Summary
A P element enhancer trap screen was conducted to identify genes involved in dorsal-ventral boundary formation in Drosophila. The son of Notch (son) gene was identified by the son2205 enhancer trap insertion, which is a partial loss-of-function mutation. Based on son2205 mutant phenotypes and genetic interactions with Notch and wingless mutations, we conclude that son participates in wing development, and functions in the Notch signaling pathway at the dorsal-ventral boundary in the wing. Notch signaling pathway components activate son enhancer trap expression in wing cells. son enhancer trap expression is regulated positively by wingless, and negatively by cut in boundary cells. Ectopic Son protein induces wingless and cut expression in wing discs. We hypothesize that there is positive feedback regulation of son by wingless, and negative regulation by cut at the dorsal-ventral boundary during wing development.
Keywords: Delta, enhancer trap, Hairless, Serrate, Suppressor of Hairless, Notch, wingless, cut, Drosophila melanogaster, dorsal-ventral boundary
Introduction
Development of higher organisms involves formation of compartment boundaries that separate cells into groups with distinct developmental fates (1, 2). Compartment boundaries were discovered because of the inability of genetically marked clones of cells to cross a boundary (3). In Drosophila, several genes that function in dorsal-ventral (DV) and anterior-posterior (AP) boundary formation have been discovered. Some genes function in boundary formation in multiple tissues, indicating that there are conserved genetic pathways that regulate compartmentalization. For instance, the engrailed gene is critical for AP boundary formation in many tissues in the developing Drosophila embryo, and the wingless and Notch signaling pathways are required for DV boundary formation in both the developing eye and wing (1, 2). Formation of the DV compartment boundary in the wing imaginal disc is initiated by wingless in 2nd instar larvae, which restricts expression of the Apterous LIM-homeoprotein to the dorsal half of the wing pouch (4). The boundary forms at the junction of Apterous-expressing and non-expressing cells (5 – 7). Apterous induces expression of Fringe (Fng) protein, and the juxtaposition of Fringe expressing and non-expressing cells activates the Serrate (Ser) Notch receptor ligand, which activates the Notch receptor at the boundary (8). There is feedback between wingless and Notch signaling, because the Notch induction initiated by wingless induces further wingless expression (9, 10).
Notch function at the boundary requires both the Delta (Dl) Notch ligand and the Suppressor of Hairless (Su(H)) DNA-binding protein (11). Notch activates the vestigial gene by binding of the Notch intracellular domain (NICD) to Su(H) bound to a vestigial boundary-specific transcriptional enhancer (12). Expression of vestigial helps activate the scalloped gene at the boundary (4), and together these genes activate the cut gene. The cut wing margin enhancer directs cut expression in a narrow band of cells at the boundary beginning in late 3rd instar development (13, 14).
The Scalloped (Sd) protein directly binds the cut wing margin enhancer, and Vestigial (Vg) interacts with Sd to transcriptionally activate cut (15 – 18). In this pathway, wingless has multiple functions, including helping to induce or maintain expression of vestigial and cut (4, 19 – 23). Induction of cut by wingless is presumably indirect, but creates regulatory loop, because cut helps maintain wingless expression at the developing margin (21).
Although many genes that regulate compartment boundary formation have been described, the genetic pathways are not fully defined. It is possible that critical components have not been discovered genetically because they are essential in early development, or because their role in boundary formation is obscured by other mutant phenotypes. We therefore undertook an enhancer trap transposon screen for genes involved in boundary formation. If an enhancer trap transposon inserts near a gene involved in boundary formation, it may be expressed in a pattern consistent with this role. For example, it might show a high level of expression in boundary cells, or in cells flanking a compartment boundary. Here we report identification of the son of Notch (son) gene by an enhancer trap screen, and present evidence that it plays a role in DV boundary formation in the developing Drosophila wing under control of the Notch signaling pathway.
Experimental Procedures
Enhancer Trap Screen
An attached-X chromosome carrying eight P{lacW} constructs (C(1)RM, y1 P{lacW}5-45fD w* P{lacW}4-5fP P{lacW}3-52d P{lacW}3-76a/0/C(1;Y)13, v1 f1, Bloomington stock #16, donated by Mel Green) was used to generate random insertions. These females were crossed to males producing P transposase (y w; Ki P{ry+t7.2 = Delta2-3}99B, Bloomington stock #4348, donated by Hugo Bellen) males. Female progeny carrying attached-X and transposase were crossed with w1118 males. Red eye male progeny of this cross were crossed with proper balancers. The balanced lines were stained for β-galactosidase activity using 3rd instar imaginal discs.
Staining of Imaginal Discs for β-galactosidase Activity
Crawling 3rd instar larvae were dissected in phosphate-buffered saline (PBS). Head cuticle regions were fixed in 1% glutaraldehyde in PBS for 15 min at room temperature, and washed twice for 10 min with PBTr (1% Triton X-100 in PBS). The washed discs were stained with 1 mg/ml 5-bromo-4-chloro-3-indolyl-beta-D-galactopyranoside (X-gal) in X-gal staining solution (10 mM NaH2PO4/Na2HPO4 (pH 7.2), 150 mM NaCl, 1 mM MgCl2, 3.1 mM K4[Fe2(CN)6], 3.1 mM K3[Fe3(CN)6], 0.3% Triton X-100) overnight at 37°C. Discs were washed twice for 10 min and mounted in 50% glycerol for microscopy.
In situ Hybridization to Whole Mount Imaginal Discs
In situ hybridization to whole-mount discs was performed using digoxigenin-labeled RNA probes (visualized as a blue alkaline phosphatase precipitate) based on previous reports (24 – 26).
RNA Probe Synthesis
1-804 bp region of CG17383 ORF was amplified by PCR using the following primer pair, son surgery-1: 5′-GGA ATT CAT GGA GAA CCT GTC GCA C-3′, son surgery-4: 5′-GCT CTA GAC TAC GGT GGC AAG GGG GCT AG-3′, and LD33329 as template. Resulting PCR product was subcloned into pGEM-Teasy vector (Promega). For sense strand, this plasmid was linearized by digestion with NcoI and transcribed with Sp6 RNA polymerase. For antisense strand, plasmid was linearized by digestion with SpeI and transcribed with T7 RNA polymerase. DIG-labeled UTP and DIG RNA labeling mix (Roche) was used to label the RNA probe. Finally, the probe was resuspended in 50% deioinized formamide, 5X SSC, 100 μg/ml salmon sperm DNA, 50 μg/ml heparin, 0.1% Tween20, and stored at −77°C until use.
Immunostaining of Imaginal Discs
Larvae at the appropriate stages were dissected in PBS. The dissected head cuticles were incubated in the fixation buffer (0.1 M PIPES pH 6.9, 1 mM EDTA, 1.0% Triton X-100, 2 mM MgSO4, 1% formaldehyde (EM grade)) for 15 minutes, and then blocking solution (50 mM Tris-HCl pH 6.8, 150 mM NaCl, 0.5% NP-40, 5 mg/ml bovine serum albumin (BSA), 0.01% Thimerosal). The cuticles were transferred to the appropriate primary antibody in wash solution (50 mM Tris-HCl pH 6.8, 150 mM NaCl, 0.5% NP-40, 1 mg/ml BSA, 0.01% Thimerosal) and incubated at 4°C overnight. After washing three times with wash solution, they were incubated with the appropriate secondary antibody at room temperature for 2 h. After washing three times with wash solution, the cuticles were dissected and mounted in Antifade solution (Molecular Probes). Mounted tissues were photographed by confocal microscopy (Zeiss LSM510).
Antibodies
The primary antibodies used in these experiments were (dilution in parentheses): mouse anti-Ct (1:25), mouse anti-Wg (1:100), and mouse anti-Dl (1:1000) antibodies obtained from the University of Iowa hybridoma bank, rabbit anti-β-galactosidase (1:1000) (Sigma), and rat anti-HA (1:1000) (Roche) antibodies. Various fluorescently-labeled secondary antibodies were purchased from Jackson Immunology and used at 1:200 dilution.
Plasmid Rescue of Enhancer Trap P Element Insertions
Five micrograms of genomic DNA prepared from the enhancer trap lines were digested with EcoRI or SacII, and then ligated and used to transform XL-1 Blue E. coli by electroporation and ampicillin selection. DNA flanking the P insertion in the rescued plasmids was determined using two primers (IR primer: 5′-CGA CGG-GAC CAC CTT ATG-TTA TTT CAT CAT G-3′, A primer: 5′-GAG TTA ATT CAA ACC CCA CGG-3′).
Yeast Two Hybrid Screen
The full length of the CG17383 open reading frame was amplified from the LD33329 EST clone (Research Genetics) and used as bait by subcloning it as an EcoRI-BamHI fragment into the pBCT vector (Panbionet), which fuses it to the Gal4 DNA binding domain. The pBCT-son vector was used to transform the PBN203 yeast strain (MATα, ura-52, his3-200, ade2-101, trp1-901 leu2-3,112, gal4d, gal80d, met-, ura3d::pGAL1-lacZ ura3:: kanMX6pGAL1-URA3 Panbionet). A Drosophila embryonic cDNA library in the pACT2 vector (Clontech) was used to transform the PBN203 yeast containing the CG17383 bait. 8 × 105 colonies were screened by selecting for growth on media lacking uracil and lacZ expression. Plasmids were recovered from positive colonies as described elsewhere (27) and sequenced.
Reverse Transcription (RT)-PCR
son transcripts were detected and quantified using RT-PCR. Total RNA was extracted from adult female flies using Trizol (GIBCO). For RT-PCR, the RNA was reverse transcribed using MMLV reverse transcriptase (Promega) and oligo-dT (18mer) primers. The resulting cDNA was amplified by PCR (rp49 primer set : 5′-CAC CAG TCG GAT CGA TAT GC-3′, 5′-CAC GTT GTG CAC CAG GAA CT-3′, son primer set: 5′-AAC TTC ACC AAA CAG AAC CG-3′, 5′-AAA ATG AGC GGA AAA TGC CC-3′).
P Element Excision
Enhancer trap lines were crossed to y w; Ki P{ry+t7.2 = Delta2-3}99B flies. Progenies with mosaic eye colors were crossed to w;; TM3/TM6B. Progeny flies with white eyes were selected, and each of them was crossed to w;; TM3/TM6B to establish individually separate lines.
Ectopic Expression of NICD, Ser, Dl, HA-Son, H, Wg, and Su(H)
The Gal4-UAS driver system (28) was used to ectopically express several proteins in the wing imaginal discs by crossing flies containing P element insertions with the appropriate UAS constructs to flies containing appropriate Gal4 drivers. The Gal4 drivers used were Ser-Gal4 (w*; P{w+mC = Ser-GAL4.GF}1 P{Ser-GAL4.GF}2, Bloomington stock #6791, donated by Lucy Cherbas and Robert Fleming) and ptc-Gal4 (w*; P{w+mW.hs = GawB}ptc559.1, Bloomington stock #2017, donated by Norbert Perrimon). Jaeseob Kim provided the UAS-NICD (29), UAS-Ser (30) and UAS-Dl (31) expression constructs. The UAS-Wg construct (32) was provided by Roele Nusse, and the UAS-H construct was obtained from Yuh Nun Jan. To create a UAS-HA-Son vector, the son open reading frame was amplified by PCR from the LD33329 EST (primer sequences 5′-GGA ATT CAT GGA GAA CCT GTC GCA C, 5′-GCT CTA GAC TAC TCG TCC TCC TCC TG) and cloned into the EcoRI and XbaI sites of the pUAST-HA vector, which contains three copies of the HA antigen coding sequence upstream of the multiple cloning site sequence. This construct was introduced into the germline of w1118 mutant flies by P element-mediated germline transfer (33).
Su(H)D47 Somatic Mosaic Clones
The FLP-FRT system (34) was used to generate Su(H)D47 mutant somatic mosaic clones. FRT40A Su(H)D47/Bc Gla; son1883/TM6B flies were crossed to FRT40A, ubi-GFP/Bc Gla; hs-FLP, MKRS/TM6B flies (w1118; MKRS, P{ry+t7.2 = hsFLP}/TM6B, Tb1, Bloomington stock #279 and w1118; P{w+mC = Ubi-GFP(S65T)nls}, P{ry+t7.2 = neoFRT} 40A/CyO, Bloomington stock #5629, donated by Bruce Edgar were balanced and crossed to make this line) and progeny were heat shocked for 1 h at 37°C from 48 – 60 hour after egg-laying, and then dissected and immunostained as described at the 3rd instar stage. The FRT40A Su(H)D47 chromosome (35) was provided by Jaeseob Kim.
Results
Identification of son (son of Notch), a Novel Gene Expressed along the DV Boundary in Wing Imaginal Discs
To identify novel genes potentially involved in dorsal-ventral boundary formation, we performed an enhancer trap screen with P{lacW} transposon (36). β-Galactosidase expression was examined in larval imaginal discs of nearly 7,500 enhancer trap lines. Two lines, 1883 and 2205, revealed virtually identical patterns in eye and wing discs of 3rd instar larvae (Fig. 1A, B). Notably, the expression in wing discs was detected along the DV boundary, suggesting that the gene(s) represented by these enhancer traps might play functional roles in this region during wing development. We identified the insertion site of each P element by plasmid rescue and both were located in the CG17383 gene (Fig. 1E). Based on the results presented in this study, we named this gene ‘son of Notch (son)’ and the enhancer trap lines will be referred to as son1883 and son2205. The son1883 insertion is in the first exon of three of the four predicted transcripts, and the son2205 insertion is in the first intron of all four transcripts. Both are upstream of the coding regions. The open reading frame is the same in all predicted transcripts, and it encodes a putative winged-helix DNA-binding protein of 336 amino acids (Fig. 1F).
Figure 1.

Structure and expression of the son of Notch (son) gene. (A, B) X-gal staining for β-galactosidase (β-gal) activity: (A) 3rd instar wing disc, (B) 3rd instar eye-antenna disc. (C, D) In situ hybridization of son transcripts: (C) 3rd instar wing disc, (D) 3rd instar eye-antenna disc. (E) Map of the son gene (CG17383), enhancer trap P element insertions, and four transcripts described by the Drosophila genome project. All transcripts encode the same protein (coding regions are denoted by filled boxes). (F) Map of the encoded Son protein showing the putative winged-helix and bipartite nuclear localization domains. (G) son transcript levels in wild-type (WT), homozygous son2205, and son1883 adult female flies measured by RT-PCR. Virtually identical results were obtained using primers that detect exon 1 in the CG17383-RB transcript or exon 4 to 5 in the CG17383-RA transcript (data not shown). rp49 transcripts detected by RT-PCR provide an internal standard.
In situ hybridization of son in wild-type imaginal discs revealed expression patterns identical to that of β-Gal in son1883 and son2205 (hereafter son-lacZ1883 and son-lacZ2205, respectively), showing that the enhancer trap lines can serve as reliable markers of son expression (Fig. 1C, D). To check whether son expression is affected by these P insertions, the transcript level of son was examined by semi-quantitative RT-PCR. In both fly lines, the transcript level of son was significantly reduced, but not abolished (Fig. 1G), suggesting that these alleles are hypomorphic mutants of son.
Son Encodes a Putative DNA Binding Protein that Self Interacts
We conducted a yeast two-hybrid screen to find proteins that interact with the Son protein. Using Son as bait, and screening some 800,000 colonies transformed with a Drosophila embryonic cDNA library (Clontech), we recovered five interacting clones, two of which encoded N terminal portions of the Son protein, and three that encoded the entire Son protein. The Son self-interaction was also reported in a large scale genome-wide yeast two-hybrid screen (37). These results imply that Son functions as a homo-multimer.
Analysis of Son Expression in Wing Imaginal Discs
In 2nd instar wing discs, son-lacZ2205 was expressed in a stripe at the presumptive DV boundary, weakly at the hinge region, and in a circle just outside of the presumptive wing pouch. The DV band of son-lacZ2205 coincided with wingless (wg) expression at the DV boundary (Fig. 2A – C). In 3rd instar wing discs, the DV band of son-lacZ2205 resolved into two narrowly separated bands, and double immunolabeling showed that cut (ct) is expressed in the narrow gap between the two bands (Fig. 2D – F). These bands coincided with the expression domain of Delta (Dl), the Notch receptor ligand, that flanks the DV boundary (Fig. 2G – I). At this stage, the AP band of son-lacZ2205 expression lied between the AP bands of Dl expression. Such expression patterns of son further support the possibility that this gene might play a role in DV boundary formation by being involved with wg or Notch signaling pathways.
Figure 2.

Expression of the son enhancer traps in wing imaginal discs. (A – C) 2nd instar wing imaginal disc: (A) β-gal immunostaining, (B) Wingless (Wg) immunostaining, (C) merge of panels A and B. (D – F) 3rd instar wing imaginal disc: (D) β-gal immunostaining, (E) Cut (Ct) immunostaining, (F) merge of panels D and E. (G – I) Late 3rd instar wing imaginal disc: (G) β-gal immunostaining, (H) Delta (Dl) immunostaining, (I) merge of panels G and H.
The Son2205 Insertion alters Eye and Wing Development
We observed that the ventral posterior section of eyes in flies homozygous for the son2205 insertion was reduced in size (Fig. 3B). To test if the mutant phenotype was caused by the son2205 insertion, we carried out precise-excision of the P element. We recovered several viable excisions, and none displayed the mutant phenotype. For ten of these excisions, we sequenced the genomic region covering the original insertion site and confirmed that they were reverted to wild-type or contained small duplications of the insertion site. These results suggest that the son2205 insertion causes the mutant eye phenotype.
Figure 3.

Effects of the son2205 mutation on eye and wing development. Adult eyes from (A) wild type, (B) homozygous son2205 mutants, (C) Nnd-1 (nd1) mutants and (D) nd1; son2205 double mutants. Adult wings from (E) wild type, (F) nd1 mutants, (G) nd1; son2205/+ double mutants, and (H, I) wgGla-1/+; son2205 homozygous mutants. (I) Rare extreme notching phenotype of wgGla-1/+; son2205 homozygous mutants. Heterozygous or homozygous son2205 mutants do not display a mutant wing margin phenotype in the absence of nd1 or wgGla-1 mutations (data not shown).
The Notch receptor plays multiple roles in eye development, including definition of the DV boundary, and cell differentiation within each ommatidium. Some mutations affecting Notch function can cause a preferential loss of the ventral domain (38). We observed that Nnd-1 (nd1), the temperature-sensitive hypomorphic Notch allele, has a synergistic genetic interaction with son2205 (Fig. 3C, D). At 18°C, nd1 eyes are slightly smaller and rougher than wild-type (39), but when combined with homozygous son2205, eyes were strongly reduced in size with virtually complete loss of the ventral half. These results suggest that son interacts with the Notch signaling pathway in the developing eye.
The son2205 insertion also provided genetic evidence demonstrating that son participates in the DV boundary formation in the developing wing. The slight adult wing margin-nicking phenotype displayed by nd1 mutants (Fig. 3F) was dominantly enhanced by son2205 (Fig. 3G). Moreover, combining the gain-of-function wgGla-1 allele of wingless with homozygous son2205 caused a nicking phenotype in some 2/3 of the adult flies (Fig. 3H). In rare cases, these flies display a strong notching phenotype (Fig. 3I). The wgGla-1 and son2205 homozygotes by themselves did not display loss of wing margin (data not shown). The dramatic genetic interactions between son2205 and mutant alleles of Notch or wg strongly support the idea that this gene has functional roles in DV boundary formation by interacting with Notch or wg signaling pathways.
Notch Receptor Signaling Induces Son Enhancer Trap Expression
In order to examine the relationship between son and the Notch signaling pathway, we checked the effects of Notch signaling activity on son expression. When a Notch ligand binds to the Notch receptor, the intracellular fragment of Notch (NICD) is produced in response and functions as a transcriptional activator (40). To ectopically activate Notch signaling, NICD was expressed in the entire dorsal wing compartment using a Ser-Gal4 driver, and as a result, son-lacZ1883 expression was induced in the corresponding region (Fig. 4A). Such effect on son-lacZ2205 expression was also observed (data not shown). Ectopic Ct expression was detected in this region as well, showing induction of Notch signaling activity (Fig. 4B).
Figure 4.

Activation of son-lacZ2205 by Notch signaling in wing discs. (A – I) 3rd instar wing imaginal discs immunostained for β-gal and Ct proteins. (A – C) Ectopic expression of the Notch receptor intracellular domain (NICD) in the dorsal compartment using a Ser-Gal4 driver. (D – F) Ectopic expression of the Serrate Notch ligand (Ser) at the anterior-posterior (AP) compartment boundary using a patched (ptc)-Gal4 driver. (G – I) Ectopic expression of the Delta Notch ligand (Dl) at the AP boundary using the ptc-Gal4 driver. (J – L) 3rd instar wing imaginal disc with a Su(H)D47 mutant mosaic clone: (J) GFP expression showing the Su(H) mutant clone lacking GFP, (K) β-gal immunostaining, (L) merge of panels J and K. The clone border is marked by a white dashed line.
Ectopic expression of Notch ligands also induced son enhancer trap expression. Panel D – F of Figure 4 shows that ectopic expression of Ser by a patched (ptc)-Gal4 along the anterior-posterior (AP) boundary induced son-lacZ1883 expression along the boundary, along with ct expression. A similar induction occurred when Dl ligand was expressed along the AP boundary (Fig. 4G – I).
NICD interacts with the Su(H) DNA-binding protein to activate gene transcription (41). We tested if Su(H) is required for induction of son-lacZ1883 by generating mitotic clones homozygous for the Su(H)D47 null allele (35) in wing imaginal discs. Su(H) mutant clones in the wing imaginal disc lost expression of son-lacZ1883 (Fig. 4J – L). Thus, son expression in the DV boundary region is likely induced by a NICD-Su(H) complex.
Wg Potentiates Son Enhancer Trap Expression
The wg signaling pathway potentiates and refines expression of genes required for DV boundary formation in the wing. Ectopic expression of Wg protein in the dorsal compartment using a Ser-Gal4 driver by itself did not induce son-lacZ1883 or ct expression (Fig. 5A – C). Also, ectopic expression of the Su(H) protein alone in the dorsal compartment did not induce son-lacZ1883 or ct expression (Fig. 5D – F). In combination, however, ectopic dorsal expression of both Su(H) and Wg induced son-lacZ1883 throughout most of the dorsal compartment, and ct expression in some dorsal cells neighboring the DV boundary (Fig. 5G – I). These results imply that ectopic Wg enhances the induction of the son enhancer trap by the Notch signaling pathway.
Figure 5.
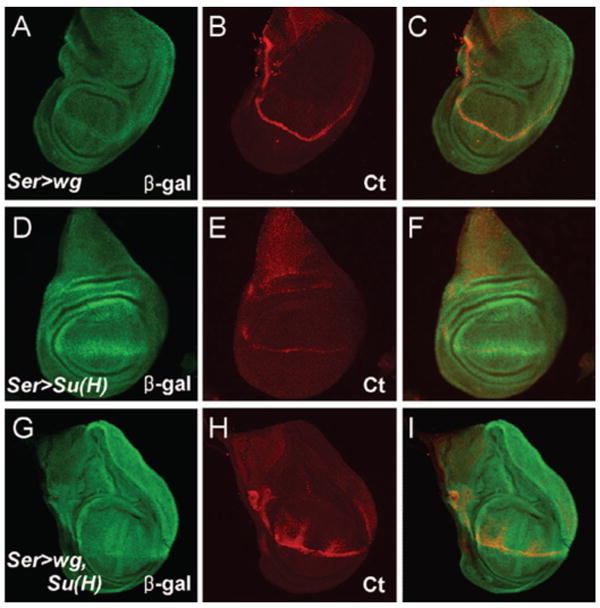
Coexpression of wg and Su(H) induces son-lacZ1883 expression. Regulatory interactions between wg, Su(H) and son. (A – C) Wg protein ectopically expressed in the dorsal compartment of a 3rd instar wing imaginal disc using the Ser-Gal4 driver: (A) β-gal immunostaining, (B) Cut (Ct) immunostaining, (C) merge of panels A and B. (D – F) Su(H) ectopically expressed in the dorsal compartment: (D) β-gal immunostaining, (E) Ct immunostaining, (F) merge of panels D and E. (G – I) Ectopic expression of Su(H) and Wg in the dorsal compartment: (G) β-gal immunostaining, (H) Ct immunostaining, (I) merge of panels G and H. Anterior is to the left and dorsal is to the top.
HA-Son Protein Induces ct, Dl, and wg Expression
The above results suggest that son is downstream of Notch and wg during wing margin development. To examine the function of son in these pathways, we ectopically expressed Son protein in the wing disc. We used a fusion protein containing the hemagglutinin epitope (HA-Son) to allow detection of the protein by immunostaining. Interestingly, ectopic expression of HA-Son along the AP boundary using the ptc-Gal4 induced expression of ct, Dl, and wg (Fig. 6A – I), suggesting that son might be involved in supporting the activation of signaling pathways involved in DV boundary formation.
Figure 6.

Crossregulation between ct, Dl, wg, and son. (A – J) HA-Son protein ectopically expressed at the AP boundary using the ptc-Gal4 driver: (A, D, G) HA-Son immunostaining, (B) Ct immunostaining, (C) merge of panels A and B, (E) Dl immunostaining, (F) merge of panels D and E, (H) Wg immunostaining, (I) merge of panels H and I, (J) β-gal immunostaining. (K – N) Ectopic expression of Su(H) and HA-Son at the AP boundary. (K) HA-Son immunostaining, (L) Ct immunostaining, (M) merge of panels K and L, (N) β-gal immunostaining.
Ectopic expression of HA-Son alone was insufficient to cause misexpression of son-lacZ1883 (Fig. 6J). In Figure 5, we showed that coexpression of Wg and Su(H) leads to son-lacZ1883 expression. Since ectopic HA-Son can cause wg expression, it seemed probable that coexpression of Su(H) with HA-Son could induce endogenous son expression. As expected, combined ectopic expression of Su(H) and HA-Son along the AP boundary induced expression of son-lacZ1883 (Fig. 6N). Collectively, these results imply that son, wg, and the Notch signaling pathway support activation of each other.
Ct Negatively Regulates Son Enhancer Trap Expression
The Hairless (H) protein interacts with Su(H) and recruits co-repressor proteins such as Groucho and dCtBP (42). We reasoned that ectopic expression of H along the AP boundary might therefore reduce son enhancer trap expression at the intersection where the DV boundary crosses the AP boundary. However, while ptc-Gal4-driven expression of H caused downregulation of ct at the intersection, the expression of son-lacZ1883 was not reduced (Fig. 7A – C). Notably, son enhancer trap expression appeared to increase in the cells where ct expression was reduced, and the two bands of son-lacZ1883 expression that flank the DV boundary, which was marked by Ct staining, were fused at this intersection.
Figure 7.

Ct functions to downregulate the transcriptional activity of son. (A – C) Hairless protein ectopically expressed at the AP boundary: (A) β-gal immunostaining, (B) Ct immunostaining, (C) merge of panels A and B. (D – G) Hairless and HA-Son protein ectopically expressed at the AP boundary. (D) β-gal immunostaining, (E) Ct immunostaining, (F) merge of panels D, E, and G, (G) HA-Son immunostaining. (H-J) ct2s mutant discs lacking ct gene expression at the dorsal-ventral boundary (wing margin). (H) β-gal immunostaining, (I) Ct immunostaining, (J) merge of panels H and I.
The loss of ct expression caused by ectopic H expression along the AP boundary was partially reversed by simultaneous ectopic expression of HA-Son (Fig. 7D – G). The ct expression was not fully restored, and it appeared that the son-lacZ1883 expression remained as a single band at the intersection. The AP bands of HA-Son and ct expression overlapped along the AP boundary, as when HA-Son was expressed alone (Fig. 6A – C). These results suggest that in the DV boundary cells, where ct expression is the highest, son expression may be repressed by ct activity. Indeed, in ct2s mutant discs, which lack ct expression in the boundary cells, the two bands of son-lacZ1883 expression were fused into one band (Fig. 7H – J). Thus, it appears that high levels of ct expression in the DV boundary cells inhibit son expression. Notably, when ectopic expression of Ct or Son was induced in cells outside the DV boundary in previous results, ct expression and son expression were not always mutually exclusive. Thus, it is likely that high levels of Ct and/or Ct in combination with other factors that function in the DV boundary are responsible for repressing son expression in the cells at the DV boundary.
Discussion
Son Participates in the Notch Signaling Pathway at the Dorsal-ventral Boundary in Wing Development
We named the gene defined by the son2205 and son1883 enhancer trap insertions son of Notch (son) because several lines of evidence support the idea that it participates in the Notch signaling pathway at the DV boundary in the developing wing: First, the son enhancer traps are expressed at the dorsal-ventral boundary in wing discs. Second, heterozygous and homozygous son2205 strongly enhance the mutant wing margin phenotype displayed by the hypomorphic Nnd-1 mutation of Notch, and these effects are reverted by precise excision of the P element. Third, son enhancer trap expression in the wing disc is induced by expression of Notch pathway components, including Notch ligands, and the intracellular fragment of Notch. Fourth, the Su(H) DNA-binding protein that forms a transcriptional activator complex with Notch is essential for son enhancer-trap expression at the wing DV boundary, and together with Wg, it can induce son enhancer trap expression. Fifth, ectopic expression of Son protein in the wing disc induces expression of ct, Dl and wg, which are normally induced by Notch signaling at the DV boundary. Sixth, son enhancer trap expression is negatively regulated by ct at the DV boundary. Altogether, these observations provide compelling evidence that son is a component of the genetic pathway induced by Notch at the wing margin, and we hypothesize that son functions downstream of Notch at the wing margin, participating in a positive regulatory loop with wg, and a negative regulatory loop with ct (Fig. 8).
Figure 8.

Possible regulatory interactions between son and components of the Notch signaling pathway at the wing dorsal-ventral boundary. The interactions involving son are hypothesized based on the results presented in this study. Previously known direct interactions are indicated with solid arrows.
Analysis of the roles of son in development would be aided by a null mutant allele of son, but we were unsuccessful in generating such an allele, despite extensive efforts. The Df(3R)Su9 allele that lacks a genomic region containing son is haplolethal (43). It may be that a son null allele may be haploinsufficient or haplolethal, or there may be a haplolethal gene near son, thus making it difficult to generate deletion mutants of this gene.
What is the Function of Son at the Wing Dorsal-ventral Boundary?
The precise role of son at the wing dorsal-ventral boundary is yet unclear, but the pattern of son enhancer-trap expression raises certain possibilities. The expression of son-lacZ1883 and Dl overlap in cells flanking the DV boundary where ct is expressed. Although misexpression of Son can ectopically induce expression of ct and Dl, the patterns of son-lacZ1883 and ct expression at the DV boundary appear to be mutually exclusive. Moreover, loss of ct expression augments son-lacZ1883 expression and leads to fusion of the two son stripes into a single broad band. Thus one possibility is that son and ct function in a loop to control ct levels, in which son helps induce ct expression, but at a high level and/or in combination with other factors, ct represses son expression. We also speculate that son could help maintain Dl expression along the DV boundary, and that ct blocks Dl expression in the boundary cells by repressing son. This scenario could explain why the son2205 mutation enhances the defective wing margin phenotype of a hypomorphic Notch mutation, since reducing Dl levels would further decrease Notch signaling activity. It is also possible that Son supports wg expression at the DV boundary, since wg is also induced by HA-Son. Although the underlying mechanisms are currently unresolved, this study presents clear evidence that son plays a definite role involved with the Notch signaling pathway in regulating DV boundary formation. Moreover, the significant effects between this novel component and several other components in this pathway suggest that further analyses of such cross-regulations will contribute to understand the intricate process of DV boundary formation.
Son encodes a self-interacting protein with a winged-helix DNA-binding domain, and it seems likely that Son acts as a transcriptional activator or repressor. For example, it could directly contribute to activation of Dl or other genes that function at the DV boundary, such as vestigial, scalloped, or wg.
In addition to the eye and wing discs, the son enhancer traps are also expressed in leg discs and the central/peripheral nervous systems of developing embryos, and thus it is likely that son has several other developmental roles.
Acknowledgments
We thank Jaeseob Kim (KAIST) for providing the fly stocks used in the enhancer trap screen, the Gal4 drivers and helpful discussions. Jae-eun Jung helped with Drosophila embryo microinjection. We thank the University of Iowa hybridoma bank for providing antibodies. We also thank Eunjung Kang for assistance with confocal microscopy. This work was supported by the BK21 Research Fellowship from the Ministry of Education and Human Resources Development, a grant (M103KV010002 04K2201 00230) from Brain Research Center of the 21st Century Frontier Research Program funded by the Ministry of Science and Technology, Republic of Korea to J.Y., and NIH grant GM055683 to D.D.
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article maybe used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
References
- 1.Irvine KD. Fringe, Notch and making developmental boundaries. Curr Opin Genet Dev. 1999;9:434–441. doi: 10.1016/S0959-437X(99)80066-5. [DOI] [PubMed] [Google Scholar]
- 2.Vincent JP. Compartment boundaries: where, why and how. Int J Dev Biol. 1998;42:311–315. [PubMed] [Google Scholar]
- 3.Garcia-Bellido A. Cell affinities in antennal homoeotic mutants of Drosophila melanogaster. Genetics. 1968;59:487–499. doi: 10.1093/genetics/59.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams JA, Paddock SW, Carroll SB. Pattern formation in a secondary field: a hierarchy of regulatory genes subdivides the developing Drosophila wing disc into discrete sub-regions. Development. 1993;117:571–584. doi: 10.1242/dev.117.2.571. [DOI] [PubMed] [Google Scholar]
- 5.Blair SS. Mechanisms of compartment formation: evidence that non-proliferating cells do not play a critical role in defining the D/V lineage restriction in the developing wing of Drosophila. Development. 1993;119:339–351. doi: 10.1242/dev.119.2.339. [DOI] [PubMed] [Google Scholar]
- 6.Blair SS, Brower DL, Thomas JB, Zavortink M. The role of apterous in the control of dorsoventral compartmentalization and PS integrin gene expression in the developing wing of Drosophila. Development. 1994;120:1805–1815. doi: 10.1242/dev.120.7.1805. [DOI] [PubMed] [Google Scholar]
- 7.Diaz-Benjumea FJ, Cohen SM. Interaction between dorsal and ventral cells in the imaginal disc directs wing development in Drosophila. Cell. 1993;75:741–752. doi: 10.1016/0092-8674(93)90494-b. [DOI] [PubMed] [Google Scholar]
- 8.Kim J, Irvine KD, Carroll SB. Cell recognition, signal induction, and symmetrical gene activation at the dorsal-ventral boundary of the developing Drosophila wing. Cell. 1995;82:795–802. doi: 10.1016/0092-8674(95)90476-x. [DOI] [PubMed] [Google Scholar]
- 9.Diaz-Benjumea FJ, Cohen SM. Serrate signals through Notch to establish a wingless-dependent organizer at the dorsal/ventral compartment boundary of the Drosophila wing. Development. 1995;121:4215–4225. doi: 10.1242/dev.121.12.4215. [DOI] [PubMed] [Google Scholar]
- 10.Rulifson EJ, Blair SS. Notch regulates wingless expression and is not required for reception of the paracrine wingless signal during wing margin neurogenesis in Drosophila. Development. 1995;121:2813–2824. doi: 10.1242/dev.121.9.2813. [DOI] [PubMed] [Google Scholar]
- 11.de Celis JF, Garcia-Bellido A, Bray SJ. Activation and function of Notch at the dorsalventral boundary of the wing imaginal disc. Development. 1996;122:359–369. doi: 10.1242/dev.122.1.359. [DOI] [PubMed] [Google Scholar]
- 12.Kim J, Sebring A, Esch JJ, Kraus ME, Vorwerk K, Magee J, Carroll SB. Integration of positional signals and regulation of wing formation and identity by Drosophila vestigial gene. Nature. 1996;382(6587):133–138. doi: 10.1038/382133a0. [DOI] [PubMed] [Google Scholar]
- 13.Dorsett D. Distance-independent inactivation of an enhancer by the suppressor of Hairywing DNA-binding protein of Drosophila. Genetics. 1993;134:1135–1144. doi: 10.1093/genetics/134.4.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jack J, Dorsett D, Delotto Y, Liu S. Expression of the cut locus in the Drosophila wing margin is required for cell type specification and is regulated by a distant enhancer. Development. 1991;113:735–747. doi: 10.1242/dev.113.3.735. [DOI] [PubMed] [Google Scholar]
- 15.Guss KA, Nelson CE, Hudson A, Kraus ME, Carroll SB. Control of a genetic regulatory network by a selector gene. Science. 2001;292(5519):1164–1167. doi: 10.1126/science.1058312. [DOI] [PubMed] [Google Scholar]
- 16.Halder G, Polaczyk P, Kraus ME, Hudson A, Kim J, Laughon A, Carroll S. The vestigial and scalloped proteins act together to directly regulate wing-specific gene expression in Drosophila. Genes Dev. 1998;12:3900–3909. doi: 10.1101/gad.12.24.3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morcillo P, Rosen C, Dorsett D. Genes regulating the remote wing margin enhancer in the Drosophila cut gene. Genetics. 1996;144:1143–1154. doi: 10.1093/genetics/144.3.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simmonds AJ, Liu X, Soanes KH, Krause HM, Irvine KD, Bell JB. Molecular interactions between vestigial and scalloped promote wing formation in Drosophila. Genes Dev. 1998;12:3815–3820. doi: 10.1101/gad.12.24.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Couso JP, Bishop SA, Martinez Arias A. The wingless signaling pathway and the patterning of the wing margin in Drosophila. Development. 1994;120:621–636. doi: 10.1242/dev.120.3.621. [DOI] [PubMed] [Google Scholar]
- 20.Couso JP, Knust E, Martinez Arias A. Serrate and wingless cooperate to induce vestigial gene expression and wing formation in Drosophila. Curr Biol. 1995;5:1437–1448. doi: 10.1016/s0960-9822(95)00281-8. [DOI] [PubMed] [Google Scholar]
- 21.Micchelli CA, Rulifson EJ, Blair SS. The function and regulation of cut expression on the wing margin of Drosophila: Notch, wingless and a dominant negative role for Delta and Serrate. Development. 1997;124:1485–1495. doi: 10.1242/dev.124.8.1485. [DOI] [PubMed] [Google Scholar]
- 22.Neumann CJ, Cohen SM. A hierarchy of cross-regulation involving Notch, wingless, vestigial and cut organizes the dorsal/ventral axis of the Drosophila wing. Development. 1996;122:3477–3485. doi: 10.1242/dev.122.11.3477. [DOI] [PubMed] [Google Scholar]
- 23.Neumann CJ, Cohen SM. Long-range action of wingless organizes the dorsal-ventral axis of the Drosophila wing. Development. 1997;124:871–880. doi: 10.1242/dev.124.4.871. [DOI] [PubMed] [Google Scholar]
- 24.O'Neill JW, Bier E. Double-label in situ hybridization using biotin and digoxigenin-tagged RNA probes. Biotechniques. 1994;17:870, 874–875. [PubMed] [Google Scholar]
- 25.Sturtevant MA, Roark M, Bier E. The Drosophila rhomboid gene mediates the localized formation of wing veins and interacts genetically with components of the EGF-R signaling pathway. Genes Dev. 1993;7:961–973. doi: 10.1101/gad.7.6.961. [DOI] [PubMed] [Google Scholar]
- 26.Tautz D, Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila reveals translational control of the segmentation gene hunchback. Chromosoma. 1989;98:81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- 27.Chien CT, Bartel PL, Sternglanz R, Fields S. The two-hybrid system: A method to identify and clone genes for proteins that interact with a protein of interest. Proc Natl Acad Sci USA. 1991;88:9578–9582. doi: 10.1073/pnas.88.21.9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 29.Doherty D, Feger G, Younger-Shepherd S, Jan LY, Jan YN. Delta is a ventral to dorsal signal complementary to Serrate, another Notch ligand, in Drosophila wing formation. Genes Dev. 1996;10:421–434. doi: 10.1101/gad.10.4.421. [DOI] [PubMed] [Google Scholar]
- 30.Speicher SA, Thomas U, Hinz U, Knust E. The Serrate locus of Drosophila and its role in morphogenesis of the wing imaginal discs: control of cell proliferation. Development. 1994;120:535–544. doi: 10.1242/dev.120.3.535. [DOI] [PubMed] [Google Scholar]
- 31.Seugnet L, Simpson P, Haenlin M. Transcriptional regulation of Notch and Delta: requirement for neuroblast segregation in Drosophila. Development. 1997;124:2015–2025. doi: 10.1242/dev.124.10.2015. [DOI] [PubMed] [Google Scholar]
- 32.Lawrence PA, Bodmer R, Vincent JP. Segmental patterning of heart mesoderm in Drosophila. Development. 1995;121:4303–4308. doi: 10.1242/dev.121.12.4303. [DOI] [PubMed] [Google Scholar]
- 33.Rubin GM, Spradling AC. Genetic transformation of Drosophila with transposable element vectors. Science. 1982;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- 34.Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- 35.Morel V, Schweisguth F. Repression by Suppressor of Hairless and activation by Notch are required to define a single row of single-minded expressing cells in the Drosophila embryo. Genes Dev. 2000;14:377–388. [PMC free article] [PubMed] [Google Scholar]
- 36.Bier E, Vaessin H, Shepherd S, Lee K, McCall K, Barbel S, Ackerman L, Carretto R, Uemura T, Grell E, et al. Searching for pattern and mutation in the Drosophila genome with a P-lacZ vector. Genes Dev. 1989;3:1273–1287. doi: 10.1101/gad.3.9.1273. [DOI] [PubMed] [Google Scholar]
- 37.Giot L, Bader JS, Brouwer C, Chaudhuri A, Kuang B, Li Y, Hao YL, Ooi CE, Godwin B, Vitols E, Vijayadamodar G, Pochart P, Machineni H, Welsh M, Kong Y, Zerhusen B, Malcolm R, Varrone Z, Collis A, Minto M, Burgess S, McDaniel L, Stimpson E, Spriggs F, Williams J, Neurath K, Ioime N, Agee M, Voss E, Furtak K, Renzulli R, Aanensen N, Carrolla S, Bickelhaupt E, Lazovatsky Y, DaSilva A, Zhong J, Stanyon CA, Finley RL, Jr, White KP, Braverman M, Jarvie T, Gold S, Leach M, Knight J, Shimkets RA, McKenna MP, Chant J, Rothberg JM. A protein interaction map of Drosophila melanogaster. Science. 2003;302(5651):1727–1736. doi: 10.1126/science.1090289. [DOI] [PubMed] [Google Scholar]
- 38.Chern JJ, Choi KW. Lobe mediates Notch signaling to control domain-specific growth in the Drosophila eye disc. Development. 2002;129:4005–4013. doi: 10.1242/dev.129.17.4005. [DOI] [PubMed] [Google Scholar]
- 39.Shellenbarger DL, Mohler JD. Temperature-sensitive mutations of the Notch locus in Drosophila melanogaster. Genetics. 1975;81:143–162. doi: 10.1093/genetics/81.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393(6683):382–386. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- 41.Fortini ME, Artavanis-Tsakonas S. The suppressor of hairless protein participates in notch receptor signaling. Cell. 1994;79:273–282. doi: 10.1016/0092-8674(94)90196-1. [DOI] [PubMed] [Google Scholar]
- 42.Barolo S, Stone T, Bang AG, Posakony JW. Default repression and notch signaling: hairless acts an adaptor to recruit the corepressors groucho and dCtBP to suppressor of hairless. Genes Dev. 2002;16:1964–1976. doi: 10.1101/gad.987402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gonzalez C, Molina I, Casal J, Ripoll P. Gross genetic dissection and interaction of the chromosomal region 95E;96F of Drosophila melanogaster. Genetics. 1989;123:371–377. doi: 10.1093/genetics/123.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]


