Abstract
Background
Y-chromosomal haplogroup (Y-HG) Q is suggested to originate in Asia and represent recent founder paternal Native American radiation into the Americas. This group is delineated into Q1, Q2 and Q3 subgroups defined by biallelic markers M120, M25/M143 and M3, respectively. Recently, a novel subgroup Q4 has been identified which is defined by bi-allelic marker M346, representing HG Q (0.41%, 3/728) in Indian population. With scanty details of HG Q in Asia, especially India, it was pertinent to explore the status of the Y-HG Q in Indian population to gather an insight to determine the extent of diversity within this region.
Results
We observed 15/630 (2.38%) Y-HG Q individuals in India with an ancestral state at M120, M25, M3 and M346 markers, indicating an absence of already known Q1, Q2, Q3 and Q4 sub-haplogroups. Interestingly, we further observed a novel 4 bp deletion/insertion polymorphism (ss4 bp, rs41352448) at 72,314 position of human arylsulfatase D pseudogene, defining a novel sub-lineage Q5 (in 5/15 individuals, i.e., 33.3 % of the observed Y-HG Q) with distributions independent of the social, cultural, linguistic and geographical affiliations in India.
Conclusion
The study adds another sublineage Q5 in the already existing arrangement of Y-HG Q in literature. It was quite interesting to observe an ancestral state Q* and a novel sub-branch Q5, not reported elsewhere, in Indian subcontinent, though in low frequency. A novel subgroup Q4 was identified recently which is also restricted to Indian subcontinent. The most plausible explanation for these observations could be an ancestral migration of individuals bearing ancestral lineage Q* to Indian subcontinent followed by an autochthonous differentiation to Q4 and Q5 sublineages later on. However, other explanations of, either the presence of both the sub haplogroups (Q4 and Q5) in ancestral migrants or recent migrations from central Asia, cannot be ruled out till the distribution and diversity of these subgroups is explored extensively in Central Asia and other regions.
Background
In the past, markers on the non-recombining region of the Y chromosome (NRY) have been used extensively as a male complement to mtDNA to study the colonization or migration histories within the different regions of the world. Y-chromosomal haplogroup (Y-HG) Q, defined by either of the binary markers P36/MEH2 [1] or M242 [2], has been suggested to originate in Asia and represent recent founder paternal Native American radiation into the Americas. In Eurasia, haplogroup Q chromosomes have been reported with the highest frequency in Siberian populations, distributed primarily across Northwest and Northeast Siberia with the vast majority in only two Siberian populations, the Kets (93.8%) and the Selkups (66.4%) [3]. Other population groups from the region bearing this haplogroup include, Kyrgyz (2%), Kazak (6%), Kallar (1%), Shiraz (8%), Bartangi (13%), Korean (2%), Yagnobi (3%), Esfahan (6%), Turkmen (10%), Dungan (8%), Tuvinian (17%), Uzbek/Kashkadarya (5%), Shugnan (11%), Uzbek/Bukhara (2%), Uzbek/Surkhandarya (4%), Yadhava (3%), Kazan Tatar (3%), Tajik/Samarkand (5%), Uighur (5%), Uzbek/Khorezm (9%), Uzbek/Tashkent (14%), Arab/Bukhara (14%), Uzbek/Fergana Valley (5%) and Uzbek/Samarkand 7%) [2]. Interestingly, Y-HG Q is the youngest paternal haplogroup observed with less frequent subgroups which are geographically restricted. These have been delineated into Q1, Q2 and Q3 subgroups defined by biallelic markers M120, M25/M143 and M3, respectively[1]. Recently, a novel subgroup Q4 was identified, defined by the bi-allelic marker M346, representing HG Q (0.41%, 3/728) in Indian populations [4].
With very less details of HG Q in Asia, especially India, it was pertinent to explore the status of the Y-HG Q in Indian populations to gather an insight to determine the extent of diversity within this region.
Results and Discussion
In the present study, we screened 630 samples belonging to different regions of India and observed 2.38% (15/630) individuals bearing Y-HG Q. It was interesting to observe that 14/15 samples did not show any of the already known Y-HG Q sub-haplogroups (Q1, Q2, Q3 and Q4), defined by biallelic markers M120, M25/M143, M3 and M346, respectively (Table 1). Only one individual was observed with the presence of M120 polymorphism, representing Q1 lineage.
Table 1.
Indian populations screened for Y-HG Q and its distribution.
| India Regions | Social Category | Linguistic Category | No. of Samples | No. of Samples in Haplogroup | |||
| North: | Q (xQ5) | Q5 | |||||
| J&K Kashmiri Pandits | Caste | high | Indo-European | 51 | * | 3 | |
| J&K kashmir Gujars | Tribe | Indo-European | 61 | * | 1Q1 | ||
| Dalits+ | Caste | low | Indo-European | 18 | # | ||
| Muslim | Religious | group | Indo-European | 19 | # | ||
| Rajput | Caste | high | Indo-European | 29 | # | ||
| Uttar Pradesh Brahmin | Caste | high | Indo-European | 14 | # | 1@ | |
| Uttar Pradesh Brahmin | Caste | high | Indo-European | 31 | * | 1 | 1 |
| Uttar Pradesh Mixed | Tribe | Austro-Asiatic | 9 | * | |||
| Punjab Brahmin | Caste | high | Indo-European | 28 | * | ||
| Himachal Brahmin | Caste | high | Indo-European | 19 | * | ||
| Himachal Rajputs | Caste | high | Indo-European | 35 | * | 1 | |
| Central: | |||||||
| Uttar Pradesh Kols | Tribe | Indo-European/Austro-Asiatic | 30 | * | |||
| Uttar Pradesh gonds | Tribe | Indo-European/Dravidian | 38 | * | |||
| Madhya Pradesh Brahmins | Caste | high | Indo-European | 42 | * | 1 | 1 |
| Madhya Pradesh Gonds | Tribe | Dravidian | 17 | * | 1 | ||
| Madhya Pradesh Saharia | Tribe | Indo-European | 89 | * | 1 | 2 | |
| Halba | Tribe | Indo-European | 21 | # | 1@ | ||
| Kamar | Tribe | Dravidian | 30 | # | |||
| Muria | Tribe | Dravidian | 20 | # | |||
| East: | |||||||
| Bihar Brahmins | Caste | high | Indo-European | 38 | * | 1 | 1 |
| Paswan | Caste | low | Indo-European | 29 | * | ||
| Agharia | Caste | middle | Indo-European | 10 | # | ||
| Bagdi | Caste | low | Indo-European | 11 | # | ||
| Gaud | Caste | middle | Indo-European | 5 | # | ||
| Ho | Tribe | Austro-Asiatic | 30 | # | |||
| Lodha | Tribe | Austro-Asiatic | 20 | # | |||
| Mahishya | Caste | middle | Indo-European | 13 | # | ||
| Santal | Tribe | Austro-Asiatic | 14 | # | |||
| Tanti | Caste | low | Indo-European | 7 | # | ||
| West Bengal Brahmin | Caste | high | Indo-European | 18 | # | ||
| Northeast: | |||||||
| Chakma | Tribe | Tibeto-Burman | 4 | # | |||
| Jamatia | Tribe | Tibeto-Burman | 30 | # | |||
| Mog | Tribe | Tibeto-Burman | 5 | # | |||
| Mizo | Tribe | Tibeto-Burman | 27 | # | |||
| Tripuri | Tribe | Tibeto-Burman | 21 | # | |||
| West: | |||||||
| Maharashtra Brahmins | Caste | high | Indo-European | 30 | * | ||
| Gujarat Bhils | Tribe | Indo-European | 12 | * | |||
| Gujarat Brahmins | Caste | high | Indo-European | 63 | * | ||
| Sourashtran | Indo-European | 46 | $ | ||||
| Koknasth Brahmin | Caste | high | Indo-European | 25 | # | ||
| Maratha | Caste | middle | Indo-European | 20 | # | ||
| Nav Buddha | Caste | low | Indo-European | 14 | # | ||
| South: | |||||||
| Andhra Brahmins | Caste | high | Dravidian | 6 | * | ||
| Kallar | Caste | Dravidian | 84 | $ | |||
| Yadhava | Caste | Dravidian | 129 | $ | 3 | ||
| Ambalakarar | Caste | middle | Dravidian | 29 | # | ||
| Irula | Tribe | Dravidian | 30 | # | |||
| Iyengar | Caste | high | Dravidian | 30 | # | ||
| Iyer | Caste | high | Dravidian | 29 | # | ||
| Koya | Tribe | Tribe | Dravidian | 27 | # | ||
| Kota | Tribe | Dravidian | 16 | # | |||
| Konda | Tribe | Tribe | Dravidian | 30 | # | ||
| Kurumba | Tribe | Dravidian | 19 | # | |||
| Pallan | Caste | low | Dravidian | 29 | # | ||
| Toda | Tribe | Dravidian | 8 | # | |||
| Vanniyar | Caste | middle | Dravidian | 25 | # | ||
| Vellalar | Caste | middle | Dravidian | 31 | # | 1@ | |
| Total | 1615 | 16 | 5 | ||||
* Present Study, # Sengupta et al. 2006, $ Seielstad et al. 2003.
'Dalits+' used instead of 'Chamar', as per Indian Constitution.
@found as 'Q4' by Sengupta et al.4
Q1Found as Q1 in present study.
Further, a novel 4 bp del/ins polymorphism (rs41352448, details provided in the Additional file 1) at 72,314 position of human arylsulfatase D pseudogene (ARSDP gene), in 5/15 individuals (33.3% of the observed Y-HG Q in the study) indicated the presence of a novel sub-group Q5 of Y-HG Q. In order to establish the exclusiveness of this polymorphism to Y-HG Q, we screened some of our samples already categorized in other unrelated haplogroups like R1a1, R2, L, H1, J, C, etc. The presence of an ancestral allele (without insertion at 72,314 position of ARSDP gene) in these samples confirmed the restriction of this novel polymorphism within the Y-HG Q. We also screened the sample with a derived state at M120 marker in the present study (representing Q1), using ss4 bp marker and found an absence of the polymorphism. In order to assign an independent status to designated Q5 and to confirm the placement of M346 derived samples as Q4 [4], it was necessary to study the novel ss4 bp marker (Q5) in M346 derived samples (Table 1). Three samples provided on request were screened for the ss4 bp polymorphism. The absence of this polymorphism in these three samples not only confirmed the authenticity of Q4 lineage but also validated the independent status of Q5 observed by us (Figure 1). In addition to the Y-HG Q5 (5/15) and Q1 (1/15) samples, there were 9/15 (60%) individuals who did not show any of the known Q subgroup signature and were designated as Q*.
Figure 1.
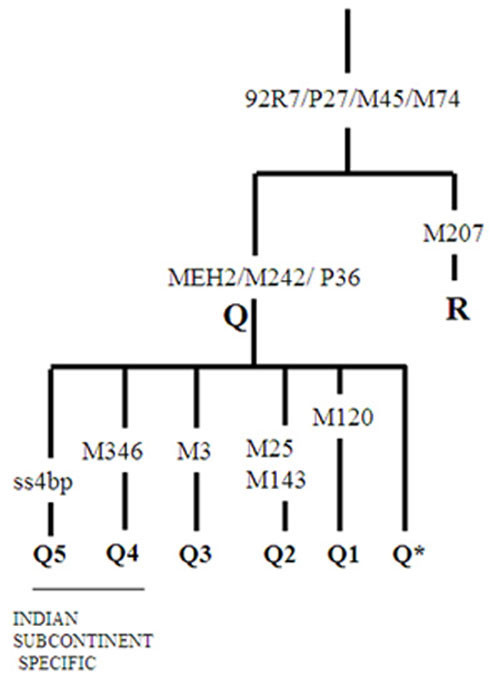
Partial YCC tree redrawn with the addition of M346 marker defining Y-HG Q4 and ss4 bp marker defining Y-HG Q5.
In order to put together our observations along with those made in literature earlier, we pooled data of 1615 Y- chromosomes (630 present study and 985 from literature) (Table 1) for analyses, of which 21/1615 (1.3%) samples represented Q lineage in India. All of our 15 samples and 3 samples belonging to Y-HG Q4 [4] were genotyped for 12 Y-microsatellite markers. However, to keep uniformity in evaluation, data of 10 overlapping Y-STR markers from present study and from different population groups of central Asia and India was used to construct median joining network [5], although 12 Y-STR markers were analysed by us (Additional file 2). For most of the Indian Y-HG Q and its sub-lineages, three clusters of Y-STR haplotypes were observed (Figure 2). One cluster included all the three Q4 and one Q*, another with all the Q5 and the third with most of the Q* bearing individuals. It was interesting to find that most of the Indian Q (Q4, Q5 and Q*) associated Y-STR haplotypes were separated from the bulk of Central Asian Q* associated haplotypes. Further, the clustering of the Y-STR haplotypes reassured the findings by the bi-allelic markers. This could either be due to population differentiation or because of the presence of these clusters in the ancestral migration from Central Asia, not clear at the moment. The increased diversity within the Indian population clusters could be interpreted as an overall effect of geographical differentiation, population expansions and severe bottlenecks resulting in loss of many of the in-between haplotypes thus, reducing the reticulation and increasing the branch lengths. It could also be as a result of independent migrations and admixture.
Figure 2.
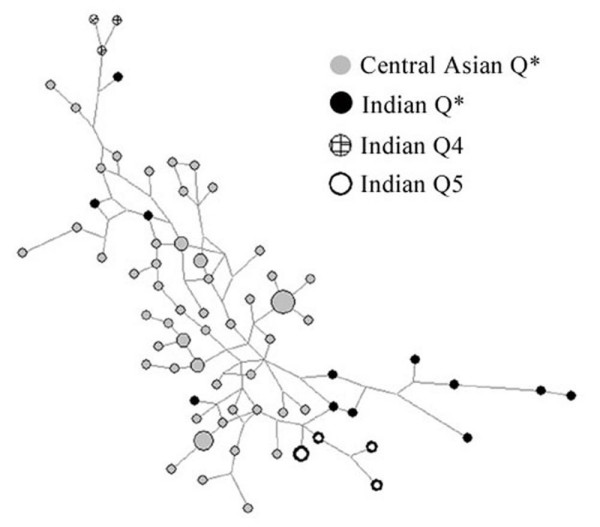
Median joining network-showing relationship of individuals bearing Y-HG Q and its subgroups in population groups of Central Asia and India.
The age estimations made using a small sample size need to be increased which is not feasible at the moment, keeping in mind a very low frequency of Y-HG Q in India. The age estimation for haplogroup Q in India was carried out on the bigger cluster bearing Q* and Q5 in the median joining network. The calculated age of 47,101.5 (34,210.5 – 75,581.4) Years at 95% CI appears to be an over estimate than the age of haplogroup Q (15,000–18,000 Years Before Present) in literature[2,3,6]. This probably has occurred due to the enhanced diversity, probably as an effect of population expansions and severe bottlenecks or might be due to later migrations and admixture. A further estimation of the age of Y-HG Q5 alone, using similar parameters, provided an age estimate of 14,492.7 (10,526.3 – 23,255.8) Years at 95% CI.
The compilation of distribution pattern of Y-HG Q in Indian population (Table 1) from the present study as well as from literature, points out that this HG is distributed widely, ranging from Indo-European castes and tribes to their Dravidian counterparts, despite its low frequency. These observations could be explained either on the basis of the ancestral relationship of different Indian population groups, irrespective of linguistic and social divisions or alternatively by some degree of recent gene flow between these groups, not clear at the moment.
Conclusion
Interestingly, apart from assigned Y-HG Q5 (33.3%, 5/15) samples and the only one individual representing Q1 lineage, there were Y-HG Q bearing (60%, 9/15) samples, representing none of the known Q subgroups and designated as Q*. It was quite interesting to observe Y-HG Q in Indian subcontinent though in low frequency but with ancestral Q* state and a novel sub-branch Q5 not reported elsewhere. A novel subgroup Q4 was identified recently which is also restricted to Indian subcontinent [4]. The most plausible explanation for these observations could be an ancestral migration of individuals bearing ancestral lineage Q* to Indian subcontinent with an autochthonous differentiation to Q4 and Q5 sublineages later on. However, other explanations of either the presence of both the sub haplogroups (Q4 and Q5) in ancestral migrants or recent migrations from central Asia, cannot be ruled out till the distribution and diversity of these subgroups is explored extensively in Central Asia and other regions. To conclude, this study presents a novel polymorphism, adding another sublineage Q5 in the already existing arrangement of Y-HG Q in literature.
Methods
Samples
We screened 630 Y-chromosomes belonging to various linguistic families, castes and tribes throughout the Indian region (Table 1) to know the status of Y-HG Q and its sublineages and compared it with the data available in literature for different regions of the world [2-4,6,7] and also for Indian population groups [2,4,8].
Markers and their analysis
The binary markers: M45, 92R7, P36, MEH2, M120, M3, M25 [1], M242 [2] and M346 [4] were used to dissect out known Y-HG Q upto its subgroups. Samples were amplified, checked in 2% agarose gel and then sequenced to detect polymorphisms (using ABI 3100 sequencer, USA). We used primer set [Forward: ttgtccagagaaacagccaat and Reverse: atccatctacctacatacctgtcatc] to define the novel 4 bp (GGAT) deletion/insertion polymorphism 'ss4 bp' [details submitted in the dbSNP (BUILD 127); NCBI Assay ID: ss65713825; refSNP ID: rs41352448 and amplicon sequence provided as Additional file 1] at 72,314 position of human arylsulfatase D pseudogene (ARSDP gene). The total PCR reaction mix made was 12.5 μl, containing 50 ng of template DNA, 6.25 pmoles of each primer, 200 μM of dNTPs, 1.5 mM MgCl2, 1× reaction buffer and 0.3 units of Taq pol enzyme (Bangalore Genei, India). The cycling conditions were: denaturation at 94°C for 1 min, followed by annealing at 56°C for 1 min, and then extension at 72°C for 1 min, repeated for 30 cycles followed by a final extension step at 72°C for 5 min. PCR products were initially checked in 2% agarose gel, sequenced (using ABI Prism 3100-Avant Genetic Analyzer, Applied Biosystems, USA) and analyzed in SeqScape software v2.1.1 (Applied Biosystems, USA). These samples were also genotyped for 12 Y-microsatellite markers (Y-STRs): DYS388, DYS389I, DYS389II, DYS390, DYS391, DYS392, DYS393, DYS394, DYS426, DYS437, DYS439, DYS448 described elsewhere[9,10] to estimate haplotype variation within the HG defined by binary markers (Additional file 2).
Statistical and Phylogenetic analysis
Although 12 Y-STR markers were analysed by us (Additional file 2), however, to keep uniformity in evaluation, data of 10 overlapping Y-STR markers from present study and from different population groups of central Asia and India bearing Y-HG Q was used to construct median joining network[5]. Age estimation[11] was carried out using averaged variance (calculated by MICROSAT software, version 1.5d) of the Y-STRs and mutation rate[12] (μ) = 6.9 × 10-4 and 95% CI [9.5 × 10-4 - 4.3 × 10-4] per generation (g = 25 years).
List of abbreviations
arylsulfatase D pseudogene (ARSDP)
Confidence Interval (CI)
Micro litre (μl)
Micro molar (μM)
Y-Chromosome Haplogroup (Y-HG)
Y-Chromosome Short Tandem Repeats (Y-STR)
Authors' contributions
SS and ER carried out the molecular genetic studies, sequence alignment, statistical and phylogenetic analyses and drafted the manuscript. AKB participated in the sequence alignment and helped in the statistical analysis. AJSB participated in the design of the study. RNKB conceived of the study, and participated in its design, coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Full amplicon sequence information of the novel 'ss4 bp' polymorphism.
Number of repeats observed at 12 Y-STRs loci in the individuals with Y-HG Q* and Q5, in the present study.
Acknowledgments
Acknowledgements
The financial assistance to SS as SRF (CSIR) and ER as JRF (UGC) is acknowledged. Authors acknowledge Prof. Toomas Kivisild (Tartu University, Estonia) for his kind suggestions, in shaping the paper and Dr. Chris Tyler-Smith (Wellcome Trust Sanger Institute, UK) for kindly providing published data for comparison and his suggestions. Authors acknowledge with thanks the procurement of 3 samples (of Q4 lineage) from Prof. PP Majumder of ISI Kolkata. The authors also acknowledge the financial support given to the National Centre of Applied Human Genetics by UGC and DBT (BT/PR6982/MEB/12/266/2005) India.
Contributor Information
Swarkar Sharma, Email: sawerkar@yahoo.com.
Ekta Rai, Email: ektarai80@yahoo.com.
Audesh K Bhat, Email: audeshs2002@yahoo.co.in.
Amarjit S Bhanwer, Email: ajsbhanwer@yahoo.com.
Rameshwar NK Bamezai, Email: bamezai@hotmail.com.
References
- Y-ChromosomeConsortium A nomenclature system for the tree of human Y-chromosomal binary haplogroups. Genome Res. 2002;12:339–348. doi: 10.1101/gr.217602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seielstad M, Yuldasheva N, Singh N, Underhill P, Oefner P, Shen P, Wells RS. A novel Y-chromosome variant puts an upper limit on the timing of first entry into the Americas. Am J Hum Genet. 2003;73:700–705. doi: 10.1086/377589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karafet TM, Osipova LP, Gubina MA, Posukh OL, Zegura SL, Hammer MF. High levels of Y-chromosome differentiation among native Siberian populations and the genetic signature of a boreal hunter-gatherer way of life. Hum Biol. 2002;74:761–789. doi: 10.1353/hub.2003.0006. [DOI] [PubMed] [Google Scholar]
- Sengupta S, Zhivotovsky LA, King R, Mehdi SQ, Edmonds CA, Chow CE, Lin AA, Mitra M, Sil SK, Ramesh A, Usha Rani MV, Thakur CM, Cavalli-Sforza LL, Majumder PP, Underhill PA. Polarity and temporality of high-resolution y-chromosome distributions in India identify both indigenous and exogenous expansions and reveal minor genetic influence of central asian pastoralists. Am J Hum Genet. 2006;78:202–221. doi: 10.1086/499411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandelt HJ, Forster P, Rohl A. Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol. 1999;16:37–48. doi: 10.1093/oxfordjournals.molbev.a026036. [DOI] [PubMed] [Google Scholar]
- Bortolini MC, Salzano FM, Thomas MG, Stuart S, Nasanen SP, Bau CH, Hutz MH, Layrisse Z, Petzl-Erler ML, Tsuneto LT, Hill K, Hurtado AM, Castro-de-Guerra D, Torres MM, Groot H, Michalski R, Nymadawa P, Bedoya G, Bradman N, Labuda D, Ruiz-Linares A. Y-chromosome evidence for differing ancient demographic histories in the Americas. Am J Hum Genet. 2003;73:524–539. doi: 10.1086/377588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinnioglu C, King R, Kivisild T, Kalfoglu E, Atasoy S, Cavalleri GL, Lillie AS, Roseman CC, Lin AA, Prince K, Oefner PJ, Shen P, Semino O, Cavalli-Sforza LL, Underhill PA. Excavating Y-chromosome haplotype strata in Anatolia. Hum Genet. 2004;114:127–148. doi: 10.1007/s00439-003-1031-4. [DOI] [PubMed] [Google Scholar]
- Sahoo S, Singh A, Himabindu G, Banerjee J, Sitalaximi T, Gaikwad S, Trivedi R, Endicott P, Kivisild T, Metspalu M, Villems R, Kashyap VK. A prehistory of Indian Y chromosomes: evaluating demic diffusion scenarios. Proc Natl Acad Sci U S A. 2006;103:843–848. doi: 10.1073/pnas.0507714103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha A, Sharma S, Bhat A, Pandit A, Bamezai R. Genetic affinity among five different population groups in India reflecting a Y-chromosome gene flow. J Hum Genet. 2005;50:49–51. doi: 10.1007/s10038-004-0219-3. [DOI] [PubMed] [Google Scholar]
- Butler JM, Schoske R, Vallone PM, Kline MC, Redd AJ, Hammer MF. A novel multiplex for simultaneous amplification of 20 Y chromosome STR markers. Forensic Sci Int. 2002;129:10–24. doi: 10.1016/S0379-0738(02)00195-0. [DOI] [PubMed] [Google Scholar]
- Quintana-Murci L, Krausz C, Zerjal T, Sayar SH, Hammer MF, Mehdi SQ, Ayub Q, Qamar R, Mohyuddin A, Radhakrishna U, Jobling MA, Tyler-Smith C, McElreavey K. Y-chromosome lineages trace diffusion of people and languages in southwestern Asia. Am J Hum Genet. 2001;68:537–542. doi: 10.1086/318200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhivotovsky LA, Underhill PA, Cinnioglu C, Kayser M, Morar B, Kivisild T, Scozzari R, Cruciani F, Destro-Bisol G, Spedini G, Chambers GK, Herrera RJ, Yong KK, Gresham D, Tournev I, Feldman MW, Kalaydjieva L. The effective mutation rate at Y chromosome short tandem repeats, with application to human population-divergence time. Am J Hum Genet. 2004;74:50–61. doi: 10.1086/380911. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Full amplicon sequence information of the novel 'ss4 bp' polymorphism.
Number of repeats observed at 12 Y-STRs loci in the individuals with Y-HG Q* and Q5, in the present study.


