Abstract
Cocaine addiction is associated with long-term cognitive alterations including deficits on tests of declarative/spatial learning and memory. To determine the extent to which cocaine exposure plays a causative role in these deficits, adult male Long-Evans rats were given daily injections of cocaine (30 mg/kg/day × 14 days) or saline vehicle. Three months later, rats were trained for 6 sessions on a Morris water maze protocol adapted from Gallagher et al. (1993). Rats given prior cocaine exposure performed similarly to controls on training trials, but searched farther from the platform location on probe trials interpolated throughout the training sessions and showed increased thigmotaxis. The results demonstrate that a regimen of cocaine exposure can impair Morris water maze performance as long as 3 months after exposure. Although the impairments were not consistent with major deficits in spatial learning and memory, they may have resulted from cocaine-induced increases in stress responsiveness and/or anxiety. Increased stress and anxiety would be expected to increase thigmotaxis as well as cause impairments in searching for the platform location, possibly through actions on ventral striatal dopamine signaling.
Keywords: Cocaine, Psychostimulant, Morris water maze, Learning, Stress, Anxiety
1. Introduction
Addiction to psychostimulant drugs such as cocaine and amphetamine is associated with a wide range of cognitive deficits (Everitt, Dickinson, & Robbins, 2001; Jentsch & Taylor, 1999; Robinson & Berridge, 2003; Volkow & Fowler, 2000). Impairments in decision-making abilities linked to prefrontal cortical function have been most frequently reported (Bechara, Dolan, Denburg, Hindes, Anderson, & Nathan, 2001; Clark & Robbins, 2002; Dom, Sabbe, Hulstijn, & van den Brink, 2005; Grant, Contoreggi, & London, 2000; Jentsch, Olausson, De La Garza, & Taylor, 2002; Jentsch & Taylor, 1999). However, emerging evidence shows that drug addiction is also associated with impaired declarative learning and memory functions. These impairments, which have been observed after as long as 6 months of drug abstinence, include problems in complex figure, pattern, and verbal recall, as well as paired-associate learning (Berry, Gorp, Herzberg, Hinkin, Boone, Steinman, & Wilkins, 1993; Di Sclafani, Tolou-Shams, Price, & Fein, 2002; Ersche, Clark, London, Robbins, & Sahakian, 2005; Fein, Di Sclafani, & Meyerhoff, 2002; Mittenberg & Motta, 1993; Santucci, Capodilupo, Bernstein, Gomez-Ramirez, Milefsky, & Mitchell, 2004; van Gorp, Wilkins, Hinkin, Moore, Hull, Horner, & Plotkin, 1999; Volkow, Fowler, Wang, & Goldstein, 2002). Moreover, structural and functional alterations in drug (particularly psychostimulant) addicts occur in brain regions linked to declarative learning and memory, including medial temporal lobe and ventral striatum (Bartzokis, Beckson, Newton, Mandelkern, Mintz, Foster, Ling, & Bridge, 1999; Breiter, Gollub, Weisskoff, Kennedy, Makris, Berke, Goodman, Kantor, Gastfriend, Riorden, Mathew, Rosen, & Hyman, 1997; Jacobsen, Giedd, Gottschalk, Kosten, & Krystal, 2001; Leland, Arce, Feinstein, & Paulus, 2006).
Despite the evidence for associations between drug addiction and impaired declarative learning and memory, it is unclear whether these impairments are caused by exposure to drugs of abuse, or if poor declarative memory is associated with a predisposition to use and abuse drugs. This question of causation can be addressed in animal models, in which drug exposure can be tightly controlled. The experiment described here was designed to determine whether cocaine can cause deficits in a spatial learning and memory task in rats that last well beyond the cessation of drug exposure (i.e., after several months of drug abstinence), using a cocaine exposure regimen that induces cognitive deficits in other settings (Burke, Franz, Gugsa, & Schoenbaum, 2006; Schoenbaum, Ramus, Shaham, Saddoris, & Setlow, 2004; Schoenbaum & Setlow, 2005; Simon, Mendez, & Setlow, 2007), and a highly sensitive water maze task which includes multiple probe trials interpolated throughout training (Gallagher et al., 1993).
2. Materials and methods
2.1 Subjects
The subjects were 24 male Long-Evans rats weighing 300−325g upon arrival (Charles River Laboratories, Wilmington, NC, USA). Rats were housed individually in a climate controlled vivarium (25° C) in the Department of Psychology at Texas A&M University. Rats had food and water available ad lib and were tested during the light cycle of a 12-hour light/dark schedule (lights on 0800−2000). Animal testing was conducted according to the “Principles of Laboratory Animal Care” (National Academy of Sciences, USA) and met all NIH and institutional animal care and use guidelines. Rats were allowed to acclimate to vivarium conditions for at least one week before the start of cocaine exposure. Two months prior to training in the water maze (one month after cocaine exposure), the rats underwent training for an unrelated experiment in a food-motivated go/no-go discrimination task in behavioral test chambers in a different laboratory from that used for water maze testing. However, all rats had been returned to ad lib food for at least one month prior to the start of water maze testing. The experiment was run in two cohorts (n = 16 and n = 8, with equal numbers of rats in each drug group in each cohort).
2.2 Behavioral Apparatus
Cocaine exposure was conducted in six identical rat behavioral test chambers (Coulbourn Instruments, Allentown, PA, USA) located in sound-attenuating cubicles and equipped with overhead infrared activity monitors. The monitors were equipped with an array of lenses focused on two infrared sensors. Changes in the relative infrared energy focused on the sensors were recorded as movement units, but this system could not discriminate between different types of movements (e.g., horizontal vs. vertical). The chambers were interfaced with a computer running Graphic State 3.01 software, which recorded locomotor activity counts.
The water maze consisted of a large circular tank (diameter 183 cm) painted white and filled with water (27° C) made opaque with non-toxic white tempera paint. For the hidden platform task, an escape platform (diameter 10 cm) was located in the southwest quadrant of the maze and submerged 2 cm below the surface of the water. The platform (Atlantis Platform, HVS Image, UK) could be retracted to the bottom of the maze during probe trials. For the visible platform task, the platform was painted black and protruded 2 cm above the water's surface. Large white geometric shapes affixed to black curtains that completely surrounded the maze provided extramaze visual cues. Rats' performance in the task was assessed using a video camera mounted above the maze and interfaced with a computerized tracking system (Water 2020, HVS Image, UK).
2.3 Procedures
Prior to the start of the cocaine exposure sessions, rats were given a pre-treatment session to assess baseline activity. In this session, rats were placed in the test chambers and left undisturbed for one hour. Rats were divided into two groups (n = 12 each) matched for baseline activity during this session. The following day, rats began drug treatment in which they received daily i.p. injections of cocaine HCl (30 mg/kg measured as the weight of the salt, 1.5 ml/kg) or the same volume of 0.9% saline vehicle for 14 consecutive days. Immediately following each injection, locomotor activity was monitored for one hour in the test chambers before the rats were returned to the vivarium. This cocaine dose/regimen was chosen because previous work has shown that it induces a range of long-lasting cognitive deficits that are similar to those observed in human cocaine addicts, as well as long-lasting sensitization to the locomotor stimulant effects of cocaine (Burke et al., 2006; Roesch, Takahashi, Gugsa, Bissonette, & Schoenbaum, 2007; Schoenbaum et al., 2004; Schoenbaum & Setlow, 2005; Simon et al., 2007).
Three months after cocaine exposure, rats were trained in the Morris water maze on a modified version of the hidden platform task (Gallagher et al., 1993; LaSarge, Montgomery, Tucker, Slaton, Griffith, Setlow, & Bizon, 2007). Rats received three trials a day over six consecutive days, with every sixth trial being a probe trial. On each training trial, rats were placed into the water facing the wall, with the start locations varying pseudorandomly (N, S, E, or W), and permitted to swim until they reached the escape platform. A maximum of 90 s was allowed before the rats were assisted to the platform. Once on the platform, rats remained there for 30 s before being removed for a 30 s inter-trial interval. For probe trials, the escape platform was retracted to the bottom of the maze for the first 30 s of the trial. After 30 s, the platform was raised and the rats were allowed to escape as on training trials. This water maze protocol was developed to be highly sensitive to small variations in performance both between and within groups. In particular, the use of multiple probe trials interpolated throughout the course of training allows numerous repeated measures of performance that would be unattainable from training trials, while minimizing the risk of extinction (Gallagher et al., 1993). Although the placement of probe trials immediately following training trials does not provide a “pure” measure of long-term memory (i.e., uncontaminated by recent learning), performance on each probe trial does reflect the accumulated memories of the previous days' training.
To assess sensorimotor abilities and motivation to escape the water independent of spatial learning ability, rats received one session consisting of six trials of visible platform training following the last day of hidden platform training. In this session, rats were trained to escape to a visible platform, which was moved to a different maze quadrant on each trial. On each trial, rats were placed into the water at a start position distal from the platform and were given 90 s to escape to the platform, with a 30 s intertrial interval.
2.4 Data analysis
In the hidden platform task, performance was primarily assessed using two proximity measures: a cumulative search error measure was calculated from training trials (and from probe trials by using the first cross of the platform location as an artificial trial endpoint), and mean search error was calculated from probe trials. Both measures assess the rats' proximity to the platform location. To compute these measures, the rats' distance from the platform location was sampled 10 times/s during each trial and these distances were averaged into 1 s bins. Cumulative search error is the sum of these 1 s averages across the training trial minus the optimal path from start position to platform location. Mean search error is the cumulative search error divided by the duration (30 s) of the probe trial (Gallagher et al., 1993; LaSarge et al., 2007). Lower values on these measures indicate searches closer to the platform location. Additional measures of performance were also calculated, including latency to reach the platform location, pathlength (distance swum), percent time in the target quadrant, swim speed (in cm/s) and thigmotaxis (calculated as percentage of time spent within 10 cm of the maze wall). Statistical analyses were conducted in SPSS 12.0 and p values less than .05 (except as noted below) were considered significant. Outliers, defined as values outside the range of ±2 SD from the group mean, were excluded prior to statistical analysis. One rat from the cocaine group on the probe trial and activity analyses and one rat from each group on the thigmotaxis analysis were excluded for this reason.
3. Results
There were no locomotor activity differences between groups during the baseline session prior to drug exposure (t(21) = .48, n.s.). During the 14 days of drug exposure, a two-factor ANOVA (day × drug condition) revealed that cocaine exposed rats had significantly greater activity levels than saline controls (F(1,21) = 80.36, p < .001, Figure 1). Cocaine-exposed rats did not exhibit sensitization of locomotor activity across the 14 days of treatment. This was likely due to a ceiling effect in locomotor activity resulting from the high dose of cocaine, such that no sensitization-induced enhancement in locomotor activity could be observed. However, it should be noted that this cocaine dose/regimen does produce robust sensitization upon testing several months later with lower challenge doses of cocaine (Schoenbaum et al., 2004; Schoenbaum & Setlow, 2005).
Figure. 1.

Locomotor activity in cocaine- and saline-exposed rats during the 2 week drug exposure period 3 months prior to water maze training. Mean activity counts are shown for 1 h sessions which immediately followed each cocaine or saline injection. Counts were measured in 5 min blocks and averaged across the session. The groups did not differ in their activity in a pre-exposure baseline session (labeled “BL” on the X axis), but thereafter cocaine significantly increased locomotor activity compared to saline controls (* p < .05).
During training in the hidden platform water maze task, two-factor ANOVAs (training trials × drug condition) revealed a significant main effect of training on cumulative search error across 5-trial blocks (F(2,44) = 64.60, p < .001, Figure 2), indicating that, across groups, rats were able to locate the platform more effectively with increased training. However, there were no main effects or interactions involving drug condition (Fs < .74, n.s., see also Table 1).
Figure. 2.
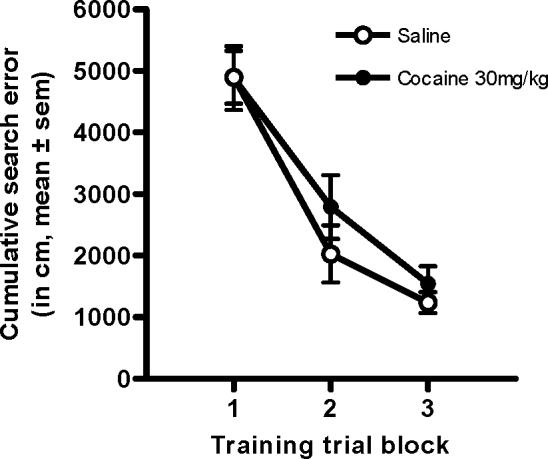
Performance on training trials in cocaine- and saline-exposed rats. Graph shows cumulative search error across training trials averaged into 5 trial blocks. There were no differences in performance between the groups.
Table 1.
Means and p values for additional comparisons between saline- and cocaine-exposed rats on water maze performance measures (two-factor ANOVAs, with prior drug exposure condition as the between-subjects variable, and trial (or trial block) as the within-subjects variable). Only the p values for main effects of drug condition are reported here.
| Measure | Mean-saline | Mean-cocaine | p value |
|---|---|---|---|
| Training Trials | |||
| latency to platform (s) | 35.1 | 37.6 | .61 |
| pathlength to platform (cm) | 850.6 | 856.1 | .91 |
| Probe trials | |||
| % time in target quadrant | 43.6 | 41.0 | .57 |
| latency to 1st platform cross (s) | 14.4 | 15.8 | .67 |
| cumulative search error to 1st platform cross (cm) | 957.2 | 1120.7 | .51 |
| pathlength during entire probe trial (cm) | 700.8 | 735.0 | .38* |
| Cue trials | |||
| latency (s) | 23.8 | 24.1 | .94 |
| pathlength (cm) | 455.7 | 400.0 | .37 |
Denotes a significant interaction between probe trial and drug condition
Improved spatial performance across training was also evident on the interpolated 30 s probe trials (Figure 3A). A two-factor ANOVA (probe trial × drug condition) revealed a significant main effect of probe trial on the mean search error measure, such that all rats' performance on this measure improved across the three probe trials (F(2,42) = 30.04, p < .001). Most importantly, there was a main effect of drug condition on this measure, such that rats given prior cocaine exposure had significantly greater mean search errors (i.e., did not concentrate their search as close to the platform location) compared to saline controls (F(1,21) = 5.39, p < .05). There was no interaction between drug treatment and probe trial (F(2,42) = .26, n.s.). Thigmotaxis during probe trials was assessed by measuring the percentage of time rats spent swimming in the maze periphery. A two-factor ANOVA (probe trial × drug condition) revealed a significant main effect of probe trial (F(2,40) = 5.54, p < .01), such that rats decreased their time spent in the maze periphery over the course of the three probe trials (Figure 3B). Importantly, there was a near-significant effect of drug condition (F(1,20) = 4.33, p = .05) on this measure, such that cocaine exposed rats spent more time in the maze periphery than controls (Figure 3B). Because this increased thigmotaxis in cocaine-exposed rats could potentially account for their increased mean search errors, the degree of relatedness between these two measures was assessed using both partial correlations (controlling for drug condition) and correlations within each drug condition. However, there were no significant correlations between mean search error and thigmotaxis (rs < .24, ps > .45, n.s.), indicating that the two measures were not reliably related.
Figure. 3.

Performance on interpolated probe trials in cocaine- and saline-exposed rats. (A.) Mean search error. Cocaine-exposed rats had greater mean search errors (i.e., searched farther from the platform location) than saline-exposed rats (* p < .05). (B.) Mean percentage of time spent in the periphery (within 10 cm of the wall) of the water maze (i.e. thigmotaxis). Cocaine-exposed rats spent more time in the maze periphery than saline-exposed rats († p = .05).
The total distance swum (pathlength) during the 30 s probe trials was also compared between groups. A two-factor ANOVA (probe trial × drug condition) revealed a main effect of probe trial (F(2,44) = 3.36, p < .05), such that rats swam shorter pathlengths over the course of the three probe trials. There was no main effect of drug condition (F(1,22) = .81, n.s.), but there was a significant interaction between the factors of probe trial and drug condition (F(2,44) = 7.42, p < .01). Post-hoc comparisons between drug conditions on each probe trial using independent sample t-tests showed that cocaine exposed rats had significantly longer pathlengths than controls on probe trial 1 (t(22) = 2.45, p < .05). However, there were no significant differences between groups on probe trials 2 and 3. Finally, rats' ability to initially locate the platform on probe trials was assessed by measuring cumulative search error to the first cross of the platform location. There was a main effect of probe trial, such that performance on this measure improved over the course of training (F(2,42) = 6.99, p < .05). However, as was found on training trials using this measure, there were no main effects or interactions involving drug condition (Fs < 2.84, n.s., see also Table 1).
In the visible platform task, a two-factor ANOVA (trial × drug condition) revealed a main effect of trial on latency to reach the platform, such that rats reached the visible platform more quickly across trials (F(5,110) = 34.74, p < .001), but no main effect or interaction involving drug condition (Fs < 2.36, n.s., Figure 4, Table 1). Likewise, there were no differences in swim speed between rats given prior cocaine or saline exposure, either on visible platform training trials or on the first training trial of the hidden platform task (Fs < .41, n.s.).
Figure. 4.

Cue (visible platform) training performance in cocaine- and saline-exposed rats. There were no differences between groups in their latency to reach the visible platform.
To assess the degree to which performance on the water maze was related to responsiveness to cocaine administration, both partial correlations (controlling for drug condition) and correlations within each drug condition were performed between the water maze measures described above and in Table 1, and both the absolute and change-from-baseline levels of locomotor activity on days 1, 7, and 14 of drug exposure. Using a more stringent p value of .01 due to the large number of analyses, there were no significant correlations between measures of water maze performance and locomotion (p > .01 on all 180 correlations).
4. Discussion
The results of this experiment demonstrate that cocaine exposure can cause long-lasting deficits in water maze performance. Compared to saline controls, rats exposed to cocaine 3 months earlier searched farther from the platform location and spent more time swimming in the maze periphery on probe trials. These impairments were not likely due to sensorimotor or motivational alterations, as cocaine-exposed rats did not differ from controls in their swim speed or ability to locate a visible platform in the water maze. Studies of cocaine addicts suggest that cognitive deficits on declarative learning and memory tasks can persist long after cessation of drug use (Beatty, Katzung, Moreland, & Nixon, 1995; Berry et al., 1993; Strickland, Mena, Villanueva-Meyer, Miller, Cummings, Mehringer, Satz, & Myers, 1993). Indeed, in one such study, deficits were observed after as long as 6 months of abstinence (Di Sclafani et al., 2002). Our results are in agreement with these studies and suggest that cocaine itself may cause long-lasting cognitive impairments observed in cocaine addicts.
Several prior animal studies have shown impairments on declarative learning and memory tasks following exposure to other stimulants, but only a few have investigated effects of cocaine on spatial task performance and none have identified long-lasting deficits (Belcher, O'Dell, & Marshall, 2006; Kantak, Udo, Ugalde, Luzzo, & Di Pietro, 2005). Quirk et al. (2001) found that cocaine administered shortly prior to, as well as during, water maze training impaired acquisition. However, it is difficult to determine whether these impairments were due to acute or chronic effects of cocaine exposure. Del Olmo et al. (2006) found that rats that had self-administered cocaine for 3 weeks were unimpaired when tested soon after the last self-administration session (although only the platform latency and quadrant time measures were reported in this study, which are consistent with the results presented here – see Table 1). The only previous study of which we are aware that examined water maze performance after long periods of abstinence found that cocaine-exposed rats were impaired after 10, but not after 105 days of abstinence (Santucci et al., 2004). Differences between these and the present results may be attributable to differences in the dose, duration, and route of cocaine administration; Santucci et al. (2004) administered 20 mg/kg cocaine subcutaneously for 8 days whereas rats in the present study received 30 mg/kg cocaine intraperitoneally for 14 days. In particular, subcutaneous administration and the resulting slower absorption of cocaine might mitigate some of the long-term effects of cocaine exposure (Samaha & Robinson, 2005). Furthermore, a large difference in the age of the animals during cocaine exposure (26 days in the Santucci et al. study, vs. 70 days in the current study) could also be a significant contributing factor, as cocaine exposure can have different long-lasting effects in adolescents vs. adults (Balda, Anderson, & Itzhak, 2006). Importantly, however, it should be noted that the cocaine exposure regimen used here does cause long-lasting deficits in other cognitive functions in adult rats that mimic those observed in cocaine addicts (Roesch et al., 2007; Schoenbaum et al., 2004; Schoenbaum & Setlow, 2005; Simon et al., 2007), thus providing a context of cocaine-induced cognitive deficits in which the present results can be placed.
The major findings of this study were that on probe trials, cocaine-exposed rats searched farther from the platform location (greater mean search error) and spent more time in the maze periphery (greater thigmotaxis) than saline controls. However, despite these effects, (particularly on the search error measure, in which the effect of cocaine exposure was as robust as some age-related deficits observed using this protocol (Bizon, LaSarge, Montgomery, McDermott, Setlow, & Griffith, 2007)), the two groups did not differ on several measures of their ability to initially find the platform location, either on training or probe trials (i.e. – the groups were able to locate the platform equally well). These latter data suggest that the effects of cocaine (at least at this dose and duration of exposure) on water maze performance are relatively subtle. However, it should be noted that the greater mean search error in cocaine-exposed rats was not a direct result of their greater thigmotaxis, as there were no significant correlations between these two measures. This was particular evident in the data from probe trial 3, on which there was a large difference between groups on the mean search error measure but no difference in thigmotaxis (compare Figures 3A and 3B).
It is not clear how cocaine exposure exerted its long-lasting effects on water maze performance. However, it is possible that alterations in stress responsiveness and/or anxiety played a role. Exposure to cocaine and other psychostimulants can induce lasting increases in responsiveness to stressors, and the water maze is a highly stressful task for rats (Engelmann, Ebner, Landgraf, & Wotjak, 2006). For example, enhanced stressor-induced stress hormone levels in cocaine-exposed rats could adversely affect retention of the platform location via acute effects on medial temporal lobe and other brain systems or via longer-term effects on processes such as hippocampal neurogenesis or LTP (Barr, Hofmann, Weinberg, & Phillips, 2002; de Quervain, Roozendaal, & McGaugh, 1998; Dominguez-Escriba, Hernandez-Rabaza, Soriano-Navarro, Barcia, Romero, & Garcia-Verdugo, 2006; Eisch & Harburg, 2006; Levy, Hopkins, & Squire, 2004; Thompson, Swant, Gosnell, & Wagner, 2004). In addition, enhanced anxiety resulting from prior cocaine exposure could increase the propensity for thigmotaxis (Erb, Funk, Borkowski, Watson, & Akil, 2004; Treit & Fundytus, 1988).
Another route through which cocaine-induced alterations in stress/anxiety could have caused the observed pattern of results is through enhancements in stressor-induced ventral striatal dopamine levels (Kalivas & Duffy, 1989; Setlow & McGaugh, 1999a). Performance on the water maze and other spatial tasks is known to involve ventral striatum, which receives direct projections from the hippocampal formation and which undergoes a wide range of neuroadaptations in response to cocaine exposure (Groenewegen, Vermeulen-Van der Zee, te Kortschot, & Witter, 1987; Hyman, Malenka, & Nestler, 2006; Kelley & Domesick, 1982; Setlow, 1997). Notably, immediate post-training dopaminergic manipulations of ventral striatum (particularly the core subregion of the nucleus accumbens) induce a pattern of deficits in the water maze that are similar to those observed here following cocaine exposure: that is, impaired searching for the platform and increased thigmotaxis on probe trials, but no deficits in initially locating the platform (although it should be noted that the interval between the last training trial and the probe trial differed substantially between these and the present experiments (Setlow & McGaugh, 1999a; Setlow & McGaugh, 1999b)). This previous work suggests that long-lasting cocaine-induced alterations in ventral striatal dopamine signaling, particularly under the stressful conditions of the water maze, may account in part for the results observed here (Anderson & Pierce, 2005; Hyman et al., 2006; Kalivas & Duffy, 1995).
The results from this study show that cocaine exposure impaired performance in the Morris water maze after 3 months of abstinence. These effects did not appear to be due to general learning or sensorimotor deficits, as performance on a cued version of the water maze was unaffected by cocaine. Future research directed toward uncovering the neural mechanisms of induction and expression of these and other long-lasting drug-induced impairments will be crucial for development of effective treatments for reversing and even preventing them, and ultimately for improving the long-term outcome of addiction.
Acknowledgements
We thank Deepa Ramamurthi, Hillary Owen, Chris Schaefer, Melanie Holsaeter, Simona Slaton, and Valerie Newman for their support in conducting these experiments, Dr. Gerianne Alexander for statistical consulting, and the Drug Supply Program at the National Institute on Drug Abuse for kindly providing cocaine HCl. Supported by the Office of the Vice President for Research at Texas A&M University (JLB), DA018764 (BS) and MH65728 (IAM).
We have no conflicts of interest regarding the contents of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson S, Pierce R. Cocaine-induced alterations in dopamine receptor signaling: Implications for reinforcement and reinstatement. Pharmacology and Therapeutics. 2005;106:389–403. doi: 10.1016/j.pharmthera.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Balda M, Anderson K, Itzhak Y. Adolescent and adult responsiveness to the incentive value of cocaine reward in mice: role of neuronal nitric oxide synthase (nNOS) gene. Neuropharmacology. 2006;51:341–349. doi: 10.1016/j.neuropharm.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Barr AM, Hofmann CE, Weinberg J, Phillips AG. Exposure to repeated, intermittent d-amphetamine induces sensitization of HPA axis to a subsequent stressor. Neuropsychopharmacology. 2002;26:286–294. doi: 10.1016/S0893-133X(01)00308-6. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Newton T, Mandelkern M, Mintz J, Foster J, Ling W, Bridge T. Selegiline effects on cocaine-induced changes in medial temporal lobe metabolism and subjective rating of euphoria. Neuropsychopharmacology. 1999;20:582–590. doi: 10.1016/S0893-133X(98)00092-X. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Katzung VM, Moreland VJ, Nixon SJ. Neuropsychological performance of recently abstinent alcoholics and cocaine abusers. Drug and Alcohol Dependence. 1995;37:247–253. doi: 10.1016/0376-8716(94)01072-s. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Belcher A, O'Dell S, Marshall J. A sensitization regimen of methamphetamine causes impairments in a novelty preference task of object recognition. Behavioural Brain Research. 2006;170:167–172. doi: 10.1016/j.bbr.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Berry J, Gorp W. v., Herzberg D, Hinkin C, Boone K, Steinman L, Wilkins J. Neuropsychological deficits in abstinent cocaine abusers: preliminary findings after two weeks of abstinence. Drug and Alcohol Dependence. 1993;32:231–237. doi: 10.1016/0376-8716(93)90087-7. [DOI] [PubMed] [Google Scholar]
- Bizon JL, LaSarge CL, Montgomery KS, McDermott AN, Setlow B, Griffith WH. Spatial reference and working memory across the lifespan of male Fischer 344 rats. Neurobiology of Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter H, Gollub R, Weisskoff R, Kennedy D, Makris N, Berke J, Goodman J, Kantor H, Gastfriend D, Riorden J, Mathew R, Rosen B, Hyman S. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Burke KA, Franz TM, Gugsa N, Schoenbaum G. Prior cocaine exposure disrupts extinction of fear conditioning. Learning and Memory. 2006;13:416–421. doi: 10.1101/lm.216206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Robbins TW. Decision-making deficits in drug addiction. Trends in Cognitive Sciences. 2002;6:361–363. doi: 10.1016/s1364-6613(02)01960-5. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ-F, Roozendaal B, McGaugh J. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- Del Olmo N, Higuera-Matas A, Miguéns M, García-Lecumberri C, Borcel E, Solís J, Ambrosio E. Hippocampal synaptic plasticity and water maze learning in cocaine self-administered rats. Annals of the New York Academy of Sciences. 2006;1074:427–437. doi: 10.1196/annals.1369.043. [DOI] [PubMed] [Google Scholar]
- Di Sclafani V, Tolou-Shams M, Price LJ, Fein G. Neuropsychological performance of individuals dependent on crack-cocaine, or crack-cocaine and alcohol, at 6 weeks and 6 months of abstinence. Drug and Alcohol Dependence. 2002;66:161–171. doi: 10.1016/s0376-8716(01)00197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dom G, Sabbe B, Hulstijn W, van den Brink W. Substance use disorders and the orbitofrontal cortex: Systematic review of behavioural decision-making and neuroimaging studies. Br J Psychiatry. 2005;187:209–220. doi: 10.1192/bjp.187.3.209. [DOI] [PubMed] [Google Scholar]
- Dominguez-Escriba L, Hernandez-Rabaza V, Soriano-Navarro M, Barcia J, Romero F, Garcia-Verdugo J. Chronic cocaine exposure impairs progenitor proliferation but spares survival and maturation of neural precursors in adult rat dentate gyrus. European Journal of Neuroscience. 2006;24:586–594. doi: 10.1111/j.1460-9568.2006.04924.x. [DOI] [PubMed] [Google Scholar]
- Eisch A, Harburg G. Opiates, psychostimulants, and adult hippocampal neurogenesis: Insights for addiction and stem cell biology. Hippocampus. 2006;16:271–286. doi: 10.1002/hipo.20161. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Ebner K, Landgraf R, Wotjak C. Effects of Morris water maze testing on the neuroendocrine stress response and intrahypothalamic release of vasopressin and oxytocin in the rat. Hormones and Behavior. 2006;50:496–501. doi: 10.1016/j.yhbeh.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Erb S, Funk D, Borkowski S, Watson S, Akil H. Effects of chronic cocaine exposure on corticotropin-releasing hormone binding protein in the central nucleus of the amygdala and bed nucleus of the stria terminalis. Neuroscience. 2004;123:1003–1009. doi: 10.1016/j.neuroscience.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Ersche K, Clark L, London M, Robbins T, Sahakian B. Profile of executive and memory function associated with amphetamine and opiate dependence. Neuropsychopharmacology. 2005;31:1036–1047. doi: 10.1038/sj.npp.1300889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behavior. Brain Research Reviews. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Meyerhoff DJ. Prefrontal cortical volume reduction associated with frontal cortex function deficit in 6-week abstinent crack-cocaine dependent men. Drug and Alcohol Dependence. 2002;68:87–93. doi: 10.1016/s0376-8716(02)00110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behavioral Neuroscience. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38:1180–1187. doi: 10.1016/s0028-3932(99)00158-x. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Vermeulen-Van der Zee E, te Kortschot A, Witter MP. Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus Vulgaris leucoagglutinin. Neuroscience. 1987;23:103–120. doi: 10.1016/0306-4522(87)90275-2. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: The Role of Reward-Related Learning and Memory. Annual Review of Neuroscience. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Jacobsen L, Giedd J, Gottschalk C, Kosten T, Krystal J. Quantitative morphology of the caudate and putamen in patients with cocaine dependence. American Journal of Psychiatry. 2001;158:486–489. doi: 10.1176/appi.ajp.158.3.486. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Olausson P, De La Garza R, Taylor JR. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology. 2002;26:183–190. doi: 10.1016/S0893-133X(01)00355-4. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kalivas P, Duffy P. Similar effects of daily cocaine and stress on mesocorticolimbic dopamine neurotransmission in the rat. Biological Psychiatry. 1989;25:913–928. doi: 10.1016/0006-3223(89)90271-0. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress. Brain Research. 1995;675:325–328. doi: 10.1016/0006-8993(95)00013-g. [DOI] [PubMed] [Google Scholar]
- Kantak K, Udo T, Ugalde F, Luzzo C, Di Pietro N. Influence of cocaine self-administration on learning related to prefrontal cortex or hippocampus functioning in rats. Psychopharmacology. 2005;181:227–236. doi: 10.1007/s00213-005-2243-1. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB. The distribution of the projection from the hippocampal formation to the nucleus accumbens in the rat: an anterograde- and retrograde-horseradish peroxidase study. Neuroscience. 1982;7:2321–2335. doi: 10.1016/0306-4522(82)90198-1. [DOI] [PubMed] [Google Scholar]
- LaSarge CL, Montgomery KS, Tucker C, Slaton GS, Griffith WH, Setlow B, Bizon JL. Deficits across multiple cognitive domains in a subset of aged Fischer 344 rats. Neurobiology of Aging. 2007;28:928–936. doi: 10.1016/j.neurobiolaging.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Leland D, Arce E, Feinstein J, Paulus M. Young adult stimulant users increased straital activation during uncertainty is related to impulsivity. Neuroimage. 2006;33:725–731. doi: 10.1016/j.neuroimage.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Hopkins R, Squire L. Impaired odor recognition memory in patients with hippocampal lesions. Learning and Memory. 2004;11:794–796. doi: 10.1101/lm.82504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittenberg W, Motta S. Effects of chronic cocaine abuse on memory and learning. Archives of Clinical Neuropsychology. 1993;8:477–483. [PubMed] [Google Scholar]
- Quirk PL, Richards RW, Avery DD. Subchronic cocaine produces training paradigm-dependent learning deficits in laboratory rats. Pharmacology, Biochemistry and Behavior. 2001;68:545–553. doi: 10.1016/s0091-3057(01)00462-2. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annual Review of Psychology. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Takahashi Y, Gugsa N, Bissonette GB, Schoenbaum G. Previous Cocaine Exposure Makes Rats Hypersensitive to Both Delay and Reward Magnitude. Journal of Neuroscience. 2007;27:245–250. doi: 10.1523/JNEUROSCI.4080-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha A, Robinson T. Why does rapid delivery of drugs to the brain promote addiction? Trends in Pharmacological Sciences. 2005;26:82–87. doi: 10.1016/j.tips.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Santucci AC, Capodilupo S, Bernstein J, Gomez-Ramirez M, Milefsky R, Mitchell H. Cocaine in adolescent rats produces residual memory impairments that are reversible with time. Neurotoxicology and Teratology. 2004;26:651–661. doi: 10.1016/j.ntt.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Ramus SJ, Shaham Y, Saddoris MP, Setlow B. Cocaine-experienced rats exhibit learning deficits in a task sensitive to orbitofrontal cortex lesions. European Journal of Neuroscience. 2004;19:1997–2002. doi: 10.1111/j.1460-9568.2004.03274.x. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B. Cocaine makes actions insensitive to outcomes but not extinction: implications for altered orbitofrontal-amygdalar function. Cerebral Cortex. 2005;15:1162–1169. doi: 10.1093/cercor/bhh216. [DOI] [PubMed] [Google Scholar]
- Setlow B. The nucleus accumbens and learning and memory. Journal of Neuroscience Research. 1997;49:515–521. doi: 10.1002/(SICI)1097-4547(19970901)49:5<515::AID-JNR1>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Setlow B, McGaugh J. Differential effects of immediate post-training sulpiride infusions into the nucleus accumbens core and shell on retention in the Morris water maze. Psychobiology. 1999a;27:248–255. [Google Scholar]
- Setlow B, McGaugh JL. Involvement of the posteroventral caudate-putamen in memory consolidation in the Morris water maze. Neurobiology of Learning and Memory. 1999b;71:240–247. doi: 10.1006/nlme.1998.3874. [DOI] [PubMed] [Google Scholar]
- Simon NW, Mendez IA, Setlow B. Cocaine Exposure Causes Long-Term Increases in Impulsive Choice. Behavioral Neuroscience. 2007;121:543–549. doi: 10.1037/0735-7044.121.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland T, Mena I, Villanueva-Meyer J, Miller B, Cummings J, Mehringer C, Satz P, Myers H. Cerebral perfusion and neuropsychological consequences of chronic cocaine use. The Journal of Neuropsychiatry and Clinical Neurosciences. 1993;5:419–427. doi: 10.1176/jnp.5.4.419. [DOI] [PubMed] [Google Scholar]
- Thompson AM, Swant J, Gosnell BA, Wagner JJ. Modulation of long-term potentiation in the rat hippocampus following cocaine self-administration. Neuroscience. 2004;127:177–185. doi: 10.1016/j.neuroscience.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Treit D, Fundytus M. Thigmotaxis as a test for anxiolytic activity in rats. Pharmacology Biochemistry and Behavior. 1988;31:959–962. doi: 10.1016/0091-3057(88)90413-3. [DOI] [PubMed] [Google Scholar]
- van Gorp WG, Wilkins JN, Hinkin CH, Moore LH, Hull J, Horner MD, Plotkin D. Declarative and procedural memory functioning in abstinent cocaine abusers. Archives of General Psychiatry. 1999;56:85–89. doi: 10.1001/archpsyc.56.1.85. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cerebral Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang G-J, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiology of Learning and Memory. 2002;78:610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]


