Abstract
Cervical cancer is one of the most common cancers in women worldwide. Persistent infection with human papillomavirus (HPV) is considered to be the etiological factor for cervical cancer. Therefore, an effective vaccine against HPV infections may lead to the control of cervical cancer. An ideal HPV vaccine should aim to generate both humoral immune response to prevent new infections as well as cell-mediated immunity to eliminate established infection or HPV-related disease. In the current study, we have generated a potential preventive and therapeutic HPV DNA vaccine using human calreticulin (CRT) linked to HPV16 early proteins, E6 and E7 and the late protein L2 (hCRTE6E7L2). We found that vaccination with hCRTE6E7L2 DNA vaccine induced a potent E6/E7-specific CD8+ T cell immune response, resulting in a significant therapeutic effect against E6/E7 expressing tumor cells. In addition, vaccination with hCRTE6E7L2 DNA generated significant L2-specific neutralizing antibody responses, protecting against pseudovirion infection. Thus, the hCRTE6E7L2 DNA vaccines are capable of generating potent preventive and therapeutic effects in vaccinated mice. Our data has significant clinical implications.
Keywords: HPV, DNA vaccine, E6, E7, L2
Introduction
Cervical cancer is the 2nd leading cause of cancer deaths in women worldwide. The primary factor in the development of cervical cancer is infection by the human papillomavirus (HPV) [1]. HPV is one of the most common sexually transmitted diseases in the world. It is now known that cervical cancer is a consequence of persistent infection with high-risk type HPV [1-5]. HPV infection is a necessary factor for the development and maintenance of cervical cancer and thus, effective vaccination against HPV represents an opportunity to control cervical cancer.
Vaccination could be implemented to prevent infection by generating neutralizing antibodies (preventive vaccines) or to eliminate infection by inducing a virus-specific cellular response (therapeutic vaccines). Current strategies for the development of safe and effective preventive vaccines are based on the induction of neutralizing antibodies against the major and minor capsid proteins, L1 and L2 of human papillomavirus. The newly licensed preventive HPV vaccine, Gardasil, which is an L1-based vaccine, has both a remarkable safety profile and clinical efficacy against the HPV genotypes from which it was derived. However, Gardasil is likely only effective against a subset of HPV genotypes, types 16, 18, 6 and 11, and therefore does not provide complete protection against all cervical cancers. As an alternative approach, the minor capsid protein L2 has arisen as a possible target for vaccine development. Importantly, vaccination with L2 induces broadly cross-neutralizing antibodies that mediate broader protection [6]. This is a significant advantage for L2-based vaccines and thus this approach is emerging as a promising candidate for the development of a broadly protective vaccine.
However, preventive HPV vaccines are unlikely to be effective in the elimination of pre-existing infection and HPV-related disease. This is an important issue since there is a considerable burden of HPV infection worldwide. Thus, in order to accelerate the effect of vaccination on the incidence of cervical cancer and help currently infected patients, it is essential to continue efforts to develop therapeutic vaccines. The choice of target antigen is extremely important for designing therapeutic vaccines. Since, the HPV-encoded E6 and E7 are essential for transformation and are co-expressed in HPV-associated lesions (For a review, see [5]), these proteins represent ideal targets for the development of HPV therapeutic vaccines.
An ideal HPV vaccine should be able to generate both humoral and cell-mediated immunity to prevent new infections as well as to eliminate established infection or HPV-related disease. Therefore, preventive and therapeutic HPV vaccines should target both early proteins such as HPV E6 and E7 and late antigens such as L2 to induce both HPV neutralizing antibodies and therapeutic cellular immunity. Such combination approaches could potentially be implemented for higher efficacy of vaccination and could potentially prevent most cervical cancers and treat HPV infections and HPV-associated lesions.
DNA vaccines pose as an attractive approach for HPV vaccine development. DNA vaccines generate effective CTL and antibody responses by delivering foreign antigen to APCs that stimulate CD4+ and CD8+ T cells. Compared to live viral or bacterial vectors, naked DNA plasmid vaccines are relatively safe and can be easily administered. Furthermore, DNA vaccines are easy to prepare on a large scale with high purity and high stability and can be engineered to express antigenic peptides or proteins (for reviews, see [7, 8]). Additionally, DNA has the unique ability to be maintained long term in an episomal form [9]. This enables prolonged expression of antigens and enhancement of immunologic memory. Using DNA vaccines to express proteins bypasses MHC restriction and thus maintains higher CTL responses than current protein vaccines. DNA vaccines can also be repeatedly applied to the same patient safely and effectively, unlike live vectors vaccines. These features make DNA vaccines a potentially plausible approach for HPV vaccine development. While DNA has considerable advantages, one major limitation is its limited potency. This is due to the fact that DNA vaccines, unlike viral vectors lack the intrinsic ability to amplify in transfected cells.
The use of calreticulin (CRT) in the context of DNA vaccines represents a promising approach for enhancing antigen-specific T cell–mediated immune responses as well as humoral responses for DNA vaccine development. CRT is an abundant 46 kDa Ca2+-binding protein which is located in the endoplasmic reticulum (ER)[10]. CRT is considered to be related to the family of heat shock proteins (HSPs) [11, 12]. This protein has been shown to associate with peptides delivered into the ER by transporters associated with antigen processing (TAP-1 and TAP-2) [13] and with MHC class I-β2 microglobulin molecules to aid in antigen presentation [14]. We have previously employed this CRT-based enhancement strategy to create effective DNA vaccines using HPV-16 E6 [15], E7 [16] or SARS-Co-V [17] as target antigens. We have shown that DNA vaccines encoding calreticulin (CRT) linked to the target antigen generate the high levels of antigen-specific CD8+ T-cell responses as well as significant antigen-specific humoral immunity [15-17]. Thus, we employed CRT in our HPV DNA vaccine developement in order to generate strong T cell specific responses as well as humoral responses against the HPV antigens, E6, E7 and L2. The employment of E6, E7 and L2 protein of HPV16 has been previously explored in protein-based vaccines to generate a chimeric L2E7E6 fusion protein (also called TA-CIN [18, 19]). Vaccination of healthy volunteers with TA-CIN induced serum antibody that neutralizes across papillomavirus species [20].
In the current study, we have created an HPV DNA vaccine encoding calreticulin linked to HPV16 early proteins, E6 and E7 and the late protein L2 (hCRTE6E7L2). We found that vaccination with hCRTE6E7L2 DNA vaccine induced a potent E6/E7-specific CD8+ T cell immune response and resulted in a significant therapeutic effect against E6/E7 expressing tumor cells. Furthermore, vaccination with hCRTE6E7L2 generated significant L2-specific neutralizing antibody responses against HPV-16 pseudovirion infection. Thus, the hCRTE6E7L2 DNA vaccines are capable of generating potent preventive and therapeutic effects in vaccinated mice. The clinical implications of the hCRTE6E7L2 DNA vaccines are discussed.
Materials and Methods
Plasmid DNA constructs
To generate pNGVL4a-hCRTE6E7L2, L2 was first amplified with PCR using pET-28a-HPV16L2 (11-200) as a template and a set of primers, 5'-aaatctagaatgaagagggccagcgccaa-3' and 5'-aaagcggccgctcaggtgtccatgggatctc-3'. The 11-200aa L2 DNA was amplified from codon modified full length L2 (obtained from Dr. M Muller) and further cloned into pET28a Vector [21]. The amplified product was then cloned into the XbaI/NotI sites of pNGVL4a vector (National Gene Vector Laboratory). hCRT was PCR amplified by primers 5'-aaagtcgacatgctgctatccgtgccgctgc-3' and 5'-gaattcgttgtctggccgcacaatca-3' using a human CRT plasmid as a template (which was kindly provided by Dr David Llewellyn of Department of Medical Biochemistry at University of Wales College of Medicine at Cardiff, UK). The PCR product was cut with Sal I/Eco RI and cloned into the Sal I/Eco RI sites of pNGVL4a-L2. HPV-16 E6 and E7 were codon optimized to enhance the translation of these genes. Codon optimized HPV-16 E6 and E7 was synthesized by GenScript Corporation (Piscataway, NJ) was cloned into EcoRI/XbaI sites of pNGVL4a-hCRTL2. The promoter driving the expression of the pNGVL4a vector is CMV. For generation of pNGVL4a-hCRTL2, L2 was amplified again with another set of primers, 5'-tttgaattcatgaagagggccagcgcca-3' and 5'-tttagatcttcaggtgtccatggggatct-3'. The PCR product was cut with EcoRI/BglII and cloned into the EcoRI/BglII sites of pNGVL4a-hCRT. The accuracy of DNA constructs was confirmed by DNA sequencing. The various genes were amplified and cloned such that each subsequent gene was “in frame” and stop codons were removed.
Mice and cell line
Female C57BL/6 mice (6 to 8 weeks old) were purchased from the National Cancer Institute (Frederick, MD, USA) and kept in the animal facility of the Johns Hopkins Hospital (Baltimore, MD, USA). All animal procedures were performed according to approved protocols and in accordance with the recommendations for the proper use and care of laboratory animals. The production and maintenance of TC-1 cells has been described previously [22].
Western Blot Analysis
BHK21 cells (ATCC, 2 ×106) were transiently transfected with 2 μg of each plasmid construct using Lipofectamine 2000 (Invitrogen) according to the vendor's manual. Cells were grown at 37°C and 5% CO2. At 24h after transfection, the expression of transfected genes in cells was characterized by Western blot analysis. The cells were lysed in Mammalian Protein Extraction Reagent (M-PER) (Pierce, Rockford, IL) and the lysate protein concentrations were determined by spectrophotometry based on the manufacturer's instruction. Equal amounts (50ug) of protein were loaded and separated by SDS-PAGE using a 10% polyacrylamide gel and blotted onto a nitrocellulose membrane. After blocking, the membrane was incubated with mouse anti-HPV-16 E6, E7 and rabbit anti-HPV 16 L2 polyclonal antibody for 1hr at room temperature, washed and incubated with HRP-conjugated sheep anti-mouse Igs and donkey anti-rabbit Igs (Amersham Biosciences, UK), respectively. Membranes were detected with chemiluminescence ECL kit. (Amersham, Arlington Heights, IL).
DNA vaccination
For the gene gun-mediated intradermal vaccination, DNA-coated gold particles (1ug DNA/bullet) were delivered to the shaved abdominal region of C57BL/6 mice using a helium-driven gene gun (BioRad, Hercules, CA, USA) with a discharge pressure of 400 p.s.i., according to a previously described protocol [23]. C57BL/6 mice were vaccinated via gene gun with 2ug/mouse of pNGVL4a, pNGVL4a-hCRT, pNGVL4ahCRTL2, pNGVL4a-hCRTE6E7 or pNGVL4a-hCRTE6E7L2. These mice received three boosters with the same dose and regimen at 1-week intervals. For the i.m.-mediated DNA vaccination, 50 ug/mouse of pNGVL4a, pNGVL4a-hCRT, pNGVL4a-hCRTL2, pNGVL4a-hCRTE6E7 and pNGVL4a-hCRTE6E7L2 DNA vaccines were delivered intramuscularly by syringe needle injection. These mice received three boosters with the same dose and regimen at 1-week intervals.
Intracellular cytokine staining and flow cytometry analysis
Cell surface marker staining of CD8 and intracellular cytokine staining for IFN-γ as well as FACScan analysis was performed using conditions described previously [24]. Prior to FACScan, splenocytes from different vaccinated groups of mice were collected, pooled and incubated for 16 h with 1ug/ml of E7 peptide (aa 49-57) or E6 peptide (aa 50-57) in the presence of GolgiPlug (1ul/ml) for detecting E6 and E7-specific CD8+T-cell precursors [25]. The stimulated splenocytes were then washed twice with FACScan buffer. Cell surface marker staining for CD8 and intracellular cytokine staining for IFN-γ were performed using conditions described previously [24]. The number of IFN-γ secreting CD8+ T cells was analyzed using flow cytometry. Analysis was performed on a Becton-Dickinson FACScan with CELLQuest software (Becton-Dickinson Immunocytometry System, Mountain View, CA, USA).
In vivo tumor protection and treatment experiments using TC-1 tumors
For in vivo tumor protection experiments, C57BL/6 mice (5 per group) were vaccinated with 2 μg/mouse of pNGVL4a, pNGVL4a-hCRT, pNGVL4a-hCRTL2, pNGVL4a-hCRTE6E7 and pNGVL4a-hCRTE6E7L2 DNA vaccines by gene gun injection twice at a 1-week interval. One week after the last vaccination, mice were challenged with 5 × 104 TC-1 tumor cells/mouse [22] by subcutaneous injection in the right leg. Tumor growth was monitored by visual inspection and palpation twice a week as described previously [22]. For in vivo tumor treatment experiments using an E6, E7-expressing tumor (TC-1), mice (5 per group) were intravenously challenged through the tail vein with 1 × 10 TC-1 cells /mouse. At 3 days after tumor challenge, mice were administered 2ug/mouse of pNGVL4a, pNGVL4a-hCRT, pNGVL4a-hCRTL2, pNGVL4a-hCRTE6E7 and pNGVL4a-hCRTE6E7L2 via gene gun. One week after the first vaccination, these mice were boosted with the same dose and regimen. Mice were killed and lungs were explanted on day 28. The pulmonary nodules on the surface of the lungs in each mouse were counted by experimenters blinded to sample identity as described previously [26].
ELISA
The full length L2 protein was expressed and purified as described previously [27]. Briefly, the codon modified full length L2 was cloned into pET 28a vector and the His tagged fusion protein was expressed in BL21 (Rosetta cells, Novagen). The recombinant protein was purified on a Ni-NTA coumn under denaturing conditions following suggested manufacturer's protocol (Qiagen). The presence of anti-HPV-16 L2 Ab's in the sera was characterized by a direct ELISA as described previously [28]. C57BL/6 mice were immunized with gene gun with 2μg/mouse of the various DNA vaccines and received three boosters with the same dose and regimen at 1-week intervals. For i.m.-mediated DNA vaccination, 50ug/mouse of each DNA vaccine was delivered intramuscularly by syringe needle injection. These mice received three boosters with the same dose and regimen at 1-week intervals. Sera were prepared from mice 7 days after last immunization and pooled. Full length of L2 protein (100ng/well) was coated in a 96-microwell plate and incubated at 4°C overnight. The wells were then blocked with PBS containing 1% BSA for 1hour at 37°C. After washing with PBS containing 0.05% Tween-20, the plate was incubated with serially diluted sera (1:100, 1:500, 1:1000) for 2hr at 37°C. Serum from vaccinated rabbit with full length of L2 protein was used as the positive control. After washing twice with PBS containing 0.05% Tween-20, The plate was further mixed with 1:1,000 dilution of a HRP-conjugated donkey anti-rabbit IgG Ab for standard control and rabbit anti-mouse IgG Ab for mouse serum (Amersham Pharmacia Biotech, Piscataway, New Jersey, USA) as secondary antibody, respectively and was incubated at room temperature for 1 hour. The ELISA plate was read with a standard ELISA reader at 450 nm.
Neutralization Assay
HPV16 pseudovirions with encapsulated secreted alkaline phosphatase (SEAP) were generated by co-transfection of 293TT cells with plasmids encoding HPV16 L2 and a SEAP reporter plasmid as described previously by Buck et al [29]. Cells collected after transfection were treated overnight with Brij 58 (0.5%), Benzonase (0.5%) and purified by centrifugation on an Optiprep step gradient (27, 33, and 39%) at 40,000 rpm for 4.5 h. Pseudovirus neutralization assays were carried out as outlined previously [30, 31]. Briefly, the pseudovirus and the pooled mouse immune sera were incubated for 1 h and the mixture was used to infect 293TT cells. 68 to 72 h post-infection, the supernatants were collected and SEAP activity in the supernatants was measured by colorimetric assay. Serum neutralization titers were defined as the highest dilution that caused at least a 50% reduction in SEAP activity, compared to control pre-immune serum samples. The minimum neutralization would be the wells where the virus is incubated with either pre-bled or PBS immunized serum and maximum neutralization would be the wells where the virus is completely neutralized and so there is no SEAP activity.
Statistical analysis
All data expressed as means ±s.d. are representative of at least two different experiments. Data for intracellular cytokine staining with flow cytometry analysis and tumor treatment experiments were analyzed by analysis of variance (ANOVA). Comparisons between individual data points were made using a Student's t-test. Kaplan Meier survival curves for tumor protection experiments were applied; for differences between curves, P-values were calculated using the log-rank test. The value of P<0.05 was considered significant.
Results
Cells transfected with the various recombinant DNA constructs express E6, E7 and/or L2 proteins
We generated the various DNA constructs pNGVL4a, pNGVL4a-L2, pNGVL4a-hCRT, pNGVL4a-hCRTL2, pNGVL4a-hCRTE6E7 and pNGVL4a-hCRTE6E7L2. In order to determine whether the BHK 21 cells transfected with each of recombinant DNA constructs expressed the encoded genes, E6, E7 and/or L2, we performed Western blot analysis with the cell lysates 24 hours after transfection using polyclonal antibodies to E6, E7 or L2 proteins. As shown in Figure 1, expression of E6 (upper panel) was observed at comparable levels in the lysates of cells transfected with 4a-hCRTE6E7 (lane 5) and the 4a-hCRTE6E7L2 (lane 6) constructs. Similarly, the expression of E7 (middle panel) protein was observed at comparable levels in the lysates of cells transfected with 4a-hCRTE6E7 (lane 5) and the 4a-hCRTE6E7L2 (lane 6) constructs. Comparable level of expression of L2 protein (lower panel) was also observed in the lysates of cells transfected with 4a-L2 (lane 2), 4a-hCRTL2 (lane 4) and the 4a-hCRTE6E7L2 (lane 6) constructs. Furthermore, the proteins derived from each of the constructs were of the expected sizes. Thus, our data indicate that the BHK 21 cells transfected with the DNA constructs express the encoded genes for E6, E7 and/or L2.
Figure 1. Characterization of the expression of E6, E7 and L2 in DNA transfected cells.
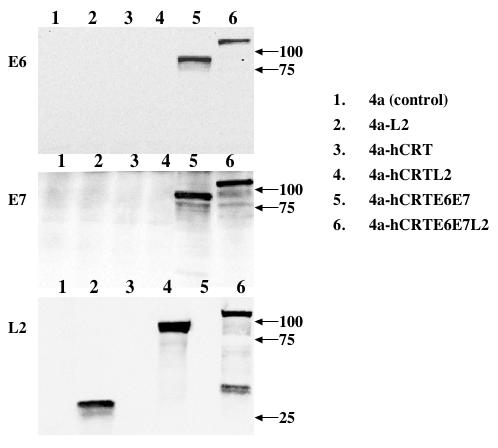
BHK 21 cells were transfected with 2 μg of the various DNA constructs, pNGVL4a, pNGVL4a-L2, pNGVL4a-hCRT, pNGVL4a-hCRTL2, pNGVL4a-hCRTE6E7 or pNGVL4a-hCRTE6E7L2. Western blot analysis was performed using 50 μg of protein from the cell lysates 24 hours after transfection. Protein expression was determined using mouse anti-HPV 16 E6 (upper panel), E7 (middle panel) or rabbit anti-HPV L2 (lower panel) polyclonal antibody. Western blot analysis showing the expression of HPV 16 E6, E7 and L2 proteins in BHK 21 cells transfected with each of the six DNA constructs. The arrows indicate the molecular weights. Note: Expression of E6 and E7 proteins were observed in the 4a-hCRTE6E7 (lane 5) and the 4a-hCRTE6E7L2 (lane 6) constructs. Expression of L2 protein was observed in the 4a-L2 (lane 2), 4a-hCRTL2 (lane 4) and the 4a-hCRTE6E7L2 (lane 6) constructs.
Vaccination with DNA vaccines expressing hCRTE6E7 and hCRTE6E7L2 generates significant number of E6 and E7-specific CD8+ T cells
In order to assess the level of E6-specific T cell immune responses generated by the recombinant DNA vaccines, we first performed intracellular cytokine staining and flow cytometry analysis to determine the number of E6-specific IFN-γ secreting CD8+ T cells generated in response to stimulation with E6 peptide. C57BL/6 mice (3 per group) were immunized intradermally via gene gun (left panel) or intramuscularly (right panel) with the various DNA vaccines. The splenocytes from vaccinated mice were incubated with H-2Kb-restricted E6 CTL peptide (aa 50-57) overnight and the number of E6-specific CD8+ T cells was determined. As shown in Figure 2A, significant numbers of IFN-γ secreting CD8+ T cells were observed after stimulation with E6 peptide in the mice immunized with DNA vaccines expressing hCRTE6E7 and hCRTE6E7L2, although the immunization with DNA vaccines expressing hCRTE6E7L2 generates lower number of E6-specific IFN-γ+ CD8+ T cells compared to immunization with DNA vaccines expressing hCRTE6E7. A graphical representation of the number of E6-specific IFN-γ+ CD8+ T cells in mice immunized intradermally via gene gun and intramuscularly is depicted in Figure 2B and C respectively. In addition, this data suggests that immunization via gene gun generates a greater number of E6-specific IFN-γ+ CD8+ T cells compared to intramuscular immunization. Thus, our data indicates that mice immunized with the DNA vaccines expressing hCRTE6E7 and hCRTE6E7L2 generate a higher frequency of E6-specific activated CD8+ T cells as compared to the hCRTL2 control.
Figure 2. Characterization of IFNγ-secreting E6-specific CD8+ T cell precursors in mice vaccinated with recombinant DNA vaccines.
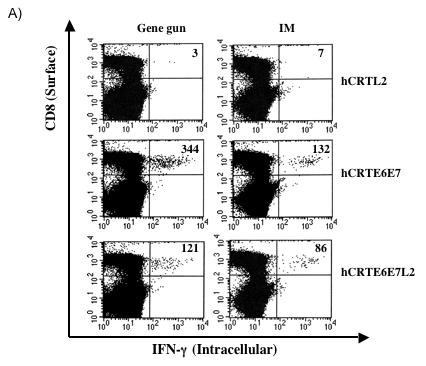
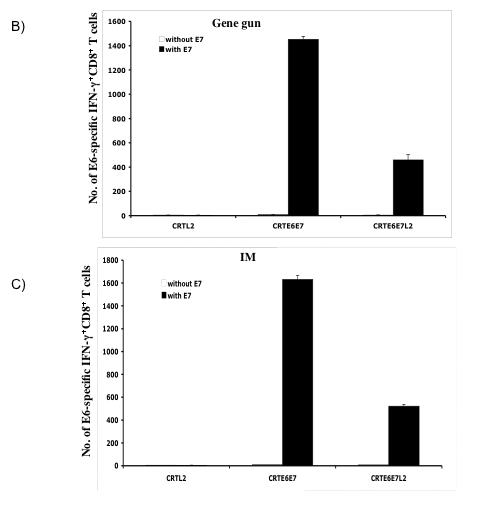
One set of C57BL/6 mice (3 per group) was immunized intradermally via gene gun with 2μg per mouse of the various recombinant DNA vaccines four times with one-week intervals. Another set of C57BL/6 mice (3 per group) was immunized intramuscularly with 50μg per mouse of the various recombinant DNA vaccines four times with one-week intervals. The splenocytes from vaccinated mice were incubated with H-2Kb-restricted E6 CTL peptide (aa 50-57) overnight. Determination of the E6-specific CD8+ T cells was performed by intracellular IFN-γ staining followed by flow cytometry analysis. A) Representative flow cytometry data showing the number of E6-specific IFNγ+ CD8+ T cells in the mice vaccinated either intradermally via gene gun (left panel) or intramuscularly (right panel) with the various DNA vaccines. B) Bar graph showing the number of E6-specific IFNγ+ CD8+ T cells from mice immunized intradermally with the various DNA vaccines via gene gun with (shaded bars) or without (empty bars) stimulation with the E6 peptide. The data was shown as mean±s.d.(p<0.05). C) Bar graph showing the number of E6-specific IFNγ+ CD8+ T cells from mice immunized intramuscularly with the various DNA vaccines with (shaded bars) or without (empty bars) stimulation with the E6 peptide. The data was shown as mean±s.d.(p<0.05).
We further characterized the level of E7-specific T cell immune responses generated by the recombinant DNA vaccines using intracellular cytokine staining and flow cytometry analysis. The splenocytes from vaccinated mice were incubated with H-2Db-restricted E7 CTL peptide (aa 49-57) overnight and the number of E7-specific CD8+ T cells was determined. As shown in Figure 3A, significant number of IFN-γ secreting CD8+ T cells were observed after stimulation with E7 peptide in the mice immunized with DNA vaccines expressing hCRTE6E7 and hCRTE6E7L2, although the immunization with DNA vaccines expressing hCRTE6E7L2 generates lower number of E7-specific IFN-γ+ CD8+ T cells compared to immunization with DNA vaccines expressing hCRTE6E7. A graphical representation of the number of E7-specific IFN-γ+ CD8+ T cells in mice immunized intradermally via gene gun and intramuscularly is depicted in Figure 3B and C respectively. In addition, this data suggests that immunization via gene gun and intramuscular immunization generate a similar number of E7-specific IFN-γ+ CD8+ T cells. Thus, our data indicates that the DNA vaccines expressing hCRTE6E7 and hCRTE6E7L2 generate a higher frequency of E7-specific activated CD8+ T cells as compared to the hCRTL2 control.
Figure 3. Characterization of IFNγ-secreting E7-specific CD8+ T cell precursors in mice vaccinated with recombinant DNA vaccines.
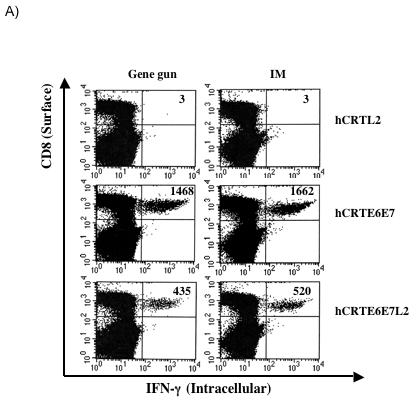
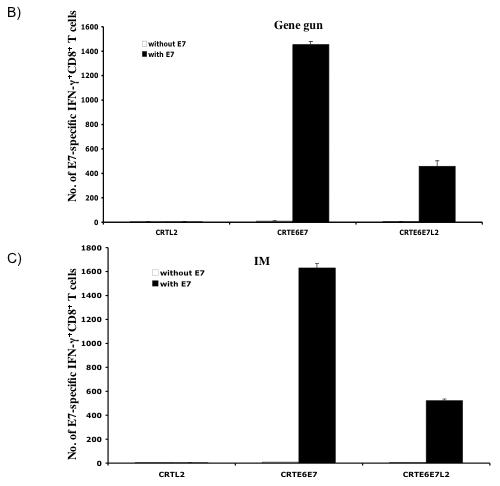
One set of C57BL/6 mice (3 per group) was immunized intradermally via gene gun with 2μg of the various recombinant DNA vaccines four times with one-week intervals. Another set of C57BL/6 mice (3 per group) was immunized intramuscularly with 50μg of the various recombinant DNA vaccines four times with one-week intervals. The splenocytes from vaccinated mice were incubated with H-2Db-restricted E7 CTL peptide (aa 49-57) overnight. Determination of the E7-specific CD8+ T cells was performed by intracellular IFN-γ staining followed by flow cytometry analysis. A) Representative flow cytometry data showing the number of E7-specific IFNγ+ CD8+ T cells in the mice vaccinated either intradermally via gene gun (left panel) or intramuscularly (right panel) with the various DNA vaccines. B) Bar graph showing the number of E7-specific IFNγ+ CD8+ T cells from mice immunized intradermally with the various DNA vaccines via gene gun with (shaded bars) or without (empty bars) stimulation with the E7 peptide. The data was shown as mean±s.d. (p<0.05). C) Bar graph showing the number of E7-specific IFNγ+ CD8+ T cells from mice immunized intramuscularly with the various DNA vaccines with (shaded bars) or without (empty bars) stimulation with the E7 peptide. The data was shown as mean±s.d. (p<0.05).
Taken together, our data indicates that the DNA vaccines expressing hCRTE6E7 and hCRTE6E7L2 generate detectable E6/E7-specific CD8+ T cell responses.
Vaccination with DNA vaccines expressing hCRTE6E7 and hCRTE6E7L2 generates significant protection and anti-tumor effect
In order to assess the anti-tumor immunity generated by DNA vaccines expressing hCRTE6E7 and hCRTE6E7L2, we performed an in vivo tumor protection assay. C57BL/6 mice (5 per group) were immunized intradermally with the various DNA vaccines via gene gun twice at a one-week interval. Seven days after the last immunization, the mice were challenged subcutaneously with 5 × 104/mouse of TC-1 tumor cells. Tumor growth was monitored by visual inspection and palpation twice a week. As shown in Figure 4A, immunization with DNA vaccines expressing hCRTE6E7 and hCRTE6E7L2 induced a higher percentage of tumor-free mice compared to immunization with the other DNA vaccines. In order to test the therapeutic effects of vaccination with DNA vaccines expressing hCRTE6E7 and hCRTE6E7L2, we challenged C57BL/6 mice (5 per group) intravenously with 1 × 104/mouse of TC-1 cells. Three and ten days later, the mice were treated intradermally with the various recombinant DNA vaccines via gene gun. Mice were sacrificed 28 days after tumor challenge and the numbers of pulmonary tumor nodules were quantified and compared. As shown in Figure 4B, there was a significant reduction in the number of pulmonary tumor nodules of mice treated with DNA vaccines expressing hCRTE6E7 and hCRTE6E7L2 compared to the mice treated with the other DNA vaccines (p<0.01). Thus taken together, our data suggest that mice vaccinated with the DNA vaccines expressing hCRTE6E7 or hCRTE6E7L2 generate significant anti-tumor effects against E6/E7 expressing tumors.
Figure 4. In vivo tumor protection and treatment experiments.
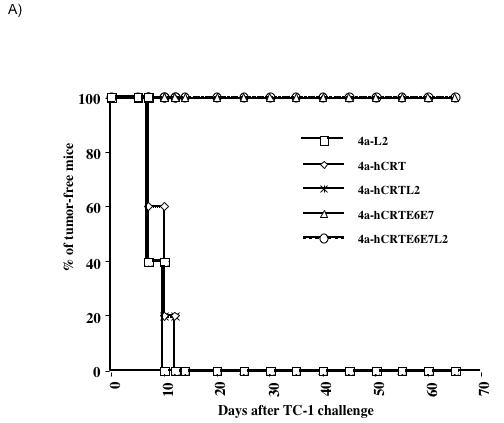
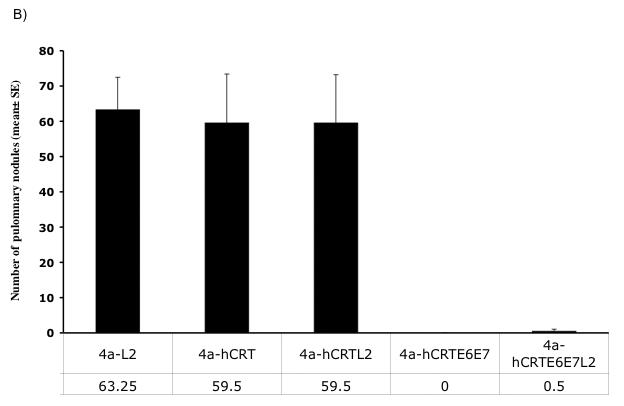
A) In vivo tumor protection experiment. C57BL/6 mice (5 per group) were immunized intradermally with the various DNA vaccines via gene gun twice with a one-week interval. Seven days after the last immunization, the mice were challenged subcutaneously with 5 × 104 /mouse of TC-1 tumor cells. Tumor growth was monitored by visual inspection and palpation twice a week and examined by Kaplan-Meier analysis. B) Bar graph depicting the quantification of the number of pulmonary nodules in mice treated with the various DNA vaccines. C57BL/6 mice (5 per group) were challenged with 1 × 104/mouse of TC-1 cells via tail vein. Three and ten days later, the mice were treated intradermally with the various recombinant DNA vaccines via gene gun. Mice were sacrificed 28 days after tumor challenge and the numbers of pulmonary tumor nodules were quantified and compared. The data was shown as mean±SE. (p<0.01).
Vaccination with DNA vaccines expressing hCRTE6E7L2 generates significant levels of L2-specific neutralizing antibody responses
In order to determine the antibody levels against L2 obtained in the mice vaccinated with the various DNA vaccines, we performed ELISA using full length L2 protein. C57BL/6 mice (3 per group) were vaccinated intradermally via gene gun or intramuscularly with the various recombinant DNA vaccines. ELISA analysis was performed to determine the levels of L2-specific antibody in vaccinated mice. As shown in Figure 5A and B, there was a significant level of L2-specific antibody response in the mice vaccinated with DNA vaccine expressing hCRTE6E7L2 intradermally via gene gun or intramuscularly compared to that in mice vaccinated with DNA vaccine expressing hCRTE6E7. Furthermore, L2-specific antibody responses in mice vaccinated with the hCRTE6E7L2 DNA were comparable to the L2-specific antibody responses in mice vaccinated with 4a-L2 or 4a-hCRTL2 DNA (p>0.05). In addition, the L2-specific antibody responses in the mice vaccinated intramuscularly were comparable to that in mice vaccinated intradermally. Thus, our data suggest that mice vaccinated with the DNA vaccine expressing hCRTE6E7L2 are capable of generating significant levels of L2-specific antibody in vaccinated mice.
Figure 5. Characterization of antibody levels against full length L2 protein by ELISA.
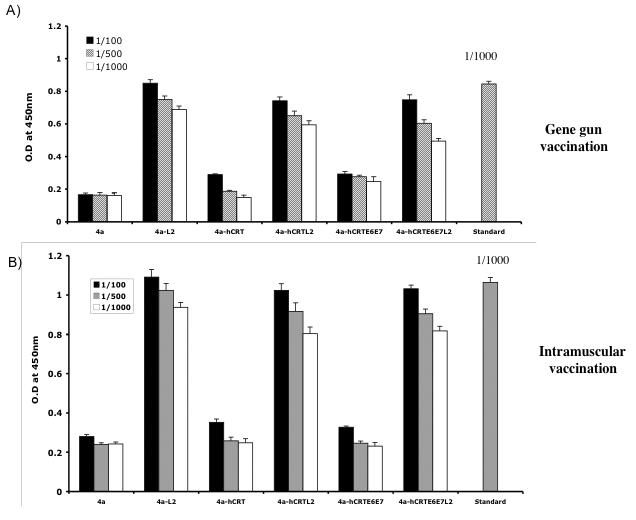
One group of C57BL/6 mice (3 per group) was immunized intradermally via gene gun with 2μg of the various recombinant DNA vaccines four times with one-week intervals. Another group of C57BL/6 mice (3 per group) was immunized intramuscularly with 50μg of the various recombinant DNA vaccines four times with one-week intervals. ELISA analysis was performed to determine the L2-specific antibody responses in vaccinated mice. A) Bar graph depicting the L2-specific antibody responses in mice vaccinated intradermally with the various DNA vaccines via gene gun. B) Bar graph depicting the L2-specific antibody responses in mice vaccinated intramuscularly with the various DNA vaccines. The results from 1: 100, 1:500 and 1:1000 dilutions are represented, showing mean absorbance (OD 450 nm) ± s.d. The data collected from all of the above experiments are from one representative experiment of two performed. Serum from rabbit vaccinated with L2 as standard control.
In order to determine the neutralizing antibody responses against HPV-16 pseudovirion infection in mice vaccinated with the various DNA vaccines, we performed neutralization assays using HPV-16 pseudovirions. C57BL/6 mice (3 per group) were vaccinated either intradermally via gene gun or intramuscularly with the various recombinant DNA vaccines. As shown in Figure 6A and B, there was a significant neutralizing antibody response in mice vaccinated with DNA vaccine expressing hCRTE6E7L2 compared to that in mice vaccinated with DNA vaccine expressing hCRTE6E7 (p<0.05). Furthermore, the neutralization activity observed in mice vaccinated with the hCRTE6E7L2 DNA was comparable to that in mice vaccinated with 4a-L2 DNA or 4a-hCRTL2 DNA. Interestingly, intramuscular administration of the DNA vaccines showed that vaccination with hCRTE6E7L2 DNA generated a slightly lower titer of HPV-16 neutralizing antibodies compared to vaccination with 4a-L2 DNA vaccine. Thus, our data suggest that hCRTE6E7L2 DNA is capable of generating significant levels of neutralizing antibody responses in vaccinated mice.
Figure 6. Neutralization assays using HPV-16 pseudovirion.
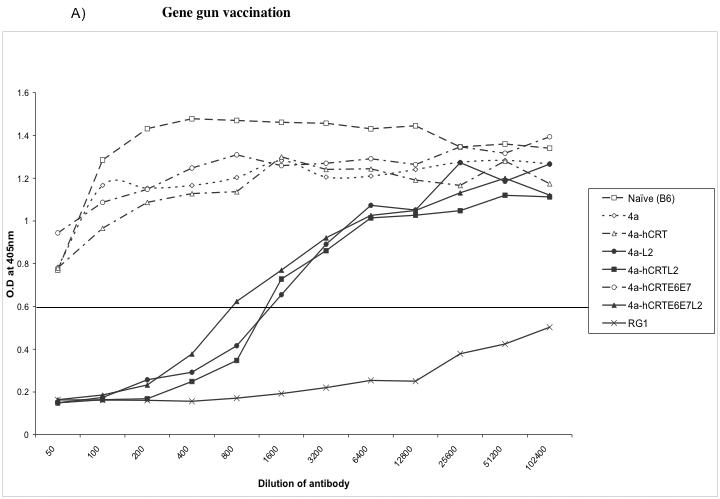
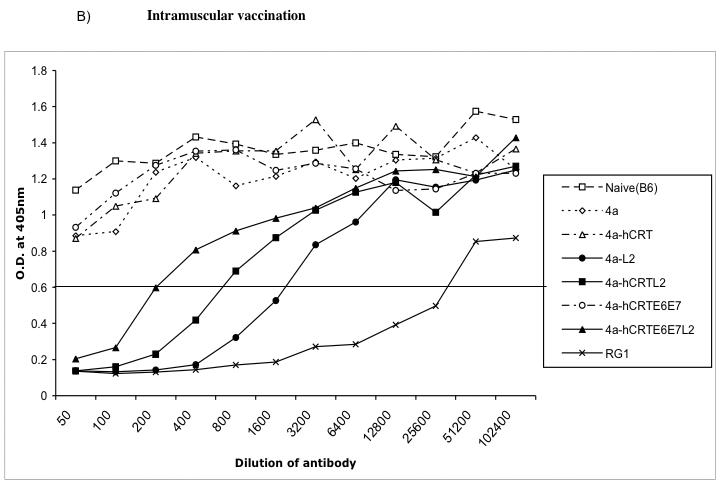
Graphical representation of the neutralization activity in mice vaccinated with the various DNA vaccines. One set of C57BL/6 mice (3 per group) was immunized intradermally via gene gun with 2μg of the various recombinant DNA vaccines four times with one-week intervals. Another set of C57BL/6 mice (3 per group) was immunized intramuscularly with 50μg of the various recombinant DNA vaccines four times with one-week intervals. Neutralizing assays were performed using HPV-16 pseudovirion to determine the L2-specific neutralizing antibody responses in vaccinated mice. RG1 is the 17-36aa anti-peptide serum that is used as a positive control antibody that generates a neutralizing response. B6I is the non-neutralizing negative control antibody.
Discussion
In the current study, we have created an HPV DNA vaccine encoding calreticulin linked to HPV16 early proteins, E6 and E7 and the late protein L2 (hCRTE6E7L2). Our data indicated that vaccination with hCRTE6E7L2 DNA vaccine was capable of generating a potent E6/E7-specific CD8+ T cell immune response and resulted in a significant therapeutic effect against E6/E7 expressing tumor cells. Furthermore, our data suggested that vaccination with hCRTE6E7L2 generated significant L2-specific neutralizing antibody responses against pseudovirion infection. Thus, we have created a potentially promising preventive and therapeutic HPV-16 DNA vaccine. Our data serves as an important foundation for future clinical translation. Currently, a therapeutic HPV DNA vaccine, pNGVL4a-hCRTE7 using the clinical grade gene gun device is being prepared for phase I clinical trials in patients with stage 1B1 HPV-16 positive cervical cancer (Trimble, personal communication). The current study represents a further advancement of studies using hCRTE7 because the vaccine used in our current study targets both E6/E7 as well as L2.
For future clinical translation, it is necessary to address the safety concerns regarding the potential for oncogenicity associated with administration of E6 and E7 as DNA vaccines into the body. Thus, it is important to use attenuated (detox) versions of E6 and E7 that have been mutated. It has been demonstrated that a mutation at E7 position 24 and/or 26 will disrupt the Rb binding site of E7, abolishing the capacity of E7 to transform cells [32]. Furthermore, mutation at E6 positions 63 or 106 has been shown to destroy several HPV-16 E6 functions, preventing the mutated E6 protein from immortalizing human epithelial cells [33, 34]. The constructs used in the current study employ attenuated (detox) forms of E6 and E7, thus eliminating any safety concerns that may arise.
Since the E6 and E7 proteins represent the ideal targets for the development of HPV therapeutic vaccines, DNA vaccines targeting both E6 and E7 may generate a more potent immune response and anti-tumor effect than those targeting either E6 or E7 alone. Indeed, a previous study by Peng et al has demonstrated that simultaneous vaccination of C57BL/6 mice or HLA-A2 transgenic mice with both CRT/E6 and CRT/E7 DNA vaccines generates significant E6- and E7-specific T-cell immune responses in vaccinated mice. Furthermore, combined vaccination with both CRT/E6 and CRT/E7 DNA generates significantly better therapeutic antitumor effects against HPV E6- and E7-expressing tumors than vaccination with either CRT/E6 DNA or CRT/E7 DNA alone [35]. These data suggest that it may be desirable to target both E6 and E7 to generate better therapeutic effect against E6/E7 expressing tumors.
The data generated in the current study suggests that the gene gun-based immunization technique may generate higher number of antigen-specific CD8+ T cells compared to intramuscular immunization, particularly in case of E6 antigen. This is in agreement with previous studies by Trimble et al that indicate that vaccine administered via gene gun generated the highest number of antigen-specific CD8+ T cells. In addition, DNA vaccination via gene gun required the least dose to generate similar or slightly better antitumor effects compared to needle intramuscular (i.m.) and biojector administrations [36]. Thus, our data suggest that DNA vaccination via gene gun represents the most effective regimen for DNA administration.
Our previous studies showed that DNA vaccines encoding CRT linked to several antigens including HPV-16 E6 [15], E7 [16] or SARS-Co-V [17] were capable of generating significantly higher levels of antigen-specific antibody responses compared to DNA vaccines encoding target antigen without linkage to CRT in vaccinated mice. However, in the current study, we observed that vaccination with DNA vaccine encoding CRT linked to L2 or E6E7L2 generated only comparable levels of L2-specific antibody responses compared to vaccination with DNA vaccines encoding wild-type L2. Thus, the strategy of employing CRT to enhance the antigen-specific antibody responses in DNA vaccines in clearly depends on the nature of the antigen. Although, the CRT strategy did not enhance the L2-specific antibody responses, it did improve the antigen-specific CD8+ T cell-mediated immune response.
In summary, we have created a preventive and therapeutic HPV DNA vaccine that is capable of generating significant antigen-specific therapeutic antitumor effects as well as neutralizing antibody response against HPV pseudovirions. Our preclinical observations serve as an important foundation for future clinical translation.
Acknowledgements
We gratefully acknowledge Ms. Talia Hoory for assistance in preparation of the manuscript. This work was supported by the Flight Attendant Medical Research Institute and National Cancer Institute SPORE in Cervical Cancer P50 CA098252 and the 1 RO1 CA114425-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 2.Ho GY, Studentsov YY, Bierman R, Burk RD. Natural history of human papillomavirus type 16 virus-like particle antibodies in young women. Cancer Epidemiol Biomarkers Prev. 2004;13(1):110–6. doi: 10.1158/1055-9965.epi-03-0191. [DOI] [PubMed] [Google Scholar]
- 3.Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55(4):244–65. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferenczy A, Franco E. Persistent human papillomavirus infection and cervical neoplasia. Lancet Oncol. 2002;3(1):11–6. doi: 10.1016/s1470-2045(01)00617-9. [DOI] [PubMed] [Google Scholar]
- 5.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nature Rev Cancer. 2002;2(5):342–50. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 6.Roden RB, Yutzy WHt, Fallon R, Inglis S, Lowy DR, Schiller JT. Minor capsid protein of human genital papillomaviruses contains subdominant, cross-neutralizing epitopes. Virology. 2000;270(2):254–7. doi: 10.1006/viro.2000.0272. [DOI] [PubMed] [Google Scholar]
- 7.Gurunathan S, Klinman DM, Seder RA. DNA vaccines: immunology, application, and optimization. Annu Rev Immunol. 2000;18:927–74. doi: 10.1146/annurev.immunol.18.1.927. [DOI] [PubMed] [Google Scholar]
- 8.Donnelly JJ, Ulmer JB, Shiver JW, Liu MA. DNA vaccines. Annu Rev Immunol. 1997;15:617–48. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 9.Seder RA, Gurunathan S. DNA vaccines--designer vaccines for the 21st century. The New England journal of medicine. 1999;341(4):277–8. doi: 10.1056/NEJM199907223410410. [DOI] [PubMed] [Google Scholar]
- 10.Nash PD, Opas M, Michalak M. Calreticulin: not just another calcium-binding protein. Molecular and cellular biochemistry. 1994;135(1):71–8. doi: 10.1007/BF00925962. [DOI] [PubMed] [Google Scholar]
- 11.Basu S, Srivastava PK. Calreticulin, a peptide-binding chaperone of the endoplasmic reticulum, elicits tumor- and peptide-specific immunity. The Journal of experimental medicine. 1999;189(5):797–802. doi: 10.1084/jem.189.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conway EM, Liu L, Nowakowski B, Steiner-Mosonyi M, Ribeiro SP, Michalak M. Heat shock-sensitive expression of calreticulin. In vitro and in vivo up-regulation. The Journal of biological chemistry. 1995;270(28):17011–6. doi: 10.1074/jbc.270.28.17011. [DOI] [PubMed] [Google Scholar]
- 13.Spee P, Neefjes J. TAP-translocated peptides specifically bind proteins in the endoplasmic reticulum, including gp96, protein disulfide isomerase and calreticulin. Eur J Immunol. 1997;27(9):2441–9. doi: 10.1002/eji.1830270944. [DOI] [PubMed] [Google Scholar]
- 14.Sadasivan B, Lehner PJ, Ortmann B, Spies T, Cresswell P. Roles for calreticulin and a novel glycoprotein, tapasin, in the interaction of MHC class I molecules with TAP. Immunity. 1996;5(2):103–14. doi: 10.1016/s1074-7613(00)80487-2. [DOI] [PubMed] [Google Scholar]
- 15.Peng S, Ji H, Trimble C, He L, Tsai YC, Yeatermeyer J, et al. Development of a DNA vaccine targeting human papillomavirus type 16 oncoprotein E6. J Virol. 2004;78(16):8468–76. doi: 10.1128/JVI.78.16.8468-8476.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng WF, Hung CF, Chai CY, Hsu KF, He L, Ling M, et al. Tumor-specific immunity and antiangiogenesis generated by a DNA vaccine encoding calreticulin linked to a tumor antigen. J Clin Invest. 2001;108(5):669–78. doi: 10.1172/JCI12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim TW, Lee JH, Hung CF, Peng S, Roden R, Wang MC, et al. Generation and characterization of DNA vaccines targeting the nucleocapsid protein of severe acute respiratory syndrome coronavirus. J Virol. 2004;78(9):4638–45. doi: 10.1128/JVI.78.9.4638-4645.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Burg SH, Kwappenberg KM, O'Neill T, Brandt RM, Melief CJ, Hickling JK, et al. Pre-clinical safety and efficacy of TA-CIN, a recombinant HPV16 L2E6E7 fusion protein vaccine, in homologous and heterologous prime-boost regimens. Vaccine. 2001;19(27):3652–60. doi: 10.1016/s0264-410x(01)00086-x. [DOI] [PubMed] [Google Scholar]
- 19.de Jong A, O'Neill T, Khan AY, Kwappenberg KM, Chisholm SE, Whittle NR, et al. Enhancement of human papillomavirus (HPV) type 16 E6 and E7-specific T-cell immunity in healthy volunteers through vaccination with TA-CIN, an HPV16 L2E7E6 fusion protein vaccine. Vaccine. 2002;20(2930):3456–64. doi: 10.1016/s0264-410x(02)00350-x. [DOI] [PubMed] [Google Scholar]
- 20.Gambhira R, Gravitt PE, Bossis I, Stern PL, Viscidi RP, Roden RB. Vaccination of healthy volunteers with human papillomavirus type 16 L2E7E6 fusion protein induces serum antibody that neutralizes across papillomavirus species. Cancer Res. 2006;66(23):11120–4. doi: 10.1158/0008-5472.CAN-06-2560. [DOI] [PubMed] [Google Scholar]
- 21.Leder C, Kleinschmidt JA, Wiethe C, Muller M. Enhancement of capsid gene expression: preparing the human papillomavirus type 16 major structural gene L1 for DNA vaccination purposes. J Virol. 2001;75(19):9201–9. doi: 10.1128/JVI.75.19.9201-9209.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin KY, Guarnieri FG, Staveley-O'Carroll KF, Levitsky HI, August JT, Pardoll DM, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56(1):21–6. [PubMed] [Google Scholar]
- 23.Chen CH, Wang TL, Hung CF, Yang Y, Young RA, Pardoll DM, et al. Enhancement of DNA vaccine potency by linkage of antigen gene to an HSP70 gene. Cancer Res. 2000;60(4):1035–42. [PubMed] [Google Scholar]
- 24.Hung CF, Cheng WF, Hsu KF, Chai CY, He L, Ling M, et al. Cancer immunotherapy using a DNA vaccine encoding the translocation domain of a bacterial toxin linked to a tumor antigen. Cancer Res. 2001;61(9):3698–703. [PubMed] [Google Scholar]
- 25.Feltkamp MC, Smits HL, Vierboom MP, Minnaar RP, de Jongh BM, Drijfhout JW, et al. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur J Immunol. 1993;23(9):2242–9. doi: 10.1002/eji.1830230929. [DOI] [PubMed] [Google Scholar]
- 26.Ji H, Wang T-L, Chen C-H, Hung C-F, Pai S, Lin K-Y, et al. Targeting HPV-16 E7 to the endosomal/lysosomal compartment enhances the antitumor immunity of DNA vaccines against murine HPV-16 E7-expressing tumors. Human Gene Therapy. 1999;10(17):2727–40. doi: 10.1089/10430349950016474. [DOI] [PubMed] [Google Scholar]
- 27.Gambhira R, Jagu S, Karanam B, Gravitt PE, Culp TD, Christensen ND, et al. Protection of Rabbits against Challenge with Rabbit Papillomaviruses by Immunization with the N Terminus of Human Papillomavirus Type 16 Minor Capsid Antigen L2. J Virol. 2007;81(21):11585–92. doi: 10.1128/JVI.01577-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng WF, Hung CH, Chai CY, Hsu KF, He L, Ling M, et al. Enhancement of sindbis virus self-replicating RNA vaccine potency by linkage of herpes simplex virus type 1 VP22 protein to antigen. J Virol. 2001;75(5):2368–76. doi: 10.1128/JVI.75.5.2368-2376.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buck CB, Pastrana DV, Lowy DR, Schiller JT. Efficient intracellular assembly of papillomaviral vectors. J Virol. 2004;78(2):751–7. doi: 10.1128/JVI.78.2.751-757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pastrana DV, Buck CB, Pang YY, Thompson CD, Castle PE, FitzGerald PC, et al. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology. 2004;321(2):205–16. doi: 10.1016/j.virol.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 31.Pastrana DV, Gambhira R, Buck CB, Pang YY, Thompson CD, Culp TD, et al. Cross-neutralization of cutaneous and mucosal Papillomavirus types with anti-sera to the amino terminus of L2. Virology. 2005;337(2):365–72. doi: 10.1016/j.virol.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 32.Munger K, Basile JR, Duensing S, Eichten A, Gonzalez SL, Grace M, et al. Biological activities and molecular targets of the human papillomavirus E7 oncoprotein. Oncogene. 2001;20(54):7888–98. doi: 10.1038/sj.onc.1204860. [DOI] [PubMed] [Google Scholar]
- 33.Gao Q, Singh L, Kumar A, Srinivasan S, Wazer DE, Band V. Human papillomavirus type 16 E6-induced degradation of E6TP1 correlates with its ability to immortalize human mammary epithelial cells. J Virol. 2001;75(9):4459–66. doi: 10.1128/JVI.75.9.4459-4466.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dalal S, Gao Q, Androphy EJ, Band V. Mutational analysis of human papillomavirus type 16 E6 demonstrates that p53 degradation is necessary for immortalization of mammary epithelial cells. J Virol. 1996;70(2):683–8. doi: 10.1128/jvi.70.2.683-688.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng S, Tomson TT, Trimble C, He L, Hung CF, Wu TC. A combination of DNA vaccines targeting human papillomavirus type 16 E6 and E7 generates potent antitumor effects. Gene Ther. 2006;13(3):257–65. doi: 10.1038/sj.gt.3302646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trimble C, Lin CT, Hung CF, Pai S, Juang J, He L, et al. Comparison of the CD8+ T cell responses and antitumor effects generated by DNA vaccine administered through gene gun, biojector, and syringe. Vaccine. 2003;21(2526):4036–42. doi: 10.1016/s0264-410x(03)00275-5. [DOI] [PubMed] [Google Scholar]


