Abstract
Purpose
To determine central keratocyte and subbasal nerve densities after penetrating keratoplasty (PK) in clear grafts and in grafts with late endothelial failure (LEF).
Methods
Ninety-nine clear grafts of 74 patients and 21 grafts with LEF in 19 patients were examined by confocal microscopy at 1 to 31 years after PK. Keratocyte density and number of keratocytes in a full-thickness column of stroma with frontal area of 1 mm2 were determined from images. Subbasal nerve fiber bundle density was measured in the central cornea as the visible length of nerve per frame area. Comparisons were made to normal corneas.
Results
Full-thickness keratocyte density in clear grafts (22,101 ± 3,799 cells/mm3, mean ± SD, n = 99) was lower than in normal corneas (26,610 ± 3,683 cells/mm3, n = 63, P < .001) but did not differ from grafts with LEF (21,268 ± 3,298 cells/mm3, n = 12, P = .47). The number of keratocytes in clear grafts (10,325 ± 1,708 cells, n = 99) was lower than in normal corneas (11,466 ± 1,503 cells, n = 63, P < .001) but did not differ from grafts with LEF (10,778 ± 1,760 cells, n=12, P = .39). Median subbasal nerve density in clear grafts (150 μm/mm2, n = 94) was lower than in normal corneas (7,025 μm/mm2, n = 76, P < .001), and nerve recovery correlated with time after PK (r = 0.36, P < .001, n = 94).
Conclusions
Keratocyte density and number of keratocytes are decreased in penetrating grafts compared to normal corneas, but not compared to grafts with LEF. Subbasal nerve density does not recover to normal through 30 years after PK.
INTRODUCTION
Penetrating keratoplasty (PK) involves replacement of host stroma and endothelium by donor tissue and requires transection of all host corneal nerves. Changes in the endothelium after PK are well documented and include a faster-than-normal rate of cell loss1,2 and decreased permeability.3 Chronic endothelial dysfunction after PK has been associated with stromal haze, and, collectively, these findings constitute late endothelial failure (LEF),4,5 which is the leading cause of graft failure.1,2
The etiology of stromal haze in LEF is not known, but changes in keratocyte density and function, or in the extracellular matrix, might increase corneal backscatter. Little is known about the role of keratocytes and corneal nerves after PK and whether changes in these cell populations affect graft clarity. Confocal microscopy enables visualization of stromal keratocytes and corneal nerve fiber bundles in vivo, and we have devised methods for quantifying keratocyte density and subbasal nerve fiber density from confocal images of corneas.6–8 In a preliminary study that used this method, we found that keratocyte density was decreased in clear penetrating grafts compared to normal corneas.9 In the present study, we expanded our series of clear penetrating grafts and also report keratocyte and subbasal nerve densities in grafts with LEF.
METHODS
SUBJECTS
Patients who had received a PK were recruited from the cornea service at Mayo Clinic between May 2000 and January 2007. Subjects were examined by slit-lamp biomicroscopy to determine whether the graft was clear or hazy from LEF.4 The study was approved by our institutional review board and adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from all participants after a detailed explanation of the nature and possible consequences of the study.
Ninety-nine clear grafts in 74 patients and 21 grafts with LEF in 19 patients were enrolled. Sixty-three normal (unoperated) corneas of 63 subjects who were enrolled in 3 other studies conducted between 2000 and 2006 were used as concurrent controls for keratocyte density,10–12 and 76 normal corneas of 76 different subjects enrolled in 2 studies conducted in 1998 were used as controls for subbasal nerve density.8,13 The annual rate of change of keratocytes was estimated from clear grafts examined at least 5 years after PK on 2 occasions separated by 4 or more years.
CONFOCAL MICROSCOPY
A Tandem Scanning confocal microscope (Tandem Scanning, Reston, Virginia) was used to examine corneas in vivo as described previously.6,14 Briefly, proparacaine hydrochloride 0.5% (Bausch & Lomb Pharmaceuticals, Inc, Tampa, Florida) was instilled into the eye to be examined. A drop of an optical coupling medium (Goniosol, CIBA Vision Ophthalmics, Atlanta, Georgia, for examinations before 2001, or GenTeal Gel, Novartis Pharmaceuticals Corp, East Hanover, New Jersey, for examinations after 2001) was placed on the tip of the objective. The position of the objective was adjusted to provide an en face view of the central cornea. The patient fixated on a target with the contralateral eye to minimize eye movements. Digital images of the central cornea were recorded with the optical section advancing through the full-thickness cornea from anterior to the epithelium to posterior to the endothelium. Each image represented a coronal section of cornea that was approximately 475 μm × 350 μm (horizontal × vertical), and the average z-depth separation between adjacent images was 2.4 μm.6,11 A “through-focus” series of images of 1 cornea constituted 1 “scan” and typically consisted of less than 300 video frames, depending on the thickness of the cornea. Two to 4 scans were acquired per eye.
KERATOCYTE DENSITY
The best-quality scan without axial movement of the cornea relative to the objective was selected for each cornea. For density measurement, the corneal stroma was divided into 5 layers6: the anterior, middle, and posterior thirds of the stroma, and the anterior and posterior thirds were further subdivided into 2 unequal layers so that the anterior 10% and posterior 10% of the stroma were represented. Two images without motion artifact were selected from each layer; for the anterior 10% layer, 1 of the 2 images was always the most anterior image containing keratocytes. Images were analyzed by a custom automated program, which objectively identified bright objects (presumed to represent keratocyte nuclei) and calculated keratocyte density.15,16
SUBBASAL NERVE DENSITY
Subbasal nerve fiber bundles are visible at the basal aspect of the basal epithelial cell layer in confocal microscopy images of normal corneas.17,18 All confocal scans for each penetrating graft were reviewed by one observer (J.C.E.), who was masked to the clinical status and the postoperative age of the graft. The total length of all visible subbasal nerve fiber bundles and their branches longer than 50 μm in each scan was measured by using a custom program.8 Each nerve fiber bundle was measured only once, but if its length extended across several adjacent images, the total length was measured as if it were projected onto 1 image. Subbasal nerve density for each scan was calculated as the total length of nerve (μm) divided by the area of the image (0.187 mm2), and the subbasal nerve density for each cornea was the mean of the densities in all useable scans for that examination.
DATA ANALYSIS
Because the central corneal thickness of PKs increases with time after transplantation,1,2 we calculated the total number of keratocytes in a full-thickness column of central stroma with 1 mm2 of frontal surface area6; for brevity, this parameter is referred to as “number of keratocytes.” Full-thickness keratocyte density was calculated as a weighted mean by dividing the number of keratocytes in the full-thickness stroma by the central stromal thickness, which was also measured by confocal microscopy.6 Mean keratocyte density of full-thickness stroma, keratocyte density for each layer of stroma, number of keratocytes, stromal thickness, and subbasal nerve density were compared between clear grafts and controls, and between clear grafts and grafts with LEF. Differences were examined by using unpaired t tests if the data were distributed normally or Wilcoxon rank-sum tests if the data were not distributed normally. P < .05 was considered statistically significant. Correlations between keratocyte density or subbasal nerve density and time after keratoplasty were assessed by using Pearson correlation coefficient if the data were distributed normally or Spearman test if the data were not distributed normally. A paired analysis was performed on 16 grafts of 14 patients who were examined twice 4 or more years apart and 5 or more years after keratoplasty. The annual rate of keratocyte loss (percent lost per year) was calculated from the number of keratocytes at each examination and the interval between examinations by assuming that the number of keratocytes decreased as a simple first-order loss. Generalized estimating equation models were used to adjust for potential correlation between fellow eyes of the same patient.19 These model results are not reported because they did not alter any of the conclusions.
RESULTS
CLEAR GRAFTS AFTER PK
Clear penetrating grafts were examined 12.8 ± 8.7 years after surgery (n = 99, mean ± standard deviation; range, 1–31 years; median, 15 years). The most common preoperative diagnoses were keratoconus and Fuchs endothelial dystrophy (Table 1). Mean keratocyte density of the full-thickness stroma was 17% lower in clear grafts than in controls (P < .001, Table 2). Keratocyte density in each layer of stroma was also lower in clear grafts compared to controls (Table 2, Figure 1). The number of keratocytes in the full-thickness central stroma in clear grafts was 10% lower than in controls (P < .001, Table 2). The central stroma of clear grafts (470 ± 46 μm) was thicker than in controls (433 ± 36 μm, P < .001). Median subbasal nerve density in clear grafts (150 μm/mm2; range, 0–5,846 μm/mm2; n = 94) was lower than in controls (7,025 μm/mm2; range, 2,371–12,448 μm/mm2; n = 76; P < .001); nerves often regenerated in a random and disordered pattern (Figure 2). No subbasal nerves were detected in 45 clear grafts (48%). Median subbasal nerve density in clear grafts for keratoconus (897 μm/mm2; range, 0–5,846 μm/mm2; n = 50) was higher than in clear grafts for Fuchs dystrophy (377 μm/mm2; range, 0–2,981 μm/mm2; n = 31; P = .04).
TABLE 1.
PREOPERATIVE DIAGNOSES IN CLEAR GRAFTS AND GRAFTS WITH LATE ENDOTHELIAL FAILURE
| PREOPERATIVE DIAGNOSIS | CLEAR PENETRATING GRAFTS (n=99) | GRAFTS WITH LATE ENDOTHELIAL FAILURE (n=21)* |
|---|---|---|
| Keratoconus | 52 | 13 |
| Fuchs dystrophy | 33 | 5 |
| Pseudophakic corneal edema | 6 | 2 |
| Aphakic corneal edema | 2 | 0 |
| Traumatic scar | 2 | 0 |
| Other | 4† | 1‡ |
Quantitative analysis of confocal microscopy images was possible in only 12 eyes (10 eyes with keratoconus, 1 eye with Fuchs dystrophy, and 1 eye with pseudophakic corneal edema).
Includes 1 eye each with herpes simplex keratitis, interstitial keratitis, granular dystrophy, and central cloudy dystrophy of François.
Failed graft; original diagnosis was keratoconus.
TABLE 2.
KERATOCYTE DENSITY AND NUMBER OF KERATOCYTES IN NORMAL CORNEAS, CLEAR GRAFTS, AND GRAFTS WITH LATE ENDOTHELIAL FAILURE (MEAN ± STANDARD DEVIATION)
| NORMAL (UNOPERATED) CORNEAS (n=63) | CLEAR PENETRATING GRAFTS (n=99) | LATE ENDOTHELIAL FAILURE (n=12) (MDD)* | |
|---|---|---|---|
| Full-thickness keratocyte density (cells/mm3, weighted mean) | 26,610 ± 3,683 | 22,101 ± 3,799† | 21,268 ± 3,298† [3,250] |
| KERATOCYTE DENSITY BY PERCENT DEPTH OF STROMA (CELLS/MM3) | |||
| Anterior 0–10% | 42,513 ± 8544 | 33,598 ± 7,967† | 34,586 ± 7,791‡ [6,900] |
| 11%–33% | 28,093 ± 4,918 | 24,855 ± 5,289† | 26,170 ± 5,883§ [4,650] |
| 34%–66% | 23,614 ± 4,744 | 21,441 ± 5,129‡ | 19,215 ± 5,618‡ [4,500] |
| 67%–90% | 23,943 ± 4,561 | 17,281 ± 5,580† | 15,372 ± 4,187† [4,750] |
| Posterior 91%–100% | 24,141 ± 4,704 | 17,654 ± 6,089† | 17,985 ± 6,966† [5,350] |
| No. of keratocytes in full- thickness central stroma with frontal area of 1 mm2 (cells) | 11,466 ± 1,503 | 10,325 ± 1,708† | 10,778 ± 1,760§ [1,490] |
MDD, minimum detectable difference between clear grafts and grafts with late endothelial failure because no significant differences existed for any variable (α = 0.05, β = 0.20).
P< .001 vs normal (unpaired t tests).
P< .01 vs normal (unpaired t tests).
P> .05 vs normal (unpaired t tests). The minimum detectable difference for keratocyte density in the 11%–33% region of stroma was 4,550 cells/mm2, and for the number of keratocytes was 1,400 cells (α = 0.05, β = 0.20 for both analyses).
FIGURE 1.
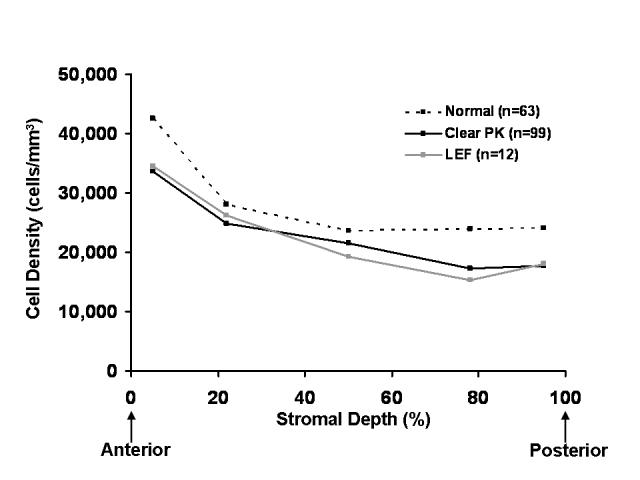
Keratocyte density in normal (unoperated) corneas, clear penetrating grafts, and grafts with late endothelial failure (LEF) is shown as a function of stromal depth. Keratocyte densities in all layers of the stroma after penetrating keratoplasty (clear grafts or grafts with LEF) were significantly lower than in normal corneas (P < .01). No differences were detectable between clear grafts and grafts with LEF.
FIGURE 2.
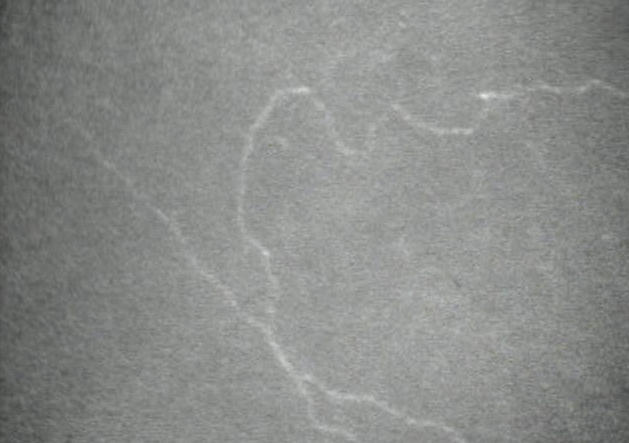
Confocal microscopy image of subbasal nerve fiber bundle 26 years after penetrating keratoplasty. Subbasal nerve fiber bundles were not detected in 48% of clear grafts, but when regeneration was evident, nerve fiber bundles were often tortuous and disordered.
Mean keratocyte density correlated weakly with time after PK (r = −0.20, P = .05, n = 99), whereas there was no correlation between the number of keratocytes and time after PK (r = −0.04, P = .68, n = 99, Figure 3, Table 3). Correlations between keratocyte density in each layer and time after PK are shown in Table 3. Stromal thickness correlated with time after PK (r = 0.26, P = .009, n = 99). Recovery of subbasal nerve density also correlated with time after PK (r = 0.36, P < .001, n = 94, Table 3, Figure 4).
FIGURE 3.
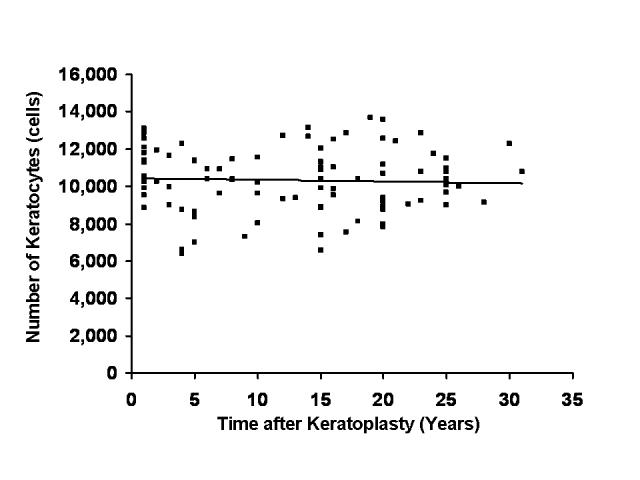
Relationship between number of keratocytes and time after penetrating keratoplasty (PK). Cross-sectional analysis of 99 clear penetrating grafts revealed no correlation between the number of keratocytes in the full-thickness central stroma with time after PK (r = −.04, P = .68).
TABLE 3.
CORRELATIONS WITH TIME AFTER KERATOPLASTY IN CLEAR GRAFTS*
| r | P Value | |
|---|---|---|
| Full-thickness keratocyte density | −0.20 | .05 |
| Keratocyte density by percent depth of stroma | ||
| Anterior 0 – 10% | 0.35 | < .001 |
| 11%– 33% | −0.09 | .37 |
| 34%– 66% | −0.27 | .008 |
| 67%– 90% | −0.33 | < .001 |
| Posterior 91%–100% | −0.14 | .18 |
| Number of keratocytes in full-thickness central stroma with frontal area of 1 mm2 | −0.04 | .68 |
| Subbasal nerve density (n=94) | 0.36 | < .001 |
n = 99 for all tests unless stated.
FIGURE 4.
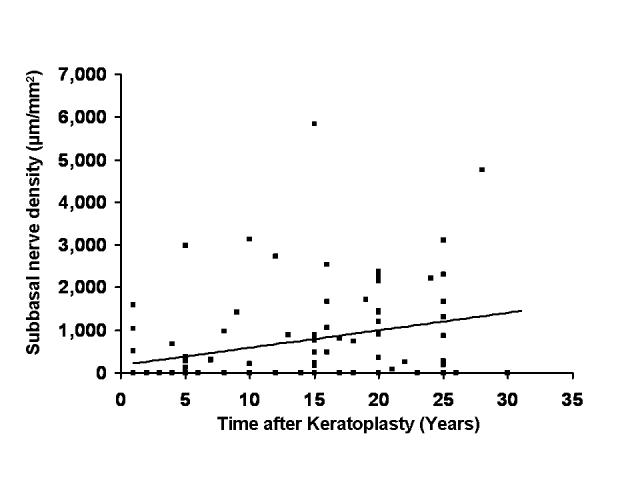
Relationship between subbasal nerve fiber bundle density and time after penetrating keratoplasty (PK). Cross-sectional analysis of 94 clear penetrating grafts suggested that subbasal nerve fiber bundle density increased with time after PK (r = 0.36, P < .001). The increase was not clinically significant because density remained decreased compared to subbasal nerve density in normal corneas (median, 7,025 μm/mm2, n = 76).
Sixteen clear grafts of 14 eyes were examined on 2 occasions separated by 5.0 ± 0.6 years (range, 4–6 years). The first examination was 17.6 ± 4.8 years after keratoplasty (range, 5–24 years), and the second examination was 22.6 ± 5.2 years after keratoplasty (range, 9–30 years). The preoperative diagnosis was keratoconus in 13 eyes and Fuchs dystrophy in 3 eyes. Mean keratocyte density for the full-thickness stroma was decreased 13% at the later examination compared to the earlier examination (P = .02, Table 4). Keratocyte density decreased only in the middle and posterior thirds of the stroma between the earlier and later examinations (Table 4, Figure 5). The number of keratocytes was decreased 13% at the later examination compared to the earlier examination (P = .03, Table 4). The rate of loss of keratocytes was 2.9 ± 5.0% per year. Central stromal thickness did not differ between the earlier (461 ± 40 μm) and later (473 ± 61 μm) examinations (P = .48); the minimum detectable difference was 50 μm (α = .05, β = .20, n = 16).
TABLE 4.
KERATOCYTE DENSITY AT REPEATED EXAMINATIONS OF CLEAR PENETRATING GRAFTS (MEAN ± STANDARD DEVIATION)*
| FIRST EXAMINATION (n=16) | SECOND EXAMINATION (n=16) | P VALUE [MDD]† | |
|---|---|---|---|
| Full-thickness keratocyte density (cells/mm3, weighted mean) | 21,631 ± 3,605 | 18,840 ± 5,792 | .02 |
| Keratocyte density by percent depthof stroma (cells/mm3) | |||
| Anterior 0– 10% | 34,096 ± 8,464 | 33,548 ± 8,393 | .84 [7,750] |
| 11%– 33% | 23,864 ± 5,329 | 21,674 ± 7,219 | .22 [5,150] |
| 34%– 66% | 21,472 ± 4,889 | 16,688 ± 5,075 | < .001 |
| 67%– 90% | 16,169 ± 3,771 | 13,085 ± 4,963 | .02 |
| Posterior 91%–100% | 18,503 ± 5,811 | 13,143 ± 5,335 | .009 |
| Number of keratocytes in full-thickness central stroma with frontal area of 1 mm2 (cells) | 9,959 ± 1,797 | 8,749 ± 2,260 | .03 |
Data are included for 16 grafts of 14 patients examined twice ≥ 4 years apart and ≥ 5 years after penetrating keratoplasty. The first examination was 17.6 ± 4.8 years after keratoplasty (range, 5–24 years) and the second examination was 22.6 ± 5.2 years after keratoplasty (range, 9–30 years).
MDD, minimum detectable difference between the first and second examinations (paired analysis, α = .05, β = .20, n = 16).
FIGURE 5.
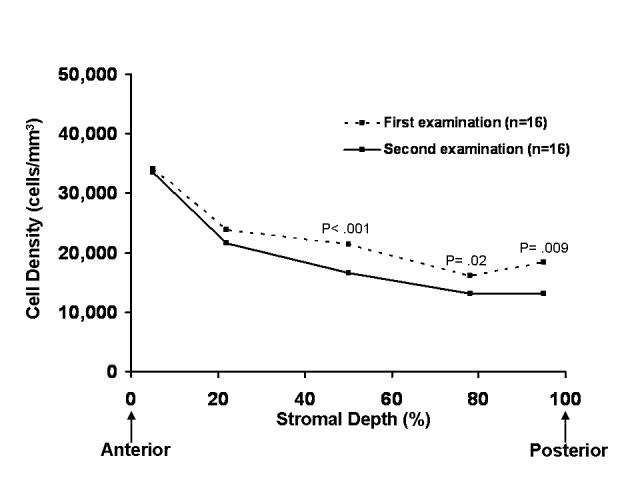
Keratocyte density at repeated examinations of clear penetrating grafts. Sixteen clear grafts of 14 patients were examined twice ≥ 4 years apart and ≥ 5 years after penetrating keratoplasty. The first examination was 17.6 ± 4.8 years after keratoplasty (range, 5–24 years), and the second examination was 22.6 ± 5.2 years after keratoplasty (range, 9–30 years). Keratocyte density decreased in the middle and posterior thirds of the stroma between the first and second examinations.
GRAFTS WITH LEF
Grafts with LEF were examined 19.2 ± 5.3 years after surgery (n = 21; range, 4–28 years; median, 20 years). The most common preoperative diagnoses were keratoconus and Fuchs endothelial dystrophy (Table 1). Keratocyte density could be measured in only 12 grafts with LEF (12 patients); images of the remaining grafts with LEF were hazy from stromal edema or an interdigitating network of keratocyte processes in the anterior stroma prevented identification of individual keratocytes (Figure 6).
FIGURE 6.
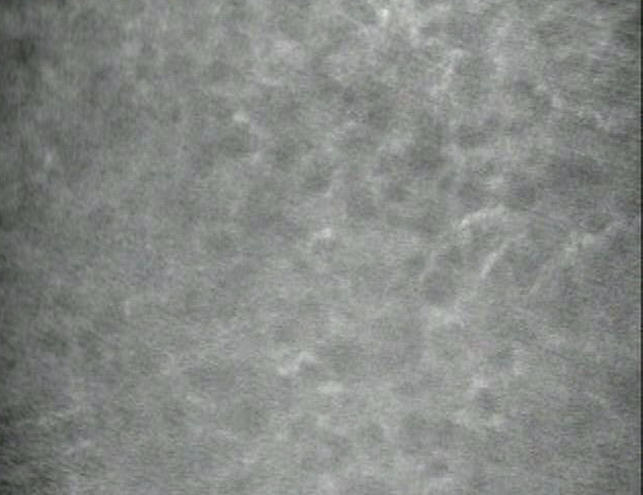
Anterior keratocytes in late endothelial failure (LEF). Confocal microscopy image of anterior keratocytes in a penetrating graft with LEF shows visible cell processes, suggesting activation of keratocytes and preventing identification of individual cells for quantitative analysis in some penetrating grafts. In normal corneas, keratocyte nuclei, and not cell processes, are visible by confocal microscopy.
Keratocyte density and keratocyte number per mm2 of full-thickness stroma did not differ between grafts with LEF and clear grafts (Table 2, Figure 1). The central stroma in grafts with LEF (508 ± 48 μm) was thicker than in clear grafts (470 ± 46 μm, P = .008). Median subbasal nerve density in grafts with LEF (340 μm/mm2, n = 11) did not differ from clear grafts (150 μm/mm2, n = 94, P = .95).
DISCUSSION
Keratocyte density and subbasal nerve fiber bundle density after PK were significantly lower than in normal corneas when measured by using confocal microscopy in vivo. Although keratocytes after PK were lost at a faster-than-normal rate,6 we were unable to detect a difference between keratocyte density in clear grafts compared to grafts with LEF. Subbasal nerve fiber bundles did not appear to regenerate to any clinically significant extent through 30 years after PK.
In normal corneas, keratocyte density is highest in the most anterior layers of the stroma, and full-thickness density declines with age at a rate similar to normal corneal endothelial and trabecular meshwork cells.6 Given that central corneal thickness increases with time after keratoplasty1,2 and that in the present study, the stroma of penetrating grafts was thicker than normal, it is not surprising that keratocyte density in clear grafts was decreased compared to normal because of redistribution of cells over a larger volume of stroma. However, we also calculated the absolute number of keratocytes in a column of full-thickness stroma, and we confirmed our preliminary report that the number of keratocytes was reduced in penetrating grafts compared to normal, contributing to decreased keratocyte density.9 Paired analysis showed that the number of keratocytes in the central stroma continued to decrease many years after keratoplasty at a rate of 2.9 ± 5.0% per year. Keratocyte density therefore also decreased over the 5-year period, but only in the middle and posterior thirds of the cornea. The latter can be explained by the posterior stroma swelling more than the anterior stroma,20 and although we did not detect an increase in stromal thickness over the 5-year period between repeated examinations, with the sample size, we were unable to detect differences smaller than 50 μm. The rate of decrease of keratocytes of 2.9% per year in the paired analysis was not supported by the cross-sectional analysis (Figure 3) and was much higher than the rate of endothelial cell loss in the second decade after keratoplasty.1,2 We analyzed confocal image contrast between the first and second examinations in the paired analysis and found that contrast was significantly lower at the second examination compared to the first examination. However, even after adjusting keratocyte density for variations in contrast, keratocyte density and the number of keratocytes were still 10% lower at the second examination compared to the first examination. We are unaware of other studies that have quantified keratocyte loss after PK, but it is conceivable that keratocytes are lost at a faster rate than endothelial cells and that keratocyte loss is higher within certain subgroups after PK.
Donor keratocytes are lost by apoptosis and necrosis during tissue preservation,21 but continued keratocyte losses several years after transplantation have not been previously documented. Keratocyte loss in penetrating grafts might be caused by starvation from decreased endothelial permeability,3 chronic apoptosis,22 or other undefined mechanisms, and may result in a gradual decrease in corneal transparency. Although we were unable to detect a difference in the number of keratocytes between grafts with LEF (with decreased transparency) and clear grafts, this study had limited power to detect small differences, was cross-sectional and not prospective in design, and the sample may have been biased because only 12 of 21 grafts with LEF had images that could be analyzed quantitatively. Because clear grafts lost keratocytes years after keratoplasty, grafts with LEF, which were examined longer after keratoplasty than the clear grafts, would have been expected to have a lower number of keratocytes, possibly contributing to decreased stromal clarity. Histologic analysis of excised grafts for LEF will help to confirm or refute the latter hypothesis.
In clear penetrating grafts, it is unclear whether transplanted keratocytes survive for decades or whether they are replaced by host keratocytes that migrate from the periphery. Donor keratocytes survive in clear grafts for at least a year after keratoplasty in rabbits23,24 and for as long as 4 years after keratoplasty in failed grafts in humans.25 Keratocytes also have migratory potential and can repopulate epikeratophakia lenticules,26–29 anterior lamellar grafts,30 and acellular collagen implants.31 Our study was not designed to determine whether host keratocytes migrate into the donor, but if that were the case, the results indicate that the rate of repopulation would be inadequate to maintain or increase the number of keratocytes in the corneal stroma.
Alterations in stromal transparency after PK could result from keratocyte dysfunction with or without decreased keratocyte density. The transparency of keratocytes appears to be related to the expression of intracellular crystallins, and decreased crystallin expression has been associated with repair cell phenotypes and increased backscatter from cells.32,33 We frequently noted keratocyte activation in grafts with LEF (Figure 6), and chronic keratocyte activation after PK might result in increased corneal backscatter,34 which would clinically manifest as stromal translucency. Evaluation of grafts excised for LEF may help determine whether keratocyte dysfunction and altered levels of crystallin expression are associated with LEF.
Decreased keratocyte density after PK has been found by other investigators using different confocal microscopes. Hollingsworth and colleagues35 used a scanning slit confocal microscope and found that anterior and posterior keratocyte densities were decreased but stable over the first year after keratoplasty compared to normal corneas, similar to our prospective data during the first year after PK.9 Niederer and colleagues36 used a laser scanning confocal microscope and found that keratocyte density was decreased in the anterior, middle, and posterior thirds of penetrating grafts several years after keratoplasty. The confocal microscopes used in the 2 latter studies are limited in their ability to measure the depth of confocal images accurately, and therefore volumetric keratocyte density and the absolute number of keratocytes were not calculated, preventing direct comparison to our study. However, the confocal microcopy images in the studies by Hollingsworth and colleagues and by Niederer and colleagues are of higher contrast compared to images from our Tandem Scanning confocal microscope, and identifying objects as keratocytes is easier than with our system. Nevertheless, Mikek and colleagues37 also used a scanning slit confocal microscope with high contrast images and found no difference in keratocyte density after PK compared to normal corneas. Because interpretation of the low contrast images from our confocal microscope can be subjective, we used an automated program to identify keratocytes objectively and calculate keratocyte density.16
Subbasal nerve fiber bundles are transected during PK, and regeneration of nerve fiber bundles in the donor is slow and incomplete, even 3 decades after surgery. In fact, no subbasal nerve fiber bundles could be detected in almost half of the clear grafts that were examined. Our cross-sectional data suggest an increase in subbasal nerve density with time after keratoplasty, but the increase was not clinically significant in the context of normal subbasal nerve density. Slow and limited subbasal nerve regeneration after PK in humans has been previously demonstrated by using confocal microscopy36,38 and by using acetylcholinesterase staining ex vivo.39 Corneal sensation was not measured in the present study, but sensitivity is known to remain significantly reduced, if not absent, for decades after PK.40–42 Tervo and colleagues39 showed that epithelial innervation was re-established after PK in humans, but stromal nerve regeneration was essentially absent, and they suggested that the discontinuity of Schwann cell channels at the graft-host junction impaired stromal nerve regeneration. Indeed, Tervo’s hypothesis is supported by evidence of significant stromal nerve regeneration after close apposition of limbal incisions in rabbits,43 and also by recovery of normal subbasal nerve density by 2 and 5 years after photorefractive keratectomy and laser in situ keratomileusis, respectively.13,44–46 The reason for higher subbasal nerve density in grafts for keratoconus compared to grafts for Fuchs dystrophy is not known, but our results confirm the findings of Niederer and colleagues.36
Whether corneal nerves are required to sustain keratocyte density and function is uncertain, but anterior keratocyte losses have been noted after keratorefractive surgery, in which there is an extended period of denervation postoperatively.13,45,47 No specific physiologic relationships between nerves and keratocytes have been established, but anatomically, human corneal nerve fibers invaginate the cytoplasm of keratocytes, suggesting that keratocytes might receive trophic factors from corneal nerves.17 After PK, chronic graft denervation might contribute to keratocyte loss or dysfunction and subsequent decreased transparency.
Further studies are necessary to determine the mechanisms of keratocyte loss after PK and the relationships between corneal nerves, keratocytes, endothelial cells, and stromal transparency. Preventing loss of stromal transparency could improve graft longevity. Furthermore, similar physiologic processes might affect the transparency of the host stroma after posterior lamellar keratoplasty, which is currently popular among corneal surgeons,48 and could affect the long-term success of these procedures.
PEER DISCUSSION
DR DANIEL S. DURRIE
I would like to thank the authors for the opportunity to discuss this well-organized, important paper. This work builds on the years of important research and publications that Drs Bourne, Erie, Patel, and associates have contributed to our knowledge of the anatomy of the cornea in normal and postsurgical eyes.
The corneal keratocytes make up 3% to 5% of the total stromal volume in normal eyes1 and have a higher density in the layers just below the epithelium.2 They secrete proteoglycans, collagen, and proteases and appear to be responsible for some of the embryologic stromal deposition and have a major role in stromal wound healing.3 The keratocytes’ role in normal corneal health is not well understood. This is also true of postsurgical (PK, PRK, LASIK) eyes.4,5
This study adds to our knowledge of the potential role of keratocytes in late endothelial failure (LEF). Since the number of keratocytes decreases over time in both normal and post keratoplasty (PK) eyes, does this decrease cause the endothelium to fail? This study shows that there is no significant difference in the density of keratocytes between normal and LEF eyes. Also, this study confirms previous studies that show a marked decrease in corneal innervation after PK. Forty-five clear grafts (48%) did not have any detectable innervation. The authors do not report if the number corneal nerves have any relationship to the number of keratocytes present in PK eyes.
There are a few weaknesses in this study, which the authors acknowledge. There was limited clinical material available for study, especially for longitudinal measurements. Also, in only 12 of 21 LEF eyes were reliable keratocyte densities measurable.
IT equipment and automated analysis software used in this study is not readily available for confirmatory studies. I would hope that the authors would work with the equipment manufacturers to make this important type of clinical analysis part of routine practice. Dr Bourne’s early work in endothelial analysis led to this measurement becoming a standard in corneal evaluations.
ACKNOWLEDGMENTS
Funding/Support: None.
Financial Disclosures: None.
REFERENCES
- 1.Patel SV, McLaren JW, Hodge DO, Bourne WM. Normal human keratocyte density and corneal thickness measurement by using confocal microscopy in vivo. Invest Ophthalmol Vis Sci. 2001;42:333–339. [PubMed] [Google Scholar]
- 2.Wilson SE, Mohan RR, Mohan RR, et al. The corneal wound healing response: cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog Retinal Eye Res. 2001;20:625–637. doi: 10.1016/s1350-9462(01)00008-8. [DOI] [PubMed] [Google Scholar]
- 3.Watsky MA, Olsen TW, Edelhauser HF. Cornea and sclera. In: Tasman W, Jaeger EA, editors. Duane’s Foundations of Clinical Ophthalmology. Vol. 2. Philadelphia: JB Lippincott; 1995. chap 4. [Google Scholar]
- 4.Vesaluoma M, Perez-Santonja J, Petroll WM, et al. Corneal stromal changes induced by myopic LASIK. Invest Ophthalmol Vis Sci. 2000;41:369–376. [PubMed] [Google Scholar]
- 5.Mitooka K, Ramirez M, Maguire LJ, et al. Keratocyte density of central human cornea after laser in situ keratomileusis. Am J Ophthalmol. 2002;133:307–314. doi: 10.1016/s0002-9394(01)01421-0. [DOI] [PubMed] [Google Scholar]
DR. JAMES CHODOSH
I have no conflicts. Have you looked at the keratocyte density in patients prior to surgery to determine if it is really different and if there is not some underlying intrinsic factor in those individuals that is responsible for reducing keratocyte density in their grafts? What is the rate of change in keratocyte density, if any, in normal corneas over time? Is keratocyte loss in any way related to the underlying disease for which the transplant was performed?
DR. GARY C. BROWN
Have you considered that this may be very similar to the late graft failure that occurs after kidney and other organ transplantation? This certainly sounds very much like the type of idiopathic failure is observed in many organs usually after several years. What type of corneal sensitivity is present after corneal transplantation and do corneal nerves really grow into and innervate the grafted tissue? How is corneal sensitivity measured and how can the stimulus be quantified?
DR. SANJAY V. PATEL
I would like to thank Dr. Durrie for leading the discussion. He makes a very valid point that, unfortunately, this study does not help us to determine if changes in keratocyte density were related to late endothelial failure. One hypothesis is that with decreased endothelial permeability after keratoplasty, there is starvation of stromal keratocytes and ultimately a reduction in their density with resultant diminished corneal transparency. I believe the relationship between late endothelial failure and keratocyte density is still unanswered and that we must perform histological examinations of these corneas to better understand the condition. In this study, 9 of the 21 eyes with late endothelial failure were excluded because we could not identify cells in confocal images, and this might have biased our results. Dr. Durrie makes another good point; the clinical implications of keratocyte loss are still unknown. We have detected keratocyte deficits after corneal excimer laser surgery; however, we have not seen any adverse clinical effects from this deficit, even at seven years after surgery. We do have new clinical studies in progress, which are designed to detect subtle clinical changes over the long-term. As discussed earlier, even if the density of keratocytes is not clinically important, keratocyte function, or dysfunction, might be very important. Dr. Chodosh, we have examined keratocyte density in pre-transplant patients, but I do not have reliable data at this time. We have studied groups of patients with corneal endothelial dystrophy most extensively, but unfortunately, the associated corneal edema limits visualization of the stromal keratocytes, and causes problems counting those cells. The rate of reduction of keratocyte density in normal patients, approximately 0.6%/year, was published several years ago and is somewhat similar to the rate of loss of endothelial cells and trabecular meshwork cells. Regarding the comment that keratocyte loss might be related to the underlying disease, this is certainly a possibility. I did not present the demographics in this cohort; more than 50 of the grafts were in patients with keratoconus and approximately 33 of the grafts were in patients with Fuchs dystrophy. We have previously shown regional losses of keratocytes in keratoconus patients, so there is a possibility that some of the keratocyte loss might occur as a result of the underlying disease process, but we have not investigated that further. With regard to late onset graft failure in other organ transplants, we have not made any direct comparisons with penetrating keratoplasty, so I cannot comment. Regarding nerve regeneration, we did not assess corneal sensitivity in this group of patients; however, multiple studies have documented a markedly reduced corneal sensitivity decades after penetrating keratoplasty by using Cochet-Bonnet esthesiometry. More sensitive esthesiometers are now available to help quantify the stimulus and test different sensory modalities. After penetrating keratoplasty, regrowth of nerves has been shown to occur mainly at the basal and subepithelial level, with some limited anterior stromal reinnervation.
ACKNOWLEDGMENTS
Funding/Support: Supported in part by grant EY 02037 from the National Institutes of Health; Research to Prevent Blindness, Inc (S.V.P. as Olga Keith Wiess Scholar and an unrestricted grant to the Department of Ophthalmology, Mayo Clinic); and the Mayo Foundation.
Financial Disclosures: None.
Author Contributions: Design and conduct of the study (S.V.P., J.C.E, J.W.M, W.M.B.); Collection, management, analysis, and interpretation of the data (S.V.P., J.C.E, J.W.M, W.M.B.); Preparation, review, and approval of the manuscript (S.V.P., J.C.E, J.W.M, W.M.B.).
REFERENCES
- 1.Patel SV, Hodge DO, Bourne WM. Corneal endothelium and postoperative outcomes 15 years after penetrating keratoplasty. Trans Am Ophthalmol Soc. 2004;102:57–65. 65–66. [PMC free article] [PubMed] [Google Scholar]
- 2.Patel SV, Hodge DO, Bourne WM. Corneal endothelium and postoperative outcomes 15 years after penetrating keratoplasty. Am J Ophthalmol. 2005;139:311–319. doi: 10.1016/j.ajo.2004.09.045. [DOI] [PubMed] [Google Scholar]
- 3.Bourne WM, Brubaker RF. Decreased endothelial permeability in transplanted corneas. Am J Ophthalmol. 1983;96:362–367. doi: 10.1016/s0002-9394(14)77828-6. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura JK, Hodge DO, Bourne WM. Initial endothelial cell density and chronic endothelial cell loss rate in corneal transplants with late endothelial failure. Ophthalmology. 1999;106:1962–1965. doi: 10.1016/S0161-6420(99)90409-8. [DOI] [PubMed] [Google Scholar]
- 5.Bell KD, Campbell RJ, Bourne WM. Pathology of late endothelial failure. Late endothelial failure of penetrating keratoplasty: study with light and electron microscopy. Cornea. 2000;19:40–46. doi: 10.1097/00003226-200001000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Patel SV, McLaren JW, Hodge DO, Bourne WM. Normal human keratocyte density and corneal thickness measurement by using confocal microscopy in vivo. Invest Ophthalmol Vis Sci. 2001;42:333–339. [PubMed] [Google Scholar]
- 7.Patel SV, McLaren JW, Hodge DO, Bourne WM. Confocal microscopy in vivo in corneas of long-term contact lens wearers. Invest Ophthalmol Vis Sci. 2002;43:995–1003. [PubMed] [Google Scholar]
- 8.Erie JC, McLaren JW, Hodge DO, Bourne WM. The effect of age on the corneal subbasal nerve plexus. Cornea. 2005;24:705–709. doi: 10.1097/01.ico.0000154387.51355.39. [DOI] [PubMed] [Google Scholar]
- 9.Bourne WM. Cellular changes in transplanted human corneas. Cornea. 2001;20:560–569. doi: 10.1097/00003226-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Erie JC, Patel SV, McLaren JW, Nau CB, Hodge DO, Bourne WM. Keratocyte density in keratoconus. A confocal microscopy study. Am J Ophthalmol. 2002;134:689–695. doi: 10.1016/s0002-9394(02)01698-7. [DOI] [PubMed] [Google Scholar]
- 11.McLaren JW, Nau CB, Erie JC, Bourne WM. Corneal thickness measurement by confocal microscopy, ultrasound, and scanning slit methods. Am J Ophthalmol. 2004;137:1011–1020. doi: 10.1016/j.ajo.2004.01.049. [DOI] [PubMed] [Google Scholar]
- 12.Patel SV, Maguire LJ, McLaren JW, Hodge DO, Bourne WM. Femtosecond laser versus mechanical microkeratome for LASIK: a randomized controlled study. Ophthalmology. 2007;114:1482–1490. doi: 10.1016/j.ophtha.2006.10.057. [DOI] [PubMed] [Google Scholar]
- 13.Calvillo MP, McLaren JW, Hodge DO, Bourne WM. Corneal reinnervation after LASIK: prospective 3-year longitudinal study. Invest Ophthalmol Vis Sci. 2004;45:3991–3996. doi: 10.1167/iovs.04-0561. [DOI] [PubMed] [Google Scholar]
- 14.Erie JC, Patel SV, McLaren JW, et al. Effect of myopic laser in situ keratomileusis on epithelial and stromal thickness: a confocal microscopy study. Ophthalmology. 2002;109:1447–1452. doi: 10.1016/s0161-6420(02)01106-5. [DOI] [PubMed] [Google Scholar]
- 15.Patel SV, McLaren JW, Camp JJ, Nelson LR, Bourne WM. Automated quantification of keratocyte density by using confocal microscopy in vivo. Invest Ophthalmol Vis Sci. 1999;40:320–326. [PubMed] [Google Scholar]
- 16.McLaren JW, Patel SV, Nau CB, Bourne WM. Automated assessment of keratocyte density in clinical confocal microscopy of the corneal stroma. J Microsc. doi: 10.1111/j.1365-2818.2007.01870.x. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller LJ, Pels L, Vrensen GF. Ultrastructural organization of human corneal nerves. Invest Ophthalmol Vis Sci. 1996;37:476–488. [PubMed] [Google Scholar]
- 18.Muller LJ, Vrensen GF, Pels L, Cardozo BN, Willekens B. Architecture of human corneal nerves. Invest Ophthalmol Vis Sci. 1997;38:985–994. [PubMed] [Google Scholar]
- 19.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 20.Lee D, Wilson G. Non-uniform swelling properties of the corneal stroma. Curr Eye Res. 1981;1:457–461. doi: 10.3109/02713688109019986. [DOI] [PubMed] [Google Scholar]
- 21.Komuro A, Hodge DO, Gores GJ, Bourne WM. Cell death during corneal storage at 4 degrees C. Invest Ophthalmol Vis Sci. 1999;40:2827–2832. [PubMed] [Google Scholar]
- 22.Beauregard C, Huq SO, Barabino S, Zhang Q, Kazlauskas A, Dana MR. Keratocyte apoptosis and failure of corneal allografts. Transplantation. 2006;81:1577–1582. doi: 10.1097/01.tp.0000209503.62204.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polack FM, Smelser GK, Rose J. Long-term survival of isotopically labeled stromal and endothelial cells in corneal homografts. Am J Ophthalmol. 1964;57:67–78. doi: 10.1016/0002-9394(64)92033-1. [DOI] [PubMed] [Google Scholar]
- 24.Polack FM, Smelser GK. The persistence of isotopically labeled cells in corneal grafts. Proc Soc Exp Biol Med. 1962;110:60–61. doi: 10.3181/00379727-110-27422. [DOI] [PubMed] [Google Scholar]
- 25.Wollensak G, Green WR. Analysis of sex-mismatched human corneal transplants by fluorescence in situ hybridization of the sex-chromosomes. Exp Eye Res. 1999;68:341–346. doi: 10.1006/exer.1998.0611. [DOI] [PubMed] [Google Scholar]
- 26.Baumgartner SD, Binder PS. Refractive keratoplasty. Histopathology of clinical specimens. Ophthalmology. 1985;92:1606–1615. [PubMed] [Google Scholar]
- 27.Grossniklaus HE, Lass JH, Jacobs G, Margo CE, McAuliffe KM. Light microscopic and ultrastructural findings in failed epikeratoplasty. Refract Corneal Surg. 1989;5:296–301. [PubMed] [Google Scholar]
- 28.Katakami C, Sahori A, Kazusa R, Yamamoto M. Keratocyte activity in wound healing after epikeratophakia in rabbits. Invest Ophthalmol Vis Sci. 1991;32:1837–1845. [PubMed] [Google Scholar]
- 29.Wang RG, Hjortdal JO, Ehlers N, Krogh E. Histopathological findings in failed human epikeratophakia lenticules. Acta Ophthalmol. 1994;72:363–368. doi: 10.1111/j.1755-3768.1994.tb02774.x. [DOI] [PubMed] [Google Scholar]
- 30.Kratz-Owens KL, Hageman GS, Schanzlin DJ. An in-vivo technique for monitoring keratocyte migration following lamellar keratoplasty. Refract Corneal Surg. 1992;8:230–234. [PubMed] [Google Scholar]
- 31.Liu Y, Gan L, Carlsson DJ, et al. A simple, cross-linked collagen tissue substitute for corneal implantation. Invest Ophthalmol Vis Sci. 2006;47:1869–1875. doi: 10.1167/iovs.05-1339. [DOI] [PubMed] [Google Scholar]
- 32.Jester JV, Moller-Pedersen T, Huang J, et al. The cellular basis of corneal transparency: evidence for 'corneal crystallins. ' J Cell Sci. 1999;112:613–622. doi: 10.1242/jcs.112.5.613. [DOI] [PubMed] [Google Scholar]
- 33.Pei Y, Reins RY, McDermott AM. Aldehyde dehydrogenase (ALDH) 3A1 expression by the human keratocyte and its repair phenotypes. Exp Eye Res. 2006;83:1063–1073. doi: 10.1016/j.exer.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 34.Moller-Pedersen T. Keratocyte reflectivity and corneal haze. Exp Eye Res. 2004;78:553–560. doi: 10.1016/s0014-4835(03)00208-2. [DOI] [PubMed] [Google Scholar]
- 35.Hollingsworth JG, Efron N, Tullo AB. A longitudinal case series investigating cellular changes to the transplanted cornea using confocal microscopy. Cont Lens Anterior Eye. 2006;29:135–141. doi: 10.1016/j.clae.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 36.Niederer RL, Perumal D, Sherwin T, McGhee CN. Corneal innervation and cellular changes after corneal transplantation: an in vivo confocal microscopy study. Invest Ophthalmol Vis Sci. 2007;48:621–626. doi: 10.1167/iovs.06-0538. [DOI] [PubMed] [Google Scholar]
- 37.Mikek K, Hawlina M, Pfeifer V. Comparative study of human keratocyte density after corneal grafting by using confocal microscopy in vivo. Klin Monatsbl Augenheilkd. 2003;220:830–834. doi: 10.1055/s-2003-812554. [DOI] [PubMed] [Google Scholar]
- 38.Richter A, Slowik C, Somodi S, Vick HP, Guthoff R. Corneal reinnervation following penetrating keratoplasty—correlation of esthesiometry and confocal microscopy. Ger J Ophthalmol. 1996;5:513–517. [PubMed] [Google Scholar]
- 39.Tervo T, Vannas A, Tervo K, Holden BA. Histochemical evidence of limited reinnervation of human corneal grafts. Acta Ophthalmol. 1985;63:207–214. doi: 10.1111/j.1755-3768.1985.tb01535.x. [DOI] [PubMed] [Google Scholar]
- 40.Rao GN, John T, Ishida N, Aquavella JV. Recovery of corneal sensitivity in grafts following penetrating keratoplasty. Ophthalmology. 1985;92:1408–1411. doi: 10.1016/s0161-6420(85)33857-5. [DOI] [PubMed] [Google Scholar]
- 41.Mathers WD, Jester JV, Lemp MA. Return of human corneal sensitivity after penetrating keratoplasty. Arch Ophthalmol. 1988;106:210–211. doi: 10.1001/archopht.1988.01060130220030. [DOI] [PubMed] [Google Scholar]
- 42.Richter A, Slowik C, Somodi S, Vick HP, Guthoff R. In vivo imaging of corneal innervation in the human using confocal microscopy. Ophthalmologe. 1997;94:141–146. doi: 10.1007/s003470050095. [DOI] [PubMed] [Google Scholar]
- 43.Chan-Ling T, Tervo K, Tervo T, Vannas A, Holden BA, Eranko L. Long-term neural regeneration in the rabbit following 180 degrees limbal incision. Invest Ophthalmol Vis Sci. 1987;28:2083–2088. [PubMed] [Google Scholar]
- 44.Linna TU, Perez-Santonja JJ, Tervo KM, Sakla HF, Alio y Sanz JL, Tervo TM. Recovery of corneal nerve morphology following laser in situ keratomileusis. Exp Eye Res. 1998;66:755–763. doi: 10.1006/exer.1998.0469. [DOI] [PubMed] [Google Scholar]
- 45.Erie JC. Corneal wound healing after photorefractive keratectomy: a 3-year confocal microscopy study. Trans Am Ophthalmol Soc. 2003;101:293–333. [PMC free article] [PubMed] [Google Scholar]
- 46.Moilanen JA, Vesaluoma MH, Muller LJ, Tervo TM. Long-term corneal morphology after PRK by in vivo confocal microscopy. Invest Ophthalmol Vis Sci. 2003;44:1064–1069. doi: 10.1167/iovs.02-0247. [DOI] [PubMed] [Google Scholar]
- 47.Erie JC, Patel SV, McLaren JW, Hodge DO, Bourne WM. Corneal keratocyte deficits after photorefractive keratectomy and laser in situ keratomileusis. Am J Ophthalmol. 2006;141:799–809. doi: 10.1016/j.ajo.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 48.Patel SV. Keratoplasty for endothelial dysfunction. Ophthalmology. 2007;114:627–628. doi: 10.1016/j.ophtha.2007.01.001. [DOI] [PubMed] [Google Scholar]


