Abstract
Important insights have recently been gained in our understanding of how host immune responses mediate resistance to parasitic helminths and control associated pathological responses. Although similar cells and cytokines are evoked in response to infection by helminths as diverse as nematodes and schistosomes, the components of the response that mediate protection are dependent on the particular parasite. In this Review, we examine recent findings regarding the mechanisms of protection in helminth infections that have been elucidated in murine models and discuss the implications of these findings in terms of future therapies.
More than two billion people are infected with parasitic helminths1. Although infections by these pathogens are generally not fatal, they are associated with high rates of morbidity, with chronic infection often leading to anaemia and malnourishment1. Developed countries have controlled these infections through primary health-care programmes and effective public sanitation, but helminth diseases are still widespread in developing nations and often drug treatment does not protect against rapid re-infection. The need for effective vaccines to control these infections is compelling and at least a few clinical trials are currently underway2,3; however, one impediment towards development of an effective vaccine is a lack of understanding of the actual components of the immune response that mediate protection against helminths. In this Review, we examine the host protective mechanisms with regard to the cell types and molecules involved in helminth expulsion and in control of pathological inflammation that is associated with infection.
Helminth infections and the corresponding host immune responses are products of a prolonged dynamic co-evolution between the host and parasite. For parasites, it is advantageous to trick the host into developing an ineffective immune response, to find a suitable niche for maturation and propagation, and to do so without killing or unduly harming the host. Conversely, the host has to ideally generate an effective immune response to expel the parasite, and minimize its harmful effects, while not sacrificing its ability to effectively respond to other pathogens. The host immune system evolved in the context of a parasite-replete environment, and the balance of immune effector and regulatory cell populations are at least partly a consequence of ongoing responses to infectious organisms that can often simultaneously invade host tissues. Although such dynamic host–pathogen interactions exist throughout much of the world, in industrialized countries infectious diseases are better controlled as a result of increased hygiene, the administration of vaccines at an early age and the widespread use of antibiotics. Although this has resulted in marked reductions in chronic and severe disease, recent studies indicate a possible adverse effect of this enhanced control of infectious diseases that leads to an increase in inflammatory disorders. One mechanism by which this unfavourable result occurs has been suggested by an extension of the hygiene hypothesis, which proposes that a dysregulated immune response might develop in individuals who were not exposed to chronic helminth infections, increasing the likelihood of the development of allergic and autoimmune diseases4,5. Studies have supported this model as the administration of helminths can downmodulate autoimmune and allergic inflammation, whereas clearance of the helminth infection can result in a resurgence of these diseases5–9. Understanding the helminth-induced immune regulatory mechanisms that control certain inflammatory diseases might point the way towards future treatments for these increasingly common immune disorders.
The protective immune response against many helminth parasites has been variously referred to as the type 2 response, TH2 (T helper 2) response or TH2-type response. Here, we use the term TH2-type response to refer to the combined immune response, which includes both innate and adaptive components, to clearly distinguish it from the adaptive TH2-cell response. TH2-type responses are typically characterized by increases in the levels of interleukin-4 (IL-4) and other TH2-type cytokines (including IL-5, IL-9, IL-13 and IL-21), activation and expansion of CD4+ TH2 cells, plasma cells secreting IgE, eosinophils, mast cells and basophils, all of which can produce several types of TH2-type cytokine. The IL-17-related cytokine IL-25 (also known as IL-17E) is also associated with the TH2-type response and can promote TH2-cell differentiation and nematode parasite expulsion10,11. Whether IL-25 is also a TH2-type cytokine or identifies another TH-cell subset remains unclear11. By contrast, interferon-γ (IFNγ)-dominant TH1-type responses are typically evoked by microbial infections, including bacteria and viruses, and are associated with increases in the numbers of TH1 cells, cytotoxic CD8+ T cells, neutrophils and macrophages. Although IL-10 was initially characterized as a TH2-type cytokine, recent findings show that this cytokine is also produced 12,13 in vivo, and can by TH1 cells and regulatory T cells downregulate both TH1-type and TH2-type responses14. Several cytokines that are preferentially expressed during the TH2-type response, including IL-4, IL-13, IL-21 and IL-25, can also downregulate TH1-type and TH17-type responses and their associated inflammation.
In this Review we examine recent findings concerning the TH2-type responses elicited by parasitic helminths with some emphasis on studies in mice infected with the intestinal nematode parasite Heligmosomoides polygyrus and the trematode parasite Schistosoma mansoni. Infection with these parasites triggers a TH2-type response, although the resultant mechanisms of protection differ greatly, with each providing unique insights. In the highly polarized TH2-type response to the hookworm model parasite, H. polygyrus, protective responses mediate parasite stress that leads to expulsion of the worm. In S. mansoni infection, the protective TH2-type response primarily downregulates an otherwise pathological TH1-type response. Therefore, the immune responses to these two helminth species exemplify distinct functions of protective TH2-type immune responses, one leading to worm expulsion and the other contributing to the control of pathological inflammation.
Recent advances have provided new insights into our understanding of the protective TH2-type response that occurs during infectious disease. In particular, macrophages and neutrophils, which are typically associated with antimicrobial immunity, are key players. It is also becoming apparent that the crosstalk between innate and adaptive components throughout the course of the immune response is important. Typically, studies have focused on the role of innate immunity in instructing and directing the adaptive effector immune response, but it is increasingly evident that T cells reciprocally provide signals that amplify and fine-tune the function of innate effector cells. Here, the inflammatory infiltrate that occurs at the host–parasite interface of these two tissue-dwelling parasite species and the possible mechanisms by which immune cells in the infiltrate contribute to the overall host protective response is discussed.
Granuloma formation in helminth infections
Granulomas are traditionally associated with localized TH1-type inflammatory responses that develop around a nidus, such as an invading microorganism or parasite. In schistosomiasis, perioval granulomatous inflammation is mediated by CD4+ T cells that are specific for antigens derived from parasite eggs. The initial TH1-type response to the acute schistosome infection targets adult parasites, but typically transitions to a TH2-type response after the parasite’s eggs are produced. Both co-stimulatory molecules and B cells are required for this switch15. The failure to develop an effective TH2-type response after egg deposition results in exacerbated granulomatous inflammation driven by TH1 and TH17 cells; this causes substantial damage to the surrounding hepatic parenchyma, and can result in death16. Typically, a well-established TH2-type response prevails and is associated with mild lesions consisting of small and well-circumscribed granulomas composed of eosinophils, macrophages and lymphocytes with an increasingly fibrotic extracellular matrix (referred to herein as mild pathology). In chronic schistosomiasis, even the subsequent TH2-type response eventually subsides and newly forming egg granulomas are diminished in size; this regulation of the immune response is termed ‘immunomodulation’16. However, tissue fibrosis, which is largely stimulated by the cytokine IL-13, can also become pathological and therefore represents a potential downside of the ongoing TH2-type response to chronic infection (reviewed in REF. 17).
Granulomas can also develop during polarized TH2-type responses, including those to infection with H. polygyrus18,19. The life cycle of H. polygyrus begins with the ingestion of third-stage larvae by the host; the larvae then travel to the small intestine, where they invade the submucosa and continue to develop for 8 days. The final stage of the life cycle involves the migration of the mature adult female and male worms back to the intestinal lumen where they mate to produce eggs that can then leave the host for subsequent transmission15 (FIG. 1). Primary infection is chronic with established adult parasites in the gut lumen that can be eliminated by the administration of a helminth-specific drug. Subsequent challenge (secondary infection) triggers a CD4+ TH2-type memory response that mediates worm expulsion within 14 days15. It should be noted that other gut-residing nematode parasites, including Trichuris muris, induce a more mixed TH1- and TH2-type response, suggesting that other characteristics besides generalized enteric infection are responsible for the highly polarized TH2-type response to H. polygyrus15,20.
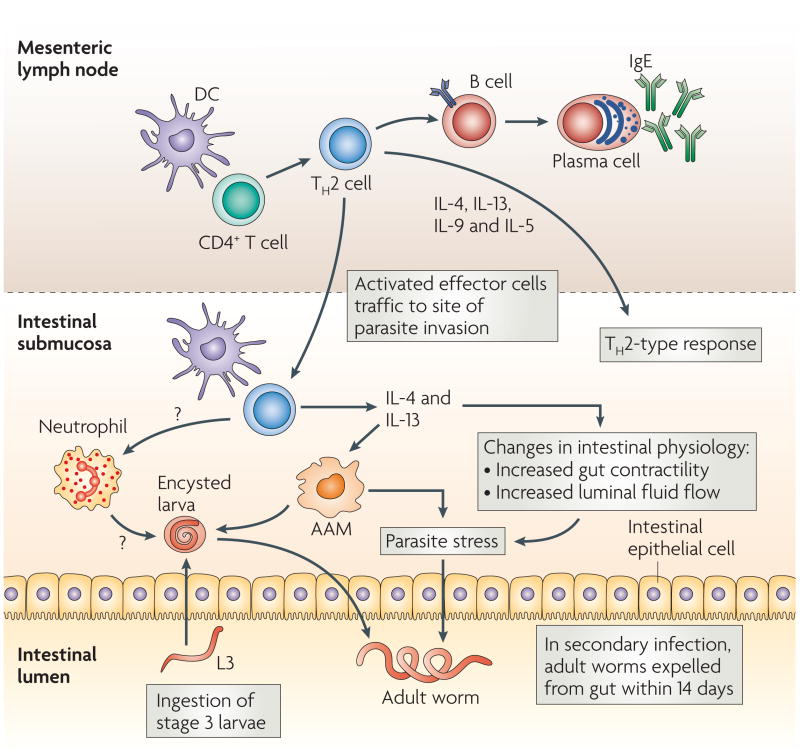
Figure 1. Protective TH2-type response during intestinal nematode infection.
Infective third-stage larvae (L3) are ingested by the host. They then travel to the duodenum, invade the epithelia, and reside in the submucosa for 8 days, after which they re-enter the lumen of the duodenum as adult nematodes. Primary infections become established and chronic, but can be cleared by antihelminthic drug treatment. Challenge (secondary) infections are naturally cleared by the host by day 14 post-infection, making this an excellent model of protective memory T helper 2 (TH2)-type responses. Parasite antigens are presented to CD4+ T cells in mesenteric lymph nodes and other gut-associated lymphoid tissues, driving the induction of TH2 effector cells. These cells exert their effector functions through the production of a number of cytokines, including interleukin-4 (IL-4), IL-13, IL-9 and IL-5. TH2 cells induce B-cell immunoglobulin class-switching to IgE. Shortly after activation, TH2 effector cells migrate to the site of parasite residence in the submucosa. Within several days, a distinct immune-cell infiltrate appears which can damage the larval parasite after secondary, but not primary, inoculation. The infiltrate following secondary inoculation includes TH2 cells, dendritic cells (DCs), neutrophils and alternatively activated macrophages (AAMs). The TH2 cytokines IL-4 and IL-13 might also facilitate expulsion of adult parasites in the lumen by inducing changes in intestinal physiology.
As the H. polygyrus larva develops in the gut submucosa, an immune-cell infiltrate with a defined architecture rapidly forms around it19 (TABLE 1). The memory immune response, which occurs by 4 days after H. polygyrus ingestion, is characterized by neutrophils immediately surrounding the invading larva. Alternatively activated macrophages, which are present in larger numbers than neutrophils, enclose the parasite and neutrophils forming a granulomatous structure. At the border of the granuloma there is an accumulation of CD4+ T cells, CD11c+ dendritic cells (DCs) and eosinophils; however, basophils, mast cells, CD8+ T cells, γδT cells and B cells are absent18,19,21. Cytokine gene-expression analysis using laser capture microdissection has shown the development of a highly polarized TH2-type response (high expression levels of IL-4 and IL-13, in the absence of IFNγexpression). By contrast, 4 days after primary inoculation with H. polygyrus, similar but markedly reduced populations of infiltrating cells are observed, with few CD4+ T cells. Taken together, these findings indicate that a potent inflammatory response can rapidly develop around an invading nematode parasite during the memory TH2-type response. These results indicate that, as in the TH1-type response to many different microorganisms, highly activated neutrophils and macrophages are among the first cells to respond, which results in the rapid development of a granulomatous structure dependent on CD4+ TH2 cells. It has been proposed that the formation of granulomas around schistosome eggs facilitates the migration of the eggs through the intestinal wall22. Although the formation of a parasite-enveloping granuloma secludes the parasite, the immune cells contained in the granuloma still appear to cause parasite damage and probably also contribute to the healing process after the adult parasite migrates back to the intestinal lumen18,23.
Table 1.
Cellular composition of helminth-induced granulomas
| Cell type Present in the granuloma | Heligmosomoides polygyrus | Schistosoma mansoni | ||
|---|---|---|---|---|
| Day 4 after primary innoculation | Day 4 after secondary innoculation | Mild pathology | Severe pathology | |
| AAM | − | +++ | ++ | − |
| CAM | − | − | − | ++ |
| Neutrophil | ++ | ++ | − | ++ |
| Dendritic cell | + | ++ | ++ | ++ |
| Eosinophil | ++ | ++ | +++ | +++ |
| TH1 cell | − | − | − | ++ |
| TH2 cell | − | ++ | ++ | −/++ |
| TH17 cell | − | − | − | ++ |
| CD8+ T cell | − | − | ++ | + |
| B cell and/or plasma cell | − | − | ++ | + |
AAM, alternatively activated macrophage; CAM, classically activated macrophage; TH, T helper.
These granulomas, which are induced by very different parasite species, therefore share a number of similarities, including macrophages as the key infiltrating cell types and the dependence on a TH2-type response for protective effects (TABLE 1). However, the immune response to H. polygyrus is strictly a TH2-type response that develops over several days, whereas the response to S. mansoni is a more mixed-type response that develops over a prolonged period of time.
Innate effector cells in helminth infections
Innate immune cells are essential for both the initiation and effector phases of TH2-type immune responses. CD4+ TH2 effector cells instruct and amplify the innate effector-cell response primarily through the secretion of cytokines; once activated, innate-cell populations in turn help to sustain and promote expansion of the TH2 effector-cell population. This crosstalk results in an overall effector response composed of interacting cells that coordinate and fine-tune targeted effector functions against the invading helminth parasite.
Neutrophils and macrophages: first responders in TH2-type inflammation?
Macrophages are conventionally associated with TH1-type responses to infectious bacteria and viruses. Classically activated macrophages engulf and destroy microorganisms through pathways that are dependent on inducible nitric oxide synthesis (iNOS)24. Until recently, macrophages were thought to be relatively quiescent during TH2-type responses, having a secondary role to eosinophils, basophils and mast cells. However, several studies have now shown pronounced alternative macrophage activation and rapid recruitment to sites of infection during TH2-type responses to helminths 18,24–29. IL-4, IL-13, IL-10 and IL-21 trigger the alternative activation of macrophages24,30–32, which can readily be distinguished by the high expression levels of markers such as arginase-1, IL-4 receptor α-chain (IL-4Rα), the mannose receptor CD206 and the absence of iNOS expression18,33.
Alternatively activated macrophages appear to have at least three principal functions: regulation of the immune response, wound healing and resistance to parasite invasion (FIG. 2). With regard to immune regulation, previous studies showed that macrophages from granulomas of schistosome eggs can downregulate TH1 cells 34; other studies have identified regulatory Gr1+F4/80+ macrophages35. More recently, in vivo studies have shown that life-threatening acute inflammation is largely controlled by alternatively activated macrophages, demonstrating their regulatory potential26. Similarly, alternatively activated macrophages that are elicited in response to filarial parasites preferentially downregulate the TH1-cell response, through an IL-10-independent mechanism mediated partially by transforming growth factor-β (TGFβ)28.
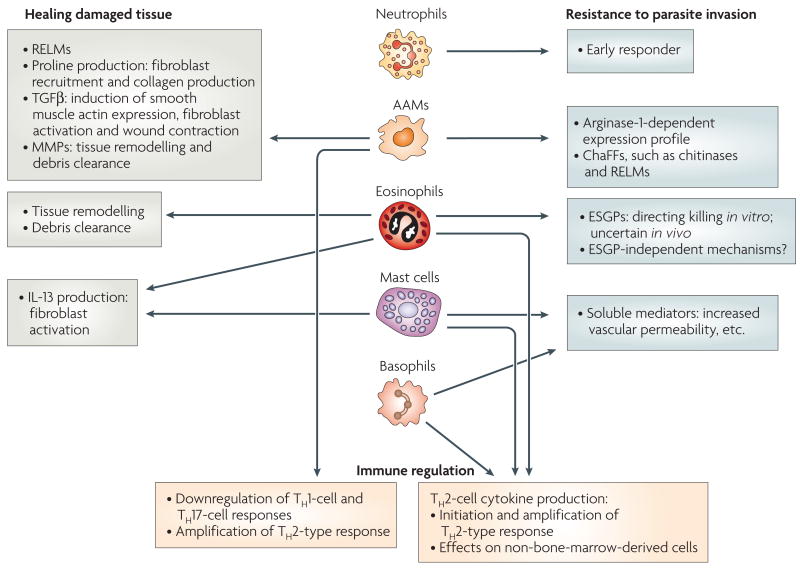
Figure 2. Functions of innate effector cells during the TH2-type response.
In general, the effector functions of innate immune cells during protective T helper 2 (TH2)-type responses can include healing damaged tissue, mediating resistance to parasite invasion and regulating the immune response. Alternatively activated macrophages (AAMs) and eosinophils might contribute to the healing of tissue damage caused by invasive helminth parasites through the production of factors that initiate and promote the remodelling and restructuring of tissue. AAMs, eosinophils, basophils, mast cells and neutrophils can contribute to resistance to parasite invasion. AAMs and eosinophils can directly stress tissue-dwelling parasites. Basophils and mast cells produce soluble mediators (including leukotrienes, prostaglandins and histamine) that promote luminal fluid flow, nerve stimulation and gut contractility. Neutrophils are early responders to invasive helminths, and are localized adjacent to the parasites. AAMs can regulate the immune response by downregulating TH1 and TH17 cells and promoting TH2 cells. Eosinophils, basophils and mast cells can produce TH2-type cytokines including interleukin-4 (IL-4) and IL-13, and thereby amplify the TH2-type response. ChaFFs, chitinase and FIZZ family members; ESGPs, eosinophil secondary granule proteins; MMPs, matrix metalloproteases; RELMs, resistin-like molecules, TGFβ, transforming growth factor-β.
Given their large size, parasitic helminths can potentially cause significant tissue damage by migrating through and residing within specific sites. Alternatively activated macrophages might contribute to wound healing by clearing matrix and cell debris and by releasing cytokines, growth factors and angiogenic factors that promote fibroplasia and angiogenesis even at sterile sites36 (FIG. 2). Some genes expressed by alternatively activated macrophages are associated with wound healing37,38 (BOX 1), these include resistin-like molecules and extracellular matrix proteins39.
In addition to wound repair, alternatively activated macrophages may also mediate more direct effects on tissue-dwelling helminths, for example, by targeting the glycan chitin that is frequently expressed by helminths, but not by mammals. The chitinase and fizz family member proteins (ChaFFs), which include chitinase and chitinase-like secreted proteins and are secreted by alternatively activated macrophages (BOX 1), are prime candidates for mediating host resistance40. Although antiparasitic properties for many of these proteins have not been described, some have surprising roles in mediating TH2-type inflammation (BOX 1). Therefore, ChaFFs have evolved multiple functions that may contribute to resistance to helminth infection, including enzymatic and other activities that potentially damage certain parasites and promote the TH2-type inflammatory response.
Alternative macrophage activation is important for protection against the tissue-dwelling life-cycle stage of H. polygyrus18 (FIG. 1; TABLE 1); protective immunity against the parasite was blocked when hosts were depleted of macrophages or arginase-1 function was inhibited18. In this system, CD4+ T cells and IL-4 are required for both alternative macrophage activation and parasite expulsion15. These studies underscore the inter-play between TH2 cells and innate effector cells during protective TH2-type responses, and suggest that TH2-cell-derived IL-4 drives alternative macrophage activation, which then contributes to parasite clearance through an arginase-1-dependent pathway (FIGS 2,3).
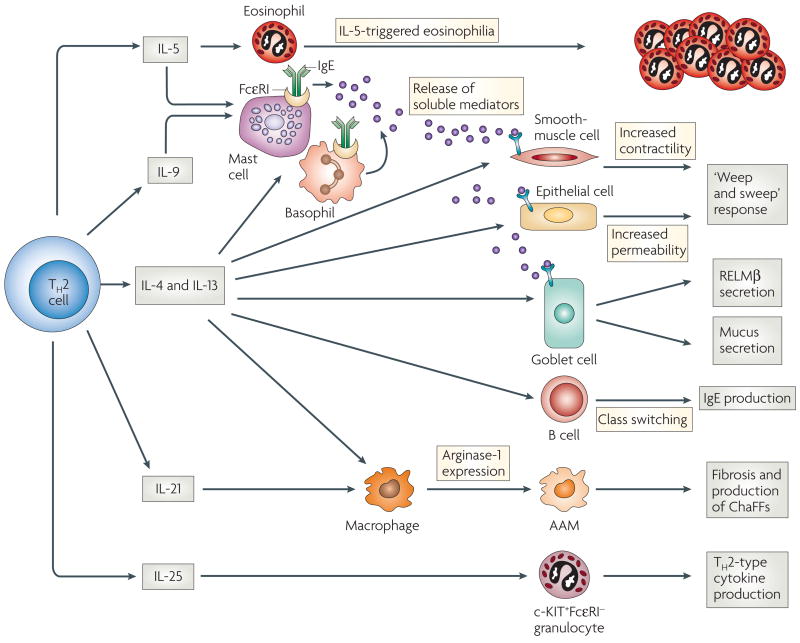
Figure 3. TH2-cell functions during helminth infection.
T helper 2 (TH2) cells orchestrate the immune response primarily through the production of cytokines in the lymph nodes and periphery. Interleukin-5 (IL-5) triggers eosinophilia, and in conjunction with IL-4, IL-9, and IL-13, and the crosslinking of FcεRIs (high-affinity Fc receptors for IgE), can result in enhanced mast-cell and basophil development and release of mediators. IL-4 and IL-13 stimulate increased smooth-muscle-cell contractility, increased intestinal permeability and elevated goblet-cell mucous secretion. IL-4 and IL-13 also enhance responsiveness of these cell types to mast-cell-derived mediators. Collectively, these effects can contribute to the ‘weep and sweep’ response to intestinal helminths. IL-4, in conjunction with other signals, can induce class switching in B cells, leading to IgE production. IL-4, IL-13 and IL-21, can drive development of alternatively activated macrophages (AAMs), leading to upregulation of arginase-1 expression, and in some cases this might lead to fibrosis, as in chronic schistosomiasis. IL-25 expression stimulates a c-KIT+FcεRI− population to migrate to lymph nodes and upregulate TH2-type cytokine mRNAs. It is unclear whether IL-25 is a TH2-type cytokine, or whether it is expressed by a distinct TH-cell lineage. IL-21, also produced by TH17 cells, is instrumental in the development of a TH17-like response (not shown). ChaFFs, chitinase and FIZZ family members; RELM, resistin-like molecule.
Neutrophils are also activated and are recruited to sites of infection during tissue invasion by helminths18,19 (FIG. 2). Classically and alternatively activated neutrophils have recently been distinguished following bacterial infections41 and it will be important for future studies to determine the phenotypes of the neutrophils that are associated with helminth infection. Studies have suggested a possible role for neutrophils in mediating resistance to H. polygyrus in vivo and damaging parasites in vitro. More recent reports indicate an important role for neutrophils in the killing of the larval stages of Strongyloides stercoralis. It was shown that neutrophils were rapidly recruited to diffusion chambers containing S. stercoralis larvae, where they killed larvae in the absence of other cell types, although eosinophils were also required for optimal killing42. During infection with S. mansoni, neutrophils seem to have little effect, as their depletion did not influence the severity of disease26. Generally, however, neutrophils are increasingly recognized as important components of the TH2-type response elicited during helminth infection. Following their rapid recruitment to sites of parasitic helminth invasion, neutrophils, working in coordination with other cell populations, including eosinophils and macrophages, can potentially directly damage tissue-dwelling helminths.
Box 1 ChaFFs and helminth infection.
Chitinase and FIZZ (found in inflammatory zone) family members (ChaFFs) are recently described secretory proteins that are induced during T helper 2 (TH2)-type responses and contribute to allergic asthma, fibrosis or helminth immunity40,121. Some of these molecules are related to enzymes that degrade chitin, a molecule that is common to some pathogens, including some helminth larvae and fungi40,121. Resistin-like molecules (RELMs) have sequence homology to resistin (also known as FIZZ3), a hormone released by adipocytes that mediates insulin resistance122. The descriptions below of specific ChaFFs are primarily based on studies in murine models.
AMCase
AMCase (acidic mammalian chitinase) is a functional chitinase that is upregulated during TH2-type inflammatory responses by interleukin-13 (IL-13) in lung epithelium and pulmonary macrophages in a model of allergic asthma123, and at host–parasite interfaces during helminth infections18,29,124. Macrophages have been shown to express AMCase during Heligmosomoides polygyrus infection18, Brugia malayi implantation121 and in Schistosoma mansoni egg granulomas32. AMCase plays an effector role in promoting TH2-type responses by inducing eotaxin and CC-chemokine ligand 2 (CCL2; also known MCP1)123.
Ym1
This is a lectin with affinity for chitin. It lacks chitinase activity and is one of the most highly upregulated transcripts in macrophages during nematode and schistosome infection32,125. It is also found forming spontaneous crystals in asthmatic lungs126. Its induction is dependent on IL-4 and signal transducer and activator of transcription 6 (STAT6) signalling127. It also binds heparin136, suggesting that it mediates interactions between cells and the extracellular matrix. Ym1 may also be an eosinophil chemotactic factor137.
RELMα
RELMα (also known as FIZZ1) has a diverse expression profile, including by lung, intestinal, and adipose tissues, as well as by macrophages. Macrophages express very high levels of RELMα mRNA when alternatively activated by infection with S. mansoni, B. malayi, Nippostrongylus brasiliensis or Litomosoides sigmodontis, as do other antigen-presenting cells18,29,32,121. RELMα seems to have multiple functions, such as inhibition of adipocyte differentiation (perhaps due to its homology with resistin), inhibition of the action of nerve growth factor (suggesting a role in immunoregulation) and stimulation of collagen production in myofibroblasts (providing a potential link between alternatively activated macrophages, fibrosis, and tissue repair40).
RELMβ
RELMβ (also known as FIZZ2) has a more restricted expression than RELMα, and is found expressed almost exclusively in the gastrointestinal tract where it is important for normal colonic function and for the TH2-type response to helminth infection128,129. Upon infection with Trichuris muris, Trichuris spiralis or N. brasiliensis, intestinal goblet cells upregulate and secrete RELMβ under the control of IL-13 signalling through the type II IL-4 receptor. Immunofluorescent staining has shown direct interactions between RELMβ and the helminths T. muris and Strongyloides stercoralis, in which it binds to structures on the lateral alae, thereby interfering with helminth chemotaxis towards host tissues128.
RELMγ
This is the least understood member of the family. Expressed in the lungs and in haematopoietic tissues, it might have a role in regulating promyelocytic development130. This protein is also found upregulated in nematode-infected intestines131, although a role for RELMγ in these types of infection is unknown.
Eosinophils: in search of a function?
The innate immune cell populations typically associated with TH2-type responses, eosinophils, basophils and mast cells, have an integral role in antihelminth responses and in the allergic cascade15,43. Following helminth infection, eosinophil numbers increase dramatically in the blood44, and these eosinophils rapidly migrate to the site of infection, where they degranulate, releasing eosinophil secondary granule proteins (ESGPs) (FIG. 2).
Until recently, eosinophil biology was examined by blocking or inhibiting IL-5, a CD4+ T-cell-derived cytokine that is required for eosinophilia (FIG. 3). These studies generally showed continued resistance to a variety of helminths despite the reduced eosinophil numbers45,46. However, not all eosinophils were eliminated using these approaches, and other cell populations, including neutrophils47, might also respond to IL-5, and their function might therefore have been altered by these treatments. More recently, several eosinophil-deficient mouse models have been developed. These include mice in which the eosinophil peroxidase promoter drives expression of the diphtheria toxin (referred to as TgPHIL mice), and mice that have deletion of a GATA1 binding sequence (referred to as ΔdblGATA mice)48,49. In these mouse models, primary responses to schistosomes48 and to the intestinal nematode parasite Nippostrongylus brasiliensis49 are unaffected. However, eosinophils mediated parasite damage during N. brasiliensis challenge, even in the absence of CD4+ T cells50. Depletion of eosinophils with antibodies specific for CC-chemokine receptor 3 (CCR3) enhanced susceptibility to primary infection with Strongyloides stercoralis42. Similar results were obtained from a model of filarial disease, although mice deficient in ESGPs remained resistant51, suggesting that eosinophils also mediate protection through an ESGP-independent mechanism. Eosinophils can also have a regulatory role through the production of cytokines, including IL-4 and IL-13 (REFS 52,53)(FIG. 2), and can function in vitro to present antigens to T cells in a S. stercoralis model54; however, in general, eosinophil depletion does not appear to markedly impair the development of the in vivo TH2-type response to most parasitic helminths45,48,55. A major effector function of eosinophils and their ESGPs might be in tissue remodelling and debris clearance following tissue injury, a general activity that might help to mediate the wound healing response following parasite tissue invasion56 (FIG. 2).
Basophils and mast cells: they are activated, but are they primary players?
Following helminth infection, basophils increase in number in the blood and tissues. Following N. brasiliensis infection, IL-4-producing basophils are readily detected in the lungs, liver and spleen, but have not been detected in the lymph nodes or Peyer’s patches57,58; it is possible that they infiltrate lymph nodes at an unidentified time. Basophils and eosinophils might be a major source of IL-4 at early stages of the TH2-cell response (FIG. 2), suggesting that they promote the development of TH2 cells or their recruitment to sites of inflammation57,58. To further understand basophil biology and the role of these cells in helminth infection, it will be important to develop an effective method for selectively depleting basophils in vivo.
Mast cells share many characteristics with basophils, including the cell-surface expression of the high-affinity Fc receptor for IgE (FcεRI) and the Toll-like receptors (TLRs) TLR2 and TLR4, the release of mediators upon activation and IL-4 secretion, they have also been shown recently to derive from a common progenitor59. However, unlike basophils, which circulate in the blood, mast cells reside in peripheral tissues and are thus well situated to respond immediately to invasive agents. Increased numbers of mucosal mast cells are often observed in affected tissues during helminth infections and this increase is dependent on TH2-type cytokines that are primarily derived from CD4+ T cells; specifically, IL-4-dependent mucosal mastocytosis is associated with a resistant phenotype to H. polygyrus60. Although mastocytosis is pronounced in the small intestine of mice inoculated with H. polygyrus, interestingly, increased numbers of mast cells are not observed in or near the granuloma in which the larval parasite develops19. Furthermore, previous studies have shown that mice lacking mast cells (referred to as w/wv mice) are able to clear H. polygyrus infection (J.F.U. Jr, unpublished observations). Mast cells also do not appear to have a key role in schistosomiasis, although they might contribute to the immune response at early stages of infection when the cercariae penetrate the skin61.
A role for mast cells in helminth immunity has been described in Trichinella spiralis; studies using w/wv mice or stem-cell factor (SCF)-specific antibodies that impair mast-cell function showed that SCF is required for mucosal mastocytosis during helminth infection, and is important for effective helminth expulsion62,63. Furthermore, mice deficient in mouse mast-cell protease 1 (mMCP-1; also known as β-chymase and Mcpt1) are unable to expel T. spiralis indicating the antihelminth properties of mast-cell cytoplasmic granules (FIG. 2); however, mMCP-1 has little effect on N. brasiliensis host resistance64. mMCP-1 might function to impair epithelial-cell barriers by degrading the tight junction protein occludin and thereby increasing luminal fluid flow65,66.
Although most helminths induce pronounced basophil and mast-cell responses, the importance of these cells in mediating resistance to these parasites varies greatly. This variation may be related to the distinct microenvironments that are occupied by different intestinal nematodes. For example, adult T. spiralis — unlike adult H. polygyrus and N. brasiliensis, which inhabit the intestinal lumen — reside in intestinal epithelial-cell syncytia, raising the possibility that mast-cell mediators might be more important in expelling tissue-dwelling nematodes, possibly by increasing vascular permeability67.
Other cell populations
Several non-leukocyte populations have recently been described as having a role in helminth infections. In vitro studies suggest that intestinal epithelial cells (IECs) secrete factors, including thymic stromal lymphopoietin (TSLP), that block the production of IL-12 by DCs and thereby inhibit the ability of DCs to promote TH1-type responses68. These findings have been extended in vivo to show that IECs, at least partly through their production of TSLP, help to sustain the protective TH2-type response to T. muris by inhibiting TH1-type inflammatory responses69. As T. muris resides in epithelial-cell syncytia, physiological changes in this cell type may have particularly important implications with regard to resistance to this parasite. Indeed, studies have shown that the epithelial-cell turnover that is induced by TH2-type cytokines acts as an ‘escalator’, expunging whipworms from the syncytia into the gut lumen70.
Although natural killer (NK) cells have been shown to be activated during helminth infection71,72, including gut-resident NK cells that were shown to produce IL-13 during T. muris infection of scid (severe combined immunodeficient) mice73, the role of these cells in protective responses is unclear. Recent studies have implicated IL-25 in the promotion of host protective TH2-type immune responses during intestinal nematode infections and also in the recruitment of a newly identified non-B-cell, non-T-cell population that expresses elevated levels of TH2-type cytokine mRNAs to draining mesenteric lymph nodes. These cells express c-KIT but not FcεRI, and might represent a distinct lineage or perhaps a precursor population of mast cells or basophils that contributes to the development of the TH2-type response11 (FIG. 3).
Box 2 IL-4 and IL-13 in helminth infections.
Direct effects of interleukin-4 (IL-4) and IL-13 on gut tissue during helminth infection
Sources
T cells are a primary source of IL-4 and IL-13, but B cells, basophils, eosinophils and mast cells might also contribute significantly at both the initiation and effector phases of the response.
Receptors
Type I IL-4 receptor. Type I IL-4 receptor (IL-4R) binds IL-4, it is a heterodimer of IL-4Rα and the common cytokine-receptor γ chain (γc), it is expressed on bone-marrow-derived cells, including T and B cells, mast cells, basophils, eosinophils and macrophages. It signals through Janus kinase-dependent tyrosine phosphorylation of the IL-4R and signal transducer and activator of transcription 6 (STAT6).
Type II IL-4R. This receptor binds IL-4 and IL-13, it is a heterodimer of IL-4Rα and IL-13Rα1, it is expressed on non-bone-marrow-derived cells, including epithelial cells, smooth muscle cells, endothelial cells and fibroblasts and also various bone-marrow-derived cells but not T cells132. It signals through Janus-kinase-dependent tyrosine phosphorylation of the IL-4R and STAT6.
A second IL-13R chain, IL-13Rα2, is soluble, not linked to STAT6, and might function as a decoy receptor to modulate IL-13 activity in vivo133, with recent studies suggesting that it modulates IL-13-mediated muscle contractility134, and downregulates fibrosis in schistosome granulomas17.
Effects on non-bone-marrow-derived tissues
IL-4 and IL-13 induce increased contractility of intestinal longitudinal smooth muscle and also increased intraluminal fluid through increased intestinal permeability and responsiveness to mediators such as prostaglandin E2, which stimulates fluid secretion135. Similar STAT6-dependent effects are observed following infection with nematodes (such as Heligmosomoides polygyrus, Trichuris spiralis and Nippostrongylus brasiliensis )85. The secreted fluids may also include toxins which damage the intestinal parasite. Increased mucus secretion has been observed in N. brasiliensis infection15, and IL-4 has been shown to stimulate Paneth-cell growth and secretion of antibacterial products which may also harm helminths67. In addition, IL-13 induces goblet-cell secretion of resistin-like molecule-β (RELMβ), which may bind cuticle structures thereby impairing chemosensory function128.
IL-4 and IL-13 effector mechanisms may differ between helminth parasites
H. polygyrus. The IL-4-mediated protective response involves both macrophage effects on tissue-dwelling larvae and also probably direct effects of IL-4 (and also IL-13) on small intestinal tissue, thereby interfering with adult worm adherence and feeding.
N. brasiliensis. The IL-13-mediated response involves primarily non-bone-marrow derived cells, probably directly affecting the small intestine, which leads to modified worm adherence and feeding.
T. spiralis. IL-4 and IL-13 stimulate B-cell production of IgE, mastocytosis and basophilia. Mast-cell and basophil degranulation and release of IL-4 and IL-13, as well as other mediators, induces changes in the small intestinal tissue, creating an inhospitable environment for both luminal larvae and adults that dwell in intestinal tissues.
T. muris. IL-13 and, to a lesser extent, IL-4 stimulate rapid epithelial-cell turnover in the large intestine, essentially expelling parasites from their habitat (which are syncytia of epithelial cells)70.
Schistosoma mansoni. IL-4 and IL-13 induce alternatively activated macrophages, which help to control the development of an underlying pathological TH1- and/or TH17-type inflammation26,88. IL-13, in particular, can contribute to fibrosis17.
Adaptive effector cells: T and B cells
Helminths potently induce the development of effector TH2 cells following infection. The ensuing cytokine response by TH2 cells has broad effects, promoting antihelminth effector functions in a variety of bone-marrow-derived and non-bone-marrow-derived cell populations and tissues.
CD4+ T helper effector cells: heterogeneous and essential
Effector TH2 cells induced by helminths are characterized by the production of the cytokines IL-4, IL-5, IL-9, IL-13 and the recently identified TH2-type cytokine IL-21, and the absence of IFNγ and IL-17 production (FIGS 3,4). In immune responses to S. mansoni, but usually not to H. polygyrus, TH2 cells can also produce IL-10 (REF. 15). Following infection with different species of bacteria and viruses, naive T cells differentiate along a reciprocal pattern, with high levels of IFNγ and low levels of IL-4, that is largely shaped by adjuvant microbial pathogen-associated molecular patterns (PAMPs) that bind to TLRs and other innate receptors expressed by DCs and other antigen-presenting cells74. Recent studies show that helminths can also provide an adjuvant function, driving TH2-cell differentiation in vivo to non-parasite antigens75,76. In this context, IL-2 mediates initial TH2-cell differentiation whereas IL-4 is required for expansion of antigen-specific TH2 cells. The source of IL-4 may include non-T cells, bystander T cells and the antigen-specific TH2 cells that produce IL-4, promoting proliferation through an autocrine feedback loop. Indeed, autocrine IL-4 produced by antigen-specific CD4+ T cells is sufficient to support TH2-cell differentiation and expansion during N. brasiliensis infection75. The adjuvant structures on helminths that drive TH2-cell differentiation are not yet clearly defined; however, intriguing data suggest that glycans77,78, lipids and lipoproteins79,80, and perhaps proteases81 expressed by certain helminths, might function as PAMPs to drive TH2-cell differentiation. In a recent important study, chitin was identified as a potential TH2-cell-stimulating PAMP82. The role of DCs in preferentially promoting TH2-cell differentiation during helminth infection remains unclear, although several studies indicate that DCs activated following exposure to schistosome egg antigen (SEA) preferentially support TH2-cell differentiation83,84.
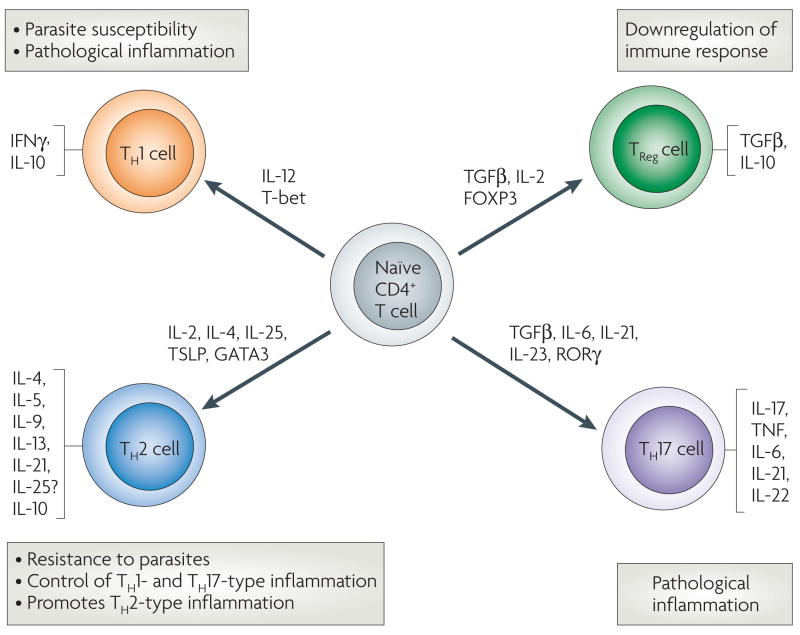
Figure 4. T helper and regulatory cells.
Naive CD4+ T cells can differentiate into several different types of effector and regulatory cells during helminth infection. Specific cytokines and transcription factors contribute to differentiation and expansion of these cell populations, and their differential activation plays a major role in determining whether an immune response will contribute to host protection or pathological inflammation. Exposure to interleukin-12 (IL-12) produced by antigen-presenting dendritic cells (DCs) induces T-bet expression, driving T helper 1 (TH1)-cell differentiation. These cells produce interferon-γ (IFNγ), and are unable to clear helminth parasites. The circumstances leading to TH2-cell differentiation are not as clear but recently, IL-2, IL-25 and thymic stromal lymphopoietin (TSLP), and associated transcription factors, have been implicated. TH2 cells produce a panel of cytokines that contribute to antihelminth immunity by instructing innate bone-marrow- and non-bone-marrow-derived cells, which in turn can instruct and amplify TH2 cells. Strong TH2-type responses often lead to increased resistance to parasites, as seen in Heligmosomoides polygyrus infection, or control of harmful inflammation mediated by TH1 cells and TH17 cells, as in schistosomiasis. However, chronic potent TH2-cell responses can also lead to harmful TH2-type inflammatory responses including fibrosis. Expression of the transcription factor forkhead box P3 (FOXP3) along with the cytokines transforming growth factor-β (TGFβ) and IL-2, leads to differentiation of regulatory T cells (TReg cells), which can inhibit the immune response. Several factors, including retinoic-acid-receptor-related orphan receptor-γ (RORγ), TGFβ, IL-6, IL-21 and IL-23, have been shown to promote TH17-cell differentiation, and these effector cells can mediate harmful inflammation. IL-10, which is no longer considered to be exclusively a TH2-type cytokine, can be produced by TH1, TH2, and TRegcells and downregulates both TH1- and TH2-type responses. TNF, tumour-necrosis factor.
During helminth infections, TH2 cells orchestrate the activation and expansion of leukocytes primarily through the production of cytokines, an essential function that serves to amplify and sustain the TH2-type response. In addition, IL-4 and IL-13 can directly affect cell populations that express IL-4R but that are not derived from the bone marrow, such as small-intestine smooth-muscle cells, epithelial cells and myenteric neurons67 (BOX 2; FIG. 3). IL-4R signalling is largely dependent on the actions of signal transducer and activator of transcription 6 (STAT6). H. polygyrus infection induces STAT6-dependent changes in epithelial-cell function, increased smooth-muscle contractility, increased mucus production, and enhanced fluids in the gut lumen 85–87 (BOX 2; FIG. 3). These studies suggest TH2-cell derived IL-4 and IL-13 contribute in a STAT6-dependent manner to the ‘weep and sweep’ response that is characteristic of many intestinal helminth infections, in which increased luminal fluids (weep) and muscle contractility (sweep) are speculated to make the intestinal lumen an inhospitable environment for the helminth parasite. The weep and sweep response functions to decrease the fecundity of adult worms and increase the likelihood of live parasite expulsion, rather than to kill them. So, multiple host protective mechanisms might contribute to an effective H. polygyrus response, with macrophages targeting developing parasites during the tissue-dwelling phase, and cytokine-induced changes in gut physiology affecting adult worms in the intestinal lumen (BOX 2; FIG. 1). Blockade of the TH2-type response during H. polygyrus infection does not default to a TH1-type response; instead, the immune response is generally inhibited21. By contrast, infection with schistosomes elicits an early TH1-type response, which typically changes to a TH2-type response following parasite egg deposition16,88.
The host-specific CD4+ T-cell response to schistosome infection stimulates parasite development89, and results in an inflammatory environment that is required for translocation of parasite eggs from the intravascular compartment into the intestinal lumen22. The controlled hepato-intestinal granulomatous inflammation around the eggs in this TH2-type-polarized milieu might represent a compromise between the parasite and the vertebrate host, allowing the parasite to complete its life cycle while at the same time protecting the host from severe, lethal disease. In certain mouse strains, the host–parasite balance is not achieved, and this results in a severe pathological inflammatory response mediated by CD4+ TH1 and TH17 cells16,88,90.
TH17 cells represent a recently discovered CD4+ T-cell subpopulation, defined by their production of IL-17, that can mediate chronic inflammation in autoimmune diseases such as encephalitis and arthritis91 (FIG. 4). TH17 cells are stimulated by IL-23, a heterodimeric cytokine composed of the IL-12p40 and IL-23p19 subunits (in contrast to IL-12, which is an IL-12p40–IL-12p35 heterodimer)92. In schistosomiasis, exacerbation of egg-induced pathology that is associated with elevated IL-17 levels occurs after immunization with SEA in complete Freund’s adjuvant of infected wild-type or IL-12p35-deficient C57BL/6 mice, and fails to occur in mice lacking IL-12p40 (REFS 90,93). These findings suggest that the IL-17–IL-23 pathway is critical for development of severe schistosomiasis, although a role for the IL-12–IFNγ pathway cannot be completely excluded. Consistent with this interpretation, CBA mice, which naturally develop extensive pathology, also have elevated IL-17 responses and the egg-induced pathology can be reduced using IL-17-specific antibodies90. More recent studies have shown that IL-6, TGFβ and IL-21 (REF. 94) are the key inducers of TH17-cell differentiation, and that IL-23 serves as a survival and expansion factor for this subset of T cells95–97.
These studies suggest that, in schistosomiasis, TH2 cells primarily downregulate harmful TH1- and TH17-type inflammatory responses either directly or through innate immune cell populations (FIGS 2,4). By comparison, the TH2-type immune response to H. polygyrus, which is not associated with an underlying TH1-type response, instead contributes to parasite resistance and eventual helminth expulsion from the gut. The lack of harmful TH2-cell-mediated pathology following H. polygyrus infection is probably a consequence of the relatively short 8 day period of tissue residence.
Regulatory T cells
Nematode infections can induce and expand naturally occurring regulatory T cells (TReg cells) in humans and mice98–101, suggesting a role for these TReg cells in helminth-induced modulation of inflammatory diseases102 (FIG. 4). Hyporesponsiveness associated with infection of mice with the filarial parasite Litomosoides sigmodontis was blocked following in vivo depletion of TReg cells, which resulted in increased killing of parasites103. These studies suggest that TReg cells induced during filarial parasite infection have an important role in immunosuppression induced by filarial parasites and in increased worm survival. Recently, CD8+ lamina propria T cells induced by infection with H. polygyrus were shown to inhibit T-cell proliferation and the development of experimentally induced colitis104, suggesting that other regulatory T-cell populations are also important in nematode infections. TReg cells have been suggested to have an important role in the suppression of the TH1-type inflammatory response to SEA. Initial reports have suggested this effect was mediated through IL-10 (REFS 105,106); however, more recent findings indicate that these TReg cells can function through as yet unidentified IL-10-independent mechanisms as well107–109. TReg cells might also be instrumental in controlling TH2-type responses in chronic S. mansoni infection106,108. Future studies should examine the role of the TH2 cells, TReg cells, alternatively activated macrophages and perhaps other innate cell populations, in the control of TH1- and TH17-cell-mediated inflammation and the upregulation of the TH2-type response. It is possible that several or all of these different cell populations interact to mediate the evolution of a potentially pathological TH1-type response into a protective TH2-type response following egg deposition in chronic schistosomiasis.
B cells and antibody responses
During polarized TH2-type responses, IL-4 mediates B-cell class switching to IgE, a primary mediator of acute allergic and asthmatic reactions that binds FcεRI on mast cells and basophils (FIG. 3). Antigen crosslinking of FcRεI-bound IgE triggers mast-cell degranulation and the release of soluble mediators110. IL-4 and IL-13 signalling through the IL-4R can increase the sensitivity of target cells to basophil- and mast-cell-derived mediators. Therefore, IgE effects are amplified in the presence of these TH2-type cytokines and this results in enhanced responses including increased vascular permeability, smooth muscle contractility, and also the recruitment of TH2-type effector cells, including eosinophils and TH2 cells67.
Interestingly, neither IgE nor mast cells appear to have an essential role in the development of the host protective immune response to either H. polygyrus or N. brasiliensis87 (J.F.U. Jr, unpublished observations). By contrast, mast cells are required for protective immunity against T. spiralis; however, parasite-specific IgE does not have an essential role, as recent studies have demonstrated that effective expulsion is achieved in B-cell-deficient mice111. In these cases, TH2-type cytokines and perhaps other factors must be sufficient for mast-cell degranulation.
Antibody classes other than IgE have been described in nematode infection. Secreted IgM was essential for timely expulsion of filarial parasites112. This is the primary antibody type that recognizes larval parasites, and might be produced in a T-cell-independent manner. As macrophages express Fc receptors for IgM, IgM might also be important for macrophage recognition of filarial parasites113.
Schistosome infections elicit strong host antibody responses against multiple components of the parasite, the majority of which are glycan determinants114. Although it seems unlikely that the antibodies have a significant antiparasitic effect, B cells can generally support the establishment of the TH2-type response115, thereby contributing to reduced pathology in schistosomiasis116,117, and correlations between IgE production and protective immune responses have been reported in human schistosomiasis118. Therefore, although the humoral antibody response is typically associated with TH2-type responses during infectious disease, in many cases antibodies do not appear to have an essential role in helminth protective responses that either involve parasite expulsion or that down-regulate harmful TH1-type responses. As it is clear that specific components of the TH2-type response that actually mediate protection vary greatly with the specific parasitic helminth and developmental stage of the host119, it is very possible that an essential role for antibodies will be identified as such parasitic infections are examined.
Concluding remarks and future directions
The TH2-type response mediates protective responses to a wide variety of parasitic helminths. Activation and function of this response is dependent on ongoing crosstalk between innate and adaptive effector-cell populations. It is initiated by innate-cell populations that promote TH2-cell differentiation. As the response progresses, TH2 cells, primarily through cytokine production, amplify and instruct the innate cell populations, which reciprocate and help to sustain the TH2 cells. The result is a potent, orchestrated response, involving a diverse population of leukocytes that secrete TH2-type cytokines and other factors that promote helminth-specific immunity by directly affecting the parasite, triggering non-bone-marrow-derived cells to undergo physiological changes, and further amplify the TH2-type response. Although many of these effector cells are activated in response to most helminth infections, only certain cell types are actually effective against any one type of parasitic helminth. Individual effector-cell types might also have multiple functions that have an essential role in the response to some helminths and not others. Both the cell population and effector function mediating protection are at least partly dependent on the tissue site of parasite residence.
Current treatments for helminth infection include frequent administration of helminth-specific drugs such as praziquantel (for schistosomiasis) and benzimidazoles (for hookworm infection). Although these treatments have been effective, parasites could potentially develop resistance, and re-infect individuals shortly after drug administration. Therefore, identification of new helminth-specific therapies, with the ultimate development of effective vaccines, is urgently needed. A number of vaccine candidates are being tested in clinical trials with, as yet, little success88,120. The inefficacy of vaccines tested against helminths might in part result from too narrow a focus on single antigens and their ability to induce effective antibody responses. Ultimately, the most effective vaccines will probably elicit broad TH2-type responses including potent cell-mediated and humoral components. The choice of adjuvant may be particularly important in eliciting these kinds of multi-faceted immune responses. In some cases, successful vaccines may be directed more towards suppressing harmful pathological immune responses than expelling the helminths. The recent advances in our understanding of how various innate and adaptive components of the TH2-type response contribute to host protective responses provides a new set of compelling targets for the development of more effective therapies and vaccines.
Acknowledgments
We greatly appreciate the careful review and thoughtful suggestions provided by F.D. Finkelman and E.J. Pearce, and would like to thank T. Kreider for his help in editing the original manuscript and drawing the original figures. This work was supported by National Institutes of Health Grants AI031678 and AI066188.
- Helminths
These are worms that are characterized by their body shape into cestodes (flat worms), nematodes (round worms) and trematodes (leaf-shaped worms).
- Hygiene hypothesis
This hypothesis originally proposed that the increased incidence of atopic diseases in westernized countries was a consequence of living in an overly clean environment resulting in an under-stimulated immune system that responded inappropriately to harmless antigens. More recently it has been proposed that an absence of exposure to pathogens, in particular helminths, may predispose to both increased allergy and autoimmune disease.
- TH2-type response
(T-helper-2-type response). An immune response including innate and adaptive components that is elicited by helminth infections and many allergic reactions. Common features include expression of TH2-type cytokines (IL-4, IL-5 and IL-13), eosinophilia, basophilia, mastocytosis, goblet-cell hyperplasia and IgE production.
- TH1 cells
(T helper 1 cells). This is a CD4+ effector T cell mainly characterized by its expression of interferon-γ.
- Heligmosomoides polygyrus
A natural mouse gastrointestinal trichostrongylid nematode parasite, used as a model of human intestinal nematode infection. Primary infections become established and chronic, and can be cleared by helminth-specific drug treatment. Challenge infections are naturally cleared by the host by day 14 post-infection, making this an excellent model of protective memory T-helper-2-type responses.
- Schistosoma mansoni
A blood-dwelling trematode parasite that causes a major form of human hepato-intestinal schistosomiasis. Infection of mice with this parasite represents a well-established murine model of this disease.
- Granuloma
A circumscribed inflammatory cell infiltrate surrounding a nidus. Its composition usually includes T cells and macrophages, and in the case of helminth infection, eosinophils.
- TH17 cell
(T helper 17 cell). This is a CD4+ effector T cell that expresses interleukin-17 (IL-17), IL-6, tumour necrosis factor, IL-21 and IL-22, but does not express interferon-γ nor IL-4.
- Alternatively activated macrophage
A macrophage stimulated by interleukin-4 (IL-4) or IL-13 that expresses arginase-1, mannose receptor CD206 and IL-4 receptor α. There may be pathogen-associated molecular patterns expressed by helminths that can also drive alternative activation of macrophages.
- Classically activated macrophage
A macrophage that is activated through Toll-like receptors and interferon-γ that expresses inducible nitric oxide synthase and nitric oxide.
- Filarial parasites
These thread-like nematode parasites cause a wide range of diseases in humans, including River blindness (by Onchocerca volvulus) and elephantiasis (by Wuchereria bancrofti, Brugia malayi and Brugia timori).
- Chitinase and FIZZ family member proteins
(Chaffs). Proteins that are expressed by alternatively activated macrophages or goblet cells during TH2-type responses. They include acidic mammalian chitinase (AMCase), Ym1, Ym2, resistin-like molecule (RELMα; also known as FIZZ1), RELMβ (also known as FIZZ 2) and RELMγ.
- Strongyloides stercoralis
A parasitic roundworm (threadworm) of humans and other mammals.
- Goblet cells
Mucus-producing cells found in the epithelial-cell lining of the intestine and lungs.
- Allergic cascade
The sequence of events contributing to acute and chronic allergic reactions. Surface Fc receptors for IgE on mast cells are crosslinked, triggering the release of soluble mediators. Immediate vascular permeability and smooth muscle contractility is associated with acute allergic reactions, and eosinophils and T helper 2 cells are associated with chronic allergic reactions.
- Nippostrongylus brasiliensis
A trichostrongylid intestinal nematode rodent parasite that is widely used as a model to study T-helper-2-type responses. In most inbred mouse strains, primary infection results in rapid expulsion of the parasite within 10–12 days.
- Toll-like receptors
(TLRs). The best characterized family of receptors for pathogen-associated molecular patterns. Receptors in this family recognize bacterial cell-wall and membrane structures, flagella, bacterial DNA motifs, viral double-stranded RNA and other structures.
- Pathogen-associated molecular patterns
(PAMPs). Conserved microbial structures recognized by innate receptors, including Toll-like receptors.
- Regulatory T cell
(TReg cell). A type of CD4+ T cell that is characterized by its expression of forkhead box P3 (FOXP3) and high levels of CD25. TReg cells can downmodulate many types of immune responses.
- Class switching
The somatic-recombination process by which the class of immunoglobulin is switched from IgM to IgG, IgA or IgE. During T helper 1 (TH1)-type responses, B cells can class-switch to produce IgG2a whereas during TH2-type responses B cells can switch to produce IgE.
Footnotes
DATABASES
Entrez Gene: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene
chitinase | FcεRI | IL.4 | IL.5 | IL.9 | IL.10 | IL.13 | IL.17 | IL.21 | IL.23p19 | STAT6 | TSLP
FURTHER INFORMATION
William C. Gause’s homepage: http://njms.umdnj.edu/research/gause/research.htm
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.WHO. World Health Report. World Health Organization; Geneva, Switzerland: 1999. [Google Scholar]
- 2.Capron A, Riveau G, Capron M, Trottein F. Schistosomes: the road from host-parasite interactions to vaccines in clinical trials. Trends Parasitol. 2005;21:143–149. doi: 10.1016/j.pt.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Goud GN, et al. Expression of the Necator americanus hookworm larval antigen Na-ASP-2 in Pichia pastoris and purification of the recombinant protein for use in human clinical trials. Vaccine. 2005;23:4754–4764. doi: 10.1016/j.vaccine.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 4.Fallon PG, Mangan NE. Suppression of TH2-type allergic reactions by helminth infection. Nature Rev Immunol. 2007;7:220–230. doi: 10.1038/nri2039. [DOI] [PubMed] [Google Scholar]
- 5.Wilson MS, Maizels RM. Regulation of allergy and autoimmunity in helminth infection. Clin Rev Allergy Immunol. 2004;26:35–50. doi: 10.1385/CRIAI:26:1:35. [DOI] [PubMed] [Google Scholar]
- 6.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holt PG. Parasites, atopy, and the hygiene hypothesis: resolution of a paradox? Lancet. 2000;356:1699–1701. doi: 10.1016/S0140-6736(00)03198-6. [DOI] [PubMed] [Google Scholar]
- 8.Yazdanbakhsh M, Matricardi PM. Parasites and the hygiene hypothesis: regulating the immune system? Clin Rev Allergy Immunol. 2004;26:15–24. doi: 10.1385/CRIAI:26:1:15. [DOI] [PubMed] [Google Scholar]
- 9.Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–494. doi: 10.1126/science.296.5567.490. A review of the inverse relationship of helminth infections and atopic diseases. [DOI] [PubMed] [Google Scholar]
- 10.Owyang AM, et al. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J Exp Med. 2006;203:843–849. doi: 10.1084/jem.20051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fallon PG, et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203:1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jankovic D, et al. Conventional T-bet+Foxp3− Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med. 2007;204:273–283. doi: 10.1084/jem.20062175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson CF, Oukka M, Kuchroo VJ, Sacks D. CD4+CD25−Foxp3− Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J Exp Med. 2007;204:285–297. doi: 10.1084/jem.20061886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffman KF, Cheever AW, Wynn TA. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine response induce distinct forms of lethal immunopathology in murine schistosomiasis. J Immunol. 2000;164:6406–6416. doi: 10.4049/jimmunol.164.12.6406. [DOI] [PubMed] [Google Scholar]
- 15.Gause WC, Urban JF, Jr, Stadecker M. J The immune response to parasitic helminths: insights from murine models. Trends Immunol. 2003;24:269–277. doi: 10.1016/s1471-4906(03)00101-7. [DOI] [PubMed] [Google Scholar]
- 16.Stadecker MJ, et al. The immunobiology of Th1 polarization in high-pathology schistosomiasis. Immunol Rev. 2004;201:168–179. doi: 10.1111/j.0105-2896.2004.00197.x. [DOI] [PubMed] [Google Scholar]
- 17.Wynn TA. Fibrotic disease and the TH1/TH2 paradigm. Nature Rev Immunol. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anthony RM, et al. Memory TH2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nature Med. 2006;12:955–960. doi: 10.1038/nm1451. This study identifies alternatively activated macrophages as antihelminthic effector cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morimoto M, et al. Peripheral CD4 T cells rapidly accumulate at the host: parasite interface during an inflammatory Th2 memory response. J Immunol. 2004;172:2424–2430. doi: 10.4049/jimmunol.172.4.2424. [DOI] [PubMed] [Google Scholar]
- 20.Cliffe LJ, Grencis RK. The Trichuris muris system: a paradigm of resistance and susceptibility to intestinal nematode infection. Adv Parasitol. 2004;57:255–307. doi: 10.1016/S0065-308X(04)57004-5. [DOI] [PubMed] [Google Scholar]
- 21.Liu Z, et al. Requirements for the development of IL-4-producing T cells during intestinal nematode infections: what it takes to make a Th2 cell in vivo. Immunol Rev. 2004;201:57–74. doi: 10.1111/j.0105-2896.2004.00186.x. [DOI] [PubMed] [Google Scholar]
- 22.Doenhoff MJ. Granulomatous inflammation and the transmission of infection: schistosomiasis—and TB too? Immunol Today. 1998;19:462–467. doi: 10.1016/s0167-5699(98)01310-3. [DOI] [PubMed] [Google Scholar]
- 23.Kreider T, Anthony RM, Urban JF, Jr, Gause WC. Alternatively activated macrophages in helminth infections. Curr Opin Immunol. 2007;19:448–453. doi: 10.1016/j.coi.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–346. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez-Sosa M, et al. Chronic helminth infection induces alternatively activated macrophages expressing high levels of CCR5 with low interleukin-12 production and Th2-biasing ability. Infect Immun. 2002;70:3656–3664. doi: 10.1128/IAI.70.7.3656-3664.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herbert DR, et al. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20:623–635. doi: 10.1016/s1074-7613(04)00107-4. This paper describes the regulatory potential of alternatively activated macrophages in the context of helminth infection. [DOI] [PubMed] [Google Scholar]
- 27.Gupta R, et al. Macrophages in the development of protective immunity against experimental Brugia malayi infection. Parasitology. 2004;129:311–323. doi: 10.1017/s0031182004005682. [DOI] [PubMed] [Google Scholar]
- 28.Taylor MD, Harris A, Nair MG, Maizels RM, Allen JE. F4/80+ alternatively activated macrophages control CD4+ T cell hyporesponsiveness at sites peripheral to filarial infection. J Immunol. 2006;176:6918–6927. doi: 10.4049/jimmunol.176.11.6918. [DOI] [PubMed] [Google Scholar]
- 29.Reece JJ, Siracusa MC, Scott AL. Innate immune responses to lung-stage helminth infection induce alternatively activated alveolar macrophages. Infect Immun. 2006;74:4970–4981. doi: 10.1128/IAI.00687-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson CF, Mosser DM. A novel phenotype for an activated macrophage: the type 2 activated macrophage. J Leukoc Biol. 2002;72:101–106. [PubMed] [Google Scholar]
- 31.Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 32.Pesce J, et al. The IL-21 receptor augments Th2 effector function and alternative macrophage activation. J Clin Invest. 2006;116:2044–2055. doi: 10.1172/JCI27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hesse M, et al. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of L-arginine metabolism. J Immunol. 2001;167:6533–6544. doi: 10.4049/jimmunol.167.11.6533. This study characterizes the iNOS/arginase-1 balance in classically activated and alternatively activated macrophages in the context of infectious disease. [DOI] [PubMed] [Google Scholar]
- 34.Flores Villanueva PO, Harris TS, Ricklan DE, Stadecker MJ. Macrophages from schistosomal egg granulomas induce unresponsiveness in specific cloned Th-1 lymphocytes in vitro and down-regulate schistosomal granulomatous disease in vivo. J Immunol. 1994;152:1847–1855. [PubMed] [Google Scholar]
- 35.Atochina O, Daly-Engel T, Piskorska D, McGuire E, Harn DA. A schistosome-expressed immunomodulatory glycoconjugate expands peritoneal Gr1+ macrophages that suppress naive CD4+ T cell proliferation via an IFN-γ and nitric oxide-dependent mechanism. J Immunol. 2001;167:4293–4302. doi: 10.4049/jimmunol.167.8.4293. [DOI] [PubMed] [Google Scholar]
- 36.Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 2005;15:599–607. doi: 10.1016/j.tcb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Sakthianandeswaren A, et al. The wound repair response controls outcome to cutaneous leishmaniasis. Proc Natl Acad Sci USA. 2005;102:15551–15556. doi: 10.1073/pnas.0505630102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gratchev A, et al. Alternatively activated macrophages differentially express fibronectin and its splice variants and the extracellular matrix protein βIG-H3. Scand J Immunol. 2001;53:386–392. doi: 10.1046/j.1365-3083.2001.00885.x. [DOI] [PubMed] [Google Scholar]
- 39.Gratchev A, Schledzewski K, Guillot P, Goerdt S. Alternatively activated antigen-presenting cells: molecular repertoire, immune regulation, and healing. Skin Pharmacol Appl Skin Physiol. 2001;14:272–279. doi: 10.1159/000056357. [DOI] [PubMed] [Google Scholar]
- 40.Nair MG, Guild KJ, Artis D. Novel effector molecules in type 2 inflammation: lessons drawn from helminth infection and allergy. J Immunol. 2006;177:1393–1399. doi: 10.4049/jimmunol.177.3.1393. This is a comprehensive review of the ChaFF family of molecules during TH2-type responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsuda Y, et al. Three different neutrophil subsets exhibited in mice with different susceptibilities to infection by methicillin-resistant Staphylococcus aureus. Immunity. 2004;21:215–226. doi: 10.1016/j.immuni.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 42.Galioto AM, et al. Role of eosinophils and neutrophils in innate and adaptive protective immunity to larval Strongyloides stercoralis in mice. Infect Immun. 2006;74:5730–5738. doi: 10.1128/IAI.01958-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Broide DH. Molecular and cellular mechanisms of allergic disease. J Allergy Clin Immunol. 2001;108:S65–S71. doi: 10.1067/mai.2001.116436. [DOI] [PubMed] [Google Scholar]
- 44.Ganley-Leal LM, et al. Correlation between eosinophils and protection against reinfection with Schistosoma mansoni and the effect of human immunodeficiency virus type 1 coinfection in humans. Infect Immun. 2006;74:2169–2176. doi: 10.1128/IAI.74.4.2169-2176.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brunet LR, Sabin EA, Cheever AW, Kopf MA, Pearce EJ. Interleukin 5 (IL-5) is not required for expression of a Th2 response or host resistance mechanisms during murine schistosomiasis mansoni but does play a role in development of IL-4-producing non-T, non-B cells. Infect Immun. 1999;67:3014–3018. doi: 10.1128/iai.67.6.3014-3018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herndon FJ, Kayes SG. Depletion of eosinophils by anti-IL-5 monoclonal antibody treatment of mice infected with Trichinella spiralis does not alter parasite burden or immunologic resistance to reinfection. J Immunol. 1992;149:3642–3647. [PubMed] [Google Scholar]
- 47.Saeftel M, Arndt M, Specht S, Volkmann L, Hoerauf A. Synergism of γ interferon and interleukin-5 in the control of murine filariasis. Infect Immun. 2003;71:6978–6985. doi: 10.1128/IAI.71.12.6978-6985.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swartz JM, et al. Schistosoma mansoni infection in eosinophil lineage-ablated mice. Blood. 2006;108:2420–2427. doi: 10.1182/blood-2006-04-015933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knott ML, et al. Impaired resistance in early secondary Nippostrongylus brasiliensis infections in mice with defective eosinophilopoeisis. Int J Parasitol. 2007;37:1367–1378. doi: 10.1016/j.ijpara.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 50.Voehringer D, Reese TA, Huang X, Shinkai K, Locksley RM. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J Exp Med. 2006;203:1435–1446. doi: 10.1084/jem.20052448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramalingam T, Porte P, Lee J, Rajan TV. Eosinophils, but not eosinophil peroxidase or major basic protein, are important for host protection in experimental Brugia pahangi infection. Infect Immun. 2005;73:8442–8443. doi: 10.1128/IAI.73.12.8442-8443.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sabin EA, Kopf MA, Pearce EJ. Schistosoma mansoni egg-induced early IL-4 production is dependent upon IL-5 and eosinophils. J Exp Med. 1996;184:1871–1878. doi: 10.1084/jem.184.5.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reiman RM, et al. Interleukin-5 (IL-5) augments the progression of liver fibrosis by regulating IL-13 activity. Infect Immun. 2006;74:1471–1479. doi: 10.1128/IAI.74.3.1471-1479.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Padigel UM, Lee JJ, Nolan TJ, Schad GA, Abraham D. Eosinophils can function as antigen-presenting cells to induce primary and secondary immune responses to Strongyloides stercoralis. Infect Immun. 2006;74:3232–3238. doi: 10.1128/IAI.02067-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holland MJ, Harcus YM, Balic A, Maizels RM. Th2 induction by Nippostrongylus secreted antigens in mice deficient in B cells, eosinophils or MHC class I-related receptors. Immunol Lett. 2005;96:93–101. doi: 10.1016/j.imlet.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 56.Lee JJ, Lee NA. Eosinophil degranulation: an evolutionary vestige or a universally destructive effector function? Clin Exp Allergy. 2005;35:986–994. doi: 10.1111/j.1365-2222.2005.02302.x. [DOI] [PubMed] [Google Scholar]
- 57.Min B, et al. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med. 2004;200:507–517. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shinkai K, Mohrs M, Locksley RM. Helper T cells regulate type-2 innate immunity in vivo. Nature. 2002;420:825–829. doi: 10.1038/nature01202. [DOI] [PubMed] [Google Scholar]
- 59.Arinobu Y, et al. Developmental checkpoints of the basophil/mast cell lineages in adult murine hematopoiesis. Proc Natl Acad Sci USA. 2005;102:18105–18110. doi: 10.1073/pnas.0509148102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Behnke JM, Lowe A, Clifford S, Wakelin D. Cellular and serological responses in resistant and susceptible mice exposed to repeated infection with Heligmosomoides polygyrus bakeri. Parasite Immunol. 2003;25:333–340. doi: 10.1046/j.1365-3024.2003.00639.x. [DOI] [PubMed] [Google Scholar]
- 61.Gerken SE, Vaz NM, Mota-Santos TA. Local anaphylactic reactions to the penetration of cercariae of Schistosoma mansoni. Braz J Med Biol Res. 1990;23:275–281. [PubMed] [Google Scholar]
- 62.Grencis RK, Else KJ, Huntley JF, Nishikawa SI. The in vivo role of stem cell factor (c-kit ligand) on mastocytosis and host protective immunity to the intestinal nematode Trichinella spiralis in mice. Parasite Immunol. 1993;15:55–59. doi: 10.1111/j.1365-3024.1993.tb00572.x. [DOI] [PubMed] [Google Scholar]
- 63.Newlands GF, Miller HR, MacKellar A, Galli SJ. Stem cell factor contributes to intestinal mucosal mast cell hyperplasia in rats infected with Nippostrongylus brasiliensis or Trichinella spiralis, but anti-stem cell factor treatment decreases parasite egg production during N. brasiliensis infection. Blood. 1995;86:1968–1976. [PubMed] [Google Scholar]
- 64.Knight PA, Wright SH, Lawrence CE, Paterson YY, Miller HR. Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J Exp Med. 2000;192:1849–1856. doi: 10.1084/jem.192.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McDermott JR, et al. Mast cells disrupt epithelial barrier function during enteric nematode infection. Proc Natl Acad Sci USA. 2003;100:7761–7766. doi: 10.1073/pnas.1231488100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pennock JL, Grencis RK. The mast cell and gut nematodes: damage and defence. Chem Immunol Allergy. 2006;90:128–140. doi: 10.1159/000088885. [DOI] [PubMed] [Google Scholar]
- 67.Finkelman FD, et al. Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol Rev. 2004;201:139–155. doi: 10.1111/j.0105-2896.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- 68.Rimoldi M, et al. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nature Immunol. 2005;6:507–514. doi: 10.1038/ni1192. [DOI] [PubMed] [Google Scholar]
- 69.Zaph C, et al. Epithelial-cell-intrinsic IKK-β expression regulates intestinal immune homeostasis. Nature. 2007;446:552–556. doi: 10.1038/nature05590. This study provides the identification of the epithelial-cell inflammatory pathway as being essential to induce a polarized TH2-type response to helminth infection. [DOI] [PubMed] [Google Scholar]
- 70.Cliffe LJ, et al. Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science. 2005;308:1463–1465. doi: 10.1126/science.1108661. This paper describes a role for epithelial-cell turnover in the protective response to helminth infection. [DOI] [PubMed] [Google Scholar]
- 71.Hsieh GC, et al. A secreted protein from the human hookworm Necator americanus binds selectively to NK cells and induces IFN-γ production. J Immunol. 2004;173:2699–2704. doi: 10.4049/jimmunol.173.4.2699. [DOI] [PubMed] [Google Scholar]
- 72.Tliba O, Chauvin A, Le Vern Y, Boulard C, Sbille P. Evaluation of the hepatic NK cell response during the early phase of Fasciola hepatica infection in rats. Vet Res. 2002;33:327–332. doi: 10.1051/vetres:2002020. [DOI] [PubMed] [Google Scholar]
- 73.McDermott JR, Humphreys NE, Forman SP, Donaldson DD, Grencis RK. Intraepithelial NK cell-derived IL-13 induces intestinal pathology associated with nematode infection. J Immunol. 2005;175:3207–3213. doi: 10.4049/jimmunol.175.5.3207. [DOI] [PubMed] [Google Scholar]
- 74.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nature Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 75.Liu Z, et al. IL-2 and autocrine IL-4 drive the in vivo development of antigen-specific Th2 T cells elicited by nematode parasites. J Immunol. 2005;174:2242–2249. doi: 10.4049/jimmunol.174.4.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Holland MJ, Harcus YM, Riches PL, Maizels RM. Proteins secreted by the parasitic nematode Nippostrongylus brasiliensis act as adjuvants for Th2 responses. Eur J Immunol. 2000;30:1977–1987. doi: 10.1002/1521-4141(200007)30:7<1977::AID-IMMU1977>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 77.Okano M, Satoskar AR, Nishizaki K, Abe M, Harn DA. Jr Induction of Th2 responses and IgE is largely due to carbohydrates functioning as adjuvants on Schistosoma mansoni egg antigens. J Immunol. 1999;163:6712–6717. [PubMed] [Google Scholar]
- 78.Hokke CH, Yazdanbakhsh M. Schistosome glycans and innate immunity. Parasite Immunol. 2005;27:257–264. doi: 10.1111/j.1365-3024.2005.00781.x. [DOI] [PubMed] [Google Scholar]
- 79.Akdis CA, et al. Inhibition of T helper 2-type responses, IgE production and eosinophilia by synthetic lipopeptides. Eur J Immunol. 2003;33:2717–2726. doi: 10.1002/eji.200323329. [DOI] [PubMed] [Google Scholar]
- 80.van der Kleij D, et al. A novel host-parasite lipid cross-talk. Schistosomal lyso-phosphatidylserine activates toll-like receptor 2 and affects immune polarization. J Biol Chem. 2002;277:48122–48129. doi: 10.1074/jbc.M206941200. [DOI] [PubMed] [Google Scholar]
- 81.Phillips C, Coward WR, Pritchard DI, Hewitt CR. Basophils express a type 2 cytokine profile on exposure to proteases from helminths and house dust mites. J Leukoc Biol. 2003;73:165–171. doi: 10.1189/jlb.0702356. [DOI] [PubMed] [Google Scholar]
- 82.Reese TA, et al. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447:92–96. doi: 10.1038/nature05746. This study provides the identification of chitin as a PAMP recognized by TH2-type responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jankovic D, Kullberg MC, Caspar P, Sher A. Parasite-induced Th2 polarization is associated with down-regulated dendritic cell responsiveness to Th1 stimuli and a transient delay in T lymphocyte cycling. J Immunol. 2004;173:2419–2427. doi: 10.4049/jimmunol.173.4.2419. [DOI] [PubMed] [Google Scholar]
- 84.Cervi L, MacDonald AS, Kane C, Dzierszinski F, Pearce EJ. Cutting edge: dendritic cells copulsed with microbial and helminth antigens undergo modified maturation, segregate the antigens to distinct intracellular compartments, and concurrently induce microbe-specific Th1 and helminth-specific Th2 responses. J Immunol. 2004;172:2016–2020. doi: 10.4049/jimmunol.172.4.2016. [DOI] [PubMed] [Google Scholar]
- 85.Madden KB, et al. Enteric nematodes induce stereotypic STAT6-dependent alterations in intestinal epithelial cell function. J Immunol. 2004;172:5616–5621. doi: 10.4049/jimmunol.172.9.5616. [DOI] [PubMed] [Google Scholar]
- 86.Shea-Donohue T, et al. The role of IL-4 in Heligmosomoides polygyrus-induced alterations in murine intestinal epithelial cell function. J Immunol. 2001;167:2234–2239. doi: 10.4049/jimmunol.167.4.2234. [DOI] [PubMed] [Google Scholar]
- 87.Madden KB, et al. Role of STAT6 and mast cells in IL-4- and IL-13-induced alterations in murine intestinal epithelial cell function. J Immunol. 2002;169:4417–4422. doi: 10.4049/jimmunol.169.8.4417. [DOI] [PubMed] [Google Scholar]
- 88.Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nature Rev Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 89.Davies SJ, et al. Modulation of blood fluke development in the liver by hepatic CD4+ lymphocytes. Science. 2001;294:1358–1361. doi: 10.1126/science.1064462. [DOI] [PubMed] [Google Scholar]
- 90.Rutitzky LI, Lopes da Rosa JR, Stadecker MJ. Severe CD4 T cell-mediated immunopathology in murine schistosomiasis is dependent on IL-12p40 and correlates with high levels of IL-17. J Immunol. 2005;175:3920–3926. doi: 10.4049/jimmunol.175.6.3920. This study describes the role of TH17 cells in severe schistosomiasis. [DOI] [PubMed] [Google Scholar]
- 91.Cua DJ, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. This study identifies IL-23, instead of IL-12, as being responsible for driving harmful proinflammatory responses. [DOI] [PubMed] [Google Scholar]
- 92.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 93.Rutitzky LI, Hernandez HJ, Stadecker MJ. Th1-polarizing immunization with egg antigens correlates with severe exacerbation of immunopathology and death in schistosome infection. Proc Natl Acad Sci USA. 2001;98:13243–13248. doi: 10.1073/pnas.231258498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nurieva R, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 95.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 96.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 97.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Saunders KA, Raine T, Cooke A, Lawrence CE. Inhibition of autoimmune type 1 diabetes by gastrointestinal helminth infection. Infect Immun. 2007;75:397–407. doi: 10.1128/IAI.00664-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McInnes IB, et al. A novel therapeutic approach targeting articular inflammation using the filarial nematode-derived phosphorylcholine-containing glycoprotein ES-62. J Immunol. 2003;171:2127–2133. doi: 10.4049/jimmunol.171.4.2127. [DOI] [PubMed] [Google Scholar]
- 100.van den Biggelaar AH, et al. Decreased atopy in children infected with Schistosoma haematobium: a role for parasite-induced interleukin-10. Lancet. 2000;356:1723–1727. doi: 10.1016/S0140-6736(00)03206-2. [DOI] [PubMed] [Google Scholar]
- 101.Summers RW, et al. Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. Am J Gastroenterol. 2003;98:2034–2041. doi: 10.1111/j.1572-0241.2003.07660.x. [DOI] [PubMed] [Google Scholar]
- 102.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nature Immunol. 2005;6:353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 103.Taylor MD, et al. Removal of regulatory T cell activity reverses hyporesponsiveness and leads to filarial parasite clearance in vivo. J Immunol. 2005;174:4924–4933. doi: 10.4049/jimmunol.174.8.4924. [DOI] [PubMed] [Google Scholar]
- 104.Metwali A, et al. Induction of CD8+ regulatory T cells in the intestine by Heligmosomoides polygyrus infection. Am J Physiol Gastrointest Liver Physiol. 2006;291:G253–G259. doi: 10.1152/ajpgi.00409.2005. [DOI] [PubMed] [Google Scholar]
- 105.Hesse M, et al. The pathogenesis of schistosomiasis is controlled by cooperating IL-10-producing innate effector and regulatory T cells. J Immunol. 2004;172:3157–3166. doi: 10.4049/jimmunol.172.5.3157. [DOI] [PubMed] [Google Scholar]
- 106.Freeman CM, et al. CCR8 is expressed by antigen-elicited, IL-10-producing CD4+CD25+ T cells, which regulate Th2-mediated granuloma formation in mice. J Immunol. 2005;174:1962–1970. doi: 10.4049/jimmunol.174.4.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Baumgart M, Tompkins F, Leng J, Hesse M. Naturally occurring CD4+Foxp3+ regulatory T cells are an essential, IL-10-independent part of the immunoregulatory network in Schistosoma mansoni egg-induced inflammation. J Immunol. 2006;176:5374–5387. doi: 10.4049/jimmunol.176.9.5374. [DOI] [PubMed] [Google Scholar]
- 108.Taylor JJ, Mohrs M, Pearce EJ. Regulatory T cell responses develop in parallel to Th responses and control the magnitude and phenotype of the Th effector population. J Immunol. 2006;176:5839–5847. doi: 10.4049/jimmunol.176.10.5839. [DOI] [PubMed] [Google Scholar]
- 109.McKee AS, Pearce EJ. CD25+CD4+ cells contribute to Th2 polarization during helminth infection by suppressing Th1 response development. J Immunol. 2004;173:1224–1231. doi: 10.4049/jimmunol.173.2.1224. [DOI] [PubMed] [Google Scholar]
- 110.Boyce JA. Mast cells: beyond IgE. J Allergy Clin Immunol. 2003;111:24–32. doi: 10.1067/mai.2003.60. [DOI] [PubMed] [Google Scholar]
- 111.Dawicki W, Marshall JS. New and emerging roles for mast cells in host defence. Curr Opin Immunol. 2007;19:31–38. doi: 10.1016/j.coi.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 112.Rajan B, Ramalingam T, Rajan TV. Critical role for IgM in host protection in experimental filarial infection. J Immunol. 2005;175:1827–1833. doi: 10.4049/jimmunol.175.3.1827. [DOI] [PubMed] [Google Scholar]
- 113.Shibuya A, et al. Fcα/μ receptor mediates endocytosis of IgM-coated microbes. Nature Immunol. 2000;1:441–446. doi: 10.1038/80886. [DOI] [PubMed] [Google Scholar]
- 114.van Remoortere A, et al. Dominant antibody responses to Fucα1–3GalNAc and Fucα1-2Fucα1-3GlcNAc containing carbohydrate epitopes in Pan troglodytes vaccinated and infected with Schistosoma mansoni. Exp Parasitol. 2003;105:219–225. doi: 10.1016/j.exppara.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 115.Liu Q, et al. The role of B cells in the development of CD4 effector T cells during a polarized Th2 immune response. J Immunol. 2007;179:3821–3830. doi: 10.4049/jimmunol.179.6.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hernandez HJ, Wang Y, Stadecker MJ. In infection with Schistosoma mansoni, B cells are required for T helper type 2 cell responses but not for granuloma formation. J Immunol. 1997;158:4832–4837. [PubMed] [Google Scholar]
- 117.Jankovic D, et al. CD4+ T cell-mediated granulomatous pathology in schistosomiasis is downregulated by a B cell-dependent mechanism requiring Fc receptor signaling. J Exp Med. 1998;187:619–629. doi: 10.1084/jem.187.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hagan P, Blumenthal UJ, Dunn D, Simpson AJ, Wilkins HA. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature. 1991;349:243–245. doi: 10.1038/349243a0. [DOI] [PubMed] [Google Scholar]
- 119.Harris NL, et al. Mechanisms of neonatal mucosal antibody protection. J Immunol. 2006;177:6256–6262. doi: 10.4049/jimmunol.177.9.6256. [DOI] [PubMed] [Google Scholar]
- 120.Hotez PJ, et al. New technologies for the control of human hookworm infection. Trends Parasitol. 2006;22:327–331. doi: 10.1016/j.pt.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 121.Nair MG, et al. Chitinase and fizz family members are a generalized feature of nematode infection with selective upregulation of Ym1 and Fizz1 by antigen-presenting cells. Infect Immun. 2005;73:385–394. doi: 10.1128/IAI.73.1.385-394.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Steppan CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 123.Zhu Z, et al. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science. 2004;304:1678–1682. doi: 10.1126/science.1095336. This study describes a role for alternatively activated macrophages in TH2-type inflammation, albeit a harmful one in allergic models. [DOI] [PubMed] [Google Scholar]
- 124.Sandler NG, Mentink-Kane MM, Cheever AW, Wynn TA. Global gene expression profiles during acute pathogen-induced pulmonary inflammation reveal divergent roles for Th1 and Th2 responses in tissue repair. J Immunol. 2003;171:3655–3667. doi: 10.4049/jimmunol.171.7.3655. [DOI] [PubMed] [Google Scholar]
- 125.Loke P, et al. IL-4 dependent alternatively-activated macrophages have a distinctive in vivo gene expression phenotype. BMC Immunol. 2002;3:7. doi: 10.1186/1471-2172-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guo L, Johnson RS, Schuh JC. Biochemical characterization of endogenously formed eosinophilic crystals in the lungs of mice. J Biol Chem. 2000;275:8032–8037. doi: 10.1074/jbc.275.11.8032. [DOI] [PubMed] [Google Scholar]
- 127.Welch JS, et al. TH2 cytokines and allergic challenge induce Ym1 expression in macrophages by a STAT6-dependent mechanism. J Biol Chem. 2002;277:42821–42829. doi: 10.1074/jbc.M205873200. [DOI] [PubMed] [Google Scholar]
- 128.Artis D, et al. RELMβ/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc Natl Acad Sci USA. 2004;101:13596–13600. doi: 10.1073/pnas.0404034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hogan SP, et al. Resistin-like molecule β regulates innate colonic function: barrier integrity and inflammation susceptibility. J Allergy Clin Immunol. 2006;118:257–268. doi: 10.1016/j.jaci.2006.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schinke T, et al. Cloning and functional characterization of resistin-like molecule gamma. Biochem Biophys Res Commun. 2004;314:356–362. doi: 10.1016/j.bbrc.2003.12.100. [DOI] [PubMed] [Google Scholar]
- 131.Wang ML, et al. Regulation of RELM/FIZZ isoform expression by Cdx2 in response to innate and adaptive immune stimulation in the intestine. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1074–G1083. doi: 10.1152/ajpgi.00442.2004. [DOI] [PubMed] [Google Scholar]
- 132.Chomarat P, Banchereau J. Interleukin-4 and interleukin-13: their similarities and discrepancies. Int Rev Immunol. 1998;17:1–52. doi: 10.3109/08830189809084486. [DOI] [PubMed] [Google Scholar]
- 133.Mentink-Kane MM, et al. IL-13 receptor α2 down-modulates granulomatous inflammation and prolongs host survival in schistosomiasis. Proc Natl Acad Sci USA. 2004;101:586–590. doi: 10.1073/pnas.0305064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Morimoto M, et al. Functional importance of regional differences in localized gene expression of receptors for IL-13 in murine gut. J Immunol. 2006;176:491–495. doi: 10.4049/jimmunol.176.1.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhao A, et al. Dependence of IL-4, IL-13, and nematode-induced alterations in murine small intestinal smooth muscle contractility on Stat6 and enteric nerves. J Immunol. 2003;171:948–954. doi: 10.4049/jimmunol.171.2.948. [DOI] [PubMed] [Google Scholar]
- 136.Falcone FH, et al. A Brugia malayi homolog of macrophage migration inhibitor factor reveals an important link between macrophages and eosinophil recruitment during nematode infection. J Immunol. 2001;167:5348–5354. doi: 10.4049/jimmunol.167.9.5348. [DOI] [PubMed] [Google Scholar]
- 137.Chang NC, et al. A macrophage protein, Ym1, transiently expressed during inflammation is a novel mammalian lectin. J Biol Chem. 2001;276:17497–17506. doi: 10.1074/jbc.M010417200. [DOI] [PubMed] [Google Scholar]