Abstract
Purpose
To gain a better understanding of laser flare photometry values (“flare”) as a feature of chronic anterior uveitis in children; to identify relationships between flare and other patient and disease characteristics; to describe changes in flare during course of disease; and specifically to determine whether elevated flare is predictive of subsequent adverse events.
Methods
A retrospective review of medical records was performed for all children (aged ≤16 years at disease onset) with chronic anterior uveitis presumed to be noninfectious, who were examined by one clinician at the Jules Stein Eye Institute since laser flare photometry became available at that facility. All involved eyes were studied. Cross-sectional analysis compared initial flare to other characteristics. Relationships between potential risk factors and outcomes were studied by Kaplan-Meier analyses and Cox proportional hazards regression models.
Results
Included were 114 patients (198 involved eyes). Follow-up ranged from 0 to 154.8 months (median, 23.5 months for 82 patients with follow-up). Flare was related to the following factors: anterior chamber cells, keratic precipitates, papillitis, and various complications of uveitis, including band keratopathy, posterior synechiae, and cataract. Flare was not a function of disease duration. High flare was associated with an increased risk of vision loss and development of new vision-threatening complications, including glaucoma/increased intraocular pressure, during follow-up; risk was independent of anterior chamber cells.
Conclusions
Flare may be useful in the evaluation and management of chronic anterior uveitis in children. Flare is a marker of disease severity and is predictive of adverse events during the course of disease.
INTRODUCTION
In 1959, Hogan, Kimura, and Thygeson1 recommended that “cell” and “flare” (the reflection of light from proteins in the aqueous humor) be used to describe anterior uveitis, and they proposed a system to quantify these signs clinically during slit-lamp biomicroscopy. Since that time, most textbooks on uveitis2–6 have recommended that flare be quantified during the evaluation of patients with uveitis, yet few authors discuss a rationale for doing so. In fact, many uveitis subspecialists discount the utility of flare assessment in the evaluation of patients with anterior uveitis, concentrating instead on changes in anterior chamber cells as a measure of disease severity and response to anti-inflammatory therapies.
The clinical relevance of flare remains a subject of uncertainty. Whitcup,5 for example, has written that “Some disagreement exists as to whether the presence of flare by itself, without cells or other signs of active inflammation, should be treated. In our opinion, without objective quantification of a change in the leakage across the blood ocular barrier, chronic flare alone is not a sign of active inflammation. Damaged blood vessels may be leaky for a long time after the active inflammation has resolved. Continued treatment with drugs such as corticosteroids may do little to alter the repair of these vessels in the absence of active inflammation. There is no evidence that small amounts of increased protein in the anterior chamber are detrimental to the eye, and there appears to be no reason for continued therapy in this situation. Specifically, children with juvenile rheumatoid arthritis with flare but no cells should not be treated with topical corticosteroids. Therefore flare should be considered a marker of inflammation but not necessarily a pathognomonic finding of active inflammation.”
The introduction of laser flare photometry has again focused attention on aqueous humor protein concentration as a sign of anterior segment inflammation.7 Reports by Gonzales and associates8 and by Davis and associates,9 which describe relationships between laser flare photometry values and complications of uveitis, suggest that flare should be reconsidered as an important sign of disease in patients with uveitis. Causal relationships between aqueous humor protein and various uveitic complications were not established in these reports, and the possibility that the relationships are indirect, reflecting the occurrence of both elevated aqueous humor protein concentration and complications as signs of disease chronicity, have been raised in scientific discussions about these reports. It is well accepted, for example, that some patients with long-standing uveitis and hypotony will have marked flare, despite lack of other evidence of active inflammation.5 More recently, however, it has been reported that increased flare at presentation, as determined clinically during slit-lamp biomicroscopy, is associated with vision loss during follow-up of patients with juvenile idiopathic arthritis and uveitis.10,11 The predictive value of increased flare for disease outcomes should be confirmed with objective measures of aqueous humor protein concentration.
Laser flare photometry offers an objective tool to facilitate the reconsideration of flare and its importance in patients with uveitis. A reproducible assessment of disease status would be particularly valuable in children, as they are often unable to cooperate during prolonged examinations at the slit-lamp biomicroscope and are likely to have disease of long duration, involving the care of multiple clinicians who must share data.12
The purpose of the current study is to gain a better understanding of laser flare photometry and its clinical applications through assessment of a cohort of pediatric patients with chronic anterior uveitis, presumed to be noninfectious in nature that has been evaluated with serial laser flare photometry measurements. Specific goals include the following:
To determine whether relationships between elevated laser flare photometry values and uveitic complications reported previously are present in a larger cohort of patients. Cross-sectional studies will be expanded to include other host and disease factors, and to determine the strength of reported relationships.
To describe changes that occur in flare during the course of chronic disease, and to determine whether elevated laser flare photometry values are a reflection of increased duration of disease. Despite the reported relationships between laser flare photometry values and complications in cross-sectional studies,8,9 little is known about the changes that occur in laser flare photometry values over time.
To identify the predictive value of laser flare photometry values, if any, for development of vision loss or vision-threatening complications, and whether the predictive value is independent of other disease-associated factors. Specifically, the hypothesis that patients with flare values ≥20 photon units/millisecond (pu/msec) are at increased risk for adverse events will be tested. In doing so, outcomes for the subgroup of patients with low flare values, but elevated anterior chamber cells, will be assessed; it has been suggested that such patients represent a low-risk population, even if inflammatory cells persist in the anterior chamber.12
METHODS
Subjects included all patients with chronic anterior uveitis presumed to be noninfectious in nature, who were 16 years of age or younger at the time of uveitis onset, and who were seen by the author since 1991, when laser flare photometry became available at the Jules Stein Eye Institute. Patients who were as old as 20 years of age at first examination by the author were included if it was documented that the onset of uveitis had been at age 16 years or younger. Although all cases were consistent with noninfectious uveitis, that fact could not be confirmed for all patients; it is possible, for example, that children with unilateral uveitis and limited follow-up, whose peripheral retina could not be examined in detail, had toxoplasmosis or toxocariasis. Patients with additional evidence of intermediate uveitis (anterior vitreous inflammatory reaction out of proportion to the amount of cells seen in the anterior chamber; inflammatory condensates [“snowballs”] in the inferior, anterior vitreous humor; “snowbanking” over the inferior pars plana and inferior, anterior retina) were included if it had been determined by the author at the time of baseline examination that the anterior chamber cellular reaction was not simply “spillover” from intermediate uveitis alone (ie, the patients had “anterior and intermediate uveitis,” as described by McCannel and associates13 and by the Standardization of Uveitis Nomenclature [SUN] Working Group14). This study was approved by the Institutional Review Board at UCLA, with waiver of the need for informed patient consent to review medical records.
DATA COLLECTION
Data were collected in a retrospective review of medical records maintained in the office of the author. Data available through November 30, 2006, was included. Demographic, medical, and ophthalmic data were collected at baseline. Demographic data included age and gender. Medical data included whether or not the patient had an associated systemic inflammatory disease (juvenile idiopathic arthritis [JIA]; sarcoidosis; other) and treatment (whether or not the patient was receiving systemic immunomodulatory therapy; use of oral or topical glaucoma medications). No attempt was made to categorize patients with JIA by disease manifestations or antinuclear antibody test results. It was determined whether uveitis was unilateral or bilateral. For each patient, duration of uveitis prior to baseline was determined on the basis of records that had been obtained at initial evaluation or by patient report at examination. For purposes of analysis, both eyes of patients with bilateral disease were assumed to have the same duration of uveitis. Ophthalmic data for each involved eye included history of ophthalmic surgery (cataract extraction; glaucoma surgery); best-corrected visual acuity; intraocular pressure; the presence of keratic precipitates; laser flare photometry values; anterior chamber cell score; presence of signs indicating intermediate uveitis, as described above; presence of papillitis; and presence of complications attributable to uveitis. The following complications were specifically sought: band keratopathy; peripheral anterior synechiae; posterior synechiae; cataract; inflammatory membranes (ciliary, pupillary); glaucoma or elevated intraocular pressure not attributed to corticosteroid response; hypotony; macular edema. Other findings were also recorded as complications, if so identified in the patient’s medical record.
Additional ophthalmic data were recorded from follow-up examinations, if any. For each involved eye, the following data were collected from each examination during follow-up: laser flare photometry value, anterior chamber cell score, and intraocular pressure, with corresponding intervals since baseline. It was determined whether eyes without vision-threatening complications at baseline developed such complications during follow-up. It was also determined whether eyes with glaucoma or elevated intraocular pressure at baseline developed evidence of progressive glaucoma or additional intraocular pressure problems (“glaucoma/elevated intraocular pressure” during follow-up, as described under Study Definitions below). Also recorded were the following intervals for each involved eye from baseline, if applicable: to start of immunomodulatory therapy for patients not on such therapy at baseline; to start of additional glaucoma medications; to first new vision-threatening complication; to glaucoma/elevated intraocular pressure; to decrease of visual acuity to 20/50 or less; and to decrease of visual acuity to 20/200 or less.
For many patients, medical records did not provide quantitative descriptions of complications; thus, no attempt was made to analyze the severity or progression of complications during follow-up other than glaucoma/elevated intraocular pressure.
Data on anterior chamber cells were recorded exactly as written in medical records. These data were managed as described under Study Definitions below.
Laser flare photometry values were obtained with either the Kowa FC-1000 or the Kowa FM-500 (Kowa Co Ltd, Electronics and Optics Division, Tokyo, Japan), both of which are commercially available machines. Laser flare photometry was performed as recommended by Ladas and associates.7 Briefly, 7 acceptable laser flare photometry readings were saved at each sitting. The high and low readings were discarded, because of the fact that outliers occasionally occur. The mean of the remaining 5 readings was calculated by the photometer and used in analyses. Laser flare photometry values (referred to hereafter as flare or flare values) are expressed in the arbitrary units of photon units per millisecond (pu/msec). Mean flare values provided by the FC-1000 or FM-500 were used for all analyses. Clinical grading of flare was not recorded.
Information on systemic immunomodulatory therapy was collected because of its possible effect on the relationships between flare and disease manifestations and outcomes. The purpose of the study was not to investigate the effect of immunomodulatory therapy on disease outcomes. Patients were considered to be on immunomodulatory therapy if they were taking one or more of the following systemic agents as monotherapy or in combination therapy: adalimumab; azathioprine; cyclosporine, infliximab; methotrexate; mycophenolate mofetil. Information was not collected on specific drugs, doses, or duration of treatment. The interval between baseline and the start of immunomodulatory therapy during follow-up was determined to identify whether immunomodulatory therapy had been started before development of adverse events, if any. No patient was treated with long-term oral corticosteroids. All eyes had been treated with topical corticosteroid, and no attempt was made to quantify corticosteroid exposure.
STUDY DEFINITIONS
Baseline was considered to be the date of the patient’s first examination by the author at the Jules Stein Eye Institute. Baseline flare was a laser flare photometry measurement obtained at the baseline examination. First flare was the initial laser flare photometry measurement, if obtained at an examination after baseline. For most analyses, either baseline flare or first flare was used, depending on which value was available (baseline/first flare). Maximum flare was the highest flare value obtained at any point during the course of disease. Minimum flare was the lowest flare value obtained at any point in the course of disease. Change in flare was calculated for eyes with follow-up and 2 or more flare values by subtracting the baseline/first flare value from the most recent flare value. With regard to change in flare, increased flare was identified by a most recent flare value that was at least 10 pu/msec higher than baseline/first flare values for a given eye, while decreased flare was identified by a most recent flare value that was at least 10 pu/msec less than baseline/first flare. All other changes were considered stable. Fluctuation of flare was defined for eyes with follow-up and 2 or more flare values as the absolute difference between minimum and maximum flare.
The intensity of anterior chamber cellular reactions had been recorded during clinical examinations using the semi-quantitative grading system described by Hogan and associates1 with modifications described by Ladas and associates.7 The definitions of the semi-quantitative categories differ slightly from definitions currently recommended by the SUN Working Group14 (Table 1). In these scoring systems, the concentrations of cells within each successive category do not follow a linear progression. Some uveitis specialists believe that small changes in anterior chamber cells do not reflect change in disease activity.5 Nevertheless, it has been the author’s routine to refine measurements further during clinical examinations, as discussed by Whitcup5 for the lower end of the range associated with grade 1+ cells. For grades 1+, 2+, and 3+, within-grade approximations of cell concentration are made routinely by the author; scores are categorized further as being at the lower end of the cell range for a given category (“light”) or at the upper end of a given category (“heavy”). For example, cell concentrations at the lower end of grade 1 (5 or 6 cells per volume) would be identified as “light 1+.” Also, patients may be identified as being on the border between two categories (eg, “occasional-1+ cells”). Using these subcategorical designations, anterior chamber cell scores were converted to a linear scale for analyses in this study, using the conversions shown in Table 1. Doing so might increase statistical power to identify relationships between cells and other disease factors, by allowing anterior chamber cells to be used as a continuous variable. Converted values are referred to hereafter as anterior chamber cell counts. When categorical values were used to create subgroups, the term anterior chamber cell scores was used.
TABLE 1.
CONVERSION OF CATEGORICAL VALUES FOR ANTERIOR CHAMBER CELLS TO NUMERICAL VALUES FOR PURPOSE OF ANALYSIS
| CORRESPONDING RANGES (NO. OF CELLS)
|
|||
|---|---|---|---|
| ORIGINAL VALUE FROM PATIENT RECORDS (CATEGORIES) | Modified Definitions from Hogan and associates1 | Definitions from the SUN Research Group14 | CONVERTED VALUE FOR CURRENT ANALYSES (NO. OF CELLS) |
| 0, rare | — | <1 | 0 |
| Rare-occasional | — | — | 0.5 |
| Occasional, trace | 1 – 4 | 1 – 5 | 2.5 |
| Occasional – 1+ | — | — | 4.5 |
| 1+ | 5 – 10 | 6 – 15 | 7.5 |
| Heavy 1+; 1–2+; light 2+ | — | — | 10 |
| 2+ | 10 – 20 | 16 – 25 | 15 |
| Heavy 2+; 2–3+; light 3+ | — | — | 20 |
| 3+ | 20 – 50 | 26 – 50 | 35 |
| Heavy 3+; 3–4+ | — | — | 50 |
| 4+ | >50 | >50 | 75 |
It could not be determined whether cell concentrations for eyes identified clinically as having “occasional-1+ cells” fell within the range that defined grade 1+. Also, it was not reasonable to assume that a difference existed between eyes assigned a score of “occasional-1+” and those assigned a score of “light 1+”. Thus, for purposes of assigning eyes to subgroups based on anterior chamber cell thresholds, eyes with either of these scores were considered to be <1+ for purposes of analysis.
Because treatment aimed to lower intraocular pressure before development of glaucomatous optic nerve damage, glaucoma and elevated intraocular pressure were considered collectively as a single complication for purposes of analysis. Glaucoma/elevated intraocular pressure at baseline was defined for study purposes as the presence of one of the following criteria at baseline: intraocular pressure >21 mmHg without documentation that the patient was crying or squeezing during intraocular pressure determination, and in the absence of corticosteroid therapy; the use of glaucoma medications; history of glaucoma surgery prior to baseline; glaucomatous optic disc abnormalities; or glaucomatous visual field changes. Glaucoma/elevated intraocular pressure during follow-up was defined for study purposes as the presence of one of the following criteria during follow-up: intraocular pressure >21 mm Hg at 2 examinations separated by more than 2 months, in the absence of crying or squeezing during intraocular pressure determination at each examination, and in the setting of reduced or discontinued corticosteroid therapy between examinations; new glaucomatous optic disc abnormalities; new glaucomatous visual field changes; or the need for additional glaucoma medications to maintain intraocular pressure ≤21 mm Hg, in a patient with glaucoma/elevated intraocular pressure at baseline. These criteria are based on the following assumptions: corticosteroid treatment was tapered for all patients who had elevated intraocular pressure, if corticosteroid-response was suspected; systemic immunomodulatory therapy was started for those patients whose inflammation increased with taper of corticosteroid; an effect of immunomodulatory therapy may not be seen for as long as 2 months after start of therapy; and elevated intraocular pressure attributable to corticosteroid response will generally resolve within 2 months. Not included were eyes considered to have corticosteroid-induced ocular hypertension on the basis of an on-off phenomenon with corticosteroid rechallenge.
Postoperative inflammatory membranes on the surface of intraocular lenses were not included among the inflammatory membranes analyzed as complications in this study.
Hypotony was defined as intraocular pressure <5 mm Hg in a patient without a history of trabeculectomy or placement of a drainage device in the same eye.
Reduced visual acuity attributed to myopic shift associated with cataract was considered vision loss for purposes of analysis, if visual acuity dropped to 20/50 or less with use of the patient’s previous refractive correction.
DATA ANALYSIS
All analyses were by involved eye. Because the correlation of flare between right and left eyes of patients with bilateral disease was weak (see “Results” section), analyses were not controlled for relationships between eyes.
Factors related to the following adverse events during follow-up were sought: reduction of visual acuity to 20/50 or less; reduction of visual acuity to 20/200 or less; any vision-threatening complication, as listed above; and specifically glaucoma/elevated intraocular pressure. The following factors were considered: immunomodulatory therapy before adverse event; anterior chamber cells ≥1+ at baseline; flare ≥10 pu/msec at baseline/first flare; flare ≥20 pu/msec at baseline/first flare; rise in flare of 10 pu/msec before adverse event; flare <20 pu/msec at adverse event; and any vision-threatening complication at baseline. Cases were considered not to be at risk for development of specific vision-threatening complications other than glaucoma/elevated intraocular pressure, if the same complication was present at baseline. Likewise, patients were not evaluated for visual acuity loss to 20/50 or less or for visual acuity loss to 20/200 or less during follow-up, if best-corrected visual acuity at baseline was already at or below those thresholds.
Relationships between baseline/first flare and the following factors at baseline were sought: duration of uveitis before baseline; age; gender; presence of associated systemic disease; presence of signs indicating intermediate uveitis; keratic precipitates; papillitis; anterior chamber cells; use of immunomodulatory therapy; any vision-threatening complication at baseline; and the presence of 4 specific vision-threatening complications individually (band keratopathy; posterior synechiae; cataract; glaucoma/elevated intraocular pressure). Two separate sets of comparisons were performed, one in which flare was considered as a continuous variable, and one in which flare was considered as a categorical variable (<20 pu/msec; ≥20 pu/msec). All other factors were considered as categorical variables in these comparisons. Because initial flare was not determined at baseline for all patients, a secondary analysis was performed in which the same comparisons were performed using only eyes with baseline flare. Dose effects (and possible threshold effects) were sought by looking at the prevalence of each finding for the following categories of baseline/first flare: <10 pu/msec; 10 to 19.9 pu/msec; 20 to 49.9 pu/msec; 50 to 99.9 pu/msec; ≥100 pu/msec. In addition, the correlation between flare and anterior chamber cells was determined using both factors as continuous variables.
Change in flare associated with start of immunomodulatory therapy during follow-up was determined by subtracting the first flare value obtained after 2 months of immunomodulatory therapy (but no later than 6 months after start of therapy) from the last flare value obtained before the start of therapy.
STATISTICAL ANALYSIS
Statistical analysis was performed using statistical software SAS version 9.1 (SAS Institute, Inc, Cary, North Carolina). Fisher exact tests were performed to evaluate the relationships between 2 categorical variables. Relationships between 2 continuous variables were analyzed with Spearman correlation coefficients because the distribution of data was skewed. Relationships between one categorical variable and one continuous variable were analyzed with Wilcoxon rank-sum test (when the categorical variable had only 2 levels) or Kruskal-Wallis tests (when the categorical variable had more than 2 levels). Log-rank tests were performed to assess the difference in Kaplan-Meier curves between levels of categorical variables. To control for potential confounding factors, multivariable Cox proportional hazards regression models were used to analyze the relationships between several risk factors simultaneously. A P value of <.05 was considered to be statistically significant.
RESULTS
A total of 115 patients who met inclusion criteria were seen during the study period; medical records were available for 114 (99%) of these patients. Patient and disease characteristics are shown in Table 2. The group of children who had follow-up examinations at UCLA after baseline appears to be representative of the entire study population, based on demographic and medical factors. Data regarding flare values for all eyes at baseline and during follow-up are shown in Table 3. Laser flare photometry values were available for 169 of 198 involved eyes (96 of 114 patients). Laser flare photometry measurements were performed on noninvolved eyes of 2 additional patients with unilateral uveitis, but could not be obtained on the involved eyes. Serial measurements were available for 113 of 146 involved eyes (64 of 82 patients) that had follow-up at UCLA. There was a trend toward longer follow-up of patients with eyes having higher baseline/first flare values (r = 0.172, P = .069).
TABLE 2.
CHARACTERISTICS OF CHRONIC ANTERIOR UVEITIS IN CHILDREN
| CHARACTERISTIC | ALL PATIENTS (N = 114) | PATIENTS WITH FOLLOW-UP (N = 82) |
|---|---|---|
| Age at baseline (yr) | ||
| Mean ± SD | 10.3 ± 4.3 | 9.9 ± 4.4 |
| Median (range) | 10.7 (2.0 to 19.0) | 9.5 (2.0 to 18.7) |
| Female gender | 80 (70%) | 53 (65%) |
| Laterality of uveitis | ||
| Right eye only | 9 ( 8%) | 5 ( 6%) |
| Left eye only | 21 (18%) | 13 (16%) |
| Both eyes | 84 (74%) | 64 (78%) |
| Systemic disease association | ||
| None | 69 (61%) | 48 (59%) |
| JIA | 43 (38%) | 33 (40%) |
| Sarcoidosis | 2 ( 2%) | 1 ( 1%) |
| Classification of uveitis | ||
| Anterior | 95 (83%) | 71 (87%) |
| Anterior and intermediate | 19 (17%) | 11 (13%) |
| Duration of uveitis prior to baseline (mo) | (N = 96) | (N = 72) |
| Mean ± SD | 25.4 ± 36.3 | 23.0 ± 35.3 |
| Median (range) | 11 (0 to 180) | 6 (0 to 180) |
| Year of baseline | ||
| Median (range) | 2003 (1993 to 2006) | 2002 (1993 to 2006) |
| Use of immunomodulatory therapy prior to baseline | 33 (29%) | 19 (23%) |
| Year of most recent examination | ||
| Median (range) | — | 2006 (1999 to 2006) |
| Duration of follow-up (mo) | ||
| Mean ± SD | — | 35.0 ± 35.9 |
| Median (range) | — | 23.5 (0.43 to 154.8) |
| Duration of uveitis, including follow-up (mo) | (N = 72) | |
| Mean + SD | — | 58.1 ± 49.4 |
| Median (range) | — | 49.2 (2.4 to 301.5) |
JIA, juvenile idiopathic arthritis; SD, standard deviation.
TABLE 3.
LASER FLARE PHOTOMETRY VALUES FOR CHILDREN WITH CHRONIC ANTERIOR UVEITIS
| MEASUREMENT | ALL EYES (N = 198) |
|---|---|
| Baseline flare* | (N = 130)† |
| Mean ± SD | 44.0 ± 61.3 |
| Median (range) | 22.9 (1.9–345.4) |
| Proportion ≥20 pu/msec | 70 (54%) |
| First flare‡ | (N = 39)† |
| Mean ± SD | 36.8 ± 57.6 |
| Median (range) | 15.1 (3.1–274.3) |
| Proportion ≥ 20 pu/msec | 17 (44%) |
| Interval since baseline (mo) | 21.0 ± 21.5 |
| Median (range) | 15.9 (1.6–82.6) |
| Most recent flare§ | (N = 113)† |
| Mean ± SD | 32.2 ± 50.0 |
| Median (range) | 13.6 (2.5–350.1) |
| Proportion ≥ 20 pu/msec | 41 (36%) |
| Change in flare during follow-up¶ | (N = 113)† |
| Mean ± SD | −12.4 ± 39.2 |
| Median (range) | −3.7 (−185.4 to +97.4) |
| Interval during which change occurred (mo) | |
| Mean ± SD | 34.3 ± 28.4 |
| Median (range) | 26.7 (0.7–127.9) |
| Fluctuation of flare|| | (N = 113) † |
| Mean ± SD | 49.4 ± 59.4 |
| Median (range) | 19.2 (1.1–260.8) |
| Maximum flare** | (N = 169) † |
| Mean ± SD | 58.9 ± 76.5 |
| Median (range) | 23.2 (1.9–462.5) |
| Interval since onset of uveitis (mo) †† | |
| Mean ± SD | 32.3 ± 36.8 |
| Median (range) | 24.0 (0–180) |
Values obtained during initial examination (baseline). Laser flare photometry measurements were not obtained on every patient at initial examination.
Number of eyes for which data were available. For some patients with bilateral disease, measurements could not be obtained for both eyes.
Initial values obtained at an examination after baseline, if laser flare photometry was not performed at baseline.
For patient who had follow-up and who had laser flare photometry measurements at multiple examinations.
Calculated by subtracting baseline flare value or first flare value from most recent flare value.
The absolute difference between the highest and lowest flare values for each patient with follow-up who had multiple laser flare photometry measurements.
The highest flare value obtained for each patient, regardless of when during the course of disease.
Calculated by adding duration of disease before baseline and interval from baseline to examination at which maximum flare obtained. If the duration of uveitis before baseline was unknown, the patient was assigned the value “0” for study purposes.
Laser flare photometry measurements were performed successfully on patients as young as 2 years, 4 months of age. Laser flare photometry measurements were not performed on 18 patients for the following reasons. Measurements could not be obtained for 3 eyes because of central corneal opacification (calcific band keratopathy; corneal haze) and could not be obtained for 1 eye because of a small pupil (caused by extensive posterior synechiae) and white cataract. Measurements could not be obtained in 5 patients because of poor cooperation with the testing procedure. With one exception, poor cooperation was attributable to patient age (range, 2 years 6 months to 4 years 6 months). The other uncooperative patient (aged 8 years) had a developmental disorder that resulted in behavioral disturbances. No explanation for lack of measurement was provided in the records of 9 patients. All were older than 9 years of age, and none had central corneal opacities. Seven of 9 were examined during 1999–2000, suggesting the possibility that technical or logistic problems during that period accounted for the lack of testing, although a reason for lack of testing was not stated specifically in the medical records.
Among children with bilateral disease, there was a significant relationship between flare values for the right and left eyes when all examinations were considered, but the correlation was weak (r = 0.293, P < .001). As a result, per-eye cross-sectional analyses were not controlled for relationships between eyes of patients with bilateral disease.
Maximum flare values occurred relatively early in the course of disease (median, 24.0 months; Table 3), when compared to the duration of disease at most recent examination (median, 49.2 months; Table 2). The highest recorded flare value was 462.5 pu/msec; it was obtained during follow-up of an eye with hypotony at baseline; the eye was found to have ciliary body atrophy by direct observation at the time of cataract extraction and vitrectomy. The patient’s other eye, also with hypotony at baseline, had a maximum flare of 282.3 pu/msec. Flare was available for 1 other of the 5 total eyes with hypotony at baseline; maximum flare were 345.4 pu/msec.
CROSS-SECTIONAL RELATIONSHIPS WITH FLARE
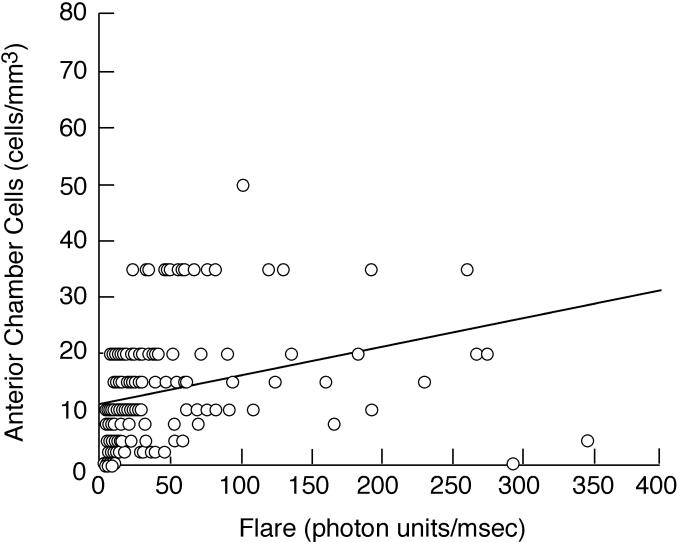
Tables 4 and 5 show the relationships between baseline/first flare values and other factors at baseline. When flare was considered either as a continuous variable (Table 4) or a categorical variable (<20 pu/msec vs ≥20 pu/msec; Table 5), flare was strongly associated with anterior chamber cells and keratic precipitates. The correlation between baseline/first flare and anterior chamber cells (r = 0.565, P < .001; Figure 1) was only moderate, however. Flare was also associated with evidence of intermediate uveitis and with papillitis, although the associations were less strong.
TABLE 4.
RELATIONSHIPS BETWEEN INITIAL FLARE VALUES AND OTHER HOST AND DISEASE FACTORS AT BASELINE FOR CHILDREN WITH CHRONIC ANTERIOR UVEITIS
| BASELINE/FIRST FLARE† | ||
|---|---|---|
| FACTOR* | MEAN ± SD | MEDIAN (RANGE) |
| Age | ||
| <10 yr (n = 81) | 44.2 ± 61.2 | 15.1 (31–292.5) |
| ≥10 yr (n = 88) | 40.6 ± 59.9 | 22.9 (1.9–345.4) |
| P value‡ | .539 | |
| Gender | ||
| Female (n = 120) | 42.1 ± 56.3 | 22.1 (3.1–292.5) |
| Male (n = 49) | 43.0 ± 70.1 | 17.9 (1.9–345.4) |
| P value‡ | .292 | |
| Presence of systemic disease association§ | ||
| None (n = 103) | 45.0 ± 66.4 | 20.3 (1.9–345.4) |
| JIA (n = 64) | 38.0 ± 50.4 | 17.0 (3.1–274.3) |
| P value‡ | .714 | |
| Duration of disease¶ | ||
| <1 yr (n = 74) | 44.2 ± 64.8 | 21.7 (3.6–345.4) |
| ≥1 yr (n = 95) | 40.9 ± 57.0 | 20.0 (1.9–274.3) |
| P value‡ | .996 | |
| Immunomodulatory therapy | ||
| Yes (n = 66) | 47.0 ± 65.3 | 22.7 (3.5–292.5) |
| No (n = 103) | 39.4 ± 57.1 | 18.7 (1.9–345.4) |
| P value‡ | .255 | |
| Cells|| | ||
| < 1+ (n=51) | 25.0 ± 61.6 | 7.5 (1.9–345.4) |
| ≥1+ (n=116) | 49.1 ± 57.6 | 26.7 (4.1–274.3) |
| P value‡ | <.001 | |
| Keratic precipitates | ||
| Yes (n = 47) | 60.0 ± 67.3 | 32.5 (3.3–292.5) |
| No (n = 122) | 35.5 ± 56.3 | 15.2 (1.9–345.4) |
| P value‡ | <.001 | |
| Evidence of intermediate uveitis | ||
| Yes (n = 33) | 46.3 ± 51.9 | 28.1 (4.1–229.8) |
| No (n = 136) | 44.4 ± 62.4 | 16.4 (1.9–345.4) |
| P value‡ | .027 | |
| Papillitis | ||
| Yes (n = 31) | 45.2 ± 43.2 | 28.1 (3.3–160.2) |
| No (n = 130) | 42.2 ± 65.1 | 17.6 (1.9–345.4) |
| P value‡ | .079 | |
| Any vision-threatening complication** | ||
| Yes (n = 89) | 66.7 ± 74.2 | 39.0 (1.9–345.4) |
| No (n = 80) | 15.3 ± 14.6 | 8.7 (3.1–90.5) |
| P value‡ | <.001 | |
| Specific complications | ||
| Band keratopathy | ||
| Yes (n = 39) | 111.2 ± 85.4 | 76.1 (12.3–345.4) |
| No (n = 130) | 21.7 ± 27.1 | 13.6 (1.9–229.8) |
| P value‡ | <.001 | |
| Posterior synechiae | ||
| Yes (n = 50) | 84.4 ± 85.5 | 51.7 (4.5–345.4) |
| No (n = 119) | 24.8 ± 33.4 | 13.0 (1.9–229.8) |
| P value‡ | <.001 | |
| Cataract | ||
| Yes (n = 54) | 80.4 ± 88.4 | 48.8 (1.9–345.4) |
| No (n = 115) | 24.5 ± 27.2 | 14.4 (3.1–160.2) |
| P value‡ | <.001 | |
| Glaucoma/elevated intraocular pressure | ||
| Yes (n = 20) | 42.3 ± 44.1 | 30.6 (9.0–192.7) |
| No (n = 149) | 42.4 ± 62.4 | 19.1 (1.9–345.4) |
| P value‡ | .075 | |
JIA, juvenile idiopathic arthritis.
Factor at baseline unless otherwise specified.
Laser flare photometry value obtained at baseline or initial laser flare photometry value obtained after baseline (“first flare”), if none available at baseline.
Wilcoxon rank-sum test
Comparison excludes 2 patients with sarcoidosis (flare values of 38.8 pu/msec and 52.3 pu/msec).
Duration of disease prior to baseline, based on history, and interval from baseline to first flare, if applicable.
Anterior chamber cell determination from same examination as flare determination. Categorical scores converted to numerical values, as described in text.
Includes the following complications: band keratopathy; posterior synechiae; peripheral anterior synechiae; cataract; inflammatory membranes; glaucoma or elevated intraocular pressure; hypotony; macular edema; other.
TABLE 5.
HOST AND DISEASE FACTORS AT INITIAL FLARE DETERMINATION FOR CHILDREN WITH CHRONIC ANTERIOR UVEITIS GROUPED BY INITIAL FLARE VALUE
| SUBGROUPS BASED ON BASELINE/FIRST FLARE† (n = 169) | |||
|---|---|---|---|
| FACTOR* | <20 pu/msec | ≥20 pu/msec | P value‡ |
| Age < 10 yr | 44 (53.7%) | 37 (42.5%) | .167 |
| Gender (female) | 56 (68.3%) | 64 (73.6%) | .500 |
| Presence of systemic disease association | 33 (40.2%) | 33 (37.9%) | .535 |
| Duration of disease§ (n = 165) | 34 (43.0%) | 43 (50.0%) | .435 |
| Immunomodulatory therapy | 29 (35.4%) | 37 (42.5%) | .349 |
| Cells ≥1+¶ (n = 167) | 41 (50.6%) | 75 (87.2%) | <.001 |
| Keratic precipitates | 13 (15.9%) | 34 (39.1%) | .001 |
| Evidence of intermediate uveitis | 10 (12.2%) | 23 (26.4%) | .021 |
| Papillitis (n = 161) | 10 (12.8%) | 21 (25.3%) | .048 |
| Any vision-threatening complication** | 25 (30.5%) | 64 (73.6%) | <.001 |
| Specific complication | |||
| Band keratopathy | 2 (24%) | 37 (42.5%) | <.001 |
| Posterior synechiae | 9 (11.0%) | 41 (47.1%) | <.001 |
| Cataract | 16 (19.5%) | 38 (43.7%) | <.001 |
| Glaucoma/elevated intraocular pressure | 7 (8.5%) | 13 (14.9%) | .238 |
Factor at baseline unless otherwise specified. Listed for each factor is the number of eyes (percentage of eyes in subgroup with factor); n = 169 unless otherwise stated.
Comparison using laser flare photometry value obtained at baseline or initial laser flare photometry value obtained after baseline (“first flare”), if none available at baseline.
Fisher exact test.
Duration of disease prior to baseline, based on history, and interval from baseline to first flare, if applicable.
Anterior chamber cell determination from same examination as flare determination.
Includes the following complications: band keratopathy; posterior synechiae; peripheral anterior synechiae; cataract; inflammatory membranes; glaucoma or elevated intraocular pressure; hypotony; macular edema; other.
FIGURE 1.
Scatter plot showing baseline/first flare value vs number of anterior chamber cells from the same examination date for eyes of children with chronic anterior uveitis. See text for explanation of conversion from categorical cell score to numerical cell value.
Vision-threatening complications were present in 107 eyes of 74 patients at baseline. Band keratopathy was present in 50 eyes of 37 patients; posterior synechiae were present in 61 eyes of 50 patients; peripheral anterior synechiae were present in 3 eyes of 2 patients; cataract was present in 67 eyes of 48 patients; inflammatory membranes were present in 13 eyes of 8 patients; glaucoma/elevated intraocular pressure was present in 25 eyes of 20 patients; hypotony was present in 5 eyes of 4 patients; macular edema was present in 7 eyes of 5 patients; and other complications were present in 3 eyes of 2 patients (retinal detachment in 2 eyes of 1 patient; retinoschisis in 1 eye). Baseline/first flare was strongly associated with any vision-threatening complication at baseline and was associated with the specific complications of band keratopathy, posterior synechiae, and cataract, whether flare was considered as a continuous (Table 4) or as a categorical (Table 5) variable. A weak association between flare and glaucoma/elevated intraocular pressure was noted only when flare was considered as a continuous variable. There were no relationships between flare and the following factors: age, gender, the presence of an associated systemic disease, duration of disease before laser flare photometry measurement, or use of immunomodulatory therapy at baseline. The same relationships were identified when only eyes with baseline flare were evaluated, although the relationships were less strong because of smaller sample sizes (data not shown).
In trend analyses, there appeared to be variable threshold effects, with increased, but stable, prevalence of the following factors above the indicated thresholds: evidence of intermediate uveitis (5% for patients with flare <10 pu/msec vs 26% for eyes with flare ≥10 pu/msec); presence of keratic precipitates (16% for eyes with flare <20 pu/msec vs 39% for eyes with flare ≥20 pu/msec); presence of any complications at baseline (39% for eyes with flare <50 pu/msec vs 95% for eyes with flare ≥50 pu/msec); and possibly glaucoma/elevated intraocular pressure (2% for patients with flare <10 pu/msec vs 17% for eyes with flare ≥10 pu/msec). There may have been thresholds at high flare values for other factors, but the small number of events and the small sample sizes for categories of flare ≥20 pu/msec made assessment difficult.
FLARE DURING THE COURSE OF DISEASE
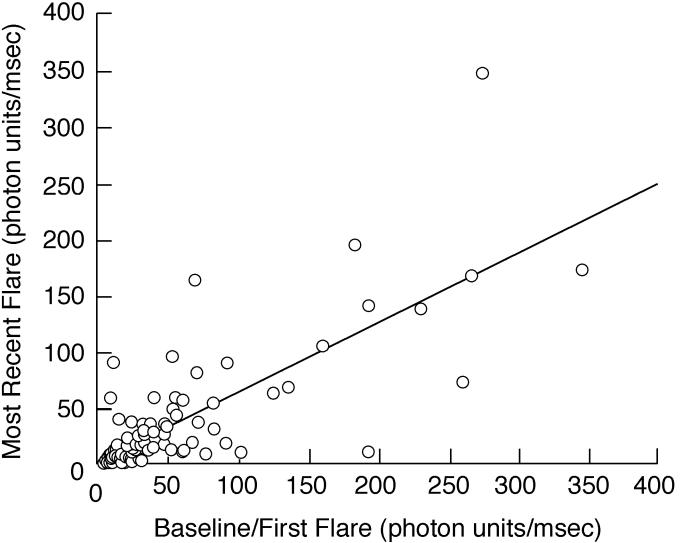
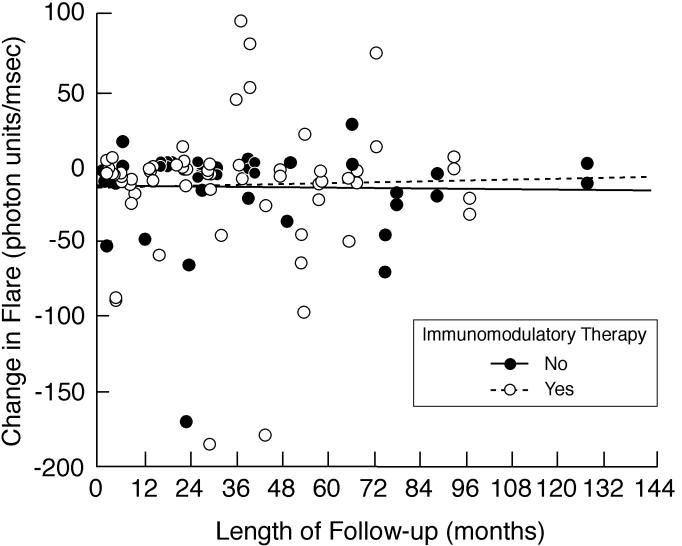
There was little change between baseline/first flare and most recent flare when all eyes were considered (Table 3; Figure 2), but there was substantial variation for individual patients. There was also substantial fluctuation of flare (difference between maximum and minimum flare values) during the course of disease (Table 3). Factors related to change from baseline/first flare to most recent flare values are shown in Table 6. Both increased and decreased flare during follow-up was more closely associated with female gender, with baseline/first flare ≥20 pu/msec, and with any vision-threatening complications at baseline than was stable flare. Increased flare during follow-up was also associated with the presence of a systemic disease, and decreased flare was also associated with development of vision-threatening complications during follow-up. Among eyes that had an increase of flare during follow-up, 80% had received immunomodulatory therapy, whereas those with decreased or stable flare were equally distributed between treated and untreated eyes, but the association did not reach statistical significance (also see Figure 3).
FIGURE 2.
Scatter plot showing baseline/first flare value vs most recent flare value for eyes of children with chronic anterior uveitis. There was little overall change in flare values during the course of disease, regardless of length of follow-up.
TABLE 6.
HOST AND DISEASE FACTORS FOR CHILDREN WITH CHRONIC ANTERIOR UVEITIS GROUPED BY CHANGE OF FLARE DURING FOLLOW-UP
| SUBGROUPS BASED ON CHANGE OF FLARE DURING FOLLOW-UP† (n = 113) | ||||
|---|---|---|---|---|
| FACTOR* | DECREASED | STABLE | INCREASED | P VALUE‡ |
| Age < 10 yr (n = 113) | 19 (51.4%) | 33 (50.0%) | 7 (70.0%) | .530 |
| Gender (female) | 26 (70.3%) | 35 (53.0%) | 10 (100.0%) | .005 |
| Presence of systemic disease association | 12 (32.4%) | 31 (47.0%) | 8 (80.0%) | .028 |
| Immunomodulatory therapy at baseline or during follow-up | 21 (56.8%) | 36 (54.6%) | 8 (80.0%) | .339 |
| Baseline/first flare ≥20 pu/msec§ | 34 (91.9%) | 20 (30.3%) | 7 (70.0%) | <.001 |
| Evidence of intermediate uveitis | 9 (24.3%) | 7 (10.6%) | 1 (10.0%) | .159 |
| Any vision-threatening complication at baseline¶ | 26 (70.3%) | 26 (39.4%) | 7 (70.0%) | .006 |
| Any vision-threatening complication during follow-up¶ | 19 (51.4%) | 18 (27.3%) | 3 (30.0%) | .049 |
Factor at baseline unless otherwise specified. Listed for each factor is the number of eyes (percentage of eyes in subgroup with factor);
Calculated by subtracting most recent flare value from value obtained at baseline or initial laser flare photometry value obtained after baseline (“first flare”), if none available at baseline. Increase of flare was defined as most recent flare value ≥10 pu/msec higher than baseline/first flare. Stable flare was defined as most recent flare ≤9.9 pu/msec higher or lower than baseline/first flare. Decrease of flare was defined as most recent flare value ≥10 pu/msec lower than baseline flare.
Fischer exact test.
Laser flare photometry value at baseline or initial flare value obtained after baseline (“first flare”), if none at baseline.
Includes the following complications: band keratopathy; posterior synechiae; peripheral anterior synechiae; cataract; inflammatory membranes; glaucoma/elevated intraocular pressure; hypotony; macular edema; other.
FIGURE 3.
Scatter plot showing length of follow-up vs change in flare values for eyes of children with chronic anterior uveitis. The difference was determined by subtracting baseline or first flare value from the most recent flare value. There was an overall decline in flare during the course of disease for involved eyes for children with follow-up. Shown are subgroup analyses based on use of immunomodulatory therapy.
Factors related to fluctuation of flare during follow-up are shown in Table 7. Greater fluctuation was associated with female gender, immunomodulatory therapy, baseline/first flare, evidence of intermediate uveitis, presence of vision-threatening complications at baseline, and development of vision-threatening complications during follow-up. Fluctuation was correlated with longer follow-up (r = 0.467, P < .001). In contrast, change in flare during follow-up was not a function of duration of follow-up, regardless of whether or not patients received immunomodulatory therapy (Figure 3).
TABLE 7.
RELATIONSHIPS BETWEEN FLUCTUATION OF FLARE VALUES AND OTHER HOST AND DISEASE FACTORS AT BASELINE FOR CHILDREN WITH CHRONIC ANTERIOR UVEITIS
| FLUCTUATION OF FLARE† | ||
|---|---|---|
| FACTOR* | MEAN ± SD | MEDIAN (RANGE) |
| Age | ||
| <10 yr (n = 59) | 58.9 ± 67.2 | 19.4 (1.3–260.8) |
| ≥10 yr (n = 54) | 39.1 ± 48.1 | 17.8 (1.1–231.3) |
| P value‡ | .265 | |
| Gender | ||
| Female (n = 71) | 53.1 ± 58.6 | 251 (1.1–260.8) |
| Male (n = 42) | 43.2 ± 60.8 | 8.5 (2.0–231.3) |
| P value‡ | .032 | |
| Presence of systemic disease association§ | ||
| None (n = 62) | 50.9 ± 64.6 | 17.8 (1.1–260.8) |
| JIA (n = 49) | 47.5 ± 53.9 | 17.0 (1.3–188.2) |
| P value‡ | .771 | |
| Immunomodulatory therapy | ||
| Yes (n = 65) | 65.9 ± 65.4 | 64.2 (2.0–260.8) |
| No (n = 48) | 27.1 ± 41.3 | 10.0 (1.1–231.3) |
| P value‡ | <.001 | |
| Baseline/first flare¶ | ||
| <20 pu/msec (n = 52) | 14.5 ± 24.0 | 6.1 (1.3–114.9) |
| ≥20 pu/msec (n = 61) | 79.2 ± 64.3 | 66.3 (1.1–260.8) |
| P value‡ | <.001 | |
| Evidence of intermediate uveitis | ||
| Yes (n = 17) | 73.4 ± 66.7 | 69.8 (2.2–231.9) |
| No (n = 96) | 45.2 ± 57.3 | 15.5 (1.1–260.8) |
| P value‡ | .031 | |
| Any vision-threatening complication at baseline|| | ||
| Yes (n = 59) | 73.4 ± 68.7 | 65.2 (2.2–260.8) |
| No (n = 54) | 23.3 ± 31.0 | 10.7 (1.1–130.7) |
| P value‡ | <.001 | |
| Any vision-threatening complications during follow-up|| | ||
| Yes (n = 40) | 76.3 ± 62.4 | 69.0 (4.5–260.8) |
| No (n = 73) | 34.7 ± 52.5 | 12.0 (1.1-231-3) |
| P value‡ | <.001 | |
Factor at baseline unless otherwise specified.
Absolute difference between maximum and minimum flare values during course of disease for individual eyes for which at least 2 laser flare photometry measurements were made during follow-up.
Wilcoxon rank-sum test
Comparison excludes 2 patients with sarcoidosis (fluctuation of 31.3 pu/msec and 69.8 pu/msec).
Laser flare photometry value obtained at baseline or initial laser flare photometry value obtained after baseline (“first flare”), if none available at baseline.
Includes the following complications: band keratopathy; posterior synechiae; peripheral anterior synechiae; cataract; inflammatory membranes; glaucoma or elevated intraocular pressure; hypotony; macular edema; other.
PREDICTIVE VALUE OF FLARE
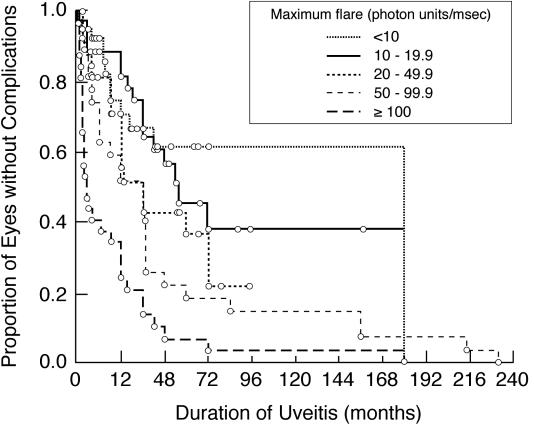
Vision-threatening complications occurred in 49 eyes of 31 patients during follow-up. Band keratopathy occurred in 3 eyes of 3 patients; posterior synechiae occurred in 9 eyes of 7 patients; peripheral anterior synechiae occurred in 6 eyes of 5 patients; cataract occurred in 18 eyes of 11 patients; inflammatory membranes occurred in 1 eye; glaucoma/elevated intraocular pressure occurred in 37 eyes of 23 patients; and other complications occurred in 1 eye (subretinal neovascular membrane). With the exception of 1 eye, glaucoma/elevated intraocular pressure was identified before, or concurrently with, other complications during follow-up. One eye of a patient with bilateral uveitis developed band keratopathy before intraocular pressure rose. No patient developed hypotony or clinical apparent macular edema during follow-up. Higher maximum flare values were associated with increasing risk of complications during the course of disease (P < .001, Figure 4).
FIGURE 4.
Kaplan-Meier analysis showing duration of uveitis vs the proportion of involved eyes of children with chronic anterior uveitis that are without any vision-threatening complications. Shown are subgroup analyses based on maximum flare value (<10 pu/msec; 10–19.9 pu/msec; 20–49.9 pu/msec; 50–99.9 pu/msec; ≥100 pu/msec). For study purposes, complications identified at baseline were assumed to have occurred at baseline. Duration of uveitis for each patient is from onset of disease, as determined by history.
Tables 8 and 9 show the predictive values of various factors for development of vision-threatening complications or vision loss during follow-up. On univariate analyses (Table 8), increased baseline/first flare (both ≥10 pu/msec and ≥20 pu/msec) was associated with development of any vision-threatening complication and glaucoma/elevated intraocular pressure. Baseline/first flare ≥20 pu/msec was also associated with loss of visual acuity to 20/50 or less. Kaplan-Meier analyses also showed the associations between baseline flare ≥20 pu/msec and any vision-threatening complications (Figure 5), glaucoma/elevated intraocular pressure (Figure 6), and visual acuity loss to 20/50 or less (Figure 7). In univariate analyses (Table 8), the presence of any vision-threatening complication at baseline was also associated with development of additional vision-threatening complications during follow-up and with loss of visual acuity to 20/50 or less. Increased baseline cell count was only weakly associated with the development of vision-threatening complications during follow-up. The relative risk of the factors for loss of visual acuity to 20/200 or less could not be determined statistically, as no patient with baseline/first flare <20 pu/msec had loss of visual acuity to this level during follow-up.
TABLE 8.
RISK FACTORS FOR VISION LOSS OR DEVELOPMENT OF VISION-THREATENING COMPLICATIONS DURING FOLLOW-UP IN CHILDREN WITH CHRONIC ANTERIOR UVEITIS: UNIVARIATE ANALYSES
| VISION-THREATENING COMPLICATIONS DURING FOLLOW-UP | ||||||
|---|---|---|---|---|---|---|
| Any | Glaucoma/Elevated Intraocular Pressure* | VISUAL ACUITY LOSS (≤20/50) DURING FOLLOW-UP | ||||
| RISK FACTOR | RR (95% CI) | P Value† | RR (95% CI) | P Value† | RR (95% CI) | P Value† |
| Immunomodulatory therapy‡ | 1.60 (0.81–3.18) | .180 | 1.39 (0.64–3.02) | .412 | 2.92 (0.83–10.21) | .094 |
| Baseline cells | 2.34 (0.96–5.66) | .060 | 1.68 (0.68–4.20) | .264 | 0.76 (0.23–2.57) | .662 |
| Baseline/first flare | ||||||
| ≥10 pu/msec | 2.92 (1.20–7.08) | .018 | 4.84 (1.45–16.17) | .010 | 3.46 (0.77–15.54) | .105 |
| ≥20 pu/msec | 3.15 (1.47–6.79) | .003 | 4.62 (1.74–12.28) | .002 | 5.87 (1.31–26.30) | .021 |
| Any vision-threatening complication at baseline | 2.18 (1.06–4.49) | .035 | 1.99 (0.88–4.46) | .097 | 3.89 (1.20–12.59) | .023 |
CI, confidence interval; RR, relative risk.
Includes eyes with glaucoma/elevated intraocular pressure at baseline, if there was evidence of progressive optic disc or visual field changes or loss of control with medication regimen used at baseline.
Cox proportional hazards regression models.
Use at any time point during follow-up, before event.
TABLE 9.
RISK FACTORS FOR VISION LOSS OR DEVELOPMENT OF VISION-THREATENING COMPLICATIONS DURING FOLLOW-UP IN CHILDREN WITH CHRONIC ANTERIOR UVEITIS: MULTIVARIATE ANALYSES
| VISION-THREATENING COMPLICATIONS DURING FOLLOW-UP | ||||||
|---|---|---|---|---|---|---|
| Any | Glaucoma/Elevated Intraocular Pressure* | VISUAL ACUITY LOSS (≤20/50) DURING FOLLOW-UP | ||||
| RISK FACTOR | RR (95% CI) | P Value† | RR (95% CI) | P Value† | RR (95% CI) | P Value† |
| Immunomodulatory therapy‡ | 1.09 (0.51–2.33) | .821 | 0.82 (0.35–1.96) | .658 | 1.42 (0.38–5.31) | .606 |
| Baseline cells | 1.59 (0.62–4.06) | .337 | 0.81 (0.30–2.23) | .685 | 0.27 (0.07–1.07) | .062 |
| Baseline/first flare ≥20 pu/msec | 2.25 (0.86–5.88) | .097 | 4.73 (1.42–15.77) | .012 | 6.06 (1.04–35.23) | .045 |
| Any vision-threatening complication at baseline | 1.18 (0.48–2.92) | .717 | 1.03 (0.39–2.70) | .954 | 2.19 (0.52–9.26) | .287 |
CI, confidence interval; RR, relative risk.
Includes eyes that had glaucoma/elevated intraocular pressure at baseline, if there was evidence of progressive optic disc or visual field changes during follow-up.
Cox proportional hazards regression models. Also included in the model was duration of disease prior to baseline/first flare determination.
Use at any time point during follow-up, before event.
FIGURE 5.
Kaplan-Meier analysis showing the proportion of involved eyes of children with chronic anterior uveitis that are without any vision-threatening complication during follow-up after initial flare determination. Shown are subgroup analyses based on baseline/first flare value (<20 pu/msec vs 20 ≥pu/msec).
FIGURE 6.
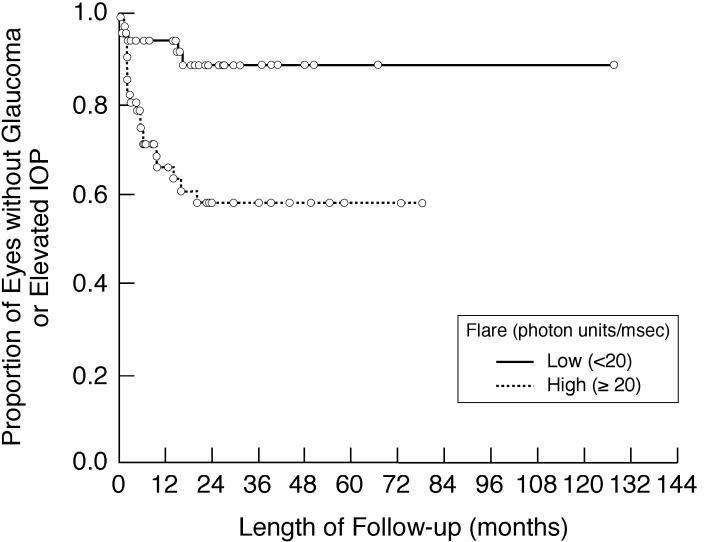
Kaplan-Meier analysis showing the proportion of involved eyes of children with chronic anterior uveitis that are without new evidence of glaucoma or loss of intraocular pressure control during follow-up after initial flare determination. Shown are subgroup analyses based on baseline/first flare value (<20 pu/msec vs 20 ≥pu/msec). IOP, intraocular pressure.
FIGURE 7.
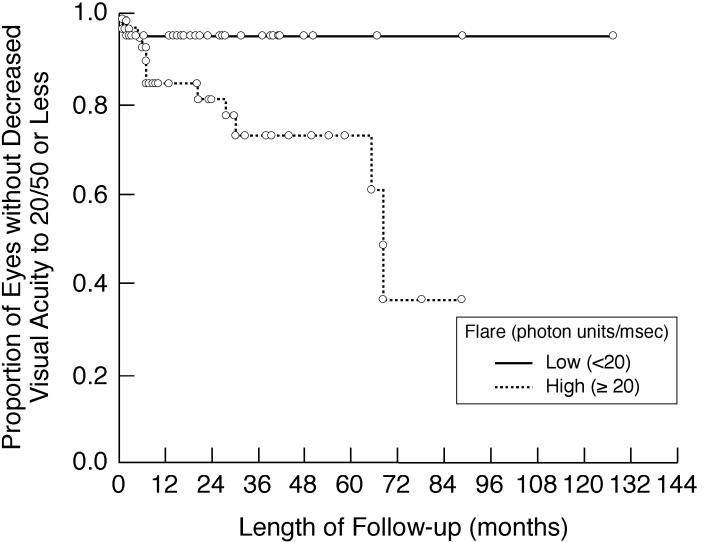
Kaplan-Meier analysis showing the proportion of involved eyes of children with chronic anterior uveitis that are without decreased visual acuity to 20/50 or less during follow-up after initial flare determination. Shown are subgroup analyses based on baseline/first flare value (<20 pu/msec vs 20 ≥pu/msec).
On multivariate analyses that included the same factors, as well as the use of immunomodulatory therapy before an event and duration of disease from onset of disease to event (Table 9), baseline/first flare ≥20 pu/msec retained its association with development of glaucoma/elevated intraocular pressure during follow-up and loss of visual acuity to 20/50 or less, whereas the presence of any vision-threatening complications at baseline did not. Also, increased baseline cell count was no longer associated with development of vision-threatening complications; it remained weakly associated with visual acuity loss to 20/50 or less, but the relative risk (RR) was 0.27 (95% confidence interval [CI], 0.07–1.07, P = .062), consistent with a protective effect.
The same multivariate analyses were repeated using baseline/first flare ≥10 pu/msec as threshold. The pattern of results was similar (data not shown), but baseline/first flare was significantly associated only with glaucoma/elevated intraocular pressure during follow-up (RR = 4.34 [95% CI, 1.09–17.20], P = .037).
When flare was considered as a continuous variable, baseline/first flare was associated with visual acuity loss to 20/200 or less, both in univariate (RR = 1.91 [1.19–3.05], P = .007) and multivariate (RR = 2.02 [1.03–3.95], P = .039) analyses. Other associations were nonsignificant, but all were in the same direction (increased flare associated with adverse events) as found in the analyses using categorical data for baseline/first flare. Kaplan-Meier analysis also showed an increased risk of visual acuity loss to 20/200 or less for eyes with baseline/first flare ≥20 pu/msec (Figure 8).
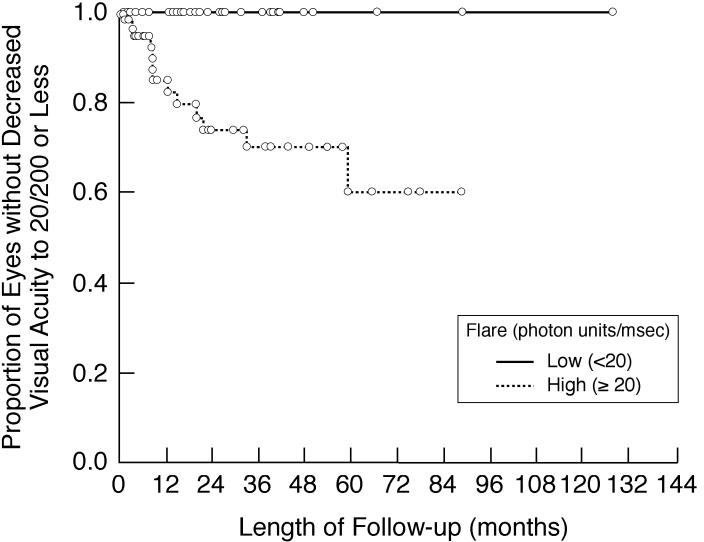
FIGURE 8.
Kaplan-Meier analysis showing the proportion of involved eyes of children with chronic anterior uveitis that are without decreased visual acuity to 20/200 or less during follow-up after initial flare determination. Shown are subgroup analyses based on baseline/first flare value (<20 pu/msec vs 20 ≥pu/msec).
Shifts of flare values before occurrence of events were also investigated to determine whether they would provide additional predictive values for development of complications or vision loss. For the subgroup of patients with baseline/first flare ≥20 pu/msec, those with persistence of flare ≥20 pu/msec during follow-up were at higher risk for vision-threatening complications than those whose flare fell below 20 pu/msec, but the difference was not statistically significant (RR = 1.55 [0.62–3.89], P = .349). Conversely, a rise of flare ≥10 pu/msec above baseline/first flare values did not provide evidence of additional risk for vision-threatening complications during follow-up. Those whose flare values fell from ≥20 pu/msec at baseline/first flare determination to below 20 pu/msec during follow-up appeared still to have a higher risk for vision-threatening complications on Kaplan-Meier plots than those with baseline/first flare values <20 pu/msec whose flare remained below 20 pu/msec during follow-up (data not shown), but the difference was not statistically significant.
SUBGROUP ANALYSES BASED ON FLARE AND CELLS
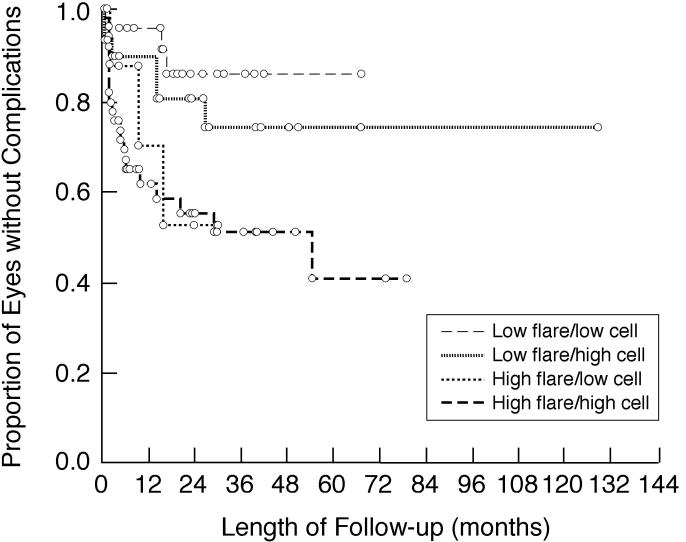
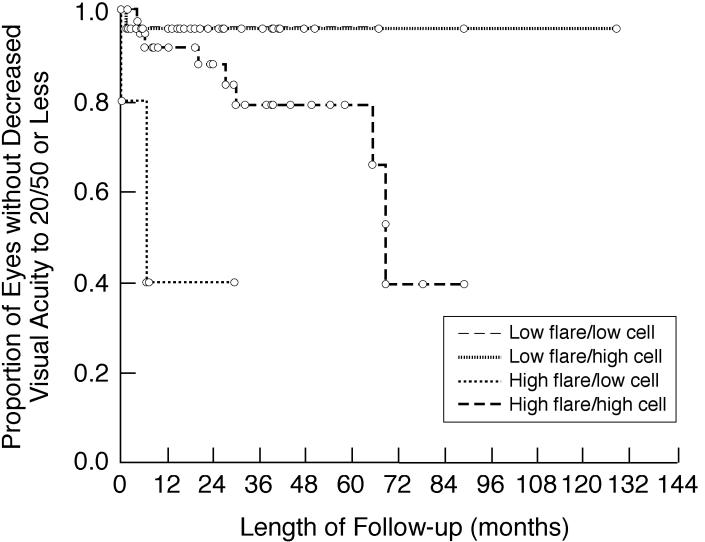
Patients distributed by baseline/first flare and corresponding anterior chamber cell scores are shown in Table 10. Among those with discordant flare and cell scores, those with low flare and high (≥1+) cell scores were more prevalent than those with high flare and low cell scores, regardless of the threshold used to define high flare (≥10 pu/msec or ≥20 pu/msec). Subgroups with low flare (<20 pu/msec) had lower risks of vision-threatening complications (Figure 9) and visual acuity loss to 20/50 or less (Figure 10) during follow-up than those with high flare, regardless of cells scores.
TABLE 10.
INVOLVED EYES OF CHILDREN WITH CHRONIC ANTERIOR UVEITIS GROUPED ON THE BASIS OF FLARE AND CELLS
| CELLS† | ||
|---|---|---|
| BASELINE/FIRST FLARE* | <1+ (n =51) | ≥1+ (n = 116) |
| Lower threshold | ||
| <10 pu/msec (n =54) | 33 (20%) | 21 (13%) |
| ≥10 pu/msec (n = 113) | 18 (11%) | 95 (57%) |
| Higher threshold | ||
| <20 pu/msec (n =81) | 40 (24%) | 41 (25%) |
| ≥20 pu/msec (n = 86) | 11 (7%) | 75 (45%) |
Laser flare photometry value at baseline or initial flare value obtained after baseline (“first flare”), if none at baseline.
Anterior chamber cell score assigned clinically from the same examination at which flare value obtained. Patients scored clinically as having cells below mid-range for the 1+ category (eg, “occasional-1+ cells”) were included in groups categorized as having <1+ cells. Listed for each group is proportion of all eyes.
FIGURE 9.
Kaplan-Meier analysis showing the proportion of involved eyes of children with chronic anterior uveitis that are without any vision-threatening complications during follow-up after initial flare determination. Shown are subgroup analyses based on baseline/first flare and corresponding anterior chamber cell score. Low flare is defined as <20 pu/msec; high flare is defined as ≥20 pu/msec. Low cells is defined as <1+; high cells is defined as ≥1+.
FIGURE 10.
Kaplan-Meier analysis showing the proportion of involved eyes of children with chronic anterior uveitis that are without decreased visual acuity to 20/50 or less during follow-up after initial flare determination. Shown are subgroup analyses based on baseline/first flare and corresponding anterior chamber cell score. Low flare is defined as <20 pu/msec; high flare is defined as ≥20 pu/msec. Low cells is defined as <1+; high cells is defined as ≥1+.
Among patients with low flare (<20 pu/msec) and high cells, there was no statistical difference in risk of vision-threatening complications or visual acuity loss to 20/50 or less during follow-up, whether cells remained ≥1+ during the first 30 months of follow-up (the longest interval from baseline to a complication for any individual in this subgroup) or decreased to <1+ during the first 30 months of follow-up. The small sample sizes and low event rates limit the ability to draw conclusions regarding a lack of effect from the persistence of cells in some patients, however.
IMMUNOMODULATORY THERAPY
Immunomodulatory therapy was being used by 33 children at baseline (56 involved eyes), and was started on an additional 24 patients during follow-up. Patients not on immunomodulatory therapy at baseline with eyes that had baseline/first flare ≥20 pu/msec were more likely to begin therapy during follow-up (P = .006). Change in flare associated with start of therapy during follow-up ranged from −89.0 pu/msec to +33.9 pu/msec (mean, −7.3 ± 27.6 pu/msec; median, −0.5 pu/msec). Immunomodulatory therapy was associated with change in flare and fluctuation in flare during the entire course of follow-up, as described above.
DISCUSSION
The term flare refers to the appearance of light reflected from solutes in the aqueous humor (Tyndall effect) and seen during slit-lamp biomicroscopy. It has become synonymous with increased concentration of aqueous humor protein, which is caused by a “breakdown of the blood-ocular barrier” (increased vascular permeability of iris and ciliary body blood vessels), as one feature of inflammation involving the anterior segment. Concepts regarding flare have evolved over the past half-century. Hogan and associates1 assumed that flare was a sign of active intraocular inflammation (except in atrophic eyes with hypotony). Duke-Elder and Perkins2 agreed and also considered flare to be the “glue” that caused posterior synechiae. Schlaegel,3 however, stated that flare “cannot be cured” and that clinicians must rely on other signs for the assessment of disease activity. More recently, uveitis specialists have assumed that the vascular damage responsible for flare will at least be long-standing, if not permanent,5,6 and thus, increased flare is a marker of past inflammation but should not be considered a sign of active inflammation.5,15 The presence of cells in the anterior chamber, rather than flare, is now generally considered to be the factor most responsible for risk of uveitic complications,5 and treatment is directed towards the elimination of anterior chamber cells.16,17
The current lack of attention to flare may be related to the practical difficulty in assessing changes in flare clinically, despite the criteria that have been proposed for categorizing flare during examinations. A change in cells can be identified more easily than a change in flare, and anterior chamber cells appear to change more dramatically with exacerbations of inflammation, and in response to treatment, than does flare.
Laser flare photometry provides a technique to identify changes in aqueous humor protein objectively.7 The technique and its applications have been reviewed in detail by Ladas and associates7; a brief summary of information relevant to the current study is summarized below. The Kowa FC-1000 and the Kowa FM-500 (Kowa Co Ltd, Electronics and Optics Division, Tokyo, Japan) determine flare by projecting a laser through the anterior chamber, and then measuring light that is scattered by proteins in the aqueous humor. The ability of these machines to determine protein concentrations accurately and reproducibly has been confirmed in patient-based and laboratory studies.7 Photometry measurements have been compared in several studies to clinical grades of flare assigned by experienced uveitis specialists; there is a general increase in laser flare photometry values with increasing clinical grades, but ranges overlap substantially between assigned categorical grades, indicating the lack of precision of clinical assessments.
Laser flare photometry values in healthy children without eye disease are <5 pu/msec.7 A variety of factors can affect laser flare photometry measurements, including time of day, age, mydriasis, cataract, and topical medications, but the effect of these factors is fairly small compared to the changes seen with disease. Of possibly more importance with regard to uveitis is the fact that protein content, as well as protein concentration, can alter laser flare photometry values.7 Higher-molecular-weight proteins will increase values, despite a constant concentration. As vascular permeability increases, there will be a relatively larger proportion of high-molecular-weight proteins in the aqueous humor. Thus, rising values may indicate increased protein concentration, a change in protein composition, or both.
Ladas and associates7 also reviewed published experience (through 2002) with laser flare photometry in the evaluation of patients with uveitis. Most reports have attempted to characterize various forms of uveitis on the basis of flare levels. There has been some study of changes in laser flare photometry values during the early course of uveitic diseases and exacerbations of episodic disease, but there is little information about long-term changes in laser flare photometry values among patients with chronic uveitis. Ladas and associates7 also cited their clinical experience in suggesting that fluctuations of less than 10 pu/msec may be clinically unimportant during the course of disease, and that values <20 pu/msec may be associated with a low risk of uveitic complications. Thresholds used in this study were based on those suggestions.
Chronic anterior uveitis in children, although less common than uveitis in adults,12 can be a devastating disease because of severe complications that can lead to vision loss.9,12,18–26 The incidence of these complications is believed to be greater in children than in adults. From a practical standpoint, laser flare photometry may be a useful method for evaluating uveitis in children, as it is possible to obtain reliable measurements even on very young patients.
Current results confirm the relationships between baseline flare and uveitic complications reported previously in a cross-sectional study of children with various forms of chronic anterior uveitis by Davis and associates.9 In that study, elevated flare was associated with any complication and specifically with posterior synechiae. Cataracts were more common in children with elevated flare, but the relationship did not reach statistical significance. Glaucoma/ocular hypertension was not associated with flare. Although a subgroup of the current cohort was included in the investigation by Davis and associates,9 there are several differences between the two studies. Davis and associates9 studied a smaller population that also included patients with isolated intermediate uveitis and patients with panuveitis. They defined glaucoma/ocular hypertension using a threshold intraocular pressure of 24 mm Hg. The current study also identified relationships between flare and band keratopathy, keratic precipitates, and papillitis, and confirmed the relationship with cataracts statistically. There was an apparent relationship between flare and glaucoma/elevated intraocular pressure, as defined for this study, although the association was weak statistically. Flare was higher in children who also had a component of intermediate uveitis, providing support for an observation by Duke-Elder and Perkins2 in 1966 that clinical flare was highest among patients with “cyclitis.”
These relationships cannot be explained on the basis of disease chronicity alone. Although the cumulative risk of complications increases over time, results show that flare does not invariably rise during the course of disease. Overall, there was a small decrease in flare during the course of disease. Davis and associates9 reported a small increase in median flare among their patients with serial examinations, but in both studies, differences were small and not related to duration of follow-up. The fact that flare fell overall in this study might be attributable, in part, to selection bias. One would logically expect, however, that the population with high flare (if it is a reflection of more severe disease) would be followed longer than those with low flare (if it was a reflection of less severe disease). The stability of flare in patients with chronic anterior uveitis is in contrast to findings in patients with limited or episodic forms of uveitis. Herbort and associates27 have shown that patients with HLA-B27-associated “acute anterior uveitis” have marked elevations of flare at the sudden onset of disease and that flare decreases rapidly to normal levels with treatment. The stability of laser flare photometry values in patients with chronic anterior uveitis is consistent with the clinical observation that flare is persistent in this population. The reason for persistence of elevated flare is unknown. In a study of rabbits, O’Connor28 showed a persistence of vascular permeability to serum proteins in eyes after resolution of toxoplasmic retinochoroiditis, despite the absence of clinically active disease. Similar changes may account for the persistence of elevated flare in children with chronic anterior uveitis, despite anti-inflammatory treatment. The nature of the vascular damage will require future study.
The observed relationships between flare and complications may be causal. Gonzales and associates8 have speculated that fibrin, cytokines, and other proteins in the aqueous humor of inflamed eyes play either direct or regulatory roles in the development of complications. Maximum flare values, which occurred at variable points during the course of disease, appeared to segregate patients into distinct subgroups with different risks for complications. This observation may reflect the fact that chronic anterior uveitis is a heterogeneous group of disorders that may have unique disease features; for example, the mix of aqueous humor proteins may differ between patient subgroups.
A number of previous studies19–24,26,29,30 have identified clinical factors that predict an increased risk of vision loss or vision-threatening complications in children with chronic anterior uveitis. They include an association with JIA (and with specific disease manifestations, including the presence of antinuclear antibodies); younger age; gender (female in some studies, male in others); ethnicity other than white Caucasian; duration of disease and interval before treatment; and the presence of complications at initial examination. Posterior synechiae, in particular, have been shown to be predictors of poor outcome. 19,20 Edelsten and associates20 used posterior synechiae as a surrogate for disease severity at initial examination. In their publication, they stated that “anecdotal reports claim that flare itself places the eye at increased risk of glaucoma,” but they did not cite the source of those reports.20
Davis and associates9 identified a weak association between baseline laser flare photometry values and subsequent complications, but the association was not independent of baseline complications. In contrast, multivariate analysis in this study found that baseline/first flare was an independent predictor of vision loss and vision-threatening complications, whereas baseline complications were not. The finding supports the observation by Thorne and associates11 that increased flare, as determined by slit-lamp biomicroscopy during clinical examination, is associated with a risk of complications and vision loss during follow-up. Edelsten and associates20 have suggested that the presence of complications at disease onset may cause irreversible anterior segment changes that increase the chance of cataract and glaucoma during the subsequent course of disease. The fact that only flare was an independent predictor of complications suggests that complications throughout the course of disease may actually be a direct result of mechanisms related to the presence of aqueous humor proteins.
It was difficult to study the effect of change in flare levels during follow-up, because of fluctuations, differential lengths of follow-up, and irregular follow-up schedules. There was a suggestion that a fall in flare reduced the risk of complications, but it did not seem to eliminate altogether the risk associated with earlier elevations of flare. If true, this observation would support the hypothesis by Edelsten and associates20 that tissue damage early in the course of disease does, in fact, have an influence on subsequent events.
Glaucoma/elevated intraocular pressure was the most common complication of uveitis in this cohort, as reported previously for children with uveitis.18 The association between flare and glaucoma/elevated intraocular pressure was the least strong of the relationships identified between flare and complications at baseline. In contrast, the association between baseline/first flare and development of glaucoma/elevated intraocular pressure during follow-up was significant. Ladas and associates31 showed a relationship between elevated laser flare photometry values and reduced aqueous outflow facility in patients with uveitis, which may provide an explanation for the relationship between elevated flare and the eventual development of glaucoma/elevated intraocular pressure. In their study, elevated flare was not associated with elevated intraocular pressure at the time of examinations, however, which was attributed possibly to compensatory mechanisms that reduce intraocular pressure in patients with uveitis. The difficulty in identifying the relationship between elevated flare and glaucoma/elevated intraocular pressure in a cross-sectional comparison may be attributable to the complex nature of aqueous humor flow dynamics, including such compensatory mechanisms, and the complex interactions between elevated intraocular pressure and optic nerve damage. Also, the difficulty with which uveitic glaucoma is distinguished from secondary glaucoma associated with corticosteroid response, especially in a retrospective review, may have affected results.
The fact that associations between flare and complications during follow-up were significant when flare was considered as a categorical variable, but not when it was considered as a continuous variable, suggests the possibility of a threshold effect. (With linear relationships, use of continuous variables should be a statistically more powerful technique.) Trend analyses in the cross-sectional portion of this study did suggest threshold effects for some, but not all, relationships between flare and complications seen at baseline. Thresholds seemed to vary between different complications, with glaucoma/elevated intraocular pressure having a low threshold (10 pu/msec of flare).
Some investigators consider a fall of anterior chamber cells to <1+ to be a sign of successful treatment.16,17 A threshold of ≥1+ was therefore used to define elevated cells for this study. We were unable to divide patients strictly on the basis of categories, as defined by Hogan and associates1 or by the SUN Working Group,14 however, as explained in the “Methods” section (patients with cell concentrations in the lower range of the 1+ category were defined as being <1+ for purposes of analysis). With the large variability of clinical assessments known to occur between observers,7 it is unlikely that this discrepancy led to results that would be any less precise than results from studies in which all patients with cells at the lower range for the 1+ category were included in the ≥1+ group.
The fact that flare was only moderately correlated with cells suggests that flare can provide clinically useful information that is independent of cell scores. Although cells are used clinically in the management of uveitis, baseline/first flare was a stronger predictor of vision loss and vision-threatening complications than cells. In multivariate analysis, flare was associated with these outcomes, whereas cells were not. Also, in subgroup analyses, baseline/first flare was associated with a decreased risk of these outcomes, regardless of cells. The low risk of adverse events associated with the group having low flare and high cells could not be attributed to a drop in cells during follow-up. This finding supports the observation by Holland and Stiehm12 that there may be a subgroup of patients with low flare values who have a good prognosis despite the persistence of observable anterior chamber cells.
Among patients with discordant signs, those with low flare/high cells were more common at initial flare determination than those with high flare/low cells. The latter combination of signs would traditionally be considered to represent long-standing, but inactive, disease and might not be treated.5 Patients in this subgroup did develop complications during follow-up, however. Of concern is whether treatment will alter the course of disease for such patients.
The long-term effects of immunomodulatory therapy on flare have not been studied. There was some suggestion that treatment alters flare, which may be a mechanism by which disease outcomes are affected by treatment. The association between immunomodulatory therapy and both increased and decreased flare during follow-up, and its association with increased fluctuation of flare, could be a reflection of either treatment effect, selection bias, or both. It is possible that some drugs or combinations have more beneficial effects than others. Rising flare values among treated patients may be a continuation of changes that led to the initiation of immunomodulatory therapy; in the absence of an appropriate control group, the possibility of a beneficial effect of therapy, despite a rise in flare, cannot be ruled out. The fact that immunomodulatory therapy had a weak positive relationship with vision loss during follow-up may be another reflection of selection bias. Edelsten and associates32 found that systemic treatment was an independent predictor of vision loss in children with uveitis, attributed to the fact that patients with more severe disease were more likely to be treated. It is well accepted, however, that immunomodulatory therapy has a beneficial effect in children with chronic anterior uveitis,33 and in a study by Thorne and associates,11 use of immunomodulatory therapy was associated with a decreased risk of some complications and blindness in the better eye of children with JIA-associated uveitis.
The relationship between flare and adverse events was independent of treatment. If a fall in flare has only limited effect on risk after disease is established, early, aggressive control of inflammation may be necessary to improve disease outcomes. Yu and associates17 found that immunomodulatory therapy for patients with JIA-associated uveitis was related to prevention of vision loss only when given early in the course of disease.
This study suffers from the limitations of a retrospective analysis, including imprecision of data, nonstandardized data collection, assessment at irregular intervals, and differential follow-up. The fact that treatment was not randomized, and not administered in a standard manner, made it difficult to control for this variable. Differential follow-up of subjects in longitudinal studies can lead to erroneous interpretation of results.34 Survival analyses, which take into account differential follow-up, were therefore used; however, these techniques assume consistent level of risk during follow-up, which may not have been true in the current population. Results from this study and from previous reports suggest that complications may be more common during the early course of uveitis in children.
A strength of the study is the fact that evaluations were provided by a single observer. Although flare values were determined objectively, the observer was not masked to treatment status or previous clinical findings while recording examination data. Initial laser flare photometry measurements were not performed at baseline examination for some children. Because flare was fairly stable during follow-up, combining baseline and first flare values for analysis appeared to be acceptable; results of the cross-sectional comparisons were the same whether baseline/first flare values or baseline values alone were studied.
Poor patient cooperation, especially among younger patients, affected the completeness and accuracy of recorded information. Visual acuity testing was difficult in young children. Those who did not know the alphabet were tested with a chart containing pictures rather than letters; this chart did not measure visual acuity better than the 20/30 level, but this limitation should not have affected results, as a threshold of ≤20/50 was used in analyses for vision loss.
Complications were not quantified and progression of complications was not consistently recorded, which limited ability to assess continuing risk of adverse events. Assessment of vision loss did, however, provide some measure of progression for complications such as cataract. During chart reviews, it was difficult to distinguish between corticosteroid-induced ocular hypertension and elevated intraocular pressure attributable to uveitis. Likewise, the relative contributions of corticosteroid therapy and uveitis to cataract formation were not considered. Immunomodulatory therapy was considered a dichotomous variable for study, but patients were receiving a variety of medications in different combinations, at different doses, and for different durations. Treatment was not randomized.
Another limitation is the fact that our statistical analyses did not take into account the multiple comparisons made in the exploratory portions of this study; thus, some significant relationships may be spurious, on the basis of chance occurrence alone, and will need to be confirmed in future investigations.
Because all patients were seen in a tertiary referral center, this cohort may not be representative of all children with chronic anterior uveitis. Because this study included only pediatric patients, it may not be possible to generalize the results to all patients with uveitis. The fact that cross-sectional relationships between flare and other disease factors were similar to those found for adults in a previous study8 suggests that the findings regarding the predictive value of flare may in fact be applicable to adults as well.
Risk for complications might actually be greater than reflected in these results, as patients with complications at baseline could not, by design of the study, develop the same complication (except glaucoma/elevated intraocular pressure) during follow-up, yet were not removed from the denominator in Kaplan-Meier analyses as long as they were being followed (to allow identification of other complications).
In summary, the results of this study provide a more detailed understanding of flare in children with chronic anterior uveitis. Observations suggest that flare is not simply a sign of blood-aqueous break-down in patients with a history of intraocular inflammation. The relationships between flare and other disease-related factors indicate that flare is at least a dynamic sign of disease severity and that aqueous humor protein may in fact play a role in disease pathogenesis. Results have led to the following conclusions:
Flare is related to a variety of disease manifestations, including uveitic complications.
Typically, flare changes little overall after the onset of a patient’s disease, although it can fluctuate throughout its course. After the early period of disease, elevation of flare may, therefore, not be reversible to normal levels.
The level of flare appears to segregate patients into distinct groups with different risks of vision-threatening complications.
Early levels of flare appear to influence outcomes more than changes in flare during the course of disease.
The relationship between flare and vision loss or vision-limiting complications is independent of anterior chamber cells and is not simply a reflection of treatment effects. Patients with low flare have a reduced risk of vision loss and vision-threatening complications, whether or not they have high anterior chamber cell levels or persistence of anterior chamber cells during the course of disease.
Current results, in conjunction with previous observations, are consistent with a hypothesis that initial events in the pathogenesis of chronic anterior uveitis cause the eye’s flare to rise to a certain level early; elevated flare will then continue to contribute to the eye’s risk for vision-threatening complications. Because aqueous humor protein remains at an elevated level (accounting for the clinical observation that flare persists), treatment or other factors may modulate flare somewhat but cannot prevent complications altogether. Certain complications (eg, ciliary body atrophy with hypotony) could increase flare even further, late in the course of disease. The highest flare recorded in this cohort was for an eye with hypotony.
The current results raise questions for additional study that may help to test this hypothesis. When in the course of disease does flare rise to its stable level? To address the issues raised by Whitcup,5 and quoted in the “Introduction” to this article, what is the effect of various treatments on flare? Can early, aggressive drug therapy return flare to normal levels and thereby reduce the risk of subsequent complications? Is increased flare causal or a surrogate for other changes that cause complications? If causal, what components of the aqueous humor are responsible for tissue damage and vision loss? It will be particularly important in future studies to investigate flare closer to the onset of uveitis. To address this issue, it might be appropriate to include laser flare photometry measurements as a part of ophthalmic screening evaluations of children with JIA, to determine whether changes in flare are a harbinger of uveitis onset.
ACKNOWLEDGMENTS
Funding/Support: Supported by the Maggi Kelly Vision Fund, Jules Stein Eye Institute; the Skirball Foundation, New York, New York; Research to Prevent Blindness, Inc; and Mr Al Pacino through Al’s Irrevocable Charitable Trust, New York, New York. Additional support was provided by the Emily Plumb Estate and Trust Gift for resources utilized in the Jules Stein Eye Institute Clinical Research Center. Dr Holland is recipient of a Physician-Scientist Award from Research to Prevent Blindness, Inc.
Financial Disclosure: The FC-1000 and FM-500 (laser flare photometers) used to generate laser flare photometry data analyzed in this study were originally provided to the author by Kowa Company, Ltd, Electronics and Optics Division, Tokyo, Japan, for research purposes. Dr Holland was an unpaid member of the Kowa Laser Flare-Cell Photometry Medical Advisory Board from 1991 to 1995. The author has no other interests in the products or techniques described in this report or in competing techniques. The author has no other conflicts of interest with any other aspects of this study. Funding entities had no role in the conduction or presentation of this study.
Other Acknowledgments: Mr Christopher S. Denove, Research Assistant, Jules Stein Eye Institute Clinical Research Center, was principally responsible for data collection. Additional assistance with data collection was provided by Emma Clay, MD, Sophie X. Deng, MD, PhD, Peter J. Kappel, MD, and Deborah C. Parish, BA, Department of Ophthalmology, David Geffen School of Medicine at UCLA. I am indebted to Fei Yu, PhD, Senior Statistician and Assistant Scientist, Department of Biostatistics, UCLA School of Public Health and Department of Ophthalmology, David Geffen School of Medicine at UCLA, who provided assistance with all statistical analyses of data. Joseph Caprioli, MD, provided assistance in the formulation of study criteria for the identification of patients with the complication of glaucoma/elevated intraocular pressure. Yen H. Tran, BA, of the Ocular Inflammatory Disease Center, Jules Stein Eye Institute, provided assistance with background research and organization of the study.
REFERENCES
- 1.Hogan MJ, Kimura SJ, Thygeson P. Signs and symptoms of uveitis. I. Anterior uveitis. Am J Ophthalmol. 1959;47:155–170. doi: 10.1016/s0002-9394(14)78239-x. [DOI] [PubMed] [Google Scholar]
- 2.Duke-Elder S, Perkins ES. The clinical signs and symptoms of uveitis. In: Duke-Elder S, editor. System of Ophthalmology. St Louis: CV Mosby; 1966. pp. 132–150. [Google Scholar]
- 3.Schlaegel TF. Essentials of Uveitis. 1. Boston: Little, Brown; 1967. p. 7. [Google Scholar]
- 4.Smith RE, Nozik RA. Uveitis. A Clinical Approach to Diagnosis and Management. 2. Balitmore: Williams & Wilkins; 1989. Signs of uveitis; pp. 16–17. [Google Scholar]
- 5.Whitcup SM. Examination of the patient with uveitis. In: Nussenblatt RB, Whitcup SM, editors. Uveitis. Fundamentals and Clinical Practice. 3. Philadelphia: Mosby; 2004. pp. 57–69. [Google Scholar]
- 6.Harper SL, Chorich LJ, Foster CS. Diagnosis of uveitis. In: Foster CS, Vitale AT, editors. Diagnosis and Treatment of Uveitis. Philadelphia: Saunders; 2002. p. 91. [Google Scholar]
- 7.Ladas JG, Wheeler NC, Morhun PJ, et al. Laser flare-cell photometry: methodology and clinical applications. Surv Ophthalmol. 2005;50:27–47. doi: 10.1016/j.survophthal.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Gonzales CA, Ladas JG, Davis JL, et al. Relationships between laser flare photometry values and complications of uveitis. Arch Ophthalmol. 2001;119:1763–1769. doi: 10.1001/archopht.119.12.1763. [DOI] [PubMed] [Google Scholar]
- 9.Davis JL, Dacanay LM, Holland GN, et al. Laser flare photometry and complications of chronic uveitis in children. Am J Ophthalmol. 2003;135:763–771. doi: 10.1016/s0002-9394(03)00315-5. [DOI] [PubMed] [Google Scholar]
- 10.Woreta F, Thorne JE, Jabs DA, et al. Risk factors for ocular complications and poor visual acuity at presentation among patients with uveitis associated with juvenile idiopathic arthritis. Am J Ophthalmol. 2007;143:647–655. doi: 10.1016/j.ajo.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thorne JE, Woreta F, Kedhar SR, et al. Juvenile idiopathic arthritis-associated uveitis: incidence of ocular complications and visual acuity loss. Am J Ophthalmol. 2007;143:840–846. doi: 10.1016/j.ajo.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 12.Holland GN, Stiehm ER. Special considerations in the evaluation and management of uveitis in children. Am J Ophthalmol. 2003;135:867–878. doi: 10.1016/s0002-9394(03)00314-3. [DOI] [PubMed] [Google Scholar]
- 13.McCannel CA, Holland GN, Helm CJ, et al. Causes of uveitis in the general practice of ophthalmology. UCLA Community-Based Uveitis Study Group. Am J Ophthalmol. 1996;121:35–46. doi: 10.1016/s0002-9394(14)70532-x. [DOI] [PubMed] [Google Scholar]
- 14.The Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–516. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotaniemi K, Kautiainen H, Karma A, Aho K. Occurrence of uveitis in recently diagnosed juvenile chronic arthritis: a prospective study. Ophthalmology. 2001;108:2071–2075. doi: 10.1016/s0161-6420(01)00773-4. [DOI] [PubMed] [Google Scholar]
- 16.Samson CM, Waheed N, Baltatzis S, Foster CS. Methotrexate therapy for chronic noninfectious uveitis: analysis of a case series of 160 patients. Ophthalmology. 2001;108:1134–1139. doi: 10.1016/s0161-6420(01)00576-0. [DOI] [PubMed] [Google Scholar]
- 17.Yu EN, Meniconi ME, Tufail F, et al. Outcomes of treatment with immunomodulatory therapy in patients with corticosteroid-resistant juvenile idiopathic arthritis-associated chronic iridocyclitis. Ocul Immunol Inflamm. 2005;13:353–360. doi: 10.1080/09273940590951061. [DOI] [PubMed] [Google Scholar]
- 18.Kanski JJ, Shun-Shin GA. Systemic uveitis syndromes in childhood: an analysis of 340 cases. Ophthalmology. 1984;91:1247–1252. doi: 10.1016/s0161-6420(84)34177-x. [DOI] [PubMed] [Google Scholar]
- 19.Wolf MD, Lichter PR, Ragsdale CG. Prognostic factors in the uveitis of juvenile rheumatoid arthritis. Ophthalmology. 1987;94:1242–1248. doi: 10.1016/s0161-6420(87)80007-6. [DOI] [PubMed] [Google Scholar]
- 20.Edelsten C, Lee V, Bentley CR, et al. An evaluation of baseline risk factors predicting severity in juvenile idiopathic arthritis associated uveitis and other chronic anterior uveitis in early childhood. Br J Ophthalmol. 2002;86:51–56. doi: 10.1136/bjo.86.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chia A, Franco, Lee V, et al. Factors related to severe uveitis at diagnosis in children with juvenile idiopathic arthritis in a screening program. Am J Ophthalmol. 2003;135:757–762. doi: 10.1016/s0002-9394(03)00225-3. [DOI] [PubMed] [Google Scholar]
- 22.Sijssens KM, Rothova A, Berendschot TT, de Boer JH. Ocular hypertension and secondary glaucoma in children with uveitis. Ophthalmology. 2006;113:853–859. doi: 10.1016/j.ophtha.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg KD, Feuer WJ, Davis JL. Ocular complications of pediatric uveitis. Ophthalmology. 2004;111:2299–2306. doi: 10.1016/j.ophtha.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Kump LI, Castaneda RA, Androudi SN, et al. Visual outcomes in children with juvenile idiopathic arthritis-associated uveitis. Ophthalmology. 2006;113:1874–1877. doi: 10.1016/j.ophtha.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 25.Ozdal PC, Vianna RN, Deschenes J. Visual outcome of juvenile rheumatoid arthritis-associated uveitis in adults. Ocul Immunol Inflamm. 2005;13:33–38. doi: 10.1080/09273940590909220. [DOI] [PubMed] [Google Scholar]
- 26.Kump LI, Cervantes-Castaneda RA, Androudi SN, Foster CS. Analysis of pediatric uveitis cases at a tertiary referral center. Ophthalmology. 2005;112:1287–1292. doi: 10.1016/j.ophtha.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 27.Herbort CP, Guex-Crosier Y, de Ancos E, Pittet N. Use of laser flare photometry to assess and monitor inflammation in uveitis. Ophthalmology. 1997;104:64–71. doi: 10.1016/s0161-6420(97)30359-5. [DOI] [PubMed] [Google Scholar]
- 28.O’Connor GR. The influence of hypersensitivity on the pathogenesis of ocular toxoplasmosis. Trans Am Ophthalmol Soc. 1970;68:501–547. [PMC free article] [PubMed] [Google Scholar]
- 29.Dana MR, Merayo-Lloves J, Schaumberg DA, Foster CS. Visual outcomes prognosticators in juvenile rheumatoid arthritis-associated uveitis. Ophthalmology. 1997;104:236–244. doi: 10.1016/s0161-6420(97)30329-7. [DOI] [PubMed] [Google Scholar]
- 30.Zulian F, Martini G, Falcini F, et al. Early predictors of severe course of uveitis in oligoarticular juvenile idiopathic arthritis. J Rheumatol. 2002;29:2446–2453. [PubMed] [Google Scholar]
- 31.Ladas JG, Yu F, Loo R, et al. Relationship between aqueous humor protein level and outflow facility in patients with uveitis. Invest Ophthalmol Vis Sci. 2001;42:2584–2588. [PubMed] [Google Scholar]
- 32.Edelsten C, Reddy MA, Stanford MR, Graham EM. Visual loss associated with pediatric uveitis in English primary and referral centers. Am J Ophthalmol. 2003;135:676–680. doi: 10.1016/s0002-9394(02)02148-7. [DOI] [PubMed] [Google Scholar]
- 33.Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol. 2000;130:492–513. doi: 10.1016/s0002-9394(00)00659-0. [DOI] [PubMed] [Google Scholar]
- 34.Jabs DA. Improving the reporting of clinical case series. Am J Ophthalmol. 2005;139:900–905. doi: 10.1016/j.ajo.2004.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]