Abstract
Plasmodium falciparum malaria causes 1–2 million deaths per year. Most deaths occur as a result of complications such as severe anemia and cerebral malaria (CM) (coma). Red cells of children with severe malaria-associated anemia (SMA) have acquired deficiencies in the complement regulatory proteins complement receptor 1 (CR1, CD35) and decay accelerating factor (DAF, CD55). We investigated whether these deficiencies affect the ability of erythrocytes to bind immune complexes (ICs) and regulate complement activation. We recruited 75 children with SMA (Hb ≤ 6 g/dL) from the holoendemic malaria region of the Lake Victoria basin, western Kenya, and 74 age- and gender-matched uncomplicated malaria controls. In addition, we recruited 32 children with CM and 52 age- and gender-matched controls. Deficiencies in red cell CR1 and CD55 in children with SMA were accompanied by a marked decline in IC binding capacity and increased C3b deposition in vivo and ex vivo. Importantly, these changes were specific because they were not seen in red cells of children with CM or their controls. These data suggest that the declines in red cell CR1 and CD55 seen in children with SMA are of physiologic significance and may predispose erythrocytes to complement-mediated damage and phagocytosis in vivo.
INTRODUCTION
Plasmodium falciparum is an intracellular parasite of humans that is transmitted by the bite of Anopheles mosquitoes. It is responsible for 1–2 million deaths per year, the majority of which occur in sub-Saharan Africa (1). The invasion and growth of the parasite in erythrocytes is a prominent part of the life cycle and is associated with most of the morbidity and mortality. Severe anemia is one of the major complications of infection with P. falciparum malaria (2). The pathogenesis of this anemia is not understood well. Although destruction of erythrocytes takes place by the direct effect of the parasite, the degree of anemia in severe cases cannot be explained solely on this basis(3–5). Therefore, uninfected erythrocytes must be affected and destroyed as well. Several studies have documented that the life span of uninfected erythrocytes is decreased in persons infected with P. falciparum and in animal models (3,4). Earlier studies by Facer et al. (6,7) reported the presence of C3d on the surface of erythrocytes from children with malaria. These observations motivated us to determine whether there is a defect in the complement regulatory protein machinery of red cells in children with severe malaria associated anemia (SMA).
Red cell complement regulatory proteins protect the cells from autologous complement attack. Complement receptor 1 (CR1, CD35), decay accelerating factor (DAF, CD55), and the membrane inhibitor of reactive lysis (MIRL, CD59) are erythrocyte surface proteins that promote the inactivation and binding of C3b in immune complexes (ICs) (CR1), promote inactivation of C3b convertases (CR1 and CD55), and interfere with the assembly of the membrane attack complex C5b-9 (CD59)(8,9). Red cells are able to bind C3b-bearing ICs via CR1 and carry them to the liver and spleen where they are removed from circulation (10,11). Consequently, complement regulatory proteins may play an important role in protecting red cells from complement-mediated destruction as a result of IC formation and complement activation that occur during malaria infection (12–15).
We have shown that red cells of children with SMA have decreased levels of CR1 and CD55 (14,16,17). We hypothesized that these changes could translate into a decreased functional capacity to bind ICs and prevent complement deposition, which could result in their increased rate of destruction. To test our hypothesis we carried out a case-control study in children with SMA and age and gender-matched symptomatic uncomplicated malaria controls and determined their levels of erythrocyte CR1 and CD55, their erythrocyte IC binding capacity, and the susceptibility of their red cells to complement deposition in vivo and ex vivo. As an additional comparison group, we recruited children with cerebral malaria (CM) and age- and gender-matched symptomatic uncomplicated malaria controls.
MATERIALS AND METHODS
Study Design and Populations
Participants were recruited under a human use protocol approved by the Human Use Research Committee, the Walter Reed Army Institute of Research, and the National Ethics Review Committee of the Kenya Medical Research Institute. Informed consent was obtained from all parents or guardians. The study had a matched case-control design. SMA cases, defined as children with asexual P. falciparum parasitemia by Giemsa-stained thick and thin blood smear and Hb ≤ 6 g/dL, were recruited from the pediatric ward of the Nyanza Provincial General Hospital (NPGH), Kisumu, Kenya, where malaria is holoendemic. Because CM is uncommon in this area, CM cases were recruited from the pediatric ward of the Kisii District Hospital (KDH), as well as from the NPGH. KDH is located in the highlands of western Kenya where transmission is seasonal and, consequently, it receives many more CM cases than the NPGH(18). CM was defined as asexual P. falciparum parasitemia by Giemsa-stained blood smear and a Blantyre coma score of ≤ 2(19), lasting at least 30 min if there was a history of convulsions. Symptomatic uncomplicated malaria controls matched by gender and age ± two months were assigned to each case at a case:control ratio of 1:1 for SMA and 1:2 for CM, and were identified from the outpatient clinic of the same hospital where the corresponding case was recruited. Controls were defined as children with a normal mental status, a Hb > 6 g/dL, a Giemsa-stained blood smear positive for asexual P. falciparum, and an axillary temperature ≥ 37.5°C. In the absence of fever, we required two of the following signs or symptoms: nausea/ vomiting, irritability, poor feeding, myalgias, or headache. General exclusion criteria also included evidence of concomitant serious infections (i.e. meningitis excluded by lumbar puncture when indicated, pneumonia, sepsis), chronic illness, or a history of blood transfusion in the three months preceding enrollment to avoid the influence of donor erythrocytes in our measurements.
All children were evaluated in a standardized fashion at enrollment and at follow-up two months later. If a child failed to return for follow-up, a field worker traveled to his/her last known domicile to determine his/her status. During follow-up, a blood sample was obtained once it was confirmed that the child was asymptomatic and free of parasitemia. If malaria persisted at the first follow-up visit, the child was re-treated and re-evaluated two weeks later. Inpatient treatment for malaria consisted of IV quinine (2) and outpatient therapy was with artemether/lumefantrine (20).
To compare the results to a normal population we also present data on a set of 79 normal individuals 12 to 45 years of age who were recruited in a recent cross-sectional study (manuscript in preparation).
Blood Samples and Smears
Giemsa-stained thick and thin blood smears, and thin reticulocyte smears stained with new methylene blue Sigma-Aldrich, St. Louis, MO, USA), were prepared from capillary blood obtained by finger prick. A 2.5 mL sample of venous blood was obtained at enrollment and 5 mL at follow-up. Hemoglobin levels were measured from EDTA-anticoagulated blood using a hematology analyzer (Coulter, Hialeah, FL, USA). The EDTA-anticoagulated blood was centrifuged and the plasma was stored at −70°C for use as part of other ongoing studies. The erythrocyte pellet was washed once with 50 volumes of Alsevers buffer and a portion resuspended in the same volume of buffer for storage at 4°C until used. The remaining pellet was washed with ten volumes of phosphate buffered saline pH 7.4 (PBS) and resuspended in glycerolyte (50% glycerol, 16 g/L sodium lactate, 300 mg/L KCl, 25 mM sodium phosphate pH 6.8) for cryopreservation in liquid nitrogen (21).
Flow Cytometric Measurement of CR1 and CD55
Erythrocyte CR1 and CD55 levels were determined using frozen samples. In preliminary experiments, we observed no significant effect of freezing on the level of CR1 or CD55. All primary antibodies were titered to saturation. The following primary antibodies were used in dilutions of 1:20: anti-CR1 clone E11, anti-CD55 clone IA10, and isotype controls for each (Becton-Dickinson, Belgium). A secondary FITC-conjugated goat anti-mouse IgG (Becton-Dickinson) was used at a dilution of 1:50. Ten μL of thawed erythrocyte pellet was washed twice in 1 mL of Alsevers buffer, and re-suspended in the same volume of buffer. Unless otherwise stated, all procedures were as described previously (16,17). Flow cytometry was carried out using a FACScan flow cytometer (Becton-Dickinson, San José, CA, USA). Red cells were gated on the basis of their forward and side scatter characteristics using logarithmic amplification. The median fluorescence intensity (MFI) of each sample was measured using logarithmic amplification. The MFI values for CR1 and CD55 were normalized to the mean of the MFI of the red cell standard using the formula,
where “CorrMFIs” and “MFIs” are the corrected and uncorrected sample MFI respectively, “MFIcmean” is the mean of all the MFI values of the standard control, and “MFIc” is the MFI of the control obtained in parallel with the sample. The number of molecules of CR1 per red cell was derived from a fluorescence standard curve created using cells with known CR1 numbers. Red cell anti-CD55 antibody binding capacities (ABC) were derived from a standard curve created using beads of known ABC (Bangs Lab, Fishers, IN, USA).
Preparation of ICs, IC Opsonization, and IC Binding Capacity
Fifty μL of 49 mg/mL rabbit anti-BSA (Accurate Chemical and Scientific Corp., Westbury, NY, USA) and 3 μL of 5 mg/mL BSA-FITC (Sigma-Aldrich) were added to 950 μL of RPMI 1640 (Sigma-Aldrich). This combination was noted to be the point of equivalence in preliminary experiments. The mixture was incubated at 37°C for 1 h and overnight at 4°C. The next day, the precipitate was centrifuged at 10,000g for 10 min. The pellet was washed twice with RPMI and resuspended in 500 μL of RPMI. The IC preparation was made fresh weekly, aliquoted, and stored at −20°C.
For IC opsonization, 5 μL of stock IC was incubated in a total volume of 100 μL containing 30% AB + serum ± 20 mM EDTA (negative control) at 37°C for 30 min with constant rocking motion. In the meantime, 100 μL of Alsevers buffer containing thawed or freshly collected erythrocytes from study participants or from an aparasitemic standard control was added to individual wells of a separate 96-well plate. After centrifugation at 500g for 5 min, the cells were resuspended in 100 μL of plain RPMI1640. This was followed by the addition to separate wells of IC opsonized with or without EDTA, or of plain RPMI 1640 (unstained control). To determine to what extent the opsonized ICs were binding to red cell CR1, some red cell samples were pre-incubated with RPMI 1640 containing a 1:20 dilution of chicken anti-CR1 Fab (Accurate) or an irrelevant chicken Fab prepared from whole IgY (Accurate) using a Fab preparation kit (Pierce Biotechnology Inc., Rockport, IL, USA) followed by the addition of IC and incubation as above. Following the incubation with IC, the erythrocytes were washed twice in 200 μL of ice cold RPMI1640, resuspended in PBS containing 1% paraformaldehyde, and stored at 4°C until acquisition. After gating, the erythrocyte FITC fluorescence was measured using logarithmic amplification and the cut off for the positive red cells was set using the unstained cells. The percent of positive red cells (IC binding capacity) was calculated based on this cut off. To control for day-to-day variation, the IC binding capacity was normalized to the mean IC binding capacity of the red cell standard used throughout using a formula similar to the one used for correction of the CR1 and CD55 MFI (above).
Measurement of Complement Susceptibility
All centrifugation steps were at 500g for 5 min. Rabbit polyclonal anti-C3a (negative control antibody) and anti-C3b (Accurate) were pre-adsorbed × 3 by adding a 1:50 dilution of antibody in PBS to an equal volume of packed pre-washed erythrocytes from the normal standard control. The cells were incubated for 1 h at 37°C with constant rocking followed by centrifugation. The pre-adsorbed antibody was frozen at −20°C in single-use aliquots. 100 μL pre-washed fresh or thawed erythrocytes in Alsevers buffer was added to wells of a 96-well plate and resuspended in PBS (baseline C3b determination) or PBS containing 30% AB + serum ± 20 mM EDTA plus IC to induce complement activation. The IC was used at a dilution that induced a predetermined amount of C3b deposition on standard cells, which in most cases was 1:20. After incubation for 10 min at 37°C, the cells were washed twice in PBS and resuspended in 50 μL of pre-adsorbed rabbit anti-C3b, anti-C3a, or in PBS (unstained control), and incubated for 10 min at 37°C. After two washes, the cells were resuspended in 1:50 anti-rabbit PE (Sigma-Aldrich) for 30 min at room temperature, washed twice, resuspended in PBS. Acquisition was carried out as above. Red cells with IC deposition (FITC-positive) were excluded from the analysis gate. The baseline C3b MFI was adjusted by subtracting the C3a MFI. To subtract the baseline C3b contribution after complement activation with IC, C3b MFI obtained in samples incubated with EDTA was subtracted from the C3b MFI in samples without EDTA.
Statistical Analysis
Statistical analysis was carried out using SPSS v11.5 (Spss Inc., Chicago, IL, USA). To compare the clinical parameters at enrollment or follow-up between cases and controls or between SMA and CM cases, we used the paired or independent samples t-test respectively. For the complement regulatory protein levels, IC binding capacity, and C3b MFI, the primary comparisons were between cases at enrollment and follow-up, and between cases and controls at enrollment. For this, we used the general linear model (GLM) to carry out matched one-way analysis of variance (ANOVA) across the three levels (cases at enrollment and follow-up, and controls at enrollment) including a term when appropriate for sample type (fresh or frozen). Dunnett’s test was used to adjust for multiple comparisons using the cases at enrollment as the comparator. To compare controls at enrollment and follow-up, we used the paired t-test. Categorical variables were compared using the Pearson chi-square. All tests were two-sided with α≤0.05.
RESULTS
Demographics and Clinical Characteristics of the Participants
The demographic and clinical characteristics of the participants at the time of enrollment are summarized in Table 1. Consistent with previous observations (17,22), CM cases tended to be older than SMA cases (P = 0.001). The corrected reticulocyte counts were similar between cases and controls at enrollment as well as between SMA and CM cases (P = 0.82). At follow-up, the mean (SD) corrected reticulocyte count for SMA cases (N = 64) and controls (N = 61) was 1.6 (2.5) and 1.2 (1.4) respectively, P = 0.21 by paired t-test. The same parameter for CM cases (N = 26) and controls (N = 43) were 1.1 (1.7) and 1.0 (1.0) respectively, P = 0.71 by matched ANOVA. There were no significant differences between cases and controls in the distribution of districts of origin (P = 0.23 for SMA cases and controls, and P = 0.10 for CM cases and controls) or in the distribution of ethnic groups (P = 0.46 for SMA cases and controls, and P = 0.29 for CM cases and controls). There were five deaths among patients with SMA (6.7% mortality) all of which occurred during the initial hospitalization. There was one death among the SMA controls (1.3% mortality) which was due to an episode of severe diarrhea. One in-hospital death occurred among CM cases recruited at KDH (4.2% mortality) and no deaths occurred among CM cases at NPGH.
Table 1.
Clinical and demographic characteristics of the study groups
| Nyanza Provincial General Hospital (NPGH)
|
Kisii District Hospital (KDH)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Severe Anemia
|
Cerebral Malaria
|
Cerebral Malaria
|
|||||||
| Variable | Cases (N = 75) | Controls (N = 74) | P | Cases (N = 8) | Controls (N = 15) | P | Cases (N = 24) | Controls (N = 37) | P |
| Mean age (SD) in months | 16.9 (13.7) | 16.8 (13.3) | NSb | 35.9 (15.9) | 35.7 (15.7) | NS | 32.2 (20.4) | 32.7 (16.8) | NS |
| No. Females (%) | 34 (45.3) | 33 (44.6) | NS | 4 (50) | 8 (53.3) | NS | 12 (50) | 19 (51.4) | NS |
| Mean Haemoglobin (SD) g/dL | 4.7 (0.9) | 8.7 (1.5) | NAc | 8.6 (1.6) | 9.1 (1.3) | 0.45 | 9.2 (1.8) | 9.6 (1.7) | 0.45 |
| %Corrected Reticulocyte (SD)d | 1.5 (1.6) | 1.4 (1.9) | NS | 1.6 (1.6) | 1.4 (1.4) | NS | 1.5 (1.4) | 1.5 (1.8) | NS |
| Mean # Parasites/μL ×10−3 (SD) | 71.1 (27.1) | 50.4 (32.1) | 0.12 | 49.5 (46.8) | 49.2 (24.6) | 0.88 | 67.7 (76.4) | 40.4 (60.9) | 0.10 |
| Home District (%) | |||||||||
| Kisumu | 30 (40.0) | 38 (51.3) | 4 (50) | 6 (40) | 0 | 0 | |||
| Bondo | 7 (9.3) | 4 (5.4) | 0 | 4 (26.7) | 0 | 0 | |||
| Nyando | 15 (20.0) | 5 (6.7) | 0 | 2 (13.3) | 0 | 0 | |||
| Siaya | 12 (16.0) | 11 (14.9) | 1 (12.5) | 1 (6.7) | 0 | 0 | |||
| Vihiga | 3 (4.0) | 3 (4.1) | 1 (12.5) | 1 (6.7) | 0 | 0 | |||
| Rachuonyo | 2 (2.7) | 4 (5.4) | 0 | 0 | 0 | 0 | |||
| Kisii | 0 | 0 | 0 | 0 | 6 (25) | 10 (27.0) | |||
| Kisii Central | 0 | 0 | 0 | 0 | 10 (41.7) | 24 (64.9) | |||
| Gucha | 0 | 0 | 0 | 0 | 3 (12.5) | 2 (5.4) | |||
| Otherse | 6 (8.0) | 9 (12.2) | 2 (25) | 1 (6.7) | 5 (20.8) | 1 (2.7) | |||
| Ethnic group (%) | |||||||||
| Luo | 66 (88.0) | 65 (87.8) | 7 (87.5) | 14 (93.3) | 1 (4.2) | 1 (2.7) | |||
| Luhya | 6 (8.0) | 9 (12.2) | 1 (12.5) | 1 (6.7) | 2 (8.3) | 0 | |||
| Abagusii | 0 | 0 | 0 | 0 | 21 (87.5) | 36 (97.3) | |||
| Othersf | 3 (4.0) | 0 | 0 | 0 | 0 | 0 | |||
All P values were obtained using paired t-test.
NS = Not Significant.
NA = Not applicable.
Corrected % reticulocyte was calculated as = % reticulocyte × (Subject Hct/Normal Hct) where normal Hct was 35% for age 0 to ≤6 months, 33% for age >6 to ≤12 months, 30% for age >12 to ≤24 months, 33% for age >24 to ≤ 48 months, and 36% for age >48 months.
Levels of Red Cell Complement Regulatory Proteins CR1 and CD55
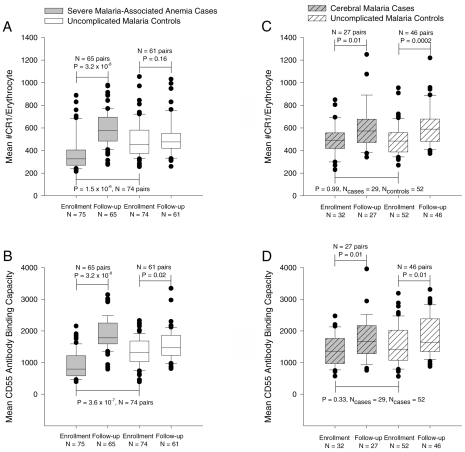
SMA cases had lower levels of erythrocyte CR1 and CD55 than their age and gender-matched controls at enrollment (Figures 1A and 1B). These observations are consistent with our previous findings (14,17). At follow-up, the CR1 and CD55 levels rebounded above the control levels. The nature of the rebound is not clear but could not be accounted by the effect of blood transfusion because it also was seen among children who did not receive transfusion. It also could not be due to reticulocytosis because the corrected mean(SD) reticulocyte count for SMA cases and controls did not differ at follow-up, 1.6 (2.5) versus 1.2 (1.4) P = 0.21 by paired t-test. However, the rebound effect is temporary because we showed in a longer follow-up study that this difference disappears over time (14). By contrast (Figures 1C and 1D), children with CM had similar level of CR1 and CD55 as their uncomplicated malaria controls. The level of erythrocyte CR1 and CD55 was significantly lower in children with SMA than in children with CM upon enrollment (CR1 P = 0.001, 95% CI of mean difference 47 to 180 CR1 molecules/erythrocyte; CD55 P < 0.001, 95% CI of mean difference 236 to 620 ABC).
Figure 1.
CR1 and CD55 expression on red cells of children with SMA, CM, and their age and gender-matched controls. Data are presented as box plots using all the available values, where the boundary of the box indicates the values between the 25th and 75th percentiles, a line within the box marks the median, and the whiskers indicate the 90th and 10th percentiles. The outlying points are represented as dots. P values for the comparisons between cases at enrollment with cases at follow-up and controls at enrollment are based on matched analysis using Dunnett’s test for multiple comparisons. P values for the comparison between controls at enrollment and follow-up are based on a paired t-test.
IC Binding Capacity
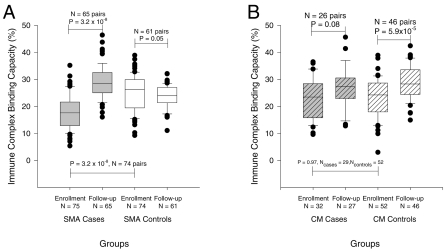
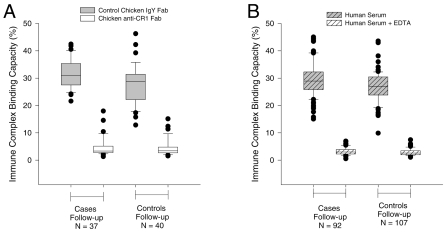
To determine if the red cell complement regulatory protein deficiencies led to declines in IC binding capacity, we measured this parameter in the same populations. Both fresh and frozen samples were used in these assays. There were no significant differences in the proportions of fresh versus frozen samples used between cases and controls or between enrollment and follow-up (data not shown) and we found no significant difference between the IC binding capacity of fresh and frozen samples. However, to control for any effect of the sample type, the final model included a term for this factor. Figure 2A shows that the IC binding capacity of erythrocytes from children with SMA was low at enrollment and also was much lower than that of corresponding controls. As with CR1 and CD55, we also observed a rebound effect of IC binding capacity in children with SMA after treatment. The IC binding capacity of SMA controls did not change with infection. The IC binding capacity of red cells from children with CM (Figure 2B) and their controls was low at enrollment but there was no difference between the two groups at enrollment or follow-up. These observations are in keeping with the changes in CR1 levels observed (Figure 1). Finally, the IC binding capacity of SMA cases was lower than that for CM cases at enrollment, even after adjustment for sample type (P < 0.001, 95% CI of the mean difference 2.6% to 8.3%). To demonstrate the specificity of our assay, we used chicken anti-human CR1 Fab or EDTA to block binding of C3b-oposonized IC to red cells. Either reagent resulted in near complete inhibition of IC binding to erythrocytes, confirming that most of the IC was binding directly to CR1, and that this binding was complement-dependent (Figure 3).
Figure 2.
IC binding capacity of red cells of children with SMA, CM, and their age and gender-matched controls. The data represent the percent of red cells with surface IC (IC binding capacity) when red cells were incubated with opsonized ICs in plain RPMI 1640 without anti-CR1. P values for the comparisons between cases at enrollment with cases at follow-up and controls at enrollment are based on matched analysis using Dunnett’s test for multiple comparisons. P values for the comparison between controls at enrollment and follow-up are based on a paired t-test.
Figure 3.
Effect of EDTA and anti-CR1 on the IC binding capacity of red cells of SMA and CM cases, and their age and gender-matched controls. Anti-CR1 Fab or total purified IgY Fab were pre-incubated with red cells prior to the addition of opsonized ICs (Panel A) or red cells were incubated with ICs opsonized in the presence or absence of EDTA (Panel B).
Complement Susceptibility
We measured complement deposition on red cells at baseline and after in vitro complement activation via the classical pathway using ICs. We present these results as the C3b MFI of the total red cell populations in the gate after exclusion of FITC-positive (IC-bearing) cells. Both fresh and frozen samples were used in these assays and therefore, we controlled for this variable in the final models.
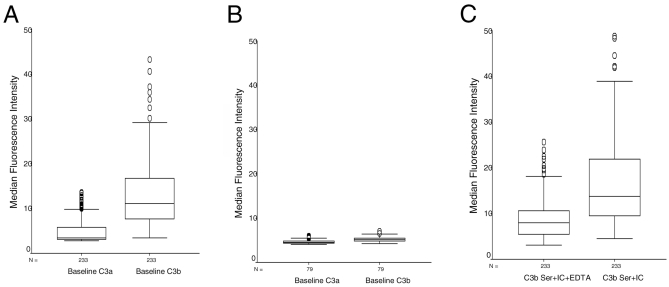
Figure 4A shows the uncorrected values for 233 samples at enrollment. The baseline C3a MFI is very low compared with the uncorrected baseline C3b MFI, suggesting there is very little nonspecific C3 deposition and low nonspecific reactivity from the anti-C3b antibody. The corrected baseline C3b MFI presented in subsequent figures represents the subtraction of the C3a MFI from the uncorrected C3b MFI. By comparison, Figure 4B shows very low reactivity of uncorrected anti-C3a and anti-C3b MFI using red cells from 79 uninfected asymptomatic individuals of age 12 to 45, further confirming that the findings in the children in this case-control study are specific. During the activation of C3 with IC (Figure 4C), inclusion of EDTA resulted in low MFI, suggesting low C3b deposition and low non-specific binding of the IC to the red cells. The final corrected C3b MFI after IC stimulation was obtained by subtraction of the C3b MFI in the presence of EDTA from that obtained in the absence of EDTA.
Figure 4.
Uncorrected values for baseline C3a and C3b, and C3b after complement stimulation. A) Baseline C3a and C3b MFI on red cells of all participants in the case-control study at enrollment. B) Baseline C3a and C3b MFI of normal aparasitemic controls from a cross-sectional study. C) C3b deposition after complement activation with IC with and without EDTA.
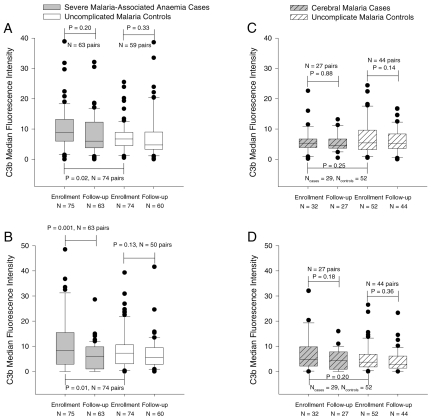
We found that red cells of children with SMA had higher C3b MFI upon enrollment than that on red cells of children with uncomplicated malaria (Figure 5A). Although the red cell C3b MFI was lower at follow-up than at enrollment for children with SMA, this difference was not statistically significant. By contrast, there were no significant differences in red cell C3b MFI between children with CM and their controls (Figures 5C). Children with SMA at enrollment had higher red cell C3b MFI than children with CM at enrollment (P = 0.02, 95% CI of the mean difference = 0.6 to 8.4).
Figure 5.
C3b deposition on red cells in vivo (panels A and C) and following complement activation ex vivo via the classical pathway (panels B and D). P values for the comparisons between cases at enrollment with cases at follow-up and controls at enrollment are based on matched analysis using Dunnett’s test for multiple comparisons. P values for the comparison between controls at enrollment and follow-up are based on a paired t-test.
Upon activation of the complement cascade via the classical pathway, C3b deposition was measured as MFI correcting for the baseline C3b deposition. The enrollment red cell C3b MFI was higher for children with SMA than for their controls (Figure 5B). The red cell C3b MFI for children with SMA dropped at follow-up but remained unchanged for their controls. On the other hand, the C3b MFI of red cells from children with CM did not differ from that of their uncomplicated malaria controls and did not change significantly between enrollment and follow-up (Figure 5D). The red cell C3b-MFI after complement activation was higher for children with SMA than for children with CM at enrollment (P = 0.02, 95% CI for the mean difference = 1.0 to 12.1).
DISCUSSION
Severe malaria-associated anemia is a complex phenomenon. Multiple pathophysiologic mechanisms have been proposed (23,24). In most cases, as in our series, the erythropoiesis is depressed as evidenced by an inappropriately low reticulocyte count. However, because of the 120-day lifespan of red cells, bone marrow depression alone cannot account for this manifestation. Central to the pathophysiology of this severe anemia is the destruction of uninfected erythrocytes (5). The mechanism of destruction remains unclear. Complement activation is a major suspect because complement deposition has been observed in red cells of children with malaria (6,7) and it is implicated in the removal of senescent and damaged erythrocytes from circulation (25). Therefore, we have concentrated on studying the complement regulatory protein machinery of red cells.
We reported previously that red cells of children with SMA have low levels of CR1 and CD55 and, in addition, have increased surface IgG and increased susceptibility to phagocytosis (16). Because CR1 and CD55 protect red cells from complement (C3b) attack, their reduced levels on red cells could result in increased C3b deposition at sites other than CR1 and CD55. We also have shown that these children have increased levels of circulating ICs (14,15). Thus, children with SMA have a source of complement activation and a defect that may sensitize erythrocytes to complement-mediated damage. In the present study, we set out to determine whether the deficiencies in erythrocyte complement regulatory proteins are physiologically relevant. We expanded the definition of severe anemia to include children with Hb ≤6 g/dL in an effort to detect children earlier in the path to severe anemia and to enhance our ability to detect early changes on erythrocytes prior to their removal from circulation. In addition, unlike previous studies of complement deposition on red cells of children with SMA (26), we included children with CM and their controls to determine the specificity of our findings.
The declines in the levels of erythrocyte CR1 and CD55 in children with SMA reported here are similar to the ones we have reported in previous studies(16,17). This decline is very specific to SMA because it is not seen in children with CM. Although the CR1 levels of CM controls at enrollment are significantly lower than at follow-up, there was no difference between CM cases and controls at enrollment (Figure 2). The high level of statistical significance for the comparison between CM controls at enrollment and follow-up is likely a reflection of the large number of controls. We have proposed that the loss of complement regulatory proteins is related to the repeated episodes of malaria attacks that are characteristic of areas of high intensity malaria transmission where severe anemia is most common, such as the Lake Victoria region of western Kenya (27). With each bout of malaria, ICs are formed and bound to CR1 on red cells. In the liver and spleen, the ICs are removed by macrophages together with membrane CR1 and possibly CD55(11,28–30).
Corresponding to the level of CR1, erythrocytes of children with SMA at enrollment have the lowest IC binding capacity of any group at any time. Although the absolute decline in IC binding capacity is small (95% CI for difference between SMA cases and controls at enrollment = 4.9%–9.5%), these changes represent declines of 25% to 38% of the median binding capacity. Thus, red cells of children with SMA have a significant decline in their ability to bind ICs. When not bound to red cells, the ICs can deposit in tissues and on the endothelium where they continue to activate complement and stimulate pro-inflammatory cytokines (30,31).
To assess the susceptibility of red cells to C3b deposition, we measured surface C3b both at baseline and after in vitro complement activation with IC. Because our detection antibody was raised against C3b, we cannot exclude the possibility that it recognizes terminal C3 components such as C3bi, and C3d,g also. However, C3b, C3bi, and C3d all can induce phagocytosis, especially in the presence of IgG (32–34), which we have shown to be present on red cells of children with SMA (16). Red cells of children with SMA had higher C3b deposition than their controls and CM cases, both at baseline and after complement activation as measured by C3b MFI. On the other hand, we saw no difference in the C3b deposition on red cells of children with CM and their controls.
Our data suggest that the deficiencies in complement regulatory proteins seen in the red cells of children with SMA translate in declines in the IC binding capacity and increases in complement susceptibility both in vivo and ex vivo. These changes, together with increased IgG deposition on red cells (16) and up-regulation of Fc and complement receptors on macrophages activated by TNF-α (23,35), are likely responsible for increased destruction of red cells in children with SMA. Further, the striking difference in findings between red cells of children with SMA and CM, despite similar parasite density, lend more strength to the hypothesis that the losses in CR1 and CD55 are significant contributors to, if not directly responsible for, the increased destruction of erythrocytes of children with SMA.
ACKNOWLEDGMENTS
We would like to thank the children who participated in our studies, as well as their parents, for their willingness to contribute to this research endeavor. We are indebted to our dedicated staff of clinicians, nurses, drivers, and field workers who made these studies possible. We also thank Craig Morrissette for helpful comments on the statistical analysis. This work is published with the permission of the Office of the Director, the Kenya Medical Research Institute.
This work was supported in part by the US Army Medical Research and Materiel Command and by a grant from the National Heart Lung and Blood Institute (R01 HL 7502). Walter Otieno was supported by a training grant from the Fogarty International Center (1 D43 TW06239).
Footnotes
Online address: http://www.molmed.org
DISCLAIMER
The views of the authors do not purport to reflect the position of the Department of the Army or the Department of Defense. The U.S. Government has the right to retain a nonexclusive, royalty-free license in and to any copyright covering this paper.
REFERENCES
- 1.World Health Organization. The World Health Report: Changing History. Geneva: WHO; 2004. [Google Scholar]
- 2.World Health Organization CDC. Severe falciparum malaria. Trans R Soc Trop Med Hyg. 2000;94(Suppl 1):S1–90. [PubMed] [Google Scholar]
- 3.Coleman RM, Rencricca NJ, Rittershaus CW, Brissette WH. Malaria: decreased survival of transfused normal erythrocytes in infected rats. J Parasitol. 1976;62:138–40. [PubMed] [Google Scholar]
- 4.Salmon MG, de Souza JB, Butcher GA, Playfair JH. Premature removal of uninfected erythrocytes during malarial infection of normal and immunodeficient mice. Clin Exp Immunol. 1997;108:471–6. doi: 10.1046/j.1365-2249.1997.3991297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jakeman GN, Saul A, Hogarth WL, Collins WE. Anaemia of acute malaria infections in non-immune patients primarily results from destruction of uninfected erythrocytes. Parasitology. 1999;119 ( Pt 2):127–33. doi: 10.1017/s0031182099004564. [DOI] [PubMed] [Google Scholar]
- 6.Facer CA, Bray RS, Brown J. Direct Coombs antiglobulin reactions in Gambian children with Plasmodium falciparum malaria. I Incidence and class specificity. Clin Exp Immunol. 1979;35:119–27. [PMC free article] [PubMed] [Google Scholar]
- 7.Facer CA. Direct Coombs antiglobulin reactions in Gambian children with Plasmodium falciparum malaria. II Specificity of erythrocyte-bound IgG. Clin Exp Immunol. 1980;39:279–88. [PMC free article] [PubMed] [Google Scholar]
- 8.Parker CJ. Regulation of complement by membrane proteins: an overview. Curr Top Microbiol Immunol. 1992;178:1–6. doi: 10.1007/978-3-642-77014-2_1. [DOI] [PubMed] [Google Scholar]
- 9.Miwa T, Song WC. Membrane complement regulatory proteins: insight from animal studies and relevance to human diseases. Int Immunopharmacol. 2001;1:445–59. doi: 10.1016/s1567-5769(00)00043-6. [DOI] [PubMed] [Google Scholar]
- 10.Madi N, Paccaud JP, Steiger G, Schifferli JA. Immune complex binding efficiency of erythrocyte complement receptor 1 (CR1) Clin Exp Immunol. 1991;84:9–15. doi: 10.1111/j.1365-2249.1991.tb08116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reinagel ML, Gezen M, Ferguson PJ, Kuhn S, Martin EN, Taylor RP. The primate erythrocyte complement receptor (CR1) as a privileged site: binding of immunoglobulin G to erythrocyte CR1 does not target erythrocytes for phagocytosis. Blood. 1997;89:1068–77. [PubMed] [Google Scholar]
- 12.Jhaveri KN, et al. Autoantibodies, immunoglobulins, complement and circulating immune complexes in acute malaria. Natl Med J India. 1997;10:5–7. [PubMed] [Google Scholar]
- 13.Tyagi P, Biswas S. Naturally occurring plasmodia-specific circulating immune complexes in individuals of malaria endemic areas in India. Indian J Malariol. 1999;36:12–8. [PubMed] [Google Scholar]
- 14.Stoute JA, Odindo AO, Owuor BO, Mibei EK, Opollo MO, Waitumbi JN. Loss of red blood cell-complement regulatory proteins and increased levels of circulating immune complexes are associated with severe malarial anemia. J Infect Dis. 2003;187:522–5. doi: 10.1086/367712. [DOI] [PubMed] [Google Scholar]
- 15.Mibei EK, Orago AS, Stoute JA. Immune complex levels in children with severe Plasmodium falciparum malaria. Am J Trop Med Hyg. 2005;72:593–9. [PubMed] [Google Scholar]
- 16.Waitumbi JN, Opollo MO, Muga RO, Misore AO, Stoute JA. Red cell surface changes and erythrophagocytosis in children with severe Plasmodium falciparum anemia. Blood. 2000;95:1481–6. [PubMed] [Google Scholar]
- 17.Waitumbi JN, Donvito B, Kisserli A, Cohen JH, Stoute JA. Age-related changes in red blood cell complement regulatory proteins and susceptibility to severe malaria. J Infect Dis. 2004;190:1183–91. doi: 10.1086/423140. [DOI] [PubMed] [Google Scholar]
- 18.Hay SI, Noor AM, Simba M, Busolo M, Guyatt HL, Ochola SA, Snow RW. Clinical epidemiology of malaria in the highlands of western Kenya. Emerg Infect Dis. 2002;8:543–8. doi: 10.3201/eid0806.010309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molyneux ME, Taylor TE, Wirima JJ, Borgstein A. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. Q J Med. 1989;71:441–59. [PubMed] [Google Scholar]
- 20.Kokwaro G, Mwai L, Nzila A. Artemether/lumefantrine in the treatment of uncomplicated falciparum malaria. Expert Opin Pharmacother. 2007;8:75–94. doi: 10.1517/14656566.8.1.75. [DOI] [PubMed] [Google Scholar]
- 21.Cockburn IA, Donvito B, Cohen JH, Rowe JA. A simple method for accurate quantification of complement receptor 1 on erythrocytes preserved by fixing or freezing. J Immunol Methods. 2002;271:59–64. doi: 10.1016/s0022-1759(02)00368-x. [DOI] [PubMed] [Google Scholar]
- 22.Snow RW, et al. Severe childhood malaria in two areas of markedly different falciparum transmission in east Africa. Acta Trop. 1994;57:289–300. doi: 10.1016/0001-706x(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 23.Akanmori BD, et al. Distinct patterns of cytokine regulation in discrete clinical forms of Plasmodium falciparum malaria. Eur Cytokine Netw. 2000;11:113–8. [PubMed] [Google Scholar]
- 24.Kurtzhals JA, Rodrigues O, Addae M, Commey JO, Nkrumah FK, Hviid L. Reversible suppression of bone marrow response to erythropoietin in Plasmodium falciparum malaria. Br J Haematol. 1997;97:169–74. doi: 10.1046/j.1365-2141.1997.82654.x. [DOI] [PubMed] [Google Scholar]
- 25.Shapiro S, Kohn D, Miller B, Gershon H. Erythrocytes from young but not elderly donors can bind and degrade immune complex- and antibody-bound C3 in vitro. Clin Exp Immunol. 1994;95:181–90. doi: 10.1111/j.1365-2249.1994.tb06034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goka BQ, et al. Complement binding to erythrocytes is associated with macrophage activation and reduced hemoglobin in Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg. 2001;95:545–9. doi: 10.1016/s0035-9203(01)90036-7. [DOI] [PubMed] [Google Scholar]
- 27.Stoute JA. Complement-regulatory proteins in severe malaria: too little or too much of a good thing? Trends Parasitol. 2005;21:218–23. doi: 10.1016/j.pt.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Cornacoff JB, Hebert LA, Smead WL, VanAman ME, Birmingham DJ, Waxman FJ. Primate erythrocyte-immune complex-clearing mechanism. J Clin Invest. 1983;71:236–47. doi: 10.1172/JCI110764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cosio FG, Shen XP, Birmingham DJ, Van Aman M, Hebert LA. Evaluation of the mechanisms responsible for the reduction in erythrocyte complement receptors when immune complexes form in vivo in primates. J Immunol. 1990;145:4198–206. [PubMed] [Google Scholar]
- 30.Waxman FJ, et al. Complement depletion accelerates the clearance of immune complexes from the circulation of primates. J Clin Invest. 1984;74:1329–40. doi: 10.1172/JCI111543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu X, Wittwer AJ, Carr LS, Crippes BA, DeLarco JE, Lefkowith JB. Cytokine-induced neutrophil chemoattractant mediates neutrophil influx in immune complex glomerulonephritis in rat. J Clin Invest. 1994;94:337–44. doi: 10.1172/JCI117326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ehlenberger AG, Nussenzweig V. The role of membrane receptors for C3b and C3d in phagocytosis. J Exp Med. 1977;145:357–71. doi: 10.1084/jem.145.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaither TA, Vargas I, Inada S, Frank MM. The complement fragment C3d facilitates phagocytosis by monocytes. Immunology. 1987;62:405–11. [PMC free article] [PubMed] [Google Scholar]
- 34.Fishelson Z. Complement C3: a molecular mosaic of binding sites. Mol Immunol. 1991;28:545–52. doi: 10.1016/0161-5890(91)90169-k. [DOI] [PubMed] [Google Scholar]
- 35.Kitagawa S, et al. Activation of human monocyte functions by tumor necrosis factor: rapid priming for enhanced release of superoxide and erythrophagocytosis, but no direct triggering of superoxide release. Exp Hematol. 1996;24:559–67. [PubMed] [Google Scholar]