Abstract
Purpose
Graves ophthalmopathy and Graves disease may be caused by the same autoimmune process. An explanation for this may be the presence of autoantibodies reacting with an autoantigen in the orbit and the thyroid gland, like the thyroid-stimulating hormone (TSH) receptor. The purpose of this study was to see if initial levels of TSH receptor antibodies, known as thyroid-stimulating immunoglobulin (TSI), in pediatric patients with Graves disease were associated with the development of Graves ophthalmopathy during the follow-up period.
Methods
This was a retrospective review of all the patients at Texas Children’s Hospital with a new diagnosis of Graves disease between the years 2000 and 2006, who had TSI titers obtained at the time of diagnosis. The ocular findings during the follow-up period were analyzed in relation to the TSI levels.
Results
Forty-nine patients were included (36 female, 13 male). The mean age was 11.3 ± 4.1 years. Fifty-three percent developed Graves ophthalmopathy during the follow-up period (24.6 ± 37.6 months). Thirty-two (65%) of the 49 children had positive TSI levels at the time of diagnosis, and 22 (69%) of them developed Graves ophthalmopathy. Only 4 (24%) of the 17 children with normal or indeterminate TSI levels developed Graves ophthalmopathy. A significant association between elevated initial TSI levels and Graves ophthalmopathy was found (χ2 = 6.94, P = .029). The most frequent ocular findings were mild proptosis (44%), exposure keratitis (4%), lid lag (2%), and motility deficits (2%).
Conclusion
A positive association exists between elevated initial levels of TSI and the development of Graves ophthalmopathy in children with Graves disease.
INTRODUCTION
Graves ophthalmopathy, also called thyroid-associated orbitopathy, is an autoimmune inflammatory process linked to Graves hyperthyroidism, described for the first time in 1835.1 It can also be present in euthyroid patients. Diagnosis of Graves ophthalmopathy currently depends solely on the clinical examination. There are no objective laboratory tests available to make the diagnosis.
Although hyperthyroidism can be successfully treated most of the time, the ophthalmopathy often produces significant problems that can lead to permanent cosmetic and functional sequelae, such as eyelid retraction, proptosis, keratopathy, compressive optic neuropathy, and strabismus. Numerous reports have reviewed the characteristics of Graves ophthalmopathy in adults, but only a few have examined the clinical features in pediatric patients.2,3
The assessment of Graves ophthalmopathy is currently based on the clinical findings and determination of systemic thyroid hormone status. The precise mechanism of thyroid eye disease still remains conjectural. Even though there are reasonable hypotheses, such as the existence of an autoantigen present in both the thyroid gland and the orbit,4 the search for an ideal test for the early diagnosis of Graves disease and Graves ophthalmopathy continues. Thyroid-stimulating hormone (TSH) receptors are present in the orbit and are expressed on orbital fibroblasts.5,6 If the orbital TSH receptor is the site of attack in Graves ophthalmopathy, it would be expected that elevated TSH receptor autoantibody titers would be associated with the clinical expression of the orbital disease.
Currently, two types of assays are used to detect TSH receptor antibodies.7,8 One type is based on the competition between the antibody and TSH for binding to the TSH receptor. The other is a functional assay that measures the production of cyclic adenosine monophosphate (cAMP) in response to a TSH receptor interaction with stimulating antibodies (thyroid-stimulating immunoglobulins, or TSIs) or blocking antibodies (thyroid-binding inhibitory immunoglobulins, or TBIIs). The competitive assay does not distinguish between the TSH receptor antibodies that stimulate or block the TSH receptor. Only functional assays can identify whether the antibody is a stimulating or blocking antibody, thereby making them much more useful.
The purpose of this study was to determine if the initial levels of TSI in children with a recent diagnosis of Graves disease were associated with the presence of Graves ophthalmopathy during the follow-up period. If these were found to be associated, then TSI levels could be used as a predictor of Graves ophthalmopathy in pediatric Graves disease.
SUBJECTS AND METHODS
This retrospective review had the approval of the Institutional Review Board of Baylor College of Medicine, Houston, Texas. All patients younger than 18 years with a new diagnosis of Graves disease between the years 2000 and 2006 were identified using the database at Texas Children’s Hospital. The search was conducted using the diagnosis codes Graves disease and hyperthyroidism. One hundred eighty-two patients were identified. To be included in the study, patients also had to have had TSI levels taken at the time of diagnosis and had to have had at least one ophthalmologic evaluation during the follow-up period. Forty-nine of the 182 patients fulfilled these criteria. A chart review was performed, and the following information was recorded: age at onset, gender, TSI levels at the time of diagnosis, antiperoxidase antibody titers at time of presentation if available, ocular findings, and time between the diagnosis of Graves disease and onset of ocular abnormalities.
AUTOANTIBODY MEASUREMENTS
Several different laboratories were used to determine TSI levels in the patients. In the first laboratory, the values were expressed as negative (or normal) if the level was less than or equal to 100%, indeterminate if it was between 101% and 129%, and positive if it was greater than or equal to 130%. In the second laboratory, the levels were defined as normal if less than 10, indeterminate if between 10 and 14, and positive if 15 or higher. For statistical analysis, only the categorical data (negative, indeterminate, and positive) were used. We also analyzed antiperoxidase antibody titers at the time of presentation, if available, as a secondary variable. These titers were also reported as normal (0 to 20 IU/mL) or elevated (> 20 IU/mL).
Data was analyzed using SPSS. Statistical significance was set at a P value of ≤ .05. To analyze the relationship between the initial TSI level and the development of Graves ophthalmopathy, we considered only the positive and negative values. The χ2 test was used to determine dependency, and descriptive parameters were used to describe the sample.
RESULTS
Seventy-four percent (36) of the 49 patients were female, and the mean age was 11.2 ± 4.1 years (range, 3–17 years). Thirty-three percent were younger than 10 years of age. Patients were followed for a mean of 24.6 ± 37.5 months. Twenty-six patients (53%) developed Graves ophthalmopathy. The group of patients who developed Graves ophthalmopathy was composed primarily of girls (69%) and patients older than 11 years (58%) (Figure 1).
FIGURE 1.
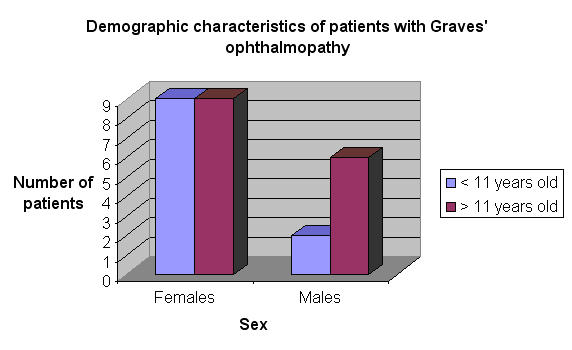
Demographic characteristics of children with Graves ophthalmopathy.
Thirty-two (65%) of the 49 patients had positive TSI levels at the time of the diagnosis, and 22 of them (69%) developed ocular findings consistent with Graves disease during the follow-up period. In the group with negative or indeterminate TSI levels, only 5 patients (29%) developed Graves ophthalmopathy (Figure 2). The most frequent finding was mild proptosis in 22 patients (Figure 3). No patients had a significant motility deficit or restriction. No patients had a vision-threatening complication.
FIGURE 2.
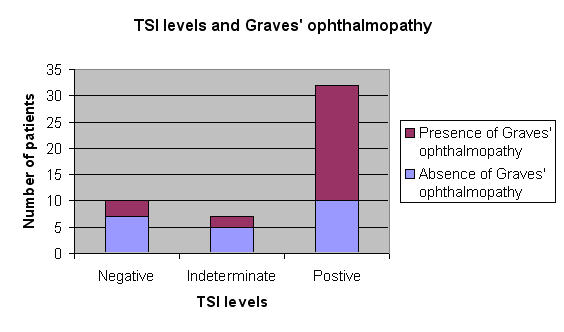
Initial thyroid-stimulating immunoglobulin (TSI) levels and the presence or absence of Graves ophthalmopathy.
FIGURE 3.
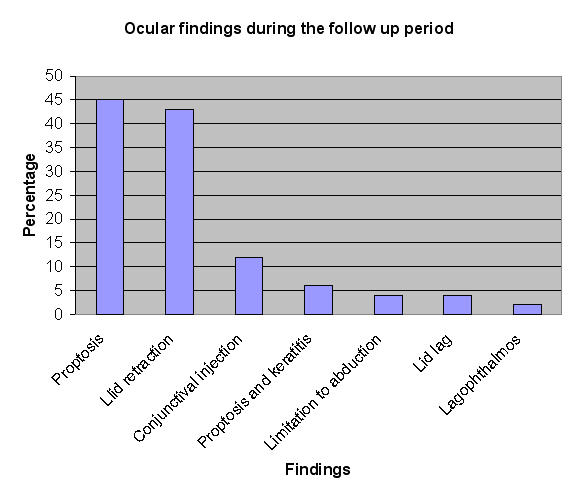
Ocular findings during the follow-up period.
There was a strong correlation between initial positive TSI levels and eventual development of Graves ophthalmopathy (χ2 = 6.94; P = .029). The temporal relationship between diagnosis of thyroid dysfunction and the onset of ophthalmic signs varied between 0 and 6 months (average, 0.5 ± 1.3 months), but most patients had some ocular signs at the time of diagnosis (Figure 4).
FIGURE 4.
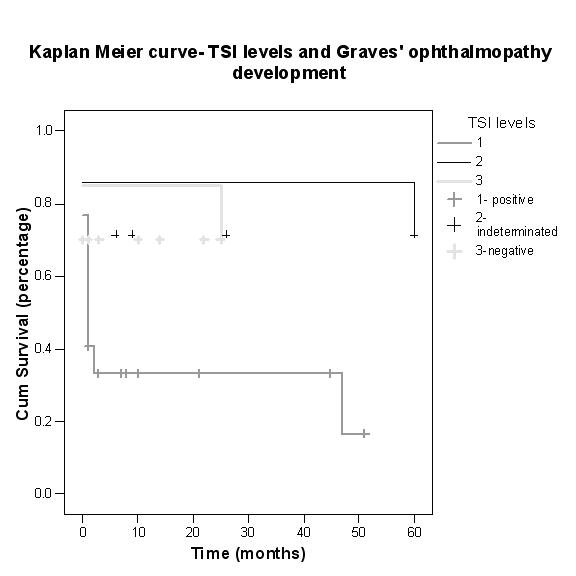
Kaplan-Meier curve showing thyroid-stimulating immunoglobulin (TSI) levels and onset of Graves ophthalmology.
Of the 49 patients, 37 (76%) had antiperoxidase antibody levels taken at the time of presentation. Twenty-seven (73%) of the 37 had positive levels, and 16 (59%) of the 27 had Graves ophthalmopathy. Unlike TSI levels, however, there was no significant association between these two parameters (χ2 = 1.09; P = .29). There was also no correlation between the levels of TSI and antiperoxidase antibodies in our series.
DISCUSSION
The incidence of Graves disease in adults has been reported to be between 15 and 20 per 100,000 per year,8 but in children it is much less common, reported to be between 0.79 and 6.5 per 100,000 children.9,10 Graves ophthalmopathy is also more benign in children.11,12
The primary thyroid target autoantigen driving the immunologic process in Graves disease is the TSH receptor, although several other antigenic targets, such as thyroglobulin, thyroid microsomal antigen, and sodium-iodine symporter, have also been identified.13 The TSH receptor is also expressed in lymphocytes, fibroblasts, and certain adipocytes in the orbit.14 A thyroid receptor autoantibody directed against the TSH receptor can activate it, resulting in cAMP-dependent stimulation of thyrocyte function and growth causing hyperthyroidism.15
The TSI level in adult patients with Graves disease has been found to be a useful marker to determine the activity of the orbital disease in order to help clinical decision making regarding treatment of thyroid orbitopathy.16 Longitudinal direct correlation of TSI level with clinical activity of disease in Graves ophthalmopathy has also been reported,16 and TSI levels have also been useful in the diagnosis of Graves ophthalmopathy in euthyroid adult patients.17 Gerding and colleagues18 demonstrated a strong direct correlation between the levels of TSI and TBII and the severity of Graves ophthalmopathy activity in adults. The levels of TSI and TBII in Gerding’s study, however, did not correlate with the duration of the orbital disease, and no association was found between the antibody levels and eye motility disturbances.
In this retrospective study, positive TSI levels did correlate with the development of Graves ophthalmopathy in children with Graves disease. This is the first study to our knowledge in the ophthalmology literature that has analyzed this potential association. If this finding holds true, TSI assays could become potentially useful to endocrinologists and pediatric ophthalmologists for their discussions with parents and affected children regarding their personal risk of developing Graves ophthalmopathy. It also may assist the ophthalmologist in deciding follow-up needs. In this study, the initial levels of basal TSH, T3, and T4 were not analyzed because it is known that Graves ophthalmopathy does not correlate with these hormone levels.19
There are some conflicting reports in the literature regarding TSI levels and Graves ophthalmopathy. Shibayama and coworkers19 did not find a relationship between TSI levels and presence of Graves ophthalmopathy in children with Graves disease. However, these investigators did not describe when the titers were obtained or when the Graves ophthalmopathy occurred. They then reported a relationship between TBII levels and Graves ophthalmopathy. The exact role of TBII titers as a marker of Graves disease (or ophthalmopathy) remains controversial. We did not review TBII titers in our study, as this test is not frequently ordered in pediatric patients with a recent diagnosis of Graves disease, and test results were not available in most of our patients. Khoo and associates20 reported a significant correlation between initial TSI levels in adult patients with Graves disease who later developed Graves ophthalmopathy, but they found no correlation with TBII. They also found a significant correlation between antiperoxidase antibody negativity and Graves ophthalmopathy. We did not find a similar association, although some of our patients had not had this laboratory test done.
The incidence of Graves ophthalmopathy in pediatric patients with Graves disease has previously been reported to be between 43%19 and 63%.3 Our results (53%) were similar. We also found that Graves disease affects females (74%) and teens (68 % older than 11 years) more often, as has been previously reported. Teenaged girls are most affected by Graves ophthalmopathy. The frequency of eye findings in our study cohort was similar to what has been previously described.21 None of our patients required surgical intervention, and none developed vision-threatening complications. These findings are in agreement with previous reports.2,21 Thus, in general, the ocular involvement in pediatric Graves patients appears to be less severe than that seen in adults.2,21
This study has a significant weakness based on its retrospective design and the potential biases that are introduced from this. Only 49 of 182 children with Graves disease in the Texas Children’s Hospital database had TSI levels and an eye examination performed and could be included in the study cohort for analysis. We have no information on the other children, who may or may not have had Graves ophthalmopathy. It would be ideal to perform a prospective study to more completely and rigorously investigate this relationship between TSI and Graves ophthalmopathy.
In conclusion, there appears to be an association between initial levels of TSI and the development of Graves ophthalmopathy in children with Graves disease. If these results are confirmed in a well-designed prospective study, the TSI titer could become a useful laboratory test to be used as a predictor of Graves ophthalmopathy in children with a recent diagnosis of Graves disease.
PEER DISCUSSION
DR DAVID T. TSE:
I want to thank the authors for sending the manuscript for review well in advance of the meeting and to congratulate them for undertaking the effort to investigate the potential utility of a laboratory test capable of substantiating clinical observation of thyroid-associated orbitopathy.
In Graves disease, the primary thyroid target autoantigen driving the immunologic process is the thyroid-stimulating hormone (TSH) receptor. Thyroid receptor auto antibodies (TSHR Ab) directed against the TSH receptors can activate the receptors to initiate cyclic adenosine monophosphate (cAMP)-dependent stimulation of thyrocyte function and growth, resulting in hyperthyroidism. It is hypothesized that in a certain immunogenic environment, circulating T cells directed against particular antigens on the thyroid follicular cells recognize antigenic epitopes also shared by tissues in the orbit. Thus, the rationale of bioassay for a measurable immunologic marker, such as the thyroid- stimulating immunoglobulin (TSI) that might reflect disease activity of the orbitopathy, is meritorious.
The stated purpose of the study was to determine whether the initial levels of TSI in pediatric patients with Graves disease were associated with the eventual development of Graves ophthalmopathy during the follow-up period. This was a retrospective case series identifying patients 18 years or younger in whom Graves ophthalmopathy was diagnosed from 2000 through 2006, and in whom TSI titers were taken at the time of diagnosis. Only 49 patients qualified for the study; thus 27% of the subjects had at least one ophthalmologic examination and measurement of TSI.
Within this study cohort, 36 were female and 13 were male patients, for a female to male ratio of 2.78:1. Thirty-two (65%) of the 49 children had positive TSI levels at the time of diagnosis, and 22 (69%) of them developed Graves ophthalmopathy. Only 4 (24%) of the 17 children with normal or indeterminate TSI levels developed Graves ophthalmopathy.
Based on the TSI results, the authors concluded that there is an apparent association between the initial levels of TSI and the development of Graves ophthalmopathy and suggested that TSI levels might be used as a predictor of ophthalmopathy in pediatric Graves disease patients.
Aside from the limitations of a retrospective analysis, which the authors have acknowledged, there is a potential bias of study design, as only patients with Graves disease who had titers of TSI taken at the time of diagnosis and an ophthalmic examination were included. This could lead to either an under-representation or an over-representation of patients with Graves ophthalmopathy. For example, the presence of Graves ophthalmopathy in a hyperthyroid patient confirms the diagnosis of Graves disease, but the physician may not order a TSI because he or she has made the diagnosis of Graves disease based on clinical grounds, thus posing a potential under-representation of patients with ophthalmopathy. Similarly, if the physician was not sure about the underlying cause of a patient’s hyperthyroidism, the physician may have ordered a TSI in patients with Graves disease but no signs of ophthalmopathy. On the other hand, a physician may decide to order a TSI because of the presence of Graves ophthalmopathy to follow the disease progression. This, in turn, could lead to an overrepresentation of patients with Graves ophthalmopathy in this study. The fact that patients needed to have at least one eye examination could also lead to an overrepresentation of patients with Graves ophthalmopathy. The primary physician may not send a patient with Graves disease devoid of clinical evidence of ophthalmopathy to the eye clinic for an evaluation. Finally, this study excluded patients with Graves disease who either did not have TSI levels ordered or who did not get an ophthalmic consult. The secondary question of the association between gender and Graves ophthalmopathy would be better addressed including those patients with an ophthalmic consult who did not get a TSI test.
In evaluating the data, the study reveals that the risk of developing Graves ophthalmopathy is higher when TSI is positive, since 69% of TSI-positive patients developed eye symptoms. However, the authors did not show whether a certain threshold level of TSI predicts the development of Graves ophthalmopathy. On the contrary, the absence of TSI reduces the risk but does not exclude the development of Graves ophthalmopathy. The study does not provide the information claimed by the authors in that they do not measure the actual level of TSI activity, but rather give a qualitative assessment of the presence or absence of TSI by designating the results as positive, indeterminate, or negative. The level of TSI activity, however, might be necessary to give the primary physician and ophthalmologist the tools to predict the clinical course.
If negative and indeterminate results were combined, then 13 of 23 cases judged to be negative for Graves ophthalmopathy were also negative by TSI, giving a specificity of 57%. Similarly, 22 of 26 cases positive for Graves ophthalmopathy were also positive on TSI screening, for a sensitivity of 85%. In this study, where the prevalence of ophthalmopathy among Graves patients is 50%, approximately 24% of those not testing positive are false negatives and about 31% of those testing positive are false positives. Thus, while a relationship between TSI and ophthalmopathy has been demonstrated, by itself it would not be an efficient screening test for ophthalmopathy among Graves disease patients. In short, TSI does not have great sensitivity or specificity.
The follow-up time for the whole group was 24.6 ± 37.5 months. The large standard deviation suggests that some patients had either very long or short follow-up times. One wonders if any of those who were negative for Graves ophthalmopathy were followed less than 6 months the maximum time for the development of ophthalmic signs in the positive cases. That is, could there have been patients who developed or will develop Graves ophthalmopathy after data collection for the study was terminated, and might they tend to be TSI negative?
If the authors had analyzed these data with Kaplan-Meier methods, which account for varying follow-up times, this would not have been a problem. This could be especially true since most of the Graves ophthalmopathy cases had ocular symptoms at the time of Graves disease diagnosis, and there was probably selective referral for an ophthalmology consultation. However, the finding is quite significant, and it would likely hold up if follow-up times were accounted for. It might be interesting to compare TSI test results among the Graves ophthalmopathy patients who did and did not present with ocular findings.
How does this study compare to the literature? The authors elected to use TSI as the benchmark test to correlate with the presence of Graves ophthalmopathy. The TSI is a functional assay with measurement of cAMP in response to TSH receptor interaction with a stimulating immunoglobulin. Not looked at in this study, but used in other studies, is the thyrotropin-binding inhibitory immunoglobulin (TBII). The TBII is a competitive assay between the antibody and thyrotropin (TSH) for binding to the TSH receptor, and that does not differentiate between stimulating and blocking antibodies. In a prospective study of 65 children, Shibayama and coworkers1 found that there was no difference in the TSI titers between patients with or without Graves ophthalmopathy. They also reported that TBII levels were significantly higher in patients with Graves ophthalmopathy.
I agree with the authors’ call for a prospective study to truly determine whether the quantitatively measured titers of TSI or TBII correlate with the development of Graves ophthalmopathy. The use of a uniform and validated thyroid-associated orbitopathy scale and a longitudinal follow-up period would be helpful to provide stronger evidence of this correlation.
ACKNOWLEDGMENTS
Funding/Support: None. Financial Disclosures: None.
REFERENCE
- 1.Shibayama K, Ohyama Y, Yokota Y, et al. Assays for thyroid-stimulating antibodies and thyrotropin-binding inhibitory immunoglobulins in children with Graves’ disease. Endocr J. 2005;52:505–510. doi: 10.1507/endocrj.52.505. [DOI] [PubMed] [Google Scholar]
DR. CAROL L. SHIELDS
No conflict of interest. Do the TSH receptor antibodies cross the placental barrier? This would be very useful to know. For the children who present with neonatal presumed thyroid disease the test could be performed not only in the child, but also in the mother.
DR. EVELYN A. PAYSSE
I would like to thank everyone for their comments. Dr. Tse, thank you for your very complete and comprehensive critique. With regard to your first question about potential bias, we had the ability to analyze the data from 49 of 182 patients. Because this was a retrospective study, we could only evaluate the data we had, and that is why a prospective study should be performed. With regard to bias in our institution, some endocrinologists always ordered TSI on all patients with Graves disease, and others obtained the test only if they suspected Graves ophthalmopathy or if the diagnosis was in question. For the most part, some never ordered the test and others always ordered it, so the selection bias may be somewhat less, but it is still present. Regarding the patients who underwent eye exams, but who were not tested for TSI levels, we did not have the ability to analyze them and I cannot answer that question. Regarding the classification of threshold TSI values versus categorical data, our laboratories reported the test results as positive, negative or indeterminate. Because two separate and unrelated labs performed the tests and reported different values with the percentiles that were recorded differently, we were unable to merge the data. Regarding the length of follow-up, most of our patients had long term follow-up. I agree with Dr. Tse in that we may have included patients with Graves ophthalmopathy who developed this condition after their follow-up period ended; however, most of them developed this finding within six months after of diagnosis. Shibayama investigated TBII, but we did not evaluate the effect of this finding since we did not have enough patients who were tested for this. Because our endocrinologists usually did not order this test, we did not have sufficient information to analyze. Regarding the question if TSI crosses the placenta, I do not know whether it does or not. I will investigate that in our neonatal patients with Graves disease.
ACKNOWLEDGMENTS
Funding/Support: Supported by an Unrestricted grant from Research to Prevent Blindness, Inc.
Financial Disclosures: None.
Author Contributions: Design and conduct of the study (O.M.A., E.A.P.); Collection of data (O.A., E.A.P.); Interpretation of data (O.M.A., I.A., E.A.P.); Preparation, review and approval of the manuscript (O.M.A., I.A., E.A.P.).
REFERENCES
- 1.Graves R. Clinical lectures (part II) London Med Surg J. 1835;7:516–517. [Google Scholar]
- 2.Uretsky SH, Kennerdell JS, Gutai JP. Graves’ ophthalmopathy in childhood and adolescence. Arch Ophthalmol. 1980;98:1963–1964. doi: 10.1001/archopht.1980.01020040815002. [DOI] [PubMed] [Google Scholar]
- 3.Chan W, Wong GW, Fan DS, et al. Ophthalmopathy in childhood Graves’ disease. Br J Ophthalmol. 2002;86:740–742. doi: 10.1136/bjo.86.7.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heufelder AE. Involvement of the orbital fibroblast and TSH receptor in the pathogenesis of Graves’ ophthalmopathy. Thyroid. 1995;5:331–340. doi: 10.1089/thy.1995.5.331. [DOI] [PubMed] [Google Scholar]
- 5.Paschke R, Vassart G, Ludgate M. Current evidence for and against the TSH receptor being the common antigen in Graves’ disease and thyroid associated ophthalmopathy. Clin Endocrinol (Oxf) 1995;42:565–569. doi: 10.1111/j.1365-2265.1995.tb02681.x. [DOI] [PubMed] [Google Scholar]
- 6.Crisp MS, Lane C, Halliwell M, Wynford-Thomas D, Ludgate M. Thyrotropin receptor transcripts in human adipose tissue. J Clin Endocrinol Metab. 1997;82:2003–2005. [PubMed] [Google Scholar]
- 7.Gupta MK. Thyrotropin receptor antibodies: advances and importance of detection techniques in thyroid diseases. Clin Biochem. 1992;25:193–199. doi: 10.1016/0009-9120(92)90302-9. [DOI] [PubMed] [Google Scholar]
- 8.Orgiazzi J. Anti-TSH receptor antibodies in clinical practice. Endocrinol Metab Clin North Am. 2000;29:339–355. vii. doi: 10.1016/s0889-8529(05)70135-3. [DOI] [PubMed] [Google Scholar]
- 9.Perrild H, Lavard L, Brock-Jacobsen B. Clinical aspects and treatment of juvenile Graves’ disease. Exp Clin Endocrinol Diabetes. 1997;105 (Suppl 4):55–57. doi: 10.1055/s-0029-1211934. [DOI] [PubMed] [Google Scholar]
- 10.Wong GW, Cheng PS. Increasing incidence of childhood Graves’ disease in Hong Kong: a follow-up study. Clin Endocrinol (Oxf) 2001;54:547–550. doi: 10.1046/j.1365-2265.2001.01252.x. [DOI] [PubMed] [Google Scholar]
- 11.Bartley GB, Fatourechi V, Kadrmas EF, et al. Chronology of Graves’ ophthalmopathy in an incidence cohort. Am J Ophthalmol. 1996;121:426–434. doi: 10.1016/s0002-9394(14)70439-8. [DOI] [PubMed] [Google Scholar]
- 12.Young LA. Dysthyroid ophthalmopathy in children. J Pediatr Ophthalmol Strabismus. 1979;16:105–107. doi: 10.3928/0191-3913-19790301-05. [DOI] [PubMed] [Google Scholar]
- 13.Bahn RS. Thyrotropin receptor expression in orbital adipose/connective tissues from patients with thyroid-associated ophthalmopathy. Thyroid. 2002;12:193–195. doi: 10.1089/105072502753600124. [DOI] [PubMed] [Google Scholar]
- 14.Saravanan P, Dayan CM. Thyroid autoantibodies. Endocrinol Metab Clin North Am. 2001;30:315–337. viii. doi: 10.1016/s0889-8529(05)70189-4. [DOI] [PubMed] [Google Scholar]
- 15.Costagliola S, Many MC, Denef JF, Pohlenz J, Refetoff S, Vassart G. Genetic immunization of outbred mice with thyrotropin receptor cDNA provides a model of Graves’ disease. J Clin Invest. 2000;105:803–811. doi: 10.1172/JCI7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dragan LR, Seiff SR, Lee DC. Longitudinal correlation of thyroid-stimulating immunoglobulin with clinical activity of disease in thyroid-associated orbitopathy. Ophthal Plast Reconstr Surg. 2006;22:13–19. doi: 10.1097/01.iop.0000192649.23508.f7. [DOI] [PubMed] [Google Scholar]
- 17.Kazuo K, Fujikado T, Ohmi G, Hosohata J, Tano Y. Value of thyroid stimulating antibody in the diagnosis of thyroid associated ophthalmopathy of euthyroid patients. Br J Ophthalmol. 1997;81:1080–1083. doi: 10.1136/bjo.81.12.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerding MN, van der Meer JW, Broenink M, Bakker O, Wiersinga WM, Prummel MF. Association of thyrotrophin receptor antibodies with the clinical features of Graves’ ophthalmopathy. Clin Endocrinol (Oxf) 2000;52:267–271. doi: 10.1046/j.1365-2265.2000.00959.x. [DOI] [PubMed] [Google Scholar]
- 19.Shibayama K, Ohyama Y, Yokota Y, Ohtsu S, Takubo N, Matsuura N. Assays for thyroid-stimulating antibodies and thyrotropin-binding inhibitory immunoglobulins in children with Graves’ disease. Endocr J. 2005;52:505–510. doi: 10.1507/endocrj.52.505. [DOI] [PubMed] [Google Scholar]
- 20.Khoo DH, Ho SC, Seah LL, et al. The combination of absent thyroid peroxidase antibodies and high thyroid-stimulating immunoglobulin levels in Graves’ disease identifies a group at markedly increased risk of ophthalmopathy. Thyroid. 1999;9:1175–1180. doi: 10.1089/thy.1999.9.1175. [DOI] [PubMed] [Google Scholar]
- 21.Durairaj VD, Bartley GB, Garrity JA. Clinical features and treatment of graves ophthalmopathy in pediatric patients. Ophthal Plast Reconstr Surg. 2006;22:7–12. doi: 10.1097/01.iop.0000195006.08929.46. [DOI] [PubMed] [Google Scholar]


