Abstract
Objective:
To examine the effect of exercise on overnight hypoglycemia in children with type 1 diabetes (T1D).
Study Design:
At 5 clinical sites, 50 subjects with T1D (11 to 17 years) were studied in a clinical research center on two separate days. One day included an afternoon exercise session on a treadmill. On both days, frequently sampled blood glucose levels were measured at the DirecNet Central Laboratory. Insulin doses were similar on both days.
Results:
During exercise, plasma glucose levels fell in almost all subjects; 11 (22%) developed hypoglycemia. Mean glucose from 10pm to 6am was lower on the exercise day versus the sedentary day (131 vs. 154 mg/dL, P=0.003). Hypoglycemia developed overnight more often on the exercise nights versus the sedentary nights (P=0.009), occurring only on the exercise night in 13 (26%), only on the sedentary night in 3 (6%), on both nights in 11 (22%), and on neither night in 23 (46%). Hypoglycemia was unusual on the sedentary night if the pre-bedtime snack glucose was >130 mg/dL.
Conclusion:
These findings indicate that overnight hypoglycemia post-exercise is common in children with T1D and support the importance of modifying diabetes management following afternoon exercise to reduce the risk of hypoglycemia.
Keywords: type 1 diabetes, hypoglycemia, exercise
Introduction
Since the days of Joslin and Allen, exercise has been recommended as one of the three cornerstones of management of diabetes. However, like many aspects of treatment of children with type 1 diabetes (T1DM), vigorous physical exercise presents clinicians, parents and patients with a dilemma. On one hand, regular exercise is encouraged in children in order to enhance psychosocial well being and cardiovascular health, as well as to achieve and maintain ideal body weight and body composition. On the other hand, prolonged exercise can make regulation of blood glucose levels more difficult both during and after the period of increased physical activity. These difficulties are compounded by the irregular pattern of physical activity that characterizes most youth who are not participating in organized sports or regimented training programs and by traditional methods of diabetes management that featured fixed and inflexible insulin replacement regimens.
The possibility that prolonged periods of aerobic exercise during the day may increase the risk of severe hypoglycemia during the following night is a very common concern. A number of studies in children and adults have demonstrated that the majority of severe hypoglycemic events occur at night and suggest that such events are more frequent following days of increased physical activity.1, 2 In addressing this issue, investigators in the Diabetes Control and Complications Trial showed that unusual physical activity was more frequent on days with severe hypoglycemic events than on randomly chosen days but the difference was not statistically significant.2 Only limited data are available regarding the role of daytime exercise on overnight hypoglycemia in children with T1DM3 and none of these studies examined the impact of exercise on asymptomatic, biochemical hypoglycemia during the overnight period using rigorously controlled research protocols.
The Diabetes Research in Children Network (DirecNet) is a multi-center study group whose objectives include the examination of factors that contribute to the risk or could possibly prevent hypoglycemia in children with T1DM. The present study was undertaken to examine the effect of late afternoon exercise on the frequency of overnight hypoglycemia by comparing glucose data collected on a day of exercise with a sedentary day in an inpatient clinical research center setting. To isolate the effects of exercise per se, meals were similar on both study days and basal and bolus insulin doses were based on the treatment algorithms that the subject used at home on sedentary days.
Methods
Subjects
Consent Procedures
The DirecNet Data and Safety Monitoring Board and the Institutional Review Boards at each of the DirecNet centers approved the study protocol, consent form and assent form. A parent or guardian and each subject gave written consent and assent respectively.
Eligibility Criteria and Assessment
To be eligible for the study the subject had to have 1) age between 10 and 18 years, 2) clinical diagnosis of type 1 diabetes mellitus of ≥18 months duration, 3) stable insulin regimen for at least one month prior involving either use of an insulin pump or multiple daily injections consisting of insulin glargine and insulin lispro or insulin aspart (five patients also used NPH in the morning and one both NPH and ultralente), 4) HbA1c ≤10.0% measured with the DCA®2000+ (Bayer Diagnostics, Tarrytown, NY), 5) body mass index (BMI) between the 5th and 95th percentile for age and gender,4 6) body weight ≥36.0 kg, 7) a normal hematocrit, and 8) normal thyroid function. Subjects were not eligible if they 1) had asthma that was medically treated in the prior year, 2) were currently using glucocorticoids or beta blockers, 3) had used pseudoephedrine within 48 hours, 4) had experienced severe hypoglycemia (seizure or loss of consciousness) within prior 2 weeks, 5) had an active infection, 6) anticipated a significant change in exercise regimen between admissions, or 7) had another medical condition or were using a medication that in the judgment of the investigator could affect completion of the exercise protocol.
Study Procedures
The study consisted of two inpatient stays in the clinical research centers at each of the DirecNet sites lasting about 24 hours each and separated by one to four weeks: one with a 75-minute exercise session in the late afternoon (“exercise day”) and one without the exercise (“sedentary day”). The order of the exercise and sedentary days was determined at random.
Prior to the first admission, the subject's daily meal plan and insulin algorithms used at home were recorded. Meals and bedtime snacks of similar caloric and carbohydrate content were consumed on both hospital days. Insulin management on both the exercise day and sedentary day were as similar as possible and followed the same routine that the subject would follow at home on a day without exercise. If the subject used an insulin algorithm with the bedtime snack (or no snack) on a sedentary day at home, this same pattern was followed on both exercise and sedentary days in the hospital. Subjects using insulin injections were instructed to give the injection in a site other than the legs on the study days. For subjects using an insulin infusion pump, the basal rate used at home in the afternoon was continued uninterrupted during the exercise session.
On both the sedentary and exercise days, the subject was admitted to the clinical research center prior to lunch. An intravenous catheter was inserted in an antecubital vein for blood sample collection. On both days, a standardized protocol was followed for checking the blood glucose at 2 p.m. and 3 p.m. and either short-acting insulin or oral carbohydrate was given if indicated, in order to titrate the 4 p.m. blood glucose prior to exercise between 100 mg/dL and 200 mg/dL.
Exercise Procedures
On the morning of the exercise day, the subjects walked on a motorized treadmill for 5 to 15 minutes to determine the settings needed to achieve a heart rate of 140 beats/minute. This was estimated to be equivalent to the heart rate achieved at 55% maximum effort (VO2max).5 These treadmill settings were used for the start of the exercise session which commenced at about 4 p.m.
The exercise session consisted of 15 minutes walking on a treadmill at a heart rate of approximately 140 beats/minute followed by a 5-minute rest period. This cycle was repeated 3 more times for a total of four 15-minute exercise periods with 5-minute rest periods in between (75 minutes total). A heart rate monitor was worn throughout the time of exercise.
Blood glucose measurements were made from venous blood both for a central laboratory sample as well as home glucose meter measurement (see below) prior to starting the exercise, during each of the 3 rest periods, immediately following the exercise session, and at 15 minute intervals for one hour following the completion of the exercise. If during exercise the blood glucose dropped to <60 mg/dL, the subject was given 15-30g of carbohydrate and after 5 to 15 minutes, the blood glucose was rechecked. Exercise did not resume until the blood glucose was >70 mg/dL.
Evening and Overnight Procedures
Dinner was consumed at about 6:15 p.m. After dinner, the blood glucose was checked at 7:00 p.m., 8:00 p.m., and 9:00 p.m. A bedtime snack was given at approximately 9:30 p.m. if the subject would normally receive one as part of a sedentary day treatment regimen.
Subjects were asked to go to sleep at approximately 10:00 p.m. and were awakened at approximately 7:00 a.m. Blood glucose measurements were made using samples from the intravenous catheter every half-hour from 10:00 p.m. through 6:00 a.m. (see below).
If at any time the blood glucose was less than 60 mg/dL, the subject was given 15-30g of carbohydrates and the blood glucose was rechecked in 15 minutes. If the blood glucose value was still <60 mg/dL after 15 minutes, another 15-30g of carbohydrates were administered, a procedure repeated at 15 minute intervals until the blood glucose value was >70 mg/dL.
Glucose Determinations
Glucose measurements were made with the One-Touch® Ultra® Meter (“Ultra”; Lifescan, Milpitas, CA) at all sampling times (as described earlier). We have previously demonstrated the accuracy of this meter.6 In addition, blood samples for central laboratory determination of serum glucose levels were obtained from the intravenous catheter during the exercise session and hourly from 10 p.m. to 6 a.m. and at other times if the Ultra glucose value was <60 mg/dL. Glucose determinations were made at the DirecNet Central Biochemistry Laboratory at the University of Minnesota using a hexokinase enzymatic method.7, 8
Statistical Methods
Overnight hypoglycemia was considered to have occurred when a central laboratory glucose level was ≤60 mg/dL between 10 p.m. and 6 a.m. For purposes of analysis, unless otherwise stated, the definition of hypoglycemia also included cases in which hypoglycemia treatment was given based on an Ultra meter glucose value but a confirmatory central lab glucose value ≤60 mg/dL was not present. Analyses using only central lab confirmed hypoglycemia cases are indicated as such.
The proportions of subjects developing hypoglycemia overnight on the exercise and sedentary nights were compared using generalized estimating equations (GEE) controlling for a possible period (1st vs. 2nd visit) effect and repeated measures from the same subject.
A hypoglycemia index was calculated for each subject in order to characterize the cumulative magnitude of hypoglycemia during the period from 10 p.m. to 6 a.m. during each admission. At each of the 17 nightly 30-min measurement points during that interval (9 reference glucose values on the hour and 8 Ultra values on the half-hour), the difference between 70 mg/dL and any obtained glucose values <70 was calculated. Glucose values ≥70 mg/dL contributed a score of ‘0’ to the index. The mean of the 17 difference scores was computed for each subject for each CRC admission. For cases where the subject was treated for hypoglycemia, the most recent prior glucose measurement was carried forward one hour in the calculation of the hypoglycemia index. Because this hypoglycemia index had a skewed distribution, a permutation test was used to compare exercise vs. sedentary results.
Mean overnight glucose values were compared using repeated measures regression. The association of hypoglycemia with the self-reported number of days with at least one hour of physical activity during a typical week (used as a surrogate measure of fitness) was evaluated separately for the exercise and sedentary visits using logistic regression and for the exercise and sedentary visits combined using the GEE regression model described above (by adding fitness as an independent variable). Associations of overnight hypoglycemia with hypoglycemia during exercise (binary) and the 9 p.m. glucose (continuous) were analyzed in a similar manner. An adjusted R2 value was calculated using the generalized coefficient of determination.9 Multivariate analysis for overnight hypoglycemia included a term for exercise vs. sedentary visits and used a stepwise procedure to select among the following factors: gender, age, HbA1c level, insulin route (pump versus multiple daily injections), total daily insulin dose, average bolus dose (carbohydrate to insulin ratio) used in daily insulin regimen, body mass index, and self-reported frequency of at home exercise. The sample size was estimated to be 50 subjects to have 90% power with an alpha level of 5% to detect a 3-fold difference in the hypoglycemia index (see definition above) comparing the exercise and sedentary nights and to have approximately 80% power for a comparison of the incidences of hypoglycemia.
Results
Fifty subjects participated in the study between June 2004 and November 2004. Their average age was 14.8 ± 1.7 years; 44% were female; 90% were Caucasian, 4% African-American, 2% Hispanic, and 4% Asian. The mean duration of diabetes was 7.0 ± 3.7 years. An insulin pump was used by 54% and multiple daily injections by the other 46%. Mean HbA1c was 7.8 ± 0.8%. A severe episode of hypoglycemia (resulting in seizure or loss of consciousness) in the 6 months prior to the study was reported by 3 subjects (6%). Half of the subjects completed the exercise admission first and the other half the sedentary admission first. The median time between the two admissions was 14 days (interquartile range 7 to 16 days, range 6 to 39 days).
Exercise Session
The full exercise session was completed by 46 (92%) of the 50 subjects. Three subjects completed the first three cycles and part of a fourth and the remaining subject completed two cycles fully and two partially. One subject did not reach the target heart rate for one of the four exercise cycles and the other 49 subjects achieved target for all four cycles. Forty-one (82%) of the subjects had a decrease in their pre-exercise glucose level of at least 25% and 11 (22%) of the subjects became hypoglycemic (blood glucose ≤60 mg/dL) either during or immediately following the exercise session. An additional 6 subjects were treated for hypoglycemia due to a falling glucose level on the Ultra meter but did not have a confirmatory central lab glucose value below 60 mg/dL.
Post-exercise Glucose Levels
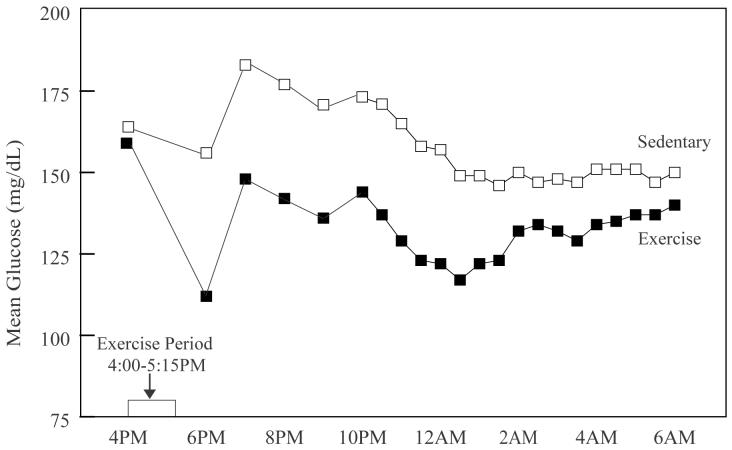
The mean glucose level was similar on the exercise and sedentary days at 4 p.m. (prior to the start of the exercise). Following the completion of the exercise and throughout the night the glucose levels were significantly lower on the exercise day than on the sedentary day (Figure 1). During the overnight period (10 p.m. to 6 a.m.) plasma glucose averaged 131 ± 58 mg/dL on the exercise nights and 154 ± 69 mg/dL on the sedentary nights (P=0.003).
Figure 1.
Mean Glucose Levels on the Exercise and Sedentary Days.
Between 10 p.m. and 6 a.m., a glucose level ≤60 mg/dL was confirmed by the central lab for 21 (42%) of the 50 exercise nights and 8 (16%) of the sedentary nights. On an additional 3 exercise nights and 6 sedentary nights, treatment was given for hypoglycemia based on an Ultra meter glucose value, but a blood sample was either not sent to the central lab (N=5, protocol violation) or the central lab value was >60 mg/dL (N=4).
As shown in Table 1, hypoglycemia (including central lab confirmed and unconfirmed cases) occurred more often on the exercise night than on the sedentary night (P=0.009 overall and P<0.001 in an analysis limited to confirmed cases). Hypoglycemia occurred only on the exercise night in 13 subjects (26%), only on the sedentary night in 3 subjects (6%), on both nights in 11 (22%), and on neither night in 23 (46%). The hypoglycemia index was greater on the exercise nights than on the sedentary nights (mean = 2.3 ± 3.0 mg/dL versus 1.5 ± 3.2 mg/dL, P=0.07 overall and 2.1 ± 2.9 versus 1.1 ± 2.7, P=0.01 in an analysis limited to central lab glucose values).
The overall result of a higher incidence of hypoglycemia on the exercise nights than on the sedentary nights was consistent in subgroups based on gender, age, HbA1c level, insulin route (pump versus multiple daily injections), total daily insulin dose, average bolus dose (carbohydrate to insulin ratio) used in daily insulin regimen, body mass index, and frequency of exercise performed at home. Only the latter factor appeared to influence the risk of nocturnal hypoglycemia). As shown in Table 2, subjects who exercised more frequently at home were at greater risk of nocturnal hypoglycemia on both the exercise night (P=0.02) and the sedentary night (P=0.05). On the exercise day, 11 (65%) of the 17 subjects who became hypoglycemic or were treated for low glucose during the exercise also developed hypoglycemia overnight compared with 13 (39%) of the 33 subjects who did not become hypoglycemic during the exercise (P=0.14).
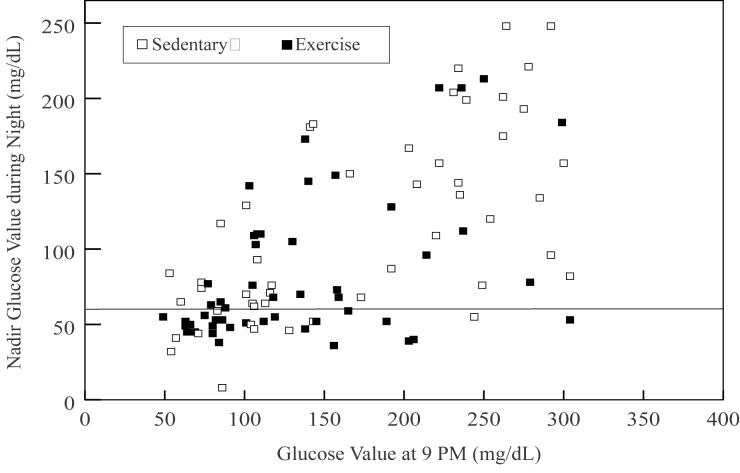
The pre-snack blood glucose (9 p.m.) was predictive of overnight hypoglycemia on both the sedentary day (P=0.002, R2=0.39) and on the exercise day (P=0.04 R2=0.12). On the sedentary day, overnight hypoglycemia was unusual if the pre-snack glucose was >130 mg/dL whereas on the exercise day, hypoglycemia occurred fairly frequently even when the glucose was above this level (Figure 2). On the sedentary day, overnight hypoglycemia developed in 12 (55%) of 22 subjects whose pre-snack glucose was ≤130 mg/dL and in only 2 (7%) of 28 subjects whose pre-snack glucose was >130 mg/dL (P=0.001). On the exercise day, overnight hypoglycemia developed in 16 (57%) of 28 subjects whose pre-snack glucose was ≤130 mg/dL and in 8 (36%) of 22 subjects whose pre-snack glucose was >130 mg/dL (P=0.15). Multivariate analysis using stepwise logistic regression (with P<0.20 as the inclusion criterion) identified only the 9 p.m. glucose value (P=0.003) and self-reported days per week of exercise (P=0.06) for inclusion in the model.
Figure 2.
Relationship between Pre-bedtime (9pm) Glucose Level and Overnight Glucose Nadir
Discussion
We designed the present study to more carefully define the effect of afternoon exercise on the relative risk of hypoglycemia during the following night in a cohort of 50 children with T1DM, who were using an intensive diabetes management regimen involving either insulin pumps or multiple daily insulin injections. A carefully controlled, cross-over design that involved a supervised and standardized exercise protocol was utilized to compare the frequency of overnight hypoglycemia following afternoon exercise with that following a sedentary day in a clinical research center setting. We specifically chose to have the subjects exercise in the late afternoon as children and adolescents often are more active at the end of the school day, when different athletic practice and game sessions take place. In addition, the duration and intensity of the exercise regimen was designed to mimic a typical length of time children are involved in such activities.
It is noteworthy that nocturnal hypoglycemia was very common in our subjects even on the days when they did not exercise. In 28 percent of the youngsters, hypoglycemia developed during the night of their sedentary day even though only 3 of the subjects had experienced a severe hypoglycemic event at home during the 6 months prior to the study. This may relate to the adverse effects of deep sleep on protective, counter-regulatory hormone responses to hypoglycemia.10, 11 In adults with T1DM reduced awakening from sleep during hypoglycemia has been observed in comparison to non-diabetic individuals.12 An even more disturbing finding was that nearly twice as many of our children had a hypoglycemic event on the night following exercise than on the night following a sedentary day. Further analysis of the differences between the exercise and sedentary days and nights demonstrated that they were consistent across subgroups, with similar results for older vs. younger subjects, those with higher vs. lower BMI, and those on insulin pumps vs. using multiple daily injections. The level of glycemic control, as measured by the HbA1c level also made no difference on the relative risk for overnight hypoglycemia following exercise. These findings extend those of MacDonald et al., who reported in a prospective though uncontrolled outpatient case series that 48 (16%) of 300 patients with T1DM (4 to 24 years old) self-reported at least one episode of moderate or severe hypoglycemia occurring 4 or more hours after exercise during a two-year period.3 Although there are a number of studies that have evaluated the incidence of hypoglycemia during or immediately following exercise,13-17 we are not aware of a prior study that has systematically evaluated the occurrence of overnight hypoglycemia following exercise in children or in adults.
The glucose level prior to the bedtime snack was a predictor of overnight hypoglycemia on both the sedentary and exercise days, although more so on the sedentary day. On the sedentary day, overnight hypoglycemia was unusual (only 7% of subjects) if the pre-snack glucose was greater than 130 mg/dL, and common (55%) when ≤130 mg/dL at bedtime. However, on the exercise day, hypoglycemia occurred fairly frequently whether the pre-bedtime snack glucose was ≤130 mg/dL (57%) or greater than 130 mg/dL (36%). These data underscore the usefulness of having somewhat higher target glucose levels at bedtime in children with T1DM, particularly on days with intense exercise.
The study procedures specified the use of similar insulin doses on both the exercise and sedentary day. Specifically, the subject's usual routine for a sedentary day was followed on the exercise day even if the subject typically would have lowered his or her overnight basal insulin replacement on days of unusually intense physical activity. This approach allowed us to examine the effect of exercise per se on the risk of nocturnal hypoglycemia and it is clinically relevant, since many youngsters on pumps or who receive pre-breakfast doses of glargine insulin do not or cannot adjust their overnight basal insulin.
Although not directly tested in this study, reports indicate that a single bout of exercise can increase glucose transport into skeletal muscle tissue for at least 16 hours post exercise in non-diabetic and diabetic subjects.18 The molecular signaling mechanisms that lead to increased glucose transport following exercise have not been completely elucidated but appear to involve both insulin-dependent and insulin-independent pathways.19 Thus, the greater frequency and magnitude of hypoglycemia observed in our subjects on the nights following exercise are likely dependent not only on the insulin administered overnight but also on increased insulin-independent glucose transport activity triggered by intense muscle contractions many hours prior.
The design of this study did not allow determination of how the insulin sensitivity was changed, whether due to changed pharmacokinetics or pharmacodynamics. Chronic exercise training in humans results in numerous beneficial effects in skeletal muscle, including an increase in the insulin-sensitive glucose transporter 4 (GLUT4) expression, the rate-limiting step in glucose metabolism in skeletal tissue. The increase in muscle GLUT4 in trained individuals, in turn, contributes to an increase in the responsiveness of muscle to insulin-stimulated glucose uptake.20 Increases in overall insulin sensitivity may be one explanation why the subjects who exercised more frequently at home were at greater risk for overnight hypoglycemia following both the sedentary and exercise days in this study.
The findings of this study support the well-recognized clinical observation that exercise has benefit in lowering plasma glucose levels both during and following exercise in children with T1DM. Hyperglycemia was more common during the sedentary night, and lower glucose levels were sustained for many hours following exercise on the exercise day compared with the sedentary day. Our findings also support the use of flexible diabetes management regimens that attempt to adjust food intake and insulin dosing on evenings following exercise to reduce the risk of overnight hypoglycemia. The currently FDA-approved continuous glucose sensing systems are not practical for day-to-day use in children and lack sufficient accuracy in the low glucose range to serve as effective guides to overnight glucose control.21-26 Consequently, frequent meter glucose testing at bedtime and in the middle of the night remains the only currently effective means to adjust treatment regimens to minimize the risk of exercise-induced, nocturnal hypoglycemia. Our data indicate that such monitoring may be especially needed in youth who are about to start or are currently participating in regular exercise, such as a high school sports program. Further carefully controlled studies are needed to systematically examine the most effective methods of adjusting insulin doses and meal composition and quantity in order to maximize the benefits and safety of exercise in children with T1DM.
Acknowledgement
Appreciation is expressed for the work performed by the CRC Nurses at the five clinical centers.
This research has been supported by the following NIH/NICHD Grants: HD041919-01; HD041915-01; HD041890; HD041918-01; HD041908-01; and HD041906-01 and by Nemours Research Programs. Clinical Centers also received funding through the following GCRC Grant Numbers M01 RR00069; RR00059; RR 06022 and RR00070-41. LifeScan, Milpitas, CA, provided the One Touch® Ultra® Blood Glucose Monitoring Systems and test strips.
Appendix
Writing Committee
Eva Tsalikian, MD; Nelly Mauras, MD; Roy W. Beck, MD, PhD; William V. Tamborlane, MD; Kathleen F. Janz, EdD; H. Peter Chase, MD; Tim Wysocki, PhD, ABPP; Stuart A. Weinzimer, MD; Bruce A. Buckingham, MD; Craig Kollman, PhD; Dongyuan Xing, MPH; Katrina J. Ruedy, MSPH
The DirecNet Study Group
Clinical Centers (Listed in alphabetical order with clinical center name, city, and state. Personnel are listed as (PI) for Principal Investigator, (I) for co-Investigator and (C) for Coordinators.) (1) Barbara Davis Center for Childhood Diabetes, University of Colorado, Denver, CO: H. Peter Chase, MD (PI); Rosanna Fiallo-Scharer, MD (I); Jennifer H. Fisher, ND, RN (C); Barbara Tallant, RN, MA (C); (2) Department of Pediatrics, University of Iowa Carver College of Medicine, Iowa City, IA: Eva Tsalikian, MD (PI); Michael J. Tansey, MD (I); Linda F. Larson, RN (C); Julie Coffey, MSN (C); (3) Nemours Children's Clinic, Jacksonville, FL: Tim Wysocki, PhD, ABPP (PI); Nelly Mauras, MD (I); Larry A. Fox, MD (I); Keisha Bird, MSN (C); Kelly L. Lofton, RN (C); (4) Division of Pediatric Endocrinology and Diabetes, Stanford University, Stanford, CA: Bruce A. Buckingham, MD (PI); Darrell M. Wilson, MD (I); Jennifer M. Block, RN, CDE (C); Paula Clinton, RD, CDE (C); (5) Department of Pediatrics, Yale University School of Medicine, New Haven, CT: Stuart A. Weinzimer, MD (PI); William V. Tamborlane, MD (I); Elizabeth A. Doyle, MSN (C); Kristin Sikes, MSN (C); Coordinating Center: Jaeb Center for Health Research, Tampa, FL: Roy W. Beck, MD, PhD; Katrina J. Ruedy, MSPH; Craig Kollman, PhD; Dongyuan Xing, MPH; Andrea Kalajian, MS; Cynthia R. Silvester; University of Minnesota Central Laboratory: Michael W. Steffes, MD, PhD; Jean M. Bucksa, CLS; Maren L. Nowicki, CLS; Carol A. Van Hale, CLS; Vicky Makky, CLS; National Institutes of Health: Gilman D. Grave, MD; Barbara Linder MD, PhD; Karen K. Winer, MD; Data and Safety Monitoring Board: Dorothy M. Becker, MBBCh; Christopher Cox, PhD; Christopher M. Ryan, PhD; Neil H. White, MD, CDE; Perrin C. White, MD
References
- 1.Davis EA, Keating B, Byrne GC, Russell M, Jones TW. Hypoglycemia: incidence and clinical predictors in a large population-based sample of children and adolescents with IDDM. Diabetes Care. 1997;20:22–5. doi: 10.2337/diacare.20.1.22. [DOI] [PubMed] [Google Scholar]
- 2.The DCCT Research Group Epidemiology of severe hypoglycemia in the diabetes control and complications trial. Am J Med. 1991;90:450–9. [PubMed] [Google Scholar]
- 3.MacDonald MJ. Postexecise Late-Onset Hypoglycemia in Insulin-Dependent Diabetic Patients. Diabetes Care. 1987;10:584–8. doi: 10.2337/diacare.10.5.584. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey BMI-for-age charts, 2 to 20 years, LMS parameters and selected smoothed BMI percentiles, by sex and age, 2000. Available from: http://www.cdc.gov/nchs/about/major/nhanes/growthcharts/datafiles.htm. Accessed February 2003.
- 5.Rowland TW. Developmental Exercise Physiology. Human Kinetics Publishers; Champaign, IL: 1996. Exercise Testing; pp. 27–47. [Google Scholar]
- 6.Diabetes Research in Children Network (DirecNet) Study Group A multicenter study of the accuracy of the OneTouch Ultra home glucose meter in children with type 1 diabetes. Diabetes Technol Ther. 2003;5:933–41. doi: 10.1089/152091503322640971. [DOI] [PubMed] [Google Scholar]
- 7.Neese JW, Duncan P, Bayse D, Robinson M, Cooper T, Stewart C. Development and evaluation of a hexokinase/glucose-6-phosphate dehydrogenase procedure for use as a national glucose reference method. Center for Disease Control; Atlanta: 1976. (HEW No. (CDC) 77-8330). [Google Scholar]
- 8.Passey RB, Gillum RL, Fuller JB, Urry FM, Giles ML. Evaluation and comparison of 10 glucose methods and the reference method recommended in the proposed product class standard (1974) Clin Chem. 1977;23:131–9. [PubMed] [Google Scholar]
- 9.Nagelkerke NJD. A note on a general definition of the coefficient of determination. Biometrika. 1991;78:691–2. [Google Scholar]
- 10.Jones TW, Porter P, Sherwin RS, et al. Decreased epinephrine responses to hypoglycemia during sleep. N Eng J Med. 1998;338:1657–62. doi: 10.1056/NEJM199806043382303. [DOI] [PubMed] [Google Scholar]
- 11.Cryer PE. Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Eng J Med. 2004;350:2272–9. doi: 10.1056/NEJMra031354. [DOI] [PubMed] [Google Scholar]
- 12.Banarer S, Cryer PE. Sleep-related hypoglycemia-associated autonomic failure in type 1 diabetes. Diabetes. 2003;52:1195–1203. doi: 10.2337/diabetes.52.5.1195. [DOI] [PubMed] [Google Scholar]
- 13.Stratton R, Wilson DP, Endres RK. Acute glycemic effects of exercise in adolescents with insulin-dependent diabetes mellitus. Phys Sportsmed. 1988;16:150–7. doi: 10.1080/00913847.1988.11709460. [DOI] [PubMed] [Google Scholar]
- 14.Riddell MC, Bar-Or O, Ayub BV, Calvert RE, Heigenhauser GJF. Glucose ingestion matched with total carbohydrate utilization attenuates hypoglycemia during exercise in adolescents with IDDM. Int J Sport Nutr. 1999;9:24–34. doi: 10.1123/ijsn.9.1.24. [DOI] [PubMed] [Google Scholar]
- 15.Riddell MC, Bar-Or O, Hollidge-Horvat M, Schwarcz HP, Heigenhauser GJF. Glucose ingestion and substrate utilization during exercise in boys with IDDM. J Appl Physiol. 2000;88:1239–46. doi: 10.1152/jappl.2000.88.4.1239. [DOI] [PubMed] [Google Scholar]
- 16.Biankin SA, Jenkins AB, Campbell LV, Choi KL, Forrest QG, Chisholm DJ. Target-seeking behavior of plasma glucose with exercise in type 1 diabetics. Diabetes Care. 2003;26:297–301. doi: 10.2337/diacare.26.2.297. [DOI] [PubMed] [Google Scholar]
- 17.Guelfi KJ, Jones TW, Fournier PA. Intermittent high-intensity exercise does not increase the risk of early postexercise hypoglycemia in individuals with type 1 diabetes. Diabetes Care. 2005;28:416–8. doi: 10.2337/diacare.28.2.416. [DOI] [PubMed] [Google Scholar]
- 18.Borghouts LB, Keizer HA. Exercise and insulin sensitivity: a review. Int J Sports Med. 2000;21:1–12. doi: 10.1055/s-2000-8847. [DOI] [PubMed] [Google Scholar]
- 19.Koistinen HA, Zierath JR. Regulation of glucose transport in human skeletal muscle. Ann Med. 2002;34:410–8. doi: 10.1080/078538902321012351. [DOI] [PubMed] [Google Scholar]
- 20.Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity. Annu Rev Med. 1998;49:235–61. doi: 10.1146/annurev.med.49.1.235. [DOI] [PubMed] [Google Scholar]
- 21.Diabetes Research in Children Network (DirecNet) Study Group A randomized multicenter trial comparing the GlucoWatch Biographer with standard glucose monitoring in children with type 1 diabetes. Diabetes Care. doi: 10.2337/diacare.28.5.1101. In Press. [DOI] [PubMed] [Google Scholar]
- 22.Diabetes Research in Children Network (DirecNet) Study Group The accuracy of the CGMS™ in children with type 1 diabetes: results of the Diabetes Research in Children Network (DirecNet) accuracy study. Diabetes Technol Ther. 2003;5:781–9. doi: 10.1089/152091503322526987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diabetes Research in Children Network (DirecNet) Study Group The accuracy of the GlucoWatch® G2™ Biographer in children with type 1 diabetes: results of the Diabetes Research in Children Network (DirecNet) accuracy study. Diabetes Technol Ther. 2003;5:791–800. doi: 10.1089/152091503322526996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diabetes Research in Children Network (DirecNet) Study Group Accuracy of the GlucoWatch G2 Biographer and the Continuous Glucose Monitoring System during hypoglycemia: experience of the Diabetes Research in Children Network. Diabetes Care. 2004;27:722–6. doi: 10.2337/diacare.27.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGowan K, Thomas W, Moran A. Spurious reporting of nocturnal hypoglycemia by CGMS in patients with tightly controlled type 1 diabetes. Diabetes Care. 2002;25:1499–1503. doi: 10.2337/diacare.25.9.1499. [DOI] [PubMed] [Google Scholar]
- 26.Metzger M, Leibowitz G, Wainstein J, Glaser B, Raz I. Reproducibility of glucose measurements using the glucose sensor. Diabetes Care. 2002;25:1185–91. doi: 10.2337/diacare.25.7.1185. [DOI] [PubMed] [Google Scholar]