Abstract
Purpose
Leber hereditary optic neuropathy (LHON) results from point mutations in mitochondrial DNA (mtDNA) present in all cells but is only manifested in retinal ganglion cells (RGCs). Given that RGCs use superoxide for intracellular signaling after axotomy, and that LHON mutations increase superoxide levels in non-RGC transmitochondrial cybrids, I hypothesized that RGCs regulate superoxide levels differently than other neuronal cells.
Methods
Superoxide production in mitochondria isolated from the RGC-5 cell line, rat brain, or neuroblastoma SK-N-AS cells was measured and correlated with levels of mitochondrial electron transport chain (METC) complexes.
Results
The rate of superoxide production in brain mitochondria was more than 5 times the rate in RGC-5 cells when complex I substrates were used. Rotenone significantly increased the rate of superoxide production in brain but not RGC-5 mitochondria. Succinate-dependent superoxide production was similar in brain and RGC-5 mitochondria, but was increased by the complex III inhibitor antimycin A only in brain cells. Neuroblastoma mitochondria demonstrated similar superoxide generation rates as brain cells. Lower rates of superoxide production probably reflected lower levels of METC components.
Conclusions
These results demonstrate that RGC-5 mitochondria produce superoxide at significantly lower rates than brain mitochondria. Tighter regulation of superoxide levels in RGCs would prevent aberrant apoptosis signaling. LHON mtDNA mutations may interfere with superoxide regulation, possibly leading to aberrant RGC death and consequent optic neuropathy.
INTRODUCTION
HISTORY
In 1871, Theodor Leber of the University of Göttingen described 55 patients in 16 families with a hereditary optic neuropathy of rapid onset,1 subsequently named Leber hereditary optic neuropathy (LHON). His patients were mostly male, had visual loss beginning in the late teens or early 20s, and did not recover. Subsequently several pedigrees with similar clinical findings were described, almost all with a peculiar mode of inheritance from mother to affected son or mother to carrier daughter. Initially considered an X-linked recessive disorder, the greater-than-expected occurrence in women and much less-than-expected occurrence in maternal grandfathers of affected males suggested an alternate mechanism for transmission.2 In retrospect, many apparent cases of transmission from father to child were probably other hereditary optic neuropathies. Cytoplasmic transmission via the ovum was suggested in 1936,3 and the knowledge that mitochondrial DNA (mtDNA) is maternally inherited4 led to the discovery by Wallace and colleagues5 that many cases of LHON are due to a mutation at position 11778 of the mitochondrial genome, which codes for the ND4 component of complex I in the mitochondrial electron transport chain (METC). Subsequently, mutations at positions 34606 (ND1) and 144847 (ND6) have been shown to be associated with LHON in multiple pedigrees. A mutation at position 14459 (ND6) is associated with LHON and dystonia.8 Many other mtDNA mutations are associated with LHON, but are not always pathogenic (eg, 152579).
CLINICAL BACKGROUND
Leber hereditary optic neuropathy is the most common hereditary optic nerve disease causing severe visual loss. As mentioned above, it is a maternally inherited optic neuropathy typically characterized by decreased visual acuity, a visual field defect involving the blind spot and central fixation (cecocentral scotoma), and a characteristic hyperemic pseudoedema of the optic disc, which does not leak on fluorescein angiography. Fine telangiectatic vessels are often present at the disc margins. Most commonly one eye is involved, and the other eye becomes involved from weeks to months later; acute bilateral disease is seen in about one-fourth of patients.10 In some patients the visual loss may be indolent.11 Instead of disc swelling, there may simply be optic atrophy, or even cupping.12,13 The visual fields may be atypical, eg, bitemporal hemianopsia.11 The degree of visual loss may be particularly severe, with most patients seeing 20/200 or worse.14,15 Visual loss is typically rapid, progressing over days to weeks. While LHON is usually painless, some patients may have discomfort reminiscent of optic neuritis.10 Occasional patients may spontaneously recover vision after a variable interval, especially those with the 14484 mutation.
There is a strong male predominance in patients with LHON,15 the degree depending on the specific mutation. The age at onset is usually in the second or third decade but has been reported as early as age 416 and as late as age 73.17 The age at onset does not differ significantly between the different mutations.10
UNUSUAL FEATURES OF LHON
From a neuro-ophthalmic point of view, LHON has several peculiarities that are not explained on the basis of a pathogenic mechanism. First, it is unclear why the disease arises most commonly at a particular age (second and third decades) and in a particular gender (male). Second, when the disease occurs, it is unclear why both eyes are affected simultaneously or sequentially in all cases. Third, why is a particular type of visual field defect (cecocentral scotoma) usually seen, unlike the more common central scotoma or nerve fiber bundle defects seen with more common optic neuropathies? Fourth, why does spontaneous improvement occur in some patients, even years after the loss of vision?
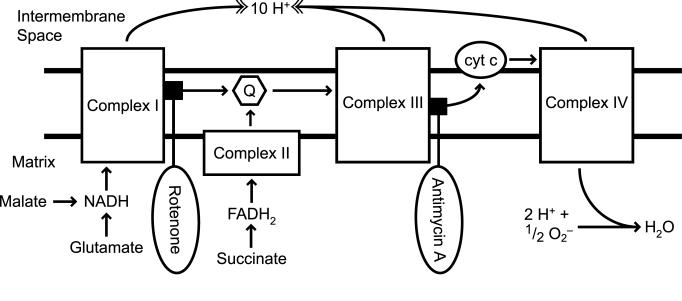
But the most peculiar feature of LHON is the relatively selective involvement of retinal ganglion cells (RGCs). Although it is not uncommon for LHON mutations to be associated with a multiple sclerosis–like illness,18–20 the primary clinical symptom complex in LHON is optic neuropathy, a disorder of RGCs. Maternally inherited, LHON results from point mutations in mtDNA. The primary mutations code for proteins in complex I of the METC, which subserves oxidative phosphorylation-mediated production of ATP (Figure 1). The fundamental paradox of LHON is that in most cases, although all cells in the body have the same mtDNA mutations, the disease is almost exclusively manifested in the RGCs, the axons of which make up the optic nerve. The reason for this is unclear, and is the subject of this thesis. I hypothesize that RGC involvement in LHON is a result of a cell type-specific susceptibility of RGCs to the effect of the complex I mutations seen in the disease.
FIGURE 1.
Mitochondrial electron transport chain (METC) substrates and inhibitors. Oxidative phosphorylation involves the creation of a concentration gradient of protons (H+) passage of electrons along complexes located in the inner membrane of the mitochondria. The source of these electrons at complexes I and III are NADH and FADH2. Intermediates of the tricarboxylic acid cycle are responsible for providing the reducing power required for METC function along with the malate-aspartate shuttle system. Rotenone blocks the passage of electrons from Fe-S clusters to ubiquinone (Q). Antimycin A blocks electron flow from cytochrome bH to a semiquinone intermediate.
THE BIOCHEMICAL EFFECTS OF LHON MUTATIONS
Complex I activity in mitochondria is critical for RGC survival. Seminal work by the laboratory of Guy and colleagues has used adeno-associated virus to overexpress or knock down (via ribozymes) critical genes in RGCs and produce a LHON phenotype.21–23 Knockdown of the mitochondrial-specific superoxide dismutase-2 (SOD-2)21 or functional interference with complex I activity by knocking down a nuclear-encoded subunit23 both produce an optic neuropathy. However, this important work does not directly address the role of the LHON mutations. To study the effect of LHON mutations, many groups have produced transmitochondrial cybrids in human cell lines. Cybrids are cytoplasmic hybrids, ie, cells that have fusions of cytoplasms from two different parental cells. Transmitochondrial cybrids are fusions where the mitochondria are derived from one type of cell and the nucleus and other organelles are from a second type of cell. Three steps are required to accomplish this process.
First, cells that contain mitochondria but no nuclei are purified. Platelets are usually used for this purpose. The platelets are usually obtained from a subject (or animal strain, in the case of nonhuman cells) that has the mtDNA mutation in question. Second, completely different cells are depleted of mtDNA by culturing them for extended periods in ethidium bromide. These cells are usually fibroblasts or derived from a cancer cell line. For ethidium bromide to deplete mtDNA, it is necessary that the cells actively divide in culture, as the drug works by causing mutations in all replicating DNA. Unlike nuclear DNA, which has excellent DNA repair mechanisms, mtDNA accumulate the ethidium bromide–induced mutations, whereas in most cases the nuclear DNA is relatively preserved. The product of this procedure is a line of cells called ρ0 (pronounced “rho-zero”). In the third step, the mutant mtDNA-containing cells from the first step are fused with the mtDNA-depleted ρ0 cells from the second step, resulting in a line of dividing cells that contain the mtDNA mutation.
Studies with LHON cybrids have demonstrated up-regulation of some mtDNA transcripts, most notably that of aldose reductase.24 In addition, LHON cybrids forced to use the METC as their primary energy source, through glucose-deficient galactose media, experienced apoptotic death and increased cytochrome C release.25 It has been suggested that apoptosis in LHON cybrids is caspase-independent.26 However, other work shows that apoptosis is blocked by the caspase inhibitor zVAD-fmk (zVal-Ala-Asp-fluoro-methyl ketone).27 Common LHON mutations reduce the rate of respiration in LHON cybrids and oxygen consumption.28,29 The production of LHON cybrids has been possible only in cells other than RGCs, because producing the ρ0 state requires a dividing cell, and RGCs are postmitotic neurons.
Given that METC complexes I and III are the main sources of basal superoxide production,30,31 it is possible that aberrant production of superoxide from mutated METC components would cause cell death. But why would abnormalities in superoxide anion production be a mechanism for the relatively specific effect of LHON mtDNA mutations on RGCs? One explanation comes from research demonstrating that RGCs use superoxide as an intracellular signal for initiating the apoptosis program after axonal injury,32,33 probably by oxidizing critical sulfhydryls in signaling macromolecules.34,35 A second line of evidence is that increasing mitochondrial superoxide levels by knocking down mitochondrial SOD-2 increases RGC death.21
HYPOTHESIS TESTED IN THE PRESENT STUDY
Given that RGCs use superoxide for intracellular signaling, that they are specifically affected in LHON, a disease of specific complex I mtDNA mutations, and that these mutations lead to elevated superoxide, it is possible that the RGC-specific involvement in LHON results from RGC-specific features of superoxide regulation within the cell. To study this, my research project tested the following hypothesis: Superoxide production in isolated RGC mitochondria differs from production in other neuronal mitochondria.
Performing these experiments took advantage of two recent advances. The first is a novel method for differentiating the RGC-5 cell line into RGCs.36 Mitochondria cannot be isolated in bulk from RGCs, because it would require hundreds of animals per experiment. Use of RGC-5 cells in bulk culture obviates this concern. A second advance was the ability to optimize a previously published microassay for superoxide from isolated mitochondria, using the conversion of superoxide into hydrogen peroxide (H2O2) by SOD-2.
If mitochondria derived from RGCs were qualitatively different from brain and neuroblastoma-derived mitochondria with respect to superoxide production in the presence of complex-specific METC substrates and inhibitors, then this tighter regulation of superoxide levels presumably might prevent aberrant apoptosis signaling.33 In accordance with this hypothesis, SOD-2 knockdown in RGCs also causes their death, a finding observed in vivo by Qi and colleagues.21 Given that superoxide production is disturbed with LHON mutations,37 its aberrant production in the specifically sensitive RGC could account for the selective RGC death seen in LHON.
METHODS
ANIMALS
Brain mitochondria were obtained from the cerebral cortex of euthanized adult Long-Evans rats. All other experiments were performed with established cell lines. Animals were used in accordance with federal, state, Association for Research in Vision and Ophthalmology (ARVO), and institutional guidelines for the use of animals in laboratory research.
MATERIALS
Staurosporine (from Streptomyces staurosporeus; ≥98% purity; catalog number 380–014) was obtained from Alexis Biochemicals (San Diego, California). Cell culture reagents, unless noted, were obtained from BioWhittaker (Rockland, Maine). Unless specified, antibodies and fluorescent dyes were obtained from Molecular Probes (Eugene, Oregon). Rotenone and dimethylsulfoxide (DMSO) were obtained from Sigma (St Louis, Missouri). Antimycin A was obtained from Fisher Scientific (Hampton, New Hampshire).
SOLUTIONS
Amplex Red solution consisted of 100 μM Amplex Red reagent, 0.2 U/mL horseradish peroxidase (HRP), and 0.05 M sodium phosphate. Mannitol, tris-HCl, and potassium chloride (MTP) solution consisted of 110 mM mannitol, 60 mM Tris-HCl, 60 mM KCl, 10 mM KH2PO4, 0.5 mM EDTA, pH 7.4.
CELL CULTURE AND DIFFERENTIATION
Dr Neeraj Agarwal of the University of North Texas generously provided the RGC-5 cell line. RGC-5 cells were cultured in DMEM (Mediatech, Inc, Herndon, California) containing 1 gm/L glucose with L-glutamine, supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were incubated at 37°C in humidified 5% CO2. To induce differentiation, RGC-5 cells were treated with staurosporine (316 nM) 24 hours prior to mitochondria isolation. The neuroblastoma SK-N-AS line was obtained from Dr Arthur Polans of the University of Wisconsin (Madison). Cells were cultured in RPMI 1640, supplemented with 10% fetal bovine serum, 10 mM HEPES, penicillin 100 U/mL, streptomycin 100 μg/mL, and amphotericin B 0.25 μg/mL.
ISOLATION OF RGC-5 AND NEUROBLASTOMA SK-N-AS MITOCHONDRIA
Cells were treated with trypsin-EDTA to allow detachment from growing flasks, centrifuged 7 minutes at 250g at 4°C. Mitochondria were isolated from the pellet using the Pierce Mitochondria Isolation Kit for Mammalian Cells (Pierce Biotechnology, Rockford, Illinois; Prod No. 89874) using the Reagent-Based Protocol. Briefly, cells were treated with reagents in the presence of Pierce Halt Protease Inhibitor Cocktail, EDTA-free (Prod No. 78415) and were subject to a series of graded centrifugations. To maintain the integrity of the mitochondria, samples were kept on ice during the isolation process. The mitochondrial pellet was suspended in MTP medium and remained on ice until analysis of mitochondrial activity was performed.
ISOLATION OF BRAIN MITOCHONDRIA
Long-Evans rats were euthanized with CO2 gas, and approximately 200 mg of the frontal cortex was removed. The tissue was washed with ice-cold phosphate-buffered saline (PBS) and a mitochondrial isolation kit for soft tissues (Pierce, Prod No. 89801) was used. Briefly, the brain tissue was cut into small pieces and added to a cold Dounce homogenizer containing PBS. The tissue was homogenized using 7 to 10 strokes of the homogenizer. The homogenate was then centrifuged per protocol and the resultant mitochondrial pellet suspended in MTP and kept on ice until analyzed.
PROTEIN CONTENT OF MITOCHONDRIAL PREPARATIONS
Isolated mitochondria pellets were suspended in MTP, and the protein content of the isolated mitochondria was determined by Bradford assay, using bovine serum albumin as a standard. For each experiment, protein concentrations were adjusted so that each mitochondrial sample had the same protein content.
MITOTRACKER GREEN FM QUANTIFICATION OF MITOCHONDRIAL PREPARATIONS
The fluorophore MitoTracker Green FM was used as a secondary method to verify that the mitochondrial content was similar. MitoTracker Green FM becomes a fluorescent thiol-conjugate (excitation 590 nm/emission 515 nm) after oxidation in the lipid membrane of mitochondria. Solutions containing mitochondria were treated with MitoTracker Green FM (0.5 μM) in a 1:1 ratio and were allowed to aggregate fluorescent product for 30 minutes in a 96-well plate. Fluorescence was then compared between cell type mitochondria using a Wallac Victor2 1420 multilabel counter with appropriate filters (excitation 545 nm/emission 580 nm).
COMPLEX I THROUGH IV AND SOD-2 CONTENT OF MITOCHONDRIAL PREPARATIONS
Mitochondrial enriched and depleted (ie, cytoplasm) samples obtained from the final supernatant of the mitochondria isolation procedure were stored in NuPage lithium dodecyl sulfate (LDS) sample buffer with reducing agent (Invitrogen, Carlsbad, California). The protein concentrations of the isolated mitochondria were determined by Bradford assay, and equal amounts of protein for each mitochondrial preparation were boiled in the presence of 4× LDS sample buffer (Invitrogen) plus 5% β-mercaptoethanol, resolved on a Bis-Tris 4% to 12% polyacrylamide gel (NuPAGE; Invitrogen), and transferred overnight at 50 mA to nitrocellulose membrane in a transfer apparatus (Mini Protean II; Bio-Rad Laboratories, Hercules, California). After transfer, the membrane was blocked with 5% nonfat milk in Tris-buffered saline (TBS; pH 8.0) for 60 minutes and then probed with one of the following mouse monoclonal antibodies, all from Invitrogen except for SOD-2, which was from Upstate Biotechnology (Lake Placid, New York):
| Antigen | Expected Size (kD) | Subunit | Concentration (μg/mL) |
|---|---|---|---|
| Complex I | 42.5 | Alpha subcomplex | 0.5 |
| Complex II | 72.7 | Flavoprotein | 0.1 |
| Complex III | 48.5 | Core II | 0.4 |
| Complex IV | 57 | COX I | 2.0 |
| SOD-2 | 24 | – | 0.5 |
Blots were rinsed 3 times with TBS containing 0.05% Tween-20 (Fisher Scientific), then washed 5 times for 10 minutes each at room temperature on an orbital shaker. Secondary antibodies used were purified HRP-conjugated goat anti-mouse IgG or HRP-conjugated goat anti-rabbit IgG (1:5000; Jackson ImmunoResearch Laboratories, West Grove, Pennsylvania), with incubations for 1 hour at room temperature, followed by 3 rinses and five 10-minute washes with TBS containing Tween-20 at room temperature on an orbital shaker. Blots were treated with freshly prepared ECL solution containing 100 mM Tris-HCl (pH 8.5), 1.25 mM luminol, 225 μM p-coumaric acid (Sigma-Aldrich), and 1 mM H2O2 (Fisher Scientific) for 1 minute, and excess solution was allowed to drip off. The blots were then exposed to film (BioMax XAR; Eastman Kodak Company, Rochester, New York) and developed. The films were scanned at 1600 dpi, and band density was determined by calculating the integrated intensity of the area containing the band of interest and subtracting the intensity of an equal sized area of background, using NIH ImageJ software. Band density readings are presented with respect to the density of the band from the control, untreated cell condition.
SUPEROXIDE MEASUREMENTS
Superoxide was indirectly measured by assaying H2O2,38,39 the product of superoxide dismutation by mitochondrial SOD-2. The fluorescent product of Amplex Red and H2O2 was analyzed in various conditions in duplicate to quadruplicate. All assays were performed in 96-well plates and read with a Wallac Victor2 1420 multilabel counter using the appropriate filters (excitation 535 nm/emission 580 nm). To each well, Amplex Red solution was added and a time-lapse fluorescent baseline was established in the absence of mitochondria. Consecutively, equal volumes of brain, RGC-5, or neuroblastoma mitochondria, a substrate (glutamate/malate or succinate), and an inhibitor of the METC (rotenone or antimycin A) were added to wells containing Amplex Red. Fluorescence determinations were made after each addition. All figures are expressed in nmol/min/mg protein, assuming full superoxide dismutation. All experiments were repeated 2 to 4 times.
STATISTICAL ANALYSIS
Comparisons between two groups were by the Student unpaired t test. Significant differences required P < .05.
RESULTS
MITOCHONDRIA CAN BE ISOLATED IN BULK FROM RGC-5 CELLS
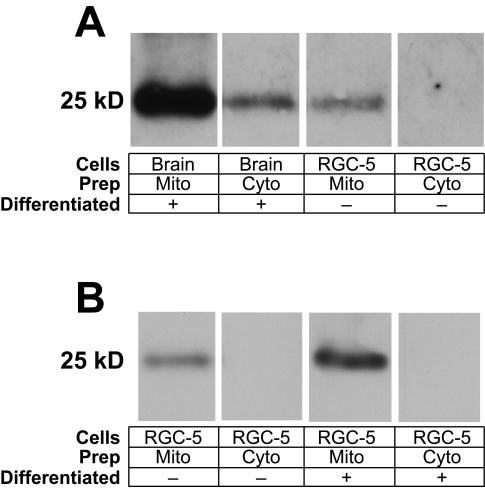
Because RGCs are postmitotic and are present in relatively small (105 cells/retina in the rat) numbers, it is impractical to biochemically study RGC mitochondria in bulk. Instead, the present study used the RGC-5 cell line, which when differentiated has many morphological similarities to RGCs.36 RGC-5 cells were grown in multiple tissue culture flasks, and mitochondria were isolated. To verify mitochondrial enrichment, mitochondrial-enriched and cytosolic fractions were immunoblotted for the METC complex IV protein cytochrome c oxidase (Figure 2A). For each tissue, there was enrichment of mitochondria, but the depletion was not complete, eg, residual immunostaining of COX in the brain cytoplasmic sample. For the same amount of loaded protein from each tissue, there was 16 times more cytochrome c oxidase in brain compared to RGC-5 cells, as measured by densitometry, suggesting that cerebral cortex may have slightly more METC complex IV than RGCs. RGC-5 cells differentiated with staurosporine had 15.2% more cytochrome c oxidase than undifferentiated RGC-5 cells (Figure 2B).
FIGURE 2.
Immunoblotting measurement of cytochrome c oxidase. Isolated mitochondrial samples standardized for protein content by Bradford assay were compared to corresponding mitochondria-depleted samples for the presence of the mitochondrial transmembrane complex IV protein cytochrome c oxidase (COX). Samples were subject to electrophoresis and transferred overnight to nitrocellulose. Blots were incubated with a mouse anti-COX antibody and then goat anti-mouse horseradish peroxidase-conjugated antibody. There is considerable purification of mitochondria compared to the mitochondria-depleted samples for brain, undifferentiated RGC-5 cells, and undifferentiated RGC-5 cells. There is a greater amount of COX per mg protein present in mitochondria isolated from brain than that from undifferentiated RGC-5 per unit weight. There is somewhat more COX present in mitochondria isolated from differentiated RGC-5 cells compared to undifferentiated RGC-5 cells. For each tissue, there is enrichment of mitochondria in the “Mito” samples and relative depletion in the “Cyto” samples. The depletion is not complete, as manifested by residual immunostaining of COX in the brain cytoplasmic sample.
Mitochondria were also quantitated using the fluorophore MitoTracker Green FM, which reacts with mitochondrial free sulfhydryls. Mitochondrial samples from RGC-5 cells and brain exhibited similar relative fluorescence values per mg protein (data not shown).
BRAIN AND RGC-5 CELLS DIFFER IN SUPEROXIDE PRODUCTION AFTER COMPLEX I INHIBITION
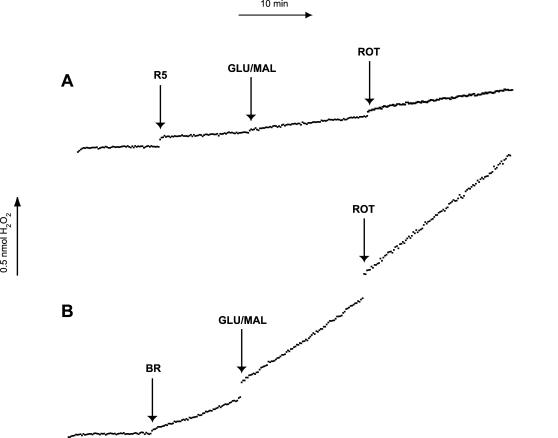
Superoxide was indirectly measured by detecting H2O2 generated as a result of dismutation of superoxide by mitochondrial SOD-2.38,39 The results of a typical experiment are depicted in Figure 3. The basal level of superoxide production by RGC-5 mitochondria in the absence of substrate ranged from 0.030 ± 0.004 nmol/min/mg protein, approximately one-seventh that measured in brain mitochondria (0.208 ± 0.033; P = .0017). Mitochondria were then incubated with glutamate (10 mM) and malate (5 mM), which yields NADH and serves as a substrate for complex I. In the presence of glutamate/malate, brain (0.262 ± 0.028; P = .0042 compared to no substrate) and RGC-5 (0.046 ± 0.004; P = .0005 compared to no substrate) mitochondria significantly increased superoxide production. They were then treated with the complex I inhibitor rotenone (6.7 μM). There was an insignificant change in the rate of superoxide production after the addition of the complex I inhibitor rotenone in brain (0.279 ± 0.035; P = .40 compared to glutamate/malate alone) and RGC-5 mitochondria (0.048 ± 0.004; P = .24). The rates of superoxide production per mg protein in all conditions were significantly lower in RGC-5 mitochondria than in brain mitochondria (Table 1).
FIGURE 3.
Superoxide production in brain and RGC-5 mitochondria after complex I inhibition. Mitochondria isolated from brain (BR) and undifferentiated RGC-5 (uR5) cells were standardized for protein content, and 50 μL was added to a well in a 96-well plate containing 50 μL Amplex Red (AR). Prior to the addition of solutions containing mitochondria, a baseline of superoxide production was established by measuring the fluorescent product of H2O2 and AR, resorufin at approximately 10-second intervals. In a similar manner, production of superoxide was analyzed after the addition of the complex I substrates glutamate/malate (GLU/MAL) and the complex I inhibitor rotenone (ROT). The mitochondrial superoxide production levels after the addition of glutamate/malate in brain cells were significantly higher than that of undifferentiated RGC-5 cells during basal metabolism, in the presence of glutamate/malate and after complex I inhibition with rotenone.
TABLE 1.
SUPEROXIDE PRODUCTION IN MITOCHONDRIA FROM BRAIN, UNDIFFERENTIATED RGC-5 CELLS, AND NEUROBLASTOMA SK-N-AS CELLS IN THE PRESENCE OF A COMPLEX I SUBSTRATE AND INHIBITOR*
| VARIABLE | BRAIN | UNDIFFERENTIATED RGC-5 CELLS | SK-N-AS NEUROBLASTOMA CELLS |
|---|---|---|---|
| N = 7 | N = 8 | N = 7 | |
| Basal respiration | 0.208 ± 0.033 | 0.030 ± 0.004 | 0.318 ± 0.035 |
| Glutamate/malate | 0.262 ± 0.028 | 0.046 ± 0.004 | 0.560 ± 0.054 |
| Glutamate/malate + rotenone | 0.279 ± 0.035 | 0.048 ± 0.004 | 0.383 ± 0.050 |
The rates of superoxide production were determined after the addition of the complex I substrates glutamate (10 mM) and malate (5 mM) and the complex I inhibitor rotenone (6.7 μM). Superoxide was indirectly measured using the H2O2 probe Amplex Red in the presence of horseradish peroxidase. The values are given in nmol H2O2/min/mg protein ± standard error of the mean, with N being the number of independent samples for each mitochondria type.
BRAIN AND RGC-5 MITOCHONDRIA DIFFER IN SUPEROXIDE PRODUCTION AFTER COMPLEX III INHIBITION
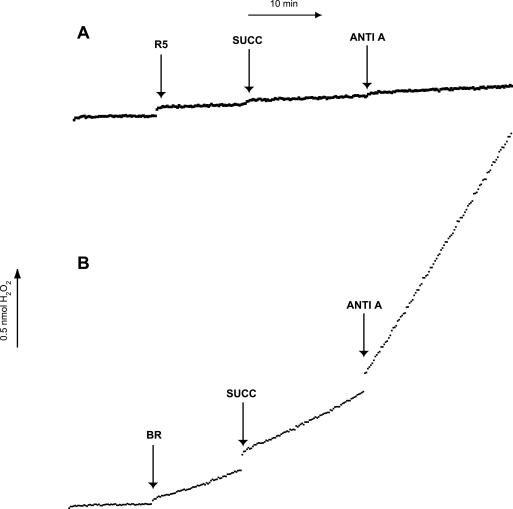
Mitochondria were incubated with succinate (10 mM), which yields FADH2 and serves as a substrate for complex II. The results of a typical experiment are depicted in Figure 4. In the presence of succinate, brain mitochondria exhibited a significant increase in superoxide production rate (0.193± 0.027 to 0.216 ± 0.022; P = .04) as did RGC-5 mitochondria (0.028 ± 0.005 to 0.031 ± 0.005; P = .024). Mitochondria were then treated with the complex III inhibitor antimycin A (0.5 μM). There was a significant increase in the rate of superoxide production after the addition of antimycin A in brain (0.540 ± 0.116; P = .023 compared to substrate alone), but not RGC-5 mitochondria (0.024 ± 0.004; P = .278). The rates of superoxide production in all conditions were substantially greater in brain mitochondria than in RGC-5 mitochondria (Table 2).
FIGURE 4.
Superoxide production in brain and RGC-5 mitochondria after complex III inhibition. Mitochondria isolated from brain (BR) and undifferentiated RGC-5 cells (uR5) were normalized for protein content, and superoxide production was analyzed after the addition of the complex II substrate succinate (SUCC) and the complex III inhibitor antimycin A (ANTI-A). The mitochondrial superoxide production levels in brain cells were significantly higher than that of undifferentiated RGC-5 cells in the presence of succinate and after complex III inhibition with antimycin A. Both succinate and antimycin A elicited minimal superoxide production in undifferentiated RGC-5 cells, whereas in brain cells there was a fourfold increase in superoxide production after the addition of antimycin A.
TABLE 2.
SUPEROXIDE PRODUCTION IN MITOCHONDRIA FROM BRAIN, UNDIFFERENTIATED RGC-5 CELLS, AND NEUROBLASTOMA SK-N-AS CELLS IN THE PRESENCE OF A COMPLEX II SUBSTRATE AND A COMPLEX III INHIBITOR*
| VARIABLE | BRAIN | UNDIFFERENTIATED RGC-5 CELLS | SK-N-AS NEUROBLASTOMA CELLS |
|---|---|---|---|
| N = 7 | N = 8 | N = 7 | |
| Basal respiration | 0.193 ± 0.027 | 0.028 ± 0.005 | 0.348 ± 0.042 |
| Succinate | 0.216 ± 0.022 | 0.031 ± 0.005 | 0.307 ± 0.024 |
| Succinate + antimycin A | 0.540 ± 0.116 | 0.024 ± 0.004 | 0.277 ± 0.034 |
The rates of superoxide production were determined after the addition of the complex II substrate succinate (10 mM) and the complex III inhibitor antimycin A (0.5 μM). Superoxide was indirectly measured using the H2O2 probe Amplex Red in the presence of horseradish peroxidase. The values are given in nmol H2O2/min/mg protein ± standard error of the mean, with N being the number of independent samples for each mitochondria type.
DIFFERENTIATION OF RGC-5 CELLS AFFECTS SUPEROXIDE PRODUCTION IN RESPONSE TO COMPLEX III BUT NOT COMPLEX I INHIBITION
Basal mitochondrial superoxide production was similar in undifferentiated and differentiated RGC-5 cells (0.030 ± 0.004 vs 0.033 ± 0.004; P = .60). Mitochondria from undifferentiated and differentiated RGC-5 were incubated with glutamate/malate and subsequently treated with rotenone. There was no significant difference between differentiated and undifferentiated RGC-5 cells in the production of superoxide after treatment with glutamate/malate (0.046 ± 0.004 vs 0.052 ± 0.003; P = .30) or rotenone (0.048 ± 0.004 vs 0.055 ± 0.002; P = .18). Mitochondria from undifferentiated and differentiated RGC-5 did not significantly differ in rates of superoxide production when incubated with the complex II substrate succinate (0.031 ± 0.005 vs 0.037 ± 0.001; P = .30). However, addition of antimycin A resulted in somewhat more superoxide production in differentiated but not undifferentiated RGC-5 cells (0.040 ± 0.002 vs 0.024 ± 0.004; P = .001). Nonetheless, mitochondria from differentiated RGC-5 cells had much lower superoxide production than cerebral mitochondria under all treatment conditions.
RGC-5 CELLS GENERATE SIGNIFICANTLY LESS SUPEROXIDE THAN NEUROBLASTOMA SK-N-AS CELLS WITH COMPLEX I BUT NOT COMPLEX II SUBSTRATES
The differences between superoxide generation from RGC-5 and brain mitochondria could theoretically reflect differences in the source of cells, the former being a cultured cell line and the latter being fresh tissue. To rule out this possibility, superoxide generation in RGC-5 mitochondria was compared to another neuronal cell line, the SK-N-AS neuroblastoma line. As with cerebral cells, the basal superoxide production was much lower in RGC-5 cells compared to neuroblastoma cells (0.030 ± 0.004 vs 0.318 ± 0.035; P = .0002). Superoxide generation from RGC-5 and SK-N-AS cells was measured after the addition of glutamate/malate. There was a much greater increase in superoxide production after the addition of glutamate/malate to SK-N-AS mitochondria (0.560 ± 0.054; P < .0001 compared to no substrate) than RGC-5 mitochondria (0.064 ± 0.008 to 0.108 ± 0.008; P = .003). After the addition of rotenone, there was a significant decrease in superoxide production in SK-N-AS cells (0.383 ± 0.050; P < .0001 compared to substrate alone) but not RGC-5 cells. SK-N-AS mitochondria experienced an insignificant decrease in superoxide production after the addition of succinate (0.348 ± 0.042 to 0.307 ± 0.024; P = .08), whereas RGC-5 mitochondria did not undergo a change in superoxide production. The addition of antimycin A to neuroblastoma mitochondria resulted in a small but significant decrease in superoxide production (0.277 ± 0.034; P = .026 compared to substrate alone), similar to RGC-5 mitochondria but different from the increase in superoxide seen in cerebral mitochondria. In all cases, the rates of superoxide production by neuroblastoma mitochondria were much higher than RGC-5 mitochondria.
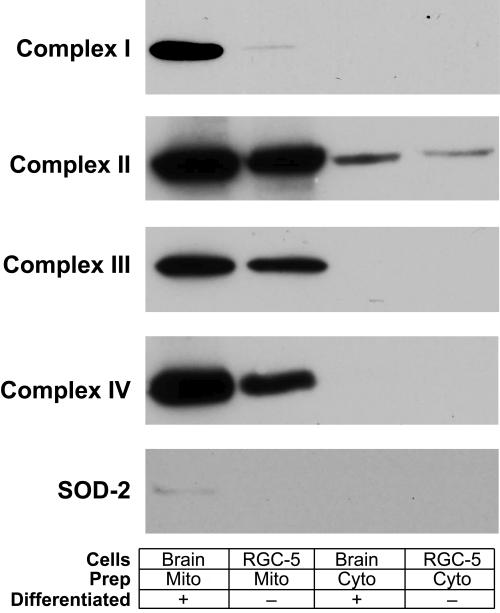
RELATIVE AMOUNTS OF METC COMPONENTS DIFFER BETWEEN RGC-5 CELLS AND BRAIN
Lower rates of superoxide production in RGC-5 mitochondria could reflect either lower superoxide production or greater scavenging activity. To differentiate these possibilities, equal amounts of mitochondrial proteins from RGC-5 cells and rat cerebral cortex were immunoblotted for components of complexes I through IV (including cytochrome c oxidase, already performed in the validation of the mitochondrial preparations) and SOD-2 (Figure 5). Overall, there were lower amounts of all METC components in RGC-5 cells compared to brain, after loading equivalent amounts of mitochondrial proteins. There was far less complex I and SOD-2 in RGC-5 cells than brain, whereas the levels of complexes II and III were less dissimilar and the level of complex IV was intermediate. Shorter exposures of the film to the blot in the chemiluminescence reaction demonstrated relatively less discrepancy between samples (data not shown), but it persisted, suggesting that RGC-5 cells have different relative proportions of METC components compared to brain.
FIGURE 5.
Differential expression of mitochondrial electron transport chain components in brain and RGC-5 cells. Isolated rat brain and RGC-5 mitochondria-enriched samples standardized for protein content by Bradford assay were compared to cytoplasmic samples for subunits of mitochondrial complexes I through IV and superoxide dismutase-2 (SOD-2). Samples were electrophoresed and transferred overnight to nitrocellulose, then incubated with appropriate primary and horseradish peroxidase-conjugated secondary antibodies. RGC-5 cells had less complex I through IV components and SOD-2 than brain, but the difference was greatest with respect to complex I and SOD-2, and to a lesser extent complex IV.
DISCUSSION
These studies demonstrate that RGC mitochondria can be isolated for metabolic studies using the RGC-like RGC-5 cell line as a source. When incubated with METC substrates and treated with complex I or III inhibitors, RGC-5, brain, and neuroblastoma mitochondria significantly differed in their production of superoxide, suggesting that neuronal mitochondrial bioenergetics differ based on the cell of origin. To see if differentiation affected reactive oxygen species production, superoxide production was compared between differentiated and undifferentiated RGC-5 cells. Differentiated and undifferentiated RGC-5 cells produced similar amounts of superoxide in the presence of complex I substrates and inhibitors but differed in superoxide production after incubation with succinate, a complex II substrate, and the complex III inhibitor antimycin A.
Overall, the rate of superoxide production of RGC-5 cell mitochondria was much less than that of brain mitochondria for a given amount of mitochondrial protein. Comparing the rates of production in the absence of substrates and inhibitors, basal brain mitochondria superoxide production was 7 times of that of RGC-5 cells. The most notable difference after METC inhibition was the increased superoxide production in brain mitochondria in the presence of the complex III inhibitor antimycin A. After treatment with antimycin A, brain mitochondria had a greater than twofold increase in superoxide production, compared to a small decrease in superoxide production rate in RGC-5 mitochondria.
These differences appear to reflect dramatic differences in levels of METC components within RGC-5 mitochondria, compared to brain mitochondria. Immunoblotting experiments comparing RGC-5 cells and brain demonstrated lower concentrations of complex subunits in RGC-5 cells, with the differences being most apparent in complex I, less so in complex IV, and least apparent in complexes II and III. Furthermore, the difference in SOD-2 expression between RGC-5 cells and brain reflected the differences in levels of METC components, probably reflecting the fact that superoxide is produced in complex I in the basal state.31
MECHANISMS FOR DIFFERENTIAL SUPEROXIDE PRODUCTION IN RGC MITOCHONDRIA
There are at least 3 mechanisms that would explain the lower superoxide production in mitochondria from RGC-5 cells:
Lower rates of transfer via the METC, eg, due to lower density of METC in RGC-5 mitochondria
Greater concentrations of the primary mitochondrial enzyme that metabolizes superoxide, ie, SOD-2, in RGC-5 cells
Greater concentrations of non-METC proteins in RGC-5 mitochondria preparations, diluting the relative concentration of METC components
The immunoblotting experiments demonstrated lower concentrations of all METC complex components, based on antibodies targeted of one subunit per complex. Those results could be consistent with either mechanism 1 or 3, but not mechanism 2. Furthermore, the finding that the levels of SOD-2 were not increased in RGC-5 mitochondria argues against mechanism 2. The finding that there were differences in the relative concentrations of some complexes, eg, a more reduced level of complex I vs complexes II or III, argues against mechanism 3. Nonetheless, it is still possible that a greater level of non-METC components in RGC-5 mitochondria could explain some of the functional (lower superoxide production) and immunoblotting (less METC components) findings. Examples of such proteins, which number in the hundreds,40 could include those associated with the tricarboxylic acid cycle, protein synthesis, and lipid metabolism. However, if the differences in superoxide production were simply a matter of lower concentration of METC components, then this could explain differences in baseline superoxide production, but would not explain differences in patterns of superoxide production, eg, why there was such a large increase in superoxide production in brain mitochondria after treatment with antimycin A.
INTERPRETATION OF SUPEROXIDE PRODUCTION EXPERIMENTS
Although the complex I mtDNA mutations of LHON are usually present in all cells (except in cases of extreme heteroplasmy), the disease usually affects only RGCs, with a small number of patients also having white matter central nervous system (CNS) disease.18–20 The reason for the RGC specificity is unclear. It is not just abnormal sensitivity of the RGC METC, because efficiencies in mitochondrial electron transport have been linked to a number of diseases affecting the CNS. For example, intoxication with the METC complex I inhibitors rotenone and 1-methyl-4-pyridinium (MPP+), the metabolic product of N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), produces characteristics of Parkinson’s disease. Similarly, treatment with the complex II inhibitor 3-nitropropionic acid generates symptoms of Huntington’s disease.
One interpretation of the present study is that the decreased RGC levels of superoxide may have a physiological rationale. RGCs use superoxide as a mitochondrial-derived intracellular messenger for signaling the initiation of apoptosis after axonal injury.33 If RGCs use superoxide to signal cell death, then it is reasonable to presume that they also tightly regulate its intracellular concentration in relevant compartments. Otherwise, there would be aberrant death of a critical class of neurons, which are postmitotic in mammals. Aberrant production of superoxide in LHON mtDNA mutations37 may abrogate this protection.
STUDYING THE EFFECT OF LHON MTDNA MUTATIONS IN RETINAL GANGLION CELLS
To understand the biological basis of the RGC-specific nature of the LHON mtDNA mutations, it would be necessary to study RGCs that have the LHON mutations and compare them to wild-type RGCs. Although it is possible to culture human RGCs postmortem or after enucleation,41 this is impractical for LHON studies. There is no strain of animals that develop spontaneous LHON. The next best step would be to develop a human RGC line, as with the rat RGC-5 line, and make transmitochondrial cybrids. However, there is no such human line, and attempts to create animal lines similar to the RGC-5 line have been unsuccessful. Therefore, the development of transmitochondrial rat cybrids from RGCs, using rat-modified LHON mtDNA mutations, would be a suitable alternative. Ongoing work in other laboratories has demonstrated the ability to target mtDNA-coded complex I subunits to mitochondria using a mitochondrial targeting sequence and has been presented at the ARVO Annual Meeting, but has not yet been published. The methodology is different from that used by Guy and colleagues,42 where the mutant 11778 was rescued with a mitochondrially targeted wild-type ND4 subunit expressed in the nucleus.
By utilizing mitotic RGC-5 cells in conjunction with long-term EtBr treatment and then differentiating them, a RGC cell line could be created that lacked functional METC components. This would allow the separation of the effects of complex I (and other complexes) on superoxide generation and death signaling, compared to other sources of cell superoxide. Functional consequences of mitochondrial stressors affecteing RGC-5 cells with damaged or partially depleted mtDNA could be studied in the same way. Ideally, creation of LHON cybrids in RGC-5 cells would elucidate the precise role of the LHON mtDNA mutations in RGC death, but this is currently impractical because of difficulties in producing xenomitochondrial (ie, rat-human) cybrids.43
ADVANTAGES AND DISADVANTAGES OF USING RGC-5 CELLS FOR MITOCHONDRIAL STUDIES
Purifying a usable quantity of mitochondria from freshly isolated RGCs is impractical. Thus the use of a differentiable RGC cell line allows otherwise unfeasible mitochondrial studies. Difficulties in isolation of mitochondria from RGCs include the large numbers of animals necessary to obtain enough RGCs (there are approximately 105 RGCs per rat retina) and subsequently obtaining mitochondria from a small number of purified RGCs. RGC-5 cells are transformed retinal neurons that exhibit properties characteristic of RGCs, including expression of Thy-1 and Brn-3c, which are not seen in other retinal cells under normal conditions.44 Low-dose staurosporine, a broad-spectrum protein kinase inhibitor, induces RGC-5 cells to differentiate to a phenotype that is similar to a mature RGC.36
EXPERIMENTAL LIMITATIONS
There are several limitations to this study. Superoxide was not measured directly, but instead indirectly measured by conversion via SOD-2 into H2O2 and using the H2O2 probe Amplex Red. Indirect superoxide measurement with a probe specific for H2O2 is commonly performed38,39 because mitochondrial SOD-2 converts superoxide to H2O2, and the latter freely crosses organelle and cell membranes. Amplex Red reacts with H2O2 in the presence of HRP and is converted to the fluorescent product resorufin. In the present study, changes in the levels of resorufin were detected and used to determine the production rates of superoxide. Nonetheless, the possibility that abnormally low SOD-2 activity in brain biased the results can be excluded, based on the results of immunoblots demonstrating higher levels of SOD-2 in brain mitochondria than RGC-5 cell mitochondria.
Superoxide production was measured in mitochondria obtained from the RGC-5 cell line, and not mitochondria from freshly isolated RGCs. It is possible that some of the differences that were measured in superoxide generation arose because the brain mitochondria were isolated from whole cerebral tissue and the RGC-5 mitochondria were isolated from a cell line. Brain mitochondria were isolated from a mixture of cell types, ie, not only neurons but also glial cells, and this could blur differences between the brain and RGC-5 mitochondria. To ensure that differences between mitochondria from RGC-5 cells and cerebral cortical cells were not due to differences in the source of the tissue (a cell line vs fresh tissue), mitochondria from the RGC-5 cell line were compared with mitochondria from a neuroblastoma cell line. Compared to the RGC-5 cells, the overall rate of superoxide production was significantly greater and slopes differed significantly in all conditions. Unfortunately, it is impractical to do mitochondrial metabolic experiments from freshly isolated RGCs, as discussed above, and whole retina cannot be used because RGCs make up only a small fraction of the tissue.
IMPLICATIONS FOR THE PATHOGENESIS OF LEBER HEREDITARY OPTIC NEUROPATHY
There are several theories to explain the mechanism of RGC death in LHON. One theory suggests that RGCs are very energy-demanding cells, and mutations affecting the METC would result in RGC-specific death because the cell cannot fulfill its energy demands. However, this theory does not address why there is little neuronal loss in other high-energy-requiring cells, such as photoreceptors or cardiac muscle (as is seen in some mtDNA deletions), nor does it explain the delayed onset of LHON. Most important, there is a discrepancy between the degree of ATP deficiency and the severity of the optic neuropathy in cells with LHON mutations.45
Instead, some have speculated that differences in ROS production and sensitivity to oxidative stress among cells types may explain the timing and the specificity of LHON. There is precedence for cell type–specific differences in mitochondrial superoxide production.46 Rat brain and liver mitochondria differ in superoxide production in the presence of METC substrates and inhibitors,47 and LHON cybrids in neuronal-like differentiated NT2 cells have greatly increased superoxide production.37 It is logical that ROS would be involved in LHON for several reasons. First, the mutations are in known superoxide production sites.48 Second, inhibition of these sites dramatically increases superoxide synthesis.38 Third, neuronal cybrids with LHON mutations have increased superoxide production.37 Pioneering work by the Guy laboratory demonstrated that knockdown of SOD-2 in RGCs caused an optic neuropathy,21 and overexpression of SOD-2 ameliorated the optic neuropathy induced by knockdown of one of the nuclear encoded complex I subunits.22,23 In brain mitochondria, complex I produces the majority of superoxide radicals,39 and the same may be true for RGCs.33 If RGC mitochondria have mechanisms for reducing levels of superoxide production, particularly in response to METC inhibitors, then mutations of critical complex I components could theoretically lead to catastrophic superoxide production, producing anomalous signaling cell death in the absence of axonal damage.33
ACKNOWLEDGMENTS
Funding/Support: This work was financially supported by grant EY12492 from the National Institutes of Health (L.A.L.), the Retina Research Foundation, the Leber’s Hereditary Optic Neuropathy Research Fund, a Dolly Green award from Research to Prevent Blindness, and an unrestricted departmental grant from Research to Prevent Blindness, Inc.
Financial Disclosures: The author is inventor on US patent 7,303,915 on the differentiation of RGC-5 cells, which has been assigned to the Wisconsin Alumni Research Foundation. There are no other financial involvements with companies or other agencies related to the topic of this thesis.
Other Acknowledgments: The research described would have been impossible without the immense contributions from students and technicians in my laboratory at the University of Wisconsin, particularly Mark J. Hoegger, BS, and Christopher J. Lieven, BS.
REFERENCES
- 1.Leber T. Über hereditäre und kongenital-angelegte Sehnervenleiden. Arch Ophthalmol (Berlin) 1871;17:249–291. [Google Scholar]
- 2.Russell WR. Hereditary aspects of Leber’s optic atrophy. With a report of cases consequent upon the mating of cousins. Trans Ophthalmol Soc U K. 1931;51:187–202. [Google Scholar]
- 3.Imai Y, Moriwaki D. A probable case of cytoplasmic inheritance in man: a critique of Leber’s disease. J Genet. 1936;33:163–167. [Google Scholar]
- 4.Giles RE, Blanc H, Cann HM, Wallace DC. Maternal inheritance of human mitochondrial DNA. Proc Natl Acad Sci U S A. 1980;77:6715–6719. doi: 10.1073/pnas.77.11.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallace DC, Singh G, Lott MT, et al. Mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy. Science. 1988;242:1427–1430. doi: 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]
- 6.Huoponen K, Vilkki J, Aula P, Nikoskelainen EK, Savontaus ML. A new mtDNA mutation associated with Leber hereditary optic neuroretinopathy. Am J Hum Genet. 1991;48:1147–1153. [PMC free article] [PubMed] [Google Scholar]
- 7.Johns DR, Neufeld MJ, Park RD. An ND-6 mitochondrial DNA mutation associated with Leber hereditary optic neuropathy. Biochem Biophys Res Comm. 1992;187:1551–1557. doi: 10.1016/0006-291x(92)90479-5. [DOI] [PubMed] [Google Scholar]
- 8.Jun AS, Brown MD, Wallace DC. A mitochondrial DNA mutation at nucleotide pair 14459 of the NADH dehydrogenase subunit 6 gene associated with maternally inherited Leber hereditary optic neuropathy and dystonia. Proc Natl Acad Sci U S A. 1994;91:6206–6210. doi: 10.1073/pnas.91.13.6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johns DR, Smith KH, Savino PJ, Miller NR. Leber’s hereditary optic neuropathy. Clinical manifestations of the 15257 mutation. Ophthalmology. 1993;100:981–986. doi: 10.1016/s0161-6420(93)31527-7. [DOI] [PubMed] [Google Scholar]
- 10.Harding AE, Sweeney MG, Govan GG, Riordan-Eva P. Pedigree analysis in Leber hereditary optic neuropathy families with a pathogenic mtDNA mutation. Am J Hum Genet. 1995;57:77–86. [PMC free article] [PubMed] [Google Scholar]
- 11.Weiner NC, Newman NJ, Lessell S, Johns DR, Lott MT, Wallace DC. Atypical Leber’s hereditary optic neuropathy with molecular confirmation. Arch Neurol. 1993;50:470–473. doi: 10.1001/archneur.1993.00540050022009. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson DM, Stone EM. Difficulty differentiating Leber’s from dominant optic neuropathy in a patient with remote visual loss. J Clin Neuro Ophthalmol. 1991;11:152–157. [PubMed] [Google Scholar]
- 13.Ortiz RG, Newman NJ, Manoukian SV, Diesenhouse MC, Lott MT, Wallace DC. Optic disk cupping and electrocardiographic abnormalities in an American pedigree with Leber’s hereditary optic neuropathy. Am J Ophthalmol. 1992;113:561–566. doi: 10.1016/s0002-9394(14)74730-0. [DOI] [PubMed] [Google Scholar]
- 14.Newman NJ, Wallace DC. Mitochondria and Leber’s hereditary optic neuropathy. Am J Ophthalmol. 1990;109:726–730. doi: 10.1016/s0002-9394(14)72445-6. [DOI] [PubMed] [Google Scholar]
- 15.Newman NJ, Lott MT, Wallace DC. The clinical characteristics of pedigrees of Leber’s hereditary optic neuropathy with the 11778 mutation. Am J Ophthalmol. 1991;111:750–762. doi: 10.1016/s0002-9394(14)76784-4. [DOI] [PubMed] [Google Scholar]
- 16.Meire GM, Cochaux P, Candaele C, Broux C. Clinical and genetical manifestations in 34 families with Leber’s hereditary optic neuropathy (LHON) Bull Soc Belge Ophtalmol. 1994;254:137–146. [PubMed] [Google Scholar]
- 17.Ajax ET, Kardon R. Late-onset Leber’s hereditary optic neuropathy. J Neuro-Ophthalmol. 1998;18:30–31. [PubMed] [Google Scholar]
- 18.Riordan-Eva P, Sanders MD, Govan GG, Sweeney MG, Da Costa J, Harding AE. The clinical features of Leber’s hereditary optic neuropathy defined by the presence of a pathogenic mitochondrial DNA mutation. Brain. 1995;118 ( Pt 2):319–337. doi: 10.1093/brain/118.2.319. [DOI] [PubMed] [Google Scholar]
- 19.Harding AE, Sweeney MG, Miller DH, et al. Occurrence of a multiple sclerosis-like illness in women who have a Leber’s hereditary optic neuropathy mitochondrial DNA mutation. Brain. 1992;115 ( Pt 4):979–989. doi: 10.1093/brain/115.4.979. [DOI] [PubMed] [Google Scholar]
- 20.Lees F, Macdonald AM, Turner JW. Leber’s disease with symptoms resembling disseminated sclerosis. J Neurol Neurosurg Psychiatry. 1964;27:415–421. doi: 10.1136/jnnp.27.5.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi X, Lewin AS, Hauswirth WW, Guy J. Optic neuropathy induced by reductions in mitochondrial superoxide dismutase. Invest Ophthalmol Vis Sci. 2003;44:1088–1096. doi: 10.1167/iovs.02-0864. [DOI] [PubMed] [Google Scholar]
- 22.Qi X, Lewin AS, Sun L, Hauswirth WW, Guy J. SOD2 gene transfer protects against optic neuropathy induced by deficiency of complex I. Ann Neurol. 2004;56:182–191. doi: 10.1002/ana.20175. [DOI] [PubMed] [Google Scholar]
- 23.Qi X, Lewin AS, Hauswirth WW, Guy J. Suppression of complex I gene expression induces optic neuropathy. Ann Neurol. 2003;53:198–205. doi: 10.1002/ana.10426. [DOI] [PubMed] [Google Scholar]
- 24.Danielson SR, Carelli V, Tan G, et al. Isolation of transcriptomal changes attributable to LHON mutations and the cybridization process. Brain. 2005;128:1026–1037. doi: 10.1093/brain/awh447. [DOI] [PubMed] [Google Scholar]
- 25.Ghelli A, Zanna C, Porcelli AM, et al. Leber’s hereditary optic neuropathy (LHON) pathogenic mutations induce mitochondrial-dependent apoptotic death in transmitochondrial cells incubated with galactose medium. J Biol Chem. 2003;278:4145–4150. doi: 10.1074/jbc.M210285200. [DOI] [PubMed] [Google Scholar]
- 26.Zanna C, Ghelli A, Porcelli AM, Carelli V, Martinuzzi A, Rugolo M. Apoptotic cell death of cybrid cells bearing Leber’s hereditary optic neuropathy mutations is caspase independent. Ann N Y Acad Sci. 2003;1010:213–217. doi: 10.1196/annals.1299.037. [DOI] [PubMed] [Google Scholar]
- 27.Danielson SR, Wong A, Carelli V, Martinuzzi A, Schapira AH, Cortopassi GA. Cells bearing mutations causing Leber’s hereditary optic neuropathy are sensitized to Fas-induced apoptosis. J Biol Chem. 2002;277:5810–5815. doi: 10.1074/jbc.M110119200. [DOI] [PubMed] [Google Scholar]
- 28.Brown MD, Trounce IA, Jun AS, Allen JC, Wallace DC. Functional analysis of lymphoblast and cybrid mitochondria containing the 3460, 11778, or 14484 Leber’s hereditary optic neuropathy mitochondrial DNA mutation. J Biol Chem. 2000;275:39831–39836. doi: 10.1074/jbc.M006476200. [DOI] [PubMed] [Google Scholar]
- 29.Vergani L, Martinuzzi A, Carelli V, et al. MtDNA mutations associated with Leber’s hereditary optic neuropathy: studies on cytoplasmic hybrid (cybrid) cells. Biochem Biophys Res Commun. 1995;210:880–888. doi: 10.1006/bbrc.1995.1740. [DOI] [PubMed] [Google Scholar]
- 30.Boveris A, Cadenas E, Stoppani AO. Role of ubiquinone in the mitochondrial generation of hydrogen peroxide. Biochem J. 1976;156:435–444. doi: 10.1042/bj1560435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turrens JF, Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem J. 1980;191:421–427. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen SM, Alexejun CN, Levin LA. Amplification of a reactive oxygen species signal in axotomized retinal ganglion cells. Antioxid Redox Signal. 2003;5:629–634. doi: 10.1089/152308603770310293. [DOI] [PubMed] [Google Scholar]
- 33.Lieven CJ, Schlieve CR, Hoegger MJ, Levin LA. Retinal ganglion cell axotomy induces an increase in intracellular superoxide anion. Invest Ophthalmol Vis Sci. 2006;47:1477–1485. doi: 10.1167/iovs.05-0921. [DOI] [PubMed] [Google Scholar]
- 34.Geiger LK, Kortuem KR, Alexejun C, Levin LA. Reduced redox state allows prolonged survival of axotomized neonatal retinal ganglion cells. Neuroscience. 2002;109:635–642. doi: 10.1016/s0306-4522(01)00493-6. [DOI] [PubMed] [Google Scholar]
- 35.Swanson KI, Schlieve CR, Lieven CJ, Levin LA. Neuroprotective effect of sulfhydryl reduction in a rat optic nerve crush model. Invest Ophthalmol Vis Sci. 2005;46:3737–3741. doi: 10.1167/iovs.05-0155. [DOI] [PubMed] [Google Scholar]
- 36.Frassetto LJ, Schlieve CR, Lieven CJ, et al. Kinase-dependent differentiation of a retinal ganglion cell precursor. Invest Ophthalmol Vis Sci. 2006;47:427–438. doi: 10.1167/iovs.05-0340. [DOI] [PubMed] [Google Scholar]
- 37.Wong A, Cavelier L, Collins-Schramm HE, et al. Differentiation-specific effects of LHON mutations introduced into neuronal NT2 cells. Hum Mol Genet. 2002;11:431–438. doi: 10.1093/hmg/11.4.431. [DOI] [PubMed] [Google Scholar]
- 38.Lambert AJ, Brand MD. Inhibitors of the quinone-binding site allow rapid superoxide production from mitochondrial NADH:ubiquinone oxidoreductase (complex I) J Biol Chem. 2004;279:39414–39420. doi: 10.1074/jbc.M406576200. [DOI] [PubMed] [Google Scholar]
- 39.Kudin AP, Bimpong-Buta NY, Vielhaber S, Elger CE, Kunz WS. Characterization of superoxide-producing sites in isolated brain mitochondria. J Biol Chem. 2004;279:4127–4135. doi: 10.1074/jbc.M310341200. [DOI] [PubMed] [Google Scholar]
- 40.Cotter D, Guda P, Fahy E, Subramaniam S. MitoProteome: mitochondrial protein sequence database and annotation system. Nucleic Acids Res. 2004;32:D463–467. doi: 10.1093/nar/gkh048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu DN, Ritch R. Tissue culture of adult human retinal ganglion cells. J Glaucoma. 1997;6:37–43. [PubMed] [Google Scholar]
- 42.Guy J, Qi X, Pallotti F, et al. Rescue of a mitochondrial deficiency causing Leber hereditary optic neuropathy. Ann Neurol. 2002;52:534–542. doi: 10.1002/ana.10354. [DOI] [PubMed] [Google Scholar]
- 43.Dey R, Barrientos A, Moraes CT. Functional constraints of nuclear-mitochondrial DNA interactions in xenomitochondrial rodent cell lines. J Biol Chem. 2000;275:31520–31527. doi: 10.1074/jbc.M004053200. [DOI] [PubMed] [Google Scholar]
- 44.Krishnamoorthy RR, Agarwal P, Prasanna G, et al. Characterization of a transformed rat retinal ganglion cell line. Brain Res Mol Brain Res. 2001;86:1–12. doi: 10.1016/s0169-328x(00)00224-2. [DOI] [PubMed] [Google Scholar]
- 45.Baracca A, Solaini G, Sgarbi G, et al. Severe impairment of complex I-driven adenosine triphosphate synthesis in Leber hereditary optic neuropathy cybrids. Arch Neurol. 2005;62:730–736. doi: 10.1001/archneur.62.5.730. [DOI] [PubMed] [Google Scholar]
- 46.St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem. 2002;277:44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- 47.Panov A, Dikalov S, Shalbuyeva N, Taylor G, Sherer T, Greenamyre JT. Rotenone model of Parkinson disease: multiple brain mitochondria dysfunctions after short term systemic rotenone intoxication. J Biol Chem. 2005;280:42026–42035. doi: 10.1074/jbc.M508628200. [DOI] [PubMed] [Google Scholar]
- 48.Degli Esposti M, Carelli V, Ghelli A, et al. Functional alterations of the mitochondrially encoded ND4 subunit associated with Leber’s hereditary optic neuropathy. FEBS Lett. 1994;352:375–379. doi: 10.1016/0014-5793(94)00971-6. [DOI] [PubMed] [Google Scholar]