Abstract
The small GTPase RhoA is activated by the angiotensin II (AngII) type 1 receptor (AT1R), which is part of the local renin-angiotensin system that is involved in podocyte injury preceding glomerular crescent formation. We demonstrated previously that inhibition of AT1R protects against crescentic glomerular injury in Fc receptor-deficient mice (γ−/−) with anti-glomerular basement membrane antibody-induced glomerulonephritis (anti-GBM GN). Here, we hypothesized that the RhoA kinase inhibitor, fasudil, attenuates AT1R-dependent crescentic GN. We examined anti-GBM GN in γ−/− mice with or without fasudil treatment, and further investigated the underlying mechanisms in cultured differentiated podocytes and leukocytes. Fasudil markedly attenuated crescentic GN with a significant decrease in proteinuria and hematuria, infiltration of T cells and monocytes/macrophages as well as their local proliferation, and preservation of podocyte-specific proteins, including WT-1 and nephrin, in glomeruli. In vitro studies showed that AngII induced the down-regulation of both nephrin and WT-1 expression in podocytes, which was reversed by fasudil in a dose-dependent manner. Additionally, fasudil blocked the AngII-induced migration of both macrophages and T cells. Furthermore, we also examined lipopolysaccharide-induced nephrotic syndrome in severe combined immunodeficiency disease mice and found that fasudil failed to block the development of proteinuria because of a B7-1-dependent podocyte injury. In conclusion, fasudil treatment prevents crescent formation and disease progression in anti-GBM GN by preventing AngII-induced podocyte injury and leukocyte migration.
Crescentic glomerulonephritis (GN) is a manifestation of severe glomerular injury with a poor clinical outcome.1 It is observed in a variety of GN of immune origin, in particular anti-glomerular basement membrane (anti-GBM) disease2 and class IV lupus nephritis.3 However, the pathogenesis of glomerular crescents remains unknown. It is generally considered that epithelial cells predominated in crescents of patients during the early phase of disease, whereas late phases were characterized by rupture of the basement membrane of Bowman’s capsule and subsequent infiltration of cellular crescents, predominantly by macrophages.4,5,6,7,8 This picture is also confirmed in animal models with anti-GBM glomerulonephritis (anti-GBM GN).9,10 However, origin of the crescentic epithelial cells is still controversial. Moeller and colleagues11 recently demonstrated by podocyte-specific 2.5P-Cre mouse with anti-GBM GN that visceral glomerular epithelial cells, podocytes, adhered to the parietal basement membrane and populated glomerular crescents during the early phase of cellular crescents. On the other hand, Tipping and Holdsworth1 demonstrated critical roles of macrophages and T cells in the crescent formation in this disease by their series of elegant studies. Therefore, podocytes and macrophages/T cells may be important players in the progression of crescent formation, and should be the targets for the treatments of crescentic GN.
The podocyte is a highly differentiated cell of the kidney glomerulus that forms multiple interdigitating foot processes.12 The neighboring foot processes derived from different podocyte plasma membranes are connected by a continuous membrane-like structure called a slit diaphragm (SD)13 or slit membrane.14 Several SD proteins, whose interactions are regulated with or without the actin cytoskeleton, have recently been identified.15 It is widely accepted that podocyte dysfunction is involved in the development of proteinuria in certain kidney diseases such as minimal change nephrotic syndrome, focal segmental glomerulosclerosis, and membranous nephropathy. In addition, cytoskeletal changes of podocytes are critically involved in the pathogenesis of GN.16 Blocking cytoskeleton rearrangement using a RhoA kinase inhibitor prevented the activation of nuclear factor (NF)-κB and Ap-1, suggesting a direct link between cytoskeleton and transcriptional regulation in podocytes.16
Small GTPases of the Rho family are key regulators of the cellular cytoskeleton. RhoA is involved in the regulation of stress fiber and focal adhesion formation, cell morphology, cell aggregation, cadherin-mediated cell-cell adhesion, cell motility, cytokinesis, membrane ruffling, neurite retraction, microvilli formation, and smooth muscle contraction.17,18,19 Fasudil and Y-27632 specifically inhibit RhoA kinase activity by competing for ATP binding, and are useful tools for evaluating the cellular function of RhoA kinase. RhoA functions in response to various heterotrimeric G protein-coupled receptor agonists.20 Agonists such as lysophosphatidic acid, thrombin, and thromboxane A2 induce cytoskeletal alteration through Gα12 and/or Gα13 subunits in nonmuscle cells.21 Receptors for the vasoconstrictive agents, angiotensin II (AngII), endothelin, and vasopressin were also recently linked to Gα12/13 and Gq activation.21
Clinical and experimental studies have implicated AngII in the regulation of expression of adhesion molecules in many diseases.22 In addition, AngII enhances chemokine expression in various tissues and cell types.23 In particular, immunocompetent cells are equipped with components of the renin-angiotensin system (RAS) and contribute to AngII generation.22,23,24 These findings suggest that RAS may influence the prognosis of many renal diseases in association with activation of the immune system. Furthermore, Th1-predominant immune responses promote crescent formation in the experimental models.1,25
We demonstrated previously the marked protective action of an AngII type I receptor (AT1R) antagonist against crescentic glomerular injury in FcR-deficient mice (γ−/− mice) with anti-GBM GN.26 Indeed, anti-GBM GN was completely attenuated in bone marrow chimeras of γ−/− and AT1R−/− mice.25 These protective outcomes were linked to attenuated infiltration of macrophages and T cells into glomeruli.26,27 Based on this, the purpose of the present study is to examine whether a RhoA kinase inhibitor ameliorates the AngII-dependent crescentic glomerular injury via podocyte protection or prevention of leukocyte recruitment.
Materials and Methods
Animals
The γ−/− mice were generated by homologous recombination, as described previously,26,28 and were fed regular chow. All animal procedures were conducted in accordance with the guidelines for the care and use of laboratory animals approved by Juntendo University School of Medicine. All experiments used age-matched 8- to 10-week-old female animals. Mice were divided into three groups, anti-GBM GN without treatment (untreated group; no Tx group), fasudil-treated anti-GBM GN (fasudil-treated group; Tx group), and delayed fasudil-treated anti-GBM GN (delayed fasudil-treated group; delayed Tx group) groups. Blood pressure was measured by the tail-cuff method using an automatic sphygmomanometer (Softron, Tokyo, Japan) before injection with nephrotoxic serum (NTS) (Kyowa Hakko Kogyo Co., Tokyo, Japan) and before sacrifice (day 14). Before each measurement, the mice were placed in a Plexiglas cage at 37°C for 5 minutes.
Experimental Protocol for Anti-GBM GN
The method used for preparation of NTS has been described previously.26 Anti-GBM GN was induced by a single intravenous injection of NTS through the tail vein of mice preimmunized with rabbit IgG and complete Freund adjuvant 5 days earlier. We administered NTS at a dose of 200 μl/20 g body weight to both groups. The selection of this dose was based on results of preliminary studies, which showed that it was sufficient to induce proteinuria and severe renal damage in γ−/− mice. None of the mice developed anaphylactic symptoms after injection of NTS. Urinary protein was determined by the Knight’s method as described previously.26 Blood samples were taken from the orbital venous plexus before sacrifice. Kidneys were perfused with cold saline and removed under general anesthesia, and then either frozen in liquid nitrogen or fixed in 4% paraformaldehyde. Fasudil (Asahi Kasei Pharma Co., Tokyo, Japan) was selected to inhibit RhoA kinase because it is the only such inhibitor practically available for long-term in vivo use.29,30 In the treatment group, fasudil (10 mg/kg body weight/day) was intraperitoneally administered daily throughout the experiment from 4 days before (n = 6) (fasudil-treated group) or 7days after (n = 5) (delayed fasudil-treated group) NTS injection. To determine the number of proliferating cells, mice were injected intraperitoneally with BrdU (5-bromo-2′-deoxyuridine; Calbiochem, La Jolla, CA) at 2 mg/20 g body weight at 16, 8, and 4 hours before sacrifice.31
Cell Culture
An immortalized murine podocyte clone was kindly provided by Dr. Peter Mundel (Mount Sinai School of Medicine, New York, NY). The preparation and characterization of these cells have been described in detail previously.32 Podocytes were maintained in RPMI 1640 medium (Sigma, Tokyo, Japan) supplemented with 10% heat-inactivated fetal calf serum (Life Technologies, Inc., Gaithersburg, MD), 100 U/ml penicillin G, and 100 μg/ml streptomycin in a humidified atmosphere with 5% CO2. During propagation, the culture medium was supplemented with 10 U/ml recombinant mouse interferon-γ (PeproTech EC, London, UK) to enhance the expression of T-antigen, and the cells were cultured at 33°C (permissive conditions). Podocytes were cultured on type I collagen-coated dishes (Asahi Techno Glass Co., Tokyo, Japan) at 37°C (nonpermissive conditions) to induce differentiation without interferon-γ. At least 10 days were required to induce differentiation. Podocytes cultured for 10 to 20 passages were used in all experiments. For starvation conditions, podocytes were cultured in fetal calf serum-free medium containing 5% bovine serum albumin for 12 hours after differentiation. Jurkat T cells (ATCC TIB-152; Sumisho Pharma, Tokyo, Japan) and human immature monocyte/macrophage U-937 cells (ATCC U-937, Sumisho Pharma) were cultured and then maintained in RPMI 1640 medium (Sigma) supplemented with 10% heat-inactivated fetal calf serum (Life Technologies, Inc.), 100 U/ml penicillin G, and 100 μg/ml streptomycin in a humidified atmosphere with 5% CO2 at 37°C.
Chemotaxis Assay
The chemotactic activity induced by AngII was evaluated in 24-well Transwell chemotaxis chambers (Costar Corning, Rochester, NY) as described previously.33 The lower wells were loaded in triplicate with 600 μl of RPMI 1640 medium containing various concentrations of fasudil and AngII. The upper compartments were loaded with 100 μl of cell suspension containing 5 × 105 Jurkat or U-937 cells, which were starved for 12 hours beforehand. The chambers were incubated with or without fasudil at 37°C for 3 hours, and then AngII was added to each chamber at the same temperature for 6 hours to assess chemotaxis of the T cells and macrophages. Migrating cells in the lower compartment were counted by flow cytometry.27 Chemotaxis data were expressed relative to the number of migrating cells in the control (no AngII or fasudil) for macrophages and in the cells treated with AngII at a concentration of 10−8 mol/L for T cells.
Proliferation Assay
Cell proliferation was evaluated in 24-well chambers (Asahi Techno Glass Co.) loaded in triplicate with 100 μl of cell suspension containing 2 × 105 Jurkat or U-937 cells, which were starved for 12 hours beforehand, followed by 600 μl of RPMI 1640. The chambers were incubated with or without fasudil treatment at 37°C for 3 hours, and then AngII was added to each chamber at the same temperature for 24 hours to assess proliferative activities. Cell count was determined by flow cytometry.25 Some of the cultured cells were also used for immunohistochemistry to examine cytoskeletal change. Proliferation data were expressed relative to the average activity in control cells.
Wound Healing Assay
Wound healing assays were conducted exactly as previously described34,35 with some modification. Differentiated wild-type podocytes (each 5 × 105) were seeded on type I collagen-coated six-well plates and wounded with a sterile 200-μl pipette tip. Wounded monolayers were washed with phosphate-buffered saline (PBS) and incubated in RPMI 1640 medium. Time-lapse images were taken with a ×10 phase-contrast objective on a microscope (Nikon, Tokyo, Japan) at 0 and 24 hours. At the indicated time points, the monolayers were photographed using the grid as a marker, and the wound width (μm) was measured at each time point using Leica FW4000 software (Leica, Wetzlar, Germany). Migratory rates were calculated as (A − B)/A × 100%, with A and B reflecting the width of the wound at 0 or 24 hours, respectively. The data represent the mean ± SEM of six independent experiments.
Histology and Immunohistochemistry
Kidney sections (3-μm thickness) were fixed in 4% paraformaldehyde and stained with periodic acid-Schiff reagent or Masson Trichrome to assess histological changes by light microscopy. Immunohistochemistry was performed on 3-μm-thick formalin-fixed paraffin sections of the kidney. Sections were first deparaffinized and rehydrated before an overnight incubation with mouse anti-human WT-1 (1:100; DAKO, Tokyo, Japan) or polyclonal guinea pig anti-mouse nephrin (1:100; Progen, Heidelberg, Germany). For WT-1, the sections were then incubated with horseradish peroxidase-labeled antibody as secondary antibody (polymer-horseradish peroxidase-labeled anti-mouse antibody; Dako Cytomation, Carpinteria, CA). For nephrin, the sections were reacted with horseradish peroxidase-labeled rabbit anti-guinea pig antibody as secondary antibody (Zymed, San Francisco, CA) at room temperature for 1 hour. Immunoreactivity was detected using an enhanced DAB kit (Dako Cytomation). Nephrin-positive areas in each glomerulus were quantified with KS-400 image analysis software (Carl Zeiss MicroImaging Japan, Tokyo, Japan). Double staining of F4/80 and BrdU was performed with phycoerythrin-labeled anti-mouse F4/80 (1:50; Cedarlane, Ontario, Canada) and fluorescein isothiocyanate-labeled anti-mouse BrdU antibodies (1:100; Santa Cruz Biotechnology, Santa Cruz, CA). Sclerotic change was defined by an increase in fibrillar material and hyalinosis containing acellular, structureless material composed of glycoproteins and lipids.36 We identified that the sclerotic glomeruli include sclerotic changes of more than 50% within a glomerulus. We checked at least 200 glomeruli in each animal by Masson Trichrome staining and assessed the percentage of pathological glomeruli.
The frozen kidney sections were prepared using a cryostat and stained with the following antibodies after acetone fixation: monoclonal rat anti-mouse CD4 (L3T4) antibody (BD PharMingen, San Jose, CA) or monoclonal rat anti-mouse CD8a (Ly-2) antibody (BD PharMingen) for 1 hour, and then horseradish peroxidase-labeled goat anti-rat IgG antibody (Simplestain MAX-PO, rat; Nichirei, Tokyo, Japan) as a secondary antibody for 1 hour. Bound antibodies were detected using an enhanced DAB kit (Dako Cytomation) as mentioned above. Cytoskeletal changes with or without fasudil treatment were analyzed by immunofluorescence. For in vitro cellular staining, Jurkat or U-937 cells were incubated on the sterile cover glasses (IWAKI brand, Scitech Division, Asahi Techno Glass). These cover glasses were directly fixed with 2% paraformaldehyde and 4% sucrose, permeabilized with 0.03% Triton X-100 for 5 minutes, and then blocked with 2% bovine serum albumin, 2% fetal calf serum, and 0.2% fish gelatin in PBS. The cells were incubated with rhodamine phalloidin (Molecular Probes, Tokyo, Japan) for 1 hour and then mounted in Prolong antifade mounting media (Thermo Shandon, Pittsburgh, PA). Fluorescence images were recorded using a confocal microscope (FV-1000; Olympus, Tokyo, Japan) with excitation at 488 nm and detection at 500 to 600 nm for fluorescein isothiocyanate, and with excitation at 543 nm and long-pass detection at 555 to 655 nm for tetramethyl-rhodamine isothiocyanate.
RNA Preparation and Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)/Real-Time Quantitative Analysis (ABI Method)
Total RNA was isolated from the frozen kidney tissues or cultured podocytes (3 × 106 cells) on 15-cm dishes37 using TRIZOL reagent (Life Technologies Inc., Carlsbad, CA) according to the instructions provided by the manufacturer. One-μg aliquots of total RNA were reverse-transcribed using oligo (dT) primers (Life Technologies) and reverse transcriptase, Superscript II (Life Technologies). The product was analyzed by real-time PCR using SYBR Green (Applied Biosystems, Warrington, UK) technology on a PRISM7500 instrument (Applied Biosystems, Foster City, CA). For most of the transcripts, forward and reverse primers were identified according to the primer information available from UniSTS at the National Center for Biotechnology Information. Primer sequences are provided below. The reaction mixture consisted of 1 μl of diluted template, 12.5 μl of SYBR Green PCR Master Mix (Applied Biosystems), and 0.25 μmol/L forward and reverse primers. PCR was performed with an initial activation of DNA polymerase at 95°C for 10 minutes, then 30 to 50 cycles of denaturation at 95°C for 15 seconds, and annealing and extension at 60°C for 1 minute. Relative quantification was accompanied by measurement of threshold cycles and use of a standard curve. Gene expression of the target sequence was normalized to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Transcript level in the control group was arbitrarily expressed as 100.
Primers
We used AF191090 (forward: 5′-CCCCAACATCGACTTCACTT-3′ reverse: 5′-GGCTGCAGACACATTAGCAA-3′) as nephrin primer probes. M32599 (forward: 5′-ACCCAGAAGACTGTGGATGG-3′, reverse: 5′-CACATTGGGGGTAGGAACAC-3′) were used as GAPDH primer probes. NM144783 (forward: 5′-CAAGGACTGCGAGAGAAGGT-3′, reverse: 5′-CCTGGTGTGGGTCTTCAGAT-3′) were used as WT-1 primer probes.
Western Blotting
To further examine changes in SD protein in this disease, Western blotting for nephrin, podocin, and CD2AP was performed with protein from isolated glomeruli with anti-GBM GN mice at 14 days in both groups (n = 4, untreated group; n = 5, fasudil-treated group). The proteins were separated on 7.5 to 12% sodium dodecyl sulfate-polyacrylamide gels and then transferred to nitrocellulose membrane (Novex, San Diego, CA) by semidry blotting. Guinea pig anti-mouse nephrin (1:1000; Progen), polyclonal anti-mouse CD2AP (1:500, Santa Cruz Biotechnology), rabbit anti-mouse podocin (1:500, kindly provided from Dr. Peter Mundel), and rabbit anti-mouse GAPDH (1:5000; Abcam Inc., Cambridge, MA) were used as primary antibodies. Horseradish peroxidase-conjugated secondary antibodies were used at 1:20,000. Blots were visualized by the enhanced chemiluminescence reaction (Amersham Life Science, Piscataway, NJ).
Lipopolysaccharide (LPS)-Induced Nephrotic Syndrome Model on SCID Mice
LPS (from Escherichia coli, Sigma)-induced nephrotic syndrome were induced in 8-week-old SCID mice (SPF/VAF C.B-17/1crCrj-scid; Charles River Laboratories Japan Inc., Tokyo, Japan) as previously described.38 We prepared two models with a low (100 μl, 1 mg/ml LPS in sterile LPS-free PBS) and a high (200 μl) dose of LPS. Four days before LPS injection fasudil (10 mg/kg/day i.p.) treatment was started. Because SCID mice with high dose failed into lethal starts within 24 hours, we evaluated proteinuria at 24 hours by Knight’s method in the low-dose model. Survival rate was evaluated in the high-dose model at day 3.
Statistical Analysis
Data are presented as mean ± SEM. Statistically significant differences in mean values were tested by analysis of variance. A P value of less than 0.05 was considered statistically significant. The data were analyzed using StatView statistical software (Hulinks, Tokyo, Japan).
Results
Fasudil Prevents Blood Urea Nitrogen Elevation Independently of Blood Pressure
Blood pressure and body weight were not significantly different in the fasudil-treated and untreated groups (Table 1). Fasudil treatment significantly suppressed elevation of blood urea nitrogen (P < 0.01).
Table 1.
Systolic Blood Pressure/Diastolic Blood Pressure, Body Weight, and Blood Urea Nitrogen (BUN) in Mice Treated or Untreated with Fasudil
| No Tx group (n = 6) | Tx group (n = 6) | |
|---|---|---|
| Blood pressure (mm Hg, before treatment) | 108.1 ± 9.4/78.2 ± 7.8 (88.1 ± 8.0) | 118.9 ± 13.1/82.4 ± 16.7 (94.5 ± 14.9) |
| Blood pressure (mm Hg, day 14) | 116.5 ± 4.8/86.4 ± 5.5 (97.7 ± 3.4) | 109.7 ± 12.1/80.4 ± 7.7 (90.1 ± 8.2) |
| Body weight (g, before treatment) | 23.2 ± 1.3 | 22.6 ± 1.7 |
| Body weight (g, day 14) | 23.2 ± 1.4 | 23.3 ± 1.5 |
| BUN (mg/dl, day 14) | 84.4 ± 2.6 | 26.1 ± 4.2* |
Data are mean ± SEM. Untreated group (no Tx group): anti-GBM GN on γ−/− mice without treatment (n = 6). Fasudil-treated group (Tx group): anti-GBM GN on γ−/− mice treated with fasudil (n = 6).
P < 0.01, compared with the untreated group.
Anti-GBM NTS Induces Crescentic GN with Proteinuria and Hematuria in γ−/− Mice
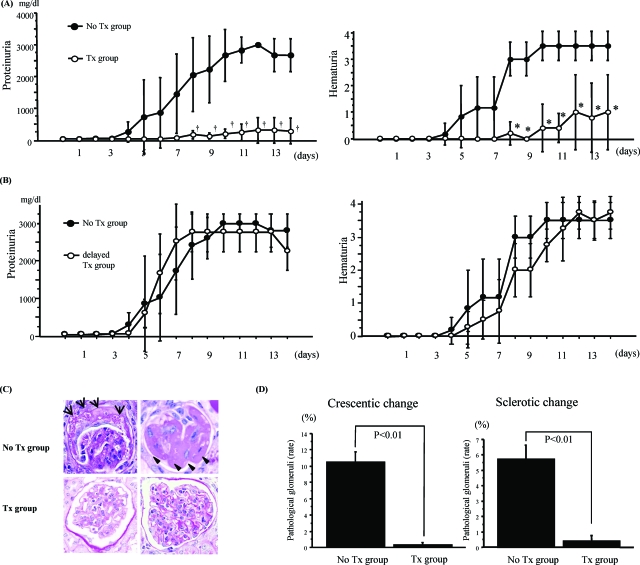
In γ−/− mice, proteinuria gradually increased from day 3 after NTS injection and continued beyond day 7 (Figure 1A, left). Sharp rises in proteinuria and hematuria appeared at days 4 to 12 in the untreated group. Mesangial proliferation and glomerular enlargement with monocyte/macrophage infiltration were observed after day 7. Crescents were also detected in glomeruli after day 14 (Figure 1C, top left).
Figure 1.
Pretreatment of fasudil prevented renal injury in γ−/− mice with anti-GBM GN. A: Disease course in γ−/− mice with or without fasudil treatment (10 mg/kg i.p. daily) starting from 4 days before NTS injection. Proteinuria increased in the untreated group, but decreased significantly with fasudil treatment (P < 0.01) (left). Hematuria was measured by stick analysis. Fasudil treatment also significantly decreased hematuria (P < 0.01) (right). Data are mean ± SEM values of six mice in each group. †P < 0.01, *P < 0.01. B: Disease course in γ−/− mice with or without fasudil treatment (10 mg/kg i.p. daily) starting from 7 days after NTS injection. Fasudil did not improve proteinuria and hematuria in each group. Data are mean ± SEM value of five mice in each group. C: Fasudil pretreatment attenuated crescent formation and sclerotic change in γ−/− mice with anti-GBM GN. In the untreated group, crescent formation (arrow) and sclerotic change (arrowhead) were seen in many glomeruli (top), but these effects were absent after fasudil treatment (bottom). D: Periodic acid-Schiff staining of at least 200 glomeruli allowed estimation of the number of pathological glomeruli and calculation of the ratio of injured glomeruli. Sclerotic glomeruli were defined by a fibrous change occupying more than half of one glomerulus. Fasudil significantly reduced the sclerotic changes (P < 0.01) (right). Crescent formation analysis was performed in each group. Fasudil markedly reduced crescent formation (P < 0.01) (left). Data are mean ± SEM values of six mice in each group. Original magnifications, ×400.
Pretreatment of Fasudil Prevents Proteinuria, Hematuria, Crescent Formation, and Sclerosis in γ−/− Mice with Anti-GBM GN
Pretreatment of fasudil could significantly suppress proteinuria and hematuria, which were seen in untreated group (Figure 1A). The incidence of severe glomerular injury was higher in the untreated group than in the fasudil-treated group. Indeed, crescent formation and mesangial proliferation with matrix expansion were clearly observed in the untreated group (Figure 1C, bottom), whereas the fasudil-treated group showed decreased crescent formation (P < 0.01) and sclerotic change (P < 0.01) (Figure 1D). In parallel with urinary findings, fasudil treatment after day7 (delayed fasudil-treated group) did not prevent progression of proteinuria and hematuria (Figure 1B) nor glomerular injury including crescentic formation and leukocyte infiltration (data not shown).
Fasudil Protects Podocyte Injury in γ−/− Mice with Anti-GBM GN
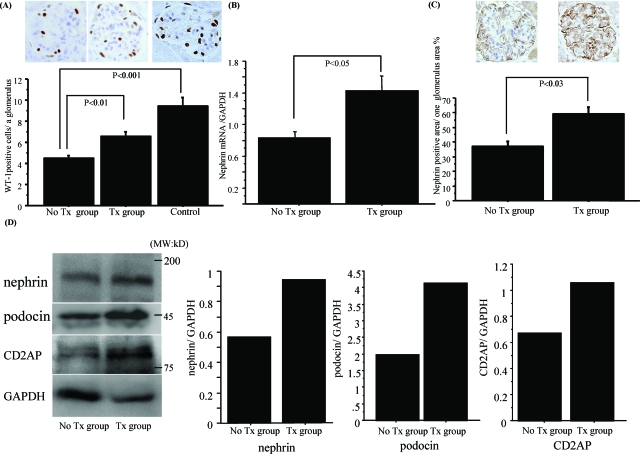
Fasudil treatment significantly protected WT-1-positive podocytes (P < 0.01) (Figure 2A). To check whether fasudil directly protected podocytes from injury, we measured nephrin mRNA expression in the kidney by real-time PCR. Nephrin mRNA in the fasudil-treated group was better preserved (P < 0.05) than that in the untreated group (Figure 2B). Immunohistochemical staining (Figure 2C) and Western blotting with isolated glomerular protein (Figure 2D) further confirmed the beneficial effect of fasudil on nephrin expression. Other SD proteins in podocytes such as podocin and CD2AP were also preserved by the treatment (Figure 2D).
Figure 2.
Fasudil treatment protected WT-1-positive podocytes and nephrin, podocin, and CD2AP expression. A: In the untreated group, WT-1-positive podocytes were decreased in number, but fasudil treatment significantly preserved these cells (P < 0.01). B: Nephrin mRNA expression was also preserved in the fasudil treatment group, but significantly decreased in the untreated group (P < 0.05). C: Nephrin staining analyzed by KS-400 software showed that fasudil treatment preserved nephrin protein expression (P < 0.03). Data are mean ± SEM values of six mice in each group. D: Nephrin, podocin, and CD2AP protein level were measured by Western blotting with protein from isolated glomeruli. Decreases of nephrin, podocin, and CD2AP expression adjusted by GAPDH were preserved by fasudil treatment. Original magnifications, ×400.
Fasudil Decreases the Number of BrdU-Positive Macrophages and CD4/CD8 Lymphocytes in γ−/− Mice with Anti-GBM GN
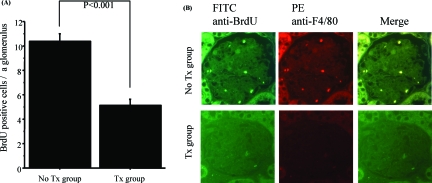
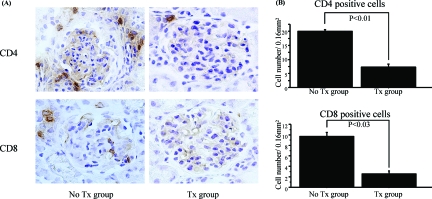
In the untreated group, BrdU-positive cells were detected in each glomerulus, whereas the fasudil-treated group showed significant decrease of these cells (P < 0.001) (Figure 3A). Double-immunofluorescence staining of F4/80 and BrdU showed that the majority of BrdU-positive cells were also F4/80-positive (Figure 3B), suggesting that fasudil decreased the number of infiltrating macrophages and their local proliferation in glomeruli. Fasudil treatment also significantly reduced the number of CD4 (P < 0.01)- or CD8 (P < 0.03)-positive cells in and around glomeruli to levels observed in the untreated group (Figure 4, A and B).
Figure 3.
Fasudil decreased BrdU-positive macrophages in glomeruli. A: Fasudil significantly decreased numbers of BrdU-positive cells in glomeruli (P < 0.001). The untreated group showed BrdU-positive cells in glomeruli. Data are mean ± SEM values of six mice in each group. B: Most of BrdU-positive cells also expressed F4/80, suggesting that most of BrdU-positive cells are mainly macrophages. Original magnifications, ×400.
Figure 4.
Fasudil significantly decreased CD4- or CD8-positive cells in renal cortex. A: Numerous CD4 (top)- or CD8 (bottom)-positive cells were noted in the untreated group, but were rarely observed in the fasudil-treated glomeruli. B: Statistical analysis of CD4- or CD8-positive cells in at least 200 fields per 0.16 mm2 (0.4 × 0.4 mm) for both groups. Fasudil significantly decreased both cell types in the cortex of the kidney. Data are mean ± SEM values of six mice in each group. Original magnifications, ×400.
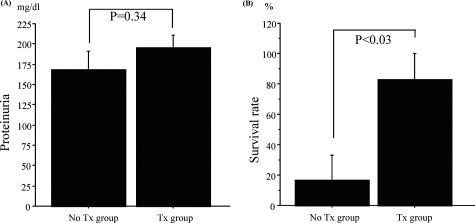
Fasudil Does Not Prevent LPS-Induced Proteinuria but Improves Survival Rate in SCID Mice
To further test the relative effects of fasudil on podocytes versus infiltrating leukocytes such as macrophages/T cells on proteinuria, we examined LPS-induced proteinuria in SCID mice. As shown in Figure 5A, fasudil could not prevent heavy proteinuria at day 1 induced by a low dose of LPS (100 μg). However, this treatment significantly improved survival rate in SCID mice with a high dose of LPS (200 μg) (Figure 5B).
Figure 5.
Fasudil improved survival rate of SCID mice with LPS injection significantly, but not proteinuria. A: Proteinuria was not improved by fasudil treatment in a low dose of LPS (100 μl) model. B: However, in a high dose of LPS (200 μl) model, fasudil significantly improved survival rate (P < 0.03). Data are mean ± SEM values of five or six mice in each group.
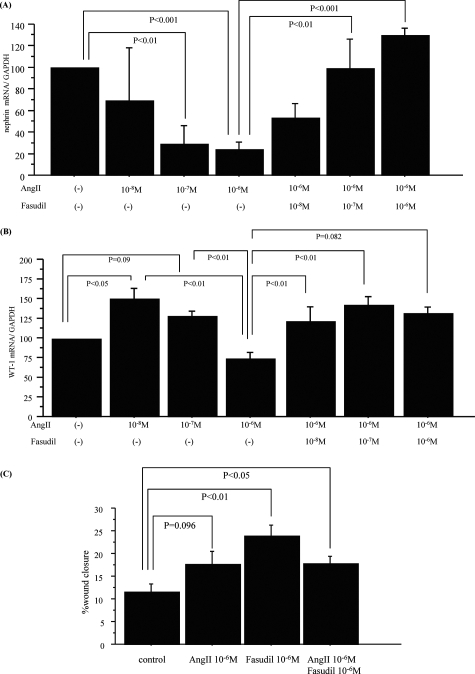
Fasudil Protects AngII-Induced Down-Regulation of Nephrin and WT-1 mRNA Expression
As shown in Figure 6A, AngII down-regulated nephrin mRNA expression in cultured podocytes, in a dose-dependent manner. Fasudil pretreatment significantly recovered the decrease by AngII. On the other hand, a high dose of AngII also decreased mRNA expression of WT-1 that was preserved by fasudil treatment, although a lower dose of AngII (10−8 mol/L) slightly enhanced the expression (Figure 6B).
Figure 6.
Fasudil protected the AngII-induced down-regulation of nephrin and WT-1 mRNA expression. A: Nephrin mRNA expression in differentiated podocytes was significantly decreased by AngII stimulation in a dose-dependent manner. AngII significantly reduced nephrin expression at 10−7 mol/L (P < 0.01) and 10−6 mol/L (P < 0.001) compared to untreated controls. Fasudil attenuated the reduction in nephrin expression induced by AngII in a dose-dependent manner at 10−7 mol/L (P < 0.01) and 10−6 mol/L (P < 0.001) fasudil. Data were expressed relative to the mean nephrin mRNA expression in untreated podocytes. B: WT-1 mRNA expression was also significantly down-regulated by high dose of AngII (10−6 mol/L) that was recovered by fasudil treatment. Data were expressed relative to the mean WT-1 mRNA expression in untreated podocytes. Data are mean ± SEM values of six procedures in each group. C: In wound-healing assays with differentiated podocytes AngII 10−6 mol/L mildly enhanced their migration (P = 0.096). Fasudil (10−6 mol/L) also increased the podocyte migration (P < 0.01), more than AngII alone, although co-incubation of AngII and fasudil did not show additive effects (P < 0.05). Data are mean ± SEM values of six experiments.
Fasudil Enhances Podocyte Migration
To assess effects of AngII and fasudil on migration activity of podocytes, a wound-healing assay with podocytes was done. Figure 6C showed that AngII mildly increased the podocyte migration. Fasudil treatment also enhanced the migration activity, although co-stimulation of AngII and fasudil did not show additive effects on the migration activity.
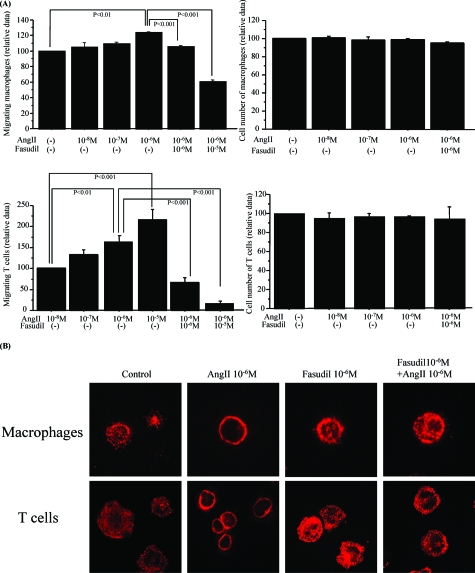
Fasudil Treatment Attenuates AngII-Enhanced Migration of Macrophages and T Cells
AngII increased the migration activity of macrophages (10−6 mol/L, P < 0.01) (Figure 7A, top left) and T cells (10−6 mol/L, P < 0.01) (Figure 7A, bottom left) in a dose-dependent manner. However, AngII did not up-regulate cell proliferation of macrophages (Figure 7A, top right) or T cells (Figure 7A, bottom right). Fasudil markedly decreased, in a dose-dependent manner, AngII-induced migration of macrophages (Figure 7A, top left) and T cells (Figure 7A, bottom left).
Figure 7.
Fasudil attenuated the AngII-induced migration activity in macrophages and T cells. A: Migration of macrophages was significantly higher in the presence of 10−6 mol/L AngII than in the absence of AngII (P < 0.01), and this effect was significantly attenuated by fasudil treatment (P < 0.001 at 10−6 mol/L and 10−5 mol/L fasudil) (top left). T cells also migrated more significantly at 10−6 mol/L AngII than at 10−8 mol/L (P < 0.01), and this migration was markedly reduced by fasudil treatment (P < 0.001 at 10−6 mol/L and 10−5 mol/L fasudil) (bottom left). Proliferation of macrophages and T cells were not influenced by AngII (top and bottom right). Data are mean ± SEM values of six wells in each group. B: AngII-induced formation of stress fibers in a polarized marginal round portion of macrophages and T cells, and this formation was reduced by fasudil treatment. Rhodamine phalloidin staining was used to visualize F-actin in the cells. Original magnifications, ×630.
Fasudil Pretreatment Attenuates AngII-Induced Stress Fiber Formation in Macrophages and T Cells
AngII (10−5 mol/L) stimulation for 6 hours induced stress fiber formation in control cells of both cell types at marginal sites (Figure 7B). Pretreatment with fasudil 10−5 mol/L for 3 hours prevented this formation.
Discussion
Podocyte numbers, evaluated by WT-1 staining, were significantly preserved in the fasudil-treated group, indicating that fasudil protected against podocyte loss or apoptosis associated with local RAS.26,27 Numerous clinical studies have shown that RAS blockade with an angiotensin-converting enzyme inhibitor and angiotensin receptor blocker improves proteinuria independently of blood pressure lowering.39 These renoprotective effects have been attributed at least in part to direct action on podocytes by blocking the RAS.39,40,41
Podocytopenia was first reported in type 2 diabetic patients42 and is often observed with severe glomerular damage, particularly with glomerular sclerosis.43 In prospective follow-up analyses, podocyte numbers provide a predictive value for albuminuria.44 Loss of podocytes may represent the decisive event that permits the affixation of parietal cells to a naked site of the GBM,45 thus leading to crescent formation and glomerulosclerosis.46 Moeller and colleagues11 nicely demonstrated by using 2.5P-Cre/ROSA26 mice that podocytes adhere to the parietal basement membrane and populate glomerular crescents during the early phase of cellular crescent formation. Interestingly, they also showed that the podocyte-derived crescentic cells did not express podocyte-specific antigens, including WT-1, nephrin, and podocin,11 suggesting that podocytes underwent profound phenotypic changes in the process of crescentic formation. Our present study showed that AngII stimulation down-regulated nephrin and WT-1 expression in the cultured podocytes that were recovered by fasudil treatment, suggesting that fasudil protected the phenotypic changes of podocytes by AngII and thus prevented the crescentic formation of this AngII-dependent disease,25,26 at least in part via this mechanism in the early phase. Indeed, not only nephrin and WT-1 but also other podocyte markers, such as podocin and CD2AP, were also preserved by fasudil treatment in association with attenuation of crescentic formation.
In podocytes, nephrin maintains the SD47,48 function including a zipper-like structure that prevents molecules in the size of albumin and larger from penetrating the filter.49 Because AngII induces a redistribution of SD protein, ZO-1, on podocytes,50 AngII may be physiologically important for cytoskeletal arrangement in these cells. Our data indicated that fasudil also influences SD proteins including nephrin via the suppression of AngII-induced cytoskeletal rearrangement. CD2AP, an adaptor actin-binding protein,37 and podocin seem to interact directly with the intracellular C-terminal domain of nephrin, and the complex can be isolated as a raft-associated component of the podocyte SD.32,37,51 Therefore, it is possible that modification to myosin light chain caused by RhoA kinase inhibitors may affect the binding of CD2AP in the complex and nephrin mRNA expression. Indeed, the present in vitro data showed that nephrin expression was protected against AngII stimulation by fasudil treatment independent of podocyte numbers. Moreover, glomerular nephrin, podocin, and CD2AP protein expression were preserved in the treatment group. These findings suggest that fasudil may preserve podocyte viability through stabilization of particular SD proteins, thus attenuating progressive podocyte injury.
Projection of multiple filopodial protrusions (microvillous transformation) in podocytes to parietal epithelial cells and subsequent bridging between GBM and parietal basement membrane may be a critical process in the early phase of the crescent formation.11 Therefore, we approached to clarify whether fasudil treatment decreased the crescent formation via preventing the podocyte’s ability to intrude to the parietal epithelium. The wound-healing assay recently reported34,35 may be most appropriate for this purpose. However, fasudil treatment unexpectedly enhanced the podocyte migration activity more than AngII alone, although co-stimulation of AngII and fasudil did not show additive effects on the migration activity. Therefore, these findings do not allow us to simply conclude that fasudil prevented AngII-induced podocyte migration to the parietal epithelium and thus subsequent crescent formation. Other factors, such as cytokine environment linking to podocyte injury by infiltrated macrophages, should be also addressed in the future study.
Mundel and co-workers38 recently demonstrated that TLR4-mediated LPS signaling induces B7-1 expression in podocytes. The activation of B7-1 in podocytes induced foot process effacement and subsequent proteinuria through reorganization of podocyte actin cytoskeleton,38 presumably including synaptopodin.52 They further demonstrated by using SCID mice with the LPS-induced nephrotic syndrome that this B7-1-mediated proteinuria is independent of T or B cells.38 Therefore, we used this model with SCID mice to approach the relative effects of fasudil on podocytes versus macrophages/T cells on proteinuria. The present study showed that fasudil treatment could not protect the LPS-induced nephrotic syndrome even in the SCID mice. This finding suggests that RhoA kinase signaling in podocytes may be not importantly involved in the B7-1-dependent foot process effacement and proteinuria, despite its major contribution to the crescent formation in anti-GBM GN. However, fasudil treatment significantly improved survival rate in this model with a high dose of LPS, suggesting that the RhoA kinase signaling may contribute to other TLR4-mediated mechanisms in other immunocompetent cells such as macrophages.53
Duffield and colleagues54 demonstrated that macrophage depletion reduced the numbers of CD4 T cells and glomerular crescents as well as improving renal function and proteinuria, implicating macrophages and CD4 T cells as key players in crescentic GN. Indeed, our data showed that attenuation of crescentic GN was associated with decreased glomerular macrophages and CD4 T cells. Our in vitro study showed that AngII directly enhanced the migration activity of both cells, but not their proliferation. However, immunohistochemical studies showed that glomerular BrdU-positive cells in the untreated group were mainly macrophages, indicating that these immune cells undergo local proliferation after migration, presumably via AngII-induced growth factors from renal resident cells.22 It is known that monocyte/macrophage infiltration and after local proliferation in glomeruli are key processes in the progression of anti-GBM GN.25,26 In addition, macrophage and T-cell infiltration in cellular crescent after rupture of parietal basement membrane are detected in late phase of crescentic formation.4,5,6,7,8 The present study demonstrated that fasudil treatment attenuated AngII-induced migrating activity of these cells, suggesting that this treatment may also protect the crescentic formation partly via blockade of the migration into the cellular crescent in the late phase. However, since fasudil treatment from day 7, presumably after the induction of early crescents, could not prevent the disease progression including proteinuria and crescent formation, initial podocyte injury by the migrated glomerular macrophage in the early stage of this disease may be critical in the subsequent glomerular injury and crescent formation.
Recent studies have indicated that RhoA kinase might be a key effector of monocyte55 and T-cell56 migration. AngII stimulates migration of rat mesangial cells and rat vascular smooth muscle cells in a dose-dependent manner,57 and statin inhibits the migration induced by AngII,58 suggesting that the effectiveness of statin depends on protein kinase C activity. A recent study further indicated that the anti-inflammatory effects of statin might depend partly on inhibition of RhoA isoprenylation.59 Therefore, the direct inhibition of RhoA isoprenylation by fasudil may underlie the migration of macrophages and CD4 T cells. Moreover, inhibition of RhoA kinase substantially prevented aggregation of the TCR/CD3 complex co-localized with lipid rafts.60 Therefore, fasudil may prevent cell migration by directly blocking the interaction of macrophages and CD4 T cells.
AngII stimulation results in the generation of actin stress fibers in rat smooth muscle cells61 and in vascular smooth muscle cells.62 AngII induced stress fiber formation in the infiltrating cells in this study. In particular, a 10−6 mol/L concentration of AngII induced thick actin bundles prominently on the round marginal portion of the macrophages and T cells. Fasudil pretreatment significantly decreased such cellular polarity and stress fibers, implicating role of RhoA in AngII-induced stress fiber formation. Stress fibers are particularly critical cytoskeletal components in leukocytes,63 being essential for chemotactic responsiveness. The fasudil-induced inhibition of cell migration observed in this study may have blocked the chemotactic responsiveness of these cells, which in turn would have attenuated the inflammation. Fasudil might also have an immunosuppressive effect, and future studies are needed to establish the complete array of its functional effects.
Conclusion
In this study, we provide evidence that a RhoA kinase inhibitor, fasudil, can markedly prevent crescentic GN, which is closely linked to local RAS activation, via podocyte protection and inhibition of macrophage and T-cell migration.
Acknowledgments
We thank Drs. Peter Mundel (Mount Sinai School of Medicine) and Katsuhiko Asanuma (Juntendo University School of Medicine) for kindly providing the podocyte cell line; Asahi Kasei Pharma Co. Ltd., for providing fasudil; Kyowa Hakko Kogyo Co. for providing anti-GBM nephrotoxin; Dr. Isao Shirato for thoughtful advice; Drs. Daisuke Sato, Yasuhiko Kanaguchi, and Tadahiro Kajiyama for technical support for in vivo experiments; and Mr. Kazutaka Yoshida for excellent technical and secretarial assistance.
Footnotes
Address reprint requests to Yasuhiko Tomino, M.D., Division of Nephrology, Department of Internal Medicine, Juntendo University School of Medicine, 2-1-1, Hongo, Bunkyo-ku, Tokyo 113-8421, Japan. E-mail: yasu@med. juntendo.ac.jp.
References
- Tipping PG, Holdsworth SR. T cells in crescentic glomerulonephritis. J Am Soc Nephrol. 2006;17:1253–1263. doi: 10.1681/ASN.2005091013. [DOI] [PubMed] [Google Scholar]
- Pusey CD. Anti-glomerular basement membrane disease. Kidney Int. 2003;64:1535–1550. doi: 10.1046/j.1523-1755.2003.00241.x. [DOI] [PubMed] [Google Scholar]
- Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, Fogo AB, Ginzler EM, Hebert L, Hill G, Hill P, Jennette JC, Kong NC, Lesavre P, Lockshin M, Looi LM, Makino H, Moura LA, Nagata M. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 2004;15:241–250. doi: 10.1097/01.asn.0000108969.21691.5d. [DOI] [PubMed] [Google Scholar]
- Morita T, Suzuki Y, Churg J. Structure and development of the glomerular crescent. Am J Pathol. 1973;72:349–368. [PMC free article] [PubMed] [Google Scholar]
- Min KW, Gyorkey F, Gyorkey P, Yium JJ, Eknoyan G. The morphogenesis of glomerular crescents in rapidly progressive glomerulonephritis. Kidney Int. 1974;5:47–56. doi: 10.1038/ki.1974.6. [DOI] [PubMed] [Google Scholar]
- Jennette JC, Hipp CG. The epithelial antigen phenotype of glomerular crescent cells. Am J Clin Pathol. 1986;86:274–280. doi: 10.1093/ajcp/86.3.274. [DOI] [PubMed] [Google Scholar]
- Boucher A, Droz D, Adafer E, Noel LH. Relationship between the integrity of Bowman’s capsule and the composition of cellular crescents in human crescentic glomerulonephritis. Lab Invest. 1987;56:526–533. [PubMed] [Google Scholar]
- Nagata M, Horita S, Shu Y, Shibata S, Hattori M, Ito K, Watanabe T. Phenotypic characteristics and cyclin-dependent kinase inhibitors repression in hyperplastic epithelial pathology in idiopathic focal segmental glomerulosclerosis. Lab Invest. 2000;80:869–880. doi: 10.1038/labinvest.3780091. [DOI] [PubMed] [Google Scholar]
- Lan HY, Nikolic-Paterson DJ, Mu W, Atkins RC. Local macrophage proliferation in the pathogenesis of glomerular crescent formation in rat anti-glomerular basement membrane (GBM) glomerulonephritis. Clin Exp Immunol. 1997;110:233–240. doi: 10.1111/j.1365-2249.1997.tb08322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Hir M, Keller C, Eschmann V, Hahnel B, Hosser H, Kriz W. Podocyte bridges between the tuft and Bowman’s capsule: an early event in experimental crescentic glomerulonephritis. J Am Soc Nephrol. 2001;12:2060–2071. doi: 10.1681/ASN.V12102060. [DOI] [PubMed] [Google Scholar]
- Moeller MJ, Soofi A, Hartmann I, Le Hir M, Wiggins R, Kriz W, Holzman LB. Podocytes populate cellular crescents in a murine model of inflammatory glomerulonephritis. J Am Soc Nephrol. 2004;15:61–67. doi: 10.1097/01.asn.0000102468.37809.c6. [DOI] [PubMed] [Google Scholar]
- Arakawa M, Tokunaga J. A scanning electron microscope study of the glomerulus. Further consideration of the mechanism of the fusion of podocyte terminal processes in nephrotic rats. Lab Invest. 1972;27:366–371. [PubMed] [Google Scholar]
- Rodewald R, Karnovsky MJ. Porous substructure of the glomerular slit diaphragm in the rat and mouse. J Cell Biol. 1974;60:423–433. doi: 10.1083/jcb.60.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada E. The fine structure of the renal glomerulus of the mouse. J Biophys Biochem Cytol. 1955;1:551–566. doi: 10.1083/jcb.1.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser J, Kriz W, Kretzler M, Mundel P. The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol. 2000;11:1–8. doi: 10.1681/ASN.V1111. [DOI] [PubMed] [Google Scholar]
- Morigi M, Buelli S, Angioletti S, Zanchi C, Longaretti L, Zoja C, Galbusera M, Gastoldi S, Mundel P, Remuzzi G, Benigni A. In response to protein load podocytes reorganize cytoskeleton and modulate endothelin-1 gene: implication for permselective dysfunction of chronic nephropathies. Am J Pathol. 2005;166:1309–1320. doi: 10.1016/S0002-9440(10)62350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Katoh K, Kano Y, Amano M, Onishi H, Kaibuchi K, Fujiwara K. Rho-kinase-mediated contraction of isolated stress fibers. J Cell Biol. 2001;153:569–584. doi: 10.1083/jcb.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seasholtz TM, Majumdar M, Brown JH. Rho as a mediator of G protein-coupled receptor signaling. Mol Pharmacol. 1999;55:949–956. doi: 10.1124/mol.55.6.949. [DOI] [PubMed] [Google Scholar]
- Gohla A, Schultz G, Offermanns S. Role for G(12)/G(13) in agonist-induced vascular smooth muscle cell contraction. Circ Res. 2000;87:221–227. doi: 10.1161/01.res.87.3.221. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Egido J. Inflammation and angiotensin II. Int J Biochem Cell Biol. 2003;35:881–900. doi: 10.1016/s1357-2725(02)00271-6. [DOI] [PubMed] [Google Scholar]
- Nahmod KA, Vermeulen ME, Raiden S, Salamone G, Gamberale R, Fernandez-Calotti P, Alvarez A, Nahmod V, Giordano M, Geffner JR. Control of dendritic cell differentiation by angiotensin II. FASEB J. 2003;17:491–493. doi: 10.1096/fj.02-0755fje. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Iturbe B, Pons H, Herrera-Acosta J, Johnson RJ. Role of immunocompetent cells in nonimmune renal diseases. Kidney Int. 2001;59:1626–1640. doi: 10.1046/j.1523-1755.2001.0590051626.x. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Gomez-Guerrero C, Shirato I, Lopez-Franco O, Hernandez-Vargas P, Sanjuan G, Ruiz-Ortega M, Sugaya T, Okumura K, Tomino Y, Ra C, Egido J. Susceptibility to T cell-mediated injury in immune complex disease is linked to local activation of renin-angiotensin system: the role of NF-AT pathway. J Immunol. 2002;169:4136–4146. doi: 10.4049/jimmunol.169.8.4136. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Shirato I, Okumura K, Ravetch JV, Takai T, Tomino Y, Ra C. Distinct contribution of Fc receptors and angiotensin II-dependent pathways in anti-GBM glomerulonephritis. Kidney Int. 1998;54:1166–1174. doi: 10.1046/j.1523-1755.1998.00108.x. [DOI] [PubMed] [Google Scholar]
- Gómez-Guerrero C, Lopez-Franco O, Suzuki Y, Sanjuan G, Hernandez-Vargas P, Blanco J, Egido J. Nitric oxide production in renal cells by immune complexes: role of kinases and nuclear factor-kappaB. Kidney Int. 2002;62:2022–2034. doi: 10.1046/j.1523-1755.2002.00653.x. [DOI] [PubMed] [Google Scholar]
- Poole A, Gibbins JM, Turner M, van Vugt MJ, van de Winkel JG, Saito T, Tybulewicz VL, Watson SP. The Fc receptor gamma-chain and the tyrosine kinase Syk are essential for activation of mouse platelets by collagen. EMBO J. 1997;16:2333–2341. doi: 10.1093/emboj/16.9.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh S, Utsunomiya T, Tsurui K, Kobayashi T, Ikegaki I, Sasaki Y, Asano T. Pharmacological profile of hydroxy fasudil as a selective rho kinase inhibitor on ischemic brain damage. Life Sci. 2001;69:1441–1453. doi: 10.1016/s0024-3205(01)01229-2. [DOI] [PubMed] [Google Scholar]
- Higashi M, Shimokawa H, Hattori T, Hiroki J, Mukai Y, Morikawa K, Ichiki T, Takahashi S, Takeshita A. Long-term inhibition of Rho-kinase suppresses angiotensin II-induced cardiovascular hypertrophy in rats in vivo: effect on endothelial NAD(P)H oxidase system. Circ Res. 2003;93:767–775. doi: 10.1161/01.RES.0000096650.91688.28. [DOI] [PubMed] [Google Scholar]
- López-Franco O, Suzuki Y, Sanjuan G, Blanco J, Hernandez-Vargas P, Yo Y, Kopp J, Egido J, Gomez-Guerrero C. Nuclear factor-kappa B inhibitors as potential novel anti-inflammatory agents for the treatment of immune glomerulonephritis. Am J Pathol. 2002;161:1497–1505. doi: 10.1016/s0002-9440(10)64425-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem MA, Ni L, Witherden I, Tryggvason K, Ruotsalainen V, Mundel P, Mathieson PW. Co-localization of nephrin, podocin, and the actin cytoskeleton: evidence for a role in podocyte foot process formation. Am J Pathol. 2002;161:1459–1466. doi: 10.1016/S0002-9440(10)64421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk W, Goodwin RH, Jr, Leonard EJ. A 48-well micro chemotaxis assembly for rapid and accurate measurement of leukocyte migration. J Immunol Methods. 1980;33:239–247. doi: 10.1016/0022-1759(80)90211-2. [DOI] [PubMed] [Google Scholar]
- Asanuma K, Yanagida-Asanuma E, Faul C, Tomino Y, Kim K, Mundel P. Synaptopodin orchestrates actin organization and cell motility via regulation of RhoA signalling. Nat Cell Biol. 2006;8:485–491. doi: 10.1038/ncb1400. [DOI] [PubMed] [Google Scholar]
- Yanagida-Asanuma E, Asanuma K, Kim K, Donnelly M, Young Choi H, Hyung Chang J, Suetsugu S, Tomino Y, Takenawa T, Faul C, Mundel P. Synaptopodin protects against proteinuria by disrupting Cdc42:IRSp53:Mena signaling complexes in kidney podocytes. Am J Pathol. 2007;171:415–427. doi: 10.2353/ajpath.2007.070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisher CC, Brenner BM. Philadelphia: J.B. Lippincott Company,; Renal Pathology with Clinical and Functional Correlations. 1994:p 355. [Google Scholar]
- Shih NY, Li J, Karpitskii V, Nguyen A, Dustin ML, Kanagawa O, Miner JH, Shaw AS. Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science. 1999;286:312–315. doi: 10.1126/science.286.5438.312. [DOI] [PubMed] [Google Scholar]
- Reiser J, von Gersdorff G, Loos M, Oh J, Asanuma K, Giardino L, Rastaldi MP, Calvaresi N, Watanabe H, Schwarz K, Faul C, Kretzler M, Davidson A, Sugimoto H, Kalluri R, Sharpe AH, Kreidberg JA, Mundel P. Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest. 2004;113:1390–1397. doi: 10.1172/JCI20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durvasula RV, Petermann AT, Hiromura K, Blonski M, Pippin J, Mundel P, Pichler R, Griffin S, Couser WG, Shankland SJ. Activation of a local tissue angiotensin system in podocytes by mechanical strain. Kidney Int. 2004;65:30–39. doi: 10.1111/j.1523-1755.2004.00362.x. [DOI] [PubMed] [Google Scholar]
- Hernández-Vargas P, Lopez-Franco O, Sanjuan G, Ruperez M, Ortiz-Munoz G, Suzuki Y, Aguado-Roncero P, Perez-Tejerizo G, Blanco J, Egido J, Ruiz-Ortega M, Gomez-Guerrero C. Suppressors of cytokine signaling regulate angiotensin II-activated Janus kinase-signal transducers and activators of transcription pathway in renal cells. J Am Soc Nephrol. 2005;16:1673–1683. doi: 10.1681/ASN.2004050374. [DOI] [PubMed] [Google Scholar]
- Yamakawa T, Tanaka S, Numaguchi K, Yamakawa Y, Motley ED, Ichihara S, Inagami T. Involvement of Rho-kinase in angiotensin II-induced hypertrophy of rat vascular smooth muscle cells. Hypertension. 2000;35:313–318. doi: 10.1161/01.hyp.35.1.313. [DOI] [PubMed] [Google Scholar]
- Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997;99:342–348. doi: 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemley KV, Lafayette RA, Safai M, Derby G, Blouch K, Squarer A, Myers BD. Podocytopenia and disease severity in IgA nephropathy. Kidney Int. 2002;61:1475–1485. doi: 10.1046/j.1523-1755.2002.00269.x. [DOI] [PubMed] [Google Scholar]
- Meyer TW, Bennett PH, Nelson RG. Podocyte number predicts long-term urinary albumin excretion in Pima Indians with type II diabetes and microalbuminuria. Diabetologia. 1999;42:1341–1344. doi: 10.1007/s001250051447. [DOI] [PubMed] [Google Scholar]
- Hoffmann S, Podlich D, Hahnel B, Kriz W, Gretz N. Angiotensin II type 1 receptor overexpression in podocytes induces glomerulosclerosis in transgenic rats. J Am Soc Nephrol. 2004;15:1475–1487. doi: 10.1097/01.asn.0000127988.42710.a7. [DOI] [PubMed] [Google Scholar]
- Kim YH, Goyal M, Kurnit D, Wharram B, Wiggins J, Holzman L, Kershaw D, Wiggins R. Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int. 2001;60:957–968. doi: 10.1046/j.1523-1755.2001.060003957.x. [DOI] [PubMed] [Google Scholar]
- Ruotsalainen V, Ljungberg P, Wartiovaara J, Lenkkeri U, Kestila M, Jalanko H, Holmberg C, Tryggvason K. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc Natl Acad Sci USA. 1999;96:7962–7967. doi: 10.1073/pnas.96.14.7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzman LB, St John PL, Kovari IA, Verma R, Holthofer H, Abrahamson DR. Nephrin localizes to the slit pore of the glomerular epithelial cell. Kidney Int. 1999;56:1481–1491. doi: 10.1046/j.1523-1755.1999.00719.x. [DOI] [PubMed] [Google Scholar]
- Tryggvason K, Wartiovaara J. Molecular basis of glomerular permselectivity. Curr Opin Nephrol Hypertens. 2001;10:543–549. doi: 10.1097/00041552-200107000-00009. [DOI] [PubMed] [Google Scholar]
- Macconi D, Abbate M, Morigi M, Angioletti S, Mister M, Buelli S, Bonomelli M, Mundel P, Endlich K, Remuzzi A, Remuzzi G. Permselective dysfunction of podocyte-podocyte contact upon angiotensin II unravels the molecular target for renoprotective intervention. Am J Pathol. 2006;168:1073–1085. doi: 10.2353/ajpath.2006.050701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz K, Simons M, Reiser J, Saleem MA, Faul C, Kriz W, Shaw AS, Holzman LB, Mundel P. Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J Clin Invest. 2001;108:1621–1629. doi: 10.1172/JCI12849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma K, Kim K, Oh J, Giardino L, Chabanis S, Faul C, Reiser J, Mundel P. Synaptopodin regulates the actin-bundling activity of alpha-actinin in an isoform-specific manner. J Clin Invest. 2005;115:1188–1198. doi: 10.1172/JCI23371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monick MM, Powers LS, Butler NS, Hunninghake GW. Inhibition of Rho family GTPases results in increased TNF-alpha production after lipopolysaccharide exposure. J Immunol. 2003;171:2625–2630. doi: 10.4049/jimmunol.171.5.2625. [DOI] [PubMed] [Google Scholar]
- Duffield JS, Tipping PG, Kipari T, Cailhier JF, Clay S, Lang R, Bonventre JV, Hughes J. Conditional ablation of macrophages halts progression of crescentic glomerulonephritis. Am J Pathol. 2005;167:1207–1219. doi: 10.1016/S0002-9440(10)61209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honing H, van den Berg TK, van der Pol SM, Dijkstra CD, van der Kammen RA, Collard JG, de Vries HE. RhoA activation promotes transendothelial migration of monocytes via ROCK. J Leukoc Biol. 2004;75:523–528. doi: 10.1189/jlb.0203054. [DOI] [PubMed] [Google Scholar]
- Liu L, Schwartz BR, Lin N, Winn RK, Harlan JM. Requirement for RhoA kinase activation in leukocyte de-adhesion. J Immunol. 2002;169:2330–2336. doi: 10.4049/jimmunol.169.5.2330. [DOI] [PubMed] [Google Scholar]
- Kohno M, Yasunari K, Minami M, Kano H, Maeda K, Mandal AK, Inoki K, Haneda M, Yoshikawa J. Regulation of rat mesangial cell migration by platelet-derived growth factor, angiotensin II, and adrenomedullin. J Am Soc Nephrol. 1999;10:2495–2502. doi: 10.1681/ASN.V10122495. [DOI] [PubMed] [Google Scholar]
- Kohno M, Shinomiya K, Abe S, Noma T, Kondo I, Oshita A, Takeuchi H, Takagi Y, Yukiiri K, Mizushige K, Ohmori K. Inhibition of migration and proliferation of rat vascular smooth muscle cells by a new HMG-CoA reductase inhibitor, pitavastatin. Hypertens Res. 2002;25:279–285. doi: 10.1291/hypres.25.279. [DOI] [PubMed] [Google Scholar]
- Blanco-Colio LM, Tunon J, Martin-Ventura JL, Egido J. Anti-inflammatory and immunomodulatory effects of statins. Kidney Int. 2003;63:12–23. doi: 10.1046/j.1523-1755.2003.00744.x. [DOI] [PubMed] [Google Scholar]
- Tharaux PL, Bukoski RC, Rocha PN, Crowley SD, Ruiz P, Nataraj C, Howell DN, Kaibuchi K, Spurney RF, Coffman TM. Rho kinase promotes alloimmune responses by regulating the proliferation and structure of T cells. J Immunol. 2003;171:96–105. doi: 10.4049/jimmunol.171.1.96. [DOI] [PubMed] [Google Scholar]
- Turner CE, Pietras KM, Taylor DS, Molloy CJ. Angiotensin II stimulation of rapid paxillin tyrosine phosphorylation correlates with the formation of focal adhesions in rat aortic smooth muscle cells. J Cell Sci. 1995;108:333–342. doi: 10.1242/jcs.108.1.333. [DOI] [PubMed] [Google Scholar]
- Fernstrom K, Farmer P, Ali MS. Cytoskeletal remodeling in vascular smooth muscle cells in response to angiotensin II-induced activation of the SHP-2 tyrosine phosphatase. J Cell Physiol. 2005;205:402–413. doi: 10.1002/jcp.20436. [DOI] [PubMed] [Google Scholar]
- Barber MA, Welch HC. PI3K and RAC signalling in leukocyte and cancer cell migration. Bull Cancer. 2006;93:E44–E52. [PubMed] [Google Scholar]