Abstract
In Saccharomyces cerevisiae, the base excision DNA repair (BER) pathway has been thought to involve only a multinucleotide (long-patch) mechanism (LP-BER), in contrast to most known cases that include a major single-nucleotide pathway (SN-BER). The key step in mammalian SN-BER, removal of the 5'-terminal abasic residue generated by AP endonuclease incision, is effected by DNA polymerase β (Polβ). Computational analysis indicates that yeast Trf4 protein, with roles in sister chromatin cohesion and RNA quality control, is a new member of the X family of DNA polymerases that includes Polβ. Previous studies of yeast trf4Δ mutants revealed hypersensitivity to methylmethane sulfonate (MMS) but not UV light, a characteristic of BER mutants in other organisms. We found that, like mammalian Polβ, Trf4 is able to form a Schiff base intermediate with a 5'-deoxyribose-5-phosphate substrate and to excise the abasic residue through a dRP lyase activity. Also like Polβ, Trf4 forms stable cross-links in vitro to 5'-incised 2-deoxyribonolactone residues in DNA. We determined the sensitivity to MMS of strains with a trf4Δ mutation in a rad27Δ background, in an AP lyase-deficient background (ogg1 ntg1 ntg2), or in a pol4Δ background. Only a RAD27 genetic interaction was detected: there was higher sensitivity for strains mutated in both TRF4 and RAD27 than either single mutant, and overexpression of Trf4 in a rad27Δ background partially suppressed MMS sensitivity. The data strongly suggest a role for Trf4 in a pathway parallel to the Rad27-dependent LP-BER in yeast. Finally, we demonstrate that Trf5 significantly affects MMS sensitivity and thus probably BER efficiency in cells expressing either wild-type Trf4 or a C-terminus-deleted form.
Keywords: DNA repair, base excision repair, Trf4, dRP lyase activity
1. Introduction
Cellular DNA is continuously damaged by endogenous and exogenous reactive species that form a variety of base lesions that threaten critical cellular processes and potentiate mutations and disease. Multiple defense mechanisms have evolved to guarantee genomic integrity, with base excision repair (BER) predominant in the repair of numerous small DNA base lesions [1-3].
BER is initiated by DNA N-glycosylases that convert diverse base lesions into a common intermediate, the abasic (AP) site. AP sites also arise directly by hydrolytic base loss or some free radical reactions on DNA. In some DNA glycosylases, an associated AP lyase activity can cleave the 3'-phosphodiester bond of the newly formed AP site. In either case, the next step is catalyzed by an AP endonuclease, either to remove the AP lyase product or incise the AP site on the 5' side, reactions generating the 3'-OH terminus necessary for gap-filling by a repair DNA polymerase. In BER initiated by a monofunctional DNA glycosylase, the AP endonuclease-generated 5'-deoxyribose-5-phosphate (5'-dRP) residue must be excised before the final repair step by DNA ligase. In vertebrates, two subpathways have been documented: single-nucleotide BER (SN-BER) that replaces only the modified nucleotide, and long-patch BER (LP-BER) that resynthesizes several (2-10) nucleotides. In SN-BER, gap-filling and 5'-dRP removal are both done by DNA polymerase β (Polβ), a member of the X family of DNA polymerases. During 5'-dRP removal by Polβ, a Schiff base intermediate is generated that can be trapped in vitro by chemical reduction. Deficiency in the dRP lyase activity of Polβ renders cells hypersensitive to the alkylating agent methyl methanesulfonate (MMS), due to the toxic accumulation of 5'-dRP residues [4]. In that context, MMS sensitivity may be considered as a hallmark of BER deficiency. Another X family DNA polymerase, Polλ, also possesses a 5'-dRP lyase activity. Cells deficient in Polλ do not show any hypersensitivity to MMS compared to wild-type [5], but Polλ is able to carry out backup BER in the absence of functional Polβ [6]. Recently, two mammalian homologues of bacterial endonuclease VIII, Neil1 and Neil2, were shown to remove 5'-dRP from DNA with an efficiency comparable to that of Polβ [7].
LP-BER provides an alternative pathway when 5'-dRP lyase activity is insufficient, or for lesions refractory to lyase-mediated excision, such as 2'-deoxyribonolactone residues [8]. In such situations, DNA polymerases (involving Polβ, Polδ and Polε), synthesize multiple nucleotides to generate a single-stranded 5' flap structure that is removed by the FEN1 nuclease prior to ligation [9-12].
Whereas evidence for a functional LP-BER in the yeast S. cerevisiae has been documented in vitro and in vivo [13,14], no SN-BER has been reported so far. The apparent absence in S. cerevisiae of the key mammalian SN-BER proteins (Polβ, XRCC1, PARP1, and DNA ligase III) did not seem to favor this pathway. SN-BER might alternatively be achieved through initial incision of an AP site by an AP lyase, followed by the action of the main yeast AP endonuclease Apn1 to generate a one-nucleotide gap. However, although S. cerevisiae possesses three AP lyases (Ogg1, Ntg1, and Ntg2), a triple mutant lacking all three enzymes is not hypersensitive to MMS [15].
No obvious Polβ homolog is present in S. cerevisiae, but three yeast proteins contain a Polβ-like nucleotidyltransferase domain: Pol4, Trf4 and Trf5 [16,17]. Pol4 is most closely related to Polλ [18-21] and shares with that enzyme a 5'-dRP lyase via a conserved helix-hairpin-helix motif needed for the activity; pol4 mutations do not confer MMS hypersensitivity even in combination with defects in the Fen1 homolog Rad27 [22-24]. Trf4 and Trf5 define a distinct family of Polβ-like nucleotidyltransferases found in all sequenced eukaryotic genomes (we note that the comparison included only a predicted nucleotidyltransferase sequence from S. pombe and not possible dRP lyase motifs [16]). The TRF4 gene was identified in a screen for mutations producing synthetic lethality with a DNA topoisomerase 1 defect [25]. TRF5 is a homolog of TRF4 (55% identity, 72% similarity) and was identified through suppression of the cold sensitivity caused by a trf4 point mutation [26]. Deletion of TRF4 causes defects in chromosome segregation, hyper-recombination in the rDNA locus, and increased sensitivity to DNA damaging agents (including MMS but not UV light), DNA replication inhibitors, and microtubule poisons [25-30]. Deletion of TRF5 did not produce any reported phenotype, but deletion of both TRF4 and TRF5 is lethal, and Trf5 overexpression suppresses top1 trf4 lethality, indicating that Trf4 and Trf5 may have overlapping functions [26]. Both proteins appear to interact with DNA polymerase ε[27] and may have a role in sister chromatid cohesion established at replication forks [31-33].
Trf4 may have DNA polymerase activity [27,33], and together with Trf5 was proposed as a catalytic subunit in a new nuclear DNA polymerase (Polσ). However, this function is controversial [34,35]. Still another proposed function for Trf4 and Trf5 is polyadenylation of defective nuclear RNA precursors to target them for degradation by the nuclear exosome [36-39].
The specific characteristics of Trf4 (putative X family DNA polymerase member, role in resistance to MMS but not UV damage), led us to investigate the possible DNA repair role of this enzyme. Here we show that Trf4 has a 5'-dRP lyase activity in vitro. Moreover, deficiency in both Trf4 and Rad27 leads to increased MMS and H2O2 sensitivity. Since the presence of Trf5 also affects MMS sensitivity, we propose that both Trf4 and Trf5 play a role in BER in S. cerevisiae, with Trf4 as a functional homolog of Polβ.
2. Materials and Methods
2.1. Growth media, yeast strains, growth conditions, transformation and chemicals
The media used in this study have been described by Sherman [40]. The yeast strains used in this study are listed in Table 1. The trf4 deletion in the FF18733 background, and the Trf4 C-terminal truncation and replacement with a c-myc tag in the CY141 and CY871 background (trf4Δ(552-584) mutant), were constructed by a PCR-mediated, one-step replacement technique using the 13Myc-KanMX6 module, with geneticin as the selection [41]. Transformations were performed using the lithium acetate procedure. Gene replacement was confirmed by PCR analysis of genomic DNA. Methyl methanesulfonate (MMS), hydroxyurea (HU) and hydrogen peroxide were purchased from Sigma.
Table 1.
S. cerevisiae yeast strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| FF18733 | MATa, his7-2, leu2-3-112, lys1-1, trp1-289, ura3-52 | F. Fabre |
| FF18-1616 | FF18733 with rad27Δ::LEU2 | F. Fabre |
| DG100 | FF18733 with trf4Δ::kanMX6Myc | This study |
| DG200 | RAD27- with trf4Δ::kanMX6Myc | This study |
| CD186 | FF18733 with ogg1Δ::URA3, ntg1 ::LEU2, ntg2Δ::TRP1 | D. Thomas |
| DG300 | CD186 with trf4Δ::kanMX6Myc | This study |
| CY141 | MATa ade2-1, ura3-1, his3-11, 15 trp1-1, leu2-3 | M. Christman |
| CY143 | CY141 with 112 rDNA ::URA3 | M. Christman |
| CY1000 | CY143 with trf4Δ::HIS3 | M. Christman |
| DG400 | CY141 with pol4Δ::kanMX6Myc | This study |
| DG500 | CY1000 with pol4Δ::kanMX6Myc | This study |
| CY871 | CY143 with trf5Δ::LEU2 | M. Christman |
| DG101 | CY141 with trf4Δ(552–584)::kanMX6Myc | This study |
| DG102 | CY871 with trf4Δ(552–584)::kanMX6Myc | This study |
| Plasmid | Genotype | Source |
| pYX212 | URA3 2u | R&D Systems |
| pTRF4 | TRF4 URA3 2u | This study |
| pTRF4-552 | trf4-552 URA3 2u | This study |
| Yeplac112 | TRP1 2u | M. Christman |
| pSH4 | TRF5 TRP1 2u | M. Christman |
2.2. Sensitivity to DNA damaging agents
Yeast strains were grown in YEPD medium at 30°C under vigorous aeration, to a density of 107 cells/ml. For H2O2 treatment, cells were pelleted and resuspended in the same volume of sterile water. Aliquots of cell suspension were then exposed to various concentrations of DNA-damaging agents for 30 min at 30°C with shaking. Untreated and treated cells were then immediately diluted and plated on YEPD agar, and colonies were scored after 3 days of incubation at 30°C. For MMS and HU treatments, cells were grown to mid-exponential phase, serially diluted in distilled water, and 10-μl aliquots were spotted on YEPD agar, or on synthetic complete medium (SC) plus glucose and lacking the appropriate supplements. The media contained the appropriate selective agents as indicated in the figure legends. After incubation at 30°C for 3 days, colonies were counted to determine survival. All experiments were independently carried out in triplicate.
2.3. Plasmid construction and site-directed mutagenesis
A list of the plasmids used is included in Table 1. The vector pYX212 (R&D Systems) was modified to express Trf4 in yeast. Plasmid pTRF4 was constructed from pCDNA3.1 (Invitrogen) by inserting a PmeI restriction fragment containing the TRF4 gene of S. cerevisiae into pYX212 at the SmaI restriction site. Correct orientation was checked by restriction digestions and confirmed by DNA sequencing. Point mutation to generate pTRF4-552 was performed by PCR-mediated mutagenesis (QuickChange site-directed mutagenesis kit, Stratagene) changing lysine-552 to alanine (K552). The construct was confirmed by DNA sequencing. Plasmid pSH4 overexpressing Trf5 and the corresponding Yeplac112 vector were provided by the M. Christman laboratory [26]. The sequences of the plasmids and oligonucleotide primers, and the details of the construction schemes are available upon request.
2.4. Biochemical reagents
Purified Trf4 protein and anti-Trf4 antibody were obtained from the M. Christman laboratory [33]. Anti-His antibody was purchased from Santa Cruz Biotechnology, Inc. [α-32P]dCTP was obtained from PerkinElmer Life Sciences. Uracil-DNA glycosylase (UDG) was purchased from New England Biolabs (Beverly, MA). E. coli endonuclease IV was prepared as described previously [42].
2.5. Oligonucleotides
For the Schiff base trapping assay, a 30-mer (5'-GTCACGTGCTGCAXACGACGTGCTGAGCCT) was used that contained either a U or a deoxyribonolactone (dL) in the X position. The complementary oligonucleotide contained an A residue opposite X and was hybridized immediately before use. For the 5'-dRP lyase assay, a 52-mer (5'-GCTTGCATGCCTGCAGGTCGAUTCTAGAGGATCCCCGGGTACCGAGCTCGAC-3') was hybridized to the complementary strand prior to enzyme reactions.
2.6. 3'-end labeling
DNA substrates containing uracil, 8-oxo-guanine (8-oxoG) or dL were 3'-end labeled by incorporation of [α32P]dCTP using the exonuclease-free Klenow fragment of DNA polymerase I. Unincorporated label was removed using Micro Biospin P-30 columns (Bio-Rad), following the manufacturer's protocols.
2.7. 5'-dRP lyase Assay
The assays employed a 52-base pair, synthetic duplex DNA substrate as described previously [43]. The 32P-labeled uracil-containing duplex DNA (10 nM) was pretreated with 5 U (supplier's units; an enzyme excess) of UDG plus 2 nM of E. coli endouclease IV. Assay mixtures were assembled at 0°C and incubated at 30°C in standard reaction buffers containing 50 mM HEPES-KOH (pH 7.5), 50 mM KCl, 10% (v/v) glycerol, 0.5 mM dithiothreitol, 0.1 mg/ml bovine serum albumin, 10 nM DNA substrate, and 5 mM EDTA or 6 mM MgCl2 as indicated. Enzyme concentrations and incubation times are indicated in the figure legends. The 5'-dRP lyase reactions were stopped by addition of 300 mM NaBH4 followed by a 30 min incubation on ice and then desalted by ethanol precipitation with the addition of glycogen as the carrier. After recovery of the precipitate, samples were resuspended in formamide loading buffer (90% formamide, 10 mM EDTA, bromophenol blue and xylene cyanol), heated 5 min at 100°C, and the products were resolved by electrophoresis in denaturing 20% polyacrylamide/7 M urea gels. The gels were dried, analyzed on a Storm 860 phosphorimager, and quantified. The percentage of total dRP excised was calculated by dividing the amount of the dRP lyase product formed in each reaction by the sum of this product and the amount of the substrate DNA containing intact 5'-dRP.
2.8. Trapping Assays
A reaction mixture (15 μl) contained 50 mM HEPES-KOH (pH7.5), 10% (v/v) glycerol, 0.5 mM dithiothreitol, 0.1 mg/ml bovine serum albumin, 50 mM KCl, 5 mM EDTA or 6 mM MgCl2 as indicated in the figures, and 200 fmol of 3'-labeled 5'-dRP-containing DNA substrate. The 5'-terminal dL-containing DNA substrate was prepared and 3'-labeled as previously described [44]. The reactions were assembled and initiated by adding the indicated amount of Trf4 or Polβ, followed by the addition of 50 mM NaBH4 to stabilize the substrate or Schiff base intermediates either immediately, or at various times as indicated in Fig. 2. After 30 min incubation on ice, reactions were terminated by the addition of SDS-PAGE loading buffer, and the samples were run on a 10% SDS-PAGE gel. Trapped complexes were visualized and quantified using a Storm 860 phosphorimager.
Figure 2. Schiff base intermediate formed with Trf4.
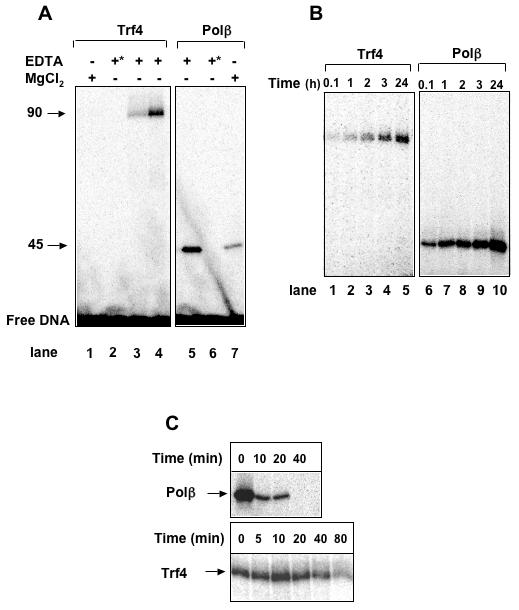
A, Trapping of Trf4-DNA and Polβ-DNA covalent complexes with NaBH4. A 5'-dRP substrate labeled at the 3' end of the damaged strand was used, and the reactions were carried out as described under Experimental Procedures. Arrows to the left indicate the mobility and Mr (in 1,000s) of the covalent complex adduct. A 40-fold excess of non-radioactive oligonucleotide substrate was added prior heating to prevent further trapping of the radioactive substrate. Lanes 1 and 7, 6 mM MgCl2 instead of 2 mM EDTA was added to the reactions; lanes 2 and 6 (marked with *), enzymes were boiled for 5 min at 100°C before the assay; lane 3, 400 nM Trf4; lanes 1, 2 and 4, 600 nM Trf4; lanes 5-7, 20 nM Polβ. B, Cross-linked adduct formed between Trf4 and a 5'-dL DNA substrate. Trf4 (lanes 1-5) or Polβ (lane 6-10), both at 600 nM, were incubated with 200 fmol 3'-end-labeled substrate for the indicated times. C, Time course of Trf4-DNA and Polβ-DNA cross-linking by NaBH4. Reactions containing an internal 5'-[α-32P]dRP duplex DNA and Trf4 or Polβ were incubated at 30°C for the indicated times before addition of 50 mM NaBH4. The lanes labeled “0 min” indicate the addition of NaBH4 immediately after assembling the reactions at 0°C. As in A, an excess of non-radioactive oligonucleotide substrate was added prior heating. Arrows on the left indicate the mobility of the Trf4-DNA and Polβ-DNA covalent adducts.
2.9. Immunoblotting
Wild-type cells containing the empty vector pYX212, or plasmids expressing wild-type Trf4 or the tag Myc-deleted version of Trf4(1-552), were grown in selective medium to mid-exponential phase before extraction. Yeast cell-free extracts were prepared under denaturing conditions [45]. Immunoblot experiments were performed as followed. Proteins were fractionated on SDS-12% polyacrylamide gels, transferred to nitrocellulose membranes, which were blocked by incubating 1 h in a PBS solution containing 5% nonfat milk and 0.1% Tween 20. The membranes were incubated with rabbit anti-yeast Trf4 polyclonal antiserum or anti-His-tag antiserum in the PBS-buffered milk/Tween solution. The membrane was washed three times in PBS containing 0.1% Tween 20 and incubated with alkaline-phosphatase-conjugated donkey anti-rabbit IgG, or with phosphatase-conjugated goat anti-mouse IgG (Santa Cruz Biotechnology), in the same buffer for 1 h. The blots were washed as described above and incubated with enhanced chemifluorescence substrate (Amersham Pharmacia Biosciences). Quantification of protein levels was performed on a fluorescence-detecting optical scanner (Storm 840, Amersham Pharmacia).
3. Results
3.1. 5'-dRP lyase activity associated with Trf4
To test the ability of Trf4 to remove a 5'-terminal dRP group, we used a 52-mer double stranded oligonucleotide containing a single uracil residue at position 22 (see 2.5. Oligonucleotides). As described under “Experimental Procedures”, the uracil-containing strand was 3'-end-labeled with [α-32P]dCTP, annealed to its complementary strand, treated with UDG to remove the uracil residue, and finally incubated with E. coli EndoIV to generate a substrate with a break bearing a 5'-terminal deoxyribose-5-phosphate residue on one side. This substrate (10 nM) was incubated with or without His-tagged Trf4 (Fig. 1A). Using His-tagged Polβ as control, substantial dRP release was observed after 10 min incubation at an enzyme-substrate ratio near 1 (Fig. 1A, compare lanes 1 and 7). However, under these reaction conditions, no dRP lyase activity was detected for recombinant Trf4 (data not shown). Therefore, we performed the dRP lyase assay with a significant excess of Trf4 over the substrate and a longer incubation time. Trf4 (900 nM) (Fig. 1A, lanes 3-6) removed most of the dRP moiety after a 40-min incubation at 30°C.
Figure 1. Detection of dRP lyase activity in Trf4.

A, Cleavage of 5'-dRP residues by Trf4. Assays were performed as described under Experimental procedures, and the relative mobilities of the DNA substrate (S) and product (P) band are indicated. Each reaction contained 10 nM of 3'-labeled, 5'-dRP-containing DNA substrate. Lanes 1-2, no enzyme; lanes 3, 5 and 6, 900 nM Trf4; lane 4, 450 nM Trf4; lanes 7-9, 20 nM Polβ. In lanes 4, 5 and 8, enzyme was pre-incubated with anti-His antibody for 10 min on ice before the repair reaction. Reactions for lanes 6 and 9 contained 6 mM MgCl2 instead of 2 mM EDTA. The repair incubation time is indicated in the figure. DNA was stabilized with NaBH4 to prevent a further degradation during electrophoresis. The percent of 5'-dRp excised is shown under each lane. The separation bar in the gel image indicates some irrelevant lanes deleted from the image; the lanes shown are from the same gel. B, Time course of dRP excision by Trf4. Trf4 (900 nM) was used in this set of assays. Error bars (±S.D) from three experiments are shown. C, Trf4 concentration dependence of the 5'-dRP lyase activity. Reactions were stopped after 40 min incubation. The combined result of two experiments is represented by a linear trendline.
Pre-incubation of purified His-Trf4 with an anti-His antibody blocked the removal of the 5'-dRP (Fig. 1A, lane 4-5). The same antibody had no effect on the His-Polβ dRP lyase activity (Fig. 1A, lane 8). This result is consistent with a 5'-dRP lyase activity that is intrinsic to Trf4, rather than due to a contaminant. As also observed for Polβ, the addition of magnesium in the reaction inhibited the Trf4 dRP lyase activity (Fig. 1A, lane 6 and 9). The dRP lyase data on the reaction time course and Trf4 concentration dependence (Fig. 1B and 1C) revealed some unusual features. The kinetics showed a lag of ∼10 min, followed by rapid product generation over the next 10 min. Significant 5'-dRP lyase activity of Trf4 was observed only at levels above a 4-fold enzyme:DNA ratio, which seemed primarily limited by the sensitivity of the assay.
In view of the foregoing observations, it was especially important to determine that the 5'-dRp lyase activity was not due to a contaminant. Trf4 was purified from an E. coli expression system, in which Fpg would be the main MgCl2-independent contaminating dRP lyase activity. To detect possible Fpg activity, we incubated Trf4 with a substrate containing an 8-oxoguanine moiety (8-oxoG), a preferred substrate of Fpg [46], in the same [enzyme-substrate] excess ratio as in the 5'-dRP lyase assay. No cleavage was detected after an incubation time of 30 minutes, compared to abundant cleavage by a control sample of purified Fpg (data not shown).
3.2. Excision of a 5'-dRP residue by Trf4 through a β-elimination mechanism
The 5'-dRP lyase reaction proceeds through a β-elimination mechanism wherein the catalytic residue forms a Schiff base intermediate that can be trapped as a covalent enzyme-DNA complex upon chemical reduction [47]. We used this feature to examine whether such was the case for Trf4. The same DNA substrate (3'-labeled) was used as in the 5'-dRP lyase assay, except that, after stopping with NaBH4, the reactions were analyzed on SDS-PAGE rather than on DNA sequencing gels. As observed with Polβ(Fig. 2A, lane 5), Trf4 could be reductively trapped on the 5'-dRp substrate to yield a product with mobility expected for a Trf4-DNA cross-link (Fig. 2A, lanes 3-4). This observation, and the lack of a band around Mr 30,000, further indicate that Trf4 and not Fpg is responsible for the observed lyase activity. Also as found for Polβ, the Trf4-dependent product was greatly diminished or absent when MgCl2 was added to the buffer (Fig. 2A, lanes 1 and 7), or when the purified Trf4 had been heated 5 min at 100°C prior to the assay (Fig. 2A, lanes 2 and 6). No trapping was observed when the Trf4 was omitted, or when the substrate had been treated with NaBH4 prior to addition of the Trf4p (data not shown).
As described previously, an oxidized AP site, 2-deoxyribonolactone (dL), forms a stable, covalent cross-link to DNA repair proteins with lyase activity; in the case of Polβ, this occurs most efficiently with the 5'-terminal dL produced by AP endonuclease cleavage [44,48,49]. With dL, this covalent cross-link formation does not require NaBH4 treatment, and the product accumulates with time. With Polβ as a positive control (Fig. 2B, lanes 6-10), we observed that Trf4 also formed a cross-link with dL, and that accumulation of the cross-link was time dependent (Fig. 2B, lanes 1-5). Again, the mobility of the Trf4 product was consistent with the size of the cross-link expected for this protein. Thus, Trf4 in vitro forms a transient covalent intermediate during its reaction with 5'-dRP, or a stable cross-link with a 5'-dL residue in DNA.
Because it has been demonstrated that initial Schiff base formation does not necessarily result in an efficient β-elimination reaction [50], we examined the kinetics of NaBH4-mediated cross-linking with Trf4. We pre-incubated the enzyme with the 5'-dRP-containing substrate (labeled at the 3' end of the dRP strand) for increasing amounts of time before adding the reducing agent. It was shown previously for Polβ that release of the non-oxidized 5'-dRP through the dRP lyase activity of the enzyme during such a pre-incubation parallels the decrease in the amount of protein that can be trapped in a covalent complex by reduction [50]. For Polβ this decrease occurs over a few minutes after the initial incubation with the substrate (Fig. 2C, upper panel). For Trf4, we observed the accumulation of the NaBH4-trapped complex up to about 10 min of pre-incubation, followed by a progressive diminution until a nearly complete disappearance of the trappable complex after 80 minutes (Fig. 2C, lower panel). Both enzymes still formed a trappable DNA-enzyme complex using a substrate pre-incubated without enzyme at 30°C for 80 minutes (data not shown). This kinetic profile of reductive trapping was consistent with the results obtained from the 5'-dRP lyase assay in which a small proportion of 5'-dRP was released after 10 min and a complete release was observed after 40 min (Fig. 1A). Thus, our biochemical results demonstrate that Trf4 possesses an intrinsic dRP lyase activity.
3.3. Inactivation of Trf4 enhances MMS toxicity in rad27 and ntg1 ntg2 ogg1 mutants
To investigate the role of Trf4 in the BER pathway in vivo, we measured the survival of a trf4 mutant in different BER backgrounds after exposure to alkylating or oxidizing agents that damage DNA (Fig. 3). We first analyzed the sensitivity in conjunction with a rad27 mutation. As shown in Figures 3A and 3B, a double rad27 trf4 mutant was more sensitive to both MMS and hydrogen peroxide treatment than was either single mutant. One can notice that a single trf4 mutant was also slightly more sensitive to the oxidative agent H2O2 compared to wild-type (WT) cells (Fig. 3B). In S. cerevisiae deficient in all three known AP lyases (Ogg1, Ntg1 and Ntg2), the repair of AP sites should be predominantly or exclusively by the Apn1-dependent pathway, which would thus enhance the amount of 5'-dRP in the DNA. The absence of Trf4 in an ogg1 ntg1 ntg2 background increased the MMS sensitivity compared to the AP lyase triple mutant (Fig. 3C), consistent with a role for Trf4 in SN-BER. Interestingly, the ogg1ntg1ntg2 triple mutant was more resistant to MMS than the WT strain (Fig. 3C). This MMS resistance was not observed for the single or double AP lyase mutants described so far [15,51-53]. The phenotype probably reflects a redundancy among the three AP lyases for AP site cleavage. The result also suggests that formation of 3'-incised abasic lesions by AP lyases may be more toxic than the 5'-incisions generated by Apn1.
Figure 3. Effect of TRF4 inactivation upon MMS and H2O2 sensitivity in different BER backgrounds.
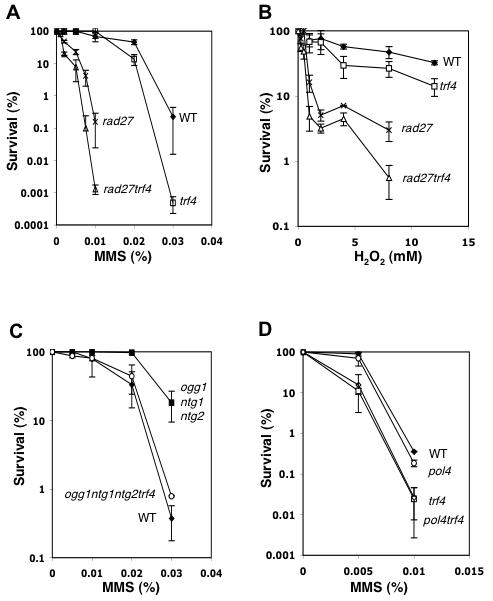
Exponentially growing cells were exposed to increasing concentrations of MMS (panels A, C and D) or H2O2 (panel B) as described in Experimental Procedures. Panel A and B strains: WT (Ʋ), trf4 (□), rad27 (×), and rad27trf4 (△). Panel C strains: WT (Ʋ), ogg1 ntg1 ntg2 (■), and ogg1 ntg1 ntg2 trf4 (○). Panel D strains: WT (Ʋ), trf4 (◇), pol4 (○), and pol4 trf4 (□). The data for each curve represent the average values (±S.D) of at least three independent experiments for each strain. Strains in panel A, B and C are isogenic to the WT FF18733. Strains in panel D are isogenic to the WT CY141.
Whereas Pol4 shows an in vitro dRP lyase activity, no MMS sensitivity phenotype is observed for the pol4 mutant. We then investigated possible redundancy between Trf4 and Pol4 by testing for MMS sensitivity. As shown in Figure 3D, the MMS sensitivity of a pol4 strain was not further enhanced by the addition of a trf4 mutation. Thus, Trf4 and Pol4 do not seem to have redundancy in their substrate preference relative to alkylation damage. The higher MMS sensitivity observed for the WT strain in this experiment may reflect background genetic differences. The MMS and hydrogen peroxide sensitivity phenotypes associated with trf4 mutations in the different BER backgrounds strongly suggests a role for Trf4 in this DNA repair pathway.
3.4. Trf4 overexpression partially complements rad27 mutant MMS sensitivity phenotype
In the absence of LP-BER dependent on Rad27, the role of a Trf4 5'-dRp lyase should be enhanced. To test this point, we overexpressed Trf4 in a rad27 strain. The results (Fig. 4) demonstrated that Trf4 overexpression can partially suppress the MMS hypersensitivity due to a rad27 mutation. Trf4 overexpression also increased MMS resistance in a WT background (Fig. 5B). Taken together, these data point to involvement of Trf4 in a BER in a pathway different from the Rad27-dependent pathway, and suggest that Trf4 activity may be limiting for efficient BER when cells are challenged with high levels of DNA damage.
Figure 4. The MMS sensitivity of a rad27 mutant strain is partially complemented by multicopy WT TRF4.
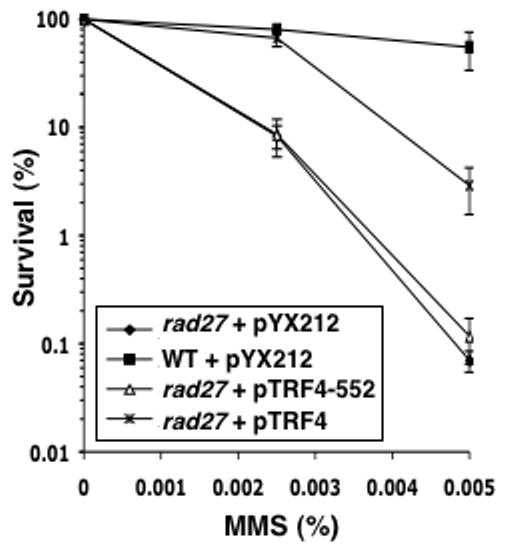
Plasmids expressing WT Trf4 (pTRF4), or a mutant derivative with alanine substitution of Lys-552 (pTRF4-552), were transformed into “rad27” strain. As controls, vector pYX212 was transformed into the rad27 and WT (FF18733) strains. MMS sensitivity was determined as described for Fig. 3 and in Experimental Procedures. Points throughout are the average ± S.D of at least three independent measurements.
Figure 5. Trf4K552A protein restores HU but not MMS resistance.
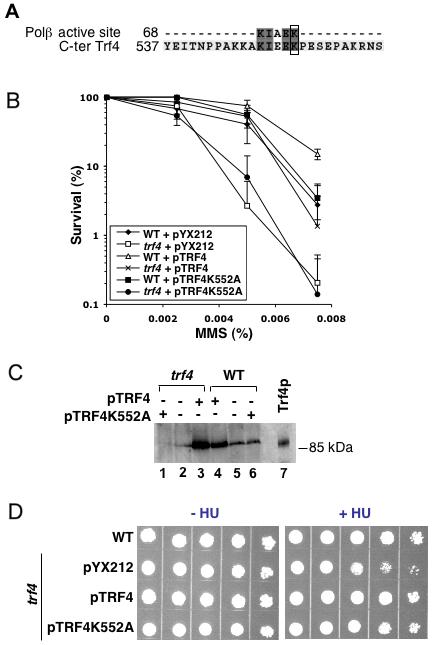
A, Sequence comparison between the Trf4 C-terminal region (C-ter) and the Polβ 5'-dRP lyase domain using the Multalin software program [73]. Only the Polβ active site sequence KIAEK is shown. Identical amino acids are highlighted with shaded boxes. The active lysine-72 of Polβ and lysine-552 of Trf4 are boxed. B, MMS sensitivity of WT (CY141) and trf4 (CY1000) strains harboring pYX212, pTRF4 or pTRF4K552A vectors. The data points represent the average ± S.D of at least three independent measurements. C, Western blot analysis of WT Trf4 and Trf4K552A expressed in CY141 (lanes 1-3) and CY100 (lanes 4-6) strains. The immunoblotting was performed as described in Experimental Procedures. The position of a size standard (Trf4p, purified Trf4 protein) is indicated. Each lane was loaded with 40 μg of total protein. Cell-free extracts were obtained from cells carrying pYX212 vector (lanes 2 and 5), pTRF4 (lanes 3 and 4), or pTRF4K552A (lane 1 and 6). D, Sensitivity of WT (CY141) strain with the pYX212 vector and a trf4 mutant strain with pTRF4 or pTRF4K552A plasmids. Strains were grown to mid-exponential phase and serially diluted at 10, 102, 103, 104 and 105 cells on YNBD medium lacking uracil with or without 125 mM HU.
3.5. The Trf4 C-terminal domain contains the dRP lyase activity
We wanted next to determine the active site of Trf4 responsible for the dRP lyase activity. An alignment made between Trf4 and Trf5 protein sequence shows an absence of homology for the N- and C-terminal region (data not shown). It has been shown previously that trf5 mutants do not show higher MMS sensitivity than WT [27]. We suspected that the N- or C-terminal region of Trf4 contains the dRP lyase domain. Sequence comparisons between the Polβ dRP lyase active domain (residues 1-82) and the N- and C-terminal regions of Trf4 showed the Polβ motif KIAEK to align with a sequence (KIEEK) in the Trf4 C-terminus, with the Polβ active site K72 matching with Trf4 K552 (Fig. 5A). A Trf4 mutant with lysine-552 changed to alanine (K552A) was generated by site-directed mutagenesis, and the mutant protein was overexpressed in a trf4Δ mutant. Overproduction of wild-type Trf4 in a trf4 background increased cellular resistance to MMS to a level similar to that of the WT strain (Fig. 5B). In contrast, the expression of the Trf4K552A protein in a trf4 mutant did not complement the MMS sensitivity phenotype. Interestingly, overexpression of Trf4 in a WT strain enhanced its MMS resistance, and this effect was not observed when Trf4K552A protein was expressed in the WT strain (Fig. 5B).
To confirm protein expression, Western blot analysis was conducted using an anti-Trf4 antiserum (Fig. 5C). While wild-type Trf4 was easily detected and clearly increased when the pTRF4 plasmid was present, no protein band was detected in the Trf4K552A extracts. We note that the anti-Trf4 antiserum employed here was generated against a C-terminal peptide of the protein [29]. In fact, an allele replacing the Trf4 C-terminus [residues 552-584] with a c-Myc tag produced protein detected by an anti-Myc antiserum in both WT and trf5 backgrounds (Fig. 6B). Thus, deletion of the C-terminus does not markedly destabilize Trf4. Moreover, the known HU hypersensitivity phenotype of a trf4 mutant was suppressed with expression of Trf4K552A protein (Fig. 5D); HU inactivates ribonucleotide reductase to block DNA synthesis indirectly [54]. Finally, a strain deficient in the C-terminal [552-584] domain of Trf4 showed hypersensitivity to MMS compared to a WT strain and similar to a trf4Δ strain (Fig. 6A). Taken together, these results strongly suggest a role for the C-terminal domain of Trf4 in DNA repair, probably its dRP lyase activity involving the lysine 552 as the active site.
Figure 6. Trf5 affects MMS sensitivity.

A, Western blot analysis of Myc-tagged Trf4. The Myc tag replaced the C-terminal domain (residues 552-584) of Trf4, and anti-Myc antibody was used. B, MMS sensitivity of WT (Ʋ), trf4 (□), trf5 (σ), trf4(1-551) (×) and trf5 trf4(1-551) (○) strains. The data for each curve represent the average values (±S.D) of at least three independent experiments for each strain. Strains are isogenic to the WT CY141.
3.6. Imbalance between Trf4 and Trf5 influences yeast BER
In view of the physical interaction between Trf4 and Trf5 proteins, we wondered whether a change in the Trf5 level could affect in vivo repair of MMS damage. To address this question, we tested the MMS sensitivity of strains deficient in Trf5. A MMS gradient plate assay did not reveal hypersensitivity in a trf5 strain compared to the WT [27]. In our MMS plate assay, we found that, contrary to a trf4Δ strain, Trf5 deficiency increased MMS resistance (Fig. 6B). Deleting the last 32 amino acids of Trf4 in the trf5 strain restored MMS sensitivity to a level similar to WT yeast. Thus, an imbalance in [Trf4-Trf5] seems to affect BER efficiency in yeast. Interestingly, overexpression of Trf5 increased MMS resistance of cells compared to the empty vector in a Trf4-deficient strain, but increased MMS sensitivity in a rad27 background (data not shown).
4. Discussion
SN-BER maintains accurate repair with time and energy saving compared to LP-BER. When this study began, there were clues to the existence of a Rad27-independent BER pathway in S. cerevisiae, such as the processing of 5'-dRP-containing or osmium tetroxide-damaged DNA in extracts of rad27 mutants [55-57], and the viability and MMS resistance of a strain deficient both in AP lyase- and Rad27-dependent BER (rad27 ogg1 ntg1 ntg2 mutant) compared to rad27 cells (Lionel Gellon, unpublished data). Spontaneous hydrolysis of 5'-dRP residues is unlikely to support efficient BER, although repair by other pathways might compensate for BER deficiencies [51,58-62].
The biochemical studies and mutant analysis presented here strongly suggest that S. cerevisiae possesses SN-BER via the 5'-dRP lyase activity of Trf4. This activity is distinct from previously reported properties of the protein. The reductive trapping profile of Trf4 on a 5'-dRp substrate was distinct from those for some Y-family DNA polymerases, such as the mitochondrial Polβ of C. fasciculate, or human and Xenopus Polγ, which accumulate the covalent Schiff base intermediate with (at most) slow dRP release [50,63]. The activity of Trf4 is intermediate, with slower accumulation of the Schiff base complex than seen for Polβ, but resolution of the complex at a faster rate than for the enzymes just mentioned (and much faster than spontaneous hydrolysis, with a half-life of about 2 h at 37°C [64]).
Our evidence indicates that the dRP lyase activity is intrinsic to Trf4. First, the 5'-dRp lyase of a His-tagged form of Trf4 was inhibited by anti-His antiserum. Second, the electrophoretic mobility of the cross-linked adduct matches that expected for Trf4 plus the dRP-containing oligonucleotide, and no other significant cross-linked species was observed. Third, a direct effort to detect possible contamination by Fpg (the predominant E. coli 5'-dRp lyase) yielded a strong negative result. Finally, although E. coli DNA polymerases I, IV, and V, and endonuclease VIII all possess a dRP lyase (AP incision) activity in vitro, in these enzymes it is quite weak [65-67]. In contrast, Trf4 has a very weak AP lyase compared to its dRp lyase (data not shown).
The Trf4 5'-dRP lyase has unusual kinetics that required longer incubations and higher Trf4 concentrations than Polβ. Similar results were obtained with two different substrates of differing length (a 31-mer and a 52-mer). Possible explanations for the slow kinetics could include effects of the opposite base [7,68,69] or the presence of the His tag on the Trf4 C-terminus where the dRP lyase activity seems to reside. Also, in its DNA polymerase mode, Trf4 might bind in a manner inappropriate for the 5'-dRp lyase. The DNA polymerase activity of the material used here was comparable to that reported previously [33], which suggests that there was no overall loss of function during storage of the protein. Cofactors might be necessary for efficient 5'-dRp lyase, as is the case for the poly(A) polymerase function of Trf4, which requires the proteins Air1 or Air2 [34,38,39,70]. Analogously, the dRP lyase activity of mammalian Polγ is mildly stimulated by the γB protein [71]. However, such a role for the Trf4-Trf5 interaction [27] would be inconsistent with the genetic data presented here.
Lysine-552 is the likely active-site residue for the Trf4 5'-dRP lyase activity, but more detailed biochemical experiments will be required to confirm this assignment. The genetic observations are consistent with such a role: although the K552A Trf4 protein suppressed the HU hypersensitivity of a trf4 strain, neither that protein nor a C-terminal truncated form restored the MMS resistance, and C-terminus-deleted Trf4 conferred viability in a trf5 background, showing that this form of the protein is active. Further experiments will be required to quantify expression of the K552A Trf4 mutant. The MMS hypersensitivity of trf4 strains was thought to reflect a deficiency in DNA repairing double-strand breaks, but only the highest MMS concentrations would produce these in any quantity [72]. A similar point applies to the H2O2 hypersensitivity of trf4 strains. A simpler explanation is the role proposed here for Trf4 in BER. Nevertheless, the higher MMS sensitivity of a rad27Δ mutant compared to a trf4Δ strain indicates that SN-BER in S. cerevisiae may not be the main BER sub-pathway. Other Trf4 functions might also contribute to the observed phenotypes.
Trf5 influences the MMS sensitivity of S. cerevisiae, with somewhat different effects in wild-type and trf4Δ backgrounds. The increased MMS resistance conferred by a trf5Δ allele implies that Trf5 has a negative impact under physiological conditions. One possible explanation is that interaction of Trf5 with Trf4 prevents the latter from functioning effectively in BER. However, the increased MMS resistance conferred by deletion of TRF5 in a trf4(1-551) strain may suggest otherwise, unless Trf5 affects still another MMS repair pathway. Since the DNA polymerase domain of Trf4 should still be present in the nucleus, it would be important to test whether the Trf4-Trf5 interaction is maintained in the trf4(1-551) strain.
Acknowledgements
We are very grateful to Agnes Chenine for invaluable support, advice and many stimulating discussions. We appreciate the helpful advice and comments of members of the Demple laboratory. We also thank Serge Boiteux, Pablo Radicella, Marc Audebert and Michael Christman for helpful comments, wise suggestions, and for improving the manuscript. This work was supported by the NIH grant GM40000 to B.D., and the Harvard Center for Medical Countermeasures against Radiation (U19A106775) from NIAID.
Abbreviations
- BER
base excision repair
- Polβ
DNA polymerase beta
- LP-BER
long-patch (multinucleotide) base excision repair
- SN-BER
single-nucleotide base excision repair
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Friedberg E, Walker G, Siede W, Wood RD, Schultz RA, Ellenberger T. DNA repair and mutagenesis. 2ND EDN ASM Press; 2005. [Google Scholar]
- 2.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–374. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 3.Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362:709–715. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- 4.Sobol RW, Prasad R, Evenski A, Baker A, Yang XP, Horton JK, Wilson SH. The lyase activity of the DNA repair protein beta-polymerase protects from DNA-damage-induced cytotoxicity. Nature. 2000;405:807–810. doi: 10.1038/35015598. [DOI] [PubMed] [Google Scholar]
- 5.Braithwaite EK, Kedar PS, Lan L, Polosina YY, Asagoshi K, Poltoratsky VP, Horton JK, Miller H, Teebor GW, Yasui A, Wilson SH. DNA polymerase lambda protects mouse fibroblasts against oxidative DNA damage and is recruited to sites of DNA damage/repair. J Biol Chem. 2005;280:31641–31647. doi: 10.1074/jbc.C500256200. [DOI] [PubMed] [Google Scholar]
- 6.Braithwaite EK, Prasad R, Shock DD, Hou EW, Beard WA, Wilson SH. DNA polymerase lambda mediates a back-up base excision repair activity in extracts of mouse embryonic fibroblasts. J Biol Chem. 2005;280:18469–18475. doi: 10.1074/jbc.M411864200. [DOI] [PubMed] [Google Scholar]
- 7.Grin IR, Khodyreva SN, Nevinsky GA, Zharkov DO. Deoxyribophosphate lyase activity of mammalian endonuclease VIII-like proteins. FEBS Lett. 2006;580:4916–4922. doi: 10.1016/j.febslet.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Sung JS, DeMott MS, Demple B. Long-patch base excision DNA repair of 2-deoxyribonolactone prevents the formation of DNA-protein cross-links with DNA polymerase beta. J Biol Chem. 2005;280:39095–39103. doi: 10.1074/jbc.M506480200. [DOI] [PubMed] [Google Scholar]
- 9.Dianov GL, Prasad R, Wilson SH, Bohr VA. Role of DNA polymerase beta in the excision step of long patch mammalian base excision repair. J Biol Chem. 1999;274:13741–13743. doi: 10.1074/jbc.274.20.13741. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto Y, Kim K, Hurwitz J, Gary R, Levin DS, Tomkinson AE, Park MS. Reconstitution of proliferating cell nuclear antigen-dependent repair of apurinic/apyrimidinic sites with purified human proteins. J Biol Chem. 1999;274:33703–33708. doi: 10.1074/jbc.274.47.33703. [DOI] [PubMed] [Google Scholar]
- 11.Pascucci B, Stucki M, Jonsson ZO, Dogliotti E, Hubscher U. Long patch base excision repair with purified human proteins. DNA ligase I as patch size mediator for DNA polymerases delta and epsilon. J Biol Chem. 1999;274:33696–33702. doi: 10.1074/jbc.274.47.33696. [DOI] [PubMed] [Google Scholar]
- 12.Prasad R, Dianov GL, Bohr VA, Wilson SH. FEN1 stimulation of DNA polymerase beta mediates an excision step in mammalian long patch base excision repair. J Biol Chem. 2000;275:4460–4466. doi: 10.1074/jbc.275.6.4460. [DOI] [PubMed] [Google Scholar]
- 13.Blank A, Kim B, Loeb LA. DNA polymerase delta is required for base excision repair of DNA methylation damage in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1994;91:9047–9051. doi: 10.1073/pnas.91.19.9047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, Wu X, Friedberg EC. DNA repair synthesis during base excision repair in vitro is catalyzed by DNA polymerase epsilon and is influenced by DNA polymerases alpha and delta in Saccharomyces cerevisiae. Mol Cell Biol. 1993;13:1051–1058. doi: 10.1128/mcb.13.2.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alseth I, Korvald H, Osman F, Seeberg E, Bjoras M. A general role of the DNA glycosylase Nth1 in the abasic sites cleavage step of base excision repair in Schizosaccharomyces pombe. Nucleic Acids Res. 2004;32:5119–5125. doi: 10.1093/nar/gkh851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aravind L, Koonin EV. DNA polymerase beta-like nucleotidyltransferase superfamily: identification of three new families, classification and evolutionary history. Nucleic Acids Res. 1999;27:1609–1618. doi: 10.1093/nar/27.7.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hubscher U, Maga G, Spadari S. Eukaryotic DNA Polymerases. Annu Rev Biochem. 2002;71:133–163. doi: 10.1146/annurev.biochem.71.090501.150041. [DOI] [PubMed] [Google Scholar]
- 18.Aoufouchi S, Flatter E, Dahan A, Faili A, Bertocci B, Storck S, Delbos F, Cocea L, Gupta N, Weill JC, Reynaud CA. Two novel human and mouse DNA polymerases of the polX family. Nucleic Acids Res. 2000;28:3684–3693. doi: 10.1093/nar/28.18.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burgers PM, Koonin EV, Bruford E, Blanco L, Burtis KC, Christman MF, Copeland WC, Friedberg EC, Hanaoka F, Hinkle DC, Lawrence CW, Nakanishi M, Ohmori H, Prakash L, Prakash S, Reynaud CA, Sugino A, Todo T, Wang Z, Weill JC, Woodgate R. Eukaryotic DNA polymerases: proposal for a revised nomenclature. J Biol Chem. 2001;276:43487–43490. doi: 10.1074/jbc.R100056200. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Diaz M, Dominguez O, Lopez-Fernandez LA, de Lera LT, Saniger ML, Ruiz JF, Parraga M, Garcia-Ortiz MJ, Kirchhoff T, del Mazo J, Bernad A, Blanco L. DNA polymerase lambda (Pol lambda), a novel eukaryotic DNA polymerase with a potential role in meiosis. J Mol Biol. 2000;301:851–867. doi: 10.1006/jmbi.2000.4005. [DOI] [PubMed] [Google Scholar]
- 21.Nagasawa K, Kitamura K, Yasui A, Nimura Y, Ikeda K, Hirai M, Matsukage A, Nakanishi M. Identification and characterization of human DNA polymerase beta 2, a DNA polymerase beta -related enzyme. J Biol Chem. 2000;275:31233–31238. doi: 10.1074/jbc.M004263200. [DOI] [PubMed] [Google Scholar]
- 22.Bebenek K, Garcia-Diaz M, Patishall SR, Kunkel TA. Biochemical properties of Saccharomyces cerevisiae DNA polymerase IV. J Biol Chem. 2005;280:20051–20058. doi: 10.1074/jbc.M501981200. [DOI] [PubMed] [Google Scholar]
- 23.McInnis M, O'Neill G, Fossum K, Reagan MS. Epistatic analysis of the roles of the RAD27 and POL4 gene products in DNA base excision repair in S. cerevisiae. DNA repair. 2002;1:311–315. doi: 10.1016/s1568-7864(02)00007-1. [DOI] [PubMed] [Google Scholar]
- 24.Prasad R, Widen SG, Singhal RK, Watkins J, Prakash L, Wilson SH. Yeast open reading frame YCR14C encodes a DNA beta-polymerase-like enzyme. Nucleic Acids Res. 1993;21:5301–5307. doi: 10.1093/nar/21.23.5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sadoff BU, Heath-Pagliuso S, Castano IB, Zhu Y, Kieff FS, Christman MF. Isolation of mutants of Saccharomyces cerevisiae requiring DNA topoisomerase I. Genetics. 1995;141:465–479. doi: 10.1093/genetics/141.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castano IB, Heath-Pagliuso S, Sadoff BU, Fitzhugh DJ, Christman MF. A novel family of TRF (DNA topoisomerase I-related function) genes required for proper nuclear segregation. Nucleic Acids Res. 1996;24:2404–2410. doi: 10.1093/nar/24.12.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edwards S, Li CM, Levy DL, Brown J, Snow PM, Campbell JL. Saccharomyces cerevisiae DNA polymerase epsilon and polymerase sigma interact physically and functionally, suggesting a role for polymerase epsilon in sister chromatid cohesion. Mol Cell Biol. 2003;23:2733–2748. doi: 10.1128/MCB.23.8.2733-2748.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walowsky C, Fitzhugh DJ, Castano IB, Ju JY, Levin NA, Christman MF. The topoisomerase-related function gene TRF4 affects cellular sensitivity to the antitumor agent camptothecin. J Biol Chem. 1999;274:7302–7308. doi: 10.1074/jbc.274.11.7302. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z, Castano IB, Adams C, Vu C, Fitzhugh D, Christman MF. Structure/function analysis of the Saccharomyces cerevisiae Trf4/Pol sigma DNA polymerase. Genetics. 2002;160:381–391. doi: 10.1093/genetics/160.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castano IB, Brzoska PM, Sadoff BU, Chen H, Christman MF. Mitotic chromosome condensation in the rDNA requires TRF4 and DNA topoisomerase I in Saccharomyces cerevisiae. Genes Dev. 1996;10:2564–2576. doi: 10.1101/gad.10.20.2564. [DOI] [PubMed] [Google Scholar]
- 31.Carson DR, Christman MF. Evidence that replication fork components catalyze establishment of cohesion between sister chromatids. Proc Natl Acad Sci U S A. 2001;98:8270–8275. doi: 10.1073/pnas.131022798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uhlmann F. Chromosome cohesion: a polymerase for chromosome bridges. Curr Biol. 2000;10:R698–700. doi: 10.1016/s0960-9822(00)00709-0. [DOI] [PubMed] [Google Scholar]
- 33.Wang Z, Castano IB, De Las Penas A, Adams C, Christman MF. Pol kappa: A DNA polymerase required for sister chromatid cohesion. Science. 2000;289:774–779. doi: 10.1126/science.289.5480.774. [DOI] [PubMed] [Google Scholar]
- 34.Haracska L, Johnson RE, Prakash L, Prakash S. Trf4 and Trf5 proteins of Saccharomyces cerevisiae exhibit poly(A) RNA polymerase activity but no DNA polymerase activity. Mol Cell Biol. 2005;25:10183–10189. doi: 10.1128/MCB.25.22.10183-10189.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saitoh S, Chabes A, McDonald WH, Thelander L, Yates JR, Russell P. Cid13 is a cytoplasmic poly(A) polymerase that regulates ribonucleotide reductase mRNA. Cell. 2002;109:563–573. doi: 10.1016/s0092-8674(02)00753-5. [DOI] [PubMed] [Google Scholar]
- 36.Kadaba S, Wang X, Anderson JT. Nuclear RNA surveillance in Saccharomyces cerevisiae: Trf4p-dependent polyadenylation of nascent hypomethylated tRNA and an aberrant form of 5S rRNA. Rna. 2006;12:508–521. doi: 10.1261/rna.2305406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LaCava J, Houseley J, Saveanu C, Petfalski E, Thompson E, Jacquier A, Tollervey D. RNA degradation by the exosome is promoted by a nuclear polyadenylation complex. Cell. 2005;121:713–724. doi: 10.1016/j.cell.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 38.Vanacova S, Wolf J, Martin G, Blank D, Dettwiler S, Friedlein A, Langen H, Keith G, Keller W. A new yeast poly(A) polymerase complex involved in RNA quality control. PLoS Biol. 2005;3:e189. doi: 10.1371/journal.pbio.0030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wyers F, Rougemaille M, Badis G, Rousselle JC, Dufour ME, Boulay J, Regnault B, Devaux F, Namane A, Seraphin B, Libri D, Jacquier A. Cryptic pol II transcripts are degraded by a nuclear quality control pathway involving a new poly(A) polymerase. Cell. 2005;121:725–737. doi: 10.1016/j.cell.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 40.Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- 41.Longtine MS, McKenzie A, 3rd, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 42.Levin JD, Johnson AW, Demple B. Homogeneous Escherichia coli endonuclease IV. Characterization of an enzyme that recognizes oxidative damage in DNA. J Biol Chem. 1988;263:8066–8071. [PubMed] [Google Scholar]
- 43.Wong D, Demple B. Modulation of the 5′-deoxyribose-5-phosphate lyase and DNA synthesis activities of mammalian DNA polymerase beta by apurinic/apyrimidinic endonuclease 1. J Biol Chem. 2004;279:25268–25275. doi: 10.1074/jbc.M400804200. [DOI] [PubMed] [Google Scholar]
- 44.DeMott MS, Beyret E, Wong D, Bales BC, Hwang JT, Greenberg MM, Demple B. Covalent trapping of human DNA polymerase beta by the oxidative DNA lesion 2-deoxyribonolactone. J Biol Chem. 2002;277:7637–7640. doi: 10.1074/jbc.C100577200. [DOI] [PubMed] [Google Scholar]
- 45.Hoffman GA, Garrison TR, Dohlman HG. Analysis of RGS proteins in Saccharomyces cerevisiae. Methods Enzymol. 2002;344:617–631. doi: 10.1016/s0076-6879(02)44744-1. [DOI] [PubMed] [Google Scholar]
- 46.Piersen CE, McCullough AK, Lloyd RS. AP lyases and dRPases: commonality of mechanism. Mutat Res. 2000;459:43–53. doi: 10.1016/s0921-8777(99)00054-3. [DOI] [PubMed] [Google Scholar]
- 47.Piersen CE, Prasad R, Wilson SH, Lloyd RS. Evidence for an imino intermediate in the DNA polymerase beta deoxyribose phosphate excision reaction. J Biol Chem. 1996;271:17811–17815. doi: 10.1074/jbc.271.30.17811. [DOI] [PubMed] [Google Scholar]
- 48.Faure V, Saparbaev M, Dumy P, Constant JF. Action of multiple base excision repair enzymes on the 2′-deoxyribonolactone. Biochem Biophys Res Commun. 2005;328:1188–1195. doi: 10.1016/j.bbrc.2005.01.082. [DOI] [PubMed] [Google Scholar]
- 49.Hashimoto M, Greenberg MM, Kow YW, Hwang JT, Cunningham RP. The 2-deoxyribonolactone lesion produced in DNA by neocarzinostatin and other damaging agents forms cross-links with the base-excision repair enzyme endonuclease III. J Am Chem Soc. 2001;123:3161–3162. doi: 10.1021/ja003354z. [DOI] [PubMed] [Google Scholar]
- 50.Haracska L, Prakash L, Prakash S. A mechanism for the exclusion of low-fidelity human Y-family DNA polymerases from base excision repair. Genes Dev. 2003;17:2777–2785. doi: 10.1101/gad.1146103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gellon L, Barbey R, Auffret van der Kemp P, Thomas D, Boiteux S. Synergism between base excision repair, mediated by the DNA glycosylases Ntg1 and Ntg2, and nucleotide excision repair in the removal of oxidatively damaged DNA bases in Saccharomyces cerevisiae. Mol Genet Genomics. 2001;265:1087–1096. doi: 10.1007/s004380100507. [DOI] [PubMed] [Google Scholar]
- 52.Hanna M, Chow BL, Morey NJ, Jinks-Robertson S, Doetsch PW, Xiao W. Involvement of two endonuclease III homologs in the base excision repair pathway for the processing of DNA alkylation damage in Saccharomyces cerevisiae. DNA Repair (Amst) 2004;3:51–59. doi: 10.1016/j.dnarep.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 53.Thomas D, Scot AD, Barbey R, Padula M, Boiteux S. Inactivation of OGG1 increases the incidence of G . C-->T . A transversions in Saccharomyces cerevisiae: evidence for endogenous oxidative damage to DNA in eukaryotic cells. Mol Gen Genet. 1997;254:171–178. doi: 10.1007/s004380050405. [DOI] [PubMed] [Google Scholar]
- 54.Koc A, Wheeler LJ, Mathews CK, Merrill GF. Hydroxyurea arrests DNA replication by a mechanism that preserves basal dNTP pools. J Biol Chem. 2004;279:223–230. doi: 10.1074/jbc.M303952200. [DOI] [PubMed] [Google Scholar]
- 55.Wu X, Wang Z. Relationships between yeast Rad27 and Apn1 in response to apurinic/apyrimidinic (AP) sites in DNA. Nucleic Acids Res. 1999;27:956–962. doi: 10.1093/nar/27.4.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Z, Wu X, Friedberg EC. Molecular mechanism of base excision repair of uracil-containing DNA in yeast cell-free extracts. J Biol Chem. 1997;272:24064–24071. doi: 10.1074/jbc.272.38.24064. [DOI] [PubMed] [Google Scholar]
- 57.Wu X, Braithwaite E, Wang Z. DNA ligation during excision repair in yeast cell-free extracts is specifically catalyzed by the CDC9 gene product. Biochemistry. 1999;38:2628–2635. doi: 10.1021/bi982592s. [DOI] [PubMed] [Google Scholar]
- 58.Boiteux S, Guillet M. Abasic sites in DNA: repair and biological consequences in Saccharomyces cerevisiae. DNA Repair (Amst) 2004;3:1–12. doi: 10.1016/j.dnarep.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 59.Guzder SN, Torres-Ramos C, Johnson RE, Haracska L, Prakash L, Prakash S. Requirement of yeast Rad1-Rad10 nuclease for the removal of 3′-blocked termini from DNA strand breaks induced by reactive oxygen species. Genes Dev. 2004;18:2283–2291. doi: 10.1101/gad.1232804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Swanson RL, Morey NJ, Doetsch PW, Jinks-Robertson S. Overlapping specificities of base excision repair, nucleotide excision repair, recombination, and translesion synthesis pathways for DNA base damage in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:2929–2935. doi: 10.1128/mcb.19.4.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun X, Thrower D, Qiu J, Wu P, Zheng L, Zhou M, Bachant J, Wilson DM, 3rd, Shen B. Complementary functions of the Saccharomyces cerevisiae Rad2 family nucleases in Okazaki fragment maturation, mutation avoidance, and chromosome stability. DNA Repair (Amst) 2003;2:925–940. doi: 10.1016/s1568-7864(03)00093-4. [DOI] [PubMed] [Google Scholar]
- 62.Johnson RE, Kovvali GK, Prakash L, Prakash S. Role of yeast Rth1 nuclease and its homologs in mutation avoidance, DNA repair, and DNA replication. Curr Genet. 1998;34:21–29. doi: 10.1007/s002940050362. [DOI] [PubMed] [Google Scholar]
- 63.Saxowsky TT, Matsumoto Y, Englund PT. The mitochondrial DNA polymerase beta from Crithidia fasciculata has 5′-deoxyribose phosphate (dRP) lyase activity but is deficient in the release of dRP. J Biol Chem. 2002;277:37201–37206. doi: 10.1074/jbc.M206654200. [DOI] [PubMed] [Google Scholar]
- 64.Bailly V, Verly WG. The multiple activities of Escherichia coli endonuclease IV and the extreme lability of 5′-terminal base-free deoxyribose 5-phosphates. Biochem J. 1989;259:761–768. doi: 10.1042/bj2590761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shen X, Woodgate R, Goodman MF. Lyase activities intrinsic to Escherichia coli polymerases IV and V. DNA Repair (Amst) 2005;4:1368–1373. doi: 10.1016/j.dnarep.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 66.Pinz KG, Bogenhagen DF. Characterization of a catalytically slow AP lyase activity in DNA polymerase gamma and other family A DNA polymerases. J Biol Chem. 2000;275:12509–12514. doi: 10.1074/jbc.275.17.12509. [DOI] [PubMed] [Google Scholar]
- 67.Graves RJ, Felzenszwalb I, Laval J, O′Connor TR. Excision of 5′-terminal deoxyribose phosphate from damaged DNA is catalyzed by the Fpg protein of Escherichia coli. J Biol Chem. 1992;267:14429–14435. [PubMed] [Google Scholar]
- 68.Girard PM, Guibourt N, Boiteux S. The Ogg1 protein of Saccharomyces cerevisiae: a 7,8-dihydro-8-oxoguanine DNA glycosylase/AP lyase whose lysine 241 is a critical residue for catalytic activity. Nucleic Acids Res. 1997;25:3204–3211. doi: 10.1093/nar/25.16.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tchou J, Bodepudi V, Shibutani S, Antoshechkin I, Miller J, Grollman AP, Johnson F. Substrate specificity of Fpg protein. Recognition and cleavage of oxidatively damaged DNA. J Biol Chem. 1994;269:15318–15324. [PubMed] [Google Scholar]
- 70.Houseley J, Tollervey D. Yeast Trf5p is a nuclear poly(A) polymerase. EMBO Rep. 2006;7:205–211. doi: 10.1038/sj.embor.7400612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pinz KG, Bogenhagen DF. The influence of the DNA polymerase gamma accessory subunit on base excision repair by the catalytic subunit. DNA Repair (Amst) 2006;5:121–128. doi: 10.1016/j.dnarep.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 72.Lundin C, North M, Erixon K, Walters K, Jenssen D, Goldman AS, Helleday T. Methyl methanesulfonate (MMS) produces heat-labile DNA damage but no detectable in vivo DNA double-strand breaks. Nucleic Acids Res. 2005;33:3799–3811. doi: 10.1093/nar/gki681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988;16:10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]


