Abstract
Purpose
To determine the prevalence of high-risk factors for hydroxychloroquine (HCQ) retinopathy and compliance with the American Academy of Ophthalmology (AAO) screening guidelines at the San Francisco Veterans Affairs Medical Center (VASF) and to develop an approach to improve the risk-benefit relationship and informed consent during HCQ treatment.
Methods
All medical records of patients receiving HCQ were reviewed, with special attention to high-risk factors for retinopathy. The results were used to develop a method of enhancing the risk-benefit relationship and improving informed consent at the VASF.
Results
Of the 109 patients taking HCQ at the VASF, 87% had at least one high-risk factor for retinal toxicity and 47% had two or more risk factors. Thirty-four percent had no evidence of an eye examination having been performed. An approach has been developed to improve the risk-benefit and informed consent for patients using HCQ at the VASF.
Conclusions
A significant number of veterans taking HCQ may be at an increased risk for retinal toxicity. More than one-third of these patients may not be managed as recommended by the AAO. Methods to minimize these risks and improve informed consent are outlined.
INTRODUCTION
Chloroquine (CQ) and hydroxychloroquine (HCQ) have been used for the treatment of systemic lupus erythematosus, rheumatoid arthritis, and other inflammatory and dermatologic diseases for many years. Irreversible retinal toxicity can occur following the use of each of these 4-aminoquinoline compounds.1,2 Recently, an American Academy of Ophthalmology (AAO) task force on screening for chloroquine and hydroxychloroquine retinopathy identified high-risk factors for retinopathy associated with HCQ treatment and recommended screening guidelines to minimize the risk of this toxicity.3 Evidence exists that a significant proportion of veterans taking HCQ many not be managed according to these AAO screening guidelines.4 The purpose of this study is to determine the prevalence of high-risk factors for HCQ retinopathy and compliance with the AAO screening guidelines at the VASF and develop an approach to improve the risk-benefit relationship and informed consent for patients during HCQ treatment.
METHODS
Following institutional review board approval, the VASF pharmacy identified the medical records of all the patients receiving HCQ between October 1, 2004, and September 30, 2005. These medical records were then reviewed with special attention to high-risk factors for retinopathy. Thereafter, the data were recorded, analyzed, and reported.
RESULTS
Between October 1, 2004, and September 30, 2005, the VASF pharmacy identified 109 patients who were using HCQ prescribed at VASF. Of the 109 patients taking HCQ, 87% had at least one high-risk factor for retinal toxicity as identified by the AAO task force, and 59% had 2 or more risk factors. Thirty-four percent of these 109 patients treated with HCQ had no evidence of an eye examination performed before or during their treatment with HCQ. There was no evidence of an eye examination performed in the records of 46% of the patients having 3 or more risk factors. A comparison between the results of the present study and a prior cohort of US veterans4 is provided in Table 1.
TABLE 1.
COMPARISON OF STUDY RESULTS OF PATIENTS WITH HIGH-RISK FACTORS FOR HYDROXYCHLOROQUINE RETINAL TOXICITY
| VARIABLE | PRESENT STUDY | PRIOR STUDY4 |
|---|---|---|
| Age >60 yr | 63 (58%) | 85% |
| Dosage >6.5 mg/kg/day | 9 (8.3%) | 19% |
| Therapy >5 yr | 15 (13.8%) | 39% |
| Liver/kidney disease | 35 (33%) | 33% |
| Retinal disease | 38 (34.9%) | 12% |
| Absence of eye examination | 37 (34%) | 40% |
DISCUSSION
The results of the present study are similar to the results from a prior study of a cohort of US veterans.4 In both studies, approximately one-third of the HCQ-treated patients were not managed as recommended by the AAO Screening Guidelines for HCQ Retinopathy. For example, 30% to 40% of the HCQ-treated patients in each study had no evidence that an eye examination had been performed prior to or during treatment with HCQ. In the present study, almost half of the patients using HCQ who had 3 or more risk factors had no evidence of an eye examination performed before or during their treatment. The absence of eye examinations in these studies not only suggests that the recorded incidence of retinal disease in each study is an underestimate, but also implies that the patients who might most benefit from eye examinations are not receiving them. Therefore, it appears that a significant number of veterans taking HCQ may be at an increased risk for retinal toxicity during their treatment with HCQ. Furthermore, these results indicate that the risk-benefit relationship for patients treated with HCQ may not be optimum. The results of both of these studies, in light of the conclusions of the AAO task force, suggest it is timely to review the risks of retinal toxicity associated with HCQ treatment and develop a method to improve the risk-benefit relationship and informed consent for patients treated with this drug.
A recent review of the literature3 emphasizes that over 1,000,000 patients have used either CQ or HCQ, whereas fewer than 20 cases of retinal toxicity occurred in individuals using appropriate doses of these aminoquinolines. In addition, all of these reported patients with retinal toxicity were using the drugs for more than 5 years. Insofar as most investigators believe that HCQ is less likely to cause retinal toxicity as compared to CQ, it seems the prevalence of HCQ-induced retinal toxicity is quite low.2,3,5 In fact, there are descriptions of large doses of HCQ without retinal toxicity that underscore the drug’s relative safety.6 Therefore, the incidence of retinal toxicity with HCQ treatment is probably even less than that (1 case in 50,000 treated) suggested by the literature review provided in the report from the AAO task force.3
Although the risk of retinal toxicity is minimal for patients using HCQ, this toxic retinopathy, when it becomes manifest, presents a major therapeutic problem for both the patient and the physician. More specifically, both HCQ- and CQ-induced retinal toxicity can be irreversible even if identified early. Furthermore, this toxicity not only often persists following its onset even if the drug is discontinued, but it can progress after cessation of HCQ therapy. Finally, the toxic retinal damage and the associated visual changes can be delayed in onset after the drug is discontinued.7–12 Therefore, it is not surprising that investigators often emphasize that even if HCQ-induced retinal toxicity is recognized at an early stage of functional loss, recovery is unlikely.3
In light of the unpredictability of the onset and subsequent behavior of HCQ-induced retinal toxicity, it is essential that both patients receiving this drug and physicians prescribing HCQ are aware of the severe limitations and true value of ophthalmologic screening examinations. It should be made clear to both patient and referring doctor that ophthalmologists may help to identify HCQ-induced retinal toxicity early, but this activity most often does not prevent further retinal damage even if the drug is discontinued. Furthermore, it must be emphasized that the screening certainly does not guarantee that there will be no subsequent retinal damage and associated visual loss even if the screening examinations are performed expertly, using expensive equipment involving prolonged testing times and with full patient cooperation.
Many elegant screening examinations continue to be developed and evaluated in an attempt to diagnose retinal toxicity early and thereby minimize the toxic effects of HCQ. For example, most recently, the potential value of early paracentral visual field testing, the detection of subtle color vision defects, and fundus autofluorescence and multifocal electroretinography each have been described and compared to prior approaches used to enhance screening examinations during HCQ treatment.13–15 However, there is no evidence that any of these newer and more elaborate tests add clinical benefit over simple Amsler grid testing at this time. Furthermore, Amsler grid testing offers many advantages. The test is inexpensive, rapid, and easy to perform for both the physician and patient. In addition, patients can perform Amsler grid testing on a regular basis in their own home. Most investigators agree that it seems reasonable to use Amsler grid testing and to continue to consider color testing, photography, and other specialized tests as optional screening devices during follow-up of patients using HCQ as reported by the AAO task force.3
Many investigators have suggested that because of the rarity of HCQ-induced retinal toxicity and because so little can be done even if the retinal damage is identified early, screening for retinal toxicity is probably not justified except in an effort to provide maximal legal protection.3,12,16 This sentiment is clearly reflected within the report by the AAO task force, which states, “It cannot be emphasized too strongly that whatever screening regimen is followed, the keys to early recognition of toxicity, and to the avoidance of liability, are first informing the patient of the risks and of the need for examinations, and second documenting these admonitions carefully in the record.”3 Therefore, it seems essential to provide a definitive and well-documented means of informing the patient about the risk-benefit relationships associated with HCQ treatment.
To address this issue and fulfill this need, an HCQ Medication Information Form has been developed that is designed to accompany HCQ as it is dispensed by the pharmacist (Appendix). This form includes a description of the high-risk factors for retinal toxicity as identified by the AAO task force and emphasizes the importance of a baseline eye examination. The form includes pertinent facts about the onset, progression, and irreversibility of HCQ-induced toxic retinopathy, and it provides an Amsler grid with a description of its proper use. Finally, a comment is provided underscoring the potential benefits of lowering the dose of HCQ as a desired therapeutic response is achieved. This Medication Information Form not only properly informs the patient about the visual risks of taking HCQ, but also encourages the patient to participate directly in the retinal screening activity. Therefore, an emphasis is placed on the patient’s ongoing responsibility in the drug toxicity screening process. Furthermore, it encourages the patient to remind the prescribing physician of the need to reevaluate the benefits of treatment in a timely fashion. Such interaction can result in decreasing the dose or even choosing to take therapeutic vacations from HCQ treatment if the symptoms for which the drug is being used are minimal or absent.
Finally, to complete the cycle of informed consent and properly document these activities, the pharmacist notifies the ophthalmologist, primary care physician, rheumatologist, and other treating physicians that the patient has received and understood the HCQ Medication Information Form at the time the patient received the medication. This procedure should provide the information and documentation suggested by the AAO task force.3
In conclusion, two independent, retrospective clinical studies suggest that a significant number of veterans in the United States who are taking HCQ may be at increased risk for retinal toxicity. The results of both of these studies report more than one-third of the patients treated with HCQ may not be managed as recommended by the AAO task force. These clinical studies, each inspired by the efforts of the AAO task force, prompted the development of an HCQ Medication Information Form. This handout can accompany the HCQ as the pharmacist dispenses it. The form emphasizes the importance of a baseline eye examination, and it provides both drug education for the patient and an Amsler grid for the patient to use at home, allowing for an earlier diagnosis of retinal toxicity. Therefore, the use of this form should not only improve informed consent, but guarantee that the patient is fully aware of the risk-benefit relationships associated with HCQ use. In addition, it makes clear that the patient is an essential and responsible member of the HCQ retinal toxicity screening process.
PEER DISCUSSION
DR SHALESH KAUSHAL
I commend Dr. Flach’s paper that attempts to provide some insights as to how ophthalmologists in general and retina specialists in particular can improve the compliance of patient screening for those taking hydroxycloroquine (HCQ).
The biological basis of chloroquine and HCQ toxicity to cells stems from their ability to dissipate the pH gradient across lysosomes by blocking the vacuolar ATPase. This leads to a significant increase in the intraluminal pH of lysosomal compartment and compromises the ability of the various proteases to cleave and degrade proteins targeted to this organelle. In addition, both antimalarials can bind to melanin and by some unknown mechanism affect cellular function. Together, these drugs lead to the eventual death of retinal pigment epithelium (RPE) cells.
The incidence of retinal toxicity of HCQ is actually quite low, and most cases are related to chloroquine. High-risk factors are (1) age; (2) dosage; (3) length of therapy; (4) presence or absence of liver/kidney disease; (5) retinal disease; and (6) body habitus/obesity.
Interestingly, the data demonstrate an inverse relationship between the number of high-risk factors a particular subgroup of patients may have and the likelihood to obtain an eye examination. Might this reflect their ability to access health care in general? It is probably the case that we, as physicians, need to empower patients to participate in their care by improving their understanding of the risks and benefits by educating them and communicating with them more effectively.
As with other screening programs, it is important to refine our diagnostic tools (mfERG, quantitative FAF, metabolic imaging) to capture disease processes when the biological process is still reversible. We also need to educate rheumatologists/internists to better recognize the high-risk factors so they can refer patients for screening similar to immunizations and breast and colon cancer screening programs.
To that end, the author presents a very nice and potentially useful consent form that educates and empowers patients to participate proactively in their management of possible HCQ toxicity. The uniqueness of the idea is that the patient receives this form from the pharmacist at the time of filling the drug prescription. Implicit to the author’s discussion is that such a form may also preempt possible lawsuits.
ACKNOWLEDGMENTS
Funding/Support: None.
Financial Disclosures: None.
DR. MALCOLM L. MAZOW
This is an excellent paper. There is no conflict. When I was a resident we were taught corneal crystals that could be seen before the retinal changes were visible. If we saw corneal crystals, in a patient on chloroquine or hydroxychloroquine, with a slit lamp examination then immediately the medication was discontinued. We believed that subsequent retinal changes would not occur. Until recently, chloroquine in high doses was used as an anti-seizure medication by pediatric neurologists. I was called as an expert in a case involving a pediatric neurologist who was treating a patient for seizures.
DR. JERRY SEBAG
I do have a financial interest in the technology that I am about to describe; unfortunately, it is presently in the red. In the past few years we have been working with Wolfgang Fink and Alfredo Sedun at University of Southern California to develop a three dimensional Amsler grid with the two standard dimensions and a third dimension of contrast sensitivity. We have detected abnormalities associated with diabetic macular edema and exudative AMD that were not detectable by conventional Amsler grid testing. We will hopefully put this on the internet for use at home. Is there any advantage to early detection of Amsler grid changes in these conditions if you add contrast sensitivity?
DR. W. BANKS ANDERSON
No conflicts. Allan, this is an excellent paper. Another way to protect yourself from medicolegal problems, as an ophthalmologist, is to ask the patients how much they weigh. The little old lady, who is referred by the rheumatologist for evaluation, may be receiving an overdose of medications. I send a letter to all the referring rheumatologists with patients in this category. I explain to them that I cannot be responsible if they treat patients with more than the recommended dose for their weight.
DR. TRAVIS A. MEREDITH
No conflicts. Your current form has two goals, the first is to protect the doctor from liability and the second is to protect the patient. I could not see the form very well, but it seems to me that there was a great deal of technical language. My wife, a patient educator, constantly stresses that if information is truly written to educate patients, then it should be at a fifth grade level and no higher. I would suggest you might wish to get a patient educator involved in designing a more effective communication tool.
DR. THOMAS D. FRANCE
No conflicts. Allan, that was a great paper. This interests me as a pediatric ophthalmologist because I have noticed our pediatric rheumatologist is more frequently prescribing hydroxychloroquine for children with juvenile rheumatoid arthritis. If Amsler grid and standard automated perimetry are the best techniques for detecting early change in the adults, then what should we use for testing three, four, and five year old children on hydroxychloroquine? I believe that the dosages used are correct for the pediatric age group. What signs should I look for when doing my screening examination that will help me to detect toxicity if the patient is unable to perform a visual field or to use an Amsler grid?
DR. PRESTON H. BLOMQUIST
No financial interest in this subject. I would like to echo the importance of calculating ideal body weight, since we want to keep our patients under the recommended 6.5 mg/kg of lean body weight/day dosage. I also ask patients if they think the hydroxychloroquine is doing them any good. I ask about their symptoms so I can communicate this information with the rheumatologist. Why should patients continue to use a potentially toxic drug that is ineffectively controlling their condition? Finally, would you please discuss the different types of Amsler grids and explain your preference: red Amsler, standard Amsler (black on white or white on black), or desaturated?
DR. RALPH C. EAGLE, JR
Nice paper. I would like to reiterate what Travis said. I am on the Institutional Review Board (IRB) at Wills and I know that your consent document would not pass our committee. It has too much technical language. Most patients really cannot relate to the milligrams per kilogram delivery concept. I strongly believe that the language should be much simpler.
DR. RICHARD P. MILLS
No conflict. Allan, I would like to congratulate you for stressing one of the few medical benefits of being noncompliant with medical therapy. Patients that become asymptomatic will tend not to use the medication regularly, and that is exactly what you are recommending.
DR. GARY C. BROWN
No conflicts. Allan, I want to thank you very much for presenting a nice paper. I have a question in regard to the older patient. This is not a difficult diagnosis to make in a twenty or thirty year old patient, but do you have any tips for detecting toxicity in a sixty or seventy year old patient? This is particularly difficult because macular degeneration can present very much like this and occurs in approximately 10% of the patients in their seventies.
DR. JOHN D. BULLOCK
No conflict. This is a great paper. If you give an Amsler grid card printed on paper to a patient who will take these medications for many years, it is likely that over the course of time the patient will lose the card. We made a magnetic Amsler grid that could be attached to the refrigerator door. With this technique patients are more likely to use this conveniently located reminder.
DR. ALLAN J. FLACH
I am overjoyed with all the responses. I will reply as best as I can. First, I would like to thank Dr. Kaushal, not only for my discussion, but for both discussions he presented at our meeting this year. I believe this is a first time that a new candidate has discussed two papers at one meeting and, although this may be a form of hazing, I am not sure this is in the bylaws. He warned me he was going to discuss the chemistry of hydroxychloroquine, and I believe it is wonderful for the discussant to supplement what the speaker does not have time to do. His comments suggest that multifocal ERG and other screening processes may have advantages, and I agree they may be of value in the future. Currently, multifocal ERG really does not add a lot to our problem solving with these patients. I know this based on what I have read, and on my personal experience. Dr. Mazow mentioned that crystals can appear in the cornea, just as they can in the lens. I recall Dr. Fritz Fraunfelder describing that sometimes the hair turns white in patients taking hydoxychloroquine and how these findings might be of some use in predicting retinal toxicity. Unfortunately, no one has ever correlated these more obvious changes with the retinal changes and I do not believe that we can reliably regard them as links to the retina toxicity. On the other hand, it is a great idea to let a patient know they are depositing crystals in their cornea or lens, but that we do not know if this is a concern for the health of the retina. I tell them that because we can see it the cornea we should watch their retina a little more carefully. Someone mentioned the work of Fink and Sadun. In the future, contrast sensitivity on the three dimensional Amsler grid along the z- axis may provide an opportunity for us to incorporate this test to assess our therapeutic approaches. I do not believe that we should use these sophisticated, possibly useful devices, or to confuse them with our current patient management. Clearly these findings do not relate to the informed consent nor do they provide any information that we can use to help us protect our patients today. Dr. Anderson emphasizes that we should ask about weight of the patient and this matter was a substantial problem for me. As Dr. Mills suggested, you must have IRB approval to perform these investigations. If you are performing a study and identify a patient who is far too thin to safely use all the medicine that has been prescribed, then you cannot call the patient as the person who detected the problem in this research study. I was so indoctrinated by our IRB about what I could and could not do that for a while I thought “Oh my gosh, I will have to wait until the study is completed”. This is nonsense. I simply called the physician who was responsible for prescribing the medication and discussed the matter with him. Obviously, this is what I should have considered from the very beginning. Dr. Meredith mentioned the issues of liability and technical language. One of the reasons I included the head of our IRB in this study was not just to obtain her approval, but to assure her involvement in implementing the suggestions expressed at this meeting. I will return the consent form to her and hopefully she will help me in the preparation of a revision. I was aware that the language of the current informed consent was formulated more for the benefit of the members of this audience than for my patients. Dr. France mentioned the problems of diagnosis in children. I am very proud of my grandchildren and know that by the age of 3 and 4 they could have performed Amsler grid testing; however, it is not fair for me to extrapolate my experience to others. I am not blessed with many children in my practice, but if I can get a glimpse of a good foveal light reflex, then I regard this as normal. I use that technique in adults and I confess that I do not dilate every patient taking hydroxychloroquine. If I see a nice foveal light reflex and it disappears, this is good enough, but if I cannot detect a foveal light reflex, then absence of this finding does not help me. In this case, I dilate the pupil and use a fundus contact lens to examine the macula for granularity. I think a clinical exam with children may be better. Dr. Blomquist also emphasized the importance of measuring body weight and testing with an Amsler grid. I believe that testing with a red Amsler grid would probably would be more sensitive. I know a superb immunologist who still uses a tangent screen with a superimposed Amsler grid. I hope he presents his technique to us at this meeting. I believe that a red Amsler grid may be more sensitive than the regular Amsler grid, but I have not used one in that way. Dr. Eagle also emphasized that the language of the informed consent should not include jargon. Dr. Mills commented that I am an optimistic to look at the brighter side and the advantages of noncompliance with medical therapy. Regarding the comments of Dr. Brown on the diagnosis of hydroxychloroquine toxicity in older patients with age-related macular degeneration, a retinal specialist in the San Francisco Bay area with an extensive military experience had cared for several such patients. Three patients with macular degeneration who took hydroxychloroquine did poorly. Was this coincidental? He refuses to follow patients with macular degeneration or macular changes in whom he does not feel capable of identifying the hydroxychloroquine retina toxicity. He tells the rheumatologist that he will not stop them from treating the patient, but that he does not want them to believe that he can do anything whatsoever to monitor these patients. This retinal specialist believes that it is impossible to do this in patients with age-related macular degeneration. I thank all of you not just for being here, but for becoming probably the best part of my presentation.
ACKNOWLEDGMENTS
Funding/Support: None.
Financial Disclosures: None.
Author Contributions: The author designed the study, collected, analyzed, and interpreted the data, and prepared the manuscript.
HYDROXYCHLOROQUINE PATIENT MEDICATION FORM
Medication Information: Hydroxychloroquine (Plaquenil)
Patient Informed Consent and Minimization of Risk of Retinal Toxicity
Those at most risk for retinal toxicity from hydroxychloroquine (HCQ) include patients:
Older than age 60 years
Taking more than 6.5mg/kg/day
Taking the HCQ more than 5 years
Presence of kidney disease
Presence of liver disease
Presence of retinal disease (PATIENT REQUIRES AT MINIMUM A BASELINE EYE EXAM)
Presence of excess weight
Although toxicity for the retina and associated vision loss is rare (1/50,000) during HCQ treatment, these are the facts associated with this retinal toxicity if it occurs:
Retinal toxicity with HCQ cannot always be prevented even if properly used.
Retinal toxicity with HCQ is usually irreversible.
Onset of retinal toxicity may occur even after the HCQ is discontinued.
Retinal toxicity may continue to progress even if HCQ is discontinued.
The best way to detect retinal toxicity with HCQ early is by you, the patient, performing Amsler Grid testing at frequent intervals on yourself. If you note changes in this test, call the eye clinic for an appointment. You should be seen within a few days.
It is important for you to keep in mind why you are using HCQ. Most patients use HCQ to relieve muscle pain (myalgias), joint pain (arthritis), skin problems (rashes) or to relieve fatigue as part of lupus or other collagen vascular diseases. You should discuss the possibility of decreasing your dose or even stopping HCQ if these symptoms improve or disappear with your primary health care provider to minimize the risk of retinal toxicity.
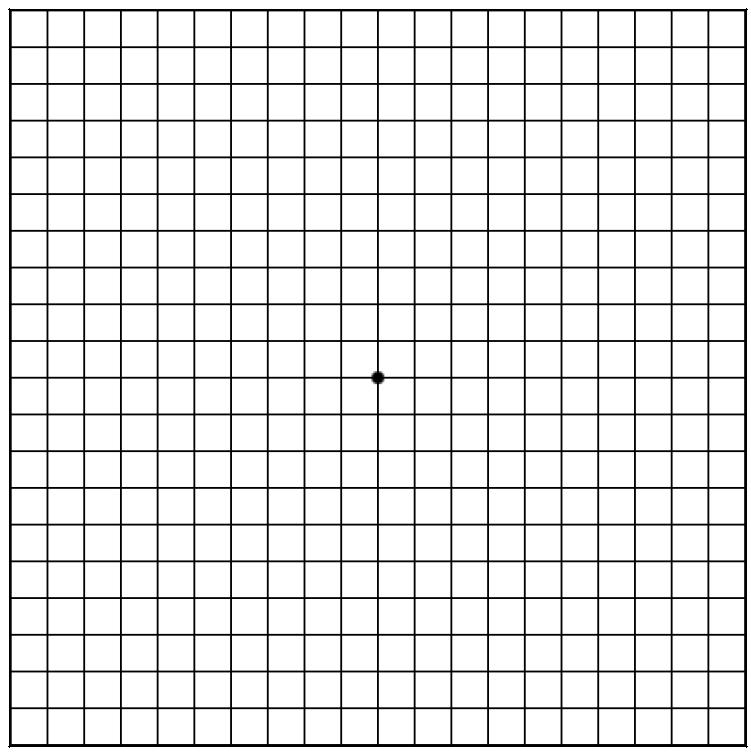
AMSLER GRID TEST
This is the best test for you to use at home to detect early signs of retinal toxicity that can accompany HCQ use. The simple test is performed in the following way:
Use the chart at eye level in a well-lit area.
Place the chart about 12 to 14 inches away from your eyes.
Wear your reading glasses if you use them to read.
Cover one eye and test the other, uncovered eye.
Look at the center dot of the Amsler Grid.
Test the other eye using the same technique.
Call the eye doctor’s office if any lines or squares on the Amsler Grid look wavy, blurred, fuzzy, or missing as observed with either eye during the test.
REFERENCES
- 1.Hobbs HE, Sorby A, Freedman A. Retinopathy following chloroquine therapy. Lancet. 1959;2:478–480. doi: 10.1016/s0140-6736(59)90604-x. [DOI] [PubMed] [Google Scholar]
- 2.Shearer RV, Dubois EL. Ocular changes induced by long-term hydroxychloroquine (Plaquenil) therapy. Am J Ophthalmol. 1967;64:245–252. doi: 10.1016/0002-9394(67)92518-4. [DOI] [PubMed] [Google Scholar]
- 3.Marmor MF, Carr RE, Easterbrook M, Farjo AA, Mieler WF. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy: a report by the American Academy of Ophthalmology. Ophthalmology. 2002;109:1377–1382. doi: 10.1016/s0161-6420(02)01168-5. [DOI] [PubMed] [Google Scholar]
- 4.Gupta G, Greenberg PB, Tsiaras WG. The prevalence of high-risk factors and adherence to screening guidelines for hydroxychloroquine retinopathy in a cohort of US veterans. Scientific poster 461. Presented at: American Academy of Ophthalmology 2005 Annual Meeting; Oct 17–18, 2005; Chicago. [Google Scholar]
- 5.Finbloom DS, Silver K, Newsome DA, Gunkel R. Comparison of hydroxychloroquine and chloroquine use and the development of retinal toxicity. J Rheumatol. 1985;12:692–694. [PubMed] [Google Scholar]
- 6.Johnson MW, Vine AK. Hydroxychloroquine therapy in massive total doses without retinal toxicity. Am J Ophthalmol. 1987;104:139–144. doi: 10.1016/0002-9394(87)90005-5. [DOI] [PubMed] [Google Scholar]
- 7.Ehrenfeld M, Nesher R, Merin S. Delayed-onset chloroquine retinopathy. Br J Ophthalmol. 1986;70:281–283. doi: 10.1136/bjo.70.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burns RP. Delayed onset of chloroquine retinopathy. N Engl J Med. 1966;275:693–696. doi: 10.1056/NEJM196609292751303. [DOI] [PubMed] [Google Scholar]
- 9.Martin LJ, Bergen RL, Dobrow HR. Delayed onset chloroquine retinopathy. Ann Ophthalmol. 1978;10:723–726. [PubMed] [Google Scholar]
- 10.Brinkley JR, Dubois EL, Ryan SJ. Long-term course of chloroquine retinopathy after cessation of medication. Am J Ophthalmol. 1979;88:1–11. doi: 10.1016/0002-9394(79)90743-8. [DOI] [PubMed] [Google Scholar]
- 11.Easterbrook M. Long-term course of antimalarial maculopathy after cessation of treatment. Can J Ophthalmol. 1992;27:237–239. [PubMed] [Google Scholar]
- 12.Coyle JT, Easterbrook M. Hydroxychloroquine retinopathy. Ophthalmology. 2001;108:2158–2159. doi: 10.1016/s0161-6420(01)00915-0. [DOI] [PubMed] [Google Scholar]
- 13.Elder M, Rahman MA, McLay J. Early paracentral visual field loss in patients taking hydroxychloroquine. Arch Ophthalmol. 2006;124:1729–1733. doi: 10.1001/archopht.124.12.1729. [DOI] [PubMed] [Google Scholar]
- 14.Vu BL, Easterbrook M, Hovis JK. Detection of color vision defects in chloroquine retinopathy. Ophthalmology. 1999;106:1799–1804. doi: 10.1016/S0161-6420(99)90338-X. [DOI] [PubMed] [Google Scholar]
- 15.Kellner U, Renner AB, Tillack H. Fundus autofluorescence and mfERG for early detection of retinal alterations in patients using chloroquine/hydroxychloroquine. Invest Ophthalmol Vis Sci. 2006;47:3531–3538. doi: 10.1167/iovs.05-1290. [DOI] [PubMed] [Google Scholar]
- 16.Lee AG. Hydroxychloroquine screening. Br J Ophthalmol. 2005;89:521–522. doi: 10.1136/bjo.2004.061473. [DOI] [PMC free article] [PubMed] [Google Scholar]


