Abstract
Purpose
To assess the findings, visual morbidity, and surgical intervention in Schnyder crystalline corneal dystrophy (SCCD).
Methods
Retrospective case series of 115 affected individuals from 34 SCCD families identified since 1989. Age, uncorrected visual acuity, best-corrected visual acuity (BCVA), corneal findings, and ocular surgery were recorded. Prospective phone, e-mail, or written contact provided updated information. Patients were divided into 3 age categories for statistical analysis: less than 26 years of age, 26 to 39 years of age, and 40 years of age and older.
Results
Mean age on initial examination was 38.8 ± 20.4 (range, 2–81) with follow-up of 55 of 79 (70%) of American patients. While there were no statistical significant correlations between logMAR visual acuity and age (logMAR BCVA =.033 + .002 × age; R =.21), the linear regression showed the trend of worse visual acuity with age. BCVA at ≥40 years was decreased compared to <40 (P < .0001), although mean BCVA was > 20/30 in both groups. Twenty-nine of 115 patients had corneal surgery with 5 phototherapeutic keratectomy (3 patients), and 39 penetrating keratoplasty (PKP) (27 patients). PKP was reported in 20 of 37 (54%) patients ≥50 years and 10 of 13 (77%) of patients ≥70. BCVA 1 year prior to PKP in 15 eyes (9 patients) ranged from 20/25 to 20/400 including 7 eyes with other ocular pathology. BCVA in the remaining 8 eyes was 20/25 to 20/70 with 3 of these 4 patients reporting preoperative glare. Chart and phone survey suggested increasing difficulty with photopic vision with aging.
Conclusion
Although excellent scotopic vision continues until middle age in SCCD, most patients had PKP by the 7th decade. SCCD causes progressive corneal opacification, which may result in glare and disproportionate loss of photopic vision.
INTRODUCTION
Schnyder crystalline corneal dystrophy (SCCD, MIM number 121800) is characterized by progressive bilateral corneal opacification resulting from deposition of abnormal cholesterol and phospholipids in the cornea. SCCD is inherited as an autosomal dominant trait with high penetrance and has been mapped to the UBIAD1 gene on 1p36.1–5
SYSTEMIC LIPID ABNORMALITIES
The incidence of hypercholesterolemia in SCCD has been reported to be up to 66% of affected patients.6–8 Although many patients with SCCD have hypercholesterolemia, most investigators agree that the severity of the dyslipidemia is not correlated to the occurrence of crystalline formation9 and that the progress of the corneal opacification is not related to the serum lipid levels.10,11 Patients affected by the corneal dystrophy may have normal or abnormal serum lipid, lipoprotein, or cholesterol levels. Likewise, serum lipid, lipoprotein, or cholesterol levels may be normal or abnormal in members of the pedigree without the corneal dystrophy.6,12–15
HISTORY
Schnyder crystalline corneal dystrophy was initially described by van Went and Wibaut16 in the Dutch literature in 1924, when they reported the characteristic corneal changes in a three generation family. In 1929, a Swiss ophthalmologist by the name of Schnyder17,18 published a report of the same disease in another 3- generation family. The disease subsequently became known as Schnyder crystalline corneal dystrophy. The dystrophy is considered rare, with less than 150 articles in the published literature.
Because the dystrophy is rare, many ophthalmologists may never examine a patient with SCCD. However, diagnosis and understanding of SCCD is made even more difficult by the number of articles published that perpetuate misinformation about the disease.
CORNEAL FINDINGS AND CONFUSION IN THE PUBLISHED LITERATURE
Corneal Crystals and SCCD
Most investigators have described the clinical appearance of SCCD to include the bilateral deposition of anterior stromal crystals early in life with subsequent appearance of corneal arcus and stromal haze10,11,13,16,17,19–33 typically suggesting that the finding of cholesterol crystals is integral to the diagnosis. However, SCCD in the absence of corneal crystal deposition has also been described.10,13,25,28,33,34 In fact, a report of 4 Swede-Finn pedigrees with 18 affected members revealed that only 50% of patients actually had corneal crystals.35 Examination of these patients demonstrated that the characteristic corneal change of SCCD was a progressive diffuse opacification of the cornea.
Despite published documentation about the varied spectrum of corneal changes in this dystrophy, more recent publications continue to emphasize the importance of crystals in the diagnosis of SCCD, reporting that “the clinical appearance of this dystrophy varies, but it is characterized by the bilateral and usually symmetric deposition of fine, needle-shaped polychromatic cholesterol crystals.”36
The presumption that most, if not all, SCCD patients have corneal crystals may increase the difficulty of making the diagnosis of SCCD in the patient who has findings typical of SCCD but does not have crystalline deposits. To emphasize the occurrence of this variation, an alternative name, Schnyder crystalline dystrophy sine crystals,37 was suggested.
CLINICAL COURSE
Although SCCD is a progressive disease,25 as recently as the last decade, one investigator wrote that the disease “is often described as stationary”38 and another indicated that the disease classically was “non-progressive… however, rare sporadic cases and individuals with progressive, panstromal Schnyder dystrophy have been described.”39
It is possible that the rarity of the dystrophy compounded by the confusion about clinical findings, has previously resulted in surgical biopsy of the SCCD cornea in order to assist the ophthalmologist in making the diagnosis.7,39 In fact, as recently as 2001, one published report indicated that the diagnosis of the disease was based on “clinical findings and corneal biopsy.”40
Penetrating Keratoplasty and Phototherapeutic Keratectomy
Most articles have suggested that the course of the dystrophy is typically benign with some indicating that “visual acuity often unaffected.”39 Although there are frequent reports of penetrating keratoplasty (PKP) in SCCD,10,15,23,26,30,32,33 the literature has reported that SCCD rarely requires corneal grafting.32 With the advent of the excimer laser, phototherapeutic keratectomy (PTK) has been successful in removal of subepithelial crystals and improving symptoms of glare and photophobia associated with the corneal opacity.36,40–43 Recurrence of the dystrophy after both PKP7,10,24,33 and PTK44 has been reported.
Questions About SCCD Not Yet Answered
Although Lisch and associates,10 in 1986, reported a 9-year follow-up of 13 patients with SCCD, there have been no recent studies documenting the actual course of visual decrease with age in a large number of patients with SCCD. The frequency of corneal surgical intervention in SCCD has never been reported. The rarity of the dystrophy has dictated that most publications have been case reports or small series that describe visual acuity in a limited number of affected patients.
FOUR LARGE SWEDE-FINN PEDIGREES WITH SCCD
In 1992, the results of clinical examinations of 18 patients affected with SCCD in 4 families from Massachusetts were published.35 Each of the 4 pedigrees had Swede-Finn ethnicity. The histopathologic findings of corneal specimens obtained from PKP surgery were described.34 Quantification of the corneal lipid was also reported.45 Subsequently, the clinical findings of 33 members of these pedigrees were published (including the 18 original affected patients).37
GENETICS: UBIAD1, THE CAUSATIVE GENE FOR SCCD
Since the initial article in 1992 to the present, the goal of isolating the genetic defect in the disease resulted in a continuation of recruitment efforts nationally and internationally to enroll additional patients with SCCD. Under Institutional Review Board (IRB) approval of the Human Investigations Committee of the University of Massachusetts Medical Center, specimens from the initial Swede-Finn families were used to map the disease to 1p36.1 With the identification of more families nationally and internationally, and using 13 families with SCCD, the genetic interval was further narrowed to 2.32 Mbp. Identity by state was present in all 13 families for two markers, which further narrowed the candidate region to 1.57 Mbp.3
At the same time that specimens were collected for the genetic mapping studies, clinical information about the affected members of the SCCD pedigrees continued to be collected. On enrollment in the genetic mapping study, information about visual acuity, corneal examination, and history of corneal surgery was requested. Since 1989, a total of 36 families worldwide with SCCD have been identified with a total of 132 affected members. Using 6 of these pedigrees, the author and coworkers recently reported that mutations in the UBAID1 gene resulted in SCCD.5
PURPOSE
The analysis of the clinical data in this large group of patients with SCCD represented an unusual opportunity to assess the visual impact of this disease. This study summarizes the clinical findings, visual acuity with age, and prevalence of corneal surgical intervention in the largest cohort of SCCD patients ever reported with the longest-term data yet reported in this disease.
METHODS
The recruitment and information gathering efforts for this study span 19 years from the recruitment of the first affected patients in 1987 to 2006. The recruitment methods varied during the 2 decades and are summarized below.
INITIAL RECRUITMENT AND SCREENING
History
Between July 1987 and October 1988, 3 unrelated individuals were referred for diagnosis of bilateral corneal opacities. Each patient was diagnosed as having SCCD. Interestingly, each of the 3 patients had a surname or maiden name of Johnson and had Swede-Finn ethnicity. Because this appeared to be a unique opportunity to study a large number of patients with this disease, a 3-part recruitment effort was begun in January 1989.35
Letters were sent to ophthalmologists in the community describing the corneal findings in SCCD and requesting that patients with these findings be referred for further evaluation. More than 600 letters were sent to patients in the local phone book with the name Johnson informing them of free ophthalmic screenings offered to identify patients with the dystrophy. In addition, articles publicizing free screenings were written for local newsletters, which were distributed in the Swede-Finn community.
Preliminary screening examinations performed from 1989 to 1995 included uncorrected visual acuity (UCVA) or best-corrected visual acuity (BCVA) and slit-lamp examination of the cornea. Patients noted to be unaffected on screening slit-lamp examination did not have complete ophthalmic examinations performed. Patients who were identified to have SCCD had dilated examination and corneal sensation testing. Testing of corneal sensation was performed before administration of eye drops by lightly touching the cornea with a wisp of cotton from a cotton swab or by performing Cochet Bonnet testing.
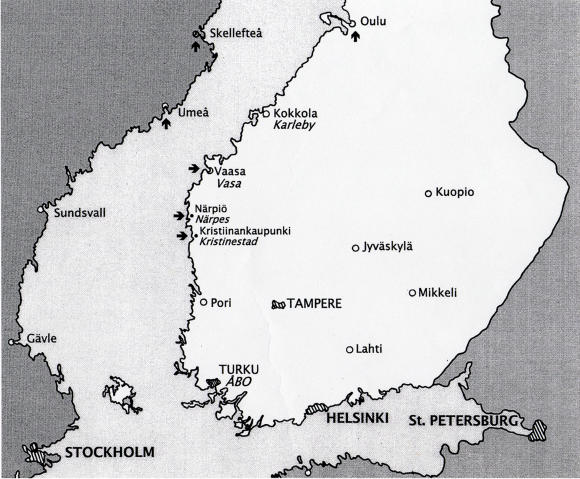
Notation was made of the location of specific corneal findings, including crystalline deposits, central disc opacity, midperipheral corneal haze, and arcus lipoides (Figure 1). Selected patients had cholesterol analysis.35 Patients were asked about family history, which allowed identification of other members of the family who could subsequently be examined. Gradually, individual pedigrees were established with indication of both the affected and unaffected individuals. The ancestors of the original 4 Swede-Finn pedigrees were found to originate from towns of Vasa, Narpes, and Kristinestad in a 60-km area on the west coast of Finland (Figure 2).
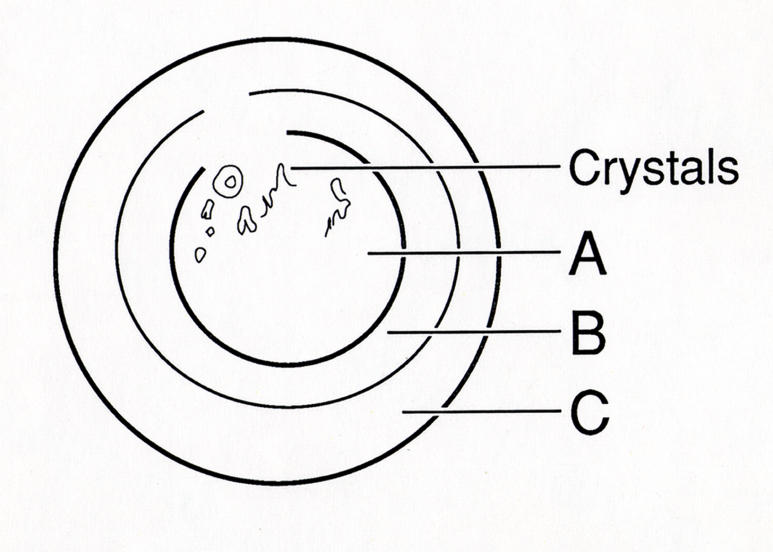
FIGURE 1.
Corneal diagram of location of corneal changes in Schnyder crystalline corneal dystrophy. Initial changes are noted in central cornea (A) with occurrence of corneal crystals and/or central haze followed by formation of arcus lipoides (C) and finally midperipheral stromal haze (B). Reprinted from Weiss JS, et al.34
FIGURE 2.
Map of Finland with arrows pointing to towns with patients identified to have Schnyder crystalline corneal dystrophy.
Aside from learning more about the corneal changes in SCCD, it appeared that examining large numbers of patients affected with SCCD could present an opportunity to isolate the genetic defect in the disease.
Present Study
Under IRB Approval of the University of Massachusetts Medical Center and the Wayne State University Medical School, different recruitment efforts were employed to attract additional SCCD patients to the study. Patients were recruited by referral from other physicians, referral from family members in affected pedigrees, or self-referral. Once an index patient agreed to participate in the study, the patient was asked to contact other family members to see if they would agree to be contacted. Throughout the years, additional pedigrees with SCCD were recruited for the study. The goal was to obtain clinical and genetic information from as many members of each SCCD pedigree as possible.
On the initial contact, patients were invited to complete a clinical data and family history form and/or submit a blood sample for genetic mapping. All studies were performed under the auspices of the IRB, and all patients who were willing to participate signed informed consent before participation.
Patients who were close enough geographically to be examined by the author underwent a complete eye examination with notation of BCVA, specific corneal findings noted on slit-lamp examination, dilated examination, and often corneal sensitivity testing. Notation was made if and when the patient had undergone corneal surgery, including PTK or PKP. Presence of genu valgum or history of prior surgery for genu valgum was indicated.
Those patients who could not be examined by the author were requested to sign medical record releases so their examining ophthalmologist could be contacted for results of their examination. The ophthalmologist was asked to complete a 1-page sheet indicating the UCVA, BCVA, intraocular pressure, motility, complete slit-lamp examination with corneal findings on either eye including crystals, arcus, central disc opacities, and midperipheral haze, and other findings such as prior PKP and dilated examination. Corneal sensitivity testing was requested.
Enrolled patients were also requested to personally complete 2 forms. The first form was a 1-page general health history, including general health questions and inquiries about hyperlipidemia and treatment. In addition, there was a 9-page family history questionnaire that asked names and ages of children, siblings, parents, grandparents, aunts and uncles, known health problems, and which members were thought to be affected with SCCD and when they were diagnosed. The family history was used to establish the individual SCCD pedigrees. Participants were also asked to provide contact information for other family members who expressed willingness to be contacted for the study.
Corneal sensation was checked by the author with cotton swab or Cochet Bonnet when the patient had no prior ocular drops. Other physicians were asked to circle if testing was done with Q-tip, Cochet Bonnet or other. Any report of reduction in sensation by the examiner or a Cochet Bonnet measurement of 5/6 or less, was recorded as decreased sensation.
Foreign patients were always referred by their own doctor. Forms were only written in English so could not be read by a non English speaker. While the doctor was asked to translate consent into the patient’s native language so informed consent could be obtained prior to study entry, the health history and family history forms were not translated and were not obtained from foreign patients. The doctor was asked to complete the examination form. However, often the form was not completed and instead, a summary of examination and/or health information was sent by e-mail or regular mail.
FOLLOW-UP FORMS
To obtain long-term information on the enrolled patients, physicians of the referring foreign families were contacted by e-mail between 2005 and 2006 requesting updated clinical information. The author had no contact information for the participating foreign families so that the referring physician was contacted directly.
Contact information was available on all of the families living in the United States from their initial study enrollment. In September 2005, using the original contact information, a medical record request form was sent to patients residing in the United States in order to obtain information about disease progression. Unfortunately, in the majority of cases, letters were either returned as undeliverable or patients did not respond. A record was made of those patients whose questionnaire was returned back stamped “return to sender” with the assumption that the patient had moved and there was no longer a forwarding address.
By April 2006, a list of corrected, current addresses for affected patients in the United States was established by using Internet search engines or by contacting known family members who could provide updated information for those family members whose address and phone numbers had changed.
Written Survey
American patients were mailed 2 separate questionnaires and a medical record release form. The eye history questionnaire was a 3-page questionnaire including questions about other ocular diseases and details about any ocular surgery, including dates and type of surgery. Specific questions included whether the patient had one or more PKP procedures and, if so, the date, postoperative vision if known, and any problems experienced. Additional questions were directed at whether there were any affected family members who were now deceased, as well as a request for contact information for any previously unaffected members who were now diagnosed as having SCCD. Medical record request form for the ophthalmologist or optometrist and HIPAA (Health Insurance Portability and Accountability Act) information were included. Patient information was updated with results of the questionnaire as well as medical records that were received. Information about date and cause of death was included for SCCD patients who were reported to die during the course of the study. Patients who were newly affected with SCCD were mailed the eye history questionnaire and medical record release form.
The 7-page health history questionnaire asked patient’s name; cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and triglyceride measurement; and whether cholesterol-lowering medication was being taken. Additional questions included whether the patient or family members had diabetes, stroke, cerebrovascular accident, myocardial infarction, and hyperlipidemia; were taking lipid-lowering drugs; or had high blood pressure.
Telephone Survey
Telephone calls to clarify survey responses and to obtain information from those patients who did not answer the survey were made to American patients in June and August of 2006.
Patients who had previously agreed to participate in the study were contacted by telephone to clarify answers supplied in written questionnaires that had been returned or to request that the questionnaire be completed and returned. In addition, during the phone call, patients were asked whether they or any affected family members had undergone PKP or other ocular surgery or had any ocular problems such as corneal graft rejection or dystrophy recurrence after PKP. Questions were also asked about systemic cholesterol and triglyceride levels, use of lipid-lowering agents, and past history of coronary artery disease, myocardial infarction, and cerebrovascular accident. Patients were also asked whether any family members had died and if so the age and cause of death.
Information from patient telephone survey that was entered into the final data set included age and cause of death for deceased affected members, whether a patient had undergone PTK or PKP, and when and whether a patient was on a cholesterol lowering medication.
DATA RECORDING
Information from the affected patient’s initial examination was recorded, including family pedigree name, patient name, date of birth, date and age at first examination, name of the doctor who performed the examination, UCVA, BCVA, corneal findings including presence of crystals, central corneal haze, midperipheral corneal haze and/or arcus lipoides; whether dilated examination was performed; presence of cataract or other ocular pathology; history of ocular surgery, including PTK or PKP; and whether there was past or present history of genu valgum, which is known to sometimes be associated with the disease.26 If clinical photographs were available, these were also used to confirm or obtain information about corneal findings such as presence of corneal crystals, midperipheral haze, or arcus lipoides. If the information was not present or was unclear on chart or photograph review, the entry was listed as unknown. Symptoms or signs such as complaints of glare or results of glare testing, as well as use of lipid-lowering medication, were recorded if available from initial or follow-up examinations. Notation was made of any additional ocular pathology found on examination, such as amblyopia or cataracts. Patients with other ocular pathology or prior ocular surgery were eliminated from UCVA and BCVA analysis for initial examination and follow-up examinations.
BCVA included vision obtained with correction (glasses or contact lenses), with pinhole, or with manifest refraction. If all 3 were listed, the vision with manifest refraction was chosen. If the vision with glasses and vision with glasses and pinhole were available, the latter was chosen. UCVA and BCVA were converted to logMAR units for statistical analysis. Patients were divided into 3 age categories for statistical analysis: less than 26 years of age, 26 to 39 years of age, and 40 years of age and older.
When available, information obtained from serial ocular examinations from chart notes was recorded for the individual patients. This information allowed long-term follow-up of ocular findings in individual patients with SCCD. For those patients who underwent corneal surgery, preoperative UCVA or BCVA within 1 year of surgery was compared to UCVA or BCVA at the most recent visit. Patients who had at least 7 years between eye examinations were used to examine the changes in visual acuity over time.
To calculate the percentage of patients in each decade who had undergone corneal surgery, data from the most recent examination, telephone, or written contact was used. The patient’s age, decade of age, and whether or not the patient reported having had PTK, photorefractive keratectomy (PRK), or PKP was recorded. The total number of patients in each decade was compared to the number of patients in that decade who had reported corneal surgery.
RESULTS
DEMOGRAPHICS
Thirty-six families with SCCD were enrolled since 1987. Two pedigrees from Finland with 20 members had no clinical information and were initially excluded. Of the remaining 34 families, 13 families originated from outside the United States and 21 of the families were recruited from the United States (Table 1). Of these, 16 families were referred by other physicians, 4 families were self-referred because of SCCD, and one family presented directly to the author for routine clinical examination, at which time SCCD was diagnosed. In total, the author examined 8 of the 21 US pedigrees.
TABLE 1.
DEMOGRAPHY AND SURGERY IN SCHNYDER CRYSTALLINE CORNEAL DYSTROPHY PEDIGREES
| FAMILY | MEMBERS | FEMALE | MALE | AVERAGE AGE | SD | PTS ≥50 WITH SURGERY | NO. PTS. PKP | NO.PTS. PTK |
|---|---|---|---|---|---|---|---|---|
| A | 19 | 4 | 15 | 30 | 19 | 2/6 | 2 | 0 |
| B | 18 | 12 | 6 | 35 | 19 | 5/9 | 5 | 1 |
| C | 2 | 2 | 0 | 56 | 23 | 1/2 | 1 | 0 |
| D | 4 | 4 | 0 | 43 | 31 | 1/2 | 1 | 0 |
| E | 3 | 1 | 2 | 22 | NI | 1/1 | 1 | 0 |
| G | 4 | 3 | 1 | 44 | 23 | 1/1 | 1 | 0 |
| H | 1 | 1 | 0 | 23 | NI | 0 | 0 | 0 |
| I | 4 | 0 | 4 | 46 | NI | 0 | 1 | 0 |
| J | 9 | 3 | 6 | 57 | 16 | 3/5 | 3 | 0 |
| K (Germany) | 4 | 2 | 2 | 37 | 14 | 0/1 | 0 | 0 |
| K1 (Germany) | 2 | 1 | 1 | 56 | 15 | 1/1 | 1 | 0 |
| L | 3 | 2 | 1 | 21 | 23 | 0 | 1 | 0 |
| M | 2 | 1 | 1 | 28 | 28 | 0 | 0 | 0 |
| N (Germany) | 2 | 1 | 1 | NI | NI | 0 | 0 | 0 |
| O | 2 | 1 | 1 | NI | NI | 1/1 | 1 | 0 |
| Q | 5 | 3 | 2 | 24 | 13 | 1/1 | 1 | 1 |
| R | 1 | 1 | 0 | 38 | 0 | 0 | 0 | 0 |
| S | 1 | 0 | 1 | NI | NI | 0 | 0 | 0 |
| T | 2 | 1 | 1 | 81 | NI | 0/2 | 0 | 0 |
| U | 1 | 0 | 1 | 44 | 0 | 1/1 | 1 | 0 |
| V | 1 | 1 | 0 | NI | NI | 0 | 0 | 0 |
| W (Turkey) | 5 | 2 | 3 | 51 | 15 | 0/1 | 0 | 1 |
| X (Taiwan) | 1 | 1 | 0 | 38 | NI | 0 | 1 | 0 |
| Y (Germany) | 5 | 3 | 2 | 41 | 18 | 1/1 | 2 | 0 |
| Z | 3 | 2 | 1 | 18 | 18 | 0 | 0 | 0 |
| AA | 1 | 0 | 1 | 63 | NI | 1/1 | 1 | 0 |
| BB (Czech) | 3 | 1 | 2 | 33 | 11 | 0 | 0 | 0 |
| BB1 (England) | 1 | NI | NI | NI | NI | 0 | 1 | 0 |
| BB2 (England) | 1 | NI | NI | NI | NI | 0 | 0 | 0 |
| BB3 (England) | 1 | NI | NI | NI | NI | 0 | 1 | 0 |
| CC (Japan) | 1 | 1 | 0 | NI | NI | 0 | 1 | 0 |
| DD (Taiwan) | 1 | 0 | 1 | NI | NI | 0 | 0 | 0 |
| EE (Taiwan) | 1 | 1 | 0 | 63 | NI | 0/1 | 0 | 0 |
| FF | 1 | 1 | 0 | 42 | NI | 0 | 0 | 0 |
| TOTAL | 115 | 56 | 56 | 39 | 20 | 20/37 | 27 | 3 |
NI, no information; PKP, penetrating keratoplasty; PTK, phototherapeutic keratectomy; Pts, patients; SD, standard deviation.
Of the 13 foreign pedigrees, 4 were from Germany,4 3 were from Taiwan, 3 from England, 1 was from Turkey,46 1 from Japan, and 1 from Czechoslovakia. The author examined patients from 2 of the 3 Taiwanese pedigrees.
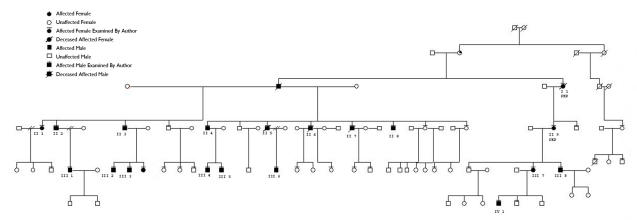
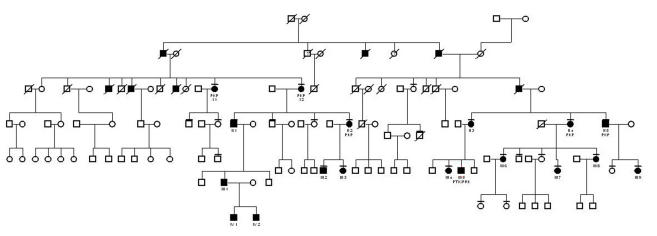
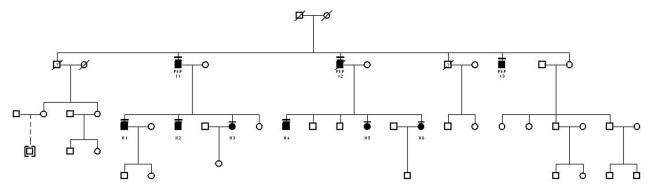
There were 115 affected patients in the 34 pedigrees. Of the 115 patients, 56 were female, 56 were male, and gender was not specified in 3 patients. Thirty of the pedigrees had 5 or fewer affected members in the family. The other 3 pedigrees were much larger: pedigree A had 19 affected members enrolled (Figure 3), pedigree B had 18 (Figure 4), and pedigree J had 9 (Figure 5).
FIGURE 3.
Pedigree A. Patients who have had penetrating keratoplasty are indicated. Individual patients are identified by a roman numeral representing the family generation and an arabic number. The unique patient identifier number and pedigree name is used to identify the patient in the text, photographs, and tables.
FIGURE 4.
Pedigree B. Key for this figure is listed in Figure 3. Individual patients are identified by a roman numeral representing the family generation and an arabic number. The unique patient identifier number and pedigree name is used to identify the patient in the text, photographs, and tables. Patients who have had penetrating keratoplasty are indicated.
FIGURE 5.
Pedigree J. Key for this figure is listed in Figure 3. Individual patients are identified by a roman numeral representing the family generation and an arabic number. The unique patient identifier number and pedigree name is used to identify the patient in the text, photographs and tables. Patients who have had penetrating keratoplasty or phototherapeutic keratectomy are indicated.
Age was specified in 93 of the 115 patients. The range of age in these patients was from 2 to 81 years of age, with a mean age of 38.8 ± 20.4. This included 46 females and 47 males.
MORTALITY
During the course of the study, it was known that at least 8 of the 115 patients died. While the exact of date of death and cause were not available for each of these patients, the information available suggested that at least 7 of the 8 patients died of causes unrelated to premature cardiovascular mortality.
Of 4 patients who died in their 9th decade, no cause of death was available for 2 patients, 1 died of pancreatic cancer, and 1 died of sepsis. Four other patients died between the 4th and 7th decade. Of these, 1 died of a brain tumor and 2 died of injuries related to auto accidents. The other patient died at age 62 of coronary artery disease, sepsis, and endocarditis.
VISUAL ACUITY
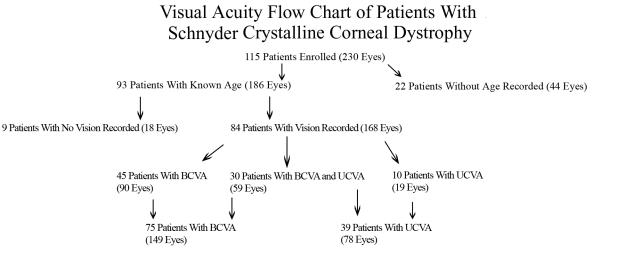
Eighty-four of 93 patients (90%) had a record of BCVA or UCVA. A patient with UCVA of 20/20 was counted as having had both UCVA of 20/20 and BCVA of 20/20 for purposes of calculation of mean visual acuity for the group. Forty-five patients had only BCVA recorded, 30 patients had BCVA and UCVA recorded, and 10 patients had only UCVA recorded (Figure 6). One patient had UCVA only in 1 eye and BCVA and UCVA in the other eye and so was counted in both categories. Because this patient was counted twice, the total number of patients appeared to add up to 85, even though only 84 patients had a record of BCVA or UCVA.
FIGURE 6.
Visual acuity flow chart of patients with Schnyder crystalline corneal dystrophy.
The mean BCVA and UCVA were analyzed in eyes that did not have prior ocular surgery or documented ocular pathology, such as cataract, amblyopia, macular degeneration, and glaucoma. To calculate the mean BCVA for each of the 3 age-groups, eyes included in the calculation had either a record of BCVA or had UCVA of 20/20 or better.
Of the 149 eyes of 75 patients that had BCVA recorded, ocular pathology excluded 5 eyes in patients <26 years of age, one eye in patients between 26 and 39 years of age, and 38 eyes in patients ≥40 years of age. The mean logMAR BCVA in patients <26 years of age (31 eyes) was .084 ± .147, at 26 to 30 years of age (39 eyes) was .076 ± .164, and at ≥40 years of age (35 eyes) was .171 ± .131.
Of the 78 eyes of 39 patients that had UCVA recorded, ocular pathology excluded 12 eyes in patients ≥40 years of age. The mean logMAR UCVA in patients <26 years of age (32 eyes ) was .173 ± .197; in those 26 to 39 years of age (22 eyes) was .125 ± .221; and in patients ≥40 years of age (12 eyes) was .258 ± .144.
The mean Snellen BCVA in affected patients with no other ocular pathology was between 20/20 and 20/25 in those <40 years of age and between 20/25 and 20/30 in those ≥40 years of age. Although there were patients in each age category who achieved BCVA of 20/20 or better, the worst BCVA reported in patients <26 years of age was 20/60, in patients 26 to 39 was 20/70, and in patients ≥40 years of age was 20/100.
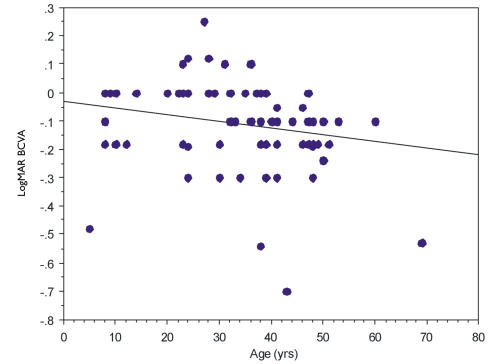
Mean Snellen UCVA was between 20/25 and 20/30 in patients <40 years of age and between 20/30 and 20/40 in patients ≥40 years of age. There were patients in all age categories with UCVA of 20/25, and the worst vision reported in all age categories was UCVA of 20/80. Regression analysis of the vision showed a weak trend of worsening vision with age y = −.033 + .002×; R2 = .046 (Figure 7). There was no statistically significant difference between patients <26 years of age and those 26 to 39 for either BCVA (P = .835) or UCVA (P = .4101). There was a statistically significant difference for both BCVA (P < .0001) and UCVA (P < .0001) between those patients <40 years of age and those ≥40 years of age.
FIGURE 7.
Regression analysis of best-corrected visual acuity (BCVA) with age in years (yrs) in Schnyder crystalline corneal dystrophy patients who have no other ocular pathology. Y-axis represents logMAR visual acuity and x-axis represents age y = −.033 + .002x; R2 = .046.
CORNEAL SENSATION
Of all eyes enrolled in the study that did not have corneal surgery, only 91 eyes had corneal sensation measurements performed (Table 2), and 47% (43 of 91) had decreased corneal sensation.
TABLE 2.
CORNEAL SENSATION IN SCHNYDER CRYSTALLINE CORNEAL DYSTROPHY
| DECREASED SENSATION | ≤25 YEARS OF AGE | 26–39 YEARS OF AGE | ≥40 YEARS OF AGE | |
|---|---|---|---|---|
| Total cohort | 43/91 (47%) | 10/26 (38%) | 6/22 (27%) | 27/43 (63%) |
| Author | 29/67 (43%) | 4/12 (33%) | 6/20 (30%) | 19/35 (54%) |
| Family A | 7/20 (35%) | 2/10 | 2/10 | 3/7 |
| Family B | 8/18 (44%) | 2/8 | 0/6 | 6/12 (50%) |
Decreased sensation was recorded in 10 of 26 eyes (38%) in patients <26 years of age, in 6 of 22 eyes (27%) of patients between 26 and 39 years of age, and in 27 of 43 eyes (63%) in patients ≥40 years. There was a statistically significant decrease in corneal sensation between those patients ≥40 years of age compared to patients <40 years of age (P = .004).
The findings in the total cohort were similar to those in the cohort examined by the author. Sixty-seven eyes that did not have prior corneal surgery had corneal sensation measurements that the author personally performed. Twenty-nine of 67 eyes (43%) had decreased corneal sensation measurements. Decreased sensation was recorded in 4 of 12 eyes (33%) of patients <26 years of age, 6 of 20 eyes (30%) of patients 26 to 39, and 19 of 35 eyes (54%) in patients ≥40 years.
These statistics were similar to those found in pedigrees A and B. For patients <26 years of age, decreased corneal sensation was recorded in 2 of 10 patients in family A and 2 of 8 patients in family B. Between 26 and 39 years of age, decreased sensation was recorded in 2 of 10 patients in family A and none of the 6 patients in family B. In patients ≥40 years of age, decreased corneal sensation was noted in 3 of 7 eyes in family A and 6 of 12 eyes in family B.
CORNEAL FINDINGS
Crystals
The prevalence of corneal crystal deposition was examined in the total cohort, those patients examined by the author and also in pedigrees A, B, and J. The number of eyes that had documentation of crystalline deposits was compared to the total number of eyes that had a record of presence or absence of crystalline deposits.
In the entire cohort, of the 160 eyes that had no prior corneal surgery and that had notation of presence or absence of corneal crystals, 119 of 160 (74%) had crystalline deposition. The percentage of eyes with crystals varied little among the different age categories with crystals noted in 38 of 50 eyes (76%) of patients <26 years of age, 23 of 36 (64%) of patients 26 to 39 years of age, and 58 of 74 eyes (78%) of patients ≥40 years of age. Four patients had crystalline deposits in only one eye. There was no statistically significant difference in the frequency of crystals reported between the individual age-groups (P = .25).
If only those patients examined by MDs other than the author were reviewed, 71 of 76 (93%) of eyes had crystal deposits. This compares to crystalline deposits noted in 48 of 84 (57%) of eyes examined by the author with the deposits occurring in 11 of 20 (55%) of eyes in patients <26 years of age, 7 of 20 eyes (35%) of patients 26 to 39 years of age, and 30 of 44 (68%) of eyes of patients ≥40 years of age.
There was a statistically significant higher prevalence of crystals in patients examined by other physicians compared to the prevalence of crystals in patients examined by the author (P < .0001).
Those pedigrees with 5 or more patients were also examined for crystal prevalence in those patients who had notation of either presence or absence of crystals. Families A, B, and J were examined by the author and had crystalline deposits in 12 of 19 (63%), 11 of 18 (61%), and 3 of 8 (36%), respectively. Both families W and Y, pedigrees from Turkey and Germany, were not examined by the author. Each of these families had 5 affected patients, all of whom had crystalline deposits.
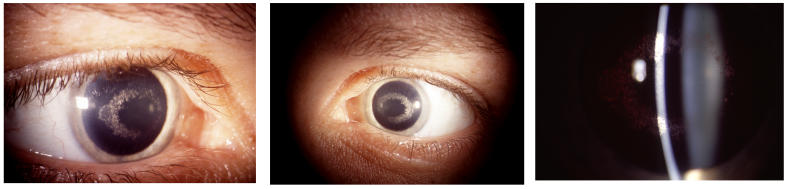
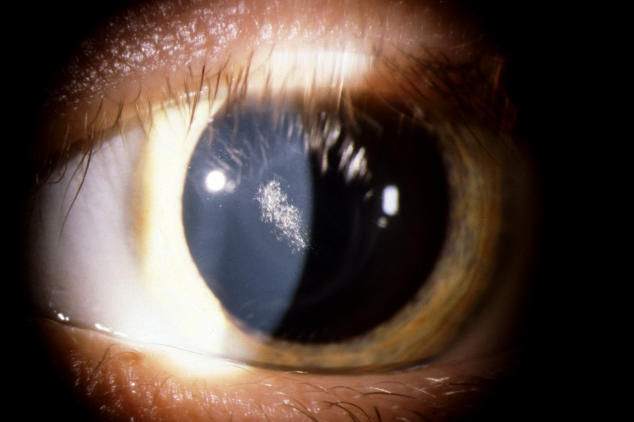
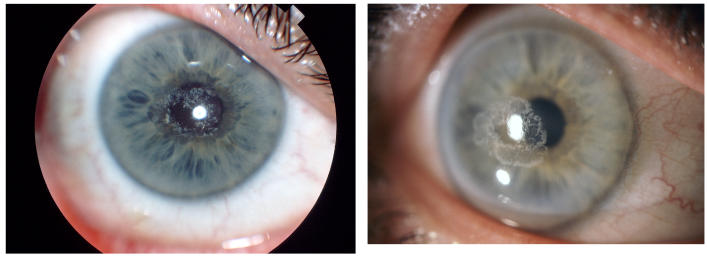
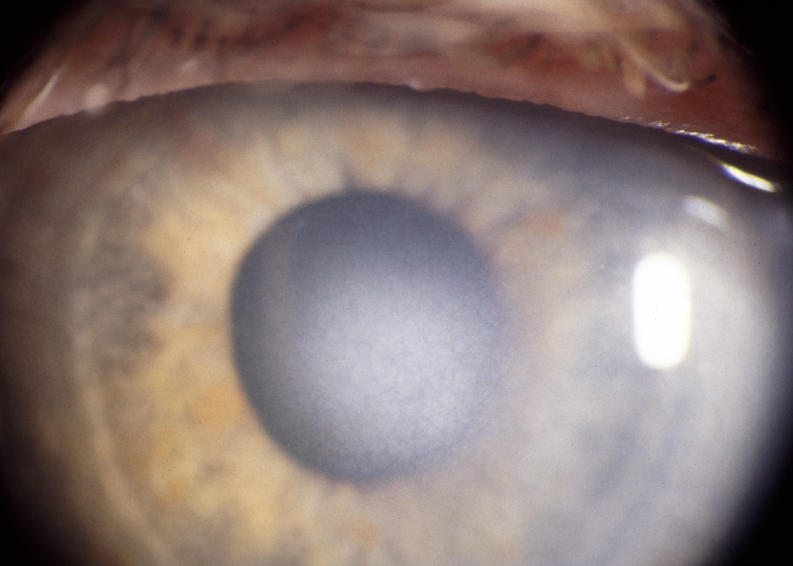
In the younger patients, the crystal configurations were initially often mirror images between the 2 eyes, but the deposits were always subepithelial (Figure 8). In younger patients, it appeared that the crystals initially formed an arc (Figure 9) and continued to deposited in ring formation, but by middle age crystals could maintain ring formation (Figure 10) or be scattered more diffusely (Figure 11).
FIGURE 8.
The corneas of a 28-year-old female in family G, with UCVA 20/15 OD and 20/20 OS, which demonstrate an almost complete circle of crystalline deposition that appears to be bilaterally symmetric. OD and OS appear to have a mirror image crystalline deposit. Left, External photograph of OD. Middle, External photograph of OS. Right, Slit-lamp photograph demonstrating subepithelial crystalline deposits.
FIGURE 9.
External photograph of the cornea of a 14-year-old male, III 2, in family B, with UCVA of 20/20 and partial arc deposition of subepithelial crystals. A symmetrical mirror image crystalline deposit was seen in the other eye.
FIGURE 10.
External photograph of the cornea of a 38-year-old male, II 7, in family A, with central haze, central ring of crystals, midperipheral clouding, and arcus lipoides. BCVA was 20/25.
FIGURE 11.
External photograph of the cornea of a 37-year-old male, III 5, in family B, with central plaque of subepithelial crystals in visual axis and BCVA of 20/50. Six months later, PRK/PTK was performed with improvement of UCVA to 20/25.
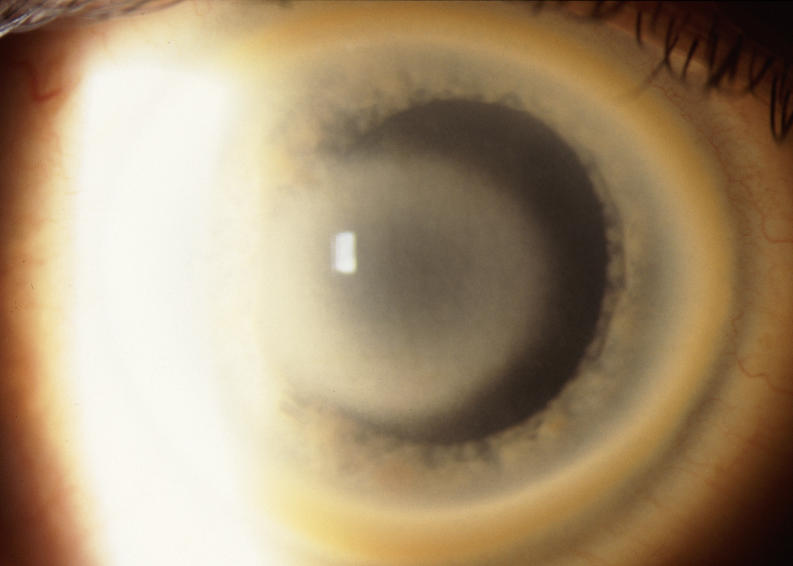
Central Corneal Haze
Of the eyes examined by all physicians who did not have prior corneal surgery and who had a record of either having presence or absence of central haze, central haze was noted in 11 of 43 eyes (26%) in patients <26 years of age, 28 of 38 eyes (74%) in patients between 26 and 39 years of age, and 71 of 75 eyes (95%) in patients ≥40 years. There was a statistically significant increase in the prevalence of haze between patients <26 years of age and those ≥26 years of age (P < .0001) and also a statistically significant increase in prevalence of haze between those patients 26 to 39 years of age compared to patients ≥40 years of age (P = .004)
Of the eyes examined by the author in which a notation was made as to presence or absence of central haze, central haze was present in 6 of 20 eyes (30%) in patients <26 years of age, 18 of 22 eyes (82%) of patients 26 to 39 years, and 47 of 47 eyes (100%) in patients ≥40 years of age. There was an increase in the prevalence of central corneal haze with age, which was statistically significant (P < .0001).
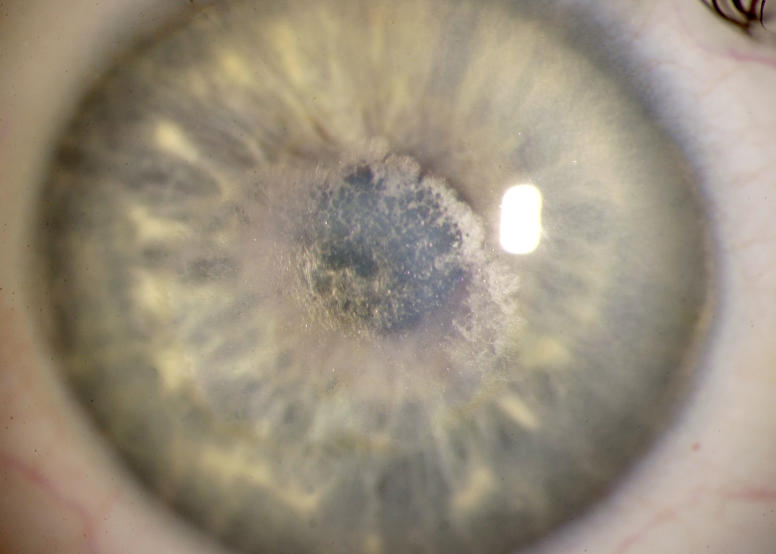
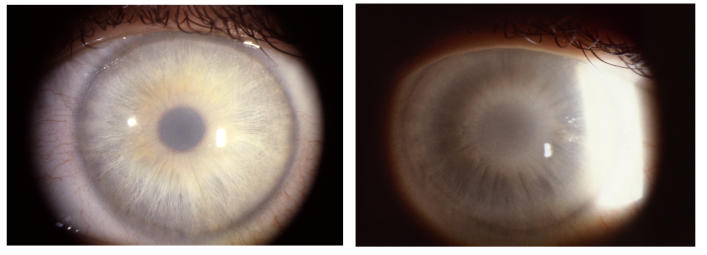
Similar to the ring formation that could occur with crystalline deposition, the central haze could appear in ring formation (Figure 12), or it could appear as a central disc (Figure 13). If retroillumination was used, it became apparent that the disc was more lucent centrally (Figure 14).
FIGURE 12.
Slit-lamp photograph of the cornea of a 23-year-old female, III 9, in family B, with BCVA 20/20 and central corneal ring opacity slightly inferiorly displaced in the visual axis. No subepithelial crystals were present.
FIGURE 13.
External photograph of the cornea of a 40-year-old male, II 5, in family A, with BCVA 20/25 and central disc-shaped stromal opacity and arcus lipoides. The central opacity is panstromal and is slightly inferiorly displaced in the visual axis. No subepithelial crystals were present.
FIGURE 14.
Slit-lamp photograph of the cornea of a 47-year-old male, II 1, in family B, with BCVA 20/30. Retroillumination reveals that the central opacity is more lucent in its middle and the opacity appears to be tessellated. Midperipheral haze and prominent arcus lipoides are also noted.
Crystals/Central Haze
Virtually all patients in each age category had evidence of crystals, central corneal haze, or a combination of both (Figure 10). In patients without corneal surgery examined by the author, in all age-groups, 15 eyes had only crystals, 33 eyes had crystals and corneal haze, and 33 eyes had only corneal haze. Three eyes had neither crystal deposition nor corneal haze. The 3 eyes with no central corneal findings belonged to 2 patients, a 4-year-old boy and a 22-year-old man. The 4-year-old child (patient III 4 in Figure 3) had SCCD crystals in the central cornea of one eye but no manifestations of the disease in his second eye. The 22-year-old man (patient III 1 in Figure 3) was not diagnosed as having SCCD on his first clinical examination, when his corneas were reported as being clear. Ten years later he was noted to have a subtle central corneal haze in the absence of crystalline deposition, and the diagnosis of SCCD was made.
Of patients without corneal surgery examined by other doctors, in all age-groups, 23 had crystals alone, 32 had crystals and corneal haze, and 11 had only corneal haze. The 3 eyes of the previously described patients had neither crystal deposition nor corneal haze.
Consequently, at all ages, virtually every SCCD patient had either corneal crystals, central corneal haze, or both findings. There was a statistically significant greater number of eyes that had only central corneal haze in patients examined by the author, 33 of 81 eyes (41%), compared to patients examined by other physicians, 11 of 66 (17%) (P = .0015).
Midperipheral Haze
In patients examined in the entire cohort and whose chart notes or photos indicated either the presence or absence of midperipheral haze, none of 44 eyes of patients <26 years of age had midperipheral haze, 9 of 20 eyes (45%) of patients 26 to 39 years of age had midperipheral haze, and 55 of 65 (85%) had midperipheral haze. There was a statistically significant increased prevalence of midperipheral haze in patients ≥40 compared to those <40 (P < .0001).
Of patients examined by the author, in which chart notes or photos indicated either the presence or absence of midperipheral haze, there was no midperipheral haze in any of the 25 eyes of patients <26 years of age, and midperipheral haze was noted in 2 of 12 eyes (17%) of patients 26 to 39 years of age. The 2 eyes with midperipheral haze belonged to a 39-year-old affected patient. Thirty-five of 39 eyes (90%) of patients ≥40 years of age had midperipheral haze.
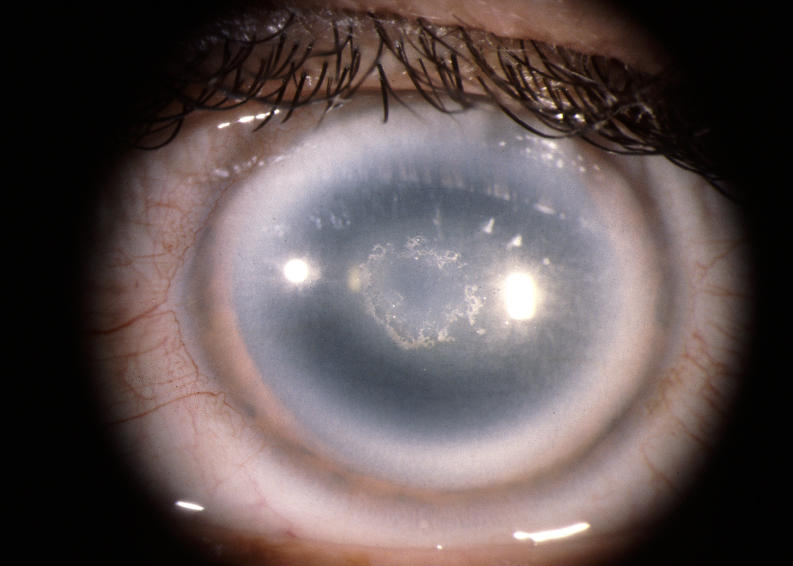
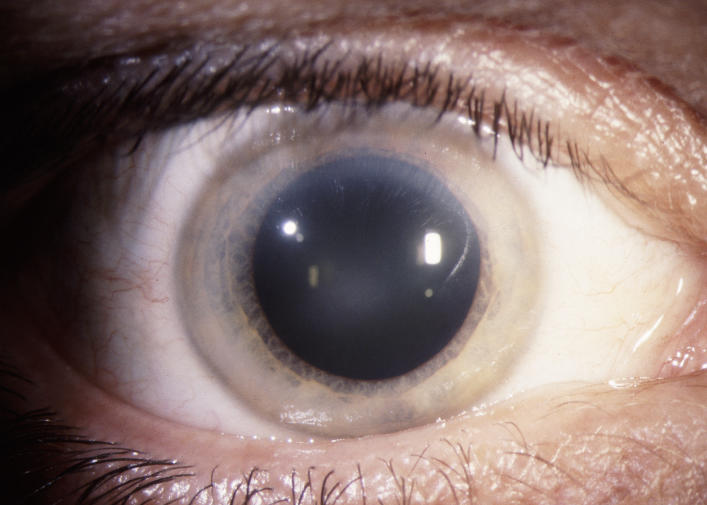
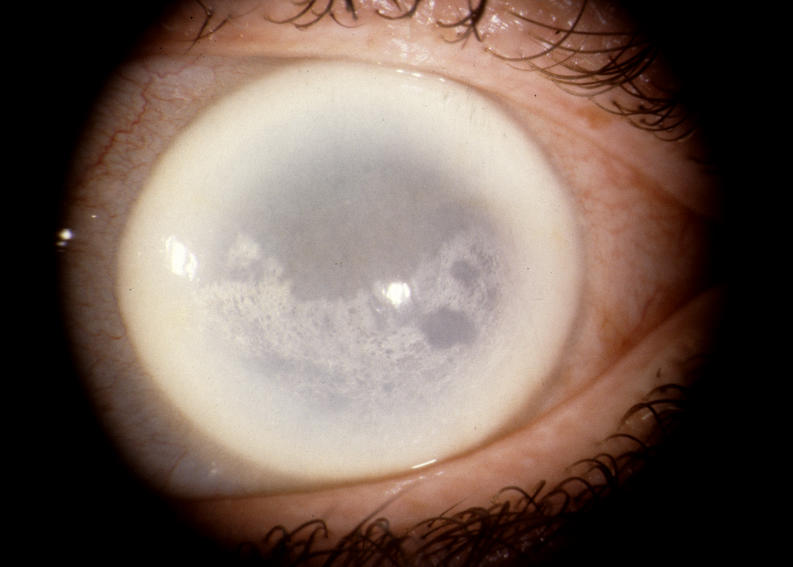
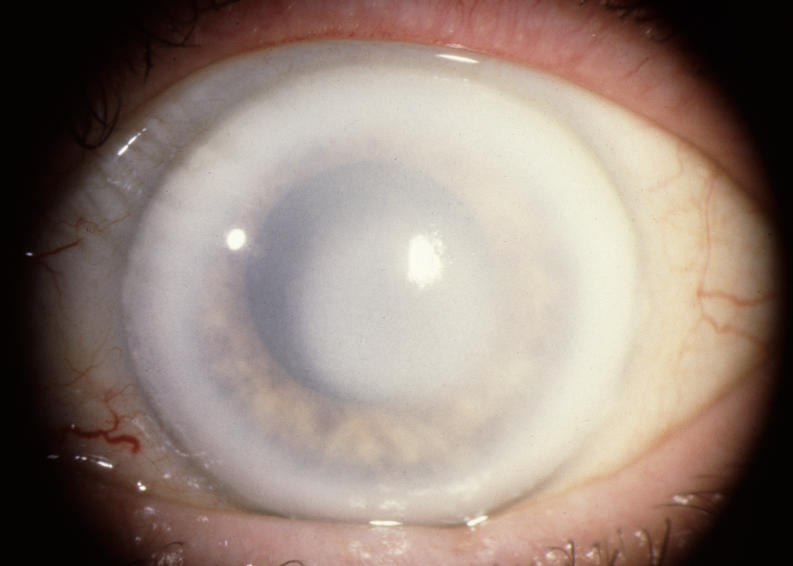
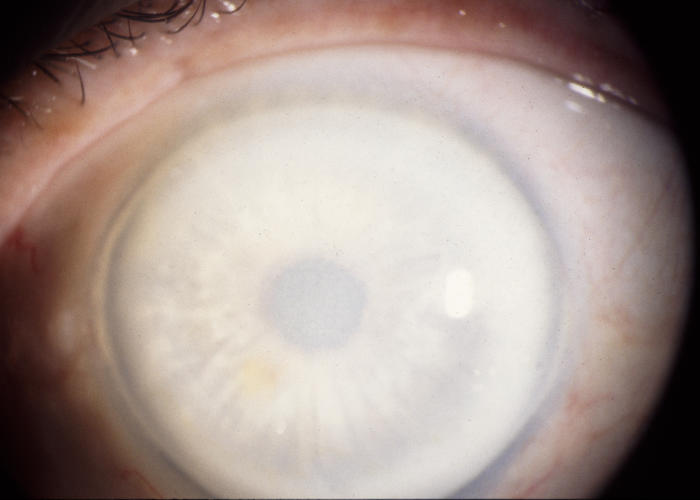
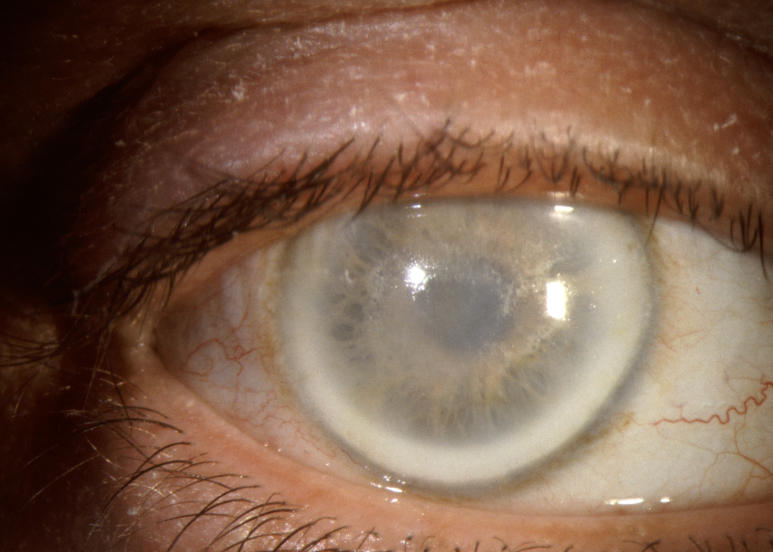
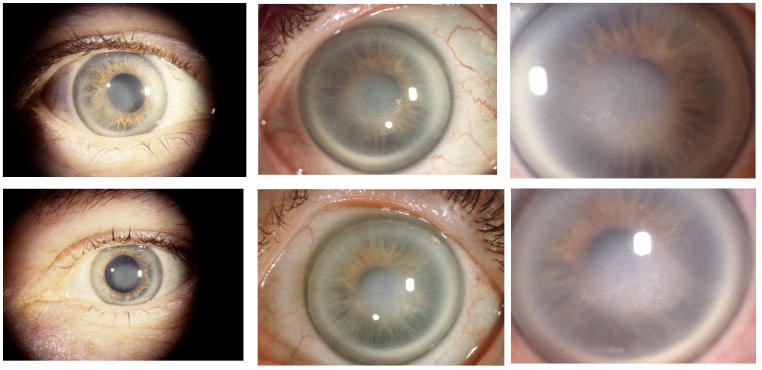
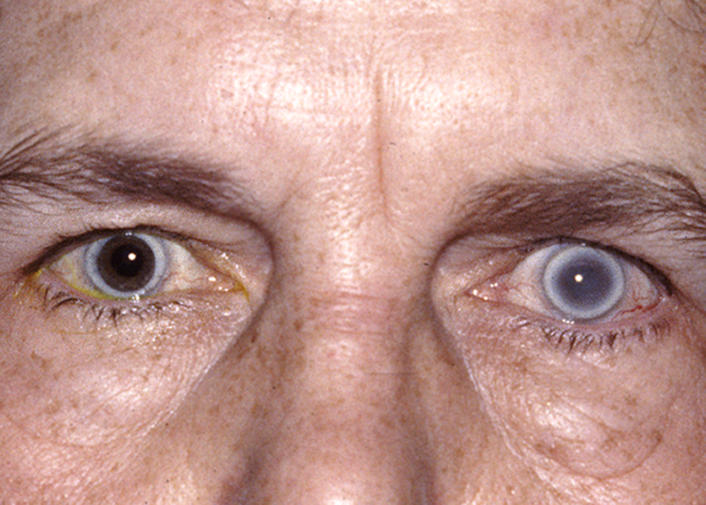
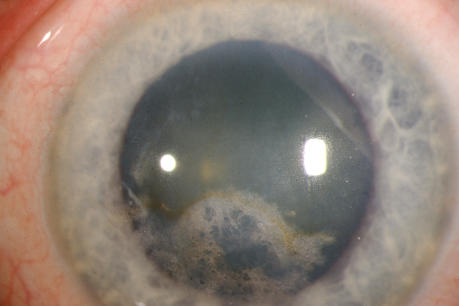
The prevalence of midperipheral haze increased from youngest to oldest age-groups with the majority of patients ≥40 years of age demonstrating this finding. In the older patients sometimes the cornea appeared diffusely hazy with prominent arcus and crystals (Figure 15) or diffusely hazy with prominent central disc opacity (Figure 16). There were cases where the most prominent finding was dense diffuse corneal haze (Figure 17), and it was not possible to delineate a central disc opacity. In such cases, the visual acuity could be surprisingly good considering the degree of corneal opacity. In some cases, retroillumination of the diffuse haze revealed that the opacity was not confluent in that there was a denser opacification in the central cornea (Figure 18).
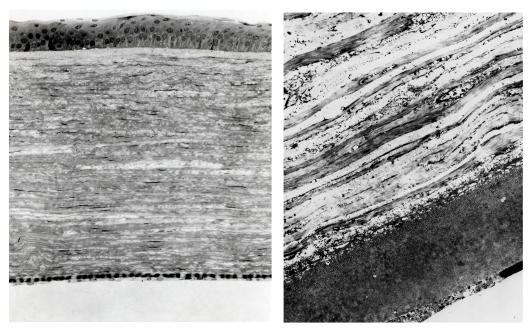
FIGURE 15.
External photograph of the cornea OD of a 63-year-old female, I 1, in family B, with BCVA 20/70 with subepithelial crystals, diffuse corneal haze, and arcus lipoides. OD underwent PKP, CE and IOL surgery within the year. Figure 28 demonstrates change in appearance of eyes after PKP.
FIGURE 16.
External photograph of the cornea of a 72-year-old female in family C, patient 2, with BCVA 20/40 with dense central opacity, midperipheral haze, and arcus lipoides that underwent PKP, cataract extraction, and IOL within the year.
FIGURE 17.
External photograph of the cornea of a 74-year-old male, I 1, in family J, with BCVA 20/25 and diffuse corneal opacification and arcus lipoides.
FIGURE 18.
Left, External photograph of the cornea of a 39-year-old female, II 2, in family B, with BCVA of 20/20 with diffuse corneal opacification that makes the entire cornea appear hazy. Patient had PKP 18 years later. Right, With use of retroillumination, a denser central opacity is apparent.
Arcus
Of the all the patients examined whose chart notes or photos indicated either the presence or absence of arcus lipoides, arcus was noted in 10 of 46 eyes (22%) of patients <26 years of age, 36 of 36 eyes (100%) of patients 26 to 39 years of age, and 71 of 73 eyes (97%) of patients ≥40 years of age. There was a statistically significant increased incidence of arcus in patients ≥26 years of age compared to those <26 years of age (P < .0001).
Of the patients examined by the author whose chart notes or photos indicated the presence or absence of arcus lipoides, in patients <26 years of age, no eyes (0 in 20) had evidence of arcus, while arcus was noted in 20 of 20 eyes (100%) of patients aged 26 to 39 and 47 of 47 (100%) of eyes of patients ≥40 years of age.
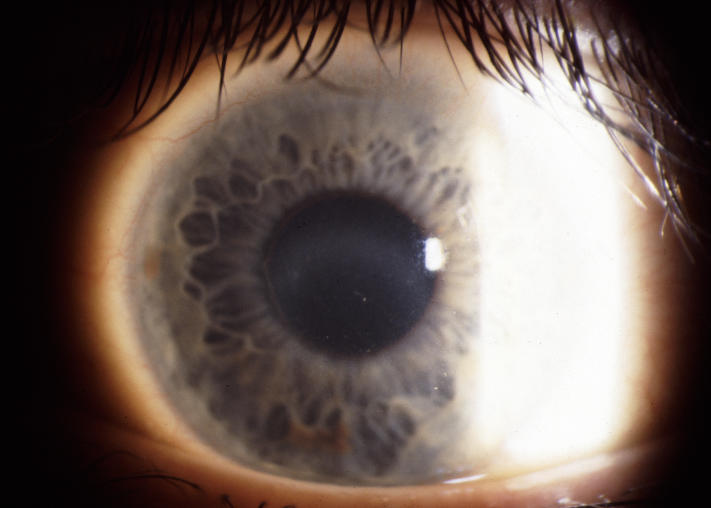
The results indicate that virtually all SCCD patients had arcus formation at ≥26 years of age. As the patient aged, the arcus became prominent enough to be easily seen without the aid of a slit lamp (Figure 19).
FIGURE 19.
External photograph of the cornea of a 49-year-old male, II 5, from family B, with BCVA of 20/30 and central and midperipheral corneal haze, central crystals, and arcus lipoides. Arcus was prominent enough to see without the aid of a slit lamp. Patient subsequently had PKP for complaints of decreased vision and glare.
LONG-TERM FOLLOW-UP OF SCCD PATIENTS
Foreign
The attempt to obtain follow-up data from foreign sources was largely unsuccessful. Two of the 13 families were enrolled in the last 6 months (K and K1) of the study, so the referring doctor was not contacted for more recent data. Of the remaining 11 foreign families, all but one (referral physician for family W) of the referring physicians answered the e-mail request for further information. However, the physicians were unable to obtain more recent information for families N, BB, BB1, BB2, BB3, CC, DD, and Y. Of the 13 foreign families, follow-up examination information was available on only 2 families, X and EE.
American
Of the 87 patients affected with SCCD in US pedigrees, at least 8 patients were known to die during the course of the study. An eye and health history questionnaire and medical request form was created to obtain follow-up information on the 79 living American patients.
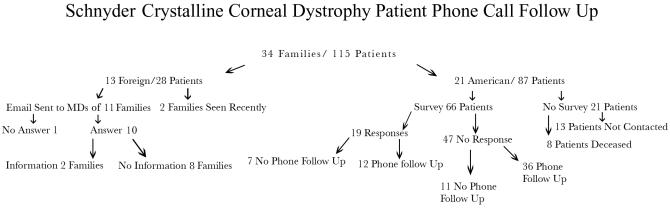
Thirteen of these patients were not sent a request for follow-up data. These included 3 patients from family Z who were examined for the first time after the survey was mailed, one patient from family H who had requested to withdraw from the study, and 9 patients from families L, M, S, V, AA, and FF who did not have current addresses or had not answered multiple prior phone or mail requests for information previously (Figure 20).
FIGURE 20.
Flow chart of Schnyder crystalline corneal dystrophy patient survey and phone call follow-up.
The remaining 66 patients were mailed an eye and health history questionnaire as well as a medical record release request. Only 19 patients returned the completed forms and/or the medical record request, which was used to obtain medical records. Twelve of these 19 patients were also contacted by telephone to clarify data.
Of the remaining 47 patients that did not return the written questionnaire or medical record release form, 36 patients answered a phone questionnaire asking about corneal surgery results, systemic cholesterol medication, and information about other family members, including whether any family members had undergone ocular surgery or had died.
In all, 55 of 66 SCCD living patients who were contacted in the United States (83%) answered a phone call or written survey. This represented 55 (70%) of the 79 living American SCCD patients cohort.
Pedigrees A, B, and J, had survey/phone call responses of 15 of 15 living members (100 %), 15 of 18 (83%), and 6 of 8 living members, respectively.
Visual Acuity Changes With Time in the Individual SCCD Patient
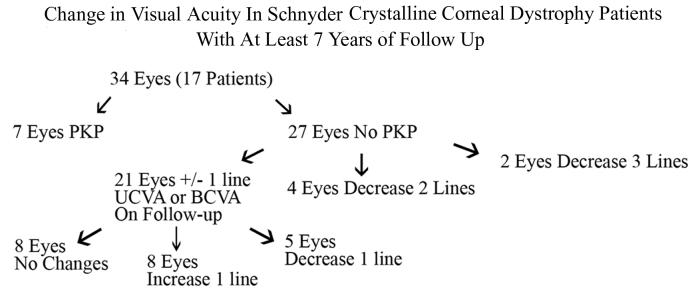
Seventeen patients (34 eyes) had at least 7 years of follow-up from their first to last ocular examination with a mean of 11.4 years ± 3.9 (range, 7–17) (Table 3). Mean age at initial examination was 33 years ± 14.7 (range, 8–60) and at last examination was 44.5 years ± 14.8 (range, 18–67) (Figure 21). All patients had UCVA or BCVA ≥20/30 on first examination except for a 40-year-old woman in pedigree C with known amblyopia and BCVA of 20/400 and a 38-year-old Taiwanese woman in pedigree X with BCVA of 20/70 OU who subsequently underwent PKP OS that year. Four of the 17 patients (24%), 7 of the 34 eyes (21%), with long-term follow-up underwent PKP in the course of the follow-up. A 41-year-old male in family Q had an unsuccessful PTK that did not improve the BCVA of 20/50, and so a PKP was performed in this eye at age 42 (Figure 22).
TABLE 3.
VISUAL ACUITY WITH LONG-TERM FOLLOW-UP IN PATIENTS WITH SCHNYDER CRYSTALLINE CORNEAL DYSTROPHY
| PATIENT NUMBER | FAMILY | AGE AT 1ST EXAM | VA OD | VA OS | AGE AT 2ND EXAM | VA OD | VA OS | YEARS FOLLOW UP | OTHER/PKP |
|---|---|---|---|---|---|---|---|---|---|
| II 1 | A | 46 | sc20/25‡ | sc20/25† | 58 | sc20/20‡ | sc20/30† | 8 | |
| III 1 | A | 23 | sc20/20‡ | sc20/20‡ | 30 | sc20/15‡ | sc20/15‡ | 7 | |
| III 7 | A | 19 | sc20/30‡ | sc20/25‡ | 36 | cc20/25‡ | sc20/20‡ | 17 | |
| III 2 | B | 14 | cc20/20§ | cc20/20* | 21 | cc20/30§ | cc20/20* | 7 | |
| III 3 | B | 10 | sc20/30‡ | sc20/30‡ | 25 | cc20/25‡ | sc20/25‡ | 15 | |
| II 3 | B | 48 | cc20/30* | cc20/25† | 62 | cc20/30* | cc20/30† | 14 | Cataract |
| III 6 | B | 29 | sc20/20¶ | sc20/20¶ | 45 | cc20/40¶ | cc20/40¶ | 16 | |
| Cataract OU | |||||||||
| 1 | C | 40 | cc20/30§ | cc20/400* | 57 | cc20/50§ | cc20/40* | 17 | |
| Amblyopia OS | |||||||||
| 1 | D | 50 | sc20/25* | sc20/25* | 61 | cc20/25* | cc20/25* | 15 | |
| 2 | D | 32 | sc20/25‡ | sc20/20§ | 43 | cc20/20‡ | cc20/30§ | 10 | |
| 1 | G | 60 | cc20/25 | cc20/25 | 67 | PKP Age 61# | PKP Age 62# | 7 | PKP |
| 1 | M | 8 | cc20/25* | cc20/25* | 18 | cc20/25* | cc20/25* | 10 | |
| 1 | Q | 33 | cc20/25 | cc20/25 | 49 | PKP Age 42# | PKP Age 43# | 16 | PKP |
| 2 | Q | 29 | cc20/20† | cc20/20† | 38 | cc20/25† | cc20/25† | 9 | |
| 1 | R | 38 | cc20/20§ | cc20/25† | 47 | cc20/30§ | cc20/30† | 10 | |
| 1 | U | 44 | cc20/20 | cc20/20 | 54 | PKP Age 45# | PKP Age 52# | 9 | PKP |
| 1 | X | 38 | cc20/70* | cc20/70 | 45 | cc20/70* | PKP Age 38# | 7 | PKP |
cc, with correction; OD, right eye; OS, left eye; PKP, penetrating keratoplasty; sc, without correction; VA, visual acuity.
Same VA.
Loss 1 line.
Gain 1 line.
Loss 2 lines.
Loss 3 lines.
PKP eye.
FIGURE 21.
Flow chart of change in visual acuity in Schnyder crystalline corneal dystrophy (SCCD) patient with at least 7 years of follow-up.
FIGURE 22.
Left, External photograph of the cornea of a 33-year-old male, patient 1, in family Q, with BCVA of 20/25, central subepithelial crystals, and arcus lipoides. (Photograph has been lightened to increase contrast and allow best visualization of crystal deposition). Right, 8 years later, patient is 43 years old with BCVA of 20/50 with increased central crystalline opacity, midperipheral haze, and arcus lipoides. PTK, which was subsequently performed within the year, did not increase BCVA and patient subsequently underwent PKP.
Of 27 eyes that did not undergo surgery, 21 eyes stayed within 1 line of the initial recorded visual acuity, 8 eyes improved by 1 line of vision, 8 eyes maintained the same UCVA or BCVA, and 5 eyes lost 1 line of UCVA or BCVA. Four additional eyes lost 2 lines of BCVA. Three of these eyes had final BCVA of 20/30. In a fourth patient, a 39-year-old woman from family C; progressive cornea opacification that occurred over a 17-year follow-up caused the BCVA to decrease from 20/30 to 20/50 in her nonamblyopic eye. PKP was reported as being planned in the near future (Figure 23). Only one patient had a loss of 3 lines of BCVA over 16 years with final BCVA of 20/40 at age 45 (patient III 6 in family B, Figure 4).
FIGURE 23.
Serial external photos of the eyes of a 39-year-old woman, patient 1, in family C, with amblyopia OS and BCVA of 20/30 OD and 20/400 OS, demonstrating central corneal disc opacity, few inferior central subepithelial crystals, midperipheral haze, and arcus lipoides. Increasing density of corneal haze is demonstrated over 17-year follow-up. BCVA at age 56 is 20/50 OD and 20/400 OS and PKP was planned. External photos of OD at age 39 (top left), OS at age 39 (bottom left), OD at age 52 (top middle), OS at age 52 (bottom middle), OD at age 56 (top right), and OS at age 56 (bottom right).
CORNEAL SURGERY
Forty-four corneal surgical procedures were performed on 43 eyes of 29 patients. Twenty-seven patients had PKP and 3 patients had PTK. A 41-year-old male in pedigree Q had PTK on one eye, but when visual acuity did not improve; PKP was performed on the same eye 1 year later (Table 1).
Phototherapeutic Keratectomy
Five eyes of 3 patients had PTK, with bilateral PTK performed in 2 of 3 patients. Mean age was 37 years (range, 34–41). Preoperative BCVA was 20/50 to 20/60 in 4 eyes whose only pathology was SCCD and 20/100 in an eye that also had a preoperative diagnosis of anisometropic amblyopia. BCVA improved in 4 of 5 eyes, including 1 eye that had anisometropic amblyopia.
A 34-year-old Turkish man (family W) had amblyopia OS. His preoperative BCVA was 20/100 OU, which improved to postoperative BCVA of 20/20 OD and 20/50 OS. A 37-year-old man, (patient III 5 in family B, Figure 4) underwent PTK and PRK for myopia OU (Figure 11). The BCVA OD improved from 20/60 to UCVA of 20/25 OD, but postoperative results were not available for the OS. A 41-year-old in family Q with BCVA of 20/50 had unilateral PTK for corneal crystalline deposition. One year postoperatively, the BCVA was 20/50 with persistence of corneal haze, and PKP was performed (Figure 22).
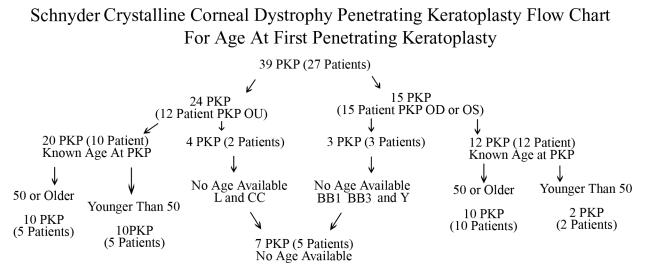
Age at First Penetrating Keratoplasty
Initial entry examination, subsequent follow-up examinations, e-mail correspondence, and written and telephone surveys revealed that 39 PKP were performed in 27 patients. Twelve patients had bilateral PKP. Of the 27 PKP patients there were 12 females and 13 males, and gender was not identified in 2 patients. Age at surgery was known in 22 patients (32 eyes) with a mean age at surgery of 60 years of age ± 13 years (range, 39–81). Age at surgery was not available in 7 eyes of 5 patients (families L, BB1, BB3, CC and Y) (Figure 24).
FIGURE 24.
Schnyder crystalline corneal dystrophy penetrating keratoplasty flow chart for age at first PKP.
Of the 22 patients whose age was known at first PKP, 15 patients (68%) had their first PKP at ≥50 years of age. The 7 patients <50 at first PKP had a mean age of 43 ± 4 years (range, 39–49). Five of the 7 patients undergoing PKP at a younger age eventually had bilateral surgery compared to the entire cohort, where 5 of 15 had bilateral PKP. There was not a statistically significant difference between the frequency of bilateral PKPs between patients <50 and patients ≥50 years of age (P = .17).
Penetrating Keratoplasty at 50 Years of Age and Above
The most recent eye examination, telephone contact, or questionnaire was used to record the patient’s age. For those patients who were deceased, the age at the last examination was recorded as the patient age. Twenty of 37 patients (54%) who were ≥50 years of age on their most recent contact reported having had unilateral or bilateral PKP surgery. For each pedigree, the number of patients ≥50 years of age who had PKP was compared to the total number of patients ≥50 years of age who were members of the pedigree (Table 1). The total number of patients in each pedigree who underwent PKP and PTK were listed in separate columns in Table 1. While information was obtained for each pedigree, only the largest pedigrees A, B, and J had at least 5 patients who were ≥50 years. The prevalence of PKP in the older age-group ranged from 2 of 6 in pedigree A, to 5 of 9 in pedigree B and 3 of 5 in pedigree J. The mean age of those patients in the ≥50 years of age cohort was 62 for A, 67 for B, and 70 for J. Each successive pedigree had both a higher percentage of patients ≥50 who had PKP and a higher mean age for this cohort. However, there was no statistical difference (P = .79) between the prevalence of PKP in each of the 3 pedigrees.
Prevalence of Corneal Surgery With Aging
To determine the prevalence of corneal surgery, PTK or PKP, as the SCCD patient aged, the age of most recent contact (including examination, written survey, or telephone contact) and whether or not the patient reported having had PKP or PTK for SCCD was recorded. In the few cases where the only information available was the age at PKP or PTK, this age was recorded as the actual patient age (Figure 24).
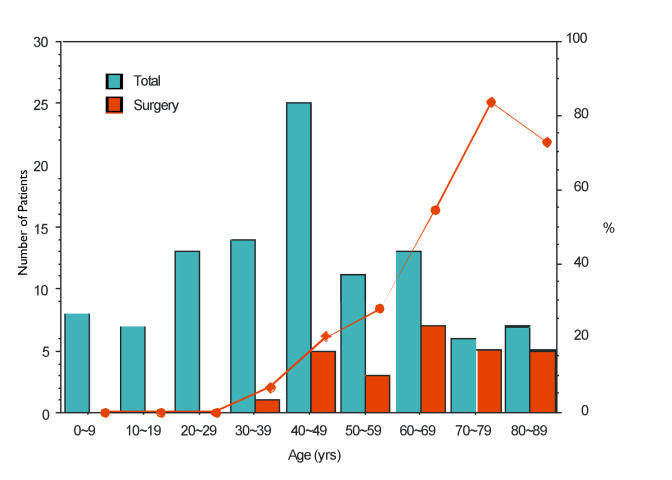
For each decade of age, the number of patients who reported corneal surgery at their last examination was compared to the total number of patients in that age-group. The percentage of patients reporting corneal surgery increased markedly after middle age, with PKP or PTK reported in 1 of 14 patients (7%) in the 4th decade, 5 of 25 (20%) in the 5th decade, 3 of 11 (27%) in the 6th decade, 7 of 13 (53%) in the 7th decade, 5 of 6 in the 8th decade, and 5 of 7 in the 9th decade. There was a statistically significant increase in the prevalence of corneal surgery with age (P = .002) (Figure 25).
FIGURE 25.
Age vs corneal surgery prevalence in Schnyder crystalline corneal dystrophy.(SCCD). Left y-axis represents number of patients, right y-axis represent percentage of patients. X-axis represents decade of age in years (yr) on most recent contact. Blue columns represent total number of patients in each decade of age. Red columns represent number of patients reporting prior corneal surgery on the most recent contact. Red line indicates percentage of patients in each decade of age with history of corneal surgery.
There were 10 patients in the 8th and 9th decade who had PKP and 3 who had not. The 3 who did not have surgery included a 78-year-old male who lived in Turkey (pedigree W), and no chart notes were available. The 2 additional patients in the 9th decade who did not undergo PKP were siblings in pedigree T. Review of the chart notes indicated that the examining ophthalmologist recorded that PKP was under consideration for both patients because of decreased vision or glare.
Penetrating Keratoplasty
Twenty-two patients underwent PKP and had information available about their age at PKP. Preoperative BCVA within 1 year of PKP was available in 9 patients (15 eyes).
Preoperative Vision
Preoperative visual data was unavailable in 13 patients because of the following reasons: Five patients did not sign medical record release forms sent to them although all did communicate medical information by phone or letter, including 3 patients informing us that they had undergone PKP surgery. Three patients died and old medical records could not be obtained. For the remaining 5, either the patient or physician did not return the follow-up data and there was no other communication. In some cases, while BCVA was available, it was obtained more than 1 year prior to PKP, typically 5 or more years, and so these patients/eyes were excluded from the calculations because they might not give accurate reflection of the level of visual decrease that necessitated surgical intervention.
Preoperative BCVA within 1 year of PKP was available in 15 eyes of 9 patients. Preoperative visual acuity ranged from 20/25 to 20/400 (Table 4), However, 6 of the 15 eyes (4 patients) had evidence of cataract formation and/or macular degeneration, and one eye had prior PTK In the remaining 8 eyes of 5 patients with no other ocular pathology, preoperative BCVA ranged from 20/20 to 20/70 with complaints of glare or decreased contrast recorded for 3 patients from pedigrees, A, E, and G. An additional 2 patients, from pedigrees B and C, had cataract formation with documentation of decrease in vision with glare testing. In total, 5 of the 9 patients (7 PKP eyes) had a chart note indicating either subjective complaint of glare or objective decrease in contrast sensitivity.
TABLE 4.
PREOPERATIVE BEST-CORRECTED VISUAL ACUITY IN PATIENTS UNDERGOING PENETRATING KERATOPLASTY
| PREOPERATIVE BCVA | NO. OF EYES | PATIENT NO. | PEDIGREE | AGE AT PKP | OCULAR PATHOLOGY | PHOTOTOPIC VISION COMPLAINTS |
|---|---|---|---|---|---|---|
| 20/25 | 2 | 1 | G | 61 | No | Lights on BCVA 20/400 |
| 1 | G | 62 | No | Lights on BCVA 20/400 | ||
| 20/30 | 2 | II 9 | A | 47 | No | Glare |
| 1 | Q | 43 | No | |||
| 20/40 | 1 | 1 | E | 50 | No | Glare |
| 20/50 | 4 | II 9 | A | 51 | No | Glare |
| 1 | E | 51 | No | |||
| 1 | Q | 42 | Prior PTK | |||
| 1 | AA | 63 | Cataract | |||
| 20/70 | 2 | I 1 | B | 64 | Cataract | Lights on BCVA of count fingers |
| 1 | X | 38 | No | |||
| 20/200 | 1 | 2 | C | 74 | Cataract | Lights on BCVA of count fingers |
| 20/400 | 2 | 2 | C | 72 | Cataract | Lights on BCVA of count fingers |
| 3 | D | 76 | SMD | |||
| Count fingers | 1 | 3 | D | 81 | SMD |
BCVA, best-corrected visual acuity; PTK, photherapeutic keratectomy; PKP, penetrating keratoplasty; SMD, senile macular degeneration.
An additional patient who underwent PKP with BCVA 20/30 3 years prior to PKP was not included in the calculations because visual acuity 1 year prior to surgery was not available but was also recorded as having a chief complaint of photophobia preoperatively (Figure 19).
Postoperative Vision
Postoperative information was available in 14 patients and 22 eyes. Range of postoperative follow-up was from 1 to 22 years with mean of 6.4 ± 6.7 years. Sixteen of 22 eyes attained BCVA of 20/50 or better. Six eyes attained visual acuity of 20/70 or worse. Five of these eyes had other pathology, including 2 with senile macular degeneration, 1 with Hollenhorst plaque, 1 with graft vascularization, and 1 with a suture abscess at the time of the examination.
Seven patients (11 eyes) recorded had a record of both preoperative BCVA within 1 year of PKP and postoperative BCVA more than 1 year after PKP (Table 5) with a mean follow-up of 5.3 years ± 2.0 years (range, 1–8). Five eyes had increase of BCVA, 3 eyes maintained same BCVA, and 3 eyes had decrease of 1 line of BCVA. Of the eyes with visual acuity loss, 2 eyes had evidence of cataract postoperatively and a third had a suture abscess.
TABLE 5.
CHANGE IN VISUAL ACUITY AFTER PENETRATING KERATOPLASTY SURGERY
| INCREASE BCVA (LINES) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PEDIGREE | PATIENT NUMBER | PREOP. BCVA | 1 | 2 | 3 | >4 | NO CHANGE | DECREASE BCVA (LINES) | ADD. SURG. † | FOLLOW UP (YRS) | POST-OPERATIVE PATHOLOGY |
| A | II 9 | 20/30 | X | 5 | |||||||
| II 9 | 20/50 | X | 1 | ||||||||
| B | I 1 | 20/70 | X | 4 | Suture Abscess | ||||||
| C | 2 | 20/200 | X | CE IOL | 5 | ||||||
| 2 | 20/400 | X | CE IOL | 4 | |||||||
| D | 3 | CF | X | CE IOL | 4 | SMD | |||||
| G | 1 | 20/25 | X | 6 | Cataract | ||||||
| 1 | 20/25 | X | 7 | Cataract | |||||||
| Q | 1 | 20/30 | X | 7 | Cataract | ||||||
| 1 | 20/50 | X | 8 | Cataract | |||||||
| X | 1 | 20/70 | X | 7 | |||||||
CE IOL, cataract extraction and intraocular lens; CF, count fingers; Preop BCVA, preoperative best-corrected visual acuity; SMD, senile macular degeneration.
Each patient in the individual pedigree has a unique identifying patient number. Patient identification numbers for pedigrees A and B are also listed on the individual pedigree for family A (Figure 3) and family B (Figure 4).
Additional ocular surgical procedures, such as CE IOL.
Two patients (3 eyes) had BCVA listed as ≥20/30 preoperatively with a presenting complaint of glare or objective decrease in vision on glare testing (Table 4 patients in pedigree A and G). Postoperative BCVA after PKP was the same in 2 eyes and 1 line worse in the third because of postoperative cataract formation.
Recurrence
Five of the 27 patients, 8 of the 39 eyes (21%), who underwent PKP, had evidence of recurrence of the dystrophy in the graft postoperatively. While all of these patients had bilateral PKP, recurrence occurred unilaterally in 2 patients and bilaterally in 3 patients
Visual acuity after recurrence was only available in 2 patients (3 eyes). Two eyes with recurrence had BCVA of 20/40, and the third had BCVA of 20/200 with graft vascularization. The remaining patients with recurrence reported maintenance of good visual acuity despite the recurrence of the dystrophy. There were no cases of repeated PKP performed for dystrophy recurrence.
Impact of Hypercholesterolemia in Patients With Corneal Surgery
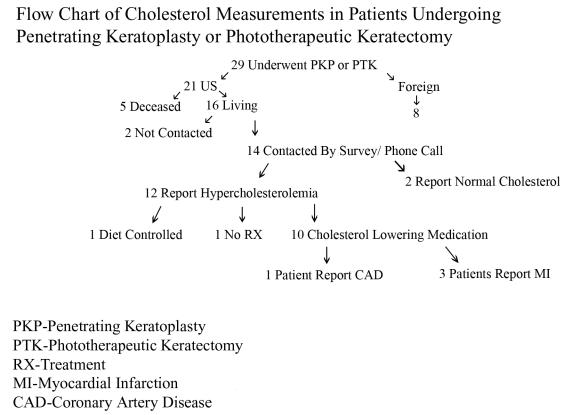
The American cohort who had PTK or PKP was contacted through written and telephone questionnaire to determine the prevalence of hyperlipidemia in those patients who had prior corneal surgery (Figure 26). Of the 21 American patients who had reported PTK or PKP, 5 patients were deceased. Two additional patients did not receive a mailing or telephone call because of inability to contact them on multiple prior occasions.
FIGURE 26.
Flow chart of cholesterol measurements in patients undergoing penetrating keratoplasty or phototherapeutic keratectomy.
Of the 5 deceased patients, 4 were 81 years of age or older at the time of their death. One patient died of pancreatic cancer, 1 patient died of sepsis, and cause of death for the other 2 patients was not available. Two of the four patients in their 9th decade had history of myocardial infarction and congestive heart failure. A fifth patient died at age 62 of coronary artery disease, bacterial endocarditis, and sepsis.
All of the remaining 14 patients were successfully contacted by written or phone questionnaire. Seven patients responded to phone and written questionnaire, and 7 patients responded to phone query alone. Twelve of the 14 patients reported elevated cholesterol (86%). The mean age of the patients with hypercholesterolemia was 68 ± 10.5 years (range, 52–82). Two patients, a 37-year-old and a 52-year-old reported normal cholesterol levels.
Of the 12 patients with hypercholesterolemia, 1 was on diet control, 1 was not using any treatment, and 10 were taking oral cholesterol-lowering medications. Ten of 14 patients (71%) contacted were using an oral medication to lower cholesterol. Cardiovascular disease was reported in 4 of 14 patients (29%) contacted. One patient reported coronary artery disease and three additional patients had a history of prior myocardial infarction
To try to compare prevalence of hypercholesterolemia of patients who had corneal surgery to those who had not undergone PTK or PKP, the frequency of cholesterol-lowering medications in SCCD patients ≥50 years who had not had corneal surgery was compared.
There were 17 patients ≥50 years who had not reported undergoing any corneal surgery. No information on cholesterol values or use of cholesterol medication was available for 4 of these patients, including 1 American patient and 3 foreign patients. Of the 13 patients with information about cholesterol medications, the mean age was 62 ± 10.3 years (range, 50–83). Seven of 13 patients (54%) were taking cholesterol-lowering agents. There was no statistically significant difference between the percentage of patients ≥50 years who were taking cholesterol-lowering agents in the group that had corneal surgery compared to the group that had no surgery (P = .34).
Genu Valgum
While information about genu valgum was not listed for all patients, 5 patients from 3 families (family A, Z, and M) were documented to have genu valgum. This finding occurred in at least 5 of 115 patients enrolled, or approximately 4% of patients.
DISCUSSION
LIMITATIONS OF THE STUDY
Long-term studies are necessary to understand disease progression. In the case of a rare disease, these studies can provide scientific rather than anecdotal evidence about the typical disease course. While much has been written about SCCD, the rarity of the disease has precluded the collection of long-term data on the extensive number of patients reported in this study.
The large number of pedigrees with SCCD collected for the purpose of genetic mapping provided an opportunity to obtain clinical information about the disease. However, this work was vexed by the challenges inherent to retrospective study. These limitations included missing data at the time of study entry, variability among examining doctors in whether UCVA or BCVA was obtained or the completeness of documentation, inability to standardize how a particular test like corneal sensitivity was actually performed, and the challenge in obtaining follow-up information because patients had moved or did not respond to written requests. The difficulty of obtaining accurate and detailed information was multiplied because this study spanned 2 decades and 3 continents. In addition, it was typically impossible to recapture the missing information after years had passed.
Physicians who referred patients from outside the United States were the initial source and primary contact for foreign patients. If the patient was no longer obtaining care from the same physician, information could not be obtained because the physician rather than the patient had been the contact. Both in and outside the United States, older records were frequently unavailable if the physician had relocated or retired.
For this reason, patients from the United States, who could be contacted directly through mail and phone, were the group that was targeted in order to obtain follow-up information. However, many patients had relocated within the 2 decades from their initial examination, and many of the initial requests for information mailed in 2005 were sent back stamped “return to sender.”
Further research uncovered current addresses, which were then used for the second mailing but no incentives were offered for form completion, and response was still poor with only 19 of 66 (29%) of patients returning their forms. Consequently, patients were then called by telephone, which was successful in bringing the response rate to 55 of 66 (83%) of the group contacted and 55 of 79 (70%) of the entire American cohort. With mail and telephone contact, the response rate in the largest cohorts A, B, and J was 15 of 15 (100%), 15 of 18 (83%), and 6 of 8, respectively. Although telephone contact increased the response rate, the type of information obtained was necessarily limited because chart notes were unavailable. However, the information provided directly from the patient about cholesterol medication, ocular surgery, and deaths in their family could still be used to provide helpful information about the disease course.
Data Analysis
To meet the challenges of incomplete information and poor follow-up; different cohorts were analyzed to confirm or refute trends to minimize the possibility of bias.
For trends involving changes of visual acuity, corneal findings, or surgical intervention with age, there were 4 types of cohorts used. The entire cohort of patients with ages specified (93 patients) was always analyzed because this provided the largest cohort and increased statistical power. Data was compared to the cohort of patients examined by the author personally (47 patients) because this cohort provided consistency of examination technique as all patients were examined by the same doctor. The largest pedigrees, A, B, and J were also examined because the follow-up of all available members of an individual family might decrease selection bias. Finally, analysis of the cohort of patients examined by physicians other than the author (46 patients) provided a means to detect a difference in examination technique by the author versus other physicians or, alternatively, detect a difference in type of patients seen by the author versus other physicians.
When there were similarities between the findings among the groups, conclusions appeared to be confirmed, but when there was a difference among the groups, the data was further analyzed. For example, comparison of the cohorts revealed that 57% of patients examined by the author had crystals compared to crystalline deposits noted in 93% of patients examined by other physicians.
To clarify this large difference in findings, the largest pedigrees were examined. Pedigrees A, B, and J had crystalline deposition in 12 of 19 (63%), 11 of 18 (61%), and 3 of 8 patients, respectively, but most patients were examined by the author. The only pedigree that had 5 or more members with data about crystals that was not examined by the author was pedigree W from Turkey and Y from Germany. In both families, 5 of the 5 family members (100%) had crystalline deposits. The possible explanations for this variation in findings were either that the families the author examined had different clinical manifestations than those examined by others physicians or that the author has a higher index of suspicion to make the diagnosis of SCCD in patients who lacked the characteristic crystalline deposition.37
The second challenge was determination of the incidence of PKP in SCCD.A critical question to address initially was whether the selection of the study population had introduced unacceptable bias. Perhaps patients with the most severe disease were referred for entry into the study.
If this was the case, the number of patients undergoing PKP would be inordinately high. The unwanted result of this preselection could be an inaccurately dismal prediction of the natural history of the disease by suggesting a higher surgical intervention than actually occurs. However, it was also possible that an insufficient follow-up of the cohort could result in the underreporting of PKPs. This could result in a falsely optimistic picture of the disease course.
An attempt to answer this challenge was the separate analysis of the 3 largest pedigrees, which had not only the greatest number of patients examined in each family but also the highest response to the phone and written follow-up questionnaires.
Pedigrees A, B, and J had long-term follow-up ranging from 75% to 100%. Consequently, the prevalence of PKP in these large pedigrees with better long-term follow-up was compared to the entire cohort to see if the results were consistent. In the entire cohort, 20 of 37 patients (54%) aged ≥50 years reported prior PKP. The prevalence of PKP in patients aged ≥50 ranged from 2 of 6 in pedigree A, 5 of 9 in pedigree B and 3 of 5 in pedigree J with the pedigrees with higher PKP incidence having a higher mean age. There was no statistically significant difference between the frequency of PKP in these 3 pedigrees (P =.79)
Despite the many limitations of this study, there appeared to be a consistency of trends of corneal surgical intervention, BCVA, and corneal findings with age, which suggest the accuracy of the conclusions drawn.
THE BASICS
Genetics
Schnyder crystalline corneal dystrophy is inherited as autosomal dominant trait with high penetrance and has been mapped to the UBIAD1 gene on 1p36.1–5 Although most cases of SCCD have a clear pattern of heredity, sporadic cases have been reported.7,32,33,38,47–49 Three of the 34 families, families E, G, and H , reported no history of the disease in prior generations. Although this could not be confirmed because both parents of the proband were not available for examination, the disease appeared to be sporadic by history in these 3 families.
Ethnicity
While the ethnicity of the patients in the literature with SCCD is largely Caucasian, Oriental patients with SCCD have also been reported.14,15,50 In this study, patients were Caucasian, Oriental, and African American. For convenience, family W from Turkey was classified as Caucasian. There are no published articles reporting the occurrence of SCCD in the African American population. Although the initial pedigrees examined, A, B, C, and D were Swede-Finn, the majority of the other US pedigrees did not have Swede-Finn ethnicity. Pedigrees E and J reported Hungarian ancestry, and pedigree Z was from Kosovo. The other pedigrees did not provide information about their ancestry.
THE CHALLENGE OF DIAGNOSING SCCD
Corneal Biopsy
The corneal findings in SCCD are well described in the literature. Nevertheless, determining whether an individual patient has the disease may be difficult, not only because of the rarity of the disease, but also because confusion is introduced by misinformation published about diagnostic criteria. Despite the predictable clinical findings in this dystrophy, as recently as the last decade, 2 articles were published using corneal biopsy rather than slit-lamp examination in order to establish the diagnosis.7,39 As recently as 2001, Ciancaglini40 wrote that “the diagnosis of SCCD is usually based on clinical findings and corneal biopsy.” It is the author’s sincere hope that this current extensive report on SCCD will not only clarify the long-term history of this disease but serve to further clarify the clinical findings of this disease so that corneal biopsy will not be required.
Schnyder crystalline corneal dystrophy causes progressive corneal opacification with age. Grop25 described 17 patients ranging in age from 7 to 82 and observed that patients developed an arcus by age 20, a central opacity at age 30, and a diffuse opacity at age 40. Despite the increasing corneal opacification, he reported that good vision was maintained until the 50s or 60s.
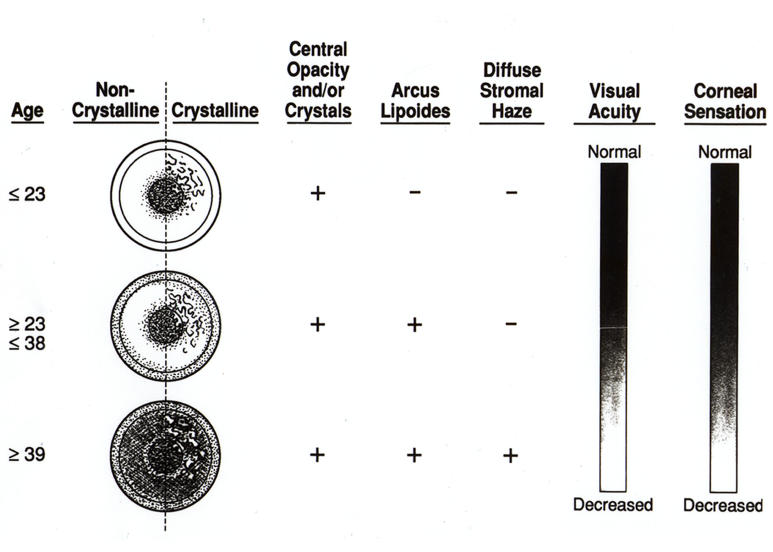
A slightly different schema was published based on the initial examination of 18 affected patients with SCCD in the 4 large Swede-Finn pedigrees35 included in this report. In this article, the central opacity was described to occur first in patients less than 23 years of age, the arcus was present in affected patients between 23 and 37, and those patients older than 37 developed a midperipheral corneal opacification (Figure 27). The present report corroborates most of these prior findings on the course of progression of the corneal findings in the disease. The earliest finding was either a central corneal opacity and/or crystalline deposition. Virtually all patients had one or both of these findings in all age-groups.
FIGURE 27.
Diagram of corneal changes with age. Reprinted from Weiss JS.35
Often the central opacity would have a ringlike formation that allowed the central visual axis to be spared until later in life. Crystals initially appeared to deposit as a ring. The central corneal haze could also be deposited as a ring or as a disc. In early SCCD with central corneal disc like opacification; retroillumination often revealed that the opacity was less dense centrally. Even later in life, the central opacity appeared to be the least dense at its center, when viewed with retroillumination. Delleman and Winkelman33 described different patterns of corneal opacification in SCCD, including a ringlike central deposit.
While arcus lipoides was recorded in 10 of 26 eyes (22%) of the patients <26 years of age in the entire cohort and none of the patients <26 years of age examined by the author; 71 of 93 eyes (97%) of patients in the entire cohort and 47 of 47 eyes (100%) examined by the author in patients who were ≥26 years of age had arcus lipoides.
Quantification of midperipheral haze was more challenging because information about this finding was often not recorded, but examination revealed that no patients <26 years of age had midperipheral haze, 9 of 20 eyes (45%) had arcus between ages 26 and 39. By ≥40, 55 of 65 eyes (85%) had midperipheral haze. This finding was more difficult to determine in the individual patient because it represented the overall progression of corneal opacification that occurs with time in the SCCD cornea. However, there was a statistically significant increase of midperipheral haze in patients ≥40 compared to those < 40 (P < .0001).
This clarification of the corneal changes that developed with age underscores that the major clinical finding in SCCD was a diffuse progressive corneal opacification. Progressive diffuse corneal opacification in SCCD has been previously reported.31,42 As the corneal opacity became more dense, even patients in SCCD pedigrees could observe the corneal opacification with their naked eye. The progressive corneal changes allowed patients to report which family members had “cloudy” corneas (Figure 28).
FIGURE 28.
External photograph of eyes of 68-year-old female, I 1, from family B, with clear cornea after PKP OD and “cloudy” cornea OS from SCCD. Bilateral arcus lipoides is apparent. Figure 15 demonstrates magnified view of corneal changes OD before PKP.
Crystals in the Difficulty of Diagnosing SCCD
For decades, the literature has reflected that an integral part of SCCD diagnosis was the deposition of cholesterol crystals. The importance of crystals in making the diagnosis of SCCD was first challenged in 1993, when examination of 4 large SCCD pedigrees revealed only 50% of patients had cholesterol crystal deposition. Nevertheless, the majority of published articles about SCCD describe the corneal crystalline change10,11,13,16–32 although diagnosis of the disease in absence of crystals is also described.10,13,19,25,28,33,34
McCarthy9 and coworkers described a 62-year-old with bilateral corneal clouding with history of poor vision in both of the deceased parents and corneal opacification in the patient’s daughter. There were no crystals present, and despite the apparent autosomal dominant inheritance, the patient received a diagnosis of macular dystrophy, which is an autosomal recessive inherited corneal dystrophy. Histopathology demonstrated lipid infiltration characteristic of SCCD but absence of alcian blue staining. Alcian blue stains mucopolysaccharides, which are deposited in macular dystrophy. Consequently, the histopathological staining pattern was characteristic of SCCD, not macular dystrophy, despite the initial misdiagnosis on clinical examination.
Previously, many thought that the presence of crystals was integral to the diagnosis of SCCD. In 1972, Garner and Tripathi24 wrote about a SCCD case described by Offret51 that “must be accepted with some reservation since cholesterol crystals were not demonstrated.” Unfortunately, the incorrect presumption that a patient cannot have SCCD unless crystals are present is still fairly prevalent. Even more recent literature indicates that the disease is characterized by presence of crystals and that while a noncrystalline form occurs, it is much less common36 or that “the main features…crystalline spindle shaped deposits.”40
Perhaps, then, it should not have been surprising to discover the large difference in the prevalence of crystalline deposition between patients examined by the author, who found crystals in 57% of the eyes examined, compared to the other physicians, who reported crystals in 93% of eyes they examined. While one possible explanation was that the Swede-Finn pedigrees of A, B, C, and D examined by the author could have had different manifestations of the dystrophy than the majority of the pedigrees; pedigree J with Hungarian ancestry was also examined by the author. The majority of members of this pedigree also did not have crystals. Typically, photographs of the patients who were not examined by the author appeared to have similar changes as those patients examined by other physicians. For example, the slit-lamp photo of the corneal changes in 38-year-old Taiwanese female were similar to the changes in a 38-year-old American male (Figure 10).
Another possible reason why the author saw more patients with SCCD without crystals is that cases of SCCD without crystals were not diagnosed by others. The challenge of making the diagnosis of SCCD in these patients has been previously reported.36 The detection of early central panstromal haze in a patient with early SCCD without crystals is very difficult. The author initially misdiagnosed a 23-year-old male in pedigree A (Patient III 1 in Figure 3) as being unaffected because no corneal opacification or crystals were detected on slit-lamp examination. Genetic testing subsequently revealed that the patient had the defect on chromosome 1 indicating he was affected with SCCD. Repeated slit-lamp examination when the patient was age 30 revealed extremely subtle signs of central corneal clouding and arcus bilaterally. Even at that age, it would have been easy to dismiss the subtle corneal clouding that was noted on examination if the examiner had not prior knowledge about the history.
It is not possible to determine whether the pedigrees examined by the author had different disease manifestations or whether the acrystalline form of the disease was not diagnosed by referring physicians. However, other findings, such as average BCVA, loss of BCVA over time, and age at surgical intervention, did not seem to vary between the pedigrees.
The increased incidence of PKP with age was associated with the progressive corneal opacification that is characteristic of the disease. It is important to emphasize that despite the emphasis on corneal crystalline deposition in SCCD, which may or may not be present in an individual patient, all patients manifest the finding of progressive corneal clouding. Some patients with SCCD who lack the characteristic corneal crystals consult with many ophthalmologists, including corneal specialists, in their quest for a diagnosis. The difficulties experienced by multiple members of family J who did not obtain a definitive diagnosis for the corneal clouding even after undergoing PKP, illustrate the problem.
Two Families With Clinical and Histopathologic Misdiagnosis
A 74-year-old male from family J (patient I 1 in Figure 5) sought the author’s opinion because of an inability to find out why family members had “cloudy cornea” despite examinations over the past 10 years by multiple well-respected corneal specialists. Both he and 2 brothers had even undergone successful PKPs, but no conclusive diagnosis was obtained from the histopathologic examination of corneal specimens. The patient was taking a cholesterol-lowering agent for hypercholesterolemia and reported a strong family history of “cloudy eyes.” Despite diffuse cornea clouding OD, which made it difficult to examine anterior segment structures (Figure 17), the BCVA was surprisingly good at 20/25 OD. He had a clear corneal transplant OS, but BCVA was reduced to 20/40 in this eye because of a Hollenhorst plaque. Although the corneal haze was diffuse without a clearly defined central opacity and an arcus which appeared to blend into the diffuse cornea haze, the corneal findings were consistent for SCCD without crystal deposition
Other members of the patient’s family (pedigree J) were examined. The patient’s 80-year-old brother ( patient I 2 in Figure 5) had a PKP OD 3 years previously for corneal clouding, and chart notes revealed the corneal specialist listed the diagnosis in this eye as central cloudy dystrophy of Francois (CCDF). On postoperative examination, BCVA was 20/30 OD and 20/40 OS. The PKP OD was clear, whereas the corneal examination OS showed diffuse corneal clouding slightly more prominent centrally and no crystalline deposits (Figure 29). The stromal opacification was tessellated, which was similar to that seen in CCDF or posterior crocodile shagreen.
FIGURE 29.
External photograph of cornea of an 80-year-old male, I 2, in family J, with BCVA of 20/30 OD and diffuse corneal haze with tessellations reminiscent of central cloudy dystrophy of Francois or posterior crocodile shagreen. OS had undergone PKP 3 years before.
Tessellation of the corneal opacity in SCCD has been previously reported.50 Review of slit-lamp photos of the patients with SCCD examined in this study revealed members of pedigrees A, B, C, G, J, and X (Figure 14) with a central opacity that contained polygonal opacities similar to posterior crocodile shagreen or CCDF. It was not possible to determine whether the polygonal opacities represented an additional corneal degeneration, posterior crocodile shagreen, or just another pattern of morphology of lipid deposit. In addition, the fact that the 80-year-old patient was having visual disability associated with the corneal clouding argued against CCDF, because CCDF is reported to cause no visual disability.52–55
The histopathology report from the 74-year-old’s prior PKP surgery was requested. The preoperative pathology diagnosis was corneal opacity. Postoperative pathology diagnosis was endothelial corneal degeneration with bullous keratopathy and central corneal leukoma. The slide was reviewed, and it appeared that the endothelium could have been stripped in processing, which gave the misdiagnosis of bullous keratopathy; no central scarring was noted. It was difficult to make any specific diagnosis on basis of re-review of the specimen because the prior routine processing of the slide prevented subsequent stains for lipid.
The son (patient II 1 in Figure 5) of the initial patient was examined with BCVA of 20/25 OD and 20/50 OS. There was a history of amblyopia OS and evidence of cataract formation OU. Corneal examination revealed bilateral central corneal opacity, subepithelial corneal crystals, midperipheral haze, and arcus (Figure 30).
FIGURE 30.
53-year-old male, II 1, in family J (son of patient I 1 in Figure 17) with BCVA 20/25 OU, central corneal haze and crystals, midperipheral haze, and arcus lipoides.
In total, the author found that 9 members of the pedigree had SCCD with bilateral corneal opacification with 3 of 9 patients having cholesterol crystalline deposition on initial examination.
Should the diagnosis of SCCD have been apparent initially? The 80-year-old proband reported that he had seen 5 corneal specialists throughout the prior decades and was unable to obtain a definitive diagnosis. While the constellation of clinical findings in the 2 brothers was challenging, namely the absence of crystal deposition and the diffuseness of the corneal changes, they were within the spectrum of SCCD findings. The patients had a history suggestive of autosomal dominant inheritance, hypercholesterolemia, corneal opacification so severe that the patient himself could remember other family members with corneal clouding, and BCVA that appeared disproportionately good compared to the severity of the opacity. All of these findings were highly suggestive, if not diagnostic, of SCCD.
Why Histopathology in SCCD Does Not Always Yield the Diagnosis
Unfortunately, the histopathologic changes associated with abnormal lipid deposition in the cornea may be missed if the specimen is not processed properly. If the ophthalmologist does not suspect the disease and alert the pathologist, the opportunity to make the diagnosis may be lost because the lipid can be dissolved by routine processing.
The inability to obtain accurate pathology was also observed to occur in a patient from pedigree U, who reported that he could see the “arch around” his father’s eye “but no clouding.” Correspondence with the patient indicated that at age 30, he was initially diagnosed at “a reputable university eye clinic” to have “atypical granular dystrophy.” He wrote that “years later, it was changed to Schnyder” during an examination with “two well respected corneal specialists.” PKP was performed, but no indication of the suspected clinical diagnosis was written on the pathology specimen. The final pathology report indicated “focal loss of endothelial cells consistent with Fuchs endothelial dystrophy.” No lipid stains were performed.
Ophthalmologists are cautioned of the importance of alerting the pathologist when considering a diagnosis of sebaceous cell carcinoma because without the proper preparation of the specimen, lipid can dissolve and the opportunity to make the diagnosis with lipid stains can be lost. If tissue is not embedded properly, staining for lipids can be negative because the lipids are dissolved out during the dehydrating stage of embedding.26
Without proper preparation of the corneal specimen in SCCD to avoid fixatives that dissolve the lipid, the opportunity to do special staining in SCCD may be lost as well.
HISTOPATHOLOGY
Light and Electron Microscopy
Histopathology of SCCD has been well described with abnormal lipid deposition throughout the corneal stroma.7,23,24,32–34,47,51,56–60
Lipid deposits have been reported particularly in the superficial stroma and Bowman’s. These stain positive with oil red O or Sudan black. But these dyes are lipid-soluble and stain only esterified cholesterol, not unesterified cholesterol61 (Figure 31). Nonesterified cholesterol, cholesterol esters, and phospholipids have been found to be the predominant lipids in the SCCD cornea.9 Crystalline deposits in SCCD have been shown to be cholesterol.24,33,47,61
FIGURE 31.
Light microscopy of the SCCD cornea with reddish hue from staining of the lipid deposits with oil red O (oil red O, ×40).
The typical compounds that are used for ultrastructural studies, such as osmium tetroxide and organic solvents and resins, can dissolve lipids. However, cryoultramicroscopy allows ultra-thin sections of cryopreserved lipid-laden tissue that can then be stained with filipin, which is a fluorescent probe that specifically detects unesterified cholesterol (Figure 32). This technique reveals that the major constituent of the corneal deposit in SCCD is unesterified cholesterol with smaller amounts of other lipids.28 Electron microscopic analysis has revealed intracellular and extracellular lipid throughout the stroma with vacuoles representing dissolved lipid cholesterol in the basal epithelium, stroma, and occasionally within endothelial cells (Figure 33).34
FIGURE 32.
Fluorescence noted from stromal deposition of filipin stained lipid (filipin, ×40).
FIGURE 33.
Left, Basal epithelial cells, corneal stroma, and few endothelial cells demonstrated dissolved lipid and cholesterol (toluidine blue, ×250). Right, Electron microscopy demonstrating lipid deposits in posterior stroma and pre-Descemet’s area (×9900).
Animal models for SCCD exist. Histopathology of the condition in the animal mode is similar to that found in humans.62,63 Crystalline stromal dystrophy is the commonest canine corneal lipid deposition and is relatively common in the Cavalier King Charles Spaniel. Corneal opacities similar to SCCD have also been produced by feeding a cholestanol-enriched diet to BALB/c mice, but these are associated with corneal vascularization, which is not present in SCCD. In this animal model, the serum cholestanol was 30 to 40 times normal, and the corneal deposits were composed of calcium phosphorous and probably cholestanol.64
Chemical Analysis
Quantitative analysis of the cornea in SCCD reveals that the lipid accumulation is mostly unesterified cholesterol and phospholipids.9 Lipid analysis of the corneal specimens from patients affected with SCCD who have undergone PKP demonstrates that apolipoprotein constituents of HDL (apo A-I, A-II, and E) are accumulated in the central cornea, whereas those of the LDL (apo B) are absent. This suggests an abnormality confined to HDL metabolism.45 HDL concentrations in the serum are inversely related to the incidence of coronary atherosclerosis.65
Chemical analysis of corneas removed from patients with SCCD reveal that the cholesterol and phospholipids contents increase greater than 10-fold and 5-fold, respectively, in affected corneas compared to normal corneas. Sixty-five percent of the cholesterol is unesterified compared to the control cornea, where 50% is esterified. Unesterified cholesterol to phospholipid molar ratios (1.5 vs .5) are higher in affected compared with normal corneas. Western blots confirm increased amounts of HDL apolipoproteins, indicating that there is a specific local metabolic defect in HDL metabolism in the corneas of SCCD patients. Interestingly, human and animal atherosclerotic lesions have also been reported to stain positive for filipin, demonstrating the accumulation of unesterified cholesterol.22,45,66
Yamada and associates15 confirmed the findings of increased unesterified cholesterol in the SCCD cornea with their chemical analysis that the SCCD cornea had only 14% of cholesterol esterified in comparison 60% to 71% esterified corneal cholesterol found in controls. Sphingomyelin was found at 33 times the concentration that was found in controls. Primary lipid keratopathy is also reported to have elevated unesterified cholesterol and sphingomyelin.
Similarity to Findings in Atherosclerosis
Filipin-stained deposits of unesterified cholesterol that are found in the SCCD cornea are similar to the filipin-stained deposits of unesterified cholesterol found in atherosclerotic lesions. In the vessels, plasma lipoprotein is the source of cholesterol. It is unclear what the source of cholesterol is in the SCCD cornea.66
ADDITIONAL CHARACTERISTIC CORNEAL FINDINGS IN SCCD
Corneal Sensation
While many patients did not have assessment of corneal sensation; approximately 27 of 43 (63%) of eyes of patients ≥40 years of age had decreased corneal sensation. In patients ≥40 years of age, 3 of 7 eyes had decreased corneal sensation in pedigree A, 6 of 12 (50%) in pedigree B, and 19 of 35 (54%) in patients examined by the author. While pooling of objective measurements of corneal sensation like Cochet Bonnet, with subjective assessment of the cotton wisp test, was not ideal for statistical analysis; the studies funding is confirmed by previous published reports of decreased corneal sensation in SCCD.7,25,57
Confocal microscopy has demonstrated the deposition of highly reflective deposits in the early stages of SCCD. Lipid deposits are noted inside keratocytes and along basal epithelial/subepithelial nerve fibers. Later in the disease, deposition of large extracellular crystals and reflective extracellular matrix results in disruption of basal epithelial/subepithelial nerve plexus. This corresponds with the clinical finding of loss of corneal sensation.40,44
VISUAL LOSS IN SCCD
The literature has suggested that SCCD typically causes minimal visual morbidity, with some investigators even reporting that “visual acuity often is unaffected,”39 For purpose of statistical analysis, both UCVA and BCVA were converted to logMAR units for all analysis in this study.
To assess the actual impact of SCCD on visual acuity, a 3-pronged approach was taken. The first was determining the visual acuity on initial examination of all patients who had no other ocular pathology and plotting the BCVA with increasing patient age (Figure 7). The second approach was to determine how vision had changed in the individual patient with time (Table 3).
The third approach was to examine the number of patients who reported corneal surgical intervention. The BCVA within 1 year prior to PKP was examined to determine the indications for intervention (Table 4). The percentage of patients in each decade of age that had reported undergoing PTK or PKP was also graphed (Figure 25). Surgical intervention was assumed to be an indirect indication of visual loss, as presumably only those patients with significant visual disability would undergo PKP or PTK.
While 75 of 93 patients had BCVA on initial examination (Figure 6); 44 of these 149 eyes were eliminated from analysis because of coexisting ocular pathology, including prior corneal surgery, cataracts, amblyopia, macular degeneration, and other retinal pathology. Perhaps somewhat predictably, 38 of the eyes with coexisting ocular pathology were in patients ≥40 years of age with the most frequent exclusionary factor being cataract. Although it is possible that some of the cataracts were visually insignificant and perhaps these eyes did not have to be excluded from visual acuity analysis, stringent criteria gave more assurance that any visual decrease associated with age would most likely only be associated with increasing corneal opacification because of SCCD.
While there was a statistically significant decrease in BCVA between those patients ≥40 years and those <40 (P < .0001), the mean Snellen BCVA was excellent in all age-groups. In those patients <40 years of age, mean Snellen BCVA was between 20/20 and 20/25, and in those patients ≥40 years of age, mean Snellen BCVA was between 20/25 and 20/30. Regression analysis demonstrated a weak trend of small deterioration in BCVA with age (Figure 7).
The overall maintenance of good visual acuity and the slow deterioration of BCVA were confirmed in the small cohort of 34 eyes that had 7 or more years of follow-up with a mean follow-up of 11.4 years. While 7 of 34 eyes underwent PKP, 21 eyes stayed within 1 line of initial visual acuity. Four additional eyes lost 2 lines of BCVA. Two eyes lost 3 lines of BCVA to final BCVA of 20/40 OU. All other eyes which had no other concomitant pathology had a final BCVA of at least 20/30. In fact, a 61-year-old woman from family D who had been followed for 15 years maintained a BCVA OU of 20/25 on her most recent visit (Figure 21 and Table 3).
Lisch and associates10 reported on 13 patients affected with SCCD that were followed for 9 years. All patients who were less than 40 years of age maintained visual acuity of at least 20/30 on second examination. Of the 3 patients that were 40 years or older, a 68-year-old had PKP, with preoperative visual acuity of 20/80 but no mention was made if there was any other ocular pathology; another 65-year-old maintained 20/30 visual acuity; and a 48-year-old had visual decrease from 20/50 OU to 20/100 OU. Unfortunately, no information was provided as to other ocular pathology, such as cataract formation.
In the current study, the slow deterioration of visual acuity and the maintenance of excellent BCVA did not explain why such a large percentage of eyes (7 of 34, 21%) followed for at least 7 years had PKP. Apparently, there was a visual impairment that was not explained by the measurement of scotopic visual acuity alone. Glare testing was not included in initial protocol and was documented in only a few patients older than 40, so the percentage of patients having loss of photopic vision could not be quantified.
Scotopic Versus Photopic Visual Acuity in the SCCD Patient
However, some charts did indicate that there was a subjective complaint of glare and a marked decrease in vision in the lightened room for some patients. The difference between scotopic and photopic visual acuity in the SCCD patient was discussed by Paparo and coworkers,36 who postulated that diffraction of light from corneal crystals resulted in a loss of photopic vision in SCCD. Fagerholm42 further suggested that although the crystals could result in light diffraction causing glare and photophobia, the diffuse general haze itself was another cause of decreased vision.
An attempt to quantify the effect of SCCD on photopic vision was performed over a decade ago by Van den Berg and coworkers.67 They postulated that the phenomenon of intraocular straylight explained the reduced visual quality in SCCD. Intraocular straylight occurs “when the retina receives light at locations that do not optically correspond to the direction the light is coming from.” Straylight was increased in the 4 eyes of SCCD patients that they measured, while visual acuity was relatively spared. This light-scattering phenomenon explained why patients were frequently bothered by loss of contrast and glare. The investigators thought that the corneal opacification, rather than the crystals alone, were the cause of the abnormal light scattering, which resulted in decreased visual quality, retinal contrast reduction, and glare. In a darkened room, they noted the patient maintained “relatively well preserved visual acuity.”67
The stray light hypothesis suggested a reason for the higher numbers of PKPS in the long-term follow-up of SCCD patients in this study than would have been anticipated considering the benign visual prognosis that this dystrophy has traditionally carried. Although the level of visual deterioration was slow and good BCVA seemed to be maintained; an increasing percentage of patients still underwent PKP with age. BCVA was reported to be as good as 20/25 in one patient prior to PKP. At the same time, those few patients who had glare testing documented demonstrated a decrease in visual acuity when lights were turned on.
PREVALENCE OF PKP IN SCCD
Although there are frequent reports of PKP in SCCD10,15,23,26,30,32,33,57,60,61,68 the literature reports that SCCD “rarely requires corneal grafting.”32,49
In the current study, 39 eyes of 27 patients underwent PKP with an increasing number of PKPs reported as patients aged. The prevalence of PKP in patients ≥50 years was 20 of 37 (54%). Ten of 13 patients ≥70 years (77%) had PKP. Only 3 patients ≥70 had no history of having PKP. Chart notes of the 2 older patients who had not had corneal surgery indicated that PKP was being considered. Chart notes were unavailable for the third patient, who lived in Turkey. This analysis implied that PKP was either performed or strongly considered in every SCCD patient who was above the age of 70.
Why was PKP performed so frequently if the BCVA did not appear to be markedly decreased? The first possibility was that selection bias recruited patients with more severe disease and artificially resulted in an increased PKP prevalence in this disease. This possibility was previously discussed in the “Data Analysis” section. A second possible explanation for the large number of PKPs performed was that PKPs could have been performed earlier than usual if the corneal surgeon was more aggressive. However, each of the patients who had preoperative BCVA of 20/50 or better within 1 year prior to the PKP originated from a different pedigree and had the PKP performed by a different surgeon. Another possibility for a higher surgical intervention than anticipated was that the approach to SCCD has changed during the years with earlier intervention because of the successful results of PKP surgery. While any of these explanations could explain a higher number of PKPs than would be expected on the basis of the corneal findings and visual acuity, the analysis of the individual pedigrees that had excellent follow-up still serves to give a good estimate of PKP frequency.
Preoperative Visual Acuity and Glare Before Penetrating Keratoplasty
Although the study was limited by number of patients who had preoperative vision within 1 year of PKP, 13 eyes had preoperative BCVA within 1 year of PKP documented.
Nine eyes of 5 patients had preoperative BCVA that was ≥20/50, including one eye with cataract and another with prior PTK. Only 3 patients with preoperative BCVA ≥20/50 had no concomitant ocular pathology. However, all 3 had preoperative documentation of glare complaints or decrease in vision under photopic conditions. The combination of good BCVA prior to surgery with a documentation of a subjective complaint of glare supports the hypothesis that SCCD may disproportionately affect scotopic vision and motivate the patient to have PKP sooner than the photopic vision might indicated.
The question of subjective glare was further clarified by an attempt to repeat the phone interview of the 55 American patients who had originally responded to phone or written follow up. Forty-one patients were reached and again interviewed by phone. Patients were asked about symptoms of glare during day and night and about functional limitations such as difficulty reading, using a computer, driving during day or night because of visual problems.69
Mean patient age was 43.8 ± 21.0 years (range, 6–83 years). Subjective decrease in near and distance vision was reported by 6 of 41 patients (14.6%) Nighttime glare was reported by 26 of 41 patients (63.4%), of whom 9 stopped or limited night driving. Nighttime glare was reported in 0 of 8 patients <25 years of age, 10 of 12 patients (83.3%) ≥25 and <45 years of age, and 16 of 21 patients (76.2%) ≥45 years of age. Daytime glare was reported by 11 of 41 patients (26.8%), one of whom reported having to stop watching television because of glare problems. Daytime glare was reported in 0of 8 of patients <25 years of age, 1 of 12 (8.3%) patients ≥25 and<45 years of age, and 10 of 21 patients (47.6%) ≥45 years of age. Prevalence of reported glare increased with age both in daytime (P = .008) and nighttime (P = .0002).
The brief phone survey had many limitations, including providing subjective, not objective, information about the prevalence of glare and lack of a control group to compare the prevalence of glare to a population unaffected with SCCD. However, the data still provides some confirmation that glare appears to be a prominent complaint in patients with SCCD and that the complaint of glare increases with age. This lends support to the hypothesis of Van den Berg and coworkers67 that progressive corneal opacification in SCCD causes light scattering. In addition, this would support the hypothesis that glare symptoms could be a potential cause for the high number of PKP in the SCCD population.
INDICATIONS FOR PENETRATING KERATOPLASTY IN THE LITERATURE FOR SCCD AND OTHER STROMAL DYSTROPHIES
Most articles written about PKP in SCCD are case reports, and so there is no recommendation in the literature on when to perform PKP for the SCCD patient. In addition, case reports on PKP in SCCD often lack important data to assess indications for surgery. For example, Weller and Rodger reported PKP was performed for “unmarried woman in her 50s….who couldn’t carry out her job” but the authors did not list vision prior to PKP.32
Ingraham39 reported PKP in a 46-year-old with BCVA of 20/80 but did not indicate whether there was any other pathology that could be causing visual decrease, such as cataract. Rodrigues and coworkers61 discussed PKP OD for a 57-year-old with BCVA OD of count fingers and OS 20/50 and complaints of photophobia but the patient also had cataract formation more prominent in the OD than OS. Was the SCCD causing the visual decrease and photophobia OD, or was it the cataract? The aging patient may have concomitant ocular pathology, such as cataract formation, which can reduce vision and cause glare symptoms. Without clear information about the complete ocular examination, it is difficult to use the published literature to clearly determine the indications for surgical intervention in SCCD.
How does the preoperative level of BCVA in the patients in this report prior to PKP compare to 2 studies of patients with corneal stromal dystrophies undergoing PKP? Ellies and coworkers70 examined 110 eyes of 73 patients with BIGH3 mutations who underwent PKP. The investigators indicated that PKP was performed for BCVA that was 20/80 or worse. Another study, by Al-Swailem and coworkers,71 reports 229 PKPs that were performed in patients with macular dystrophy; 68% of patients had preoperative visual acuity of 20/100 to 20/180.
SUCCESS OF PENETRATING KERATOPLASTY IN SCCD
The present study was limited by the lack of information on preoperative vision within a year of surgery and postoperative vision in the majority of PKP eyes. The 11 eyes in with documentation of both preoperative and postoperative visual acuity appeared to do well after PKP. Five eyes improved by 1 or more lines of BCVA. One eye with 20/30 BCVA preoperatively maintained the same visual acuity postoperatively. The remaining 5 eyes had other ocular diagnoses, including suture abscess or macular degeneration, and maintained the same visual acuity or loss of 1 line of vision. Only 1 patient reported a graft rejection and no patients reported repeat PKP in the same eye.
PHOTOTHERAPEUTIC KERATECTOMY
PTK has been reported to be successful in removing crystalline opacities that are impairing vision in SCCD.36,40,42,43,46,72–78 Paparo and coworkers36 reported 4 eyes of 3 patients with SCCD and central corneal crystals who had PTK. In all cases, the patients complained of glare or photophobia, and BCVA worsened in the lighted room. When crystals were removed after PTK, there was subjective improvement in glare and photophobia and average BCVA improved from 20/175 to 20/40 in bright light, but vision was still best under scotopic conditions. However, the average hyperopic shift was +3.28.
In the present study, PTK was performed to remove the central cholesterol crystals that were causing impairment of vision. Three patients underwent PTK with an improvement in vision in 4 of 5 eyes (Figure 11). PTK in one eye of a 41-year-old patient did not improve the preoperative BCVA of 20/50, and the patient subsequently had PKP (Figure 22, right). This patient was older than the other 2 patients who had successful PTK. By age 41, it was possible that concomitant stromal opacification resulted in visual decrease even after the crystalline opacity was removed by PTK.
Recurrence
Recurrences of SCCD after PKP have been previously reported,7,10,24,33 but there is no consensus how frequently this occurs. Delleman and Winkelman indicated recurrence was common.33 In a retrospective review of all patients with stromal dystrophies undergoing PKP at Wills Eye Hospital between 1984 and 2001, only 4 eyes of 4 patients with SCCD had PKP. There was no recurrence of the dystrophy in any of the eyes in up to 4.6 years of follow-up and so the investigators concluded that the dystrophy had a low recurrence rate. This compared to a follow-up of 5 years with a recurrence rate of 88% in corneal dystrophies of Bowman’s layer, 40% recurrence rate in granular dystrophy, and a 17.8% recurrence rate in lattice dystrophy.79
In this study, 5 of the 27 patients and 8 of the 39 eyes (21%) undergoing PKP had evidence of recurrence. While all of these patients had bilateral PKP, recurrence was unilateral in 2 patients and bilateral in 3 patients. The rate of recurrence for SCCD in this study appears to be most similar to the recurrence rate for lattice dystrophy found by Marcon and associates.79
DIFFERENTIAL DIAGNOSIS OF SCCD
Crystalline Deposits, Cloudy Corneas, and Disorders of Lipid Processing
Crystalline deposits may be found in numerous diseases, including cystinosis, dysproteinemias, multiple myeloma, monoclonal gammapathy, calcium deposits, oxalosis, hyperuricemia, Tangier disease, tyrosinosis, porphyria, Bietti’s crystalline dystrophy, infectious crystalline keratopathy; instillation of sap from the Dieffenbachia plant; and in association with ingestion of drugs such as gold, indomethacin, chlorpromazine, chloroquine, and clofazimine.7,80
Primary or secondary lipid corneal degeneration is associated with corneal neovascularization with subsequent leakage of lipid into the cornea. While primary lipid corneal degeneration has no known underlying cause, secondary lipid degeneration is typically secondary to chronic inflammation. In both entities, progressive lipid deposition results in corneal opacification with potential decrease in visual acuity. Histopathology reveals lipid granules, histiocytes, vascularization, and nongranulomatous inflammation.81,82 This is easily distinguished from SCCD because corneal blood vessels are absent in SCCD.83
Familial lecithin-cholesterol acyltransferase deficiency (LCAT), fish eye disease, and Tangier disease should also be considered in the differential diagnosis of SCCD.6,84
Lecithin-Cholesterol Acyltransferase Deficiency
In LCAT, there is absence of the LCAT enzyme that is involved in cholesterol metabolism. Unlike SCCD, LCAT is inherited in an autosomal recessive mode with deficient activity of the enzyme LCAT to esterify cholesterol in the LDL and HDL particles. The plasma may appear turbid because of the elevated free cholesterol and lecithin levels. Normochromic anemia and/or renal disease may occur.
Similarly to SCCD, corneal changes may occur before puberty with a prominent arcus lipoides and minute gray diets affecting the entire corneal stroma.85. When crystals occur, they occur in the peripheral stroma near Descemet’s 86 rather than the superficial stroma like SCCD. Vacuoles are noted in Bowman’s layer and throughout the stroma.87
Fish Eye Disease
In the extremely rare disease fish eye, the LCAT enzyme has deficient activity in esterifying cholesterol in HDL particles.84 The disease is autosomal recessive with little systemic disorder except for hypertriglyceridemia and reduced HDL levels. On clinical examination of the patient with fish eye disease, there is almost complete corneal opacification, sometimes with arcus noted and significant loss of vision by age 15. Phospholipid and cholesterol are noted throughout the corneal layers except epithelium on histopathology examination.
Tangier Disease
Tangier disease results from a deficiency of HDL and apolipoprotein, apo A1, due to increased catabolism. Many associated systemic disorders may accompany this autosomal recessively inherited disease, including lymph node enlargement, peripheral neuropathy, and hepatosplenomegaly. No arcus lipoides is noted, although there is a granular stromal haze. LCAT activity is normal, triglycerides are elevated, and there is a reduction of total cholesterol, HDL and LDL.88
Although all these diseases affect cholesterol metabolism and cause corneal clouding, there are many characteristics that allow differentiation from SCCD. Whereas SCCD is inherited in an autosomal dominant mode, LCAT, fish eye, and Tangier are autosomal recessive inherited diseases. None of the diseases have the subepithelial cholesterol crystalline deposition that can occur in SCCD. HDL is not typically affected in SCCD, but low HDL levels are seen in LCAT, fish eye and Tangier disease.34
PATHOGENESIS
Hyperlipidemia and Corneal Clouding in SCCD—Independent Variables or Causative Association and the Role of UBIAD1 in Understanding Disease Mechanism
While premature occurrence of corneal arcus is reported to be associated with coronary artery disease,89–91 corneal arcus has also been reported to occur independent of abnormal lipid levels or other systemic disorders.12 Previously, the systemic hyperlipidemia in SCCD was postulated to be the primary defect resulting in corneal clouding,11,13,47 but this theory lost favor when others documented that patients affected with SCCD may have either normal or abnormal serum lipid, lipoprotein, or cholesterol levels.12,13,90
Although familial hypertriglyceridemia and dysbetalipoproteinemia have been reported, familial hypercholesterolemia is the most common lipoprotein abnormality found14,31,92 in patients with SCCD. Hypercholesterolemia has been reported in up to two-thirds of patients with SCCD.8,93,94 By comparison, the Cavalier King Charles Spaniel and rough collie breeds of dog with crystalline dystrophy usually have normal serum lipid levels.95
Lisch and associates10 followed 13 patients with SCCD for 9 years and concluded that no link could be drawn between the corneal findings and systemic hyperlipidemia, although 8 of 12 patients had elevated cholesterol or apolipoprotein B levels and 6 of 8 had dislipoproteinemia type IIa. Consequently, it is likely that the gene for SCCD results in an imbalance in local factors affecting lipid/cholesterol transport or metabolism. A temperature-dependent enzyme defect has been postulated because the initial cholesterol deposition occurs in the axial/paraxial cornea, which is the coolest part of the cornea.92
Plasminogen activator secretion was also reported as being decreased in SCCD corneal fibroblasts when compared to normal fibroblasts, but this work has not been reduplicated.96 The possibility that the gene for SCCD plays an important role in lipid/lipoprotein metabolism throughout the body is supported by an article by Battisti and coworkers,97 who cultured the skin fibroblasts obtained from a skin biopsy of a patient with SCCD. Membrane-bound spherical vacuoles with lipid materials suggesting storage lipids were present in the skin. This work has not been reproduced
Work by Burns and associates48 documented the cornea as an active uptake and storage site for cholesterol. They injected radioactively labeled 14C-cholesterol 11 days prior to removing a patient’s cornea during PKP and demonstrated that the level of radioactive cholesterol was higher in the cornea than the serum at the time of surgery. Furthermore, lipid analysis of the corneal specimens from patients affected with SCCD who have undergone PKP revealed that the apolipoprotein constituents of HDL (apo AI, A-II and E) were accumulated in the central cornea, while those of the LDL (apo B) were absent. This suggested an abnormality confined to HDL metabolism.45 Because of its smaller size, HDL would be the only lipoprotein that could freely diffuse while intact to the central cornea. The size of the larger lipoproteins would prevent their free diffusion unless they were modified.6 HDL concentrations are inversely related to the incidence of coronary atherosclerosis.65 Consequently, it appears that SCCD is directly related to a local defect of HDL metabolism, but the relevance of abnormal HDL corneal metabolism is not yet established.
Recent discovery of UBIAD1 as the causative gene for SCCD will provide the mechanism to understand the pathogenesis of this disease. UBIAD1 contains a prenyltransferase domain that could play a role in cholesterol metabolism. Prenylation reactions are involved in cholesterol synthesis, and it is possible that excess cholesterol synthesis results from a defective gene. In addition, UBIAD1 interacts with the C-terminal portion of apo E which is known to be important in reverse cholesterol transport. Consequently, another possible disease mechanism could be that decreased cholesterol removal from the cell results from an alteration in the interaction with apo E.5
Although this study was not meant to examine cholesterol issues exhaustively, we asked patients who had PKP whether or not they had hypercholesterolemia and if they were on cholesterol-lowering medication. Twenty-one of the 29 patients who had corneal surgery lived in the United States, and 5 of these were deceased. Of the remaining 16 patients, 14 were contacted by telephone.
While 12 of the 14 patients (86%) reported elevated cholesterol levels, 4 of the 14 (29%) had a history of cardiac disease and 10 of the 14 (71%) were on a cholesterol-lowering agent. The mean age of patients with hypercholesterolemia was 68 ± 10.5 years (range, 52–82). There was no statistical difference between the percentage of patients who were ≥50 and who were on cholesterol-lowering medications among patients who had corneal surgery compared to those who did not have corneal surgery (P = .34). The few studies on the effect of systemic cholesterol on progression of the dystrophy conclude that these are independent traits,10 but the numbers of patients and length of follow-up are too small to draw any definitive conclusions. None of the previously published studies have looked at cholesterol measurements specifically in an older cohort.
Coronary Artery Disease and Myocardial Infarction
Although the purpose of this study was to assess the visual morbidity of SCCD, the frequency of hypercholesterolemia in the PKP patient presented the question of whether or not there was early mortality from cardiovascular disease.
Four patients who had PKP and were on cholesterol-lowering medication reported coronary artery disease or prior myocardial infarction. The age at death and cause of mortality for the 8 patients who were known to die during the study were also assessed. Four patients died in the 9th decade. One of these patients had a history of myocardial infarction and the other congestive heart failure. Three brothers died before the 6th decade; one from brain cancer and the other two from auto accidents. Only one patient who died before the 7th decade had a cardiac-related diagnosis of coronary artery disease, bacterial endocarditis, and sepsis.
Although the study is too small to detect any increased risk of mortality from cardiovascular events in this population, it is reassuring that 7 of the 8 deaths did not appear to be a result of premature death from cardiovascular disease.
The importance of obtaining cholesterol measurements in the affected and unaffected members of SCCD pedigrees has been previously emphasized in the literature.38 Perhaps the apparent infrequency of cardiac mortality in this cohort, combined with the large numbers of patients49 undergoing corneal surgery ≥50 years who are taking cholesterol-lowering agents; underscores that appropriate diagnosis and treatment are successful interventions in this disease.
Genu Valgum
Genu valgum has been postulated to be an independent trait7,12,26,98 reported in association with SCCD. The percentage of patients with SCCD that have this finding is not known, but Delleman and Winkelman33 reported that 16 of the 21 SCCD patients in a 6-generation pedigree had genu valgum. Only 1 of 33 patients with SCCD had genu valgum98 in the 4 Swede-Finn pedigrees previously reported.. In the current study, 5 patients in three families had genu valgum.
SUMMARY
Schnyder crystalline corneal dystrophy has previously been a poorly understood disease because of its rarity and spectrum of clinical manifestations. The present study represents the largest number of patients with SCCD and the longest follow-up of patients with SCCD ever published. The information obtained from this large case series should clarify both the clinical findings and the course of SCCD.
The ophthalmologist must be aware that despite individual variations, there are predictable changes in the corneal opacification pattern that can occur with age and that the characteristic crystals may not always be seen on examination. The pathologist must be made aware prior to processing the corneal specimen that SCCD is a consideration so that the cornea be placed in fixatives that will not dissolve lipid and prevent pathologic diagnosis.
My goal in undertaking this study was to attempt to answer the most frequent question asked by a patient newly diagnosed with SCCD. “What can I expect to happen with time?” The patient can be reassured that scotopic vision can be excellent into their 5th decade and beyond. It is most likely that that the major visual disability experienced is loss of photopic vision. In this study, surgical intervention occurred in 54% of patients 50 years and above and almost 77% of patients in the 8th or 9th decade.
Another, perhaps unasked, question is the impact of systemic hypercholesterolemia on mortality. It was reassuring to discover that only 1 of the 8 deaths might have been associated with premature demise from cardiovascular disease. The majority of the nonaccidental deaths were patients in their 9th decade. Consequently, the proper concomitant monitoring and treatment of systemic hyperlipidemia is imperative and may have resulted in normal life span in the majority of patients studied.
ACKNOWLEDGMENTS
Funding/Support: This study was supported by grant EY 12972 from the National Institutes of Health and by research grants from the Eye Bank Association of America, Kresge Eye Institute, Research to Prevent Blindness and Wayne State University.
Financial Disclosures: None.
Other Acknowledgments: I cannot sufficiently express my gratitude to Chaesik Kim BSEE (Kresge Eye Institute), who worked tirelessly on the statistical analysis and spent countless hours with me reviewing the data. His assistance was invaluable. I would like to thank all the doctors who referred SCCD patients, examined SCCD patients, and supported this work over the years, who include Outi Stromsholm, Minnie Vesaluoma, William Dupps Jr, Howard Kruth, Claes Dohlman, Paul Wasson, Walter Lisch, Jay Krachmer, Morat Koksal, Timo Tervo, Chris Rapuano, Peter Laibson, Jim Reidy, John Sutphin, Steven Lane, Don Doughman, Neil Ebeneezer, Marie Hoeltzenbein, Frank Price, James Chodosh, Peter Gloor, Kathryn Colby, Jennifer Cox, Fung-Rong Hu , Da Wen Lu, Stephen Powell, Petra Liskova, and M. Yamada.
REFERENCES
- 1.Shearman AM, Hudson TJ, Andresen JM, et al. The gene for Schnyder's crystalline corneal dystrophy maps to human chromosome 1p34.1–p36. Hum Mol Genet. 1996;5:1667–1672. doi: 10.1093/hmg/5.10.1667. [DOI] [PubMed] [Google Scholar]
- 2.Aldave AJ, Rayner SA, Principe AH, et al. Analysis of fifteen positional candidate genes for Schnyder's crystalline corneal dystrophy. Mol Vis. 2005;11:713–716. [PubMed] [Google Scholar]
- 3.Theendakara V, Tromp G, Kuivaniemi H, et al. Fine mapping of the Schnyder's crystalline corneal dystrophy locus. Hum Genet. 2004;144:594–600. doi: 10.1007/s00439-004-1110-1. [DOI] [PubMed] [Google Scholar]
- 4.Riebeling P, Polz S, Tost F, et al. [Schnyder's crystalline corneal dystrophy. Further narrowing of the linkage interval at chromosome 1p34.1–p36?] Ophthalmologe. 2003;100:979–983. doi: 10.1007/s00347-003-0883-2. [DOI] [PubMed] [Google Scholar]
- 5.Weiss JS, Kruth HS, Kuvaniemi H, et al. Mutations in UBIAD1 gene on chromosome short arm 1, region 36, cause Schnyder crystalline corneal dystrophy. Invest Ophthalmol Vis Sci. 2007;48:5007–5012. doi: 10.1167/iovs.07-0845. [DOI] [PubMed] [Google Scholar]
- 6.Bron AJ. Corneal changes in the dislipoproteinaemias. Cornea. 1989;8:135–140. [PubMed] [Google Scholar]
- 7.Brownstein S, Jackson WB, Onerheim RM. Schnyder's crystalline corneal dystrophy in association with hyperlipoproteinemia: histopathological and ultrastructural findings. Can J Ophthalmol. 1991;26:273–279. [PubMed] [Google Scholar]
- 8.Sverak J, Kindernay S. [Schnyder's hereditary crystalline degeneration of the cornea] Cesk Oftalmol. 1969;25:283–287. [PubMed] [Google Scholar]
- 9.McCarthy MM, Innis S, Dubord P, White V. Panstromal Schnyder corneal dystrophy. A clinical pathologic report with quantitative analysis of corneal lipid composition. Ophthalmology. 1994;101:895–901. [PubMed] [Google Scholar]
- 10.Lisch W, Weidle EG, Lisch C, et al. Schnyder's dystrophy. Progression and metabolism. Ophthalmic Paediatr Genet. 1986;7:45–56. doi: 10.3109/13816818609058041. [DOI] [PubMed] [Google Scholar]
- 11.Sysi R. Xanthoma corneae as hereditary dystrophy. Br J Ophthalmol. 1950;34:369–374. doi: 10.1136/bjo.34.6.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barchesi BJ, Eckel RH, Ellis PP. The cornea and disorders of lipid metabolism. Surv Ophthalmol. 1991;36:1–22. doi: 10.1016/0039-6257(91)90205-t. [DOI] [PubMed] [Google Scholar]
- 13.Bron AJ, Williams HP, Carruthers ME. Hereditary crystalline stromal dystrophy of Schnyder. I. Clinical features of a family with hyperlipoproteinaemia. Br J Ophthalmol. 1972;56:383–399. doi: 10.1136/bjo.56.5.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kajinami K, Inazu A, Wakasugi T, et al. [A case of familial hypercholesterolemia associated with Schnyder's corneal dystrophy] Nippon Naika Gakkai Zasshi. 1988;77:1017–1020. doi: 10.2169/naika.77.1017. [DOI] [PubMed] [Google Scholar]
- 15.Yamada M, Mochizuki H, Kamata Y, et al. Quantitative analysis of lipid deposits from Schnyder's corneal dystrophy. Br J Ophthalmol. 1998;82:444–447. doi: 10.1136/bjo.82.4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Went JM, Wibaut F. En zeldzame erfelijke hoornvliessandoening. Niederl Tijdschr Geneesks. 1924;68:2996–2997. [Google Scholar]
- 17.Schnyder WF. Mitteilung über einen neuen Typus von familiärer Hornhauterkrankung. Schweiz Med Wochenschr. 1929;10:559–571. [Google Scholar]
- 18.Schnyder WF. Scheibenförmige Kristalleinlagerungen in der Hornhautmitte als Erbleiden. Klin Monatsbl Augenheilkd. 1939;103:494–502. [Google Scholar]
- 19.Bec P, Arne JL, Secheyron P, et al. [Schnyder's crystalline dystrophy of the cornea] Bull Soc Ophtalmol Fr. 1979;79:1005–1007. [PubMed] [Google Scholar]
- 20.Chern KC, Meisler DM. Disappearance of crystals in Schnyder's crystalline corneal dystrophy after epithelial erosion. Am J Ophthalmol. 1995;120:802–803. doi: 10.1016/s0002-9394(14)72738-2. [DOI] [PubMed] [Google Scholar]
- 21.Delogu A. [Contribution to the knowledge of Schnyder's crystalline corneal dystrophy. ] Ann Ottalmol Clin Ocul. 1967;93:1219–1225. [PubMed] [Google Scholar]
- 22.DiFerdinando R. Degeneratio cristallinea corneae hereditaria e forma affine. G Ital Oftalmol. 1954;7:476–484. [Google Scholar]
- 23.Freddo TF, Polack FM, Leibowitz HM. Ultrastructural changes in the posterior layers of the cornea in Schnyder's crystalline dystrophy. Cornea. 1989;8:170–177. [PubMed] [Google Scholar]
- 24.Garner A, Tripathi RC. Hereditary crystalline stromal dystrophy of Schnyder. Br J Ophthalmol. 1972;56:400–408. doi: 10.1136/bjo.56.5.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grop K. Clinical and histological findings in crystalline corneal dystrophy. Acta Ophthalmol Suppl (Copenh) 1973;12:52–57. doi: 10.1111/j.1755-3768.1973.tb02552.x. [DOI] [PubMed] [Google Scholar]
- 26.Hoang-Xuan T, Pouliquen Y, Savoldelli M, Gasteau J. [Schnyder's crystalline dystrophy. I. Study of a case by light and electron microscopy] J Fr Ophtalmol. 1985;8:735–742. [PubMed] [Google Scholar]
- 27.Kaden R, Feurle G. [Schnyder's corneal dystrophy and hyperlipidemia (author's transl] Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1976;198:129–138. doi: 10.1007/BF00410013. [DOI] [PubMed] [Google Scholar]
- 28.Lisch W. [The crystalline corneal degenerations with special reference to the primary hereditary crystalline corneal degeneration (author's transl)] Klin Monatsbl Augenheilkd. 1977;171:684–704. [PubMed] [Google Scholar]
- 29.Mielke J, Rohrback JM, Schlote T. [Visual impairment in bilateral arcus lipoides. Schnyder's corneal dystrophy] Ophthalmologe. 2003;100:158–159. doi: 10.1007/s00347-002-0666-1. [DOI] [PubMed] [Google Scholar]
- 30.Rodrigues MM, Kruth HS, Krachmer JH, Willis R. Unesterified cholesterol in Schnyder's crystalline dystrophy. Am J Ophthalmol. 1987;104:157–163. doi: 10.1016/0002-9394(87)90008-0. [DOI] [PubMed] [Google Scholar]
- 31.Thiel HJ, Voigt GJ, Parwaresch MR. [Crystalline corneal dystrophy (Schnyder) in the presence of familial type IIa hyperlipoproteinaemia (author's transl)] Klin Monatsbl Augenheilkd. 1977;171:678–684. [PubMed] [Google Scholar]
- 32.Weller RO, Rodger FC. Crystalline stromal dystrophy: histochemistry and ultrastructure of the cornea. Br J Ophthalmol. 1980;64:46–52. doi: 10.1136/bjo.64.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delleman JW, Winkelman JE. Degeneratio corneae cristallinea hereditaria. A clinical, genetical and histological study. Ophthalmologica. 1968;155:409–426. doi: 10.1159/000305324. [DOI] [PubMed] [Google Scholar]
- 34.Weiss JS, Rodrigues MM, Kruth HS, et al. Panstromal Schnyder's corneal dystrophy. Ultrastructural and histochemical studies. Ophthalmology. 1992;99:1072–1081. doi: 10.1016/s0161-6420(92)31848-2. [DOI] [PubMed] [Google Scholar]
- 35.Weiss JS. Schnyder dystrophy of the cornea. A Swede-Finn connection. Cornea. 1992;11:93–101. doi: 10.1097/00003226-199203000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Paparo LG, Rapuano CJ, Raber IM, et al. Phototherapeutic keratectomy for Schnyder's crystalline corneal dystrophy. Cornea. 2000;19:343–347. doi: 10.1097/00003226-200005000-00017. [DOI] [PubMed] [Google Scholar]
- 37.Weiss JS. Schnyder crystalline dystrophy sine crystals. Recommendation for a revision of nomenclature. Ophthalmology. 1996;103:465–473. doi: 10.1016/s0161-6420(96)30670-2. [DOI] [PubMed] [Google Scholar]
- 38.Kohnen T, Pelton RW, Jones DB. [Schnyder corneal dystrophy and juvenile, systemic hypercholesteremia] Klin Monatsbl Augenheilkd. 1997;211:135–136. doi: 10.1055/s-2008-1035112. [DOI] [PubMed] [Google Scholar]
- 39.Ingraham HJ, Perry HD, Donnenfeld ED, Donaldson DD. Progressive Schnyder's corneal dystrophy. Ophthalmology. 1993;100:1824–1827. doi: 10.1016/s0161-6420(93)31391-6. [DOI] [PubMed] [Google Scholar]
- 40.Ciancaglini M, Carpineto P, Doronzo E, et al. Morphological evaluation of Schnyder's central crystalline dystrophy by confocal microscopy before and after phototherapeutic keratectomy. J Cataract Refract Surg. 2001;27:1892–1895. doi: 10.1016/s0886-3350(00)00840-3. [DOI] [PubMed] [Google Scholar]
- 41.Dinh R, Rapuano CJ, Cohen EJ, Laibson PR. Recurrence of corneal dystrophy after excimer laser phototherapeutic keratectomy. Ophthalmology. 1999;106:1490–1497. doi: 10.1016/S0161-6420(99)90441-4. [DOI] [PubMed] [Google Scholar]
- 42.Fagerholm P. Phototherapeutic keratectomy: 12 years of experience. Acta Ophthalmol Scand. 2003;81:19–32. doi: 10.1034/j.1600-0420.2003.00015.x. [DOI] [PubMed] [Google Scholar]
- 43.Herring JH, Phillips D, McCaa CS. Phototherapeutic keratectomy for Schnyder's central crystalline dystrophy. J Refract Surg. 1999;15:489. doi: 10.3928/1081-597X-19990701-17. [DOI] [PubMed] [Google Scholar]
- 44.Vesaluoma MH, Linna T U, Sankila EM, et al. In vivo confocal microscopy of a family with Schnyder crystalline corneal dystrophy. Ophthalmology. 1999;106:944–951. doi: 10.1016/S0161-6420(99)00514-X. [DOI] [PubMed] [Google Scholar]
- 45.Gaynor PM, Zhang WY, Weiss JS, et al. Accumulation of HDL apolipoproteins accompanies abnormal cholesterol accumulation in Schnyder's corneal dystrophy. Arterioscler Thromb Vasc Biol. 1996;16:993–999. doi: 10.1161/01.atv.16.8.992. [DOI] [PubMed] [Google Scholar]
- 46.Koksal M, Kargi S, Gurelik G, Akata F. Phototherapeutic keratectomy in Schnyder crystalline corneal dystrophy. Cornea. 2004;23:311–313. doi: 10.1097/00003226-200404000-00017. [DOI] [PubMed] [Google Scholar]
- 47.Bonnet P, Paufique L, Bonamour G. Cristaux de cholesterine au centre de la cornee avec gerontoxon. Bull Soc Ophtalmol Fr. 1934;46:225–229. [Google Scholar]
- 48.Burns RP, Connor W, Gipson I. Cholesterol turnover in hereditary crystalline corneal dystrophy of Schnyder. Trans Am Ophthalmol Soc. 1978;76:184–196. [PMC free article] [PubMed] [Google Scholar]
- 49.Gillespie FD, Covelli B. Crystalline corneal dystrophy. Report of a case. Am J Ophthalmol. 1963;56:465–467. doi: 10.1016/0002-9394(63)93136-2. [DOI] [PubMed] [Google Scholar]
- 50.Wu CW, Lin PY, Liu YF, et al. Central corneal mosaic opacities in Schnyder's crystalline dystrophy. Ophthalmology. 2005;112:650–653. doi: 10.1016/j.ophtha.2004.11.047. [DOI] [PubMed] [Google Scholar]
- 51.Offret G, Payrau P, Pouliquen Y, et al. [The fine structure of certain corneal dystrophies] Arch Ophtalmol Rev Gen Ophtalmol. 1966;26:171–181. [PubMed] [Google Scholar]
- 52.Bramsen T, Ehlers N, Baggesen LH. Central cloudy corneal dystrophy of Francois. Acta Ophthalmol (Copenh) 1976;54:221–226. doi: 10.1111/j.1755-3768.1976.tb00435.x. [DOI] [PubMed] [Google Scholar]
- 53.Karp CL, Scott IU, Green WR, et al. Central cloudy corneal dystrophy of Francois. A clinicopathologic study. Arch Ophthalmol. 1997;115:1058–1062. doi: 10.1001/archopht.1997.01100160228013. [DOI] [PubMed] [Google Scholar]
- 54.Meyer JC, Quantock AJ, Thonar EJ, et al. Characterization of a central corneal cloudiness sharing features of posterior crocodile shagreen and central cloud dystrophy of Francois. Cornea. 1996;15:347–354. doi: 10.1097/00003226-199607000-00003. [DOI] [PubMed] [Google Scholar]
- 55.Strachan IM. Cloudy central corneal dystrophy of Francois. Five cases in the same family. Br J Ophthalmol. 1969;54:192–194. doi: 10.1136/bjo.53.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Babel J, Englert U, Ricci A. [Crystalline dystrophy of the cornea. Histological and ultrastructural study] Arch Ophtalmol Rev Gen Ophtalmol. 1973;33:721–734. [PubMed] [Google Scholar]
- 57.Ehlers N, Matthiessen ME. Hereditary crystalline corneal dystrophy of Schnyder. Acta Ophthalmol (Copenh) 1973;51:316–324. doi: 10.1111/j.1755-3768.1973.tb06009.x. [DOI] [PubMed] [Google Scholar]
- 58.Ghosh M, McCulloch C. Crystalline dystrophy of the cornea: a light and electron microscopic study. Can J Ophthalmol. 1977;12:321–329. [PubMed] [Google Scholar]
- 59.Malbran ES. Corneal dystrophies: a clinical, pathological, and surgical approach. 28 Edward Jackson Memorial Lecture. Am J Ophthalmol. 1972;74:771–809. doi: 10.1016/0002-9394(72)91199-3. [DOI] [PubMed] [Google Scholar]
- 60.Pfannkuch F. [Primary hereditary cristalline corneal dystrophy of Schnyder. Histopathology and ultrastructure (author's transl)] Klin Monatsbl Augenheilkd. 1978;173:355–358. [PubMed] [Google Scholar]
- 61.Rodrigues MM, Kruth HS, Krachmer JH, et al. Cholesterol localization in ultrathin frozen sections in Schnyder corneal crystalline dystrophy. Am J Ophthalmol. 1990;110:513–517. doi: 10.1016/s0002-9394(14)77874-2. [DOI] [PubMed] [Google Scholar]
- 62.Crispin SM, Barnett KC. Dystrophy, degeneration and infiltration of the canine cornea. J Small Anim Pract. 1983;24:63–83. [Google Scholar]
- 63.Crispin SM, Bolton CH, Downs LG. The relationships between plasma lipoproteins and corneal lipid deposition in dogs. Clin Sci. 1988;74:12. [Google Scholar]
- 64.Kim KS, Kano K, Kasama T, et al. Effects of cholestanol feeding on corneal dystrophy in mice. Biochim Biophys Acta. 1991;1085:343–349. doi: 10.1016/0005-2760(91)90139-9. [DOI] [PubMed] [Google Scholar]
- 65.Murray RK, Granner DK, Mayes PE, Rodwell VW. Vol. 26. New York: McGraw-Hill; 2005. Harpers Biochemistry: Cholesterol Synthesis, Transport and Excretion. [Google Scholar]
- 66.Kruth HS. Accumulation of unesterified cholesterol in limbal cornea and conjunctiva of rabbits fed a high-cholesterol diet. Detection with filipin. Atherosclerosis. 1987;63:1–6. doi: 10.1016/0021-9150(87)90075-x. [DOI] [PubMed] [Google Scholar]
- 67.Van den Berg TJ, Hwan BS, Delleman JW. The intraocular straylight function in some hereditary corneal dystrophies. Doc Ophthalmol. 1993;85:13–19. doi: 10.1007/BF01268096. [DOI] [PubMed] [Google Scholar]
- 68.Eiferman RA, Rodrigues MM, Laibson PR, Arentsen JJ. Schnyder crystalline dystrophy associated with amyloid deposition. Metab Pediatr Syst Ophthalmol. 1979;3:15. [Google Scholar]
- 69.Shildkrot Y, Kirzhner, Kim C, Weiss JS. Prevalence of glare in a large cohort of patients with Schnyder corneal dystroph. Poster presented at: Association for Research in Vision and Ophthalmology meeting; May 2007; Fort Lauderdale, Florida. [Google Scholar]
- 70.Ellies P, Renard G, Valleix S, et al. Clinical outcome of eight BIGH3-linked corneal dystrophies. Ophthalmology. 2002;109:793–797. doi: 10.1016/s0161-6420(01)01025-9. [DOI] [PubMed] [Google Scholar]
- 71.Al-Swailem SA, Al-Rajhi AA, Wagoner MD. Penetrating keratoplasty for macular corneal dystrophy. Ophthalmology. 2005;112:220–224. doi: 10.1016/j.ophtha.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 72.Forster W, Atzler U, Ratkay I, Busse H. Therapeutic use of the 193-nm excimer laser in corneal pathologies. Graefes Arch Clin Exp Ophthalmol. 1997;235:296–305. doi: 10.1007/BF01739639. [DOI] [PubMed] [Google Scholar]
- 73.Maloney RK, Thompson V, Ghiselli G, et al. A prospective multicenter trial of excimer laser phototherapeutic keratectomy for corneal vision loss. The Summit Phototherapeutic Keratectomy Study Group. Am J Ophthalmol. 1996;122:149–160. doi: 10.1016/s0002-9394(14)72006-9. [DOI] [PubMed] [Google Scholar]
- 74.Orndahl MJ, Fagerholm PP. Treatment of corneal dystrophies with phototherapeutic keratectomy. J Refract Surg. 1998;14:129–135. doi: 10.3928/1081-597X-19980301-11. [DOI] [PubMed] [Google Scholar]
- 75.Rapuano CJ. Excimer laser phototherapeutic keratectomy: long-term results and practical considerations. Cornea. 1997;16:151–157. [PubMed] [Google Scholar]
- 76.Rapuano CJ, Laibson P. Excimer laser phototherapeutic keratectomy. CLAO J. 1993;19:235–240. [PubMed] [Google Scholar]
- 77.Rapuano CJ, Laibson P. Excimer laser phototherapeutic keratectomy for anterior corneal pathology. CLAO J. 1994;20:253–257. doi: 10.1097/00140068-199410000-00012. [DOI] [PubMed] [Google Scholar]
- 78.Tuunanen T, Tervo T. Excimer laser phototherapeutic keratectomy for corneal diseases: a follow-up study. CLAO J. 1995;21:67–72. [PubMed] [Google Scholar]
- 79.Marcon AS, Cohen EJ, Rapuano CJ, Laibson PR. Recurrence of corneal stromal dystrophies after penetrating keratoplasty. Cornea. 2003;22:19–21. doi: 10.1097/00003226-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 80.Brooks AM, Grant G, Gillies WE. Determination of the nature of corneal crystals by specular microscopy. Ophthalmology. 1988;95:448–452. doi: 10.1016/s0161-6420(88)33149-0. [DOI] [PubMed] [Google Scholar]
- 81.Baum JL. Cholesterol keratopathy. Am J Ophthalmol. 1969;67:372–375. doi: 10.1016/0002-9394(69)92047-9. [DOI] [PubMed] [Google Scholar]
- 82.Spraul CW, Grossniklaus HE, Lang GK. [Primary lipid keratopathy] Klin Monatsbl Augenheilkd. 2002;219:889–891. doi: 10.1055/s-2002-36944. [DOI] [PubMed] [Google Scholar]
- 83.Duran JA, Rodrigues-Ares MT. Idiopathic lipid corneal degeneration. Cornea. 1991;10:166–169. doi: 10.1097/00003226-199103000-00013. [DOI] [PubMed] [Google Scholar]
- 84.McIntyre N. Familial LCAT deficiency and fish-eye disease. J Inherit Metab Dis. 1988;11 (Suppl 1):45–46. doi: 10.1007/BF01800570. [DOI] [PubMed] [Google Scholar]
- 85.Vrabec MP, Shapiro MB, Koller E, et al. Ophthalmic observations in lecithin cholesterol acyltransferase deficiency. Arch Ophthalmol. 1988;106:225–229. doi: 10.1001/archopht.1988.01060130235035. [DOI] [PubMed] [Google Scholar]
- 86.Borven I, Egge K, Gjone E. Corneal and fundus changes in familial LCAT-deficiency. Acta Ophthalmol (Copenh) 1974;52:201–210. doi: 10.1111/j.1755-3768.1974.tb00368.x. [DOI] [PubMed] [Google Scholar]
- 87.Bethell W, McCulloch C, Ghosh M. Lecithin cholesterol acyl transferase deficiency. Light and electron microscopic finding from two corneas. Can J Ophthalmol. 1975;10:494–501. [PubMed] [Google Scholar]
- 88.Schaefer EJ, Zech LA, Schwartz DE, Brewer HB., Jr Coronary heart disease prevalence and other clinical features in familial high-density lipoprotein deficiency (Tangier disease) Ann Intern Med. 1980;93:261–266. doi: 10.7326/0003-4819-93-2-261. [DOI] [PubMed] [Google Scholar]
- 89.Halfon ST, Hames CG, Heyden S. Corneal arcus and coronary heart disease mortality. Br J Ophthalmol. 1984;68:603–604. doi: 10.1136/bjo.68.8.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rouhiainen P, Salonen R, Ouhiainen H, Salonen JT. Association of corneal arcus with ultrasonographically assessed arterial wall thickness and serum lipids. Cornea. 1993;12:142–145. doi: 10.1097/00003226-199303000-00009. [DOI] [PubMed] [Google Scholar]
- 91.Virchow AR. Über parenchymatose entrzungdung. Virchow’s Arch Path Anat. 1852;4:261–372. [Google Scholar]
- 92.Crispin S. Ocular lipid deposition and hyperlipoproteinaemia. Prog Retin Eye Res. 2002;21:169–224. doi: 10.1016/s1350-9462(02)00004-6. [DOI] [PubMed] [Google Scholar]
- 93.Karseras AG, Price DC. Central crystalline corneal dystrophy. Br J Ophthalmol. 1970;54:659–662. doi: 10.1136/bjo.54.10.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Williams HP, Bron AJ, Tripathi RC, Garner A. Hereditary crystalline corneal dystrophy with an associated blood lipid disorder. Trans Ophthalmol Soc U K. 1971;91:531–541. [PubMed] [Google Scholar]
- 95.Crispin SM. Crystalline corneal dystrophy in the dog. Histochemical and ultrastructural study. Cornea. 1988;7:149–161. doi: 10.1097/00003226-198802000-00011. [DOI] [PubMed] [Google Scholar]
- 96.Mirshahi M, Mirshahi S, Soria C, et al. [Secretion of plasminogen activators and their inhibitors in corneal fibroblasts. Modification of this secretion in Schnyder's lens corneal dystrophy] C R Acad Sci III. 1990;311:253–260. [PubMed] [Google Scholar]
- 97.Battisti C, Dotti MT, Malandrini A, et al. Schnyder corneal crystalline dystrophy: description of a new family with evidence of abnormal lipid storage in skin fibroblasts. Am J Med Genet. 1998;75:35–39. doi: 10.1002/(sici)1096-8628(19980106)75:1<35::aid-ajmg8>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 98.Hoang Xuan T, Pouliquen Y, Gasteau J. [Schnyder's crystalline dystrophy. II. Association with genu valgum] J Fr Ophtalmol. 1985;8:743–747. [PubMed] [Google Scholar]