Abstract
Multiple sclerosis patients typically experience increased pain that is relatively insensitive to opiate treatment. The mechanistic basis for this increased nociception is currently poorly understood. In the present study, we utilized the Theiler’s murine encephalomyelitis virus (TMEV) model of MS to examine possible changes in spinal cord opioid receptor mRNA over the course of disease progression. TMEV infection led to significantly decreased mu, delta and kappa opioid receptor mRNA expression as analyzed by quantitative Real-Time PCR in both male and female mice at days 90, 150 and 180 post-infection (PI). Since opioid receptor mRNA expression decreased in TMEV mice, we examined whether opiate analgesia is also altered. TMEV infected female mice had significantly decreased opiate analgesia in thermal nociceptive tests beginning at day 90 PI, while TMEV-infected male mice did not display significantly decreased opiate analgesia until day 120 PI. The novel finding that opioid receptor expression is significantly decreased in the spinal cord of TMEV mice could explain the increased nociception and loss of opiate analgesia observed in both TMEV mice and multiple sclerosis patients.
Keywords: opioid receptors, CCR-5, multiple sclerosis, Theiler’s murine encephalomyelitis virus, opioid analgesia, spinal cord, antinociception
1. Introduction
It is well accepted that MS patients are debilitated by lesions affecting motor areas of the CNS, but they also suffer from sensory deficits and pain, symptoms, which until recently, have typically gone unnoticed. Recent studies have demonstrated that pain is actually an important aspect of MS with a prevalence of 40-80% among all MS patients (Archibald et al., 1994; Ehde et al, 2006; Hadjimichael et al., 2007; Indaco et al., 1994; O’connor et al., 2007; Osterberg et al., 2005; Solaro, 2006; Svendsen et al., 2003; Vermote et al., 1986). Pain severity in MS has been shown to be similar to the pain severity reported by patients with rheumatoid arthritis and osteoarthritis (Kalia and Connor, 2005). Thus, not only is pain common in MS patients, but it can also be severely debilitating (Ehde et al., 2006), with 30% of MS patients describing pain as their worst symptom (Beard et al., 2003). Unfortunately despite the high prevalence of pain, it is estimated that only 2% of patients are referred to a pain specialist and only 26.3% are actually treated for their pain (Kalia and Connor, 2005). Furthermore, classical pain treatments, particularly opiate administration, are typically ineffective in treating MS pain, with only a minority of patients receiving significant relief (Kalman et al., 2002; Morley-Forster, 2006). While newer treatments, including the use of cannabinoids hold promise for treating MS pain (Pacher et al., 2006), side effects of these drugs have been reported which may limit their use (Solaro, 2006). In general the frequency of pain-related symptoms in MS is quite high and clinical treatment guidelines are lacking, perhaps due to the fact that the mechanisms of pain in MS have yet to be understood (Solaro, 2006).
Opioids are important modulators of both the nervous and immune systems. In the nervous system opioids modulate nociceptive pathways and are analgesic. While not as well understood, it has long been appreciated that opiates can also alter the immune response. For instance, non-HIV infected heroin addicts have alterations in many immune parameters including decreased natural killer cell cytolytic activity, blood lymphocyte proliferation, and antibody production (Brown et al., 1974; Layon et al., 1984; Neri et al., 2005). Morphine given in vivo has been shown to decrease the effectiveness of several functions of both natural and adaptive immunity, and significantly reduces cellular immunity (Sacerdote, 2006). For example, morphine inhibits induction of delayed type hypersensitivity in rats (Pellis et al., 1986) and suppresses the primary antibody response in mice (Bussiere et al., 1992). Since opiates are immunosuppressive, one would predict that long-term opiate use in MS patients might be beneficial in dampening the autoimmune response that contributes to the demyelination and neurological symptoms associated with this disease. Unfortunately, there are no studies that have examined opiate use on disease progression and only a few that have examined the effects of opiates on MS-induced pain (Kalman et al, 2002).
Since MS is a disease in which immune destruction leads to neurological deficits and increased nociception and since opioids modulate both nociception and immune function, this raises the question as to whether a change in opioid peptide or receptor expression is a contributing factor to MS-induced nociception and disease progression. To address this question we examined spinal cord opioid receptor expression and opiate analgesia in the Theiler’s murine encephalomyelitis virus (TMEV) mouse model of multiple sclerosis. We hypothesized that TMEV infected mice would have decreased spinal cord opioid receptor expression compared to uninfected controls and as a result acutely administered opiates would produce significantly less analgesia in TMEV infected mice. Thus, the goal of this study was to determine whether spinal cord opioid receptor expression is altered in TMEV mice and whether opiate analgesia is diminished as a result of these alterations.
2. Results
Opioid Receptor mRNA Expression
We used quantitative real-time PCR to examine mu opioid receptor (MOR), delta opioid receptor (DOR), kappa opioid receptor (KOR) mRNA at days 90, 150 and 180 post-infection. In addition we analyzed several non-opioid, G-protein coupled receptors including two chemokine receptors, the angiotensin II, type 1 (AT1) receptor and the sigma-1 receptor in order to determine if TMEV infection resulted in a general decrease in mRNA expression of all G-protein coupled receptors similar to what was observed for opioid receptors. The number of animals used for each of these Q-PCR experiments, the overall F-values and p values for each receptor are summarized in table 1.
Table 1.
Summary of the experimental design of the quantitative Real-Time PCR study indicating the numbers of mice per group, the total number of mice in the study (N) and the ANOVA F and p values.
| Receptor | Number of mice/group | Number of conditions (time points, sex, TMEV vs control) | Total number of mice (N) | F Value | P value |
|---|---|---|---|---|---|
| MOR mRNA | 4-5 | 12 | 52 | 14.677 | <0.0001 |
| DOR mRNA | 4-5 | 12 | 51 | 13.687 | <0.0001 |
| KOR mRNA | 4-5 | 12 | 51 | 9.859 | <0.0001 |
| Sigma-1 mRNA | 4-5 | 12 | 53 | 15.251 | <0.0001 |
| CCR-1 mRNA | 4-5 | 12 | 52 | 18.756 | <0.0001 |
| CCR-5 mRNA | 4-5 | 12 | 52 | 11.272 | <0.0001 |
| AT1 mRNA | 4-5 | 12 | 53 | 2.939 | <0.0059 |
Note: Spinal cord mRNA from the same group of 53 mice was used for all Q-PCR experiments. The n varies among receptors due to lack of adequate amounts of cDNA from 2 mice.
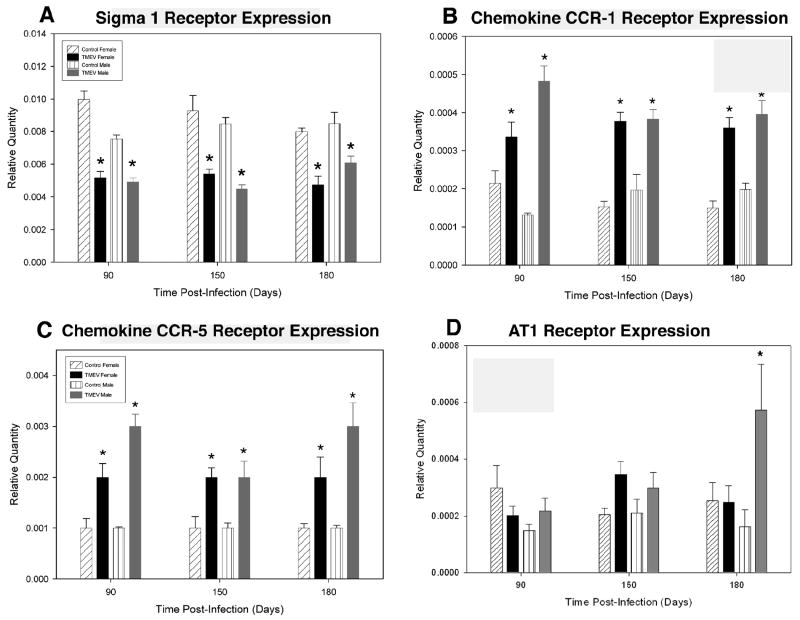
TMEV infection in both male and female mice caused a significant decrease in MOR (female mice = p<0.0001 at all time points; male mice = p<0.0001, at days 90 and 150, p<0.0023 at day 180), DOR (female mice = p<0.0002, p<0.001 and p<0.003 at days 90, 150 and 180, respectively; male mice = p<0.0001, p<0.0003 and p<0.0036 at days 90, 150 and 180, respectively) and KOR (female mice = p<0.0005, p<0.0307 and p<0.0032 at days 90, 150 and 180, respectively; male mice = p<0.0004, p<0.0001 and p<0.0408 at days 90, 150 and 180, respectively) mRNA expression at all time-points when compared to uninfected controls (figure 1). While there were no significant differences in MOR mRNA expression between TMEV infected male and female mice, male TMEV infected mice have significantly decreased expression of DOR mRNA at days 90 and 150 PI (figure 1b) and KOR mRNA at day 150 PI (figure 1c). In contrast to opioid receptors the chemokine receptors CCR1 and CCR5 were significantly increased at all time points following TMEV infection (CCR1 = p<0.0027 in females and p<0.0001 in males at day 90 and p<0.0001 at days 150 and 180 for both females and males; CCR5, female mice=p<0.0003, p<0.0002 and p<0.0001; male mice = p<0.0001 at days 90, 150 and 180, respectively). TMEV infection did not alter angotensin II receptor, type 1 (AT1) expression in TMEV-infected female mice at any time-point (figure 2). Only at day 180 PI, was there a significant increase in AT1 expression in TMEV-infected male mice (figure 2).
Figure 1. Decreased opioid receptor mRNA expression in TMEV-infected mice.
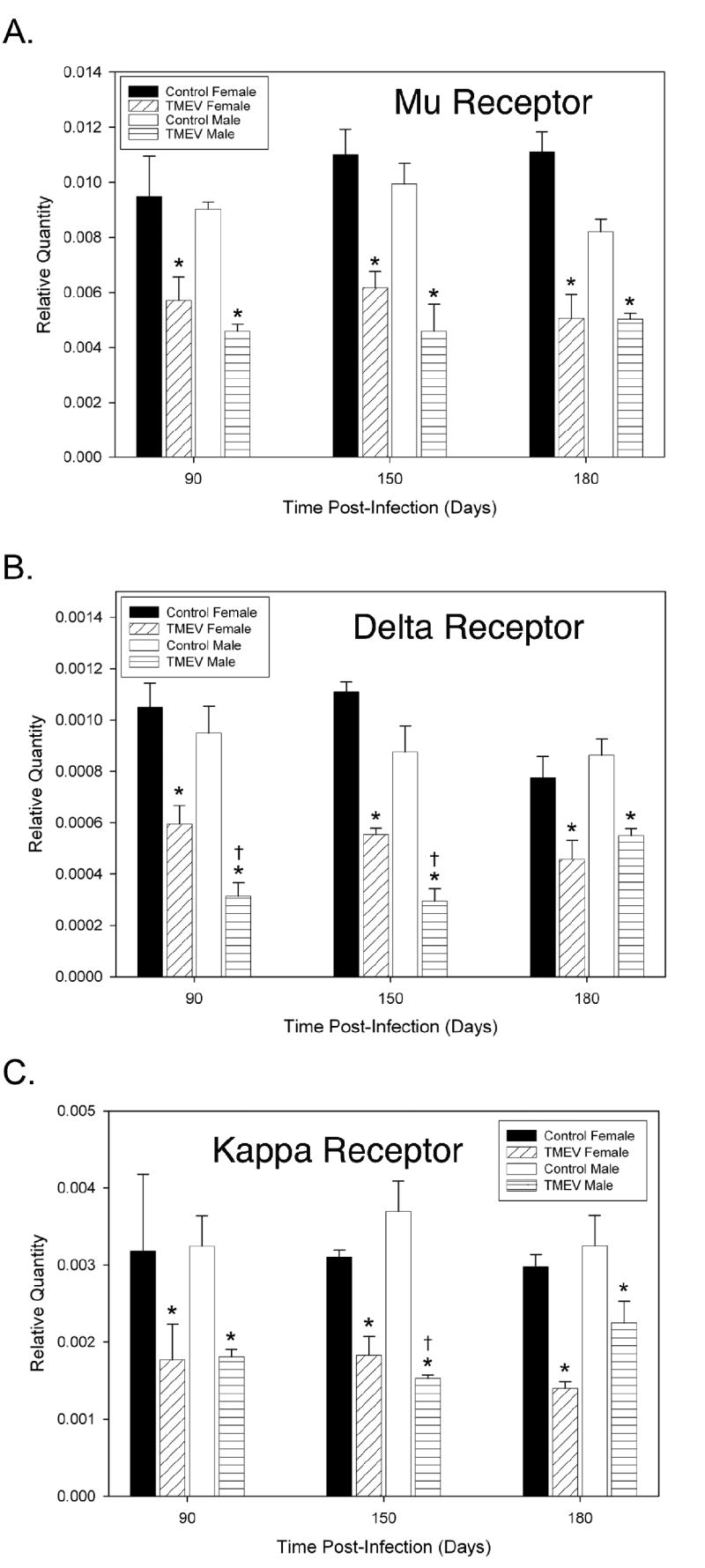
(A) Quantitative real-time PCR was utilized to analyze spinal cord opioid receptor mRNA expression in TMEV-infected and uninfected control mice. TMEV infection caused a significant decrease in mu opioid receptor mRNA expression in male and female mice compared to uninfected controls (*p<0.05). (B) TMEV-infected male and female mice had significantly decreased delta opioid receptor mRNA expression compared to uninfected controls (* p<0.05). TMEV-infected male mice had significantly decreased delta opioid receptor mRNA expression at days 90 and 150 PI († p<0.05) compared to TMEV-infected female mice. (C) Kappa opioid receptor mRNA expression was significantly decreased in TMEV infected male and female mice compared to uninfected controls (* p<0.05). TMEV-infected male mice had significantly decreased delta opioid receptor mRNA expression at day 150 PI († p<0.05) compared to TMEV-infected female mice. Results are displayed as the mean ± SEM (n = 5). Significant differences were determined using a two-way ANOVA followed by Bonferroni’s post hoc test.
Figure 2. Effects of TMEV-infection on other spinal cord G-coupled protein receptors.
Quantitative real-time PCR was utilized to analyze spinal cord Sigma-1, CCR-1, CCR-5 and angiotensin II receptor type 1 (AT1) mRNA expression in TMEV-infected and uninfected control mice. (A) TMEV infection induced a significant decrease in Sigma-1 receptor mRNA expression in both male and female mice at all time points tested (90, 150 and 180 days PI) compared to uninfected control mice (*p<0.0001). (B) TMEV infection induced a significant increase in chemokine CCR-1 receptor mRNA expression at all time points in both male and female mice at all time points tested compared to uninfected control mice (*p<0.0027). (C) TMEV infection induced a significant increase in chemokine CCR-5 receptor mRNA expression in both male and female mice at all time points tested compared to uninfected control mice (*p<0.0001). (D) TMEV infection did not alter AT1 receptor mRNA expression in female mice at any of the time points tested. Moreover, AT1 receptor mRNA expression was not altered in TMEV-treated male mice compared to uninfected controls until day 180 PI at which point there was a significant increase in expression (*p<0.0001). Results are displayed as the mean ± SEM (n = 5). Significant differences were determined using a two-way ANOVA followed by Bonferroni’s post hoc test.
Animal Behavior
We also examined animal behavior and found that TMEV infected male and female mice started to display posterior limb paralysis by day 90 PI. All of the paralyzed females showed unilateral, hind limb paralysis, but were able to walk at all time points. In contrast, 20% of male mice at days 90 and 120 PI, and 30% of male mice at days 150 and 180 PI displayed bilateral hind limb paralysis and had difficulties walking. All of the remaining male mice showed unilateral hindlimb paralysis. These visual observations of an increased disease progression in TMEV infected male mice are correlated with significant decreases in rotarod scores in male mice compared to female mice at days 90 and 120 post-infection as previously reported (Lynch et al., 2007).
Morphine Antinociception
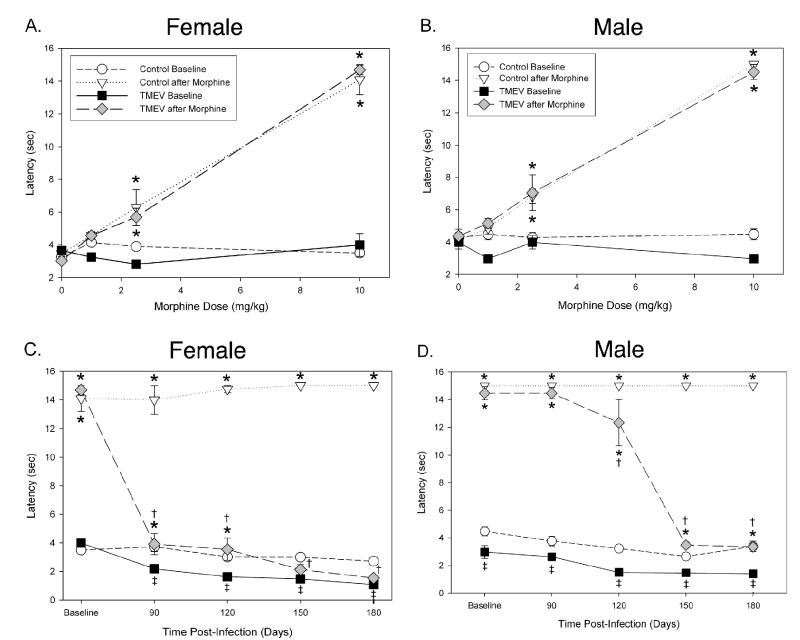
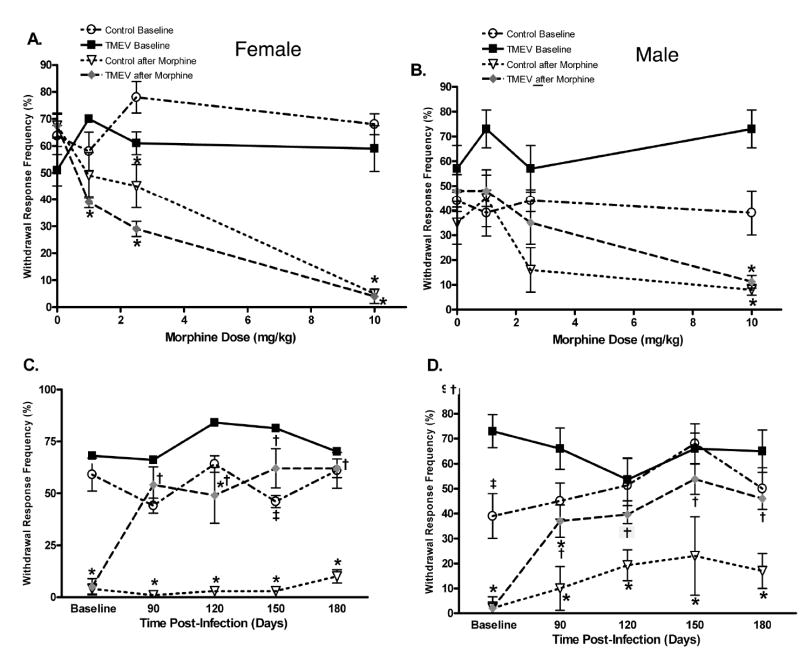
In the present study TMEV infection was found to induce a robust thermal hyperalgesia and mechanical allodynia in both male and female mice, which is similar to what we have reported previously in TMEV-treated mice (Lynch et al., 2007). Since TMEV infection reduced opioid receptor expression, we next evaluated whether TMEV infection would also produce a loss or reduction of opiate analgesia. Morphine-induced antinociception was evaluated by measuring response latencies in the thermal tail-immersion test or by measuring percent responses to von Frey stimulation (out of 10 trials). We calculated the baseline for morphine antinociception at day 60 PI, which is prior to the development of TMEV-induced neurological deficits or increased nociception. Baseline thermal latencies were determined prior to and following administration of either saline as a control or 1, 2.5 or 10 mg/kg of morphine. This produced a dose response curve in both control and TMEV infected male and female mice (see figure 3a. and 3b. for effects on thermal withdrawal latency; see figure 4a. and 4b. for effects on mechanical responses to von Frey stimulation). Based on this dose response curve, we subsequently only utilized the 10 mg/kg dose of morphine at all other time-points, because this dose produced a significant and reproducible increase in thermal withdrawal latency (antinociception) and a significant and reproducible decrease in withdrawal response frequency (antinociception) in both control and TMEV-infected animals at day 60 PI. The 10 mg/kg dose of morphine induced significant antinociception in uninfected control mice at all time points in both the thermal and mechanical nociceptive behavioral tests. In thermal testing the 10 mg/kg dose produced significantly less antinociception beginning at day 90 PI in TMEV-infected female mice when compared to uninfected controls (figure 3c.). In contrast a significant decrease in analgesia was not observed in TMEV-infected male mice until day 120 PI in comparison to uninfected controls (figure 3d.). In mechanical von Frey testing TMEV-infected female mice exhibited significantly decreased morphine analgesia when compared to morphine treated controls at days 90, 120, 150 and 180 post-infection (p<0.05, see figure 4c). TMEV-infected male mice exhibited significant morphine analgesia only at day 90 PI when compared to saline injected TMEV mice (figure 4d). TMEV-infected male mice exhibited significantly decreased morphine analgesia in the von Frey test at days 90, 120, 150 and 180 post-infection compared to uninfected controls (p<0.05).
Figure 3. TMEV-infection caused a significant decrease in morphine’s antinociceptive effect on thermal hyperalgesia.
Baseline morphine antinociception responses were determined by administering saline, 1, 2.5 or 10 mg/kg subcutaneously at day 60 PI in female (A) and male (B) TMEV-infected and uninfected controls. (C) TMEV-infected female mice exhibited thermal hyperalgesia when compared to uninfected control mice (‡ p<0.05). Morphine antinociception was determined in the chronic phase of TMEV-infection at days 90, 120, 150 and 180 PI by administering either saline or 10 mg/kg morphine subcutaneously. Uninfected control female mice exhibited significant morphine antinociception at all time points when compared to uninfected saline treated mice and TMEV infected female mice exhibited significant morphine analgesia at days 90 and 120 PI (* p<0.05). TMEV-infected female mice exhibited significantly decreased morphine analgesia when compared to uninfected controls at days 90, 120, 150 and 180 PI († p<0.05). (D) TMEV-infected male mice exhibited thermal hyperalgesia when compared to uninfected control mice (‡ p<0.05). Morphine antinociception was determined in the chronic phase of TMEV-infection at days 90, 120, 150 and 180 PI by administering either saline or 10 mg/kg morphine subcutaneously. Both TMEV-infected and uninfected control male mice exhibited significant morphine antinociception at all time points when compared to uninfected saline treated mice (* p<0.05). TMEV-infected male mice exhibited significantly decreased morphine analgesia when compared to uninfected controls at days 120, 150 and 180 PI († p<0.05).
Figure 4. TMEV-infection caused a significant decrease in morphine’s antinociceptive effect on mechanical hyperalgesia.
Baseline morphine antinociceptive responses were determined with a 9.8 mN von Frey filament 30 minutes after administering saline or 1, 2.5 or 10 mg/kg of morphine subcutaneously at day 60 PI in female (A) and male (B) TMEV-infected and uninfected controls. (C) TMEV-infected female mice exhibited mechanical hyperalgesia at day 150 PI when compared to uninfected controls (‡ p<0.05). Morphine-induced antinociception was determined during the chronic phase of TMEV-infection at days 90, 120, 150 and 180 PI following administration of 10 mg/kg morphine subcutaneously in both male and female mice. Uninfected control female mice exhibited significant morphine antinociception at all time points when compared to uninfected saline treated mice, but TMEV infected female mice exhibited significant morphine antinociception only at day 120 when compared to saline injected TMEV mice (* p <0.05). TMEV-infected female mice exhibited significantly decreased morphine analgesia when compared to morphine treated uninfected controls at days 90, 120, 150, and 180 PI († p<0.05). (D). Uninfected control male mice exhibited significant morphine antinociception at all time points when compared to uninfected saline treated mice, but TMEV-infected male mice exhibited significant morphine antinociception only at day 90 PI when compared to saline injected TMEV mice (* p <0.05). TMEV-infected male mice exhibited significantly decreased morphine analgesia when compared to uninfected controls at days 90, 120, 150 and 180 PI († p<0.05).
3. Discussion
3.1 Decreased opioid receptor expression
The present study demonstrates that TMEV infection causes a significant reduction in opioid receptor mRNA expression in the spinal cord of both male and female mice. Previous studies have shown that all 3 of the major classes of opioid receptors are present in the spinal cord (Besse et al., 1990). Mu and kappa opioid receptors are located mainly in laminae I and II, where many of the A-delta and C-fibers project, while the delta opioid receptors are more widely spread throughout the laminae of the dorsal horn and into the ventral horn (Ballet et al., 2003; Cahill et al., 2007; Gouarderes et al., 1991; Gouarderes et al., 1993). These anatomical findings, coupled with whole animal behavioral studies and electrophysiological data, indicate that opioid receptors are located on neurons, axons, and dendrites intrinsic to the spinal cord and on terminals of primary afferent fibers (Cheng et al., 1997; Inturrisi, 2002), which is consistent with the ability of opiates to reduce pain at the level of the spinal cord (Chen and Pan, 2006).
Pain is an important and frequent symptom accompanying multiple sclerosis (Svendsen et al., 2003). MS pain develops in 50-80% of MS patients, with 30% of those patients describing pain as their worst symptom Beard et al., 2003). We have previously shown that both TMEV infected male and female mice exhibited significant thermal hyperalgesia and mechanical allodynia compared to controls (Lynch et al., 2007), which is consistent with reports that most MS patients display sensory abnormalities, dominated by mechanical and thermal hyperalgesia (Osterberg et al., 1995; Svendsen et al., 2003). In the present study we have shown that TMEV infection caused a decrease in mu, delta and kappa opioid receptor mRNA expression in both males and females. Correlation analysis indicated that the loss of opioid receptor mRNA is positively correlated with the development of thermal and mechanical hyperalgesia in TMEV-infected mice. Thus it is feasible that the loss of spinal cord opioid receptors could contribute to the increased nociception observed in TMEV-infected mice (Lynch et al., 2007) and if decreases in opioid receptors also occur in MS patients over time, this may explain the increased nociception experienced by these individuals as well. In this regard it is interesting that MS pain is poorly responsive, but not totally unresponsive, to opioids (Kalman et al., 2002). This reduction in sensitivity to opiates, which is also observed in TMEV-infected mice, could also be explained by the loss of opioid receptors that occurs during disease progression.
While chemokine receptors weren’t the focus of this study is it interesting to note that the mRNA expression for both the CCR1 and CCR5 chemokine receptors increased significantly in TMEV mice at all time points examined as compared to uninfected controls. Chemokines mediate selective recruitment of leukocyte subsets into the CNS during inflammatory episodes and both the CCR1 and CCR5 receptors have been implicated in the pathogenesis of multiple sclerosis. It is interesting that recent studies have shown that polymorphisms in CCR5 genotypes modify the clinical and pathological features of multiple sclerosis (Kaimen-Maciel et al., 2007; van Venn et al., 2007). Furthermore in MOG-EAE rats, extensive up-regulation of CCR1 and CCR5 mRNA occurs in the spinal cord (Eltayeb et al., 2007), which is consistent with our current findings. Moreover, Eltayeb and coworkers (2007) showed that CCR1 and CCR5 mRNA expression is increased in cells of the macrophage/microglia lineage within EAE-induced CNS lesions, suggesting that these chemokine receptors mediate recruitment of both infiltrating macrophages and resident microglia to sites of CNS inflammation.
While angiotensin II is known to bind to AT1 receptors to cause vasoconstriction and fluid retention, both of which lead to an increase in blood pressure, recent work indicates that angiotensin II acting via the AT1 receptor controls occludin function and that inflammation-induced downregulation of angiotensin II production by astrocytes is involved in blood brain barrier dysfunction in multiple sclerosis lesions (Wosik et al., 2007). To our knowledge this is the first study showing that spinal cord AT1 receptor mRNA is significantly up-regulated in the late stages of TMEV-induced demyelination. If angiotensinogen (which is cleaved into angiotensin II) production is down-regulated in TMEV mice like it is in MS lesions from human brain samples, then one would predict a corresponding up-regulation of AT1receptors, which is what we find. Whether this is associated with altered blood-brain-barrier function in TMEV mice remains to be determined.
However, another possible explanation for the increased expression of AT1 receptor mRNA relates to immunohistochemical observations showing that the AT1 receptor is found in axonal fibers and terminals in lamina II of the dorsal horn (Ahmad et al., 2003). Lamina II is the site of termination for nociceptive fibers and there is precedence for a role of angiotensin II in modulating pain pathways—centrally administered angiotensin II attenuates morphine-induced analgesia in mice and rats (Han et al., 2000; Kaneko et al., 1985). Moreover, studies that have examined whether endogenous opioids are operative in modulating the CNS action of angiotensin II (ang II) on blood pressure have shown that endogenous opioids modulate the pressor response to intracerebral ang II and that this effect is mediated mainly through endogenous kappa opioid receptors (Rabkin, 2007). Since we have shown that male TMEV infected mice have significantly decreased expression of KOR mRNA at day 150 compared to female mice, it is possible that the link between kappa opioids and angiotensin II underlies the selective increase in AT1 receptor expression in male TMEV mice.
3.2 Decreased morphine antinociception
We observed a significant decrease in morphine-induced antinociception in TMEV-infected mice compared to non-infected controls with female mice showing an earlier reduction in morphine-induced antinociception than males. This is consistent with a recent human study in which a minority of MS patients with central pain was reported to respond to morphine and only at high doses (Kalman et al., 2002). This is also consistent with work in rodent models of neuropathic pain, where morphine has been reported to be relatively ineffective in these models compared to its effect on basal or inflammatory pain (Decosterd et al., 2004; Ossipov et al., 1995; Rashid et al., 2004). Recent work has shown that this reduction in opiate effectiveness is associated with reduced mu opioid receptor immunostaining in the spinal cord (Porreca et al., 1998) together with reduced mu opioid receptor pre- and post-synaptic action in the dorsal horn (Kohno et al., 2006). Preliminary immunohistochemical data from our lab suggest that mu opioid receptor expression is decreased primarily in laminae I and II in TMEV-infected mice, which is consistent with the findings in rodent neuropathic pain models and with the finding that MOR in the dorsal horn is expressed in almost equal amounts on the central terminals of small caliber Aδ and C fibers and on dorsal horn neurons (Abbadie et al., 2002). Thus, a decrease in spinal cord MOR expression in TMEV-infected mice would contribute to the enhanced nociception and decreased morphine antinociception observed in the present study. It should be noted that the present study relied on evaluating TMEV-induced nociception and morphine-induced antinociception using only two nociceptive behavioral tests, thermal tail withdrawal and von Frey mechanical withdrawal. While these tests can evaluate changes in reflexive behavior as it relates to nociception they fail to assess the affective-motivational dimension of pain. Such evaluations can be done using more sophisticated operant conditioning approaches such as those developed by Vierck and coworkers (Rossi et al., 2006; Wiley et al., 2007).
That we identified sex differences in the reduction of morphine-induced antinociception during TMEV disease progression is perhaps not surprising since there is considerable evidence in the literature supporting sex differences in opioid analgesia [Craft, 2003, see review by Fillingim and Gear, 2004]. In general, nonhuman animal studies suggest more robust opioid analgesic responses in males relative to females. In the present study we have identified an earlier onset in the reduction of morphine-induced antinociception in TMEV-infected females compared to infected males. Furthermore, female mice exhibited a greater loss of opiate analgesia when compared to male mice. Thus, by day 150 PI female mice did not experience an analgesic response at all, while male mice never completely lost their analgesic response to morphine in thermal nociceptive tests. In mechanical von Frey testing, both male and female TMEV mice exhibited significantly decreased morphine analgesia at all time points tested when compared to uninfected controls. It is possible that the slower reduction in loss of morphine analgesia in the thermal nociceptive tests as well as the greater reduction in female TMEV-infected mice is related to the fact that male mice typically have a more robust response to opiates than females. On the other hand examination of mu opioid receptor mRNA expression shows that male and female TMEV-infected mice have an almost equal reduction of opioid receptor message at day 90 PI. Thus, since there do not appear to be sex differences in the reduction of opioid receptor expression at day 90 PI, it is unlikely that this can explain the earlier reduction in morphine-induced antinociception in female compared to male TMEV-infected mice. Since sex hormones have been implicated in variations in disease progression in both MS patients and the EAE animal model of MS (Hoffman et al., 2006; Polanezyk et al., 2004; Tomassini and Pozzilli, 2006), it is also possible that alterations in sex hormones could occur during TMEV-induced disease progression. Sex hormones have been shown to alter mu opioid antinociception (Stoffel et al., 2005) and thus, future studies should examine whether alterations in sex hormones contribute to the observed sex differences in morphine-induced antinociception in TMEV-infected mice. Since morphine treatment was found to be more effective in male than female TMEV-infected mice, this raises the issue of whether male patients with MS may be more responsive to opiate treatment than females. This is clearly another important question that should be addressed in future clinical studies.
4. Conclusions
We utilized the Theiler’s murine encephalomyelitis virus model of MS to examine possible changes in spinal cord opioid receptor mRNA expression over the course of disease progression. TMEV infection led to significantly decreased mu, delta and kappa opioid receptor mRNA expression in both male and female mice at days 90, 150 and 180 post-infection as analyzed by quantitative Real-Time PCR. Since opioid receptor mRNA expression decreased in TMEV mice, we examined whether opiate analgesia is also altered. TMEV infected female mice had significantly decreased opiate analgesia in thermal nociceptive tests beginning at day 90 PI, while TMEV-infected male mice did not display significantly decreased opiate analgesia until day 120 PI. Both male and female TMEV infected mice showed significantly decreased opiate analgesia in von Frey tests of mechanical hyperalgesia at all time points measured (days 90-180 PI). These findings suggest that decreases in spinal cord opioid receptor expression may contribute to the increased nociception that accompanies disease progression in TMEV mice and raises the possibility that alterations in spinal opioid receptors may occur in human MS patients and contribute to the poor success of opiate treatment in these patients.
5. Experimental Procedure
Virus
Intracerebral inoculation of susceptible strains of mice with TMEV produces a biphasic disease characterized by acute encephalitis for the first 2 weeks, followed by chronic demyelination (Lipton, 1975; Rodriguez et al., 1987). Both TMEV and MS are characterized by inflammatory infiltration, demyelination and similar morphological patterns of oligodendrocyte damage and cell death, as well as, similar types of remyelination and axonal damage that correlates with neurological disability (Oleszak et al., 2004). The Daniel’s (DA) strain of TMEV was used in all experiments. The virus was grown in BHK-21 cells and titered by plaque assay in L2 cells as described previously (Alley et al., 2003).
Animals
Sixty age-matched male and female SJL/J mice (4-6 weeks old) were purchased from Jackson Laboratories (Bar Harbor, ME) and used for the Q-PCR experiments. An additional 20 mice were used for the morphine experiments described below. Mice were housed in an animal facility at the University of Minnesota in accordance with institution guidelines and were given food and water ad lib. Six-week-old SJL/J mice were anesthetized with Fluothane® and injections made using a 25-μL Hamilton syringe in the right cerebral hemisphere. Each mouse was intracerebrally injected with 20 μL of a suspension of the Daniel’s strain of TMEV virus (4 × 106 plaque forming units). To prevent fighting, male SJL/J mice were housed in individual cages. The experimental protocols for animal usage were reviewed and approved by the University of Minnesota Animal Care and Use Committee and conform to the policies outlined in the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research.
Quantitative Real-Time PCR
TMEV-infected and uninfected control male and female mice (n=4-5/group) were first anesthetized with isoflourane and then decapitated. The spinal column was removed from the mouse. The spinal cord was pushed from the vertebral column using water pressure maintained with a 22 gauge syringe filled with distilled water. After removal the cord was immediately stored in RNAlater® (Ambion, Inc., Austin, TX) at -20° C. RNA was isolated from the spinal cord using the Qiagen RNeasy mini kit protocol for difficult tissues, with a proteinase K digestion, and DNase treatment. RNA integrity was checked using an Agilent RNA 6000 Nano LabChip (Agilent Technologies, Inc. Santa Clara, CA). Total cDNA was produced by reverse transcription using the Applied Biosystems Taqman reverse transcription system (Applied Biosystems, Foster City, CA) of 0.2 μl of purified RNA, 3 μl 10X RT buffer, 6.6 μl MgCl2, 6 μl 2.5 mM dNTPs, 1.5 μl random hexamers, 0.6 μl Rnase inhibitor, and 0.75 μl Multiscribe RT. Samples were incubated for 10 minutes at 25° C, 30 minutes at 48° C, and 5 minutes at 95° C. Quantitative real-time PCR was performed in a Stratagene Mx3000P (Stratagene, La Jolla, CA). Amplification was carried out in 50 μl reaction mixtures containing 2 μl of template cDNA, 1 μl of each 5 mm primer, 25 μl 2X SYBR green (Applied Biosystems, Foster City, CA) mastermix, and 21 μl PCR water. Cycling conditions were one cycle at 95°C for 10 minutes, followed by 50 cycles of 95°C for 15 seconds, and 60° for 1 minute followed by one cycle at 95° for 15 seconds, 60° for 15 seconds and 95° for 15 seconds. Primers for quanitative real-time PCR were made using Primer 3 software (Whitehead Institute for Biomedical Research, Cambridge, MA) and primer efficiency was between 95-105%. Sequences were as follows: Mu Opioid Receptor, forward 5’-atacaggcaggggtccatag, reverse 5’-gtccataacacacagtgatgatga; Delta Opioid Receptor, forward 5’-ggaagaaattgacgcctttg, reverse 5’-gttaggtccctgctggagtg; Kappa Opioid Receptor, forward 5’-atgccctttcagagtgctgt, reverse 5’-gtagcggtccacactcat; and Beta Actin, forward 5’-ttcctccctggagaagag, reverse 5’- tgccacaggattccatac. In order to demonstrate that TMEV-infection does not alter all G-protein coupled receptors or alters them differently then opioid receptors, we also analyzed the expression of two chemokine receptors (CCR1 and CCR5), the angiotensin II receptor, type 1 (AT1) and the sigma 1 receptor. The chemokine receptors CCR1 and CCR5 were selected because they have been shown to play a pathogenic role in multiple sclerosis (Szczucinski and Losy, 2007) and therefore should be altered in TMEV-infected mice. The sigma receptor was selected because it plays a role in remyelination (Demerens et al., 1999; Palacios et al., 2003) and thus it alteration would be predicted in our TMEV model of multiple sclerosis. It is important to note that the sigma 1 receptor was initially designated a subtype of opioid receptor due to the unique psychotomimetic effects of SKF-10047 and pentazocin (Martin et al., 1976), but this receptor has now been cloned and is recognized as being a unique protein unrelated to any other mammalian proteins (Monnet, 2005). Finally the AT1 receptor was selected because it has been shown that angiotensin II acting via the AT1 receptor controls occludin function and that inflammation-induced downregulation of angiotensin II production by astrocytes is involved in blood brain barrier dysfunction in multiple sclerosis lesions (Wosik et al., 2007). Sequences for these receptors were as follows: CCR1 Receptor, forward 5’-cgagacaatcgcgaacatctac, reverse 5’-ccccggccgatatctca; CCR5 Receptor, forward 5’-atacccgatccacaggagaa, reverse 5’-ccattcctactcccaagctg; AT1 Receptor, forward 5’-aatgagcacgctctcctacc, reverse 5’-attgccagcagctttgaa; and Sigma 1 Receptor, forward 5’-cattcgggacgatactgggc, reverse 5’-cctgggatgaagacctcactttt. Specificity of amplicons was confirmed by melting curve analysis, evidence of a single band upon gel electrophoresis, authenticity of the DNA sequence of the band isolated from the gel, and resolution by BLAST analysis that the sequences of the amplicons were unique to murine opioid receptors mu, delta and kappa, respectively and to the CCR1, CCR5, Sigma 1 and AT1 receptors, which served as non-opioid receptor control genes. Real-time PCR data was analyzed using the comparative Ct method where the relative amount of gene copies was extrapolated using beta actin as a normalizer and stratagene mouse standard RNA as a calibrator. Statistical comparison between infected and uninfected age-matched mice of the same gender and between male and female mice was performed using ANOVA.
Opioid receptor mRNA expression was measured at days 90, 150 and 180 post-infection. These time points were selected because previous studies have shown that TMEV-induced neurological deficits are clearly established by day 90 PI and mice become progressively worse and are severely disabled by day 180 PI (Alley et al., 2003; Rodriguez et al., 1987). This progression in neurological deficits as measured by rotarod in TMEV mice may reflect the chronic progressive quality of MS.
Drug Administration
MS patients, particularly those patients with central or neuropathic related pain, exhibit significantly reduced opiate antinociception (Kalman et al., 2002). To examine if this phenomenon occurs in TMEV infected and uninfected control male and female mice we tested for morphine antinociception at days 60, 90, 120, 150 and 180 PI using the thermal tail-immersion assay and von Frey mechanical testing. Prior to morphine administration baseline thermal tail-immersion and von Frey mechanical stimulation scores were obtained from all mice. To test morphine antinociception, mice were subcutaneously injected with saline (n=5), 1mg/kg morphine (n=5), 2.5 mg/kg morphine (n=5) or 10 mg/kg morphine (n=5) and were acclimated for 30 minutes after injection prior to thermal tail-immersion testing or von Frey testing. Statistical comparison between infected and uninfected age-matched mice of the same gender and between male and female mice was performed using 2 way ANOVA with a Bonferroni Post-hoc test.
Thermal Tail-Immersion Assay
The thermal tail-immersion assay was performed as described previously (Lynch et al., 2007; Shimizu et al., 2005). Briefly, the tail of a gently restrained mouse was immersed in a water bath set at 50° C +/- 1°C, and the time to tail flick was recorded. A cut off time of 15 seconds was used to avoid thermal injury. After 15 sec the tail was immediately removed from the bath regardless of response. The test was repeated three times and averaged. In initial studies, no differences were found in nociceptive responses of TMEV or control female mice at different phases of the estrous cycle and thus, the data of all females were pooled for analysis of thermal hyperalgesia. Statistical comparison between infected and uninfected age-matched mice of the same gender and between male and female mice was performed using 2 way ANOVA with a Bonferroni Post-hoc test.
Von Frey Test for Mechanical Hyperalgesia
The same groups of animals used for thermal testing were also used for mechanical hyperalgesia testing. The von Frey test was modified slightly from that previously described (Lynch et al., 2007) such that a 9.8 mN filament was used for all testing. Mice were allowed to acclimate to the testing environment for at least 30 min before testing. The monofilament was applied ten times on the plantar surface of each hindpaw. The number of withdrawal responses to the monofilament was counted and expressed as percent of stimuli that evoke a response (withdrawal response frequency). Use of the 9.8 mN filament produced an average of 7-8 responses out of 10 trials (mechanical hyperalgesia) in baseline testing of male and female mice which was performed at day 60 post-TMEV injection. Establishment of mechanical hyperalgesia (7-8 responses/10) using the 9.8 mM filament was necessary in order to evaluate the analgesic effect of morphine. The monofilament was applied for 1–2 sec with an interstimulus interval of 5–10 sec. Statistical comparison between infected and uninfected age-matched mice of the same gender and between male and female mice was performed using 2 way ANOVA with a Bonferroni Post-hoc test.
Finally, we examined whether there was a correlation between the altered thermal and mechanical responses of the TMEV-infected mice from the mRNA study to the changes in opioid receptor expression. This was done using a Pearson correlation coefficient to show the strength and direction of the relationship between the mRNA expression for each of the opioid receptors and the development of hyperalgesia. As illustrated in figure 5 there is a strong correlation between the decrease in spinal cord opioid receptor mRNA expression and the decrease in response time of tail withdrawal to a thermal stimulus (the development of thermal hyperalgesia). In addition there is a weaker, but still significant, positive correlation between the development of mechanical allodynia (as indicated by increased numbers of withdrawal responses to 10 applications of a von Frey filament) and decreases in mu and delta opioid receptor mRNA expression. Only the mu receptor correlation is depicted in 5D. The correlation for the DOR receptor mRNA and mechanical allodynia was: r=-0.4454 p<0.0065.
Figure 5. Graphs demonstrating the positive correlation between the development of TMEV-induced nociception (thermal hyperalgesia and mechanical allodynia) and the decrease in opioid receptor mRNA expression.
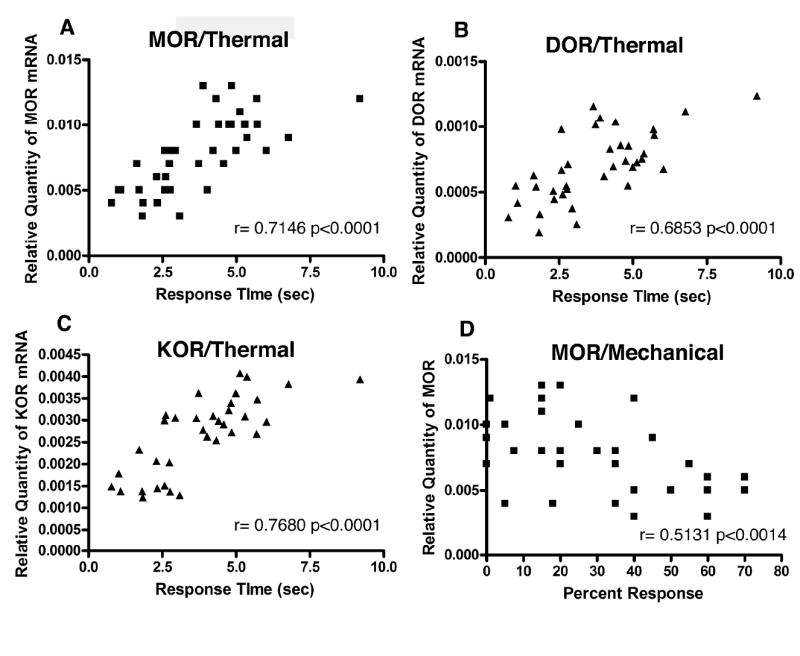
There is a strong positive correlation between the TMEV-induced decrease in tail withdrawal response time and the decrease in mu (A), delta (B) and kappa (C) opioid receptor mRNA expression. (D) There is a positive correlation between increased percent withdrawal to a von Frey mechanical stimulus (mechanical allodynia) and the decrease in mu opioid receptor mRNA expression.
Acknowledgments
This study was supported by the National Multiple Sclerosis Society (PP1125), T32 DA07097 (NIH/NIDA), and the Stark Award from the Department of Neuroscience at the University of Minnesota. We would like to thank Dr. M.K. Njenga for providing the TMEV virus.
Abbreviations
- TMEV
Theiler’s murine encephalomyelitis virus
- PI
Post-infection
- MOR
Mu opioid receptor
- DOR
Delta opioid receptor
- KOR
Kappa opioid receptor
- AT1
Angiotensin II receptor, type 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature References
- Abbadie C, Lombard MC, Besson JM, Trafton JA, Basbaum AI. Mu and delta opioid receptor-like immunoreactivity in the cervical spinal cord of the rat after dorsal rhizotomy or neonatal capsaicin: an analysis of pre- and postsynaptic receptor distributions. Brain Res. 2002;930:150–162. doi: 10.1016/s0006-8993(02)02242-4. [DOI] [PubMed] [Google Scholar]
- Alley J, Khasabov S, Simone D, Beitz AJ, Rodriguez M, Njenga MK. More severe neurologic deficits in SJL/J male than female mice following Theiler’s virus-induced CNS demyelination. Exp Neurol. 2003;180:14–24. doi: 10.1016/s0014-4886(02)00054-7. [DOI] [PubMed] [Google Scholar]
- Ahmad Z, Milligan CJ, Paton JF, Deuchars J. Angiotensin type 1 receptor immunoreactivity in the thoracic spinal cord. Brain Research. 2003;985:21–31. doi: 10.1016/s0006-8993(03)03112-3. [DOI] [PubMed] [Google Scholar]
- Archibald CJ, McGrath PJ, Ritvo PG, Fisk JD, Bhan V, Maxner CE, Murray TJ. Pain prevalence, severity and impact in a clinic sample of multiple sclerosis patients. Pain. 1994;58:89–93. doi: 10.1016/0304-3959(94)90188-0. [DOI] [PubMed] [Google Scholar]
- Ballet S, Conrath M, Fischer J, Kaneko T, Hamon M, Cesselin F. Expression and G-protein coupling of mu-opioid receptors in the spinal cord and dorsal root ganglia of polyarthritic rats. Neuropeptides. 2003;37:211–219. doi: 10.1016/s0143-4179(03)00045-3. [DOI] [PubMed] [Google Scholar]
- Beard S, Hunn A, Wight J. Treatments for spasticity and pain in multiple sclerosis: a systematic review. Health Technology Assessment. 2003;7:1–124. doi: 10.3310/hta7400. [DOI] [PubMed] [Google Scholar]
- Besse D, Lombard MC, Zajac JM, Roques BP, Besson JM. Pre- and postsynaptic distribution of mu, delta and kappa opioid receptors in the superficial layers of the cervical dorsal horn of the rat spinal cord. Brain Res. 1990;521:15–22. doi: 10.1016/0006-8993(90)91519-m. [DOI] [PubMed] [Google Scholar]
- Brown SM, Stimmel B, Taub RN, Kochwa S, Rosenfield RE. Immunologic dysfunction in heroin addicts. Arch Intern Med. 1974;134:1001–1006. [PubMed] [Google Scholar]
- Bussiere JL, Adler MW, Rogers TJ, Eisenstein TK. Differential effects of morphine and naltrexone on the antibody response in various mouse strains. Immunopharmacol Immunotoxicol. 1992;14:657–673. doi: 10.3109/08923979209005416. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Holdridge SV, Morinville A. Trafficking of delta-opioid receptors and other G-protein-coupled receptors: implications for pain and analgesia. Trends Pharmacol Sci. 2007;28:23–31. doi: 10.1016/j.tips.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Chen SR, Pan HL. Blocking mu opioid receptors in the spinal cord prevents the analgesic action by subsequent systemic opioids. Brain Res. 2006;1081:119–125. doi: 10.1016/j.brainres.2006.01.053. [DOI] [PubMed] [Google Scholar]
- Cheng PY, Liu-Chen LY, Pickel VM. Dual ultrastructural immunocytochemical labeling of mu and delta opioid receptors in the superficial layers of the rat cervical spinal cord. Brain Res. 1997;778:367–380. doi: 10.1016/s0006-8993(97)00891-3. [DOI] [PubMed] [Google Scholar]
- Craft RM. Sex differences in opioid analgesia: “from mouse to man”. Clin J Pain. 2003;19:175–186. doi: 10.1097/00002508-200305000-00005. [DOI] [PubMed] [Google Scholar]
- Decosterd I, Allchorne A, Woolf CJ. Differential analgesic sensitivity of two distinct neuropathic pain models. Anesth Analg. 2004;99:457–463. doi: 10.1213/01.ANE.0000131967.69309.4F. [DOI] [PubMed] [Google Scholar]
- Demerens C, Stankoff B, Zalc B, Lubetzki C. Eliprodil stimulates CNS myelination: new prospects for multiple sclerosis? Neurology. 1999;52:346–350. doi: 10.1212/wnl.52.2.346. [DOI] [PubMed] [Google Scholar]
- Ehde DM, Osborne TL, Hanley MA, Jensen MP, Kraft GH. The scope and nature of pain in persons with multiple sclerosis. Mult Scler. 2006;12:629–638. doi: 10.1177/1352458506071346. [DOI] [PubMed] [Google Scholar]
- Eltayeb S, Berg AL, Lassmann H, Wallstrom E, Nilsson M, Olsson T, Ericsson-Dahlstand A, Sunnemark D. Temporal expression and cellular origin of CC chemokine receptors CCR1, CCR2 and CCR5 in the central nervous system: insight into mechanisms of MOG-induced EAE. J Neuroinflammation. 2007;4:14. doi: 10.1186/1742-2094-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingim RB, Gear RW. Sex differences in opioid analgesia: clinical and experimental findings. Eur J Pain. 2004;8:413–425. doi: 10.1016/j.ejpain.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Gouarderes C, Beaudet A, Zajac JM, Cros J, Quirion R. High resolution radioautographic localization of [125I]FK-33-824-labelled mu opioid receptors in the spinal cord of normal and deafferented rats. Neuroscience. 1991;43:197–209. doi: 10.1016/0306-4522(91)90427-p. [DOI] [PubMed] [Google Scholar]
- Gouarderes C, Tellez S, Tafani JA, Zajac JM. Quantitative autoradiographic mapping of delta-opioid receptors in the rat central nervous system using [125I] [D.Ala2] deltorphin-I. Synapse. 1993;13:231–240. doi: 10.1002/syn.890130306. [DOI] [PubMed] [Google Scholar]
- Hadjimichael O, Kerns RD, Rizzo MA, Cutter G, Vollmer T. Persistent pain and uncomfortable sensations in persons with multiple sclerosis. Pain. 2007;127:35–41. doi: 10.1016/j.pain.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Han NL, Luo F, Bian ZP, Han JS. Synergistic effect of cholecystokinin octapeptide and angiotensin II in reversal of morphine induced analgesia in rats. Pain. 2000;85:465–469. doi: 10.1016/S0304-3959(99)00294-8. [DOI] [PubMed] [Google Scholar]
- Hoffman GE, Merchenthaler I, Zup SL. Neuroprotection by ovarian hormones in animal models of neurological disease. Endocrine. 2006;9:217–231. doi: 10.1385/ENDO:29:2:217. [DOI] [PubMed] [Google Scholar]
- Indaco A, Iachetta C, Nappi C, Socci L, Carrieri PB. Chronic and acute pain syndromes in patients with multiple sclerosis. Acta Neurol (Napoli) 1994;16:97–102. [PubMed] [Google Scholar]
- Inturrisi CE. Clinical pharmacology of opioids for pain. Clin J Pain. 2002;18(Suppl 4):S3–13. doi: 10.1097/00002508-200207001-00002. [DOI] [PubMed] [Google Scholar]
- Kaimen-Maciel DR, Vissoci Reiche EM, Brum Souza DG, Frota Comini ER, Bobroff F, Morimoto HK, Ehara Watanabe MA, De Oliveira J, Matsuo T, Lopes J, Donadi EA. CCR5-Delta32 genetic polymorphism associated with benign clinical course and magnetic resonance imaging findings in Brazilian patients with multiple sclerosis. Int J Mol Med. 2007;20:337–344. [PubMed] [Google Scholar]
- Kalia LV, O’Connor PW. Severity of chronic pain and its relationship to quality of life in multiple sclerosis. Mult Sclerosis. 2005;11:322–327. doi: 10.1191/1352458505ms1168oa. [DOI] [PubMed] [Google Scholar]
- Kalman S, Osterberg A, Sorensen J, Boivie J, Bertler A. Morphine responsiveness in a group of well-defined multiple sclerosis patients: a study with i.v. morphine. Eur J Pain. 2002;6:69–80. doi: 10.1053/eujp.2001.0307. [DOI] [PubMed] [Google Scholar]
- Kaneko S, Mori A, Tamura S, Satoh M, Takagi H. Intracerebroventricular administration of angiotensin II attenuates morphine-induced analgesia in mice. Neuropharmacology. 1985;24:1131–1134. doi: 10.1016/0028-3908(85)90204-7. [DOI] [PubMed] [Google Scholar]
- Kohno T, Ji RR, Ito N, Allchorne AJ, Befort K, Karchewski LA, Woolf CJ. Peripheral axonal injury results in reduced mu opioid receptor pre- and post-synaptic action in the spinal cord. Pain. 2006;117:77–87. doi: 10.1016/j.pain.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Layon J, Idris A, Warzynski M, Sherer R, Brauner D, Patch O, McCulley D, Orris P. Altered T-lymphocyte subsets in hospitalized intravenous drug abusers. Arch Intern Med. 1984;144:1376–1380. [PubMed] [Google Scholar]
- Lipton HL. Theiler’s virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect Immun. 1975;11:1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JL, Gallus NJ, Ericson ME, Beitz AJ. Analysis of nociception, sex and peripheral nerve innervation in the TMEV animal model of multiple sclerosis. Pain. 2007 doi: 10.1016/j.pain.2007.07.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan SD, Schwartz SA, Aalinkeel R, Chawda RP, Sykes DE, Nair MP. Morphine modulates chemokine gene regulation in normal human astrocytes. Clin Immunol. 2005;115:323–32. doi: 10.1016/j.clim.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine and nalorphine like drugs in the non-dependent and morphine dependent chronic spinal dog. J Pharmacol Exp Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- Monnet FP. Sigma-1 receptor as regulator of neuronal intracellular Ca2+: clinical and therapeutic relevance. Biol Cell. 2005;97:873–883. doi: 10.1042/BC20040149. [DOI] [PubMed] [Google Scholar]
- Morley-Forster P. Prevalence of neuropathic pain and the need for treatment. Pain Res Manag. 2006;11(Suppl A):5A–10A. [Google Scholar]
- Neri S, Bruno CM, Pulvirenti D, Malaguarnera M, Italiano C, Mauceri B, Abate G, Cilio D, Calvagno S, Tsami A, Ignaccolo L, Interlandi D, Prestianni L, Ricchena M, Noto R, Nair MP, Laing TJ, Schwartz SA. Randomized clinical trial to compare the effects of methadone and buprenorphine on the immune system in drug abusers. Psycohpharmacology (Berl) 2005;179:700–704. doi: 10.1007/s00213-005-2239-x. [DOI] [PubMed] [Google Scholar]
- O’connor AB, Schwid SR, Herrmann DN, Markman JD, Dworkin RH. Pain associated with multiple sclerosis: Systematic review and proposed classification. Pain. 2007 doi: 10.1016/j.pain.2007.08.024. in press. [DOI] [PubMed] [Google Scholar]
- Oleszak EL, Chang JR, Friedman H, Katsetos CD, Platsoucas CD. Theiler’s virus infection: a model for multiple sclerosis. Clin Microbiol Rev. 2004;17:174–207. doi: 10.1128/CMR.17.1.174-207.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossipov MH, Lopez Y, Nichols ML, Bian D, Porreca F. Inhibition by spinal morphine of the tail-flick response is attenuated in rats with nerve ligation injury. Neurosci Lett. 1995;199:83–86. doi: 10.1016/0304-3940(95)12026-z. [DOI] [PubMed] [Google Scholar]
- Osterberg A, Boivie J, Thuomas KA. Central pain in multiple sclerosis-prevalence and clinical characteristics. Eur J Pain. 2005;9:531–542. doi: 10.1016/j.ejpain.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Pacher P, Bátkai S, Kuno G. The Endocannabinoid System as an Emerging Target of Pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellis NR, Harper C, Dafny N. Suppression of the induction of delayed hypersensitivity in rats by repetitive morphine treatments. Exp Neurol. 1986;93:92–97. doi: 10.1016/0014-4886(86)90148-2. [DOI] [PubMed] [Google Scholar]
- Polanczyk MJ, Jones RE, Subramanian S, Afentoulis M, Rich C, Zakroczymski M, Cooke P, Vandenbark AA, Offner H. T lymphocytes do not directly mediate the protective effect of estrogen on experimental autoimmune encephalomyelitis. Am J Pathol. 2004;65:2069–2077. doi: 10.1016/S0002-9440(10)63257-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios G, Muro A, Vela JM, Molina-Holgado E, Guitart X, Ovalle S, Zamanillo D. Immunohistochemical localization of the sigma 1-receptor in oligodendrocytes in the rat central nervous system. Brain Res. 2003;961:92–99. doi: 10.1016/s0006-8993(02)03892-1. [DOI] [PubMed] [Google Scholar]
- Porreca F, Tang QB, Bian D, Riedl M, Elde R, Lai J. Spinal opioid mu receptor expression in lumbar spinal cord of rats following nerve injury. Brain Res. 1998;795:197–203. doi: 10.1016/s0006-8993(98)00292-3. [DOI] [PubMed] [Google Scholar]
- Rabkin SW. Endogenous kappa opioids mediate the action of brain angiotensin II to increase blood pressure. Neuropeptides. 2007 Nov 1; doi: 10.1016/j.npep.2007.09.003. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Rashid MH, Inoue M, Toda K, Ueda H. Loss of peripheral morphine analgesia contributes to the reduced effectiveness of systemic morphine in neuropathic pain. J Pharmacol Exp Ther. 2004;309:380–387. doi: 10.1124/jpet.103.060582. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Oleszak E, Leibowitz J. Theiler’s murine encephalomyelitis: a model of demyelination and persistence of virus. Crit Rev Immunol. 1987;7:325–365. [PubMed] [Google Scholar]
- Rossi HL, Vierck CJ, Jr, Caudle RM, Neubert JK. Characterization of cold sensitivity and thermal preference using an operant orofacial assay. Mol Pain. 2006;2:37. doi: 10.1186/1744-8069-2-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacerdote P. Opioids and the immune system. Palliat Med. 2006;20(suppl 1):s9–15. [PubMed] [Google Scholar]
- Shimizu I, Iida T, Guan Y, Zhao C, Raja SN, Jarvis MF, Cockayne DA, Caterina MJ. Enhanced thermal avoidance in mice lacking the ATP receptor P2X3. Pain. 2005;116:96–108. doi: 10.1016/j.pain.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Solaro C. Epidemiology and treatment of pain in multiple sclerosis subjects. Neurol Sci. 2006;27(Suppl 4):s291–293. [Google Scholar]
- Stoffel EC, Ulibarri CM, Folk JE, Rice KC, Craft RM. Gonadal hormone modulation of mu, kappa, and delta opioid antinociception in male and female rats. J Pain. 2005;6:261–274. doi: 10.1016/j.jpain.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svendsen KB, Jensen TS, Overvad K, Hansen HJ, Koch-Henriksen N, Bach FW. Pain in patients with multiple sclerosis: a population-based study. Arch Neurol. 2003;60:1089–1094. doi: 10.1001/archneur.60.8.1089. [DOI] [PubMed] [Google Scholar]
- Szczucinski A, Losy J. Chemokines and chemokine receptors in multiple sclerosis. Potential targets for new therapies. Acta Neurol Scand. 2007;115:137–146. doi: 10.1111/j.1600-0404.2006.00749.x. [DOI] [PubMed] [Google Scholar]
- Tomassini V, Pozzilli C. Sex hormones: a role in the control of multiple sclerosis? Expert Opin Pharmacother. 2006;7:857–868. doi: 10.1517/14656566.7.7.857. [DOI] [PubMed] [Google Scholar]
- van Veen T, Nielsen J, Berkhof J, Barkhof F, Kamphorst W, Bo L, Verweij CL, Huitinga I, Polman CH, Uitdehaag BM. CCL5 and CCR5 genotypes modify clinical, radiological and pathological features of multiple sclerosis. J Immunol. 2007;190:157–64. doi: 10.1016/j.jneuroim.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Vermote R, Ketelaer P, Carton H. Pain in multiple sclerosis patients. A prospective study using the Mc Gill Pain Questionnaire. Clin Neurol Neurosurg. 1986;88:87–93. doi: 10.1016/s0303-8467(86)80002-6. [DOI] [PubMed] [Google Scholar]
- Wiley RG, Kline RH, 4th, Vierck CJ., Jr Anti-nociceptive effects of selectively destroying substance P receptor-expressing dorsal horn neurons using [Sar9,Met(O2)11]-substance P-saporin: behavioral and anatomical analyses. Neuroscience. 2007;146:1333–1345. doi: 10.1016/j.neuroscience.2007.01.066. [DOI] [PubMed] [Google Scholar]
- Wosik K, Cayrol R, Dodelet-Devillers A, Berthelet F, Bernard M, Moumdjian R, Bouthillier A, Reudelhuber TL, Prat A. Angiotensin II controls occludin function and is required for blood brain barrier maintenance: relevance to multiple sclerosis. J Neurosci. 2007;27:9032–9042. doi: 10.1523/JNEUROSCI.2088-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]