Abstract
Theories of temporal coding by cortical neurons are supported by observations that individual neurons can respond to sensory stimulation with millisecond precision and that activity in large populations is often highly correlated. Synchronization is highest between neurons with overlapping receptive fields and modulated by both sensory stimulation and behavioral state. It is not yet clear whether cortical synchronization is an epiphenomenon or a critical component of efficient information transmission. Experimental manipulations that generate receptive field plasticity can be used to test the relationship between synchronization and receptive fields. Here we demonstrate that increasing receptive field size in primary auditory cortex by repeatedly pairing a train of tones with nucleus basalis (NB) stimulation increases synchronization, and decreasing receptive field size by pairing different tone frequencies with NB stimulation decreases synchronization. These observations seem to support the conclusion that neural synchronization is simply an artifact caused by common inputs. However, pairing tone trains of different carrier frequencies with NB stimulation increases receptive field size without increasing synchronization, and environmental enrichment increases synchronization without increasing receptive field size. The observation that receptive fields and synchronization can be manipulated independently suggests that common inputs are only one of many factors shaping the strength and temporal precision of cortical synchronization and supports the hypothesis that precise neural synchronization contributes to sensory information processing.
Keywords: Synchrony, Cross-correlation, Sensory coding, Acetylcholine, Cholinergic
1. Introduction
Understanding how neurons in the central nervous system represent and process information has challenged neuroscientists for decades. While it is commonly assumed that neurons represent information through their mean firing rates (Shadlen and Newsome, 1994), the temporal structure of spike trains likely contributes to the neuronal representation of sensory stimuli (Singer, 1999). In every sensory system, action potentials elicited in response to a stimulus can be precisely timed relative to stimulus events or to the action potentials of other neurons (Meister et al., 1995; deCharms and Merzenich, 1996; Neuenschwander and Singer, 1996; Wehr and Laurent, 1996; Johansson and Birznieks, 2004).
Both stimulus features and attention influence the synchronization of action potentials generated by different cortical neurons. For example, neurons in primary auditory cortex (A1) increase their synchronization during long continuous tones (deCharms and Merzenich, 1996). In visual cortex, neurons with non-overlapping receptive fields exhibit more synchronization when responding to a single line that extends through both receptive fields compared to two separate lines (Gray et al., 1989). When different images are presented to each eye, the image subjects perceive is correlated with greater cortical synchronization (Fries et al., 1997). The degree of cortical synchronization is also modified by selective attention (Steinmetz et al., 2000; Fries et al., 2001). These and other observations have led to the hypothesis that cortical synchronization plays an important role in sensory processing and serves to bind salient features of objects.
The degree of cortical synchronization is strongly influenced by receptive field properties (Engel et al., 1990; Nelson et al., 1992; Brosch et al., 1995, 2002; Brosch and Schreiner, 1999). Neurons with similar receptive field properties exhibit the greatest synchrony during both spontaneous and driven activity. In auditory cortex, the height of the cross-correlation function between pairs of neurons increases and the width narrows with increasing receptive field similarity (Brosch and Schreiner, 1999; Brosch et al., 2002). This relationship is observed in both awake and anesthetized conditions. The correlation in the spontaneous activity of cortical neurons with similar receptive field properties is likely a result of common inputs.
Although cortical codes based on spike timing offer many theoretical advantages, it is possible that cortical synchronization is an epiphenomenon of shared inputs. The modulation of synchrony by context and attention have been offered as evidence that synchronization is functionally relevant (Singer, 1999). However, the argument is confounded by observations that context and attention also modulate receptive field characteristics (Crist et al., 2001).
Many experimental manipulations, including aversive and appetitive conditioning, alter receptive fields. If cortical synchronization is determined primarily by receptive field structure, manipulations that increase receptive field overlap should increase synchrony and manipulations that decrease receptive field overlap should decrease synchrony. Classic plasticity studies by Merzenich and colleagues reported that receptive fields are widened by training on some tasks (Wang et al., 1995; Recanzone et al., 1992) and narrowed by training on others (Jenkins et al., 1990; Recanzone et al., 1993). Generally speaking, receptive fields narrow when animals train on fine spatial discrimination and broaden when they train on temporal judgments (Merzenich et al., 1990). Cortical synchronization was not examined in these studies.
Although changes in receptive field structure are specific to the trained stimulus, passive sensory stimulation has no long lasting effects on receptive field characteristics in adults (Recanzone et al., 1993; Zhang et al., 2001). A recent study demonstrates that reward contingencies play a critical role in gating cortical plasticity. Training two groups of animals on an identical stimulus set can result in plasticity in A1 that is specific to tone frequency or intensity depending on which parameter predicted reward (Polley et al., 2006).
Acetylcholine is one of the critical modulatory neuro-transmitters that permits long-lasting experience-dependent cortical plasticity. Drugs or lesions that interfere with the central cholinergic system block training induced map plasticity in both sensory and motor cortex, reduce learning, and prevent recovery from brain damage (Weinberger, 2003; Conner et al., 2003, 2005). Delivery of cholinergic agonists to the cortex or electrical stimulation of nucleus basalis (NB), which projects to the cortex, causes cortical receptive fields to shift toward stimuli that are associated with release of acetylcholine (Metherate and Weinberger, 1989; Rasmusson, 2000). Repeatedly pairing NB stimulation with a sound results in plasticity in A1 that mimics the changes expected after several months of behavioral training on that sound (Kilgard and Merzenich, 1998; Kilgard et al., 2001). For example, pairing a 15 Pps tone train with NB stimulation increases receptive field sizes in A1 by nearly 60%. Identical NB stimulation paired with two different randomly interleaved tone frequencies causes A1 receptive fields to decrease by more than 20%. Pairing NB stimulation with sounds that are modulated and vary in their carrier frequency results in intermediate receptive field plasticity (35% increase in bandwidth). These results suggest that release of acetylcholine marks certain sounds as behaviorally relevant, but provides little information about how to reorganize cortical circuits to optimize the representation of these sounds. The information about what specific changes to make is likely present in the pattern of stimulus evoked activity. Synaptic plasticity mechanisms, which are highly sensitive to the timing of sensory inputs (Dan and Poo, 2006) may be responsible for the differential plasticity.
In this study we analyzed cortical synchronization from a set of previously published experiments which documented plasticity induced by sensory stimulation associated with NB stimulation or enriched housing (Kilgard and Merzenich, 1998; Kilgard et al., 2001; Engineer et al., 2004).
2. Materials and methods
2.1. Subjects
Microelectrode recordings were made from small clusters of neurons at 2001 sites in A1 from 48 female adult Sprague–Dawley rats. Eighteen of the rats were experimentally naïve and housed in standard laboratory conditions. Eight of the rats were housed in an enriched auditory environment that included natural sounds and music and contained many sound sources that were activated by the rats in the cage (for more details see Engineer et al., 2004).
Twenty-two of the rats were implanted with NB electrodes and heard tones paired with electrical stimulation of NB several hundred times each day for one month (Kilgard et al., 2001). Platinum bipolar-stimulating electrodes (SNE-200, Rhodes Medical Instruments, Woodland Hills, CA) were lowered 7.0 mm below the cortical surface 3.3 mm lateral and 2.3 mm posterior to bregma and cemented into place using sterile techniques. After two weeks of recovery, rats were placed in a sound-shielded test chamber and heard: (a) two different tones, (b) seven different tones, (c) a train of six 9 k tones presented at 15 pps, or (d) tone trains of varying frequency presented at 15 Hz paired with NB electrical stimulation (Table 1).
Table 1.
Summary of experimental groups
| Group | Experiment | Receptive field size | Map expansion | Number of rats (Al sites) | Number of Al pairs |
|---|---|---|---|---|---|
| 1 | 15 pps 9 kHz tone train + NB | ↑ | ↑ | 4(244) | 112 |
| 2 | 15 pps trains of multiple tone frequencies + NB | ↑ | – | 6(223) | 89 |
| 3 | Two tone frequencies + NB | ↓ | – | 6(209) | 52 |
| 4 | Multiple tone frequencies + NB | ↓ | – | 6 (236) | 105 |
| 5 | Acoustically enriched environment | – | – | 8(397) | 159 |
| 6 | Controls | – | – | 18(692) | 250 |
| Totals | 482001 | 767 |
The first four groups heard tones paired with electrical activation of Nucleus Basalis (NB) three hundred times per day for four weeks (Kilgard et al., 2001). The fifth group was housed in an enriched environment described in (Engineer et al., 2004). Control rats were experimentally naïve and housed in standard laboratory conditions. Plasticity of frequency tuning (bandwidth and characteristic frequency) reported in previous studies (Kilgard and Merzenich, 1998; Kilgard et al., 2001; Engineer et al., 2004) is indicated for each group. Cortical synchrony was measured from multi-unit data recorded simultaneously from pairs of microelectrode penetrations into primary auditory cortex.
Rats in the two tone frequency group heard either 4 and 14 kHz or 9 and 19 kHz tones (250 ms duration) randomly interleaved every 10–30 s (70 dB SPL). The seven tone group heard randomly interleaved tones (250 ms duration) ranging from 1.3 to 14 kHz presented at 30–40 dB above rat hearing range. The tone train group heard a 15 pps train of six 70 dB 9 kHz tones (25 ms duration). The multiple frequency tone train group heard the same range of frequencies as the seven tone group the only difference was that they were presented as trains of six 70 dB 25 ms tones. All tones had 3 ms rise/fall ramps. For each of these groups, the tones were paired with identical NB stimulation (200 ms duration 100 pps train of 70–150 μA 100 μs biphasic pulses, beginning 50 ms after tone onset).
The efficacy of NB activation was continuously monitored in every rat by quantifying NB-induced EEG desynchronization during slow-wave sleep. The current level (70–150 mA) for NB stimulation was chosen for each animal to be the minimum necessary to desynchronize the EEG for 1–2 s during slow-wave sleep.
2.2. Electrophysiological recordings
After one month of NB stimulation or two months of sensory enrichment, rats were anesthetized with pentobarbital sodium (50 mg/kg). Throughout the surgical procedures and during the recording session, a state of areflexia was maintained with supplemental doses of dilute pentobarbital (8 mg/ml ip). The trachea was cannulated to ensure adequate ventilation and to minimize breathing-related noises. The skull was supported in a head holder that left the ears unobstructed. The cisternae magnum was drained of CSF to minimize cerebral edema. After reflecting the temporalis muscle, auditory cortex was exposed and the dura was resected. The cortex was maintained under a thin layer of viscous silicon oil to prevent desiccation. A1 was defined on the basis of its short latency (8–20 ms) responses and its continuous tonotopy (preferred tone frequency increased from posterior to anterior as in Kilgard and Merzenich, 1999).
Recordings were made in a shielded, double-walled sound chamber (IAC). Clusters of action potentials were recorded simultaneously from two Parylene-coated tungsten microelectrodes (FHC, 250-um separation, 1–2 MΩ at 1 kHz) that were lowered orthogonally into the cortex to a depth of 550 μm (layers IV/V). The neural signal was filtered (0.3–8 kHz) and amplified (10,000×). Action potential waveforms were recorded whenever a fixed threshold was exceeded.
Auditory frequency response tuning curves were determined by presenting 45 frequencies spanning 3–4.5 octaves centered on the approximate best frequency of the site, or 81 frequencies from 1 to 32 kHz. Each frequency was presented at 15 or 16 intensities ranging between 0 and 75 dB (either 675 or 1296 total stimuli). Tuning curve tones were randomly interleaved and separated by 500 ms. For all groups, except the two tone NB pairing, we recorded activity for 500 ms after each tone. For the two tone NB group, we only recorded responses for 100 ms after each tone and were thus unable to estimate the correlation during silence for this group.
For more detailed descriptions of the stimulation and data collection techniques, see (Kilgard et al., 2001; Engineer et al., 2004). All techniques and protocols were approved by the University of California at San Francisco and University of Texas at Dallas Animal Care and Use Committees.
2.3. Data analysis
Receptive field properties (frequency bandwidth and characteristic frequency) were quantified by a blind, experienced observer (Kilgard and Merzenich, 1998; Kilgard et al., 2001; Engineer et al., 2004). Analysis of cortical synchronization was similar to that used in (Brosch and Schreiner, 1999). Cross-correlation functions were computed for each recording pair by counting the number of spike coincidences of the two clusters for various time shifts (−50–50 ms) between the two spike trains (1 ms bin size). The cross-correlation function was normalized by dividing each of its bins by the square root of the product of the number of discharges in both spike trains. Cross-correlation functions were derived from driven activity collected with 100 ms of tone onset and spontaneous collected 250–500 ms after tone onset.
To remove correlations due to sensory stimulation, the shift predictor was subtracted from each cross-correlation function (Perkel et al., 1967). Only pairs in which both recording sites were clearly in A1 were used in this study (767 pairs total) since correlations are known to be weaker across area boundaries (Eggermont, 2000). Statistical significance was determined using two-tailed t-tests.
3. Results
The data presented here were collected from the right A1 of 48 adult rats. Cross-correlation analysis was performed on action potentials recorded from clusters of neurons at 767 pairs of sites (Table 1). Data from each pair was recorded simultaneously from two electrodes separated by 250 μm. Only pairs in which both electrodes were in A1 were analyzed. As expected given their separation, 85% of the pairs in control rats had CFs that were less than one octave apart and 50% had CFs that were less than one-third of an octave apart.
The cross-correlation function for most pairs exhibited a single symmetrical positive peak centered at 0 ms (Fig. 1a). The width of the cross-correlation function at half height was typically between 5 and 15 ms. Control rats exhibited a weak but significant negative correlation between the distance separating the CFs and the height of the normalized cross-correlation function (R = −0.20, p < 0.05). Similar relationships were also observed in three of the five experimental groups. In the two groups with significant map plasticity (one or two tone frequencies paired with NB stimulation), the median separation between CFs was only one-sixth of an octave and there was no significant relationship between CF separation and the height of the normalized cross-correlation function.
Fig. 1.
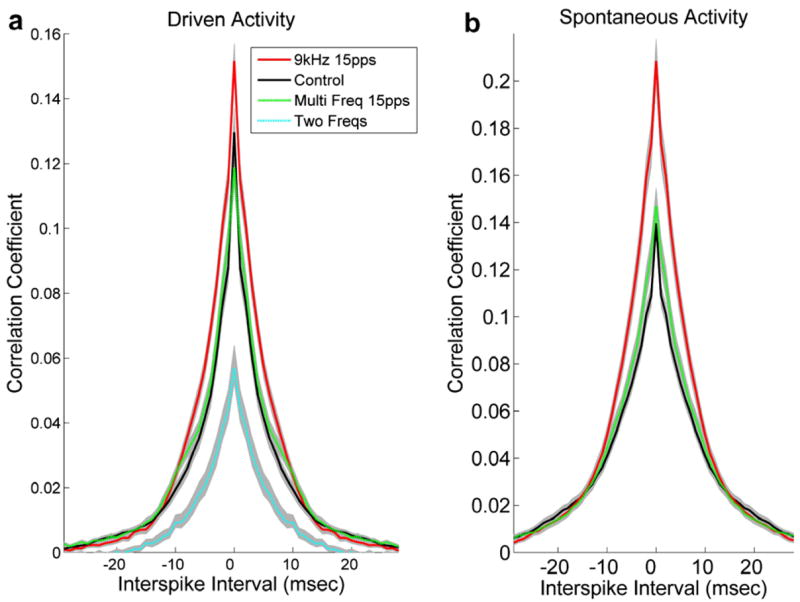
Mean cross-correlation functions derived from cortical activity in rats that had repeatedly heard one of three sets of tonal stimuli paired with electrical activation of Nucleus Basalis compared with experimentally naïve control rats. The standard error of the mean is shown for each function. Activity recorded within 100 ms of tone onset is considered driven (a) and activity recorded 250–500 ms after tone onset is considered spontaneous (b).
Repeated exposure to a 9 kHz tone train (50 dB) paired with NB stimulation several hundred times each day for four weeks generated profound receptive field plasticity such that 80% of A1 neurons responded to this sound, compared to 35% in naïve controls (Kilgard and Merzenich, 1998). The increased A1 response to 9 kHz was due to both CF shifts and increased bandwidths (Table 1). Pairing NB stimulation with 9 kHz tone trains also significantly increased the number of synchronous events compared to naïve controls during both driven (Fig. 1a) and spontaneous (Fig. 1b) activity. Sixteen percent more synchronous events were recorded during driven activity and 41% more synchronous events were recorded during spontaneous activity, compared to experimentally naïve rats.
Pairing one of two different randomly interleave tone frequencies with NB stimulation resulted in receptive field contraction (Table 1) and decreased the number of synchronous events during driven activity by 56% (Fig. 1a). Since our recordings were made 24–48 h after the last NB pairing, our results extend earlier observations (Kilgard and Merzenich, 1998; Kilgard et al., 2001) and demonstrate that sensory experience can generate enduring changes in cortical cross-correlation functions.
We also examined cross-correlation functions in a group of rats that received an identical amount of NB stimulation but heard a pattern of sounds that could be considered intermediate between the sounds used in the earlier experiments. In this experiment 15 pps tone trains were once again paired with NB stimulation but this time the tone frequency used in each train was selected randomly (1.3–14 kHz). We have previously reported that this pattern of acoustic input produces receptive field broadening but no map plasticity (Kilgard et al., 2001). This intermediate pattern of acoustic stimulation (i.e. both modulated and multiple frequencies) did not change the number of synchronous events (Fig. 1). This result demonstrates that it is possible to increase receptive field overlap without increasing cortical synchronization.
Since the peak in the cross-correlation function is not always exactly at zero, it is possible that the changes in the number of synchronous events reflect a shift toward or away from zero rather than a true change in the total correlation between recording sites. Fig. 2 shows the average peak correlation coefficient for pairs in each group. The significant increase or decrease after pairing identical NB stimulation with 9 kHz tone trains or two tone frequencies, respectively, indicates that sensory experience can alter the degree of cortical synchronization. As in earlier reports, the neural correlation coefficients were higher during spontaneous activity compared to driven activity and most cross-correlation functions had peaks at zero (Eggermont, 1994).
Fig. 2.
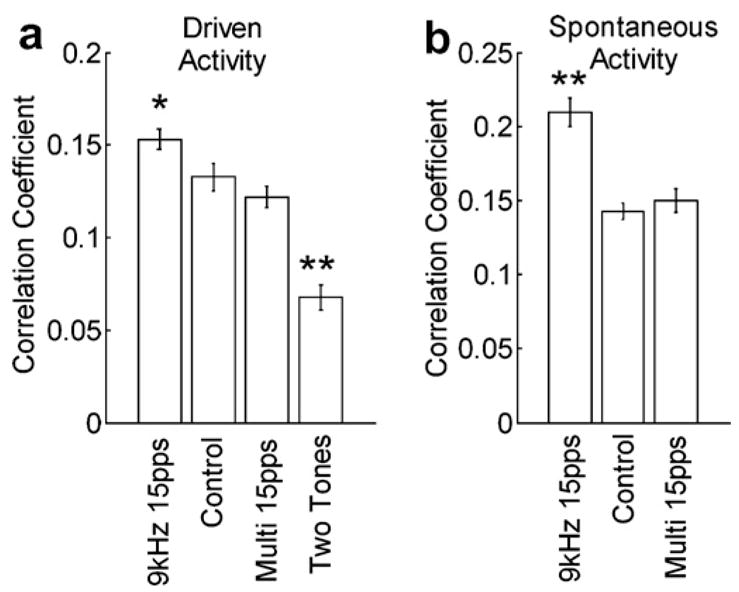
Average peak correlation between multi-unit responses from pairs of primary auditory cortex microelectrode penetrations during: (a) driven and (b) spontaneous activity. The experimental groups were exposed to one of three sets of tonal stimuli repeatedly paired with electrical activation of Nucleus Basalis. Controls were experimentally naïve rats. Error bars indicate standard error of the mean. Asterisks indicate responses are significantly different from controls (* p < 0.05; * p < 0.001).
Earlier studies observed that pairs with greater correlation coefficients tend to have narrower widths at half height (Brosch and Schreiner, 1999). Fig. 3a shows that cross-correlation width at half height was significantly narrower for rats exposed to 9 kHz tone trains compared to rats exposed to two different tone frequencies randomly interleaved. The width of the cross-correlation function during spontaneous activity was significantly narrower compared to naïve controls (Fig. 3b). Neither the height nor the width of the cross-correlation function was altered in rats that heard tone trains of multiple frequencies paired with NB stimulation.
Fig. 3.
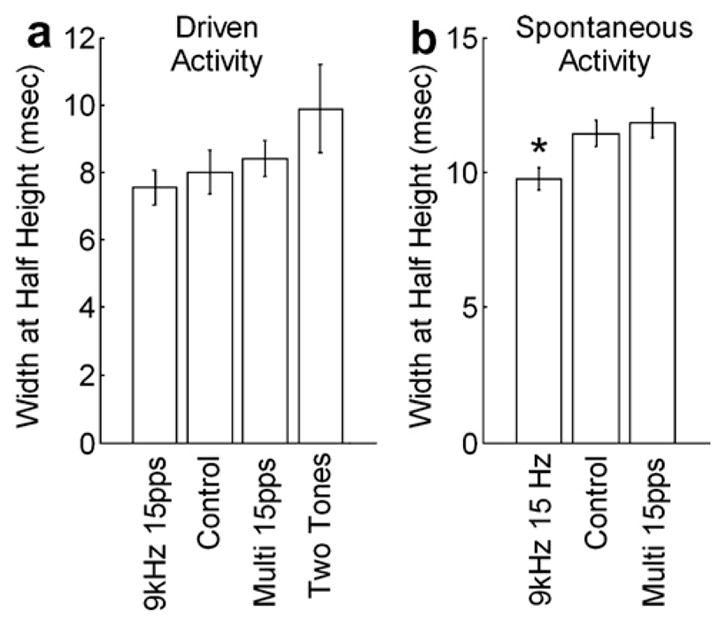
Average width of each cross-correlation function at half height during: (a) driven and (b) spontaneous activity. The experimental groups were exposed to one of three sets of tonal stimuli repeatedly paired with electrical activation of Nucleus Basalis. Controls were experimentally naïve rats.
To determine whether more naturalistic manipulations known to generate plasticity can also alter cortical synchronization, we recorded from 159 A1 pairs in eight rats that had been housed in an enriched acoustic environment from four to twelve weeks of age (Engineer et al., 2004). Although A1 responses in these rats have decreased response thresholds, shorter latencies and increased response strength and greater paired pulse depression (Engineer et al., 2004; Percaccio et al., 2005), receptive field bandwidth 10–30 dB above threshold was not different from controls. Action potentials were recorded using a different data acquisition system and a somewhat higher electrode impedance, which necessitated a new set of control data. Compared to rats housed in standard laboratory conditions, enriched rats exhibited significantly greater cross-correlation height (Fig. 4b) and narrower width (Fig. 5b) during spontaneous activity. Cross-correlation functions during driven activity were unchanged relative to controls. We also paired NB stimulation with seven tones (1.3–14 kHz) and confirmed our earlier observation that NB stimulation paired with more than one pure tone decreases receptive field size and driven cross-correlation height, but not driven width.
Fig. 4.
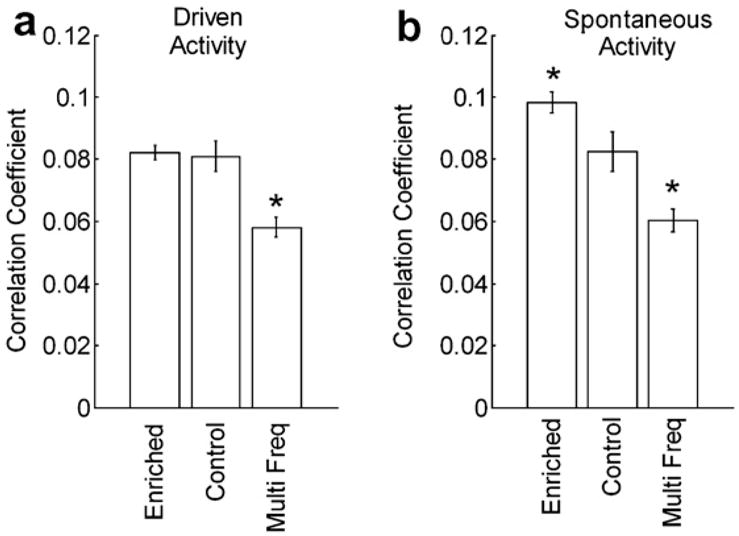
Average peak correlation between multi-unit responses from pairs of primary auditory cortex microelectrode penetrations during: (a) driven and (b) spontaneous activity. The experimental groups were either housed in an acoustically enriched environment (enriched) or exposed to one of seven pure tones repeatedly paired with electrical activation of Nucleus Basalis (Multi Freq). Controls were experimentally naïve rats housed in standard laboratory conditions.
Fig. 5.
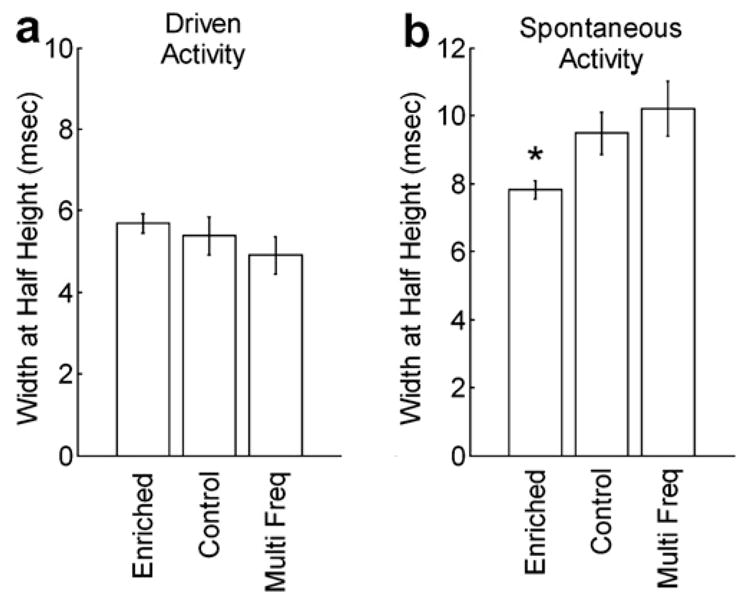
Average width of each cross-correlation function at half height during: (a) driven and (b) spontaneous activity. The experimental groups were either housed in an enriched environment (enriched) or exposed to seven different randomly interleaved pure tones repeatedly paired with electrical activation of Nucleus Basalis (Multi Freq). Controls were experimentally naïve rats housed in standard laboratory conditions.
4. Discussion
Here we report that repeated sensory stimulation coupled with chronic NB stimulation results in long lasting changes in the correlation between neurons in A1. The degree of cortical synchronization can be increased or decreased depending on the acoustic features of the sounds presented. The fact that these observed changes are specific to the sounds heard indicates that they are not an artifact of NB stimulation, which was identical in both cases. The observation that the natural sensory experiences of enriched housing can alter cortical synchronization suggests synchronization plasticity may contribute to sensory adaptation and learning.
4.1. Relationship between Receptive Field Plasticity and Cortical Synchronization
Pairing NB stimulation with a train of 9 kHz tones, which causes cortical map reorganization and receptive field expansion, increased neural synchronization in both driven and spontaneous activity up to 48 h after the last pairing session. Pairing NB stimulation with two different pure tones causes receptive field contraction and decreased neural synchronization. Since synchronization is known to be greater between neurons with similar receptive fields, it is possible that the changes in synchronization are simply an artifact of changes in receptive field overlap. Consistent with this possibility, three other experimental manipulations that increase receptive field overlap also increase cortical synchronization (Table 2 top), including intracortical microstimulation (Dinse et al., 1993; Maldonado and Gerstein, 1996), artificial scotoma (Das and Gilbert, 1995), and hearing loss (Rajan, 2000; Norena and Eggermont, 2003; Seki and Eggermont, 2002, 2003). General anesthesia, which is known to decrease receptive field size (and thus receptive field overlap), also decreases high frequency synchronization (van der Togt et al., 1998). In cat visual cortex receptive fields are 27% wider during synchronized states than they are during nonsynchronized states (Worgotter et al., 1998).
Table 2.
Summary of the effects of various experimental manipulations on cortical receptive field size and fast synchronization
Thick lines group experiments by similarity of effects. These results indicate that many factors influence cortical synchronization independent of receptive field reorganization. In many cases, the changes in receptive field size and synchronization were quantified in different studies.
Although these experiments suggest that synchrony is an artifact of receptive field overlap, other experiments have demonstrated that receptive fields and synchrony can by altered independently (Table 2 bottom). Selective attention, behavioral training, and electrical kindling can simultaneously increase synchronization and decrease receptive field size (Fries et al., 2001; Luck et al., 1997; Gassanov et al., 1985; Steinmetz et al., 2000; Vaadia et al., 1995; Schoenbaum et al., 2000; Sakurai, 1993; Schieber, 2002; Salazar et al., 2004; Valentine et al., 2004). Pairing NB stimulation with noise burst trains causes receptive fields to increase but decreases the amount of synchrony between A1 recording sites (Bao et al., 2003). Amblyopia also increases receptive field size and decreases cortical synchronization (Swindale and Mitchell, 1994; Roelfsema et al., 1994; Konig et al., 1993). Enrichment increases synchronization without causing map plasticity or receptive field broadening. Repeatedly pairing stimulation of the ventral tegmentum with a tone and pairing NB stimulation with tone trains of several different carrier frequencies both result in significant receptive field expansion without increasing cortical synchronization (Bao et al., 2001). Though the functional consequences of altered synchronization remain unclear, the present results show that cortical synchronization and receptive field plasticity can change in opposite directions. This is consistent with observations that receptive field characteristics account for less than half of the variance in the correlation strength (Brosch and Schreiner, 1999; Eggermont, 2006) and suggests that many other factors influence cortical synchronization.
Most of these studies have examined multi-unit responses recorded under general anesthesia. An important advantage of our study is that identical recording techniques were used to demonstrate that sensory experience can significantly increase or decrease cortical synchronization during spontaneous and driven activity for periods greater than 24 h. Earlier observations have shown that many minutes of auditory, somatosensory, and visual stimulation can alter cortical synchronization for several minutes (Ahissar et al., 1992; Erchova and Diamond, 2004; Das and Gilbert, 1995). In awake monkeys, the synchronization between two neurons in A1 can be altered by repeatedly presenting a tone each time one of the neurons fires (Ahissar et al., 1992). When the tone activated the second neuron, the conditioning increased the correlated firing during the conditioning period and during the several minutes of silence that followed it. When the tone inhibited the neuron and decreased the correlation during the conditioning period, the post-conditioning synchronization was decreased for several minutes. Interestingly, the conditioning had no effect on synchronization if the monkeys were not required to attend to the tones.
4.2. Potential mechanisms
The observation that training induced cortical map plasticity and synchronization plasticity both require attention (Recanzone et al., 1992, 1993; Ahissar et al., 1992) suggests that changes in synchronization and receptive field size are co-regulated. It is plausible that both forms of plasticity involve common synaptic mechanisms (Ikegaya et al., 2004; Nichols et al., 2006). Acetylcholine plays a critical role in the regulation of cortical plasticity. While presentation of unattended tones does not alter the A1 frequency map, repeated pairing of a tone with NB stimulation does, suggesting that acetylcholine plays a critical ole in the modulation of cortical plasticity (Recanzone et al., 1993; Bakin and Weinberger, 1996; Kilgard and Merzenich, 1998; Bao et al., 2001). Additionally, cholinergic antagonists and lesions prevent experience dependent cortical plasticity (Weinberger, 2003; Conner et al., 2003) and reduce fast cortical synchronization (Buzsaki and Gage, 1989).
In vitro and in vivo studies have shown that synaptic strengthening and weakening is governed by the correlated spiking of pre- and post-synaptic neurons in the cortex (Cruikshank and Weinberger, 1996). Recent experiments indicate that delays of a few milliseconds can be the difference between long-term potentiation and depression (Dan and Poo, 2006). Inputs that fire postsynaptic neurons with short latencies or act in correlated groups compete most successfully and develop strong synapses (Song and Abbott, 2001). One mechanism by which acetylcholine may influence plasticity is by transiently altering the degree of cortical synchronization (Metherate et al., 1992; Rasmusson et al., 1994). Fast cortical synchronization is increased by electrical activation of the mesencephalic reticular formation or application of cholinergic agonists and decreased by cholinergic antagonists (Munk et al., 1996; Rodriguez et al., 2004). Sensory stimulation that increases correlation in primary sensory cortex increases receptive field overlap unless the cholinergic system is blocked (Wang et al., 1995; Delacour et al., 1990). Collectively, these results suggest a close interrelationship between cortical synchronization, receptive field plasticity, and acetylcholine.
Our observation that several weeks of sound exposure paired with NB stimulation results in long lasting changes in synchronization suggests that the duration of synchronization plasticity is proportional to the duration of sensory exposure (i.e. minutes of stimulation leads to changes that last minutes, while days of stimulation leads to changes that may last for at least days). This long duration and stimulus specificity of altered cortical synchronization documented in this study suggests the possibility that these changes contribute to perceptual learning and recovery from brain damage.
Since our results only examined synchronization across separations of 250 μm, it is not clear whether similar changes in synchrony would be observed at other spatial scales. Our results describing changes in the synchronization of activity at nearby cortical locations were derived from multi-unit activity. We therefore draw no conclusions about physical connectivity (Bedenbaugh and Gerstein, 1997). Subtraction of the shift predictor did not alter the pattern of observations reported here and is not critical for our conclusions.
5. Conclusion
In summary, our results indicate that: (1) natural experiences can alter cortical synchronization; (2) changes in cortical synchronization can endure up to 48 h; and (3) cortical synchronization can be increased, decreased, or left unaltered depending on the pattern of sensory exposure associated with NB activity. Our results are inconsistent with models of cortical function which postulate that cortical synchronization is an epiphenomenon of common inputs (i.e. overlapping receptive fields). Rather, our results support the proposal that fast cortical synchronization is an independent property of cortical networks that is altered by sensory experience and may contribute significantly to information processing, memory function, and attention (Gray et al., 1989; Alloway and Roy, 2002; Sakurai, 1993; Buia and Tiesinga, 2006).
Acknowledgments
The authors thank Raluca Moucha, Matthias Munk, and Michael Brosch for valuable discussions and Vikram Jakkamsetti and Ryan Carraway for assistance in manuscript preparation. The authors also thank the anonymous reviewers for their helpful comments on an earlier draft of this paper. This work was supported by NIH Grants DC-004354 and DC-006624, and the Callier Excellence in Education Fund.
Abbreviations
- NB
nucleus basalis
- A1
primary auditory cortex
- pps
pulses per second
References
- Ahissar E, Vaadia E, Ahissar M, Bergman H, Arieli A, Abeles M. Dependence of cortical plasticity on correlated activity of single neurons and on behavioral context. Science. 1992;257:1412–1415. doi: 10.1126/science.1529342. [DOI] [PubMed] [Google Scholar]
- Alloway KD, Roy SA. Conditional cross-correlation analysis of thalamocortical neurotransmission. Behav Brain Res. 2002;135:191–196. doi: 10.1016/s0166-4328(02)00165-1. [DOI] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proc Natl Acad Sci USA. 1996;93:11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- Bao S, Chang EF, Davis JD, Gobeske KT, Merzenich MM. Progressive degradation and subsequent refinement of acoustic representations in the adult auditory cortex. J Neurosci. 2003;23:10765–10775. doi: 10.1523/JNEUROSCI.23-34-10765.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedenbaugh P, Gerstein GL. Multiunit normalized cross-correlation differs from the average single-unit normalized correlation. Neural Comput. 1997;9:1265–1275. doi: 10.1162/neco.1997.9.6.1265. [DOI] [PubMed] [Google Scholar]
- Brosch M, Schreiner CE. Correlations between neural discharges are related to receptive field properties in cat primary auditory cortex. Eur J Neurosci. 1999;11:3517–3530. doi: 10.1046/j.1460-9568.1999.00770.x. [DOI] [PubMed] [Google Scholar]
- Brosch M, Bauer R, Eckhorn R. Synchronous high-frequency oscillations in cat area 18. Eur J Neurosci. 1995;7:86–95. doi: 10.1111/j.1460-9568.1995.tb01023.x. [DOI] [PubMed] [Google Scholar]
- Brosch M, Budinger E, Scheich H. Stimulus-related gamma oscillations in primate auditory cortex. J Neurophysiol. 2002;87:2715–2725. doi: 10.1152/jn.2002.87.6.2715. [DOI] [PubMed] [Google Scholar]
- Buia C, Tiesinga P. Attentional modulation of firing rate and synchrony in a model cortical network. J Comput Neurosci. 2006;20:247–264. doi: 10.1007/s10827-006-6358-0. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Gage FH. The cholinergic nucleus basalis: a key structure in neocortical arousal. EXS. 1989;57:159–171. doi: 10.1007/978-3-0348-9138-7_16. [DOI] [PubMed] [Google Scholar]
- Conner JM, Culberson A, Packowski C, Chiba AA, Tuszynski MH. Lesions of the Basal forebrain cholinergic system impair task acquisition and abolish cortical plasticity associated with motor skill learning. Neuron. 2003;38:819–829. doi: 10.1016/s0896-6273(03)00288-5. [DOI] [PubMed] [Google Scholar]
- Conner JM, Chiba AA, Tuszynski MH. The basal forebrain cholinergic system is essential for cortical plasticity and functional recovery following brain injury. Neuron. 2005;46:173–179. doi: 10.1016/j.neuron.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Crist RE, Li W, Gilbert CD. Learning to see: experience and attention in primary visual cortex. Nat Neurosci. 2001;4:519–525. doi: 10.1038/87470. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Weinberger NM. Receptive-field plasticity in the adult auditory cortex induced by Hebbian covariance. J Neurosci. 1996;16:861–875. doi: 10.1523/JNEUROSCI.16-02-00861.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan Y, Poo MM. Spike timing-dependent plasticity: from synapse to perception. Physiol Rev. 2006;86:1033–1048. doi: 10.1152/physrev.00030.2005. [DOI] [PubMed] [Google Scholar]
- Das A, Gilbert CD. Receptive field expansion in adult visual cortex is linked to dynamic changes in strength of cortical connections. J Neurophysiol. 1995;74:779–792. doi: 10.1152/jn.1995.74.2.779. [DOI] [PubMed] [Google Scholar]
- deCharms RC, Merzenich MM. Primary cortical representation of sounds by the coordination of action-potential timing. Nature. 1996;381:610–613. doi: 10.1038/381610a0. [DOI] [PubMed] [Google Scholar]
- Delacour J, Houcine O, Costa JC. Evidence for a cholinergic mechanism of “learned” changes in the responses of barrel field neurons of the awake and undrugged rat. Neuroscience. 1990;34:1–8. doi: 10.1016/0306-4522(90)90299-j. [DOI] [PubMed] [Google Scholar]
- Dinse HR, Recanzone GH, Merzenich MM. Alterations in correlated activity parallel ICMS-induced representational plasticity. Neuroreport. 1993;5:173–176. doi: 10.1097/00001756-199311180-00020. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. Neural interaction in cat primary auditory cortex II. Effects of sound stimulation. J Neurophysiol. 1994;71:246–270. doi: 10.1152/jn.1994.71.1.246. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. Sound-induced synchronization of neural activity between and within three auditory cortical areas. J Neurophysiol. 2000;83:2708–2722. doi: 10.1152/jn.2000.83.5.2708. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. Properties of correlated neural activity clusters in cat auditory cortex resemble those of neural assemblies. J Neurophysiol. 2006;96:746–764. doi: 10.1152/jn.00059.2006. [DOI] [PubMed] [Google Scholar]
- Engel AK, Konig P, Gray CM, Singer W. Stimulus-dependent neuronal oscillations in cat visual cortex: inter-columnar interaction as determined by cross-correlation analysis. Eur J Neurosci. 1990;2:588–606. doi: 10.1111/j.1460-9568.1990.tb00449.x. [DOI] [PubMed] [Google Scholar]
- Engineer ND, Percaccio CR, Pandya PK, Moucha R, Rathbun DL, Kilgard MP. Environmental enrichment improves response strength, threshold, selectivity, and latency of auditory cortex neurons. J Neurophysiol. 2004;92:73–82. doi: 10.1152/jn.00059.2004. [DOI] [PubMed] [Google Scholar]
- Erchova IA, Diamond ME. Rapid fluctuations in rat barrel cortex plasticity. J Neurosci. 2004;24:5931–5941. doi: 10.1523/JNEUROSCI.1202-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P, Roelfsema PR, Engel AK, Konig P, Singer W. Synchronization of oscillatory responses in visual cortex correlates with perception in interocular rivalry. Proc Natl Acad Sci USA. 1997;94:12699–12704. doi: 10.1073/pnas.94.23.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Gassanov UG, Merzhanova GK, Galashina AG. Interneuronal relations within and between cortical areas during conditioning in cats. Behav Brain Res. 1985;15:137–146. doi: 10.1016/0166-4328(85)90060-9. [DOI] [PubMed] [Google Scholar]
- Gray CM, Konig P, Engel AK, Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature. 1989;338:334–337. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- Ikegaya Y, Aaron G, Cossart R, Aronov D, Lampl I, Ferster D, Yuste R. Synfire chains and cortical songs: temporal modules of cortical activity. Science. 2004;304(5670):559–564. doi: 10.1126/science.1093173. [DOI] [PubMed] [Google Scholar]
- Jenkins WM, Merzenich MM, Ochs MT, Allard T, Guic-Robles E. Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophysiol. 1990;63:82–104. doi: 10.1152/jn.1990.63.1.82. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Birznieks I. First spikes in ensembles of human tactile afferents code complex spatial fingertip events. Nat Neurosci. 2004;7:170–177. doi: 10.1038/nn1177. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Distributed representation of spectral and temporal information in rat primary auditory cortex. Hear Res. 1999;134:16–28. doi: 10.1016/s0378-5955(99)00061-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgard MP, Pandya PK, Vazquez J, Gehi A, Schreiner CE, Merzenich MM. Sensory input directs spatial and temporal plasticity in primary auditory cortex. J Neurophysiol. 2001;86:326–338. doi: 10.1152/jn.2001.86.1.326. [DOI] [PubMed] [Google Scholar]
- Konig P, Engel AK, Lowel S, Singer W. Squint affects synchronization of oscillatory responses in cat visual cortex. Eur J Neurosci. 1993;5:501–508. doi: 10.1111/j.1460-9568.1993.tb00516.x. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J Neurophysiol. 1997;77:24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- Maldonado PE, Gerstein GL. Neuronal assembly dynamics in the rat auditory cortex during reorganization induced by intracortical microstimulation. Exp Brain Res. 1996;112:431–441. doi: 10.1007/BF00227949. [DOI] [PubMed] [Google Scholar]
- Meister M, Lagnado L, Baylor DA. Concerted signaling by retinal ganglion cells. Science. 1995;270:1207–1210. doi: 10.1126/science.270.5239.1207. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Recanzone GH, Jenkins WM, Grajski KA. Adaptive mechanisms in cortical networks underlying cortical contributions to learning and nondeclarative memory. Cold Spring Harb Symp Quant Biol. 1990;55:873–887. doi: 10.1101/sqb.1990.055.01.082. [DOI] [PubMed] [Google Scholar]
- Metherate R, Weinberger NM. Acetylcholine produces stimulus-specific receptive field alterations in cat auditory cortex. Brain Res. 1989;480:372–377. doi: 10.1016/0006-8993(89)90210-2. [DOI] [PubMed] [Google Scholar]
- Metherate R, Cox CL, Ashe JH. Cellular bases of neocortical activation: modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. J Neurosci. 1992;12:4701–4711. doi: 10.1523/JNEUROSCI.12-12-04701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munk MH, Roelfsema PR, Konig P, Engel AK, Singer W. Role of reticular activation in the modulation of intracortical synchronization. Science. 1996;272:271–274. doi: 10.1126/science.272.5259.271. [DOI] [PubMed] [Google Scholar]
- Nelson JI, Salin PA, Munk MH, Arzi M, Bullier J. Spatial and temporal coherence in cortico-cortical connections: a cross-correlation study in areas 17 and 18 in the cat. Vis Neurosci. 1992;9:21–37. doi: 10.1017/s0952523800006349. [DOI] [PubMed] [Google Scholar]
- Neuenschwander S, Singer W. Long-range synchronization of oscillatory light responses in the cat retina and lateral geniculate nucleus. Nature. 1996;379:728–732. doi: 10.1038/379728a0. [DOI] [PubMed] [Google Scholar]
- Nichols J, Jakkamsetti V, Byrapureddy R, Roof B, Bui H, Thompson LT, Kilgard MP, Atzori M. Effect of enriched environment on synaptic transmission in the rat auditory cortex. Assoc Res Otolaryngol Abs. 2006:1215. [Google Scholar]
- Norena AJ, Eggermont JJ. Changes in spontaneous neural activity immediately after an acoustic trauma: implications for neural correlates of tinnitus. Hear Res. 2003;183:137–153. doi: 10.1016/s0378-5955(03)00225-9. [DOI] [PubMed] [Google Scholar]
- Percaccio CR, Engineer ND, Pruette AL, Pandya PK, Moucha R, Rathbun DL, Kilgard MP. Environmental enrichment increases paired pulse depression in rat auditory cortex. J Neurophys. 2005;94:3590–3600. doi: 10.1152/jn.00433.2005. [DOI] [PubMed] [Google Scholar]
- Perkel DH, Gerstein GL, Moore GP. Neuronal spike trains and stochastic point processes II Simultaneous spike trains. J Biophys. 1967;7:419–440. doi: 10.1016/S0006-3495(67)86597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley DB, Steinberg EE, Merzenich MM. Perceptual learning directs auditory cortical map reorganization through top–down influences. J Neurosci. 2006;26:4970–4982. doi: 10.1523/JNEUROSCI.3771-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan R. Plasticity of excitation and inhibition in the receptive field of primary auditory cortical neurons after limited receptor organ damage. Cereb Cortex. 2000;11:171–182. doi: 10.1093/cercor/11.2.171. [DOI] [PubMed] [Google Scholar]
- Rasmusson DD. The role of acetylcholine in cortical synaptic plasticity. Behav Brain Res. 2000;115:205–218. doi: 10.1016/s0166-4328(00)00259-x. [DOI] [PubMed] [Google Scholar]
- Rasmusson DD, Clow K, Szerb JC. Modification of neocortical acetylcholine release and electroencephalogram desynchronization due to brainstem stimulation by drugs applied to the basal forebrain. Neuroscience. 1994;60:665–677. doi: 10.1016/0306-4522(94)90495-2. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Jenkins WM, Grajski WM, Dinse HR. Topographic reorganization of the hand representation in cortical area 3b owl monkeys trained in a frequency-discrimination task. J Neurophysiol. 1992;67:1031–1056. doi: 10.1152/jn.1992.67.5.1031. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez R, Kallenbach U, Singer W, Munk MH. Short- and long-term effects of cholinergic modulation on gamma oscillations and response synchronization in the visual cortex. J Neurosci. 2004;24:10369–10378. doi: 10.1523/JNEUROSCI.1839-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfsema PR, König P, Engel AK, Sireteanu R, Singer W. Reduced Synchronization in the Visual Cortex of Cats with Strabismic Amblyopia. Eur J Neurosci. 1994;6:1645–1655. doi: 10.1111/j.1460-9568.1994.tb00556.x. [DOI] [PubMed] [Google Scholar]
- Sakurai Y. Dependence of functional synaptic connections of hippocampal and neocortical neurons on types of memory. Neurosci Lett. 1993;158:181–184. doi: 10.1016/0304-3940(93)90259-n. [DOI] [PubMed] [Google Scholar]
- Salazar RF, Kayser C, Konig P. Effects of training on neuronal activity and interactions in primary and higher visual cortices in the alert cat. J Neurosci. 2004;24:1627–1636. doi: 10.1523/JNEUROSCI.3200-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber MH. Training and synchrony in the motor system. J Neurosci. 2002;22:5277–5281. doi: 10.1523/JNEUROSCI.22-13-05277.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Changes in functional connectivity in orbitofrontal cortex and basolateral amygdala during learning and reversal training. J Neurosci. 2000;20:5179–5189. doi: 10.1523/JNEUROSCI.20-13-05179.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki S, Eggermont JJ. Changes in cat primary auditory cortex after minor-to-moderate pure-tone induced hearing loss. Hear Res. 2002;173:172–186. doi: 10.1016/s0378-5955(02)00518-x. [DOI] [PubMed] [Google Scholar]
- Seki S, Eggermont JJ. Changes in spontaneous firing rate and neural synchrony in cat primary auditory cortex after localized tone-induced hearing loss. Hear Res. 2003;180:28–38. doi: 10.1016/s0378-5955(03)00074-1. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. Noise, neural codes and cortical organization. Curr Opin Neurobiol. 1994;4:569–579. doi: 10.1016/0959-4388(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron. 1999;24:49–65. 111–125. doi: 10.1016/s0896-6273(00)80821-1. [DOI] [PubMed] [Google Scholar]
- Song S, Abbott LF. Cortical development and remapping through spike timing-dependent plasticity. Neuron. 2001;32:339–350. doi: 10.1016/s0896-6273(01)00451-2. [DOI] [PubMed] [Google Scholar]
- Steinmetz PN, Roy A, Fitzgerald PJ, Hsiao SS, Johnson KO, Niebur E. Attention modulates synchronized neuronal firing in primate somatosensory cortex. Nature. 2000;404:187–190. doi: 10.1038/35004588. [DOI] [PubMed] [Google Scholar]
- Swindale NV, Mitchell DE. Comparison of receptive field properties of neurons in area 17 of normal and bilaterally amblyopic cats. Exp Brain Res. 1994;99:399–410. doi: 10.1007/BF00228976. [DOI] [PubMed] [Google Scholar]
- Vaadia E, Haalman I, Abeles M, Bergman H, Prut Y, Slovin H, Aertsen A. Dynamics of neuronal interactions in monkey cortex in relation to behavioural events. Nature. 1995;373:515–518. doi: 10.1038/373515a0. [DOI] [PubMed] [Google Scholar]
- Valentine PA, Teskey GC, Eggermont JJ. Kindling changes burst firing, neural synchrony and tonotopic organization of cat primary auditory cortex. Cereb Cortex. 2004;14:827–839. doi: 10.1093/cercor/bhh041. [DOI] [PubMed] [Google Scholar]
- van der Togt C, Lamme VA, Spekreijse H. Functional connectivity within the visual cortex of the rat shows state changes. Eur J Neurosci. 1998;10:1490–1507. doi: 10.1046/j.1460-9568.1998.00200.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Merzenich MM, Sameshima K, Jenkins WM. Remodelling of hand representation in adult cortex determined by timing of tactile stimulation. Nature. 1995;378:71–75. doi: 10.1038/378071a0. [DOI] [PubMed] [Google Scholar]
- Wehr M, Laurent G. Odour encoding by temporal sequences of firing in oscillating neural assemblies. Nature. 1996;384:162–166. doi: 10.1038/384162a0. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. The nucleus basalis and memory codes: auditory cortical plasticity and the induction of specific, associative behavioral memory. Neurobiol Learn Mem. 2003;80:268–284. doi: 10.1016/s1074-7427(03)00072-8. [DOI] [PubMed] [Google Scholar]
- Worgotter F, Suder K, Zhao Y, Kerscher N, Eysel UT, Funke K. State-dependent receptive-field restructuring in the visual cortex. Nature. 1998;396:165–168. doi: 10.1038/24157. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Bao S, Merzenich MM. Persistent and specific influences of early acoustic environments on primary auditory cortex. Nat Neurosci. 2001;4:1123–1130. doi: 10.1038/nn745. [DOI] [PubMed] [Google Scholar]


