Abstract
Purpose
To describe the relationship of retinal arteriolar and venular caliber with cardiovascular risk factors, including inflammatory biomarkers, in a multiethnic population of whites, blacks, Hispanics, and Chinese.
Methods
A cross-sectional study comprising 5979 persons aged 45 to 84 years residing in six U.S. communities. Retinal vascular caliber was measured and summarized from digital retinal photographs. Standard cardiovascular risk factors, including biomarkers of inflammation (e.g., high-sensitivity C-reactive protein [hsCRP], interleukin [IL]-6, and plasma fibrinogen) and endothelial dysfunction (e.g., soluble intercellular adhesion molecule [sICAM]-1 [, plasminogen activator inhibitor [PAI]-1) were assessed.
Results
Mean retinal arteriolar caliber was 144.1 ± 14.4 (SD) μm, and venular caliber 214.0 ± 22.2 μm. In models controlling for age, gender, race-ethnicity, and center, smaller retinal arteriolar caliber was related to higher systolic and diastolic blood pressure, hypertension status, current alcohol consumption, greater body mass index, and higher levels of total homocysteine; larger retinal arteriolar caliber was related to diabetes, current cigarette smoking, and higher levels of plasma fibrin-ogen; and larger retinal venular caliber was related to diabetes, current cigarette smoking, greater body mass index and waist-hip ratio, higher levels of serum glucose, plasma triglyceride, plasma LDL-cholesterol, hsCRP, plasma fibrinogen, IL6, sICAM-1, and PAI-1 and lower levels of HDL-cholesterol. In multivariate analyses, blacks and Hispanics had larger retinal arteriolar and venular calibers than did whites and Chinese.
Conclusions
Retinal arteriolar and venular caliber is associated with a range of cardiovascular risk factors, including hypertension, diabetes, measures of obesity, and dyslipidemia. Venular caliber is also associated with systemic inflammation.
The retinal circulation allows one to visualize the microcirculation and investigate its relationship to systemic and ocular diseases. Recent studies have shown that changes in retinal vascular caliber may predict a range of cardiovascular diseases, independent of established risk factors.1,2 Generalized narrowing of retinal arterioles, for example, has been associated with incident stroke, coronary heart disease, and hypertension, independent of other risk factors.3–8 Larger retinal venular caliber has been reported to predict progression of retinopathy and nephropathy in persons with type 1 diabetes.9,10 Association between retinal vascular caliber and ocular disease, such as glaucoma, have also been reported.11
Despite these data, the pathophysiological mechanisms underlying variations in retinal vascular caliber are unclear. Retinal arteriolar narrowing is thought to reflect structural damage from chronic hypertension.2,12–17 There is evidence that retinal venular caliber may be influenced by systemic inflammation,18–20 although not all studies have found consistent relationships,21 and most have only examined associations with nonspecific inflammatory markers (e.g., white blood cell count, erythrocyte sedimentation rate).18,19 There have been no studies that have examined possible racial and ethnic differences in retinal vascular caliber.
The purpose of this present study is to examine the relationship of retinal vascular caliber to a range of cardiovascular risk factors, including specific markers of inflammation (e.g., high-sensitivity C-reactive protein, interleukin-6) and endothelial dysfunction (e.g., soluble intercellular adhesion molecule-1, plasminogen activator inhibitor-1), in a multiethnic population of whites, blacks, Hispanics, and Chinese in the United States.
Methods
Study Population
The Multi-Ethnic Study of Atherosclerosis (MESA) is a prospective cohort study of men and women aged 45 to 84 years without history of clinical cardiovascular disease (CVD) living in six U.S. communities.22 The study objective of MESA was to identify risk factors for subclinical CVD, for progression of subclinical CVD, and for transition from subclinical to clinical CVD. Selection of the study population has been reported in detail elsewhere.22 In brief, each site planned to examine approximately 1100 eligible participants, equally divided between men and women, according to site-specified racial and ethnic proportions. At the first examination, there were 6814 participants: 1086 from Baltimore; 1164 from Chicago; 1077 from Forsyth County, North Carolina; 1319 from Los Angeles County, California; 1102 from New York City; and 1066 from St. Paul, Minnesota. The tenets of the Declaration of Helsinki were observed, and institutional review board approval was granted at each study site. Written informed consent was obtained from each participant.
Fundus photography was performed at the second examination (August 2002 to January 2004), which occurred immediately after the baseline examination (July 2000 to July 2002).23,24 At the second examination, 6237 participants returned, 6147 had retinal photography, and 5979 had photographs that were suitable for measurement of retinal vascular caliber.
Retinal Photography and Measurement of Retinal Vascular Caliber
Fundus photography was performed at each site, according to a standardized protocol.23,24 Participants were seated in a darkened room. Both eyes of each participant were photographed with a 45° 6.3 megapixel digital nonmydriatic camera. Two photographic fields were taken of each eye: the first centered on the optic disc (ETDRS [Early Treatment Diabetic Retinopathy Study] field 1) and the second centered on the fovea (ETDRS field 2).25 Images were sent from the six field centers to the University of Wisconsin, Madison, for measurement of retinal vascular caliber and assessment of other retinal disease.
Retinal vascular caliber was measured with a computer-based program (IVAN, University of Wisconsin, Madison), based on a detailed protocol.26,27 Trained graders who were masked to participant characteristics performed these measurements. For this study, field 1 photographs in the right eye were selected for measurement. If retinal vascular caliber could not be measured in the right eye, the left was chosen for measurement. For each photograph, all arterioles and venules coursing through an area 0.5 to 1 disc diameter from the optic disc margin were measured and summarized as the average central arteriolar and venular equivalents, using formulas developed by Hubbard et al.26 and later modified by Knudtson.28 These equivalents are projected calibers for the central retinal vessels, measured away from the optic disc. We also calculated the arteriole-to-venule ratio (AVR), which gives the relative caliber of arterioles and venules, introducing some adjustment for variable magnification from differences in refractive errors.26 An AVR of 0.70 suggested that arteriolar caliber is, on average, 70% as large as the as venular caliber.
Reproducibility of retinal vascular measurements has been reported, with intra- and intergrader intraclass correlation coefficients ranging from 0.78 to 0.99.26,27
Assessment of Cardiovascular Risk Factors
Participants underwent an interview and assessment of cardiovascular risk factors during the course of the study.22,29 Variables for this analysis were based on data collected at the first examination. Resting blood pressure was measured three times with participants in the seated position (Dinamap model Pro 100 automated oscillometric sphygmomanometer; Critikon, GE Healthcare, Piscataway, NJ). The average of the last two measurements was used in analysis. Hypertension was defined as systolic blood pressure ≥ 140 mm Hg, diastolic blood pressure ≥90 mm Hg, or current use of antihypertensive medications. Diabetes mellitus was defined as fasting glucose ≥7.0 mmol/L (126 mg/dL) or use of insulin or oral hypoglycemic medication. Height and weight were measured with participants wearing light clothing and no shoes, and the body mass index (BMI) was calculated as kilograms per square meter. The waist-hip ratio was defined as the ratio of the waist and hip circumferences.
A detailed questionnaire was used to obtain information about past medical history (e.g., hypertension, diabetes, arthritis), cigarette smoking, and alcohol consumption (defined as current, and past/never) and medication use (e.g., antihypertensive and antidiabetic medications, oral steroids, aspirin and nonsteroidal anti-inflammatory [NSAID] agents).
Assessment of Blood Factors
Fasting (>8 hours) blood samples were drawn from participants, and aliquots were prepared for central analysis and for storage (approximately 65 aliquots per participant at the first examination) at the University of Vermont and the University of Minnesota.22 Standardized protocols were designed to allow several domains of study to be addressed, including measurements of lipids and lipoproteins, systemic inflammation, and endothelial cell function.29 Details of these methods, including coefficients of variations, are provided elsewhere.29
In the total cohort, the following were analyzed in this study: plasma total and HDL cholesterol, plasma triglycerides, serum glucose, high sensitivity C-reactive protein (hsCRP), plasma fibrinogen, IL-6, factor VIII, total homocysteine, and IgG antibody to Chlamydia pneumoniae. LDL cholesterol was calculated with the Friedewald equation.
In a subsample of participants (n = 2431), soluble intercellular adhesion molecule-1 (sICAM-1) was assayed. In a smaller subsample (n = 924), the following factors were also assayed: plasminogen activator inhibitor (PAI)-1, soluble E-selectin, von Willebrand factor (vWF), and tumor necrosis factor (TNF)-α.
Statistical Analysis
The distributions (mean ± SD) for retinal vascular caliber (central arteriolar and venular equivalents) were continuous and relatively normally distributed in the population. Therefore, we used analyses of covariance (ANCOVA) and linear regression models to determine the association of various risk factors with retinal vascular caliber.
We initially used ANCOVA to estimate mean retinal caliber in association with presence versus absence of categorical variables (e.g., diabetes) or quartiles of continuous variables (e.g., glucose levels). All models were adjusted for age, gender, race-ethnicity, and examination center. Test of trend was determined by treating categorical risk factors (e.g., quartiles of glucose) as continuous variables and the χ2 test statistic for the parameter estimate was computed.
Significant predictors (P < 0.05) in the initial models were eligible for inclusion in multivariable linear regression models examining the difference in retinal vascular caliber (regression coefficient β) in the presence versus absence of a risk factor or per-unit change in risk factor. Variables included in the final models were chosen by using a forward stepwise approach, forcing in age, gender, race-ethnicity, and center. To compare associations between arteriolar and venular calibers directly, we used the same final multivariable models for both. In these models, we also report the standardized regression coefficient (standardized β). This coefficient provides an estimate of the change in outcome (i.e., arteriolar caliber), with a 1-SD change in the risk factor level, and is useful for comparing relative strength of associations when risk factors are measured in different units.30 We performed these analyses for the total population initially, and for the four racial-ethnic groups separately. In general, because the risk factor relationships did not differ significantly between the four racial-ethnic groups, only the models for the full cohort are presented.
Finally, we examined potential explanatory factors for the racial-ethnic variations in the distribution of retinal vascular caliber. We calculated the mean difference in caliber in a general linear model (GLM), comparing blacks, Hispanics, and Chinese with whites (reference group), while adjusting for risk factors that may account for the racial and ethnic differences. All analyses were performed on computer (SPSS, ver. 12.0.1; SPSS Inc., Chicago, IL).
Results
Selected characteristics and risk factors for the full cohort and for each of the four racial-ethnic groups among participants who had retinal photographs (n = 6147) are shown in Table 1. There were significant differences in the frequency and distribution of various risk factors among the racial-ethnic groups. For example, Hispanics and Chinese were more likely to have less than 8 years of education, blacks were more likely to have hypertension and to be current cigarette smokers, and blacks and Hispanics were more likely to have diabetes and higher systolic blood pressure, serum glucose levels, and body mass index than were whites. Blacks and Hispanics had higher levels of hsCRP, plasma fibrinogen, and IL-6 than did whites and Chinese. Chinese had the highest levels of PAI-1 compared with other racial-ethnic groups.
Table 1.
Characteristics of the MESA Population
| All Persons (n = 6147)
|
White (n = 2431)
|
Black (n = 1666)
|
Hispanic (n = 1326)
|
Chinese (n = 724)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | P* | |
| Gender, male | 2931 | 47.7 | 1183 | 48.7 | 751 | 45.1 | 639 | 48.2 | 358 | 49.4 | 0.97 |
| Education, less than 8 years | 612 | 10.0 | 32 | 1.3 | 61 | 3.7 | 416 | 31.4 | 103 | 14.2 | <0.001 |
| Hypertension | 2675 | 43.5 | 906 | 37.3 | 964 | 57.9 | 544 | 41.0 | 261 | 36.0 | 0.07 |
| Diabetes | 821 | 13.4 | 164 | 6.7 | 305 | 18.3 | 247 | 18.6 | 105 | 14.5 | 0.09 |
| Cigarette smoker, current | 773 | 12.6 | 262 | 10.8 | 294 | 17.8 | 175 | 13.2 | 42 | 5.8 | 0.10 |
| Alcohol consumption, current | 3457 | 56.2 | 1753 | 79.0 | 829 | 58.7 | 645 | 64.2 | 230 | 68.0 | 0.35 |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Age (y) | 61.9 | 10.1 | 62.4 | 10.2 | 61.8 | 9.9 | 61.0 | 10.2 | 61.8 | 10.2 | 0.001 |
| Systolic blood pressure (mm Hg) | 126.1 | 21.1 | 123.0 | 20.0 | 131.4 | 21.3 | 126.4 | 21.5 | 123.7 | 21.2 | <0.001 |
| Serum glucose (mg/dL) | 103.9 | 29.6 | 98.0 | 21.2 | 106.9 | 32.4 | 110.0 | 37.0 | 105.7 | 28.5 | <0.001 |
| Body mass index (kg/m2) | 28.3 | 5.4 | 27.7 | 5.0 | 30.1 | 5.7 | 29.4 | 5.1 | 24.1 | 3.3 | <0.001 |
| Total cholesterol (mg/dL) | 194.2 | 35.6 | 195.7 | 35.2 | 189.6 | 35.9 | 198.2 | 37.3 | 192.3 | 31.3 | <0.001 |
| Triglycerides (mg/dL) | 131.2 | 87.7 | 132.0 | 88.6 | 103.6 | 61.1 | 157.4 | 103.8 | 143.7 | 86.1 | <0.001 |
| hsCRP (mg/dL)† | 1.88 | 3.38 | 1.68 | 3.36 | 2.48 | 4.48 | 2.40 | 3.71 | 0.87 | 1.31 | <0.001 |
| Plasma fibrinogen (mg/dL) | 345 | 73 | 333 | 69 | 360 | 79 | 357 | 72 | 328 | 60 | <0.001 |
| IL6 (pg/mL)† | 1.19 | 1.09 | 1.10 | 1.02 | 1.31 | 1.16 | 1.34 | 1.15 | 0.85 | 0.70 | <0.001 |
| sICAM-1 (ng/mL)‡ | 273.2 | 77.8 | 284.0 | 66.3 | 253.1 | 95.5 | 290.6 | 82.3 | 228.2 | 57.3 | <0.001 |
| PAI-1 (ng/mL)†‡ | 19.5 | 27.0 | 19.0 | 26.0 | 17.0 | 25.5 | 21.0 | 30.0 | 24.0 | 26.5 | 0.009 |
P based on χ2 (categorical), and ANOVA or Kruskal-Wallis test (continuous), comparing racial-ethnic differences for individual variables.
Results are shown as median and interquartile range.
n for PAI-1 = 924 (white = 432, black = 191, Hispanics = 210, Chinese = 91) and n for sICAM-1 = 2431 (white = 1177, black = 438, Hispanics = 524, Chinese = 292).
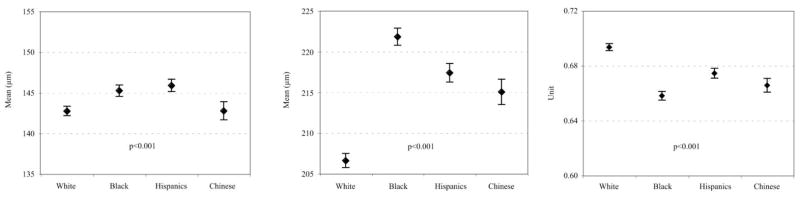
In the total cohort, the mean central retinal arteriolar caliber was 144.1 ±14.4 μm (SD) and venular caliber 214.0 ± 22.2 μm. Figure 1 shows the distribution of retinal arteriolar and venular caliber in the four racial-ethnic groups. Overall, there were significant differences in arteriolar and venular caliber and the AVR among the four racial-ethnic groups. Retinal arteriolar caliber was larger in blacks (145.2 ±14.2 μm) and Hispanics (145.9 ± 13.8 μm) than in whites (142.7 ± 14.3 μm) and Chinese (142.8 ± 15.0, P < 0.001 for overall comparison using ANOVA). Venular caliber was largest in blacks (221.8 ± 21.9 μm), intermediate in Hispanics (217.4 ± 20.9 μm) and Chinese (215.0 ± 20.8 μm), and smallest in whites (206.6 ± 21.2 μm, P < 0.001). The AVR was largest in whites, reflecting smaller venular caliber in this group.
Figure 1.
Distribution of retinal arteriolar diameter (right), venular diameter (middle), and AVR (left), by racial-ethnic group. Data are mean micrometers and 95% CI.
The associations of central retinal vascular caliber with systolic blood pressure for each of the four racial-ethnic groups are shown in Figure 2. Retinal arteriolar caliber correlated strongly and inversely with systolic blood pressure in all four racial-ethnic groups (r = −0.21 to −0.28). To a lesser extent, venular caliber also correlated inversely with systolic blood pressure (r = −0.06 to −0.13). These relationships were essentially similar among the four racial-ethnic groups (e.g., P = 0.99 comparing the correlation coefficients of arteriolar caliber and systolic blood pressure for whites versus blacks, P = 0.79 for whites versus Hispanics, and P = 0.44 for whites versus Chinese). The pattern of associations was similar for diastolic blood pressure (data not shown).
Figure 2.
Relationship of retinal arteriolar caliber (top) and venular caliber (bottom) with systolic blood pressure. Data are mean (micrometers) and 95% CI.
Table 2 shows individual risk factor associations for retinal vascular caliber, adjusted for age, gender, race-ethnicity, and center. Smaller retinal arteriolar caliber was related to older age, male gender, white and Chinese race-ethnicity, current alcohol consumption, greater BMI, and higher levels of total homocysteine. Larger retinal arteriolar caliber was related to diabetes, current cigarette smoking, and higher levels of plasma fibrinogen. Arteriolar caliber was not significantly associated with lipid, hsCRP, IL6, sICAM, and PAI-1 levels. Smaller retinal venular caliber was related to older age and white race-ethnicity. Larger retinal venular caliber was related to diabetes, current cigarette smoking, greater BMI, plasma triglyceride, hsCRP, plasma fibrinogen, IL6, sICAM-1, and PAI-1 and lower levels of HDL-cholesterol. Venular caliber was not significantly associated with gender or homocysteine. In addition, smaller arteriolar caliber was related to hypertension whereas larger venular caliber was associated with greater waist-hip ratio and higher levels of LDL-cholesterol (data not shown).
Table 2.
Relationship of Selected Cardiovascular Risk Factors with Retinal Arteriolar and Venular Caliber
| Retinal Arteriolar Caliber
|
Retinal Venular Caliber
|
|||||
|---|---|---|---|---|---|---|
| n | Mean (SE), μm* | P | n | Mean (SE), μm* | P | |
| Age (yr) | ||||||
| 45–54 | 1798 | 147.1 (0.32) | <0.001 | 1800 | 217.3 (0.49) | <0.001 |
| 55–64 | 1710 | 144.2 (0.34) | 1707 | 215.1 (0.53) | ||
| 65–74 | 1745 | 142.6 (0.35) | 1747 | 212.3 (0.54) | ||
| 75–84 | 726 | 139.8 (0.55) | 725 | 207.6 (0.90) | ||
| Gender | ||||||
| Men | 2863 | 142.5 (0.27) | <0.001 | 2858 | 214.4 (0.41) | 0.24 |
| Women | 3116 | 145.5 (0.25) | 3121 | 213.7 (0.40) | ||
| Race-ethnicity | ||||||
| White | 2373 | 142.7 (0.30) | <0.001 | 2368 | 206.6 (0.44) | <0.001 |
| Black | 1613 | 145.2 (0.35) | 1617 | 221.8 (0.54) | ||
| Hispanic | 1292 | 145.9 (0.38) | 1291 | 217.4 (0.58) | ||
| Chinese | 701 | 142.8 (0.57) | 703 | 215.0 (0.79) | ||
| Diabetes | ||||||
| Absent | 5191 | 143.9 (0.22) | <0.001 | 5192 | 214.5 (0.34) | <0.001 |
| Present | 788 | 145.9 (0.51) | 787 | 218.1 (0.77) | ||
| Cigarette smoking | ||||||
| Past/Never | 5189 | 143.7 (0.22) | <0.001 | 5187 | 213.8 (0.33) | <0.001 |
| Current | 760 | 147.3 (0.53) | 762 | 223.6 (0.78) | ||
| Alcohol consumption | ||||||
| Past/Never | 2553 | 145.1 (0.29) | <0.001 | 2553 | 215.6 (0.44) | 0.07 |
| Current | 3385 | 143.3 (0.29) | 3385 | 214.5 (0.43) | ||
| Body mass index (kg/m2) | ||||||
| 1st Quartile, <24.5 | 1457 | 145.5 (0.39) | <0.001 | 1458 | 213.2 (0.58) | <0.001 |
| 2nd Quartile, 24.5–27.5 | 1484 | 144.2 (0.38) | 1487 | 214.0 (0.57) | ||
| 3rd Quartile, 27.5–31.0 | 1497 | 143.6 (0.38) | 1494 | 215.9 (0.58) | ||
| 4th Quartile, ≥31.0 | 1541 | 143.3 (0.39) | 1540 | 217.3 (0.59) | ||
| Triglycerides (mg/dL) | ||||||
| 1st Quartile, <78 | 1532 | 144.2 (0.39) | 0.52 | 1526 | 212.2 (0.58) | <0.001 |
| 2nd Quartile, 78–111 | 1456 | 144.2 (0.38) | 1457 | 214.2 (0.57) | ||
| 3rd Quartile, 111–161 | 1507 | 143.8 (0.38) | 1507 | 215.5 (0.56) | ||
| 4th Quartile, ≥161 | 1465 | 144.6 (0.39) | 1470 | 217.9 (0.57) | ||
| HDL cholesterol (mg/dL) | ||||||
| 1st Quartile, <40 | 1519 | 144.4 (0.39) | 0.44 | 1523 | 217.6 (0.58) | <0.001 |
| 2nd Quartile, 40–48 | 1496 | 144.5 (0.38) | 1496 | 215.6 (0.56) | ||
| 3rd Quartile, 48–59 | 1528 | 144.1 (0.38) | 1522 | 214.7 (0.56) | ||
| 4th Quartile, ≥59 | 1414 | 143.7 (0.42) | 1416 | 211.5 (0.62) | ||
| Total homocysteine (μmol/L) | ||||||
| 1st Quartile, <7.2 | 1586 | 145.1 (0.38) | 0.03 | 1585 | 215.1 (0.57) | 0.69 |
| 2nd Quartile, 7.2–8.5 | 1483 | 144.1 (0.38) | 1487 | 214.4 (0.57) | ||
| 3rd Quartile, 8.5–10.5 | 1421 | 143.6 (0.39) | 1417 | 215.3 (0.59) | ||
| 4th Quartile, ≥10.5 | 1473 | 143.8 (0.39) | 1474 | 215.3 (0.59) | ||
| hsCRP (mg/L) | ||||||
| 1st Quartile, <0.81 | 1467 | 144.7 (0.38) | 0.38 | 1462 | 212.3 (0.57) | <0.001 |
| 2nd Quartile, 0.81–1.86 | 1502 | 143.8 (0.38) | 1502 | 214.3 (0.56) | ||
| 3rd Quartile, 1.86–4.17 | 1477 | 144.1 (0.38) | 1479 | 216.5 (0.57) | ||
| 4th Quartile, ≥4.20 | 1494 | 144.3 (0.40) | 1495 | 217.5 (0.59) | ||
| Plasma fibrinogen (mg/dL) | ||||||
| 1st Quartile, <294 | 1524 | 143.3 (0.38) | 0.02 | 1523 | 212.0 (0.57) | <0.001 |
| 2nd Quartile, 294–337 | 1488 | 144.2 (0.38) | 1491 | 213.8 (0.56) | ||
| 3rd Quartile, 337–387 | 1466 | 144.9 (0.38) | 1467 | 216.4 (0.57) | ||
| 4th Quartile, ≥387 | 1462 | 144.5 (0.39) | 1459 | 218.1 (0.59) | ||
| IL6 (pg/mL) | ||||||
| 1st Quartile, <0.76 | 1483 | 144.6 (0.39) | 0.34 | 1483 | 212.1 (0.57) | <0.001 |
| 2nd Quartile, 0.76–1.17 | 1455 | 143.7 (0.38) | 1454 | 214.4 (0.57) | ||
| 3rd Quartile, 1.17–1.86 | 1437 | 144.2 (0.39) | 1439 | 216.1 (0.58) | ||
| 4th Quartile, ≥1.86 | 1447 | 144.1 (0.39) | 1444 | 217.8 (0.58) | ||
| sICAM-1 (ng/mL) | ||||||
| 1st Quartile, <8.9 | 595 | 143.8 (0.60) | 0.43 | 596 | 214.2 (0.89) | 0.01 |
| 2nd Quartile, 8.9–19.5 | 593 | 145.0 (0.62) | 591 | 216.2 (0.93) | ||
| 3rd Quartile, 19.5–36.3 | 590 | 144.5 (0.64) | 591 | 216.2 (0.96) | ||
| 4th Quartile, ≥36.31 | 586 | 144.9 (0.63) | 586 | 218.3 (0.94) | ||
| PAI-1 (ng/mL) | ||||||
| 1st Quartile, <8.9 | 222 | 145.6 (1.01) | 0.44 | 225 | 213.6 (1.53) | 0.05 |
| 2nd Quartile, 8.9–19.5 | 215 | 145.6 (1.01) | 216 | 216.3 (1.53) | ||
| 3rd Quartile, 19.5–36.3 | 224 | 143.8 (0.97) | 224 | 216.5 (1.47) | ||
| 4th Quartile, ≥36.3 | 219 | 144.5 (0.99) | 218 | 219.2 (1.51) | ||
Mean (standard error) for arteriolar and venular caliber, adjusted for age, gender, race/ethnicity, and study center.
In multivariable linear regression controlling for similar risk factors (Table 3), retinal arteriolar caliber was independently related to age, gender, race-ethnicity, systolic blood pressure, serum glucose, cigarette smoking, and alcohol consumption. Among these, the strongest predictors of arteriolar caliber were systolic blood pressure, age, gender, and cigarette smoking (with standardized β of 0.10 and higher). Retinal venular caliber was independently related to age, race-ethnicity, serum glucose, cigarette smoking, alcohol consumption, body mass index, HDL and LDL cholesterol, , and hsCRP. The strongest predictors for retinal venular caliber were cigarette smoking, race-ethnicity, age, and body mass index (with standardized β of 0.10 and higher).
Table 3.
Independent Predictors of Retinal Arteriolar and Venular Caliber
| Retinal Arteriolar Caliber
|
Retinal Venular Caliber
|
||||||
|---|---|---|---|---|---|---|---|
| Risk Factors | Unit Change | β | Sβ | P | β | Sβ | P |
| Age | Per SD (10 ys) | −1.46 | −0.10 | <0.001 | −2.52 | −0.11 | <0.001 |
| Gender | Male vs. female | −3.08 | −0.11 | <0.001 | 0.17 | 0.004 | 0.78 |
| Race/ethnicity | Black vs. white | 2.65 | 0.09 | <0.001 | 13.80 | 0.30 | <0.001 |
| Hispanic vs. white | 1.13 | 0.08 | <0.001 | 4.13 | 0.18 | <0.001 | |
| Chinese vs. white | −0.58 | −0.05 | 0.03 | 2.75 | 0.16 | <0.001 | |
| Systolic blood pressure | Per SD (21 mm Hg) | −2.68 | −0.19 | <0.001 | −0.39 | −0.02 | 0.20 |
| Serum glucose | Per SD (30 mg/dL) | 0.89 | 0.06 | <0.001 | 1.73 | 0.07 | <0.001 |
| Cigarette smoking | Current vs. never/past | 4.31 | 0.10 | <0.001 | 11.36 | 0.17 | <0.001 |
| Alcohol consumption | Current vs. never/past | −2.31 | −0.08 | <0.001 | −2.14 | −0.05 | <0.001 |
| Body mass index | Per SD (5.4 kg/m2) | 0.21 | 0.01 | 0.31 | 2.21 | 0.10 | <0.001 |
| HDL Cholesterol | Per SD (15 mg/dL) | −0.17 | −0.01 | 0.42 | −1.13 | 0.03 | 0.001 |
| LDL Cholesterol | Per SD (31 mg/dL) | 0.09 | 0.01 | 0.60 | 0.63 | −0.05 | 0.02 |
| HsCRP | Per SD (5.4 mg/dL) | 0.09 | 0.01 | 0.64 | 0.93 | 0.04 | 0.002 |
β, regression coefficient, Sβ, standardized regression coefficient.
R2 for arteriolar caliber = 0.10; for venular caliber = 0.11.
In separate multivariable models without hsCRP, retinal venular caliber was independently related to plasma fibrinogen (per SD [73 mg/dL], β, 1.13; P < 0.001) and IL6 (per SD [1.19 pg/mL], β, 1.00; P < 0.001). In the subset of participants with PAI-1 and sICAM-1 measurements, neither factor was significantly associated with arteriolar or venular caliber after multivariable adjustment for other factors (data not shown).
Other risk factors examined in analyses that adjusted for age, gender, race-ethnicity, and center that were not significantly associated with either retinal arteriolar or venular caliber included a medical history of arthritis, use of oral steroids, aspirin, or NSAIDs, plasma total cholesterol, factor VIII, IgG antibodies to Chlamydia pneumoniae, soluble E-selectin, vWF, and TNF-α (data not shown).
Finally, we examined the difference in retinal vascular caliber among racial-ethnic groups (Table 4) in models that adjusted for potential explanatory risk factors. After adjustment for age, gender, and center, blacks, and Hispanics had significantly larger arteriolar caliber, and blacks, Hispanics, and Chinese had significantly larger venular caliber, than did whites. Adjustment for a range of cardiovascular risk factors and exclusion of participants with diabetes or hypertension did not alter the racial-ethnic difference in arteriolar and venular caliber between blacks and whites or the difference in venular caliber between Chinese and whites. However, the difference in arteriolar caliber between Hispanics and whites was reduced (mean difference of 3.51 μm between Hispanics and whites in age-gender-center–adjusted models decreased to 2.51 μm in multivariable models and 1.96 μm after exclusion of people with diabetes and hypertension).
Table 4.
Racial/Ethnic Differences in Retinal Arteriolar and Venular Caliber
| Retinal Arteriolar Caliber (μm)
|
Retinal Venular Caliber (μm)
|
|||
|---|---|---|---|---|
| Race-Ethnicity | Mean Difference (95% CI)* | P | Mean Difference (95% CI)* | P |
| Adjusted for age, gender, and center | ||||
| White | 0 | 0 | ||
| Black | 2.56 (1.62, 3.49) | <0.001 | 15.07 (13.67, 16.47) | <0.001 |
| Hispanic | 3.51 (2.46, 4.56) | <0.001 | 10.31 (8.73, 11.89) | <0.001 |
| Chinese | 0.51 (−0.82, 1.84) | 0.45 | 7.49 (5.51, 9.48) | <0.001 |
| Adjusted for age, gender, center, and cardiovascular risk factors† | ||||
| White | 0 | 0 | ||
| Black | 2.58 (1.30, 3.87) | <0.001 | 13.21 (11.10, 15.32) | <0.001 |
| Hispanic | 2.51 (1.15, 3.87) | <0.001 | 7.18 (4.95, 9.42) | <0.001 |
| Chinese | −0.55 (−2.17, 1.07) | 0.51 | 7.40 (4.80, 10.00) | <0.001 |
| Adjusted for age, gender, center, and cardiovascular risk factors in persons without diabetes or hypertension‡ | ||||
| White | 0 | 0 | ||
| Black | 2.45 (0.81, 4.09) | 0.004 | 12.68 (10.21, 15.15) | <0.001 |
| Hispanic | 1.96 (0.31, 3.62) | 0.02 | 7.52 (5.00, 10.04) | <0.001 |
| Chinese | −0.24 (−2.25, 1.77) | 0.81 | 6.82 (3.76, 9.87) | <0.001 |
Mean difference (95% CI) of arteriolar and venular caliber, comparing blacks, Hispanics, or Chinese with whites, adjusted for age, gender and center.
For arteriolar caliber, additional adjustment for hypertension, diabetes, smoking, alcohol consumption, systolic blood pressure, body mass index, plasma fibrinogen, total homocysteine, and education; for venular caliber, additional adjustment for systolic blood pressure, diabetes, smoking, body mass index, waist-hip ratio, glucose, triglycerides, LDL and HDL cholesterol, hsCRP, plasma fibrinogen, IL6, and education.
Adjustment for the same variables in persons without diabetes or hypertension.
Discussion
The MESA provided an opportunity to examine the relationship of retinal vascular caliber to a range of cardiovascular risk factors, including biomarkers of inflammation, endothelial dysfunction, and pathogen burden, in a large, multiethnic population free of clinical cardiovascular disease at the baseline examination. After controlling for age, gender, race-ethnicity, and center, we found that smaller retinal arteriolar caliber was related to hypertension, systolic and diastolic blood pressure, and homocysteine, whereas larger retinal arteriolar caliber was associated with diabetes, current cigarette smoking, and higher levels of plasma fibrinogen. Larger retinal venular caliber was associated with diabetes, current cigarette smoking, obesity (greater body mass index and waist-hip ratio), dyslipidemia (higher plasma triglyceride and LDL cholesterol and lower HDL cholesterol), and systemic markers of inflammation (hsCRP, plasma fibrinogen, and IL6), and endothelial dysfunction (sICAM-1 and PAI-1). These findings may provide further insights into the different systemic processes associated with retinal vascular caliber, which has now been shown to predict incident cardiovascular outcomes independently.
Inflammation is recognized as a key pathogenic process in cardiovascular disease.31,32 Thus, an important finding in the present study is the association of larger venular caliber with hsCRP, plasma fibrinogen, and IL6, independent of age, cigarette smoking, lipids, and other factors. This is consistent with recent observations of an association between systemic inflammation and retinal vascular caliber in three other cohorts.18–20 The Atherosclerosis Risk In Communities (ARIC) study reported that after adjustment for hypertension and cigarette smoking, nonspecific inflammatory factors (white blood cell count, plasma fibrinogen, and reduced albumin) were associated with smaller AVR.18 This relationship was initially thought to reflect arteriolar narrowing, but was subsequently confirmed in a reanalyses to be related to venular dilatation (Wong TY, unpublished data, 2005). The Rotterdam study also reported associations of nonspecific markers of inflammation (white blood cell count, erythrocyte sedimentation rate) with larger venular caliber,19 although no association with similar nonspecific inflammatory markers was seen in the Cardiovascular Health Study (CHS).21 The Beaver Dam Eye Study recently examined associations of specific inflammatory biomarkers with retinal vascular caliber and found similar associations of hsCRP, and IL6 with larger venular caliber independent of smoking and hypertension.20 Both the MESA and Beaver Dam Eye Study, however, found no associations of either arteriolar or venular caliber with TNF-α, another specific inflammatory marker, or to pathogen burden (IgG antibodies Chlamydia pneumoniae).
Several small clinical studies have suggested that endothelial dysfunction may also influence retinal vascular caliber.33,34 One study in 20 patients with type 2 diabetes showed that endothelium-dependent vasodilation of the retinal arteries was impaired even before clinical manifestations of microvascular disease in the kidneys.33 Another demonstrated significant differences in endothelial function in the retinal circulation in 19 subjects with hypertension, compared with that in normontensive control subjects.34 We found, however, only weak associations of larger venular caliber with some systemic markers of endothelial dysfunction (e.g., PAI-1 and sICAM-1, significant only in age-gender-race-ethnicity–adjusted models) but not others (e.g., vWF, soluble E-selectin). In the Beaver Dam Eye Study, retinal vascular caliber was not associated with either sICAM-1 or soluble E-selectin.20
The strong association between retinal arteriolar narrowing and hypertension is consistent with clinical observations and epidemiologic studies.1,2,12–17,35,36 The ARIC study first demonstrated that higher blood pressure was associated with narrower arteriolar caliber, but had little effect on venular caliber.12 This pattern has now been confirmed in five other populations,13–16,19 including the current MESA. Despite the consistency of this association in different studies, the clinical utility of measuring retinal vascular caliber to detect subtle degrees of arteriolar narrowing in an individual is of limited value at this time. In the MESA, arteriolar caliber varied by less than 10 μm (or 7%) between persons with the lowest sixth of the systolic blood pressure distribution (<106 mm Hg) compared with those in the top sixth of the systolic blood pressure distribution (>146 mm Hg, an average of 40 mm Hg difference; Fig. 2).
The cross-sectional associations of larger retinal arteriolar and venular caliber with higher serum glucose are not consistent with prospective data from the ARIC study and the Beaver Dam Eye study, which showed that smaller arteriolar caliber was associated with subsequent incidence of type 2 diabetes, independent of blood pressure.37,38 The finding of larger vascular caliber with diabetes, however, is supported by previous clinical studies on the effects of diabetes on retinal blood flow and vascular diameters.39,40 These studies postulate that in the diabetic retina, hyperglycemia and hypoxia initiate retinal vasodilation, leading to hyperperfusion. Hyperperfusion in turn interferes with autoregulation, resulting in further vasodilation. Further, data from the Wisconsin Epidemiologic Study of Diabetic Retinopathy showed that in type 1 diabetes, larger retinal arteriolar and venular caliber predicted the progression of diabetic retinopathy and incidence of proliferative retinopathy,10 and larger venular caliber predicted the incidence of gross proteinuria and nephropathy.11
Larger venular caliber, but not arteriolar caliber, was related to measures of obesity (greater body mass index and waist-hip ratio) and dyslipidemia (higher levels of plasma triglyceride and LDL-cholesterol and lower levels of HDL cholesterol). This pattern was also observed in the ARIC study,41 and may reflect a proinflammatory state associated with obesity.42,43
Most previous studies on retinal vascular caliber have been conducted in whites.1,2 However, the prevalence of cardiovascular risk factors is known to vary by race-ethnicity.44–48 There are less data regarding possible racial-ethnic variations in the frequency of microvascular disease, although there have been studies that demonstrate, for example, that microalbuminuria and skin microvascular dysfunction may be more pronounced in blacks than in whites.49–51 We now report racial-ethnic differences in mean retinal vascular caliber that were not fully explained by racial-ethnic differences in conventional cardiovascular risk factors or inflammation. In comparison with whites, after adjustment for other factors, blacks and Hispanics had larger mean arteriolar caliber and blacks, Hispanics, and Chinese had larger mean venular calibers. The significance of these findings is unclear, and there are few data for direct comparison of these results, and none in Hispanics and Chinese. In both the ARIC study and the CHS, blacks were reported to have a lower AVR than whites, which was suggested to reflect more severe degrees of arteriolar narrowing associated with chronic hypertension in blacks.18,21 Neither study, however, reported arteriolar and venular calibers separately at that time. In a reanalysis of the ARIC and CHS data, blacks had significantly larger arteriolar (P < 0.001 in ARIC and P = 0.002 in CHS, controlling for age, gender, and mean arterial blood pressure) and venular (P < 0.001 in both ARIC and CHS) calibers than did whites (Wong TY, unpublished data, 2005). Thus, the smaller AVRs in blacks are explained by larger venular caliber rather than smaller arteriolar caliber. There are several possible reasons for this observation. First, in the MESA, as in other studies, there were significant differences in the distribution of cardiovascular risk factors between the racial-ethnic groups: Blacks and Hispanics, for example, were more likely to have diabetes, obesity, hyperlipidemia, and systemic inflammation than were whites. These factors, which are associated with larger venular caliber, explained some of the differences in arteriolar caliber between Hispanics and whites, although not differences in arteriolar or venular caliber between blacks and whites (see Table 4). Second, the MESA data have shown that blacks and Hispanics were less likely to have coronary calcification than were whites,52 reflecting possible racial-ethnic differences in the susceptibility and manifestations of the coronary circulation to cardiovascular risk factors. It is possible that the racial-ethnic differences in retinal vascular caliber partly reflect variations in susceptibility of the retinal vasculature to cardiovascular risk factors or other processes not examined in this study, including genetic factors.53,54
These data may have clinical applications. First, our findings provide further evidence that an assessment of retinal vascular caliber may provide information regarding systemic cardiovascular risk.1 A quantitative measurement of retinal arteriolar caliber, for example, may allow physicians to monitor the effects of systemic blood pressure.2 Advances in automatic computer-based systems will facilitate progress in this area,55 but additional clinical studies are clearly needed.36 Second, our findings support the value of targeting the microcirculation in treatment of both systemic and ocular diseases. Retinal arteriolar narrowing has been shown to predict stroke and other cardiovascular diseases,3–8,56 and has been linked to glaucoma.11 Certain pharmacological agents (e.g., angiotensin-converting enzyme [ACE] inhibitors) have been suggested to have direct beneficial effects on microvessel structure and function57 and may therefore have added therapeutic value in the management of patients with retinal arteriolar narrowing.
The strengths of this study include the high proportion of gradable digital fundus photographs and the use of a computer-based technique to measure retinal vascular caliber. However, several limitations of this study should be noted. First, the nature of the cross-sectional analysis limits our ability to judge the temporal sequence of the associations reported. Second, although similar recruitment methods were used in all ethnic groups, the participation rate varied among those screened for the study: 70% for whites, 61% for blacks, 59% for Hispanics, and 48% for Chinese. Exclusion of persons with symptomatic cardiovascular disease could also have operated differentially across ethnic groups owing to differences in access to care and diagnosis of disease. Once they were enrolled in the study, however, there were no significant ethnic differences in participation between visit one, when the systemic variables were collected, and visit two, when retinal vascular caliber was measured. Finally, the MESA did not have data on ocular factors that may affect vascular caliber, such as intraocular pressure.12 It is unclear how these local factors may influence the relationship of vascular caliber and systemic variables in our study.
In summary, data from this study in a multiethnic cohort indicate that retinal vascular caliber is related to a range of standard and novel cardiovascular risk factors. Retinal arteriolar narrowing is associated with hypertension, whereas venular dilatation is associated with diabetes, obesity, and selected biomarkers of inflammation. Our study suggests that an assessment of retinal vascular caliber may offer insights into the contribution of subclinical vascular processes to the development of systemic and ocular diseases.
Acknowledgments
Supported by Contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169 and Grant HL69979-03 (RK, TYW) from the National Heart, Lung, and Blood Institute and a Science, Technology Innovation Grant, Australia (TYW).
The authors thank the other investigators, the staff, and the participants in the MESA study for their valuable contributions.
Footnotes
A full list of participating MESA investigators and institutions can be found at http://www.mesanhlbi.org
Disclosure: T.Y. Wong, None; F.M.A. Islam, None; R. Klein, None; B.E.K. Klein, None; M.F. Cotch, None; C. Castro, None; A.R. Sharrett, None; E. Shahar, None
References
- 1.Wong TY, Klein R, Klein BEK, et al. Retinal microvascular abnormalities, and their relation to hypertension, cardiovascular diseases and mortality. Surv Ophthalmol. 2001;46:59–80. doi: 10.1016/s0039-6257(01)00234-x. [DOI] [PubMed] [Google Scholar]
- 2.Wong TY, Mitchell P. Hypertensive retinopathy. N Engl J Med. 2004;351:2310–2317. doi: 10.1056/NEJMra032865. [DOI] [PubMed] [Google Scholar]
- 3.Wong TY, Klein R, Couper DJ, et al. Retinal microvascular abnormalities and incident strokes. The Atherosclerosis Risk in the Communities Study. Lancet. 2001;358:1134–1140. doi: 10.1016/S0140-6736(01)06253-5. [DOI] [PubMed] [Google Scholar]
- 4.Wong TY, Klein R, Sharrett AR, et al. Retinal arteriolar narrowing and incident coronary heart disease in women and men. The Atherosclerosis Risk in the Communities Study. JAMA. 2002;287:1153–1159. doi: 10.1001/jama.287.9.1153. [DOI] [PubMed] [Google Scholar]
- 5.Duncan BB, Wong TY, Tyroler HA, Davis CE, Fuchs FD. Hypertensive retinopathy independently predicts coronary heart disease. The Lipids Research Clinics Coronary Primary Prevention Trial. Br J Ophthalmology. 2002;86:1002–1006. doi: 10.1136/bjo.86.9.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong TY, Klein R, Nieto FJ, et al. Retinal microvascular abnormalities and ten-year cardiovascular mortality: a population-based case-control study. Ophthalmology. 2003;110:933–940. doi: 10.1016/S0161-6420(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 7.Wong TY, Klein R, Sharrett AR, et al. Retinal arteriolar diameter and risk for hypertension. Ann Intern Med. 2004;140:248–255. doi: 10.7326/0003-4819-140-4-200402170-00006. [DOI] [PubMed] [Google Scholar]
- 8.Wong TY, Shankar A, Klein R, Klein BEK, Hubbard LD. Prospective cohort study of retinal vessel diameters and risk of hypertension. BMJ. 2004;329:79–82. doi: 10.1136/bmj.38124.682523.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein R, Klein BEK, Moss SE, et al. The relation of retinal vessel caliber to the incidence and progression of diabetic retinopathy. XIX: The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Ophthalmol. 2004;122:76–83. doi: 10.1001/archopht.122.1.76. [DOI] [PubMed] [Google Scholar]
- 10.Wong TY, Shankar A, Klein R, Klein BE. Retinal vessel diameters and the incidence of gross proteinuria and renal insufficiency in people with type 1 diabetes. Diabetes. 2004;53:179–184. doi: 10.2337/diabetes.53.1.179. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell P, Leung H, Wang JJ, et al. Retinal vessel diameter and open-angle glaucoma. The Blue Mountains Eye Study. Ophthalmology. 2005;112:245–250. doi: 10.1016/j.ophtha.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 12.Sharrett AR, Hubbard LD, Cooper LS, et al. Retinal arteriolar diameters and elevated blood pressure. The Atherosclerosis Risk in Communities Study. Am J Epidemiol. 1999;150:263–270. doi: 10.1093/oxfordjournals.aje.a009997. [DOI] [PubMed] [Google Scholar]
- 13.Leung H, Wang JJ, Rochtchina E, Wong TY, Klein R, Mitchell P. Impact of current and past blood pressure on retinal arteriolar diameter in an older population. J Hypertension. 2004;22:1543–1549. doi: 10.1097/01.hjh.0000125455.28861.3f. [DOI] [PubMed] [Google Scholar]
- 14.Wong TY, Hubbard LD, Klein R, et al. Retinal microvascular abnormalities and blood pressure in older people. The Cardiovascular Health Study. Br J Ophthalmology. 2002;86:1007–1013. doi: 10.1136/bjo.86.9.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong TY, Klein R, Klein BEK, et al. Retinal vessel diameters and their associations with age and blood pressure. Invest Ophthalmol Vis Sci. 2003;44:4644–4650. doi: 10.1167/iovs.03-0079. [DOI] [PubMed] [Google Scholar]
- 16.Leung H, Wang JJ, Rochtchina E, et al. Relationships between age, blood pressure and retinal vessel diameters in an older population. Invest Ophthalmol Vis Sci. 2003;44:2900–2904. doi: 10.1167/iovs.02-1114. [DOI] [PubMed] [Google Scholar]
- 17.Wang JJ, Mitchell P, Leung H, et al. Hypertensive retinal vessel wall signs in the general older population. The Blue Mountains Eye Study. Hypertension. 2003;42:534–541. doi: 10.1161/01.HYP.0000090122.38230.41. [DOI] [PubMed] [Google Scholar]
- 18.Klein R, Sharrett AR, Klein BEK, et al. Are retinal arteriolar abnormalities related to atherosclerosis? The Atherosclerosis Risk in Communities Study. Arterioscler Thromb Vasc Biol. 2000;20:1644–1650. doi: 10.1161/01.atv.20.6.1644. [DOI] [PubMed] [Google Scholar]
- 19.Ikram MK, de Jong FJ, Vingerling JR, et al. Are retinal arteriolar or venular diameters associated with markers for cardiovascular disorders? The Rotterdam Study. Invest Ophthalmol Vis Sci. 2004;45:2129–2134. doi: 10.1167/iovs.03-1390. [DOI] [PubMed] [Google Scholar]
- 20.Klein R, Klein BE, Knudtson M, Wong TY, Tsai M. Are inflammatory factors related to retinal vessel caliber? The Beaver Dam Eye Study. Arch Ophthalmol. 2006;124:87–94. doi: 10.1001/archopht.124.1.87. [DOI] [PubMed] [Google Scholar]
- 21.Wong TY, Klein R, Sharrett AR, et al. The prevalence and risk factors of retinal microvascular abnormalities in older people. The Cardiovascular Health Study. Ophthalmology. 2003;110:658–666. doi: 10.1016/S0161-6420(02)01931-0. [DOI] [PubMed] [Google Scholar]
- 22.Bild DE, Bluemke DA, Burke GL, et al. The Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 23.Wong TY, Klein R, Islam FMA, et al. Diabetic retinopathy in a multi-ethnic cohort in the United States. The Multi-Ethnic Study of Atherosclerosis (MESA) Am J Ophthalmology. 2006;141:446–455. doi: 10.1016/j.ajo.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein R, Klein BE, Knudtson MD, et al. The prevalence of age-related macular degeneration in four racial-ethnic groups in the United States. The Multi-Ethnic Study of Atherosclerosis. Arch Ophthalmol. 2006;113:373–380. doi: 10.1016/j.ophtha.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 25.Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs: an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology. 1991;98(suppl):786–806. [PubMed] [Google Scholar]
- 26.Hubbard LD, Brothers RJ, King WN, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities (ARIC) Study. Ophthalmology. 1999;106:2269–2280. doi: 10.1016/s0161-6420(99)90525-0. [DOI] [PubMed] [Google Scholar]
- 27.Wong TY, Knudtson MD, Klein R, et al. Computer-assisted measurement of retinal vessel diameters in the Beaver Dam Eye Study: methodology, correlation between eyes, and effect of refractive errors. Ophthalmology. 2004;111:1183–1190. doi: 10.1016/j.ophtha.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 28.Knudtson MD, Lee KE, Hubbard LD, Wong TY, Klein R, Klein BEK. A revised method for summarizing retinal vessel diameters. Curr Eye Res. 2003;27:143–149. doi: 10.1076/ceyr.27.3.143.16049. [DOI] [PubMed] [Google Scholar]
- 29.MESA Coordinating Center. Multi-Ethnic Study of Atherosclerosis Field Center Manual of Operations. Seattle, WA: University of Washington; January 5, 2001. [Google Scholar]
- 30.Newman TB, Browner WS. In defense of standardized regression coefficients. Epidemiology. 1991;2:383–386. doi: 10.1097/00001648-199109000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Eng J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 32.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 33.Kawagishi T, Matsuyoshi M, Emoto M, et al. Impaired endothelium-dependent vascular responses of retinal and intrarenal arteries in patients with type 2 diabetes. Arterioscler Thromb Vasc Biol. 1999;19:2509–2516. doi: 10.1161/01.atv.19.10.2509. [DOI] [PubMed] [Google Scholar]
- 34.Delles C, Michelson G, Harazny J, Oehmer S, Hilgers KF, Schmieder RE. Impaired endothelial function of the retinal vasculature in hypertensive patients. Stroke. 2004;35:1289–1293. doi: 10.1161/01.STR.0000126597.11534.3b. [DOI] [PubMed] [Google Scholar]
- 35.Cuspidi C, Meani S, Salerno M, et al. Retinal microvascular changes and target organ damage in untreated essential hypertensives. J Hypertens. 2004;22:2095–2102. doi: 10.1097/00004872-200411000-00010. [DOI] [PubMed] [Google Scholar]
- 36.van den Born BJ, Hulsman CA, Hoekstra JB, Schlingemann RO, van Montfrans GA. Value of routine funduscopy in patients with hypertension: systematic review. BMJ. 2005;331:73. doi: 10.1136/bmj.331.7508.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong TY, Klein R, Sharrett AR, et al. Retinal arteriolar narrowing and risk of diabetes in middle-aged persons. JAMA. 2002;287:2528–2533. doi: 10.1001/jama.287.19.2528. [DOI] [PubMed] [Google Scholar]
- 38.Wong TY, Shankar A, Klein R, Klein BEK, Hubbard LD. Retinal arteriolar narrowing, hypertension and subsequent risk of diabetes mellitus. Arch Intern Med. 2005;165:1060–1065. doi: 10.1001/archinte.165.9.1060. [DOI] [PubMed] [Google Scholar]
- 39.Skovborg F, Nielsen AV, Lauritzen E, Hartkopp O. Diameters of the retinal vessels in diabetic and normal subject. Diabetes. 1969;18:292–298. doi: 10.2337/diab.18.5.292. [DOI] [PubMed] [Google Scholar]
- 40.Grunwald JE, Brucker AJ, Schwartz SS, et al. Diabetic glycemic control and retinal blood flow. Diabetes. 1990;39:602–607. doi: 10.2337/diab.39.5.602. [DOI] [PubMed] [Google Scholar]
- 41.Wong TY, Duncan BB, Golden SH, et al. Associations between the metabolic syndrome and retinal microvascular signs. The Atherosclerosis Risk in Communities Study. Invest Ophthalmol Vis Sci. 2004;45:2949–2954. doi: 10.1167/iovs.04-0069. [DOI] [PubMed] [Google Scholar]
- 42.Lembo G, Vecchione C, Fratta L, et al. Leptin induces direct vasodilation through distinct endothelial mechanisms. Diabetes. 2000;49:293–297. doi: 10.2337/diabetes.49.2.293. [DOI] [PubMed] [Google Scholar]
- 43.Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, Walsh K. Obesity, adiponectin and vascular inflammatory disease. Curr Opin Lipidol. 2003;14:561–566. doi: 10.1097/00041433-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Cornoni-Huntley J, LaCroix AZ, Havlik RJ. Race and sex differentials in the impact of hypertension in the United States. The National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Arch Intern Med. 1989;149:780–788. [PubMed] [Google Scholar]
- 45.Arnett DK, Rautaharju P, Crow R, et al. Black-white differences in electrocardiographic left ventricular mass and its association with blood pressure (the ARIC study). Atherosclerosis Risk in Communities. Am J Cardiol. 1994;74:247–252. doi: 10.1016/0002-9149(94)90365-4. [DOI] [PubMed] [Google Scholar]
- 46.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Stamler J. End-stage renal disease in African-American and white men: 16-year MRFIT findings. JAMA. 1997;277:1293–1298. [PubMed] [Google Scholar]
- 47.Kittner SJ, White LR, Losonczy KG, Wolf PA, Hebel JR. Black-white differences in stroke incidence in a national sample: the contribution of hypertension and diabetes mellitus. JAMA. 1990;264:1267–1270. [PubMed] [Google Scholar]
- 48.Kramer H, Han C, Post W, et al. Racial/ethnic differences in hypertension and hypertension treatment and control in the multi-ethnic study of atherosclerosis (MESA) Am J Hypertens. 2004;17:963–970. doi: 10.1016/j.amjhyper.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 49.Jacobs DR, Jr, Murtaugh MA, Steffes M, Yu X, Roseman J, Goetz FC. Gender- and race-specific determination of albumin excretion rate using albumin-to-creatinine ratio in single, untimed urine specimens: the Coronary Artery Risk Development in Young Adults Study. Am J Epidemiol. 2002;155:1114–1119. doi: 10.1093/aje/155.12.1114. [DOI] [PubMed] [Google Scholar]
- 50.Summerson JH, Bell RA, Konen JC. Racial differences in the prevalence of microalbuminuria in hypertension. Am J Kidney Dis. 1995;26:577–579. doi: 10.1016/0272-6386(95)90591-x. [DOI] [PubMed] [Google Scholar]
- 51.Strain WD, Chaturvedi N, Leggetter S, et al. Ethnic differences in skin microvascular function and their relation to cardiac target-organ damage. J Hypertens. 2005;23:133–140. doi: 10.1097/00004872-200501000-00023. [DOI] [PubMed] [Google Scholar]
- 52.Bild DE, Detrano R, Peterson D, et al. Ethnic differences in coronary calcification. The Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2005;111:1313–1320. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 53.Lee KE, Klein BE, Klein R, Knudtson MD. Familial aggregation of retinal vessel caliber in the beaver dam eye study. Invest Ophthalmol Vis Sci. 2004;45:3929–3933. doi: 10.1167/iovs.04-0462. [DOI] [PubMed] [Google Scholar]
- 54.Wang JJ, Wong TY. Genetic determinants of retinal vascular caliber: further insights into hypertension pathogenesis. Hypertension. 2006;47:644–645. doi: 10.1161/01.HYP.0000208303.74884.78. [DOI] [PubMed] [Google Scholar]
- 55.Li H, Hsu W, Lee ML, Wong TY. Automatic grading of retinal vessel caliber. IEEE Trans Biomed Eng. 2005;52:1352–1355. doi: 10.1109/TBME.2005.847402. [DOI] [PubMed] [Google Scholar]
- 56.Cooper LS, Wong TY, Klein R, et al. Retinal microvascular abnormalities and MRI-defined subclinical cerebral infarction. The Atherosclerosis Risk in Communities Study. Stroke. 2006;37:82–86. doi: 10.1161/01.STR.0000195134.04355.e5. [DOI] [PubMed] [Google Scholar]
- 57.Levy BI, Ambrosio G, Pries AR, et al. Microcirculation in hypertension: a new target for treatment? Circulation. 2001;104:735–740. doi: 10.1161/hc3101.091158. [DOI] [PubMed] [Google Scholar]