. 2.
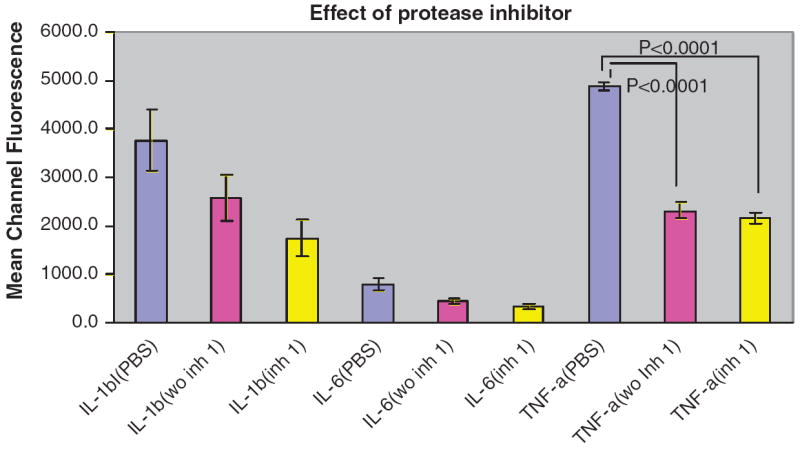
Effect of protease inhibitors added incubated 1 h at room temperature on biomarker detection in whole saliva. Results are expressed as mean channel fluorescence of each biomarkers added at 500 pg mL−1 (n = 3). Statistically significant reductions are indicated compared to PBS-TBN control.
No difference in detection of IL-1β, IL-6 and TNF-α added to saliva was observed in the presence of 1% dimethylsulfoxide and 1% PBS-TBN in whole saliva (data not shown). Results were the same for samples to which 5 or 50 pg mL−1 of each cytokine was added (data not shown).
The detection of IL-1β, TNF-α and IL-6 added after samples were incubated at different time points (0 time, 30 min and 60 min) were not significantly different (Fig. 3). Results were the same for samples to which 5 or 50 pg mL−1 of each cytokine was added (data not shown).
Fig. 3.
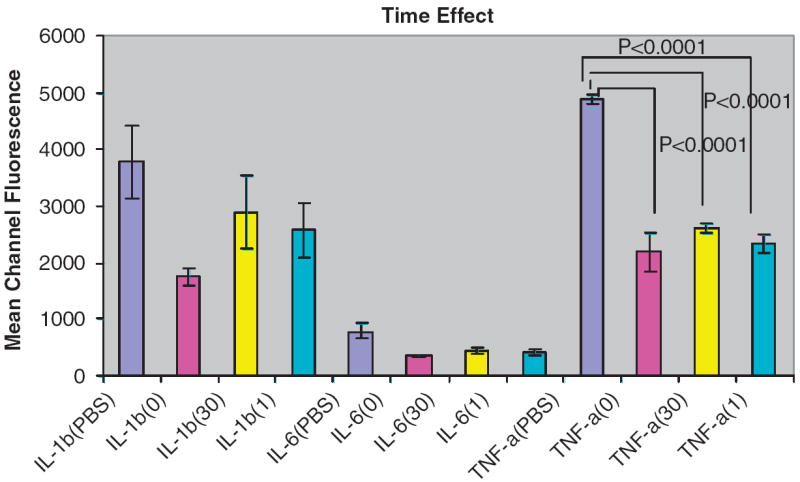
Results are expressed as mean channel fluorescence detected of each biomarker added at 500 pg mL−1 in PBS-TBN, whole saliva or parotid saliva (n = 3). Detection of the cytokines tested after saliva samples were incubated at different time points were not significantly different. Statistically significant reductions are indicated compared to PBS-TBN control.
Repeated cycles of freezing and then thawing did not significantly affect the measurement of cytokines in whole saliva samples (data not shown). Results were the same for samples to which 5 or 50 pg mL−1 of each cytokine was added (data not shown).
Discussion
The host response to periodontal pathogenic bacteria involves a complex series of events that involves both local and systemic release of cytokines that initiates and controls inflammatory reactions (Page, 1991; Kornman et al., 1997). In the present cross-sectional study, we investigated whether several candidate biomarkers in whole saliva, shown in previous studies to be elevated in gingival crevicular fluid of periodontitis patients, were associated with a history of alveolar bone loss. We developed a novel system to quantify whole mouth alveolar bone loss. This system was quite simple to implement and yielded data that could be correlated with salivary biomarker concentration. Further studies should be performed to validate this simplified bone score system by comparing it with measures of CEJ–alveolar crest distances.
Univariate analyses confirmed the association of a number of putative risk factors with periodontal disease (e.g. gender, age, history of osteoporosis, diabetes, and smoking). In addition, several individual biomarkers were also significantly correlated with bone loss score (IL-1β, IL-6, PGE2, osteocalcin and osteonectin). However, multivariate analysis revealed that IL-1β and osteonectin were the only two biomarkers studied that were significantly correlated with bone loss score. A recent study found a relationship between clinical measures of periodontal disease and salivary levels of IL-1β and matrix metalloproteinase (MMP)-8 (Miller et al., 2006). The results of the present study are consistent with that report. Unfortunately, however, this present study did not quantify (MMP)-8 in saliva.
In the present study, a higher concentration of IL-1β in patient saliva was associated with higher alveolar bone loss score. In contrast, a higher concentration of osteonectin in patient saliva was associated with a lower alveolar bone loss score. While these differences were found to be statistically significant, there were a number of discrepancies noted. For example, several subjects having high salivary levels of IL-1β showed no evidence of bone loss. The negative association between bone loss and osteonectin is somewhat surprising in light of previous studies that reported this protein to be elevated in GCF of periodontitis subjects (Bowers et al., 1989).
The concentrations of TNF-α and IL-6 in the saliva of subjects were mostly below the limit of detection (IL-6 was still, however, significantly correlated to alveolar bone loss score in the univariate analysis). There are several possible explanations for these findings. First, there might be competitive inhibitors in saliva that interfere with quantitation of the analyte. Second, the results may be influenced by the ability of the antibody pair to recognize and bind the specific epitopes on the target biomarkers. Because these antibodies are produced against recombinant cytokines, they may not exhibit exactly the same epitopes as natural cytokines. Third, saliva samples might contain proteases that could degrade the cytokines and decreased their detection.
The biomarker concentrations measured in this study are somewhat less than those reported by other recent publications, For example, while the present study reports mean TNF-α concentrations to be 1.9±0.2 pg mL−1 in whole saliva for both men and women, Wozniak et al. reported TNF-α levels to be much higher [median of 127.1 pg mL−1 (range 40.4–1594.9)] in whole saliva from a population that did not include anyone with any form of periodontitis and gingivitis (Wozniak et al., 2002). In contrast, like this present study, Aurer and coworkers found that TNF was below the level of detection in almost all samples (Aurer et al., 2005). Similarly, the IL-1β concentrations in whole saliva of 123.5±144.5 pg mL−1 reported in this study are considerably lower than those reported by Miller et al. (212.8±167.4 pg mL−1 in control subjects and 753.7±1022.4 pg mL−1 in those with moderate-severe periodontitis) (Miller et al., 2006). There are several potential explanations for these discrepancies. First, the studies used different methods to collect saliva. Wozniak et al. collected unstimulated whole saliva that was centrifuged for 5 min at 800 g and filter sterilized. Miller et al. collected unstimulated saliva, with subjects rinsing their mouths with tap water before saliva collection. Also, they did not centrifuge the saliva. In the present study, saliva was centrifuged for a longer period of time [and under greater force than the above-mentioned studies (6000 g for 10 min)]. Both actions would reduce concentrations of the target biomarkers in saliva. Obviously, there is a need for standardization of saliva collection so that the results from different studies can be compared. The important point is that the relative relationships within each study be considered. In this case, there are similar trends between studies (for example with respect to the elevation of IL-1β in subjects with alveolar bone loss).
Involvement of IL-1β in the pathogenesis of periodontal disease has long been suspected because of similarities between the known biological effects of the cytokine and the manifestations of the disease (Tatakis, 1993). Although both isoforms of IL-1, IL-1α and IL-1β have similar biological activities, IL-1β is more potent in stimulating bone resorption and is the form more frequently found in GCF (Stashenko et al., 1987, 1991; Masada et al., 1990). The specific role of osteonectin in periodontal pathogenesis has not yet been determined. However, it has been found to be the dominant noncollagenous protein of mineralized tissues and has been identified in several other tissues as well (Termine et al., 1981; Nomura et al., 1988).
It was not possible to measure carboxyterminal telopeptide of type I collagen (ICTP) levels using the ELISA kit from Nordic Bioscience Diagnostics because the background values were greater than sample values. This ELISA kit was designed to detect ICTP in serum and plasma. Several previous studies did find ICTP to be elevated in gingival crevicular fluid using radioimmunoassay (Talonpoika & Hamalainen, 1994; Bonde et al., 1997; Golub et al., 1997; Oringer et al., 1998; Al-Shammari et al., 2001).
From multivariate analysis, age was strongly correlated to alveolar bone loss in the present study. A previous study found that after age 50 years, the porosity of the mandibular alveolar cortical bone increases, while at the same time there is a decrease in bone mass (Hildebolt, 1997). It has been suggested that this increase in alveolar bone porosity in combination with local factors could be of etiologic importance in the rate of periodontal alveolar bone loss.
Smoking may be one of the most significant risk factors in the development of periodontal disease (Tomar & Asma, 2000). Our findings also indicate that subjects who smoke have higher alveolar bone loss scores. Consistent with previous studies (Palomo et al., 2005), subjects taking bisphosphonate drugs, approved for the treatment and prevention of postmenopausal osteoporosis, showed lower alveolar bone loss scores than those not taking these drugs.
The possibility of using saliva as a diagnostic tool for the determination of health status is dependent upon several factors such as sensitivity of detection and stability of biomarker in saliva and recovery of sufficient amounts to be detectable. Preliminary studies designed to evaluate the effect of whole saliva and parotid saliva on exogenous cytokines were conducted immediately following the addition of known amount(s) of cytokine. Compared to cytokines added to PBS-TBN, the detection of cytokines in whole saliva was much reduced (c. 75% compared to control), similar to findings previously reported by Wozniak and coworkers (Wozniak et al., 2002). This reduction in detection is also similar to that observed for cytokines in cervical mucus (Ginsburg et al., 1997), which, like saliva, contains substantial amounts of mucins that may sequester biomarkers to limit their detection. We have made no effort to adjust the clinical values we measured for each biomarker in saliva (Table 1).
Inhibitors of serine and cysteine proteases, as well as of aspartic and aminopeptidases, did not reverse the observed reduction in cytokines following addition to saliva. Thus, it is possible that the reduction in cytokine detection may be due to complexation of the molecules with salivary components such as mucins (Iontcheva et al., 1997).
A previous study to assess the stability of cytokines in whole saliva samples showed that addition of cytokines (IL-12, IFN-γ, IL-4 and IL-10) to whole saliva with incubation for 48 h at −80°, 4°, 21° or 37 °C showed no significant difference in biomarkers levels compared to samples tested at time 0 (Wozniak et al., 2002). Different incubation times and multiple freeze-thaw cycles might change the physiological state of whole saliva, for example viscosity, and thus might affect the detection of cytokines. However, our results suggested that incubation time and multiple freeze-thaw cycles did not affect biomarker detection.
Quantitation of biomarkers in saliva may serve as a useful tool to monitor and predict susceptibility to periodontal disease. The practical significance of this cross-sectional study remains to be determined. The sequestering and/or degradation of possible biomarkers in saliva may limit the detection of the absolute concentration of each biomarker. In the case of cytokines targeted in our study, the true concentration of biomarkers in saliva may be greater than that detected by the immunoassays used. Future, longitudinal well-controlled clinical trials are needed to find reliable salivary biomarkers for periodontal diagnosis and to determine future risk of disease.
Acknowledgments
This study was supported by a grant (DE15854) from the National Institutes of Health. The technical assistance of Mr Paul Bronson is gratefully acknowledged.
References
- Al-Shammari KF, Giannobile WV, Aldredge WA, Iacono VJ, Eber RM, Wang HL, Oringer RJ. Effect of non-surgical periodontal therapy on C-telopeptide pyridinoline cross-links (ICTP) and interleukin-1 levels. J Periodontol. 2001;72:1045–1051. doi: 10.1902/jop.2001.72.8.1045. [DOI] [PubMed] [Google Scholar]
- Aurer A, Jorgic-Srdjak K, Plancak D, Stavljenic-Rukavina A, Aurer-Kozelj J. Proinflammatory factors in saliva as possible markers for periodontal disease. Coll Antropol. 2005;29:435–439. [PubMed] [Google Scholar]
- Balbin M, Hall A, Grubb A, Mason RW, Lopez-Otin C, Abrahamson M. Structural and functional characterization of two allelic variants of human cystatin D sharing a characteristic inhibition spectrum against mammalian cysteine proteinases. J Biol Chem. 1994;269:23156–23162. [PubMed] [Google Scholar]
- Baron AC, Gansky SA, Ryder MI, Featherstone JD. Cysteine protease inhibitory activity and levels of salivary cystatins in whole saliva of periodontally diseased patients. J Periodontal Res. 1999;34:437–444. doi: 10.1111/j.1600-0765.1999.tb02279.x. [DOI] [PubMed] [Google Scholar]
- Bonde M, Garnero P, Fledelius C, Qvist P, Delmas PD, Christiansen C. Measurement of bone degradation products in serum using antibodies reactive with an isomerized form of an 8 amino acid sequence of the C-telopeptide of type I collagen. J Bone Miner Res. 1997;12:1028–1034. doi: 10.1359/jbmr.1997.12.7.1028. [DOI] [PubMed] [Google Scholar]
- Bowers MR, Fisher LW, Termine JD, Somerman MJ. Connective tissue-associated proteins in crevicular fluid: potential markers for periodontal diseases. J Periodontol. 1989;60:448–451. doi: 10.1902/jop.1989.60.8.448. [DOI] [PubMed] [Google Scholar]
- Curby WA. Device for collection of parotid saliva. J Lab Clin Med. 1953;41:493–496. [PubMed] [Google Scholar]
- Delmas PD. Biochemical markers of bone turnover. J Bone Miner Res. 1993;(Suppl 2):S549–555. doi: 10.1002/jbmr.5650081323. [DOI] [PubMed] [Google Scholar]
- Giannobile WV, Lynch SE, Denmark RG, Paquette DW, Fiorellini JP, Williams RC. Crevicular fluid osteocalcin and pyridinoline cross-linked carboxyterminal telopeptide of type I collagen (ICTP) as markers of rapid bone turnover in periodontitis. A pilot study in beagle dogs. J Clin Periodontol. 1995;22:903–910. doi: 10.1111/j.1600-051x.1995.tb01793.x. [DOI] [PubMed] [Google Scholar]
- Giannopoulou C, DiFelice R, Andersen E, Cimasoni G. Synthesis of alpha 2-macroglobulin in human gingiva: a study of the concentration of macroglobulin and albumin in gingival fluid and serum. Arch Oral Biol. 1990;35:13–16. doi: 10.1016/0003-9969(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Ginsburg KA, Wolf NA, Fidel PL. Potential effects of midcycle cervical mucus on mediators of immune reactivity. Fertil Steril. 1997;67:46–50. doi: 10.1016/s0015-0282(97)81854-7. [DOI] [PubMed] [Google Scholar]
- Golub LM, Lee HM, Greenwald RA, Ryan ME, Sorsa T, Salo T, Giannobile WV. A matrix metalloproteinase inhibitor reduces bone-type collagen degradation fragments and specific collagenases in gingival crevicular fluid during adult periodontitis. Inflamm Res. 1997;46:310–319. doi: 10.1007/s000110050193. [DOI] [PubMed] [Google Scholar]
- Hausmann E, Allen K, Clerehugh V. What alveolar crest level on a bite-wing radiograph represents bone loss? J Periodontol. 1991;62:570–572. doi: 10.1902/jop.1991.62.9.570. [DOI] [PubMed] [Google Scholar]
- Heasman PA, Collins JG, Offenbacher S. Changes in crevicular fluid levels of interleukin-1 beta, leukotriene B4, prostaglandin E2, thromboxane B2 and tumour necrosis factor alpha in experimental gingivitis in humans. J Perio Res. 1993;28:241–247. doi: 10.1111/j.1600-0765.1993.tb02090.x. [DOI] [PubMed] [Google Scholar]
- Hildebolt CF. Osteoporosis and oral bone loss. Dentomaxillofac Radiol. 1997;26:3–15. doi: 10.1038/sj.dmfr.4600226. [DOI] [PubMed] [Google Scholar]
- Ingman T, Sorsa T, Konttinen YT, Liede K, Saari H, Lindy O, Suomalainen K. Salivary collagenase, elastase- and trypsin-like proteases as biochemical markers of periodontal tissue destruction in adult and localized juvenile periodontitis. Oral Microbiol Immunol. 1993;8:298–305. doi: 10.1111/j.1399-302x.1993.tb00578.x. [DOI] [PubMed] [Google Scholar]
- Iontcheva I, Oppenheim FG, Troxler RF. Human salivary mucin MG1 selectively forms heterotypic complexes with amylase, proline-rich proteins, statherin, and histatins. J Dent Res. 1997;76:734–743. doi: 10.1177/00220345970760030501. [DOI] [PubMed] [Google Scholar]
- Kornman KS, Page RC, Tonetti MS. The host response to the microbial challenge in periodontitis: assembling the players. Periodontol 2000. 1997;14:33–53. doi: 10.1111/j.1600-0757.1997.tb00191.x. [DOI] [PubMed] [Google Scholar]
- Mandel ID, Wotman S. The salivary secretions in health and disease. Oral Sci Rev. 1976:25–47. [PubMed] [Google Scholar]
- Masada MP, Persson R, Kenney JS, Lee SW, Page RC, Allison AC. Measurement of interleukin-1 alpha and -1 beta in gingival crevicular fluid: implications for the pathogenesis of periodontal disease. J Periodontal Res. 1990;25:156–163. doi: 10.1111/j.1600-0765.1990.tb01038.x. [DOI] [PubMed] [Google Scholar]
- Miller CS, King CP, Jr, Langub MC, Kryscio RJ, Thomas MV. Salivary biomarkers of existing periodontal disease: a cross-sectional study. J Am Dent Assoc. 2006;137:322–329. doi: 10.14219/jada.archive.2006.0181. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Slots J. Salivary enzymes. Origin and relationship to periodontal disease. J Periodon Res. 1983;18:559–569. doi: 10.1111/j.1600-0765.1983.tb00393.x. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Giannopoulou C, Andersen E, Roehrich N, Brochut P, Dubrez B, Cimasoni G. A longitudinal study of various crevicular fluid components as markers of periodontal disease activity. J Clin Periodontol. 1996;23:832–838. doi: 10.1111/j.1600-051x.1996.tb00620.x. [DOI] [PubMed] [Google Scholar]
- Nomura S, Wills AJ, Edwards DR, Heath JK, Hogan BL. Developmental expression of 2ar (osteopontin) and SPARC (osteonectin) RNA as revealed by in situ hybridization. J Cell Biol. 1988;106:441–450. doi: 10.1083/jcb.106.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offenbacher S. Periodontal diseases: pathogenesis. Ann Periodontol. 1996;1:821–878. doi: 10.1902/annals.1996.1.1.821. [DOI] [PubMed] [Google Scholar]
- Oringer RJ, Palys MD, Iranmanesh A, Fiorellini JP, Haffajee AD, Socransky SS, Giannobile WV. C-telopeptide pyridinoline cross-links (ICTP) and periodontal pathogens associated with endosseous oral implants. Clin Oral Implants Res. 1998;9:365–373. doi: 10.1034/j.1600-0501.1996.090602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RC. The role of inflammatory mediators in the pathogenesis of periodontal disease. J Periodontal Res. 1991;26:230–242. doi: 10.1111/j.1600-0765.1991.tb01649.x. [DOI] [PubMed] [Google Scholar]
- Palomo L, Bissada NF, Liu J. Periodontal assessment of postmenopausal women receiving risedronate. Menopause. 2005;12:685–690. doi: 10.1097/01.gme.0000184421.50696.34. [DOI] [PubMed] [Google Scholar]
- Reinhardt RA, Masada MP, Kaldahl WB, DuBois LM, Kornman KS, Choi JI, Kalkwarf KL, Allison AC. Gingival fluid IL-1 and IL-6 levels in refractory periodontitis. J Clin Periodontol. 1993;20:225–231. doi: 10.1111/j.1600-051x.1993.tb00348.x. [DOI] [PubMed] [Google Scholar]
- Rossomando EF, Kennedy JE, Hadjimichael J. Tumour necrosis factor alpha in gingival crevicular fluid as a possible indicator of periodontal disease in humans. Arch Oral Biol. 1990;35:431–434. doi: 10.1016/0003-9969(90)90205-o. [DOI] [PubMed] [Google Scholar]
- Stashenko P, Dewhirst FE, Peros WJ, Kent RL, Ago JM. Synergistic interactions between interleukin 1, tumor necrosis factor, and lymphotoxin in bone resorption. J Immunol. 1987;138:1464–1468. [PubMed] [Google Scholar]
- Stashenko P, Jandinski JJ, Fujiyoshi P, Rynar J, Socransky SS. Tissue levels of bone resorptive cytokines in periodontal disease. J Periodontol. 1991;62:504–509. doi: 10.1902/jop.1991.62.8.504. [DOI] [PubMed] [Google Scholar]
- Taba M, Jr, Kinney J, Kim AS, Giannobile WV. Diagnostic biomarkers for oral and periodontal diseases. Dent Clin North Am. 2005;49:551–57. doi: 10.1016/j.cden.2005.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak LA. In defense of the oral cavity: structure, biosynthesis, and function of salivary mucins. Ann Rev Physiol. 1995;57:547–564. doi: 10.1146/annurev.ph.57.030195.002555. [DOI] [PubMed] [Google Scholar]
- Talonpoika JT, Hamalainen MM. Type I collagen carboxyterminal telopeptide in human gingival crevicular fluid in different clinical conditions and after periodontal treatment. J Clin Periodontol. 1994;21:320–326. doi: 10.1111/j.1600-051x.1994.tb00720.x. [DOI] [PubMed] [Google Scholar]
- Tatakis DN. Interleukin-1 and bone metabolism: a review. J Periodontol. 1993;64:416–431. [PubMed] [Google Scholar]
- Termine JD, Kleinman HK, Whitson SW, Conn KM, McGarvey ML, Martin GR. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 1981;26:99–105. doi: 10.1016/0092-8674(81)90037-4. [DOI] [PubMed] [Google Scholar]
- Tomar SL, Asma S. Smoking-attributable periodontitis in the United States: findings from NHANES III. National Health and Nutrition Examination Survey. J Periodontol. 2000;71:743–751. doi: 10.1902/jop.2000.71.5.743. [DOI] [PubMed] [Google Scholar]
- Vignali DA. Multiplexed particle-based flow cytometric assays. J Immunol Methods. 2000;243:243–255. doi: 10.1016/s0022-1759(00)00238-6. [DOI] [PubMed] [Google Scholar]
- Wozniak KL, Arribas A, Leigh JE, Fidel PL., Jr Inhibitory effects of whole and parotid saliva on immunomodulators. Oral Microbiol Immunol. 2002;17:100–107. doi: 10.1046/j.0902-0055.2001.00101.x. [DOI] [PubMed] [Google Scholar]
- Zambon JJ, Nakamura M, Slots J. Effect of periodontal therapy on salivary enzymatic activity. J Periodontal Res. 1985;20:652–659. doi: 10.1111/j.1600-0765.1985.tb00850.x. [DOI] [PubMed] [Google Scholar]


