Abstract
The heritability of blood pressure responses to dietary intervention has not been well studied. We examined the heritability of blood pressure responses to dietary sodium and potassium intake in a family feeding-study among 1,906 study participants living in rural north China. The dietary intervention included a 7-day low sodium-feeding (51.3 mmol/day), a 7-day high sodium-feeding (307.8 mmol/day), and a 7-day high-sodium plus potassium-supplementation (60 mmol/day). Blood pressure was measured 9 times during the 3-day baseline period preceding the intervention and also during the last 3 days of each intervention phase using a random-zero sphygmomanometer. Heritability was computed using maximum likelihood methods under a variance components model as implemented in the computer program SOLAR. The heritabilities of baseline blood pressure were 0.31 for systolic, 0.32 for diastolic, and 0.34 for mean arterial pressure. The heritabilities increased significantly under dietary intervention and were 0.49, 0.49, and 0.51 during low-sodium, 0.47, 0.49, and 0.51 during high-sodium, and 0.51, 0.52, and 0.53 during potassium-supplementation for systolic, diastolic, and mean arterial pressure, respectively. The heritabilities for percentage blood pressure responses to low-sodium were 0.20, 0.21 and 0.23, to high-sodium were 0.22, 0.33, and 0.33, and to potassium-supplementation were 0.24, 0.21, and 0.25, for systolic, diastolic, and mean arterial pressure, respectively. Our study indicated that the heritabilities of blood pressure under controlled dietary sodium and potassium intake were significantly higher than those under usual diet. In addition, the heritabilities of blood pressure responses to dietary sodium and potassium intake were moderate in this study population.
Keywords: blood pressure, dietary sodium, heritability, potassium supplementation, salt-sensitivity
INTRODUCTION
Observational epidemiological studies conducted in different regions of the world have shown that dietary sodium intake was positively and potassium intake was inversely associated with blood pressure (BP) (1-3). Randomized controlled trials have documented that a moderate sodium reduction or potassium supplementation was significantly associated with a reduced systolic BP (SBP) and diastolic BP (DBP) (4-6). However, despite these results there is also substantial scientific evidence suggesting that BP responses to dietary sodium and potassium intake vary considerably among individuals (6-9). For example, the reduction in BP associated with a low dietary sodium intake was significantly greater among hypertensives, African-Americans, and those who were older (9,10). Genetic factors, however, might also play an important role in determining individual's BP responses to dietary sodium intake (11). It is well established that BP levels and hypertension are heritable, with the proportion of variance explained by genetic factors ranging from 20% to 50% (12). However, the heritability of BP responses to dietary sodium and potassium intake has not been studied (13).
The Genetic Epidemiology Network of Salt Sensitivity (GenSalt) study was designed to examine the genetic influence on BP responses to dietary sodium and potassium intake in rural Chinese families who were ascertained through a proband with pre-hypertension or stage-1 hypertension (14). BP was measured in the study participants at baseline on their usual diet, and during three dietary sodium and potassium intervention periods consisting of one week each of a low-sodium, a high-sodium and a high-sodium plus potassium supplement diet. Here we present data on heritability of BP levels at the baseline observation and during dietary sodium and potassium intervention, as well as BP changes during the dietary intervention.
METHODS
Study Participants
The GenSalt study was designed to investigate the genetic factors that determine an individual's BP responses to dietary sodium and potassium intervention in families at high risk for developing hypertension. The study families were recruited from six sites in rural north China (Hebei, Henan, Shandong, Shaanxi and Jiangsu provinces). The selection of these study sites was based on homogeneity of the study population regarding their ethnicity and environmental exposures, including lifestyle and nutritional factors and habitual dietary intake of high salt and low potassium. The residents in these regions are of Han nationality, the ethnic majority in China. Probands with a mean SBP ≥130 mm Hg and/or DBP ≥85 mm Hg were identified by community-based BP screening. Both two-generation (probands, their parents and siblings) and three-generation (additionally including the probands' spouse and offspring) families were recruited for the study. All siblings, parents, spouses and offspring of the probands were eligible to participate in the baseline examination. However, only probands, siblings, offspring, and spouses of probands from three-generation families participated in the dietary intervention. We excluded the parents from intervention because decreased renal function in the elderly makes high-salt intervention unsafe. The inclusion/exclusion criteria are outlined in detail elsewhere (14). In general, individuals who had stage-2 or more severe hypertension, use of antihypertensive medications, history of clinical cardiovascular disease or diabetes, pregnancy, heavy alcohol use, or currently on a low sodium diet were excluded from the dietary intervention. Also, family structure requirements included the participation of one or more siblings (at baseline and during intervention) and at least one parent of the proband (at baseline only). Among the 1,906 eligible individuals, 1,858 (97.5%) completed the entire dietary sodium and potassium interventions.
Dietary Intervention
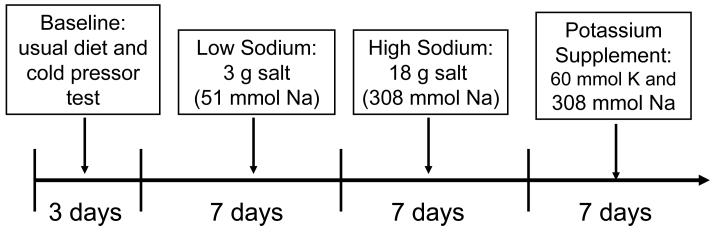
The dietary intervention included low-sodium, high-sodium, and high-potassium feeding among probands and their siblings and offspring (Figure 1). In the first 3-days at the baseline observation, the study participants consumed their usual diet. The dietary feeding study started on day 4. The intervention participants received a low sodium diet (3 grams of salt or 51.3 mmol of sodium per day) for 7 days (day 4 to day 10). Then, they received a high sodium diet (18 grams of salt or 307.8 mmol of sodium per day) for 7 days (day 11 to day 17). During the first 2 intervention phases, potassium intake remained unchanged. In the final week (day 18 to day 24), the participants maintained a high sodium diet and took a 60 mmol potassium supplement. All foods and beverages were prepared by study dieticians and provided by the study staff. The study participants came to the study kitchen for their breakfast, lunch, and dinner during the entire intervention period. The intervention participants were instructed to avoid consuming any foods that were not provided by the study. The results from 24-hour urinary excretion of sodium and potassium at the baseline examination and during each of the three dietary intervention phases showed excellent compliance. For example, the mean (standard deviation) of 24-hour urinary excretions of sodium and potassium were 241.8 (68.2) mmol and 36.8 (10.0) mmol at baseline, 46.7 (15.7) and 31.0 (8.0) during low-sodium intervention, 244.5 (40.4) and 36.2 (8.1) during high-sodium intervention, and 251.9 (36.9) and 77.3 (12.6) during high-sodium plus potassium supplementation intervention.
Figure 1.
The dietary low-sodium, high-sodium, and high-sodium plus potassium-supplementation interventions in the GenSalt study
Data Collection
Three BP measurements were obtained at each clinical visit by trained and certified observers according to a common protocol adapted from procedures recommended by the American Heart Association (15). BP was measured with the participant in the sitting position after 5 minutes of rest. In addition, participants were advised to avoid alcohol, cigarette smoking, coffee/tea, and exercise for at least 30 minutes prior to their BP measurement. A random-zero sphygmomanometer was used, and one of four cuff sizes (pediatric, regular adult, large, or thigh) was chosen on the basis of the circumference of the participant's arm (15). BP was measured each morning of the 3-day baseline observation, and on days 2, 5, 6 and 7 of each intervention period by the same BP technician using the same sphygmomanometer to avoid observation variation. Overnight fasting blood samples were drawn once by venipuncture at baseline examination and at each of the intervention periods. One 24-hour and 2 timed-overnight urinary specimens were collected at the baseline examination and on the last 3 days of each intervention period to measure sodium and potassium.
Statistical Analyses
BP levels at baseline and during intervention were calculated as the mean of 9 measurements from 3 clinical visits during the 3-day baseline observation or on days 5, 6 and 7 of each intervention period. Mean arterial pressure (MAP) = DBP + (SBP-DBP)/3. BP response to low-sodium = BP at low-sodium – BP at baseline; BP response to high-sodium = BP at high-sodium – BP at low-sodium; and BP response to potassium supplement = BP at high-sodium and potassium-supplementation – BP at high-sodium. Area-under-curve (AUC) was computed using trapezoidal method and BP from days 0, 2, 5, 6 and 7 of each intervention period. BP from the last day of the immediately prior period was used as the value on day 0 of the subsequent intervention period. AUC utilized all BP information during each intervention and should have less measurement error compared to BP response.
The BP data were adjusted for the effects of age and BP examination room temperature, separately within sex-generation-field center groups. In summary, each measure was regressed on the covariates in a stepwise manner, and only significant terms (0.05 level) were retained. The residual variance was also examined (i.e. heteroskedasticity) by regressing the squared residual from the first regression on the same covariates (stepwise) and retaining significant terms. The final adjusted phenotype was computed as the residual from the first regression, divided by the square root of the predicted score from the second regression. A final standardization step was taken to ensure a mean of zero and a standard deviation of one. These adjusted and standardized scores were used as the analysis variables.
Heritability of the adjusted and standardized scores was computed using maximum likelihood methods under a variance components model as implemented in the computer program SOLAR version 2.1.4 (16). In this model the residual phenotypic variance is partitioned into 2 components, one representing the so-called polygenic (additive) heritability, and the other representing all of the remaining nonadditive (non-familial) effects. In summary, observed covariances between pairs of subjects within a pedigree are compared to the expected values based on the product of their coefficients of relationship. A log likelihood (−2 ln L) is computed for each family and then summed across all families for an overall fit of the model to the data. To correct for ascertainment for high BP, the likelihood of each family is conditioned on the proband's value. Heritability is defined as the percentage of variance due to familial factors (computed as the variance due to the additive genetic effect divided by the total variance), and is tested for significance using a likelihood ratio test (LRT). The LRT is −2 ln L under the null hypothesis of no genetic effect (h2 = 0) compared to −2 ln L for the alternative (where h2 is estimated). The difference is asymptotically distributed as a χ2 with a single degree of freedom.
The Institutional Review Boards at all participating institutes approved the GenSalt study. In addition, the National Human Genomic Resource Administration of China approved the study. Written informed consents for the baseline observation and for the intervention were obtained from each participant prior to data collection or intervention, respectively.
RESULTS
The family structures ranged from 2 to 3 generations, with reported family relationships being verified using genome-wide microsatellite data. Table 1 shows the resulting number of individuals and relative pairs. There were a total of 3,149 individuals in 658 families having baseline BP data, and 1,871 individuals having dietary intervention data at least for the low-sodium intervention phase.
Table 1.
Sample Sizes and Family Structures
| Relative Type | Dietary Intervention |
Change during Intervention |
|||||
|---|---|---|---|---|---|---|---|
| Baseline | Low- sodium |
High- sodium |
High-sodium and potassium supplementation |
Baseline to low-sodium |
Low-to high-sodium |
High-sodium to potassium supplementation |
|
| Total Number of Individuals | 3,153 | 1,871 | 1,860 | 1,843 | 1,871 | 1,859 | 1,838 |
| Number of Relative Pairs | |||||||
| Unrelated | 1,051 | 280 | 268 | 266 | 280 | 268 | 266 |
| Parent-Offspring | 3,395 | 340 | 339 | 333 | 340 | 339 | 333 |
| Sibling | 1,527 | 1,493 | 1,478 | 1,466 | 1,493 | 1,477 | 1,460 |
| Grandparent-Grandchild | 271 | – | – | – | – | – | – |
| Avuncular | 434 | 404 | 402 | 394 | 404 | 402 | 394 |
| Half-Sibling | 16 | 16 | 16 | 16 | 16 | 16 | 16 |
| Grand Avuncular | 7 | – | – | – | – | – | – |
| Half Avuncular | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| 1st Cousins | 57 | 57 | 57 | 57 | 57 | 57 | 57 |
| 1st Cousins Once Removed | 17 | 17 | 17 | 17 | 17 | 17 | 17 |
Baseline evaluations were performed for the entire sample. The parents of the proband were not included in the sodium and potassium intervention leading to the reductions in sample sizes. There were a total of 676 probands from 658 pedigrees.
Table 2 shows the baseline characteristics in terms of age, sex, body mass index (BMI) and BP measurements, as well as BP levels during dietary sodium and potassium intervention and BP responses to the dietary intervention. As expected, the probands had higher mean baseline levels of BMI, SBP and DBP than their siblings, spouses and offsprings while the parents had the highest baseline BP levels among all groups. BP during dietary low-sodium, high-sodium, and potassium-supplementation intervention also followed the same pattern, higher in probands than their siblings, spouses and offspring. Overall, mean BP decreased from baseline to low-sodium intervention and from high-sodium to high-sodium and potassium-supplementation but increased from low-sodium to high-sodium intervention. The BP responses in every group were significantly different from zero and the responses were greater in probands compared to their siblings, spouses and offspring.
Table 2.
Blood Pressure Levels at Baseline and Intervention and Responses to Dietary Intervention
| Trait | Probands | Parents | Siblings | Spouses | Offspring |
|---|---|---|---|---|---|
| Number of subjects | 676 | 1,247 | 956 | 69 | 205 |
| Age, years | 41.0 ± 8.3 | 67.6 ± 8.5 | 39.6 ± 7.7 | 49.1 ± 6.7 | 23.5 ± 6.5 |
| Male, % | 60.4 | 48.7 | 51.1 | 33.3 | 44.4 |
| Body mass index, kg/m2 | 24.2 ± 3.3 | 22.8 ± 3.4 | 23.1 ± 2.8 | 23.4 ± 3.7 | 21.5 ± 3.3 |
| BP at baseline, mm Hg | |||||
| Systolic | 128.0 ± 11.4 | 136.7 ± 24.0 | 111.6 ± 11.5 | 112.6 ± 14.9 | 106.6 ± 10.3 |
| Diastolic | 80.3 ± 9.0 | 75.0 ± 11.7 | 71.0 ± 9.9 | 72.6 ± 10.0 | 65.3 ± 9.0 |
| BP at low-sodium, mm Hg | |||||
| Systolic | 119.9 ± 11.3 | – | 107.2 ± 9.8 | 106.1 ± 11.7 | 104.4 ± 9.7 |
| Diastolic | 76.1 ± 9.0 | – | 69.1 ± 8.6 | 69.3 ± 8.6 | 63.7 ± 8.8 |
| BP at high-sodium, mm Hg | |||||
| Systolic | 125.6 ± 12.4 | – | 111.8 ± 11.1 | 111.9 ± 13.4 | 106.9 ± 10.1 |
| Diastolic | 78.5 ± 9.4 | – | 70.8 ± 9.1 | 71.3 ± 9.5 | 64.5 ± 8.7 |
| BP at high-sodium and potassium-supplementation, mm Hg | |||||
| Systolic | 121.2 ± 12.5 | – | 108.7 ± 10.6 | 107.87 ± 12.9 | 104.9 ± 10.3 |
| Diastolic | 77.0 ± 9.1 | – | 69.5 ± 8.5 | 69.4 ± 9.6 | 63.4 ± 3.9 |
| BP responses to low-sodium, mm Hg | |||||
| Systolic | −8.0 ± 8.0 | – | −4.3 ± 6.0 | −5.2 ± 7.2 | −2.3 ± 4.9 |
| Diastolic | −4.2 ± 5.8 | – | −2.0 ± 5.1 | −2.7 ± 5.0 | −1.8 ± 5.3 |
| BP responses to high-salt, mm Hg | |||||
| Systolic | 5.7 ± 6.4 | – | 4.7 ± 5.8 | 5.7 ± 5.9 | 2.5 ± 4.3 |
| Diastolic | 2.5 ± 5.8 | – | 1.7 ± 5.2 | 2.0 ± 4.4 | 0.8 ± 5.0 |
| BP response to potassium-supplementation, mm Hg | |||||
| Systolic | −4.4 ± 5.6 | – | −3.2 ± 5.5 | −4.0 ± 5.6 | −2.0 ± 4.9 |
| Diastolic | −1.6 ± 4.6 | – | −1.3 ± 4.6 | −1.8 ± 4.0 | −1.2 ± 4.6 |
Mean ± standard deviation
Table 3 provides the heritabilities of BP at baseline, BP during the dietary intervention, and BP responses to the dietary intervention. All heritability estimates are significantly different from zero. The heritabilities of baseline BP ranged from 0.31 for systolic, 0.32 for diastolic, to 0.34 for mean arterial pressure. During dietary intervention, the heritabilities of BP increased significantly. For example, the heritabilities of SBP, DBP, and MAP were 0.49, 0.49, and 0.51 during low-sodium intervention, 0.47, 0.49, and 0.51 during high-sodium intervention, and 0.51, 0.52, and 0.53 during potassium supplementation, respectively. The variances of BP levels at baseline and dietary intervention due to additive (polygenic) and residual components are shown in table 4. The residual variances of blood pressure under a controlled diet were smaller than those at baseline.
Table 3.
Heritabilities of Blood Pressure Levels at Baseline and Intervention and Blood Pressure Responses to Dietary Intervention
| Dietary Intervention | Systolic Blood Pressure |
Diastolic Blood Pressure |
Mean Arterial Pressure |
|---|---|---|---|
| Baseline observation | 0.31 ± 0.03 | 0.32 ± 0.03 | 0.34 ± 0.03 |
| Low-sodium intervention | 0.49 ± 0.04 | 0.49 ± 0.04 | 0.51 ± 0.04 |
| High-sodium intervention | 0.47 ± 0.05 | 0.49 ± 0.05 | 0.51 ± 0.05 |
| High-sodium and potassium-supplementation | 0.51 ± 0.05 | 0.52 ± 0.04 | 0.53 ± 0.04 |
| Absolute level of BP responses to dietary intervention | |||
| Response to low-sodium (low – baseline) | 0.16 ± 0.05 | 0.20 ± 0.05 | 0.22 ± 0.05 |
| Response to high-sodium (high – low) | 0.24 ± 0.05 | 0.33 ± 0.05 | 0.33 ± 0.05 |
| Response to potassium (potassium supplementation – high) | 0.24 ± 0.05 | 0.23 ± 0.05 | 0.25 ± 0.05 |
| Percentage of BP responses to dietary intervention | |||
| Response to low-sodium (low – baseline) | 0.20 ± 0.05 | 0.21 ± 0.05 | 0.23 ± 0.05 |
| Response to high-sodium (high – low) | 0.22 ± 0.05 | 0.33 ± 0.05 | 0.33 ± 0.05 |
| Response to potassium (potassium supplementation – high) | 0.24 ± 0.05 | 0.21 ± 0.05 | 0.25 ± 0.05 |
| Area-under-Curve of BP responses to dietary intervention | |||
| Response to low-sodium (low – baseline) | 0.24 ± 0.05 | 0.34 ± 0.05 | 0.27 ± 0.04 |
| Response to high-sodium (high – low) | 0.27 ± 0.05 | 0.42 ± 0.05 | 0.35 ± 0.05 |
| Response to potassium (potassium supplementation – high) | 0.26 ± 0.05 | 0.36 ± 0.05 | 0.32 ± 0.05 |
Heritability ± standard error
Table 4.
Variances of Blood Pressure Levels at Baseline and Intervention Due to Additive (Polygenic) and Residual Components
| Dietary Intervention | Systolic Blood Pressure |
Diastolic Blood Pressure |
Mean Arterial Pressure |
|||
|---|---|---|---|---|---|---|
| Additive | Residual | Additive | Residual | Additive | Residual | |
| Baseline observation | 0.30820 | 0.67340 | 0.31470 | 0.66360 | 0.33772 | 0.65433 |
| Low-sodium intervention | 0.39527 | 0.41493 | 0.41356 | 0.43377 | 0.43246 | 0.41148 |
| High-sodium intervention | 0.39795 | 0.44743 | 0.43082 | 0.44787 | 0.44466 | 0.42615 |
| High-sodium and potassium-supplementation | 0.42730 | 0.41964 | 0.44158 | 0.40715 | 0.46294 | 0.40429 |
The heritabilities of BP responses to dietary intervention, expressed as absolute BP change, percentage change, and area-under-curve, are presented in table 3. These heritabilities were moderate and lower than BP under intervention but similar with BP at baseline. The heritabilities ranged from 0.16 to 0.33 for absolute BP changes, 0.20 to 0.33 for percentage BP changes, and 0.24 to 0.42 for the area-under-curve of BP changes.
DISCUSSION
The current study indicates that the heritability of baseline BP in these rural Chinese families is comparable to previous studies in other populations (12,17-19). More interestingly, the heritability of BP increases significantly when these study families are under a controlled diet (low-sodium, high-sodium, or potassium-supplementation). Furthermore, the current study suggests that BP responses to dietary sodium and potassium intervention are familial although the heritability is somewhat lower than that of BP under a controlled diet. This pattern of results for a smaller heritability for the BP responses than for the BP under a controlled diet is not unexpected given that each BP measure includes some degree of error which is compounded by indexing two measures simultaneously as in the response.
Previous family studies conducted in various populations reported that the heritability of usual BP ranged from 20% to 50% (12,17-19). Rotimi and colleagues reported that heritability was 45% for SBP and 43% for DBP in a population-based sample of 510 nuclear families from Nigeria (18). In the Framingham Heart Study, heritability was 33% for MAP among 204 families (19). In this large Chinese study with 658 families, the heritability of BP was 31% for systolic, 32% for diastolic and 34% for MAP. It is worth mentioning that the heritability of BP increased significantly during the dietary interventions, and the heritabilities during intervention were not significantly different from each other (47-51% for SBP, 49-52% for DBP and 51-53% for MAP). This increase in heritabilities was primarily (although not exclusively) due to a decrease in residual variances due to non-shared environmental effects. This was expected since the controlled diet among all study participants should minimize effects due to variability in dietary environments. With regard to the genetic vs. familial environmental source of the heritable component, it is known that family studies in general are unable to distinguish between the two sources since (nuclear) family members typically share both genes and environments. However, an indication that the shared effect may be primarily genetic is that the family structures in our study include not only first-degree relatives but also more distant relatives, so that the shared component is more strictly modeled according to genetic expectations. BP is a complex and multi-factorial trait that is influenced by environmental and genetic determinants, as well as interactions among multiple genetic and environmental factors. The challenge this poses to genetic epidemiological studies is the need to dissect these complex interactions, thereby identifying the underlying genes. Our findings suggest that control of environmental exposure (i.e., dietary sodium and potassium intake) should help the effort to identify genes for BP regulation.
Few studies have investigated the magnitude of heritable effects on salt-sensitivity of BP (20,21). Svetkey et al examined 20 African-American families selected through a hypertensive proband in whom salt-sensitivity was determined with an intravenous sodium-loading and furosemide volume-depletion protocol (20). The heritability estimates based on familial correlations were 26% to 84% for MAP, 26% to 74% for SBP, and 0.4% to 24% for DBP responses to the salt-sensitivity maneuver, respectively (20). In another study, 44 monozygotic twins and their families participated in a dietary sodium restriction (<75 mEq/day) intervention for a 12-week period (21). The familial correlations for the BP responses to low-sodium as compared to baseline sodium intake were significant, and heritability of BP responses was 54% for SBP and 34% for DBP using the age-weight adjusted residual variance. These estimates are somewhat larger than the current findings, which might be due to the differences in samples (twin vs. pedigree data), statistical methods (familial correlation vs. variance components model), and the study protocols. For example, Svetkey and colleagues used an acute protocol with intravenous sodium-loading and furosemide volume-depletion to measure salt-sensitivity in their study participants (20). In Miller's investigation, a twin study design was utilized (21). Both studies used a familial correction method to calculate heritability. In addition, the heritability of BP response to sodium intervention might not be very stable due to small sample size in the previous studies (20,21). The current investigation is the first large family-feeding study to estimate the heritability of BP responses to low-sodium, high-sodium, and potassium intake. Our study provides support for a heritable component of BP responses to dietary sodium and potassium intake. The limitations of our study include the dietary sodium and potassium intervention periods are relatively short. In addition, we did not test the reliability of BP responses to dietary sodium and potassium intervention in this study.
Genetic epidemiology studies have identified many biological candidate genes which were apparently related to salt-sensitivity of BP (11). In general, most biological pathways involved in BP regulation (such as renin–angiotensin system, sodium channels, noradrenergic system, signal transduction pathways, and endothelin system) are related to salt-sensitivity of BP (22). However, it is generally found that association of any one of these genes accounts for very little variance, and certainly not as much as the 25-50% of the variability noted here for the salt-sensitivity measures. Consequently, single locus models will be inadequate and oligogenic models as well as interactions among genes (gene-gene) and gene-environment interactions will likely be relevant. Several problems in searching for the salt-sensitive BP genes are overcome in the current study, as follows. First, the use of antihypertensive medications can mask the heritability and compromise the ability to detect genes. This is because individuals taking medications are usually deleted from the sample, which not only reduces the power due to smaller sample sizes, but also removes the very individuals who may exhibit the genetic evidence we are searching for. In the current rural Chinese population, pharmaceutical intervention is rare so antihypertensive medications do not constitute a problem in the GenSalt study. A second problem often encountered in genetic studies of complex traits is that of genetic heterogeneity. That is, a complex trait such as hypertension may arise via several different metabolic routes, and genetic variants leading to a disruption of function may have arisen in different paths in different subpopulations. In the GenSalt study, the population has been relatively stable for many generations and consequently is relatively homogeneous. Thus, there are likely to be fewer independent heterogeneous causes for the trait under investigation. Third, heterogeneity in the environment is also a consideration that can affect the heritability and consequently our ability to detect genes. Again, this rural Chinese population is under relatively homogeneous environments including access to processed dietary nutrients (i.e. no fast food) and activity levels (primarily farming communities). Together, these factors make the GenSalt study uniquely situated to address the question of genetic causes underlying complex traits like hypertension and salt-sensitivity.
Perspectives
In summary, the current study indicated that the heritability of usual BP was similar in Chinese families compared to other populations. Furthermore, the heritability of BP increased significantly when dietary sodium and potassium were controlled among study family members. The heritability of BP responses to low-sodium, high-sodium, and potassium-supplementation was moderate in this study population. These data suggest a heritable component of salt-sensitivity and a high prior odds of finding important novel genes for salt-sensitivity of BP and hypertension.
ACKNOWLEDGEMENT
The Genetic Epidemiology Network of Salt Sensitivity (GenSalt) is supported by a cooperative agreement project grant (U01HL072507) from the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland. The authors wish to express their sincere appreciation to the GenSalt study participants for their participation and cooperation in this project.
APPENDIX
GenSalt Collaborative Research Group
Tulane University Health Sciences Center, New Orleans, USA
Jiang He (PI), Lydia A. Bazzano, Chung-Shiuan Chen, Jing Chen, Lee Hamm, Tanika N. Kelly, Paul Muntner, Kristi Reynolds, Paul K. Whelton, and Wenjie Yang.
Washington University School of Medicine, St. Louis, USA
Dabeeru C. Rao (PI), Matthew Brown, Chi Gu, Treva Rice, Karen Schwander, and Shiping Wang.
Chinese Academy of Medical Sciences, Beijing, China
Dongfeng Gu (PI), Jie Cao, Jichun Chen, Xiufang Duan, Jianfeng Huang, Jinghan Huang, Jianxin Li, Depei Liu, Donghua Liu, Enchun Pan, Yang Wei, and Xigui Wu Wu. Shandong Academy of Medical Sciences, Shandong, China: Fanghong Lu (PI), Shikuan Jin, Qingjie Meng, Fan Wu, and Yingxin Zhao; Shandong Center for Diseases Control and Prevention, Shandong, China: Jixiang Ma (PI), Weika Li, and Jiyu Zhang; Zhengzhou University: Dongsheng Hu (PI), Yaxin Ding, Hongwei Wen, Meixi Zhang, and Weidong Zhang; Xinle Traditional Chinese Medicine Hospital, Hebei, China: Xu Ji (PI), Rongyan Li, Haijun Zu; Nanjing University of Medical Sciences, Jiangsu, China: Cailiang Yao (PI), Yongchao Li, Chong Shen, and Jiayi Zhou; Xi'an Jiaotong University, Shanxi, China: Jianjun Mu (PI), Enrang Chen, Qinzhou Huang, and Man Wang.
Chinese National Human Genome Center at Beijing
Zhi-Jian Yao (PI), Shufeng Chen, Dongfeng Gu, Hongfan Li, Laiyuan Wang, Penghua Zhang, Qi Zhao.
University of Texas Health Sciences Center at Houston
James E. Hixson (PI) and Lawrence C. Shimmin.
National Heart, Lung, and Blood Institute
Cashell E. Jaquish
REFERENCES
- 1.Elliott P, Stamler J, Nichols R, Dyer AR, Stamler R, Kesteloot H, Marmot M. Intersalt revisited: further analyses of 24 hour sodium excretion and blood pressure within and across populations. BMJ. 1996;312:1249–1253. doi: 10.1136/bmj.312.7041.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He J, Tell GS, Tang YC, Mo PS, He GQ. Relation of electrolytes to blood pressure in men. Hypertension. 1991;17:378–385. doi: 10.1161/01.hyp.17.3.378. [DOI] [PubMed] [Google Scholar]
- 3.Chobanian AV, Hill M. National Heart, Lung, and Blood Institute Workshop on Sodium and Blood Pressure. A critical review of current scientific evidence. Hypertension. 2000;35:858–863. doi: 10.1161/01.hyp.35.4.858. [DOI] [PubMed] [Google Scholar]
- 4.Cutler JA, Follmann D, Allender PS. Randomized trials of sodium reduction: an overview. Am J Clin Nutr. 1997;65(suppl):643S–651S. doi: 10.1093/ajcn/65.2.643S. [DOI] [PubMed] [Google Scholar]
- 5.Midgley JP, Matthew AG, Greenwood CM, Logan AG. Effect of reduced dietary sodium on blood pressure: a meta-analysis of randomized controlled trials. JAMA. 1996;275:1590–1597. doi: 10.1001/jama.1996.03530440070039. [DOI] [PubMed] [Google Scholar]
- 6.Whelton PK, He J, Cutler JA, Brancati FL, Appel LJ, Follmann D, Klag MJ. Effects of oral potassium on blood pressure. Meta-analysis of randomized controlled clinical trials. JAMA. 1997;277:1624–1632. doi: 10.1001/jama.1997.03540440058033. [DOI] [PubMed] [Google Scholar]
- 7.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27:481–490. doi: 10.1161/01.hyp.27.3.481. [DOI] [PubMed] [Google Scholar]
- 8.Obarzanek K, Proschan MA, Vollmer WM, Moore TJ, Sacks FM, Appel LJ, Svetkey LP, Most-Windhauser MM, Cutler JA. Individual blood pressure responses to changes in salt intake: Results from the DASH-sodium trial. Hypertension. 2003;42:459–467. doi: 10.1161/01.HYP.0000091267.39066.72. [DOI] [PubMed] [Google Scholar]
- 9.Vollmer WM, Sacks FM, Ard J, Appel LJ, Bray GA, Simons-Morton DG, Conlin PR, Svetkey LP, Erlinger TP, Moore TJ, Karanja N. for the DASH-Sodium Trial Collaborative Research Group. Effects of diet and sodium intake on blood pressure: subgroup analysis of the DASH-Sodium trial. Ann Intern Med. 2001;135:1019–1028. doi: 10.7326/0003-4819-135-12-200112180-00005. [DOI] [PubMed] [Google Scholar]
- 10.Wright JT, Jr, Rahman M, Scarpa A, Fatholahi M, Griffin V, Jean-Baptiste R, Islam M, Eissa M, White S, Douglas JG. Determinants of salt sensitivity in black and white normotensive and hypertensive women. Hypertension. 2003;42:1087–1092. doi: 10.1161/01.HYP.0000101687.89160.19. [DOI] [PubMed] [Google Scholar]
- 11.Beeks E, Kessels AG, Kroon AA, van der Klauw MM, de Leeuw PW. Genetic predisposition to salt-sensitivity: a systematic review. J Hypertens. 2004;22:1243–1249. doi: 10.1097/01.hjh.0000125443.28861.0d. [DOI] [PubMed] [Google Scholar]
- 12.Barlassina C, Lanzani C, Manunta P, Bianchi G. Genetics of essential hypertension: From families to genes. J Am Soc Nephrol. 2002;13:S155–S164. doi: 10.1097/01.asn.0000032524.13069.88. [DOI] [PubMed] [Google Scholar]
- 13.Svetkey LP, McKeown SP, Wilson AF. Heritability of salt sensitivity in Black Americans. Hypertension. 1996;287:854–858. doi: 10.1161/01.hyp.28.5.854. [DOI] [PubMed] [Google Scholar]
- 14.Gensalt Collaborative Research Group Genetic epidemiology network of salt sensitivity (GenSalt): Rationale, design, methods, and baseline characteristics of study participants. J Human Hypertens. 2007 doi: 10.1038/sj.jhh.1002207. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perloff D, Grim C, Flack J, Frohlich ED, Hill M, McDonald M, Morgenstern BZ. Human blood pressure determination by sphygmomanometer. Circulation. 1993;88:2460–2470. doi: 10.1161/01.cir.88.5.2460. [DOI] [PubMed] [Google Scholar]
- 16.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saavedra JM. Studies on genes and hypertension: A daunting task. J Hypertens. 2005;23:929–932. doi: 10.1097/01.hjh.0000166829.02323.b2. [DOI] [PubMed] [Google Scholar]
- 18.Rotimi CN, Cooper RS, Cao G, Ogunbiyi O, Ladipo M, Owoaje E, Ward R. Maximum-likelihood generalized heritability estimate for blood pressure in Nigerian families. Hypertension. 1999;33:874–878. doi: 10.1161/01.hyp.33.3.874. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell GF, DeStefano AL, Larson MG, Benjamin EJ, Chen MH, Vasan RS, Vita JA, Levy D. Heritability and a genome-wide linkage scan for arterial stiffness, wave reflection, and mean arterial pressure: the Framingham Heart Study. Circulation. 2005;112:194–199. doi: 10.1161/CIRCULATIONAHA.104.530675. [DOI] [PubMed] [Google Scholar]
- 20.Svetkey LP, McKeown SP, Wilson AF. Heritability of salt sensitivity in black Americans. Hypertension. 1996;28:854–858. doi: 10.1161/01.hyp.28.5.854. [DOI] [PubMed] [Google Scholar]
- 21.Miller JZ, Weinberger MH, Christian JE, Daugherty SA. Familial resemblance in the blood pressure response to sodium restriction. Am J Epidemiol. 1987;126:822–830. doi: 10.1093/oxfordjournals.aje.a114719. [DOI] [PubMed] [Google Scholar]
- 22.Marteau JB, Zaiou M, Siest G, Visvikis-Siest S. Genetic determinants of blood pressure regulation. J Hypertens. 2005;23:2127–2143. doi: 10.1097/01.hjh.0000186024.12364.2e. [DOI] [PubMed] [Google Scholar]