Abstract
Chronic kidney disease (CKD) is associated with inflammation and malnutrition and carries a markedly increased risk of cardiovascular disease (CVD). High Mobility Group Box Protein-1 (HMGB-1) is a 30-kDa nuclear and cytosolic protein known as a transcription and growth factor, recently identified as a proinflammatory mediator of tissue injury. Recent data implicates HMGB-1 in endotoxin lethality, rheumatoid arthritis, and atherosclerosis. The aim of this post-hoc, cross-sectional study was to determine whether HMGB-1 serum levels are elevated in CKD patients. The study groups were categorized as follows: 110 patients starting dialysis defined as CKD 5; 67 patients with moderately to severely reduced renal function or CKD 3–4; and 48 healthy controls. High-sensitivity C-reactive-protein (hs-CRP), interleukin-6 (IL-6), tumor necrosis factor (TNF), serum-albumin (S-albumin), hemoglobin A1c (HbA1c), hemoglobin, subjective global nutritional assessment (SGA), and glomerular filtration rate (GFR) were analyzed. Kruskal-Wallis test was used to compare groups and Spearman’s rank correlation test was used for continuous variables. HMGB-1, measured by Western blot, was significantly (P < 0.001) elevated in CKD 5 (146.7 ± 58.6 ng/mL) and CKD 3–4 (85.6 ± 31.8) compared with controls (10.9 ± 10.5). HMGB-1 levels were correlated positively with TNF (Rho = 0.52; P < 0.001), hs-CRP (Rho = 0.38; P < 0.001), IL-6 (Rho = 0.30; P < 0.001), HbA1c (Rho = 0.14; P = 0.02) and SGA (Rho = 0.21; P = 0.002) and negatively correlated with GFR (Rho = –0.69; P = 0.0001), Hb (Rho = –0.60; P < 0.001), S-albumin (Rho = –0.31; P < 0.001). The current study has revealed that HMGB-1 is elevated significantly in CKD patients and correlates with GFR as well as markers of inflammation and malnutrition. Future studies may delineate whether HMGB-1 is also a marker of disease activity and severity as well as a predictor of outcome in CKD.
INTRODUCTION
In recent years chronic inflammation has been the focus of numerous studies in chronic kidney disease (CKD) patients and has been shown to be associated with both malnutrition, defined as protein energy wasting (PEW) and atherosclerotic cardiovascular disease (CVD) (1,2). Inflammatory markers such as IL-6, neutrophils, and CRP have been found to be predictors of all-cause and cardiovascular mortality, independent of traditional risk factors for CVD (3,4). Age-controlled CVD mortality rates are about 10–20 times higher in dialysis patients than in the general population (5). Atherosclerosis found in uremia is studied increasingly, both in end-stage renal disease, and in earlier stages of CKD (6). Inflammation and cytokine dysregulation, oxidative stress, as well as endothelial dysfunction, are believed to be important components of cardiovascular morbidity in CKD (1). As atherosclerosis is an inflammatory disease and markedly accelerated in renal disease, it is, therefore, important to understand the nature of inflammatory factors in the circulation during renal failure. Targeting inflammation by reducing pro-inflammatory cytokines as a potential therapeutic approach is likely to be the focus of future interventional studies in CKD.
HMGB-1, a 30-kDa nuclear and cytosolic ubiquitous protein, is a DNA-binding protein known as a transcription and growth factor (7,8). Structurally, HMGB-1 is organized into two DNA-binding domains (named A-box and B-box), and a negatively charged C-terminus. Recently, however, this protein has been identified as acting as a pro-inflammatory mediator when found extracellularly in animal models and human disease. HMGB-1 is actively secreted by innate immune cells such as macrophages and monocytes. Upon endotoxin stimulation HMGB-1 is passively released by injured and necrotic cells and has been shown to stimulate necrosis-induced inflammation (9,10,11,12). Moreover, HMGB-1 induces other cytokines such as TNF-, IL-1, IL-6, and IL-8, and is also an activator of endothelial cells (HUVEC) leading to the upregulation of adhesion molecules (13,14). HMGB-1 has been shown to interact with toll-like receptor (TLR) 2, TLR 4, and the receptor for advanced glycation end products (RAGE) in established cell lines and animal models. This leads to a downstream translocation of NF-κB inducing immunostimulatory and chemotactic responses (15,16,17). Anti-HMGB-1 antibodies have been demonstrated to confer protection in animal models of sepsis, endotoxemia, and arthritis (10,18,19). Elevated HMGB-1 levels in serum have been found in clinical inflammatory conditions such as sepsis and rheumatoid arthritis (20,21,22).
However the role of HMGB-1 in CKD and the influence of renal function have not been previously investigated. Therefore the aim of this post-hoc cross-sectional study was to determine whether HMGB-1 serum levels are elevated in CKD patients with a wide range of glomerular filtration rate (GFR).
MATERIALS AND METHODS
Patients
A total of 177 CKD patients were investigated as part of a longitudinal follow-up study: 110 end-stage renal disease patients defined as CKD stage 5 patients, (GFR 7 ± 2 mL/min; mean age 53 ± 12 years) about to begin renal replacement therapy between the years 1996 and 2004; 67 patients with moderately to severely reduced renal function defined as CKD 3–4 (GFR 28 ± 10 mL/min; mean age 58 ± 14 years) included from the hospital renal outpatient clinic; and 48 healthy controls (GFR 85 ± 20 mL/min; mean age 62.1 ± 12.5 years) recruited from a population-based randomly selected group in the Stockholm region. The patients were investigated as part of an ongoing prospective study (23). The study exclusion criteria were under 20 years or over 70 years of age, clinical signs of acute infection, acute vasculitis, or liver disease at the time of evaluation; or, unwillingness to participate in the study. The causes of kidney failure in the CKD 5 group were diabetes mellitus, n = 36 (33%); chronic glomerulonephritis, n = 30 (27%); polycystic kidney disease, n = 10 (9%); nephrosclerosis, n = 6 (5%); vasculitis, n = 4 (3,6%); and 24 patients (22%) could not be classified.
After an overnight fast, venous blood samples were drawn and stored at –70°C for future biochemical analyses. High-sensitivity CRP (hs-CRP), serum albumin, HbA1c, and hemoglobin (Hb) subsequently were analyzed. hs-CRP was analyzed using an immunonephelometric procedure (Behring AG, Marburg, Germany), whereas the remaining biochemical analyses were carried out using routine methods at the Department of Clinical Chemistry at Karolinska University Hospital at Huddinge. Subjective global nutritional assessment was used for nutritional status at the time of the inclusion (24). Glomerular filtration rate (GFR) was estimated by the mean of creatinine and urea clearances from a 24-hour collection of urine, or iohexol-clearance—with the exception of eight of the CKD 5 patients where GFR was not available, and therefore was estimated with the Cock-croft-Gault formula (25). TNF and IL-6 were analyzed in serum by immunometric assays on an Immulite Analyzer (DPC, Los Angeles, CA, USA) according to the instructions of the manufacturers.
HMGB-1 Serum Determination
In brief, previously collected sera samples were thawed on ice and microfuged for 10 min at 9700g. The supernatant (100–200 μL) was ultrafiltered with Microcon YM-100 filters (Millpore, Bedford, MA, USA). Filtrate was transferred to a new tube, mixed with 5X Laemmli Sample Buffer (BioRad, Hercules, CA, USA), heated at 95°C for 5 min and spun down at 8160g for 5 min, in tabletop microfuge. 12.5 μL of heated sample (10 μL serum per lane) was fractionated through 10% to 20% Tris-HCL acrylamide gels (BioRad) and transferred to preactivated polyvinyline fluoride (PVDF) membrane (BioRad). PVDF membrane was pre-activated with methanol, rinsed and equilibrated in Tris/Glycine/20% Methanol transfer buffer. After the transfer, the membrane was rinsed briefly with wash buffer (0.2% Tween 20 in PBS) and incubated for 1 h in blocking buffer (5% non-fat dry milk in wash buffer). The membrane was probed overnight at 4°C with purified IgG from anti-HMGB1 antiserum (2 μg/mL in 1% non-fat dry milk in wash buffer). The membrane was washed two times at 10 min intervals in wash buffer and incubated with peroxidase conjugated anti-rabbit secondary antibody (Amersham Biosciences, GE Healthcare UK limited, Little Chalfont Buckinghamshire, UK) 1:7500 dilution for 1 h. The membrane was washed three times at 15 min intervals in wash buffer followed by 5 min in 1X PBS. After rinsing the membrane in distilled water, the signals were visualized using ECL Western blotting detection reagents (Amersham Biosciences). Equal volumes of ECL A and B were mixed and added to the PVDF membrane and incubated for 1 min. Excess reagent was removed by blotting the edge of the PVDF membrane on a paper towel. PVDF membrane then was exposed to Hyperfilm (Amersham Biosciences). Autoradiograph films were scanned and densitometric analyses performed using Quantity One software (BioRad) and Microsoft Excel. The levels of HMGB-1 were determined by reference to standard curves generated with purified HMGB-1.
The study protocol was approved by the Ethics Committee of Karolinska University Hospital Huddinge, Stockholm, Sweden, and informed consent was obtained from each subject.
STATISTICAL ANALYSIS
The Kruskal-Wallis test was used to compare groups; for nominal variables, the chi-square test was used. All values are expressed as mean ± SD or median (range), unless otherwise indicated. A P value < 0.05 was considered to be statistically significant. Spearman’s rank correlation test was used for continuous variables. Forward and backward stepwise multivariate regression analysis was used to assess the predictors of HMGB-1. Receiver operating characteristics (ROC) analysis was performed to estimate the cutoff points of HMGB-1. Kaplan-Meier survival analysis and Cox proportional multivariate hazards model were used for clinical outcome. The statistical analysis was performed using statistical software SAS version 9.1.4 (SAS Campus Drive, Cary, NC, USA).
RESULTS
Clinical and biochemical characteristics with regard to age, GFR, prevalence of diabetes, SGA as a marker of malnutrition or PEW, inflammation, gender distribution, BMI, lipids, and inflammation markers are presented in Table 1. CKD patients were significantly younger than the healthy controls (P < 0.001).
Table 1.
Clinical Characteristics of CKD Patients and Controls
| Controls | CKD 3–4 | CKD 5 | ||
|---|---|---|---|---|
| Number | 48 | 67 | 110 | |
| Age (years) | 62 ± 12 | 58 ± 14 | 53 ± 12 | < 0.001 |
| GFR (m/min) | 85 ± 20 | 28 ± 10 | 7 ± 2 | < 0.001 |
| Diabetes Mellitus (%) | 4 | 30 | 34 | < 0.001 |
| PEW (%)a | 4 | 3 | 32 | < 0.001 |
| Inflammation (%)b | 2 | 9 | 35 | < 0.001 |
| Males (%) | 72 | 76 | 58 | 0.02 |
| BMI (kg/m2) | 26 ± 4 | 27 ± 4 | 24 ± 4 | < 0.001 |
| Cholesterol (mmol/L) | 5.1 ± 0.9 | 5.3 ± 1.2 | 5.3 ± 1.6 | nsc |
| Triglycerides (mmol/L) | 1.3 ± 0.7 | 2.1 ± 1.2 | 1.9 ± 1.1 | < 0.001 |
| HDL-cholesterol (mmol/L) | 1.5 ± 0.5 | 1.3 ± 0.4 | 1.5 ± 0.8 | 0.01 |
| Hemoglobin, (g/L) | 143 ± 9 | 127 ± 15 | 103 ± 16 | < 0.001 |
| hs-CRP (mmol/L)d | 1.2 (0.2–32) | 4.9 (0.3–49.0) | 5.4 (0.2–119.0) | < 0.001 |
| IL-6 (pg/mL)d | 2.1 (0.4–10) | 3.2 (0.8–10.6) | 5.6 (0.75–48.4) | < 0.001 |
| TNF (pg/mL)d | 3.9 (1.3–10.6) | 7.9 (3.3–27.8) | 11.1 (3.1–49.2) | < 0.001 |
| Leukocytes (109/L) | 6.6 ± 2.0 | 7.4 ± 1.9 | 7.9 ± 2.4 | < 0.001 |
| HMGB-1 (ng/mL) | 10.9 ± 10.5 | 85.6 ± 31.8 | 146.7 ± 58.9 | < 0.001 |
Mean ± SD indicated.
Protein-energy wasting measured as SGA.
CR P ≥ 10 mg/L.
ns = not significant.
median and range.
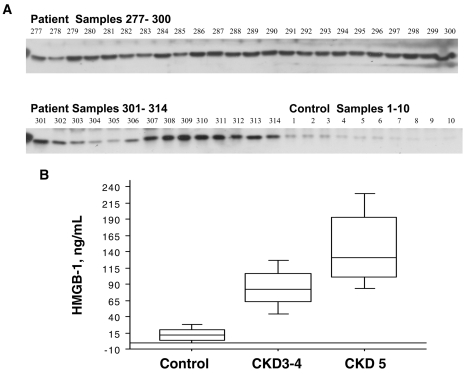
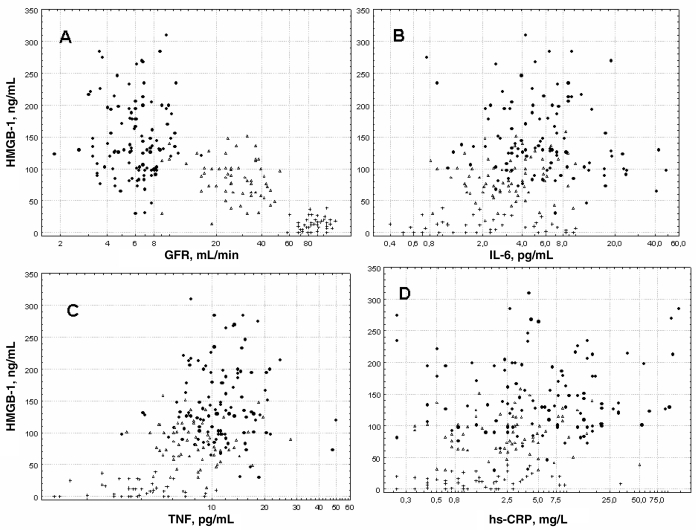
HMGB-1 serum levels were significantly (P < 0.001) elevated in CKD 5 and CKD 3–4 as compared with controls (Table 1, Figure 1B). There were no differences in HMGB-1 levels with regard to gender in any of the groups. HMGB-1 levels were positively correlated with TNF (Rho = 0.52 P < 0.001), hs-CRP (Rho = 0.35 P < 0.001), IL-6 (Rho = 0.31 P < 0.001), HbA1c (Rho = 0.14 P = 0.02), and SGA (Rho = 0.21; P = 0.002) and negatively correlated with GFR (Rho = –0.69; P = 0.0001), Hb (Rho = –0.59 P < 0.001), S-albumin (Rho = –0.31; P < 0.001) and age (Rho = –0.29; P < 0.001) (Figures 2A–D).
Figure 1.
(A). Representative HMGB1 Western Blot results in 37/110 CKD five patients versus 10/48 controls. (B) HMGB-1 significantly (P < 0.001) elevated in CKD 5 and CKD 3–4 compared with controls.
Figure 2.
(A) Spearman rank correlations: HMGB-1 negative correlation with GFR (Rho = –0.69; P = 0.0001). (B) Spearman rank correlations: positive correlation with IL-6 (Rho = 0.31 P < 0.001). (C) Spearman rank correlations: TNF (Rho = 0.52 P < 0.001). (D) Spearman rank correlations: hs-CRP (Rho = 0.35 P < 0.001). CKD 5 (filled circles), CKD 3–4 (empty triangles) and controls (crosses).
Stepwise multivariate regression analysis revealed that GFR, hs-CRP, IL-6, and age were found to be predictors of HMGB-1 levels accounting for 26% of the HMGB-1 levels in the current cohort (Table 2). There was no significant association between HMGB-1 levels and primary kidney disease in the CKD 5 cohort.
Table 2.
Forward stepwise multivariate regression analysis of predictors of the HMGB-1 ng/mL in CKD 3–5 patients (n = 177). Variables known to influence HMGB-1 ng/mL levels (sex, age, GFR, SGA, albumin, CVD, TNF, and hs-CRP) were included in the initial model.
| Partial r | Estimate | Standard error | P value | |
|---|---|---|---|---|
| Intercept | 131.1 | 34.9 | < 0.0001 | |
| Gender | 0.03 | 13.3 | 8.1 | nsb |
| Age, years | 0.06 | –0.7 | 0.3 | < 0.05 |
| GFR, 1 mL/min | 0.12 | –2.1 | 0.3 | < 0.0001 |
| hs-CRP, mg/L | 0.19 | 0.8 | 0.2 | < 0.0001 |
| S-albumin, g/L | 0.23 | 1.2 | 0.8 | ns |
Total adjusted r2 = 0.26.
ns = not significant.
Finally, 24-month survival in the CKD 5 cohort was analyzed. There were a total of 16 deaths during this time period. The serum HMGB-1 levels in this group were elevated significantly and did not overlap with healthy controls. Using a ROC analysis, a reasonable cut-off level in the CKD 5 population was determined. Surprisingly, HMGB-1 levels below 100.9 ng/mL were associated with a significantly lower survival rate (P < 0.04 by log-rank test) than for patients with higher levels when using Kaplan-Meier analysis, and also in an univariate analysis (P < 0.02 ). After adjusting for age, gender, diabetes, and IL-6 by Cox proportional hazards method, the difference remained statistically significant (P = 0.04) (Table 3). However, when controlling for SGA (as a marker for PEW), the odds ratio for the HMGB-1 cut-off level was no longer statistically significant (P = 0.16), and SGA instead demonstrated a significant odds ratio of P = 0.0004 for all-cause mortality. The majority (10/16) of deaths were attributed to CVD, the remainder to infection, malignancy, and other causes. There was no specific statistical pattern with regard to the HMGB-1 cut-off level for causes of death.
Table 3.
Univariate (HMGB-1 only) and multivariate Cox proportional hazard model for all-cause mortality for 24 months in CKD-5 patients (n = 110). HMGB-1 cut-off based on ROC analysis. IL-6 cut-off based on mean value. SGA, well versus malnourished.
| Parameter | Hazard Ratio | 95% Hazard Ratio Confidence Limits | P-value | Hazard Ratio | 95% Hazard Ratio Confidence Limits | P-value | Hazard Ratio | 95% Hazard Ratio Confidence Limits | P-value | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | 1.030 | 0.987 | 1.075 | 0.1722 | 1.011 | 0.965 | 1.059 | 0.6488 | ||||
| Gender, female | 1.008 | 0.462 | 2.199 | 0.9840 | 0.684 | 0.290 | 1.612 | 0.3858 | ||||
| HMGB-1, < 100.9 pg/mL | 2.506 | 1.175 | 5.345 | 0.0174 | 2.289 | 1.037 | 5.055 | 0.0404 | 1.812 | 0.782 | 4.198 | 0.1656 |
| IL-6, < 6.5 pg/mL | 2.935 | 1.291 | 6.674 | 0.0102 | 2.884 | 1.204 | 6.910 | 0.0175 | ||||
| Diabetes Mellitus | 2.334 | 1.074 | 5.073 | 0.0323 | 3.370 | 1.404 | 8.088 | 0.0065 | ||||
| SGA, Well versus Mal | 5.297 | 2.088 | 13.436 | 0.0004 | ||||||||
DISCUSSION
The main finding of the current study is that HMGB-1 is markedly elevated in CKD and that GFR was found to be an important predictor of HMGB-1 levels. This suggests that renal function needs to be considered when interpreting HMGB-1 levels in patient populations, as the importance of renal function has not been recognized previously. Moreover, to the best of our knowledge, no experimental animal models have yet examined the relation between loss of renal function and HMGB-1 levels. The size of the HMGB-1 protein, 30-kDa, suggests that the renal route is a major pathway of elimination and could, in part, explain the elevated circulating levels in CKD patients. It also can be assumed that current standard dialysis methods would remove only a small fraction of the circulating protein. However, not only decreased clearance, but also increased production, could contribute to the markedly elevated levels of HMGB-1 in this patient population. In recent years, it has become apparent that CKD is a state of chronic inflammation dominated by accelerated atherosclerosis with important implications for morbidity and mortality independent of traditional risk factors (1–4). Significant increases in circulating pro-inflammatory cytokines are not only a feature of CKD, but have also been demonstrated in patients with moderately reduced renal functions (26,27).
In the present study, we also could show that HMGB-1 correlated significantly with markers of inflammation and wasting. Previous studies have shown that HMGB-1, with its delayed kinetics, differs from other early innate pro-inflammatory cytokines, such as TNF and IL-1, in endotoxemia and sepsis models (10,18). A similar pattern has been found in humans with sepsis (20,21). Moreover, we have recently found elevated circulating HMGB-1 levels in chronically inflamed rheumatoid arthritis patients, as compared with healthy individuals (22). Taken together, HMGB-1 is an interesting new inflammatory marker to study in CKD in both an experimental and clinical setting. Our study, however, was aimed only at determining whether HMGB-1 serum levels are elevated in CKD patients. In spite of significant associations with other inflammatory markers, the source of this cytokine thus remains largely unknown in this patient population. Whether it is actively secreted from stimulated immune cells and/or passively released from necrotic tissue remains for future studies to determine.
The role of HMGB-1 and its effects on the endothelium, as shown in cultured human endothelial cells (HUVEC), would be an area of investigation in CKD—known for its increased risk of CVD, as HMGB-1 can induce upregulation of adhesion molecules in these experiments (14). The several-fold increase in cardiovascular morbidity and mortality associated with inflammation in CKD patients would imply that targeting pro-inflammatory cytokines, and possibly HMGB-1, may have important therapeutic implications. HMGB-1 has been implicated recently in clinically relevant cardiovascular ischemic conditions (28). Human atherosclerotic plaques, but not normal arteries, have been shown to produce extracellular HMGB-1 (29,30). Furthermore, in recent studies of auto-immune inflammatory conditions, increased expression of HMGB-1 was detected by immunohistochemical staining in skin lesions of patients with lupus erythematosus, and in the salivary glands of patients with Sjogren’s syndrome—indicating that HMGB-1 is involved in the inflammatory process of these diseases (31,32). Further studies, including tissue studies and HMGB-1 expression in urine may, therefore, clarify the role of HMGB-1, its significance in CKD, and whether its biological activity is also a marker of disease severity.
All CKD patients in our study had elevated circulating HMGB-1 levels as compared with controls. The relationship between immunodetectable levels of the cytokine in serum and bioactivity in tissue is not well known. HMGB-1 levels in serum found in the CKD 5 cohort are, nevertheless, similar to what has been found in sepsis patients, and, in CKD 3–4, to what has been found in rheumatoid arthritis patients (20,21,22). However, the survival analysis in the CKD 5 patient subgroup correlated unexpectedly with a better outcome over 24 months in patients with higher, rather than lower, levels of the cytokine. A recent sepsis study also identified higher levels of HMGB-1 as being associated with better outcome (20). However, both our study and the sepsis study were limited with regard to patient numbers and clinical events. Another important limitation of the current investigation is the post-hoc design based on a single cross-sectional sample. Patients with clinical signs of acute infection were excluded from the study, but low-grade inflammation due to subclinical infection could not be ruled out completely as a confounder. Furthermore, SGA, although widely used as a clinical index of nutritional status, might not be sensitive enough in this setting.
Thus, in the case of incident dialysis patients, it might be difficult to draw far-reaching conclusions from a single cross-sectional sample of a newly characterized cytokine as a predictive marker. Nevertheless, in previous studies, an association between other inflammatory markers, such as IL-6 and CRP, and outcome in CKD patients has been found (3,4). In the Panichi study (3), follow-up was 4 years after a single high-sensitivity CRP and IL-6 test in 162 patients, and the Honda study (4) followed 176 patients for a median of 26 months. Both these cross-sectional studies showed an association between increased levels of high-sensitivity CRP and IL-6 and outcome. Repeated measurements of cytokines are, however, likely to be more useful for outcome prediction rather than a single value. We recently have examined this with regard to repeated CRP vs. a single value, suggesting that serial measurements might increase the precision of inflammatory markers as predictors of outcome (33). In the current study, we also observed that elevated HMGB-1 levels correlate with IL-6, TNF, and CRP. Although this does not prove a causal relationship, the results are consistent with the pro-inflammatory activity of HMGB-1, which is induced both by other cytokines and can stimulate the release of TNF, IL-1, and IL-6 (12). It would certainly be of considerable interest to follow serial HMGB-1 over time in chronic inflammatory models or diseases.
Recent studies have focused on cytokine responses and outcome in CKD as well as in the elderly. Ando et al. studied TLR 4 expression in monocytes and their ex vivo cytokine synthesis in response to a standardized danger signal with bacterial lipopolysaccharide (LPS) stimulation in uremic patients (34). TLR 4 expression was lower in CKD patients as compared with controls, with reduced expression being even more severe in those CKD patients who were predisposed to infections. Infection-prone CKD patients also exhibited significantly reduced synthesis of cytokines, such as TNF and IL-1 in response to LPS. However, as SGA in the multivariate model seemed to reduce the significance of lower HMGB-1 levels and outcome in our study, the relation between wasting and immune function could be of importance as well in CKD. Indeed, as suggested by Dinarollo et al., it could be proposed that wasting may curtail the synthesis of cytokines (35). In a paper by King et al., a direct correlation of peripheral blood mononuclear cell content of IL-1Ra, as well as endotoxin-stimulated IL-1Ra production correlated directly with several indices of nutritional status, was identified in a cohort of patients on maintenance hemodialysis (36). This would suggest that PEW depresses cytokine production and potentially contributes to reduced immune responsiveness in patients on chronic hemodialysis. An additional explanation could be genetic differences in the capacity to produce HMGB-1. However, to the best of our knowledge, no such studies have yet been performed.
We could not detect any significant association between HMGB-1 levels and primary kidney disease in the CKD 5 patients. This could imply that the elevated HMGB1 levels found in incident dialysis patients reflect the correlation with GFR and the inflammation associated with uremia, rather than being associated with subgroups of inflammatory diseases such as chronic glomerulonephritis, vasculitis, or lupus.
At this time, there are no standardized assays for HMGB-1 activity. We analyzed our samples with a commonly used Western Blot method. There are a few commercially available ELISA methods. A recent paper, however, found a discrepancy between Western Blot and ELISA results suggesting that serum/plasma components bind to HMGB1 and interfere with its detection by ELISA systems (37). For the future, reliable and fast detection systems are, however, required to investigate the significance of HMGB1 in clinical samples for diagnosis and prognosis of diseases.
To conclude, the current study has revealed that HMGB-1 is significantly elevated in CKD patients and correlates with GFR and markers of inflammation and PEW. Future studies may delineate the source of this pro-inflammatory cytokine in CKD and, furthermore, whether HMGB-1 is a marker of disease severity and a consistent predictor of outcome in CKD patients.
Acknowledgments
This study has been supported by the Swedish Heart and Lung Foundation, the Swedish Medical Research Council, and, in part, by the National Institute of General Medical Sciences.
Footnotes
Online address: http://www.molmed.org
REFERENCES
- 1.Stenvinkel P, et al. IL-10, IL-6, and TNF-alpha: central factors in the altered cytokine network of uremia—the good, the bad, and the ugly. Kidney Int. 2005;67:1216–33. doi: 10.1111/j.1523-1755.2005.00200.x. [DOI] [PubMed] [Google Scholar]
- 2.Avesani CM, Carrero JJ, Axelsson J, Qureshi AR, Lindholm B, Stenvinkel P. Inflammation and wasting in chronic kidney disease. Partners in crime Kidney Int. 2006;70:S8–13. [Google Scholar]
- 3.Panichi V, et al. Interleukin-6 is a stronger predictor of total and cardiovascular mortality than C-reactive protein in haemodialysis patients. Nephrol Dial Transplant. 2004;19:1154–60. doi: 10.1093/ndt/gfh052. [DOI] [PubMed] [Google Scholar]
- 4.Honda H, et al. Serum albumin, C-reactive protein, interleukin 6, and fetuin a as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am J Kidney Dis. 2006;47:139–48. doi: 10.1053/j.ajkd.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32:S112–9. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 6.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 7.Bianchi ME, Beltrame M, Paonessa G. Specific recognition of cruciform DNA by nuclear protein HMG1. Science. 1989;243:1056–9. doi: 10.1126/science.2922595. [DOI] [PubMed] [Google Scholar]
- 8.Bianchi ME, Beltrame M. Upwardly mobile proteins. Workshop: the role of HMG proteins in chromatin structure, gene expression and neoplasia. EMBO Rep. 2000;1:109–14. doi: 10.1093/embo-reports/kvd030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–42. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–51. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Yang H, Tracey KJ. Extra cellular role of HMGB1 in inflammation and sepsis. J Intern Med. 2004;255:320–31. doi: 10.1111/j.1365-2796.2003.01302.x. [DOI] [PubMed] [Google Scholar]
- 12.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 13.Andersson U, et al. High Mobility Group 1 Protein (HMG-1) Stimulates Proinflammatory Cytokine Synthesis in Human Monocytes. J Exp Med. 2000;192:565–70. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Treutiger CJ, et al. High mobility group 1 B-box mediates activation of human endothelium. J Intern Med. 2003;254:375–85. doi: 10.1046/j.1365-2796.2003.01204.x. [DOI] [PubMed] [Google Scholar]
- 15.Kokkola R, et al. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scand J Immunol. 2005;61:1–9. doi: 10.1111/j.0300-9475.2005.01534.x. [DOI] [PubMed] [Google Scholar]
- 16.Park JS, et al. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290:C917–24. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- 17.Yu M, et al. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26:174–9. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- 18.Yang H, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci U S A. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kokkola R, et al. Successful treatment of collagen-induced arthritis in mice and rats by targeting extracellular high mobility group box chromosomal protein 1 activity. Arthritis Rheum. 2003;48:2052–8. doi: 10.1002/art.11161. [DOI] [PubMed] [Google Scholar]
- 20.Sunden-Cullberg J, et al. Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Crit Care Med. 2005;33:564–73. doi: 10.1097/01.ccm.0000155991.88802.4d. [DOI] [PubMed] [Google Scholar]
- 21.Hatada T, et al. Plasma concentrations and importance of High Mobility Group Box protein in the prognosis of organ failure in patients with disseminated intravascular coagulation. Thromb Haemost. 2005;94:975–9. doi: 10.1160/TH05-05-0316. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein RS, et al. Cholinergic Anti-Inflammatory Pathway Activity and High Mobility Group Box-1 (HMGB1) Serum Levels in Patients with Rheumatoid Arthritis. Mol Med. 2007;13:210–5. doi: 10.2119/2006-00108.Goldstein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stenvinkel P, et al. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999;55:1899–911. doi: 10.1046/j.1523-1755.1999.00422.x. [DOI] [PubMed] [Google Scholar]
- 24.Detsky AS, McLaughlin JR, Baker JP, Johnston N, Whittaker S, Mendelson RA, Jeejeebhoy KN. What is subjective global assessment of nutritional status? JPEN J Parenter Enteral Nutr. 1987;11:8–13. doi: 10.1177/014860718701100108. [DOI] [PubMed] [Google Scholar]
- 25.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 26.Pecoits-Filho R, Heimbürger O, Bárány P, Suliman M, Fehrman-Ekholm I, Lindholm B, Stenvinkel P. Associations between circulating inflammatory markers and residual renal function in CRF patients. Am J Kidney Dis. 2003;41:1212–8. doi: 10.1016/s0272-6386(03)00353-6. [DOI] [PubMed] [Google Scholar]
- 27.Landray MJ, et al. Inflammation, endothelial dysfunction, and platelet activation in patients with chronic kidney disease: the chronic renal impairment in Birmingham (CRIB) study. Am J Kidney Dis. 2004;43:244–53. doi: 10.1053/j.ajkd.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 28.Goldstein RS, et al. Elevated high-mobility group box 1 levels in patients with cerebral and myocardial ischemia. Shock. 2006;25:571–4. doi: 10.1097/01.shk.0000209540.99176.72. [DOI] [PubMed] [Google Scholar]
- 29.Kalinina N, Agrotis A, Antropova Y. Increased expression of the DNA-binding cytokine HMGB1 in human atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2004;24:2320–5. doi: 10.1161/01.ATV.0000145573.36113.8a. [DOI] [PubMed] [Google Scholar]
- 30.Porto A, et al. Smooth muscle cells in human atherosclerotic plaques secrete and proliferate in response to high mobility group box 1 protein. FASEB J. 2006;20:2565–6. doi: 10.1096/fj.06-5867fje. [DOI] [PubMed] [Google Scholar]
- 31.Popovic K, et al. Increased expression of the novel proinflammatory cytokine high mobility group box chromosomal protein 1 in skin lesions of patients with lupus erythematosus. Arthritis Rheum. 2005;52:3639–45. doi: 10.1002/art.21398. [DOI] [PubMed] [Google Scholar]
- 32.Ek M, Popovic K, Harris HE, Naucler CS, Wahren-Herlenius M. Increased extracellular levels of the novel proinflammatory cytokine high mobility group box chromosomal protein 1 in minor salivary glands of patients with Sjogren’s syndrome. Arthritis Rheum. 2006;54:2289–94. doi: 10.1002/art.21969. [DOI] [PubMed] [Google Scholar]
- 33.Qureshi AR, Snaedal Jonsdottir S, Heimbürger O, Lindholm B, Alvestrand A, Stenvinkel P, Barany P. Serial Measurements of C-reactive protein (CRP) predict outcome better than a single value in hemodialysis (HD) patients. Abstract Renal Week. American Society of Nephrology. 2006:TH-FC123. [Google Scholar]
- 34.Ando M, Shibuya A, Tsuchiya K, Akiba T, Nitta K. Reduced expression of Toll-like receptor 4 contributes to impaired cytokine response of monocytes in uremic patients. Kidney Int. 2006;70:358–62. doi: 10.1038/sj.ki.5001548. [DOI] [PubMed] [Google Scholar]
- 35.Dinarello CA, Roubenoff RA. Mechanisms of loss of lean body mass in patients on chronic dialysis. Blood Purif. 1996;14:388–94. doi: 10.1159/000170291. [DOI] [PubMed] [Google Scholar]
- 36.King AJ, Kehayias JJ, Roubenoff R, Schmid CH, Pereira BJ. Cytokine production and nutritional status in hemodialysis patients. Int J Artif Organs. 1998;21:4–11. [PubMed] [Google Scholar]
- 37.Urbonaviciute V, Fürnrohr BG, Weber C, Haslbeck M, Wilhelm S, Herrmann M, Voll RE. Factors masking HMGB1 in human serum and plasma. J Leukoc Biol. 2007;81:67–74. doi: 10.1189/jlb.0306196. [DOI] [PubMed] [Google Scholar]