Abstract
Purpose
The NeuroVision technology is a noninvasive, patient-specific, perceptual learning program based on visual stimulation and facilitation of neural connections at the cortical level, involving a computerized visual training regimen using Gabor patches, to improve contrast sensitivity and visual acuity. The efficacy of NeuroVision in enhancing uncorrected visual acuity (UCVA) and unaided contrast sensitivity function (CSF) in patients with low myopia or early presbyopia was evaluated.
Methods
Seventeen patients with low myopia (up to −1.75 D) and 21 patients with early presbyopia (up to +2.50 D add) were recruited in 2 clinical sites. Eleven myopic and 18 presbyopic patients underwent the NeuroVision program (treatment group), and 9 patients performed visual examinations only, serving as a control group.
Results
The low myopia treatment group achieved a mean improvement of 2.2 logMAR lines in unaided VA, from 0.42 to 0.20 logMAR. Unaided CSF improved at all spatial frequencies (1.5, 3, 6, 12, 18 cpd). The early presbyopia treatment group achieved a mean improvement of 2.2 logMAR lines in near UCVA, from 0.47 to 0.25 logMAR. Near unaided CSF also improved at all spatial frequencies. The control patients in both arms of the study have not shown any significant change in vision. Additionally, the mean refractive error in all groups remained unchanged after treatment.
Conclusions
Results to date suggest that the NeuroVision technology is effective in improving UCVA and unaided CSF in low myopia and early presbyopia.
INTRODUCTION
The human visual system consists of a highly sophisticated optical processing system. Optical images from the retina travel through a hierarchy of progressive levels of visual processing, from photoreceptors through several stages of spatial integration, each forming receptive fields of increasing complexity.
Contrast is one of the most important parameters triggering the neural activity in the visual cortex.1 Neural interactions determine contrast sensitivity at each spatial frequency. The combination of neural interactions at various spatial frequencies results in individual contrast sensitivity function (CSF).2
Experiments have shown that the response of individual neurons to repeated stimulus (noise) is highly variable: this high noise level imposes a fundamental limitation on the reliable detection and discrimination of visual signals by individual cortical neurons.1,3,4 The brain pools response across many neurons to average out noise activity of single cells. This creates a signal-to-noise ratio that determines detection and limits the CSF. Thus improvement of the signal-to-noise ratio leads to substantially improved visual performance.5
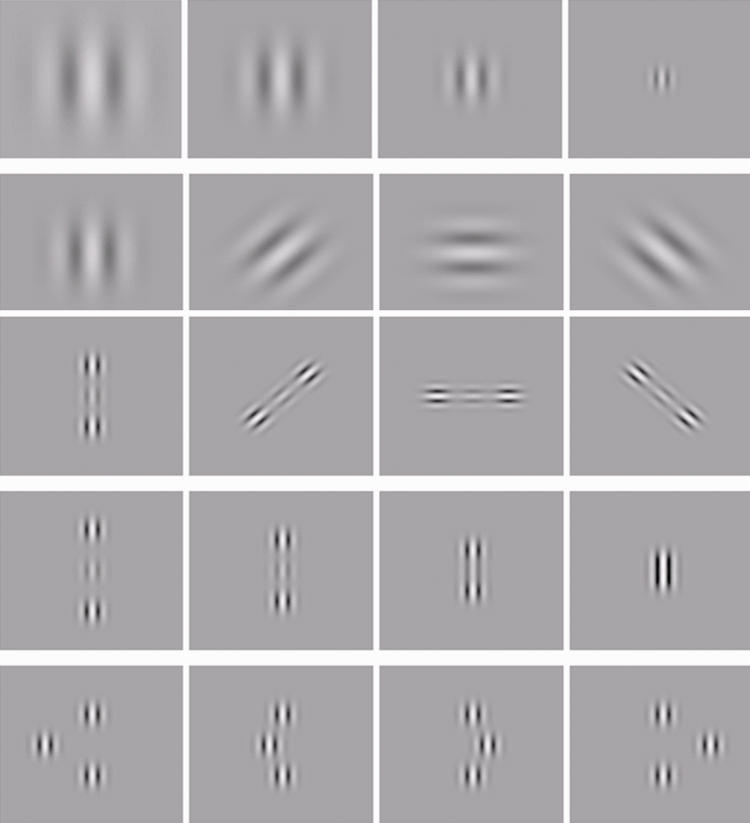
Several studies have shown that the noise of individual cortical neurons can be modulated by appropriate choice of stimulus conditions and that contrast sensitivity at low levels can be increased through control of stimulus parameters. The typical building block of the visual stimulus in the field of visual neuroscience is a Gabor patch (Figure 1), which efficiently activates and matches the shape of receptive fields in the visual cortex.6,7 Polat and colleagues8–11 demonstrated that contrast sensitivity at low levels can be increased dramatically through a “lateral masking” technique, where colinearly oriented flanking Gabors are displayed in addition to the target Gabor image (Figure 2).
FIGURE 1.
The Gabor patch.
FIGURE 2.
Lateral “masking” technique, where colinearly oriented flanking Gabors are displayed in addition to the target Gabor image.
The term perceptual learning describes a process whereby practicing certain visual talks leads to an improvement in visual performance. Brain plasticity in visual functions of adults has been shown in various studies.12–18 It was shown that visual performance improves with repetitive practice on specific controlled visual tasks. Through these precise controlled conditions, repetitive practice initiates neural modifications that lead to improvement in neuronal efficiency. These neural modifications indicate the presence of brain plasticity.
The NeuroVision technology (NeuroVision, Inc, Singapore) is a noninvasive, patient-specific, perceptual learning program based on visual stimulation. It facilitates neural connections at the cortical level through a computerized visual training regimen using Gabor patches to improve contrast sensitivity and visual acuity.
It has adopted the lateral masking technique to tailor an individualized computerized training regimen using various parameters of the stimulus (Gabors), such as spatial frequencies, spatial arrangement of the Gabor patches, contrast level, orientation (local and global), tasks order, context, and exposure duration1 (Figure 3). It improves neuronal efficiency and induces improvement of CSF by reducing the signal-to-noise ratio of neural activity in the primary visual cortex.
FIGURE 3.
Gabor patches used in different configurations, with different levels of spatial frequency, contrast, orientation, spatial location, distance, and displacement.
As visual perception depends on both the optical input received from the eye and the neural processing of that input in the visual cortex, NeuroVision technology improves quality of vision (contrast sensitivity and visual acuity) by enhancing neural processing in the primary visual cortex.
The technology has been clinically proven in the treatment of adult amblyopia,19 which until now has been considered untreatable. The technology has become available in recent years in Asia and Europe, where clinical studies showed efficacy in the treatment of amblyopia, low myopia, and early presbyopia19,20 (D. Tan and A. Fong, unpublished data, 2007; D. Tan, unpublished data, 2007).
We conducted a prospective control study to evaluate the efficacy of NeuroVision technology in improving unaided visual acuity and unaided CSF in adults with low myopia and adults with early presbyopia.
METHODS
The study was conducted in accordance with full Good Clinical Practice guidelines. It was approved by the RCRC institutional review board. Informed consent was obtained from all study subjects.
SUBJECTS
Seventeen patients with low myopia and 21 patients with early presbyopia were recruited in 2 clinical sites. Eleven myopic and 18 presbyopic patients underwent the NeuroVision program (treatment group), and 6 myopic and 3 presbyopic patients performed visual examinations only, serving as a control group. Half of the patients performed their training sessions in a clinic setting, and the other half performed their training sessions at home.
For the low myopia group, inclusion criteria were as follows: refractive error up to −1.75 D of spherical correction and up to −0.75 D of cylindrical correction in both eyes; a stable refractive state with no increase beyond ±0.5 D over the previous 6 months; distance uncorrected visual acuity (UCVA) between 0.20 and 0.60 logMAR with no more than 0.3 logMar difference between the eyes; and best spectacle-corrected visual acuity ≤0.05 logMAR (ETDRS logMAR charts).
For the early presbyopia group, inclusion criteria were age between 40 and 50 years and near UCVA between 0.20 and 0.60 logMAR with no more than 0.3 logMAR difference between the eyes. Additional criteria included a subjective report of difficulty with near vision during the preceding year.
Exclusion criteria for both groups consisted of any other ocular condition or cause for reduced visual acuity other than simple myopia, presbyopia, hyperopia, and/or astigmatism. These criteria included previous ocular surgery, pregnancy, diabetes mellitus, presence of any myopia-related ocular complications, and an altered cognitive or emotional state that might potentially impair the subject’s ability to perform treatment.
STUDY OUTLINE
Study phases included a baseline screening and enrollment visit, a series of 30 training sessions with the NeuroVision technology, and an end-of-treatment examination.
Baseline Screening and Enrollment Visit
At the time of enrollment, all patients underwent comprehensive ocular examination. For the low myopia group, manifest refraction was determined and uncorrected and best-corrected distance visual acuities were tested using an EDTRS logMAR visual acuity chart at a distance of 10 ft. Unaided and aided CSFs for distance were tested using a wall-mounted distance Functional Acuity Contrast Test (FACT) chart at a testing distance of 10 ft. For the early presbyopia group, examinations included manifest refraction for distance and near, uncorrected and corrected near visual acuity using the near EDTRS logMAR chart, and unaided and aided CSFs for near using the near FACT chart at a testing distance of 16 inches. Eligible patients were enrolled and underwent an orientation guided by the trainer.
NeuroVision Training
The NeuroVision training system is an interactive computer software that provides a series of individualized visual stimuli that are designed to enhance the neural interactions in the visual cortex. Each training session lasts for approximately 30 minutes, during which the patient needs to respond to visual perception tasks (VPTs) displayed on the computer screen. During the session the patient sits 5 ft away from a monitor in a darkened room, and a mouse is used to respond to the tasks. The training may be performed in either a clinic or a home environment.
The VPTs are patient-specific stimuli using Gabor patches displayed in lateral masking techniques directed to enhance specific neuronal inefficiencies in the visual cortex. In a typical task, the patient is exposed to 2 consecutive displays in random order. Each display has some arrangement of Gabor patches with subtle differences between the 2 displays. The patient is required to identify the correct display as determined by the instructions for the specific task. If the patient answers correctly, the target contrast will be reduced and the task will become more difficult. Incorrect answers will trigger the program to increase the contrast and the task becomes easier.
After each training session, the patient performance in each of the VPTs is recorded and sent via the Internet to the NeuroVision servers in Singapore. Special algorithms then analyze the patient performance and accordingly create the VPT parameters for the next training session. In this manner patients receive training sessions that are individually tailored to their performance and neuronal inefficiencies.
In this study each patient performed 30 training sessions. These were conducted at a pace of 2 or 3 training sessions a week over a course of 2 to 3 months. Before starting the 30 training sessions, each patient underwent 2 computerized evaluation sessions with the system to set up the baseline of individualized neural inefficiencies for the training program. This baseline information is used by the NeuroVision algorithms, together with the baseline examination results, to set the starting point for the training sessions.
Some of the patients were required by the software algorithms to use training glasses during the sessions. These were −0.5 D or −1.0 D in power and in the form of eyeglasses for patients with low myopia or emmetropic presbyopia and clip-on glasses for those with ammetropic presbyopia.
After every 5 sessions the patients underwent a periodic visual examination to monitor their progress. Distance UCVA and unaided CSF for the low myopia group and near UCVA and unaided CSF for the early presbyopia group were tested.
End-of-Treatment Examination
At the end of 30 training sessions, patients were scheduled for the end-of-treatment examination, which is a repetition of the baseline examination. Patients were requested to complete a quality of vision questionnaire upon termination of training.
Treatment Group
Patients in the treatment group completed personalized treatment sessions with the NeuroVision computerized system. These treatments were designed per NeuroVision algorithm to address the individual’s neuronal inefficiencies.
Control Group
Patients in the control group underwent the same vision testing as the treatment group with the exception that they did not perform the NeuroVision training.
RESULTS
These results are interim data from this ongoing study.
DEMOGRAPHICS
Eleven patients with low myopia, aged 19 to 39 years (mean, 31.4 ± 1.52 years), with mean refractive spherical equivalent (SE) of −0.50 D to −1.75 D (mean, −1.26 D ± 0.09 D ) completed the NeuroVision low myopia training. Eighteen presbyopic patients, aged 41 to 55 years (mean, 46.9 ± 0.56 years), with near addition range of +0.75 D to +2.50 D (mean, +1.32 D ± 0.08 D) completed the NeuroVision early presbyopia training. Nine patients (6 with myopia and 3 with presbyopia) performed visual examinations only, serving as a control group. All patients in the treatment group completed 30 sessions of training.
LOW MYOPIA GROUP
Improvement in Distance UCVA
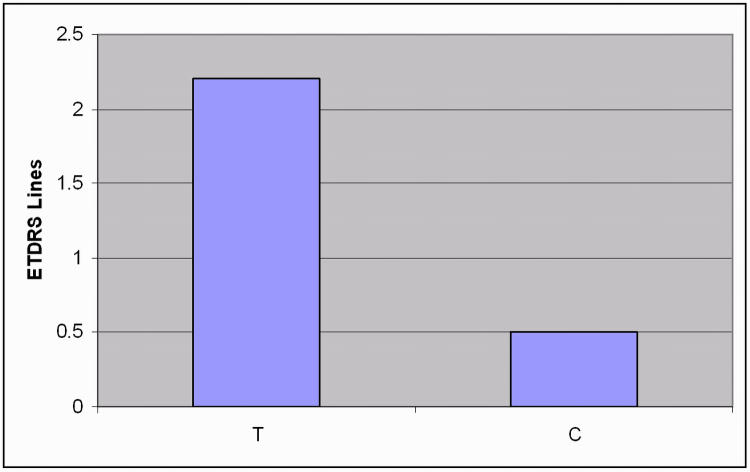
At the baseline examination, mean distance UCVA in case patients was 0.42 logMAR (95% confidence interval [CI], 0.26–0.62). At the end of treatment examination, mean distance UCVA had improved to 0.20 logMAR (95% CI, −0.06 to 0.50), demonstrating a mean improvement of 2.2 logMAR lines. No significant improvement in distance UCVA was seen in control patients. (Figure 4).
FIGURE 4.
Improvement in distance uncorrected visual acuity in patients with low myopia. T, treatment group; C, control group.
Improvement in Distance CSF
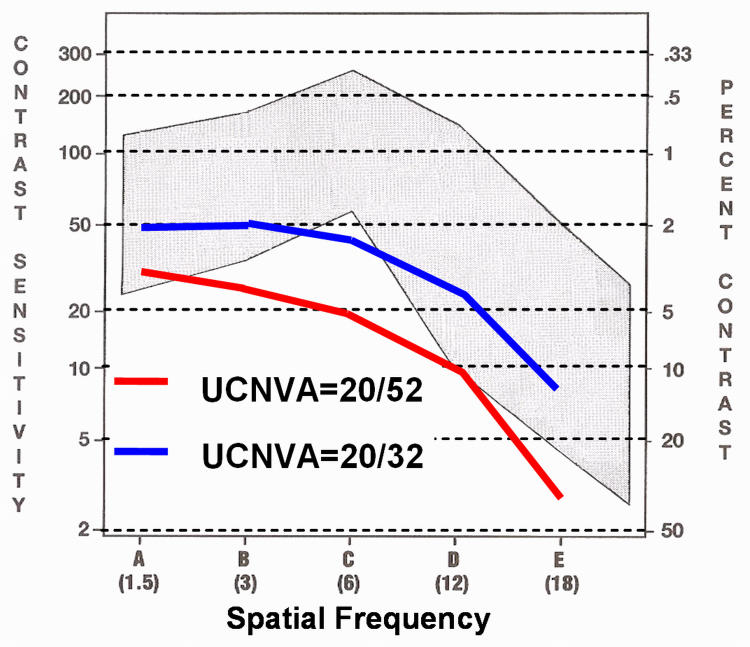
Mean distance unaided CSF improved at all spatial frequencies (1.5, 3, 6, 12, 18 cpd) to within the normal range, and sensitivity improved to a mean of 86.6% (Figure 5).
FIGURE 5.
Improvement in unaided distance contrast sensitivity function in patients with low myopia. UCNVA, uncorrected near visual acuity.
Effect on Refraction Error
Mean SE was not significantly altered after treatment. Mean baseline manifest SE and end-of-treatment mean manifest SE were −1.26 D and −1.22 D, respectively.
EARLY PRESBYOPIA GROUP
Improvement in Near UCVA
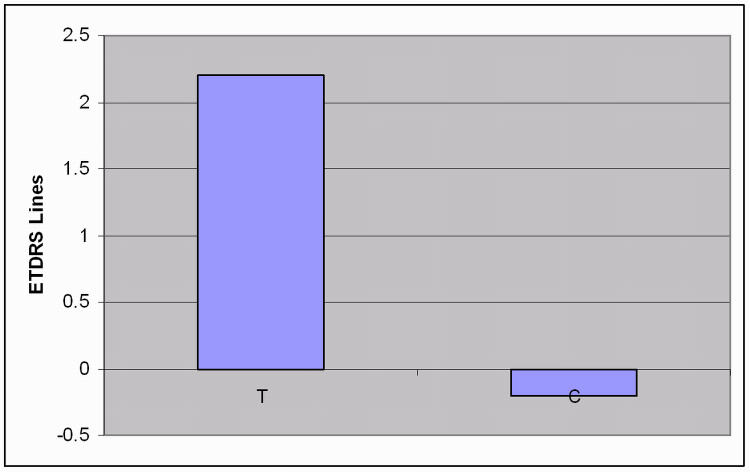
At the baseline examination, mean near UCVA (defined as near visual acuity using the best-corrected distance prescription without any addition for near) in case patients was 0.47 logMAR (95% CI, 0.22–0.62). At the end of treatment examination, mean near UCVA had improved to 0.25 logMAR (95% CI, −0.08 to 0.54), demonstrating a mean improvement of 2.2 logMAR lines. No significant improvement in near UCVA was seen in control patients (Figure 6).
FIGURE 6.
Improvement in near uncorrected visual acuity in patients with early presbyopia. T, treatment group; C, control group.
Improvement in Near CSF
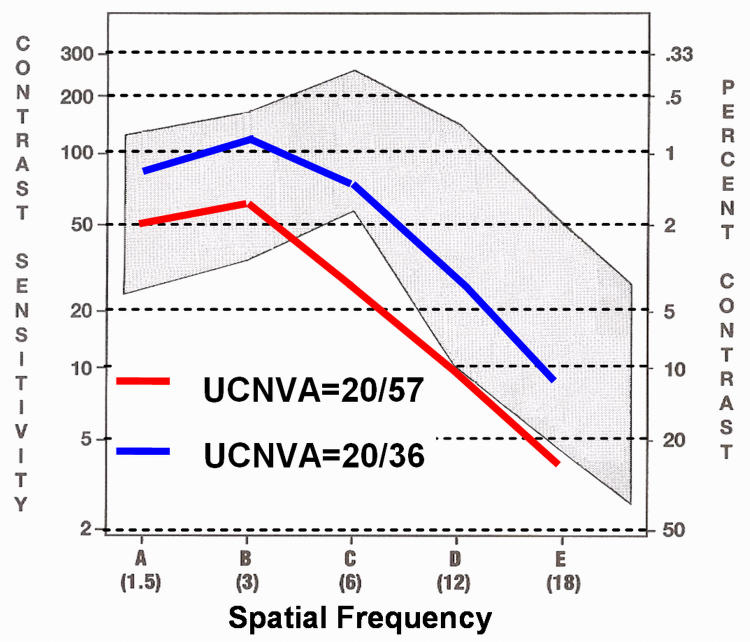
Mean unaided CSF improved at all spatial frequencies (1.5, 3, 6, 12, 18 cpd) to within the normal range, and sensitivity improved to a mean of 92.0% (Figure 7).
FIGURE 7.
Improvement in unaided near contrast sensitivity function in patients with early presbyopia. UCNVA, uncorrected near visual acuity.
Effect on Refraction Error
Mean SE was not significantly altered after treatment. Mean baseline manifest SE and end-of-treatment mean manifest SE were −0.014 D and −0.115 D, respectively. Near addition need did not change significantly posttreatment, from a mean baseline addition of +1.32 D to an end-of-treatment mean addition of +1.33 D.
COMPLICATIONS
No complications were encountered during the duration of the study. None of the eyes treated showed a drop in uncorrected or corrected VA, and no adverse effects were reported by study participants. Manifest refraction remained unchanged for the duration of the study.
DISCUSSION
The interim results of this study show preliminary evidence in the efficacy of NeuroVision Technology in improving visual acuity and CSF in adults with low myopia and early presbyopia. The improvement in UCVA and unaided CSF among the low myopic and early presbyopic groups is clinically significant. The control patients did not show any significant changes in vision, which implies that the improvement in the treatment group was not a result of memorization of the vision charts used.
The study results support as evidence that neural plasticity is retained in the adult brain. This limited plasticity in the primary visual cortex provides us room for improving our visual processing. Being more efficient and effective, the neural processing in the brain can enhance the image quality by compensating for blurred retinal images due to optical defocus of a low degree of myopia and presbyopia.
The mean improvements of 2.2 logMar lines in distance UCVA for patients with low myopia and 2.2 logMar lines in unaided near visual acuity for those with early presbyopia are clinically significant and are encouraging. The increased CSF further ratifies the acuity improvement and supports the original hypothesis of how the treatment works. With the limitation of a relatively small sample size and with the absence of a randomized double-masked control group in this study, it appears that the results of this study are similar to results reported among patients in Asia (D. Tan and A. Fong, unpublished data, 2007), which noted mean improvement of 2.8 logMar lines in distance UCVA for 55 patients with low myopia and mean improvement of 1.6 logMar lines in near UCVA for the 41 presbyopic patients (aged 41 to 55 years) after completion of the NeuroVision training. The improvements were shown to be retained for at least 12 months.
Based on these positive results, a randomized controlled trial evaluating the efficacy of NeuroVision technology in low myopia was conducted. The interim results presented by D. Tan at AsiaARVO 2007 (unpublished data, 2007) confirmed the statistically significant difference in UCVA between the masked treatment groups, and the investigators suggested that at the trial’s completion, definitive evidence would show the efficacy and safety of NeuroVision treatment in improving visual acuity and CSF in adults with low myopia.
The results of this study correlate well with reported commercial results with NeuroVision: mean improvement of 2.6 logMar lines of distance UCVA for patients with low myopia and 2.0 logMar lines near UCVA for those with early presbyopia. The commercial results show a slightly better improvement compared with the clinical trial results. NeuroVision claims that compliance and motivation play a significant role in treatment outcome. It is possible that commercial patients are more compliant with the treatment schedule, as they expect an outcome for the amount invested, whereas study patients who receive the treatment free are less motivated to comply with the treatment schedule.
In addition, variability in efficacy may be a reflection of different individuals’ “final cortical potential” that could be achieved through the perceptual learning process, in turn dependent on the state of inherent neural plasticity. Follow-up studies may be designed to address these issues and find the optimal “exposure dosing.” However, individual effort and motivation can be expected to vary, accounting for variability in the final result.
Contrast sensitivity function is an excellent representation of an individual’s spatial vision. Several studies have shown that CSF significantly correlates with some abilities associated with quality of life, such as reading speed,21,22 walking speed, mobility,23 driving performance,24 and computer task accuracy.25 In addition, contrast sensitivity declines in eyes with various ocular abnormalies26–28 and after refractive surgery.29–32
Further studies may be designed to explore the role of this novel computerized visual cortex training program in the area of rehabilitation for low vision with various ocular conditions, as well as its prospective role in the enhancement of visual potential for better quality of vision.
In conclusion, this pilot study of NeuroVision treatment in patients with low myopia and early presbyopia appears to support the hypothesis that a perceptual learning program based on visual stimulation and facilitation of neural connections at the cortical level has the ability to improve UCVA and CSF.
PEER DISCUSSION
DR STEPHEN D. McLEOD
Drs Durrie and McMinn present the results of a clinical trial that employs computer-based visual training to improve uncorrected distance and near visual acuity in patients with low myopia and early presbyopia. Thirty-eight patients underwent 30 training sessions of 30 minutes each over a 2- to 3-month period. Visual examination was performed every 5 sessions followed by a final end-of-training examination. Test results in this group were compared to 9 control subjects, who were simply examined repeatedly, presumably on a similar schedule. The schedule was consistent with the pediatric PowerVision training program offered by NeuroVision, Inc (Delaware, Rhode Island) that promotes computer-based training to address child myopia in 8 Singapore-based clinical centers, as well as a number of other sites in Asia and the United Kingdom.
The fundamental premise of the technique employed by these investigators is that visual training using Gabor images can result in improved visual performance through cortical neuronal “rewiring” that boosts the signal-to-noise ratio upon which discrimination is based.
While a number of studies have indicated that performance on specific visual tests, such as familiar object identification, spatial frequency discrimination, and Vernier offset discrimination, might show some improvement with training1, support for the idea that novel training protocols can improve performance on tests of visual acuity emanates mainly from observations of improved acuity after psychophysical training in adult amblyopic eyes.2–3
The study reported today proposes that unlike the amblyopic case, where training is presumed to improve suboptimal cortical processing of a focused retinal image, NeuroVision training, tailored to individual “neuronal inefficiencies,” is presumed to improve normal cortical processing of a defocused retinal image.
Since subject-observer interaction, effort, and coaching can have a profound influence on the outcome of visual psychophysical testing, fastidious study design is imperative. Perhaps the 2 most critical elements would include a convincing sham training exercise for control subjects, coupled with both subjects and observers masked with regards to test group assignment. Unfortunately, the current study failed to incorporate those elements and is thus inadequately controlled.
As described by NeuroVision, the neurophysiologic premise upon which the technique is based invites debate among experts; while intriguing, the current study does not lend the support of incontrovertible evidence of efficacy, and the authors are encouraged to revisit the question
ACKNOWLEDGMENTS
Funding/Support: None.
Financial Disclosures: None.
REFERENCES
- 1.Jacobs RA. Comparing perceptual learning across tasks: a review. J Vision. 2002;2:190–203. doi: 10.1167/2.2.5. [DOI] [PubMed] [Google Scholar]
- 2.Levi DM, Polat U. Neural plasticity in adults with amblyopia. Proc Natl Acad Sci U S A. 1996;93:6830–6834. doi: 10.1073/pnas.93.13.6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levi DM, Polat U, Hu YS. Visual improvement in adults with amblyopia. Invest Ophthalmol Vis Sci. 1997;38:1493–1510. [PubMed] [Google Scholar]
DR. DAVID L. GUYTON
No conflicts. Dr. Durrie, I am fascinated by these results, but I want to caution you to not readily attribute these changes to cortical changes until you certainly rule out any optical changes that might be occurring. For example, I had a Naval Academy cadet as a patient who wanted to pass the vision portion of his flight training physical. Although he was a −1.50 diopter myope by repeated manifest refractions, somehow he learned to improve his vision for brief instances from 20/40 to 20/15. By dynamic retinoscopy I could tell that he was negatively accommodating during those times. His refraction changed about 0.75 diopter in the less minus direction, he became slightly exophoric, and curiously his pupil constricted slightly. Dynamic retinoscopy or wavefront analysis before and after cycloplegic drops or alpha blockers, such as thymoxamine, or both could probably rule out optical changes as a cause before attributing the visual improvement that you have seen to changes in the cortex.
DR. ROBERT C. SERGOTT
No conflicts. This whole issue has also surfaced in neuro-ophthalmology with another company claiming improvement in visual fields. That company will not submit to unbiased methods of determining fixation or other issues. This is a real issue, and I will refer to an editorial by Jonathan Horton in the British Journal of Ophthalmology, is whether a sensory portion of the nervous system can adapt in a manner similar to a motor part of the central nervous system. To prove this is change is cortical in nature; a nontraditional magnetic resonance imaging (MRI) method or functional MRI would be very helpful.
DR. MICHAEL H. GOLDBAUM
No conflicts. The tests that you used to determine the effect of the training in these people were visual acuity, contrast sensitivity, and other such tests. These are really surrogates for performance in the real world. Does this type of training actually produce any real world improvement?
DR. RICHARD P. MILLS
No conflicts. Dr. Durrie, my very brief question is not meant to be a provocative comment. I really would like to know how this contrasts with the time honored Bates Method of improving vision.
DR. GERHARD W. CIBIS
Your presentation reminds me of the great enthusiasm 30 years ago for something called the Campbell’s striped rotating therapy for amblyopia. Dozens of papers were published about how this technique improved visual acuity by an average of two lines in children with amblyopia after six half hour sessions. I bought the machine and performed a controlled study to test the effectiveness of the treatment versus a sham treatment. I used a blank disk instead of one with stripes and determined a two line improvement in all patients who received therapy either with the striped disc or with the sham disc.
DR. GEORGE R. BEAUCHAMP
I confess to having multiple conflicts, but all derive from interests that are not financial. Dr. Durrie, I am particularly interested in the subject matter for a number of reasons. I have two questions for you. Number one, do you believe that this technology will likely be offered to children who have learning disabilities? Secondly, you specified the fundamental bases for the intervention are certain “neural inefficiencies”. This is strikingly similar to some rather general comments that are made with regard to other interventions used for learning disabilities and others. Can you specify what the “neural inefficiencies” are other than the obvious visual acuity deficits?
DR. ALLAN J. FLACH
No conflict is financial. Seldom has a presentation simultaneously stimulated so much anxiety and guilt in my opinion. You introduced your topic by stating that playing video games improves vision. I am in the process of trying to discourage my grandchildren from playing video games excessively, so can you offer us some time limits? Secondly, I was also curious about the Bates method and how it compares to what you are doing. I would like to know if I am harming my patients when they tell me that they are using the Bates method and I suggest they throw it away and do more reading instead since that will do the same for your vision. Am I lying to them? Finally, I also was concerned, like Dr. McLeod, about the lack of a control group. I agree with everything Dr. McCloud said, but you might consider reading of the classics both in and out of print as part of your control group therapy. It would be wonderful if that proved to be as effective as the therapy in improving vision.
DR. GARY C. BROWN
No conflicts. I enjoyed the presentation very much. Did you evaluate the quality of life before and after treatment in the study? I ask that is because we have studied patients with low degrees of presbyopia, up to +1.75 diopter, and we have determined that their utility with glasses is .997. This means that the average person with low degrees of presbyopia has a pretty darn good quality of life, as is.
DR. GEORGE O. WARING, III
I have no conflict of interest because of my own learning problems. I have a comment and question. Many of us do not appreciate the real contributions of Dr. Bates. He is now famous for these cockamamie schemes that are still sold on the internet after 100 years, but he was also the first person who performed refractive surgery. He observed that if you make a transverse cut in the cornea across the steep meridian you can flatten that meridian and published this finding in the Archives of Ophthalmology. He reported this finding in six cases and then disappeared from sight for ten to twelve years before returning with the Bates Method of myopia and vision improvement. My question: how would you relate your findings to the optometric world of visual training? Many patients, including a lot of high class and highly paid athletes undergo visual training. How do your findings fit in?
DR. JOHN D. BULLOCK
No conflict. I have observed several patients who had a mature cataract in one eye, normal vision in the other eye, and an afferent pupillary defect in the normal eye. I hypothesized that as the cataract was developing in the one eye, the optic nerve in that eye somehow enhanced its function to compensate. I just wondered what your comments might be.
DR. DANIEL S. DURRIE
Thank you all for your thoughtful and well received comments. I too have been extremely skeptical of this intervention. As such, I conducted my own clinical trial to prove that visual cortex training does not work. I was quite surprised by the results. As Dr. McLeod mentioned, this study certainly needs to be repeated in a large randomized clinical trial. A weakness of the current study is the lack of a subjective questionnaire which precludes determination of patient benefit. Skepticism resulting from the history of “visual training” (i.e., Bates method) is reasonable. However, this technology is quite different in that it is based on the neuroscientific theory of Gabor patches representing the receptive fields of the visual cortex. We also feel that until this neuroscientific theory is substantiated with functional imaging studies, it will continue to be met with skepticism by the ophthalmic community. The study was prospectively conducted in our research center. However, Dr. Cibis raises an excellent point; our controls in the present study were matched with visual acuity testing at the same levels, but did not undergo sham treatment. We are currently conducting a study with a sham treatment group playing a video game. The questions regarding amblyopia and visual deprivation from a dense cataract are quite interesting. The company has developed an amblyopic treatment program, and we are currently enrolling amblyopic patients in our study. In conclusion, I appreciate your comments and share your concern. I do feel this is worth scientific observation and strongly encourage further critical discussion.
ACKNOWLEDGMENTS
Funding/Support: None.
Financial Disclosure: Dr Durrie is a paid researcher for NeuroVision.
Author Contributions: Design and conduct of the study (D.D., P.S.M.); Collection, analysis, and interpretation of the data (D.D.); Preparation, review, and approval of the manuscript (D.D.).
REFERENCES
- 1.Albrecht DG, Hamilton DB. Striate cortex of monkey and cat: contrast response function. J Neurophysiol. 1982;48:217–237. doi: 10.1152/jn.1982.48.1.217. [DOI] [PubMed] [Google Scholar]
- 2.Polat U. Functional architecture of long-range perceptual interactions. Spat Vis. 1999;12:143–162. doi: 10.1163/156856899x00094. [DOI] [PubMed] [Google Scholar]
- 3.Tolhurst DJ, Movshon JA, Dean AF. The statistical reliability of signals in single neurons in cat and monkey visual cortex. Vision Res. 1983;23:775–785. doi: 10.1016/0042-6989(83)90200-6. [DOI] [PubMed] [Google Scholar]
- 4.Geisler WS, Albrecht DG. Bayesian analysis of identification performance in monkey visual cortex: nonlinear mechanisms and stimulus certainty. Vision Res. 1995;35:2723–2730. doi: 10.1016/0042-6989(95)00029-y. [DOI] [PubMed] [Google Scholar]
- 5.Geisler WS, Albrecht DG. Visual cortex neurons in monkeys and cats: detection, discrimination, and identification. Vis Neurosci. 1997;14:897–919. doi: 10.1017/s0952523800011627. [DOI] [PubMed] [Google Scholar]
- 6.Gabor D. Theory of communication. J Inst Electrical Eng. 1946;93:429–452. [Google Scholar]
- 7.Daugman JG. Two-dimensional spectral analysis of critical receptive field profiles. Vision Res. 1980;20:847–856. doi: 10.1016/0042-6989(80)90065-6. [DOI] [PubMed] [Google Scholar]
- 8.Polat U. Functional architecture of long-range perceptual interactions. Spat Vis. 1999;12:143–162. doi: 10.1163/156856899x00094. [DOI] [PubMed] [Google Scholar]
- 9.Kasamatsu T, Polat U, Pettet MW, Norcia AM. Colinear facilitation promotes reliability of single-cell responses in cat striate cortex. Exp Brain Res. 2001;138:163–172. doi: 10.1007/s002210100675. [DOI] [PubMed] [Google Scholar]
- 10.Polat U, Mizobe K, Pettet MW, Kasamatsu T, Norcia AM. Collinear stimuli regulate visual responses depending on cell’s contrast threshold. Nature. 1998;391:580–584. doi: 10.1038/35372. [DOI] [PubMed] [Google Scholar]
- 11.Polat U, Sagi D. Lateral interactions between spatial channels: suppression and facilitation revealed by lateral masking experiments. Vision Res. 1993;33:993–999. doi: 10.1016/0042-6989(93)90081-7. [DOI] [PubMed] [Google Scholar]
- 12.Polat U, Sagi D. The architecture of perceptual spatial interactions. Vision Res. 1994;34:73–78. doi: 10.1016/0042-6989(94)90258-5. [DOI] [PubMed] [Google Scholar]
- 13.Levi DM, Polat U. Neural plasticity in adults with amblyopia. Proc Natl Acad Sci U S A. 1996;93:6830–6834. doi: 10.1073/pnas.93.13.6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levi DM, Polat U, Hu YS. Visual improvement in adults with amblyopia. Invest Ophthalmol Vis Sci. 1997;38:1493–1510. [PubMed] [Google Scholar]
- 15.Kaarniranta K, Kontkanen M. Visual recovery of the amblyopic eye in an adult patient after loss of the dominant eye. Acta Ophthalmol Scand. 2003;81:539. doi: 10.1034/j.1600-0420.2003.00127.x. [DOI] [PubMed] [Google Scholar]
- 16.Simmers AJ, Gray LS. Improvement of visual function in an adult amblyope. Optom Vis Sci. 1999;76:82–87. doi: 10.1097/00006324-199902000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Fronius M, Cirina L, Cordey A, Ohrloff C. Visual improvement during psychophysical training in an adult amblyopic eye following visual loss in the contralateral eye. Graefes Arch Clin Exp Ophthalmol. 2005;243:278–280. doi: 10.1007/s00417-004-1014-8. [DOI] [PubMed] [Google Scholar]
- 18.Rahi JS, Logan S, Borja MC, Timms C, Russell-Eggitt I, Taylor D. Prediction of improved vision in the amblyopic eye after visual loss in the non-amblyopic eye. Lancet. 2002;36:621–622. doi: 10.1016/S0140-6736(02)09775-1. [DOI] [PubMed] [Google Scholar]
- 19.Polat U, Ma-Naim T, Belkin M, Sagi D. Improving vision in adult amblyopia by perceptual learning. Proc Natl Acad Sci U S A. 2004;101:6692–6697. doi: 10.1073/pnas.0401200101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim KL, Fan HB. NeuroVision treatment for low myopia following LASIK regression. J Refract Surg. 2006;22:104–408. doi: 10.3928/1081-597X-20060401-20. [DOI] [PubMed] [Google Scholar]
- 21.Whittaker SG, Loview-Kitchin J. Visual requirements for reading. Optom Vis Sci. 1993;70:54–65. doi: 10.1097/00006324-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Crossland MD, Culham LE, Rubin GS. Predicting reading fluency in patient with macular disease. Optom Vis Sci. 2005;82:11–17. [PubMed] [Google Scholar]
- 23.Marron JA, Bailey IL. Visual factors and orientation-mobility performance. Am J Optom Physiol Opt. 1982;59:413–426. doi: 10.1097/00006324-198205000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Owsley C, Ball K, McGwin G, et al. Visual processing impairment and risk of motor vehicle crash among older adults. JAMA. 1998;279:1083–1088. doi: 10.1001/jama.279.14.1083. [DOI] [PubMed] [Google Scholar]
- 25.Scott IU, Feuer WJ, Jacko JA. Impact of visual function on computer task accuracy and reaction time in a cohort of patients with age-related macular degeneration. Am J Ophthalmol. 2002;133:350–357. doi: 10.1016/s0002-9394(01)01406-4. [DOI] [PubMed] [Google Scholar]
- 26.Ansari EA, Morgan JE, Snowden RJ. Psychophysical characterization of early functional loss in glaucoma and ocular hypertension. Br J Ophthalmol. 2002;86:1131–1135. doi: 10.1136/bjo.86.10.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hawkins AS, Szlyk JP, Ardickas Z, Alexander KR, Wilensky JT. Comparison of contrast sensitivity, visual acuity, and Humphrey visual field testing in patients with glaucoma. J Glaucoma. 2003;12:134–138. [PubMed] [Google Scholar]
- 28.Bellmann C, Unnebrink K, Ruibin GS, Miller D, Holz FG. Visual acuity and contrast sensitivity in patients with neovascular age-related macular degeneration: results from the Radiation Therapy for Age-Related Macular Degeneration (ARD) Study. Graefes Arch Clin Exp Ophthalmol. 2003;241:968–974. doi: 10.1007/s00417-003-0689-6. [DOI] [PubMed] [Google Scholar]
- 29.Mutyala S, McDonald MB, Scheinblum KA, Ostrick MD, Brint SF, Thompson H. Contrast sensitivity evaluation after laser in situ keratomileusis. Ophthalmology. 2000;107:1864–1867. doi: 10.1016/s0161-6420(00)00355-9. [DOI] [PubMed] [Google Scholar]
- 30.Chan JW, Edwards MH, Woo GC, Woo VC. Contrast sensitivity after laser in situ keratomileusis: one-year follow-up. J Cataract Refract Surg. 2002;28:1774–1779. doi: 10.1016/s0886-3350(02)01499-2. [DOI] [PubMed] [Google Scholar]
- 31.Holliday JT, Dudeja DR, Chang J. Functional vision and corneal changes after laser in situ keratomileusis determined by contrast sensitivity, glare testing, and corneal topography. J Cataract Refract Surg. 1999;25:663–669. doi: 10.1016/s0886-3350(99)00011-5. [DOI] [PubMed] [Google Scholar]
- 32.Yamane N, Miyata K, Samejima T, et al. Ocular higher-order aberrations and contrast sensitivity after conventional laser in situ keratomileusis. Invest Ophthalmol Vis Sci. 2004;45:3986–3990. doi: 10.1167/iovs.04-0629. [DOI] [PubMed] [Google Scholar]