Abstract
Human defensin (HD)-5 is an antimicrobial peptide expressed in small intestinal Paneth cells, and alterations in HD-5 expression may be important in Crohn’s disease (CD) pathogenesis. Levels of HD-5 in Paneth cells and ileostomy fluid from control and CD patients were studied by quantitative immunodot analysis, immunohistochemistry, acid urea-polyacrylamide gel electrophoresis and sodium dodecyl sulfate-polyacrylamide gel electrophoresis Western blotting, reverse phase-high performance liquid chromatography, N-terminal amino acid sequencing, and ES-QToF mass spectrometry. In both control and CD patients, HD-5 in Paneth cell extracts was present almost exclusively in the precursor form. HD-5 levels in ileostomy fluid were lower in CD patients (n = 51) than in controls (n = 20): median (range), 7.9 (5.5 to 35.0) μg/ml versus 10.5 (6.0 to 30.4) μg/ml; P = 0.05; this difference was most marked in CD patients with homozygous/compound heterozygous mutations in NOD2 (P = 0.03). In control ileostomy fluid, HD-5 was present in the mature form only. In contrast, CD patient ileostomy fluid contained both precursor and mature forms of HD-5, with the majority present in a complex with trypsin, chymotrypsinogen/chymotrypsin, and α1-anti-trypsin. Pro-HD-5 was not associated with trypsin or chymotrypsinogen in Paneth cell extracts. In conclusion, pro-HD-5 in the intestinal lumen is processed by trypsin in a complex in which chymotrypsinogen is also cleaved for activation. The persistence of this complex in CD may be attributable to increased luminal levels of proteinase inhibitors such as α1-anti-trypsin.
Crohn’s disease (CD) is a chronic inflammatory condition of the gastrointestinal tract that is believed to arise from an abnormality that results in the loss of the normal immunological unresponsiveness to the resident luminal microflora.1,2 The primary abnormality may affect the surface epithelium and/or the mucosal immune system. The identification of homozygous/compound heterozygous mutations in the CARD15/NOD2 gene in a proportion of patients with CD3,4,5 has led to significant interest in the role of innate immunity in the etiopathogenesis of CD. Studies have shown that NOD2 is a cytosolic protein that binds muramyl dipeptide (derived from peptidoglycan of both Gram-positive and Gram-negative bacteria), leading to activation of nuclear factor (NF)-κB and subsequent expression of proinflammatory mediators such as interleukin-1 and tumor necrosis factor-α.6 In addition to monocytes, NOD2 has recently been reported to be expressed in Paneth cells,7,8 which are a subtype of epithelial cells normally present in the base of small intestinal crypts. Paneth cells are normally absent in the colon but are frequently seen in colonic samples of patients with inflammatory bowel disease.9,10 Because they express antimicrobial peptides of the α-defensin family, antimicrobial proteins (lysozyme, secretory phospholipase A2, angiogenin 4), and an intestinal bactericidal lectin,11 Paneth cells are believed to be important in innate immunity in the intestine.12,13 Dysregulation of Paneth cell function leading to impaired expression or activities of antimicrobial peptides and proteins may adversely affect intestinal protection against luminal microorganisms. There is a vast and complex population of microorganisms in the colon and terminal ileum, with 30 to 40 species making up the majority of the total population.14,15 CD frequently occurs in the terminal ileum, and those with mutations in the NOD2 gene frequently develop disease in this region.16
Two members of the α-defensin family, human defensin(HD)-5 and HD-6, have been identified in humans and shown to be expressed by Paneth cells17 and also infrequent cells designated intermediate cells.9 HD-5 is synthesized in its precursor form (Figure 1) and studies have shown that it is stored in this form in Paneth cells.9,18 Recombinant mature HD-5 is active against many Gram-negative and Gram-positive bacteria and the predicted mature form of HD-5 has been identified in samples from the small intestinal lumen.18,19 In vitro, trypsin has been shown to be capable of processing pro-HD-5 to the mature form and this enzyme is present in Paneth cells.18,20,21 When Paneth cell-containing isolated terminal ileal crypts were stimulated in vitro with carbamyl choline or lipopolysaccharide, a truncated form of pro-HD-5 (but not mature form) was released.9 Truncated forms of pro-HD-5 have also been identified in ileal neobladder urine.22 Thus, the in vivo site (inside or on the surface of Paneth cells or in the lumen) and mechanism(s) of processing of pro-HD-5 remain to be determined. This is in contrast to α-defensins (designated cryptdins) in murine Paneth cells, which are stored in mature form, after intracellular processing by matrilysin [also known as matrix metalloproteinase-7 (MMP-7)].23 The importance of cryptdin processing has been shown by increased susceptibility to orally administered Salmonella typhimurium in mice lacking MMP-7.23 In Trichinella spiralis-induced inflammation in murine small intestine, we have previously shown active Paneth cell degranulation,24 which would be expected to release antimicrobial peptides and proteins into the lumen. In humans, it is not known whether the precursor form of HD-5 may be processed intracellularly in Paneth cells (eg, via an induced or activated processing enzyme) during small intestinal inflammation, as in CD. Such intracellular processing would enable the enteric defensin to be active on release by the Paneth cells, as is the case in mice. To address the above issues, we have investigated the form of HD-5 present in small intestinal Paneth cells of patients with CD. Ileostomy fluid of controls and patients with CD was used to characterize luminal expression of HD-5.
Figure 1.
Predicted amino acid sequence of HD-5, based on the cDNA sequence.17 Single letter amino acid code for pre-pro-HD-5 is shown. Pro-HD-5 (with N terminus ESLQER and predicted mass of 8102 Da) was isolated from crypt cell extracts from normal and CD small intestine. In two CD crypt extracts, a smaller peptide [with N terminus ADEAT (arrow) and predicted mass of 7360 Da] was also isolated. In ileostomy fluid of controls, only the mature form of HD-5 (with N-terminal sequence ATCYCRT) was identified. In CD ileostomy fluid, the mature (with N-terminal sequence ATCYCRT and predicted mass of 3580 Da) and precursor forms of HD-5 were identified.
Materials and Methods
Patients and Samples
Human intestinal mucosal samples were obtained from operation resection specimens, after informed consent and with approval of the Nottingham Research Ethics Committee. Histologically normal terminal ileal mucosa was obtained from right hemi-colectomy specimens (n = 8) resected for colon carcinoma affecting the cecum or ascending colon. Small intestinal (seven terminal ileum, three jejunum) mucosal samples affected by CD were also obtained from operation resection specimens, in which there was active inflammation. CD was diagnosed in these patients using standard criteria (clinical, endoscopic, radiological, and histological features).
Ileostomy fluid was collected, in the presence of protease inhibitors (104 mmol/L AEBSF, 80 μmol/L aprotinin, 2 mmol/L leupeptin, 4 mmol/L bestatin, 1.5 mmol/L pepstatin A, 1.4 mmol/L E-64; Sigma, Poole, UK), from patients with an ileostomy. It was studied after centrifugation, either immediately or after storage at −80°C. Patients included 51 with CD and 20 with normal small intestine (seven ulcerative colitis, seven colonic carcinoma, two mesenteric ischemia, one intractable constipation, one familial adenomatous polyposis, one appendiceal abscess, and one ruptured ectopic pregnancy). For the ileostomy patients with CD, clinical disease activity was assessed using the Harvey-Bradshaw index,25 with the modification that the score for number of liquid stools per day was replaced with the number of stoma bags filled per day minus the usual number of stoma bags filled when in remission. Active disease was taken as a modified Harvey-Bradshaw index of 8 or more, and inactive as 3 or less, in keeping with previous reports.26
Gel Electrophoresis, Immunoblotting, and Immunohistochemistry
Acid-urea polyacrylamide gel electrophoresis (AU-PAGE) was used for the resolution of cationic peptides and proteins as previously described.9 Precast 18% Tris-tricine sodium dodecyl sulfate (SDS)-PAGE ready gels (Bio-Rad, Hemel Hempstead, UK) were also used for the resolution of peptides and small proteins. The presence of immunoreactive HD-5 in crypt cell extracts, ileostomy fluid samples, and purified fractions, was determined by dot blot and Western blot analyses using polyclonal anti-HD-5 sera.9 This anti-sera was raised against synthetic mature HD-5 (amino acids 63 to 94 in Figure 1). It will therefore recognize both mature and pro-HD-5. Sections of formalin-fixed, paraffin-embedded intestinal tissue samples were used for immunohistochemistry, using rabbit anti-HD-5 and anti-chymotrypsin (Biogenesis, Kingston, NH) antibodies, as previously described.9
Isolation and Purification of HD-5 from Crypt Cell Acid Extracts and from Ileostomy Fluid
Intestinal mucosal crypt epithelial cells were isolated from fresh small bowel tissue, lysed, and cationic peptides and proteins were extracted as previously described.9,27 HD-5 was purified from crypt acid extracts using cation exchange chromatography and reverse phase-high performance liquid chromatography (RP-HPLC), as previously described.9 Ileostomy fluid was collected as described above. After addition of acetic acid (to make a 10% v/v solution), protease inhibitors and centrifugation (150,000 × g), the supernatant was diluted 15:1 with 5 mmol/L ammonium acetate (pH 6.0) buffer. Cationic peptides and proteins were obtained using cation exchange beads (Macro-Prep CM Support, Bio-Rad) and concentrated (Vivaspin 2-kDa MWCO filters). After application to RP-HPLC (as previously described9), HD-5-containing fractions were characterized by AU-PAGE, 18% Tris-tricine SDS-PAGE, Western blot analysis, N-terminal amino acid sequencing, and mass spectrometry.
Mass Spectrometry and N-Terminal Amino Acid Sequencing
Intact protein mass determination was performed using an electrospray Q-ToF2 tandem mass spectrometer (Waters Co., Milford, MA). Spectra were processed between 3000 and 10,000 Da, at a resolution of 1 Da and a peak width of 0.75 Da. After MaxEnt1 processing, the spectra were centered with the minimum peak width at half height set at 1 and using the centroid top method at 80%. N-terminal amino acid sequencing of purified peptides was performed by Edman degradation chemistry using an automated amino acid sequencer (model 474A; Perkin Elmer Biosystems, Shelton, CT). Deduced N-terminal sequences were searched against the SwissProt protein sequence database, using the FASTA algorithm.
NOD2 Genotyping
Polymerase chain reaction (PCR) primer pairs were used to amplify regions of 2 to 400 bases around SNP 8 (sense: 5′-TTTGCTCAGACACCTCTTCAATTGTG-3′, anti-sense: 5′-AAAATGTCAACTTGAGGTGCCCAAC-3′,probe extension: 5′-TGAGTGCCAGACATCTGAGAAGGCCCTGCTC-3′), SNP 12 (sense: 5′-AGTGAGGCCACTCTGGGATTGAGT-3′, anti-sense: 5′-AGCTCCTCCCTCTTCACCTGATCTC-3′, probe extension: 5′-CCCCCTCGTCACCCACTCTGTTGC-3′) and SNP 13 (sense: 5′-GAATCTCAGACATGAGCAGGATGTGT-3′, anti-sense: 5′-CCGTCACCCCATTTTACAGATAGAAA-3′, probe extension: 5′-TTACCAGACTTCAGGATGGTGTCATTCCTTTCAAGGG-3′) of NOD2 gene. NOD2 genotyping was performed on the amplified DNA regions using the ABI Prism SNaPshot Multiplex Kit (Applied Biosystems, Foster City, CA) to perform a probe extension assay. To verify the probe extension assay, DNA samples representative of different NOD2 genotypes were sequenced using the Big Dye terminator cycle sequencing kit (Applied Biosciences, Foster City, CA) according to the manufacturer’s instructions.
Blinding
Comparative studies of HD-5 levels in ileostomy fluid samples were performed in a blinded manner. Thus, the samples were coded before study by others not involved in this research and the code broken after the results were available.
Statistical Analysis
Data are expressed as median (range) and were analyzed by Kruskal-Wallis and Mann-Whitney U-tests.
Results
Immunohistochemistry of Small Intestinal Tissue Samples
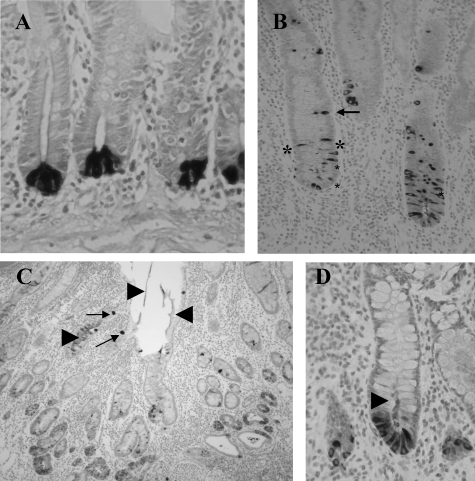
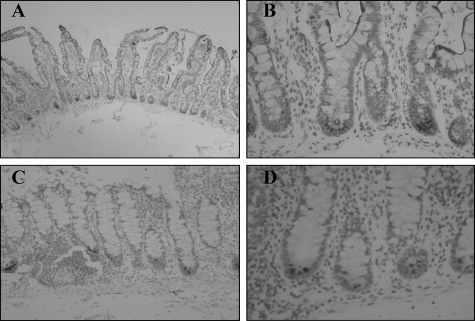
Immunohistochemistry showed HD-5 expression predominantly in Paneth cells in sections of both normal and Crohn’s ileum (Figure 2). In Crohn’s small intestine, in addition to predominant expression in Paneth cells, HD-5 immunoreactivity was also often seen in enterocytes and goblet-like intermediate cells further up the crypt, and within the crypt lumen (Figure 2), features only occasionally seen in normal small bowel.
Figure 2.
Sections of normal (A) and Crohn’s (B–D) small intestinal sections immunostained with anti-HD-5 antibody. Strongly HD-5-immunoreactive Paneth cells are shown at the base of crypts in A. In B, HD-5-immunoreactive cells with morphological appearances of enterocytes are shown close to the asterisk. Intermediate cells are arrowed in B and C and arrowheads show HD-5-immunoreactive material in the lumen in C and D.
HD-5 Is Present Predominantly in Precursor Form in CD and Control Small Intestinal Crypt Cell Extracts
Crypts were isolated from small intestinal mucosal samples of 10 CD (seven terminal ileum, six NOD2 wild type, two NOD2 SNP12 heterozygotes, one NOD2 SNP13 heterozygote, and one NOD2 SNP13 homozygote) and 8 normal terminal ileal resection specimens, and proteins extracted in acetic acid. Figure 3 demonstrates that the electrophoretic mobility on AU-PAGE of HD-5 derived from crypt epithelial cells from Crohn’s small intestine is broadly similar to that of HD-5 derived from normal small intestine. Multiple bands on AU-PAGE for control (normal) samples are similar to those previously reported9,18 for pro-HD-5 purified from normal small intestinal Paneth cells. These bands disappear on neutralization of anti-HD-5 antiserum using purified HD-5 (not shown). In contrast, mature HD-5 derived from the ileostomy fluid migrates significantly further on AU-PAGE as a single band.
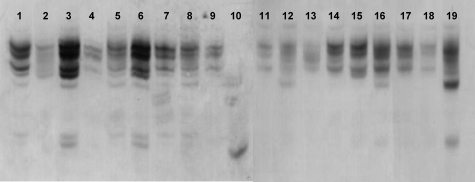
Figure 3.
HD-5 AU-PAGE Western blot of small intestinal crypt epithelial cell extracts. Acid extracts of normal and active CD small intestinal crypts were separated by AU-PAGE, and after transfer to PVDF membrane, immunoblotting was performed using anti-HD-5 antibody. Electrophoretic mobility of HD-5 in crypt extracts from 10 samples affected by active CD (lanes 2 to 9, 11 to 12) was primarily similar to that for eight separate normal ileal crypt samples (lanes 1, 13 to 19) but distinct from that of synthetic mature HD-5 (lane 10). Lane 8 shows sample from resection specimen of patient CD6, whose ileostomy fluid was collected 5 weeks after operation for characterization of intraluminal HD-5 (see Table 2).
Eleven of the small intestinal crypt epithelial cell acid extracts [six normal and five Crohn’s (two NOD2 wild type, two NOD2 SNP12 heterozygotes, and one NOD2 SNP13 heterozygote)] were fractionated using cation exchange chromatography. In all cases, HD-5 eluted at 0.63 to 0.83 mol/L NaCl, and these fractions were used for subsequent purification by RP-HPLC, in which only the fraction eluting at 39% acetonitrile contained HD-5 (Figure 4).
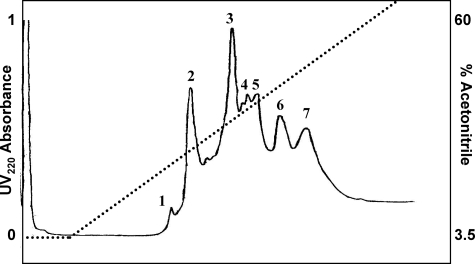
Figure 4.
RP-HPLC purification of HD-5 from HD-5-immunoreactive cation exchange fractions of ileal crypt epithelial acid extract from one representative resection specimen. Individual eluting peaks 1 to 7 were collected and analyzed by dot blot. Only peak 3, eluting at 39% acetonitrile, was immunoreactive for HD-5.
ES-Q-ToF MS of RP-HPLC-purified HD-5 peptide from six normal ileal and three Crohn’s small intestinal resection specimens showed the presence of a single mass of 8102 Da, which is identical to the predicted mass of pro-HD-5. In two samples of HD-5 purified from two CD crypt extracts (one NOD2 wild type and one NOD2 SNP12 heterozygote), a smaller peptide with mass 7360 Da was seen in addition to the mass for pro-HD-5 (8102 Da, Figure 5). This mass difference would occur if a 6-amino acid truncation (ESLQER) had occurred from the N terminus of the HD-5 pro-peptide (expected mass of truncated peptide is 7360 Da, Figure 1). These findings were confirmed by N-terminal amino acid sequencing with the sequence ESLQE for all seven samples (two normal ileum, five Crohn’s small bowel) studied and also a secondary sequence, ADEAT, for the two CD samples with a smaller HD-5 mass (7360 Da, Figure 1).
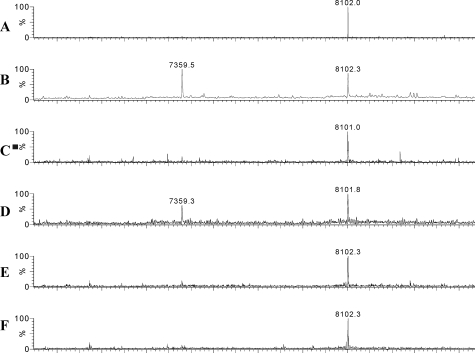
Figure 5.
Mass spectral analysis of RP-HPLC-purified HD-5 from small intestinal crypt epithelial extracts. HD-5 purified from a normal ileal resection specimen (A, representative of six studied) and from five Crohn’s small bowel specimens (B–F) was applied to a Q-ToF mass spectrometer. Each contains a peptide of mass of 8102 Da, which is identical to the expected mass for HD-5 propeptide. Two Crohn’s samples (B and D) also contain a truncated form with a mass of 7359.5 Da.
HD-5 Levels in the Small Intestinal Lumen
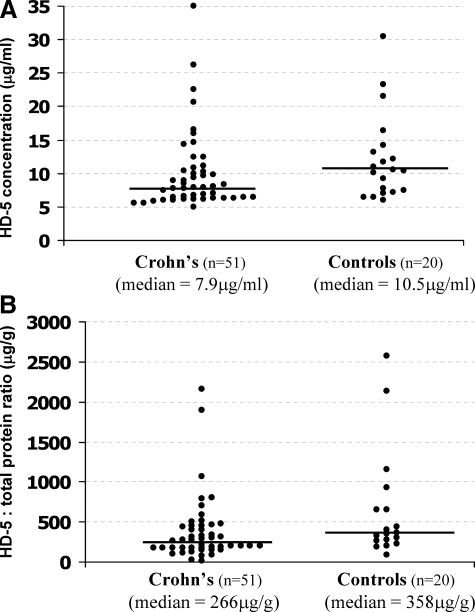
HD-5 concentration in ileostomy fluid was extrapolated from a standard curve obtained by densitometric analysis of dot blots of known concentrations of purified pro-HD-5 from normal small bowel. In a blinded manner, 71 samples (51 Crohn’s and 20 controls) were analyzed eight times, and the mean HD-5 level, and coefficient of variation, calculated for each sample. The mean coefficient of variation over all samples was 6.6%. As shown in Table 1 and Figure 6A, luminal HD-5 levels were lower in Crohn’s ileostomy fluid than control [median (range): 7.9 (5.5 to 35.0) μg/ml versus 10.5 (6.0 to 30.4) μg/ml; P = 0.05], and this was most marked in those with active disease, as defined by modified Harvey Bradshaw index ≥8 [active versus inactive CD: 6.3 (5.9 to 9.0) μg/ml versus 8.9 (5.5 to 26.2) μg/ml; P = 0.01], and in NOD2 mutant homozygotes/compound heterozygotes (P = 0.03). Two of the latter patients had jejunostomies (modified Harvey Bradshaw scores 12 and 4) and the remaining two had ileostomies (modified Harvey Bradshaw scores 4 and 13). To control for differences in intestinal fluid flux, the data were expressed as a ratio of HD-5 to total protein concentration in the ileostomy fluid, which also demonstrated lower HD-5 levels in Crohn’s versus control patients; P = 0.04 (Figure 6B).
Table 1.
Patient Data for Small Intestinal Fluid Analyses
| Control patients | Crohn’s patients | |
|---|---|---|
| Number | 20 | 51 |
| Age | 45 (35 to 70) years | 39 (19 to 85) years |
| Gender (male/female) | 9/11 | 19/32 |
| Time since ileostomy | 0.32 (0.08 to 34) years | 4 (0.17 to 28) years |
| Jejunostomies/ileostomies | 1/19 | 5/46 |
| HD-5 levels | 10.5 (6 to 30.4) μg/ml | 7.9 (5.5 to 35) μg/ml |
| HD-5 levels according to Crohn’s disease activity* | ||
| Inactive | 8.9 (5.5 to 26.2) μg/ml, n = 37 | |
| Active | 6.3 (5.9 to 9) μg/ml, n = 7 | |
| HD-5 levels according to NOD2 genotype† | ||
| WT | 10.4 (6 to 23.5) μg/ml, n = 13 | 7.9 (5.5 to 26.2) μg/ml, n = 35 |
| Heterozygote | 14.2 (7.4 to 30.4), n = 7 | 8.2 (6.2 to 35) μg/ml, n = 12 |
| Hom/Com Het | 5.9 (5.8 to 8.5) μg/ml, n = 4 |
Data given as median (range).
Active Crohn’s defined as modified Harvey Bradshaw index 8 or more. Inactive Crohn’s defined as index of 3 or less.
WT indicates NOD2 wild-type genotype. Hom/Com Het indicates NOD2 mutant homozygote (SNP13, n = 3) or compound heterozygote (SNP8/SNP13, n = 1).
Figure 6.
A: A scatter plot of HD-5 concentration in ileostomy fluid of Crohn’s (n = 51) and control (n = 20) patients. HD-5 levels are lower in Crohn’s patients than in controls [median (range): 7.9 (5.5 to 35.0) μg/g versus 10.5 (6.0 to 30.4) μg/ml; P = 0.05]. B: When expressed as a ratio of HD-5 to total protein concentration in the ileostomy fluid, to control for variation in intestinal fluid flux, the median value is lower in Crohn’s than controls [266 (28 to 2165) μg/g versus 358 (88 to 2576) μg/g; P = 0.04)].
HD-5 in Ileostomy Fluid of Patients with CD (but Not Controls) Is Present in a Complex with Chymotrypsin and Trypsin
Initial Evidence from AU-PAGE and SDS-PAGE Westerns
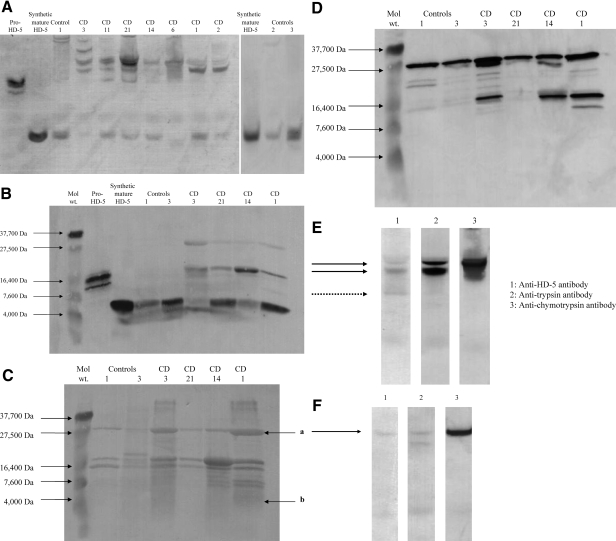
Molecular forms of HD-5 present in the ileostomy fluid of control (n = 3, reasons for colonic resection: ulcerative colitis, cancer, mesenteric ischemia) and CD patients (n = 7, Table 2) were investigated. In control concentrated ileostomy fluid samples, only one HD-5-immunoreactive band with electrophoretic mobility similar to that of synthetic mature HD-5 was seen on AU-PAGE Western blot analysis (Figure 7A). By contrast, multiple HD-5-immunoreactive bands were seen on AU-PAGE Western of ileostomy fluid samples from seven patients with CD. These included bands with similar electrophoretic mobility to those seen with mature and precursor forms of HD-5. However, the most prominent HD-5-positive bands had slower electrophoretic mobility than the precursor form of HD-5 isolated from normal small intestinal crypt cell extract (Figure 7A). When analyzed by SDS-PAGE Western under reducing/denaturing conditions, the most prominent HD-5-immunoreactive bands were seen in the regions of the mature and precursor forms of HD-5. Weak HD-5-positive bands with electrophoretic mobility slower than pro-HD-5 (similar to that for the 27.5-kDa marker) were also seen (Figure 7B), which could be attributable to remnants of a complex containing mature HD-5 and other proteins responsible for delayed electrophoretic mobility of HD-5 on AU-PAGE and which becomes dissociated under the reducing/denaturing conditions of SDS-PAGE.
Table 2.
HD5 Immunoreactivity
| Details of Crohn’s disease patients and ileostomy fluid samples | Fractions that were HD-5-positive on RP-HPLC | HD-5-positive bands on AU-PAGE Western | HD-5-positive bands on SDS-PAGE Western |
|---|---|---|---|
| CD1: 28-year-old female. Three previous resections, high ileostomy output. Ileostomy fluid obtained 1.6 years after last operation. No evidence of active small bowel disease. Homozygous for NOD2 SNP13 mutation. | 50% acetonitrile: not studied further. 59% acetonitrile: amino acid sequences matching trypsin. | Band at position consistent with mature HD-5. Also, two bands with slower electrophoretic mobility than pro-HD-5. | Bands with positions consistent with mature HD-5 and pro-HD-5. Also, weak band at greater size than pro-HD-5 (∼28 kDa). |
| CD2: 64-year-old female. Three previous resections. High ileostomy output. Ileostomy fluid obtained 4 years after last operation. No evidence of active small bowel disease. NOD2 SNP8/SNP13 compound heterozygote mutations. | 59% acetonitrile (only): not studied further. | Band at position consistent with mature HD-5 (weak). Also, two bands with slower electrophoretic mobility than pro-HD-5. | Not analyzed. |
| CD3: 25-year-old female. Previous pan-proctocolectomy. Ileostomy fluid obtained 2 years after surgery. No evidence of active small bowel disease. NOD2 wild type. | Not analyzed by RP-HPLC. | Band at position consistent with mature HD-5 (weak). Also, three bands with slower electrophoretic mobility than pro-HD-5. | Bands with positions consistent with mature HD-5 and pro-HD-5. Also, weak band at greater size than pro-HD-5 (∼28 kDa). |
| CD6: 19-year-old male. One previous resection. Ileostomy fluid collected 5 weeks after operation. No evidence of active small bowel disease. NOD2 SNP8 heterozygote mutation. | 59% acetonitrile (only): amino acid sequences matching for chymotrypsinogen, chymotrypsin (chain B) and chymotrypsin C (caldecrin). | Band with slower electrophoretic mobility than pro-HD-5 and smear below to position of pro-HD-5. | Not analyzed. |
| CD14: 32-year-old female. Previous right hemicolectomy and end ileostomy. Ileostomy fluid collected 6 months after surgery. NOD2 SNP8 heterozygote mutation. | Not analyzed by RP-HPLC | Band at position consistent with mature HD-5. Also, two bands with slower electrophoretic mobility than pro-HD-5. | Bands with positions consistent with mature HD-5 and pro-HD-5. Also, weak band at greater size than pro-HD-5 (∼28 kDa). |
| CD11: 35-year-old female. Two previous resections, loop ileostomy. Ileostomy fluid collected 2 years after last operation. No evidence of active small bowel disease. NOD2 wild type. | 39% acetonitrile: not studied further. 50% acetonitrile: not studied further. 59% acetonitrile: amino acid sequences matching for chymotrypsinogen and chymotrypsin (chain B). | Bands at positions consistent with mature HD-5 and pro-HD-5. Also, two bands with slower electrophoretic mobility than pro-HD-5. | Not analyzed. |
| CD21: 59-year-old male. Two previous resections. Ileostomy fluid collected 5 weeks after last operation. No evidence of active small bowel disease. NOD2 wild type. | 50% acetonitrile: N-terminal amino acid sequence matching mature HD-5. 59% acetonitrile: amino acid sequences matching for chymotrypsinogen and chymotrypsin (chain B). | Bands at positions consistent with mature HD-5 and pro-HD-5. Also, two bands with slower electrophoretic mobility than pro-HD-5. | Bands with positions consistent with mature HD-5 and pro-HD-5. Also weak band at greater size than pro-HD-5 (∼28 kDa). |
| Control 1: 38-year-old female. Pan-proctocolectomy and end ileostomy for ulcerative colitis. Ileostomy fluid collected years after operation. NOD2 wild type. | 50% acetonitrile: N-terminal amino acid sequence matching mature HD-5. | Band at position consistent with mature HD-5 only. | Band at position consistent with mature HD-5 only. |
| Control 2: 59-year-old male. Right hemicolectomy and loop ileostomy for cecal carcinoma. Ileostomy fluid collected 6 weeks after operation NOD2 wild type. | Not analyzed by RP-HPLC. | Band at position consistent with mature HD-5 only. | Not analyzed. |
| Control 3: 55-year-old male. Small intestinal resection for ischemic bowel. High output ileostomy. Ileostomy fluid collected 2 years after operation NOD2wild type. | Not analyzed by RP-HPLC. | Band at position consistent with mature HD-5 only. | Band at position consistent with mature HD-5 only. |
Figure 7.
A: HD-5 AU-PAGE Western blot of ileostomy fluid. After concentration using cation exchange beads, ileostomy fluid samples (and also pro-HD-5 isolated from normal ileal epithelial crypts and synthetic mature HD-5) were used for AU-PAGE. After transfer to PVDF membrane, anti-HD-5 antibody was used for immunoblotting. In control ileostomy fluid samples, only one HD-5 immunoreactive band is seen, with electrophoretic mobility similar to that for synthetic mature HD-5. However, multiple HD-5-positive bands are seen in all seven CD (CD1, CD2, CD3, CD6, CD11, CD14, CD21) ileostomy samples. HD-5-immunoreactive bands with electrophoretic mobility similar to mature and precursor forms of HD-5 are seen in many CD samples. However, the most prominent HD-5-positive bands in the CD ileostomy fluid samples have slower electrophoretic mobility than the precursor form of HD-5 isolated from normal ileal crypt epithelial extract. Varying amounts of total concentrated cationic proteins were applied per lane. B: HD-5 SDS-PAGE Western blot of ileostomy fluid. Some of the same ileostomy fluid samples as in A were used for SDS-PAGE under reducing/denaturing conditions, before transfer to PVDF membrane and immunoblotting using anti-HD-5 antibody. As expected, the control ileostomy fluid samples have similar electrophoretic mobility to that for synthetic mature HD-5. In the CD ileostomy fluid samples, the most prominent HD-5-immunoreactive bands have electrophoretic mobility similar to mature and pro-HD-5. Weakly HD-5-positive bands with slower migration than pro-HD-5 are also seen. C: Protein bands after SDS-PAGE Western blot of ileostomy fluid. The same samples as in B were separated by SDS-PAGE and PVDF membrane with transferred protein was stained with amido black. Multiple protein bands are seen in the control and CD ileostomy fluid samples. N-terminal sequencing of protein band a in sample from CD1 revealed two amino acid sequences: IVNGE and IVGGY, which match N-terminal sequences for chymotrypsinogen (expected molecular weight of 27.8 kDa with signal sequence) and trypsin (expected molecular weight of trypsinogen-1 of 26.56 kDa with signal sequence) respectively. N-terminal sequencing of weak protein band b showed the amino acid sequence: ATCYCR, which matches the N-terminal sequence for mature HD-5. D: Chymotrypsin SDS-PAGE Western blot of ileostomy fluid. SDS-PAGE gel was run with samples as in C, but the PVDF membrane with transferred proteins was used for immunoblotting using anti-chymotrypsin antibody. Chymotrypsin-immunoreactive bands are seen (in similar region to 27.5-kDa molecular weight marker) in both control and CD ileostomy fluid samples (additional chymotrypsin-immunoreactive bands with lower molecular weights are also seen in a number of the samples). E: AU-PAGE Western blots of one CD ileostomy fluid sample. Concentrated ileostomy fluid from CD patient CD21 was separated by AU-PAGE in gels runs simultaneously. After transfer of the same samples to three PVDF membranes, immunoblotting was undertaken using antibodies to HD-5 (lane 1), trypsin (lane 2), and chymotrypsin (lane 3). The dashed arrow in lane 1 is likely to represent pro-HD-5 (mature is not seen) and the two solid arrows point to HD-5-immunoreactive bands with slower electrophoretic mobility that pro-HD-5. Trypsin (blot 2)- and chymotrypsin-immunoreactive (blot 3) bands are seen in similar positions to those for the slowest two HD-5-positive bands. Mature HD-5 is not seen because this had run off the AU-PAGE gel. F: Identical experiment to E, using concentrated ileostomy fluid from CD patient CD14. Again HD-5 immunoreactivity is seen at the same position as trypsin and chymotrypsin immunoreactivity. No pro-HD-5 is seen in this sample (as also seen in CD14 lane in A), and mature HD-5 had run off the gel.
Protein staining of polyvinylidene difluoride (PVDF) membrane with the same samples (after transfer from SDS-PAGE gel similar to Figure 7B) showed multiple bands in CD and control ileostomy fluid samples (Figure 7C). N-terminal sequencing of low-molecular weight protein band of one CD sample confirmed the presence of mature HD-5 (sequence: ATCYCRT). In this sample there was also a prominent protein band in the region of the weakly HD-5-immunoreactive band (at approximately the same level as 27.5-kDa marker) in Figure 7B and N-terminal sequencing of this band revealed two sequences, IVNGE and IVGGY, which matched chymotrypsin (chain B) and trypsin sequences, respectively. These studies therefore suggest that HD-5 could be present in Crohn’s ileostomy fluid in a complex with chymotrypsin and trypsin. The presence of chymotrypsin in the ileostomy fluid samples was confirmed by SDS-PAGE Western blot analysis using anti-chymotrypsin antibody, which showed strongly immunoreactive bands at ∼27.5 kDa in both control and CD ileostomy fluid samples (Figure 7D). Additional lower molecular weight chymotrypsin-immunoreactive bands were also seen in a number of the samples.
Ileostomy fluid from two patients with CD (CD21 and CD14) were studied by application to AU-PAGE gels (run simultaneously), which were subsequently used for immunoblotting studies using antibodies to HD-5, chymotrypsin, and trypsin (Figure 7, E and F). In the sample from patient CD21, three HD-5-positive bands were seen, of which the two with the slowest electrophoretic mobility were in a similar position to trypsin- and chymotrypsin-immunoreactive bands. Based on earlier AU-PAGE Western of the same sample (Figure 7A), we believe that the HD-5-positive band with the fastest electrophoretic mobility is pro-HD-5. In ileostomy fluid from patient CD14 (Figure 7F), one band (as also seen in Figure 7A) with slow electrophoretic mobility was seen, which was in a similar position to trypsin- and chymotrypsin-immunoreactive bands. In both figures (Figure 7, E and F), mature HD-5 is not seen because it had run off the gel.
RP-HPLC Elution Profiles of HD-5 Are Different in Crohn’s Ileostomy Fluid Samples, Compared to Controls
After application of concentrated ileostomy fluid samples from control patients to a C18 RP-HPLC column, HD-5 eluted at an acetonitrile concentration of 50% and its N-terminal sequence (ATCYCRT) was identical to that predicted for mature HD-5 (Figure 8A). By contrast, after application of concentrated ileostomy fluid samples from patients with CD, the majority of HD-5 eluted at 59% acetonitrile in samples from all five patients studied (Figure 8, B and D). As summarized in Table 2, HD-5 immunoreactivity was also seen in fractions eluting at 50% acetonitrile (in three samples) and 39% acetonitrile (in one sample). HD-5 was detected in dot blots of fractions eluting at 59%, 50%, and 39% acetonitrile from one sample (CD11, Figure 8D). Mature HD-5 would be expected to elute at 50% acetonitrile and this was confirmed by N-terminal sequencing of HD-5 (ATCYCRT, Figure 1) eluting in this region from one CD sample (CD21, Figure 8B). Our earlier studies using crypt cell extracts had shown that pro-HD-5 elutes at 39% acetonitrile (Figure 4).
Figure 8.
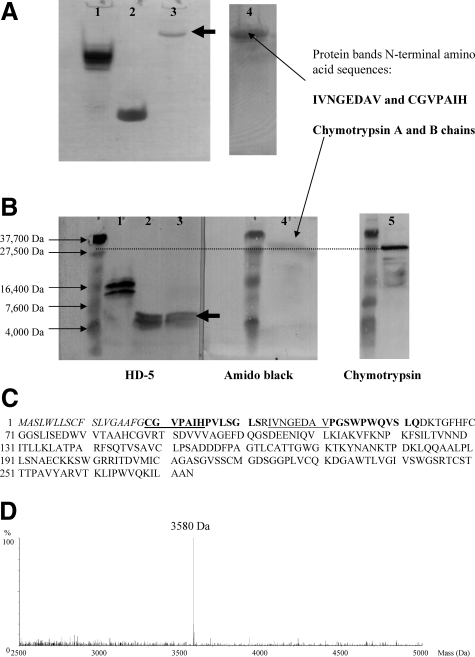
RP-HPLC fractionation. Cation exchange-concentrated ileostomy fluid, of control patient (A) and Crohn’s patients (B and D), was applied to C18 RP-HPLC column, and fractions immunoreactive (by dot blot) for HD-5 were collected (arrowed on traces). A: Control patient (formerly with ulcerative colitis). Only a single peak (arrows), at 50% acetonitrile, was immunoreactive for HD-5 and had N-terminal sequence ATCYCRT, identical to predicted mature HD-5 peptide. B: Crohn’s patient CD21. HD-5 eluted at 50% acetonitrile (small arrow) and 59% acetonitrile (large arrow). After application to PVDF membrane, N-terminal sequencing was performed on protein eluting at 50% acetonitrile, which revealed the sequence ATCYCRT, which matches that for the mature form of HD-5. The HD-5-positive 59% acetonitrile fraction was subjected to AU-PAGE Western blot analysis (see Figure 9A), SDS-PAGE Western blot analysis (see Figure 9B) and ES-QToF MS (see Figure 9, C and D). C: Predicted amino acid sequence (from cDNA) of trypsin-1 (cationic trypsin). Underlined are amino acid sequences from one CD (CD1) ileostomy fluid fraction eluting at 59% acetonitrile. D: Crohn’s patient CD11. Three fractions were immunoreactive for HD-5 (39%, 50%, and 59% acetonitrile, respectively, arrowed). The large arrowed peak (59% acetonitrile) was run on AU-PAGE, and N-terminal sequences of the immunoreactive band obtained were IVNGE (chymotrypsin chain B) and CGVPA (chymotrypsinogen).
Complex of HD-5, Chymotrypsinogen/Chymotrypsin, and Trypsin in Crohn’s Ileostomy Fluid Elutes in 59% Acetonitrile RP-HPLC Fraction
After application of CD-concentrated ileostomy fluid samples to RP-HPLC column, the majority of immunoreactive HD-5 eluted with 59% acetonitrile. AU-PAGE of the 59% acetonitrile fraction from all four CD ileostomy fluid samples showed a single protein band. N-terminal sequencing (Edman degradation after AU-PAGE) revealed amino acid sequences that matched chymotrypsinogen and chymotrypsin (chain B) in three (patients CD6, CD11, CD21; Table 2) and trypsin in the fourth sample (CD1), confirming findings using whole concentrated ileostomy fluid sample (Figure 7C), where sequences matching for chymotrypsin and trypsin were seen.
Studies by ES-QToF MS (using the same 59% acetonitrile fraction from three CD ileostomy fluid samples) confirmed the above N-terminal sequencing findings. Thus, analysis of the sample from patient CD1 revealed three sequences that matched the amino acid sequence of trypsin-1 (cationic trypsin) (Figure 8C). In the sample from CD patient CD21, amino acid sequences matching chymotrypsinogen/chymotrypsin (chain B) were seen (Figure 9C). In the 59% acetonitrile sample from patient CD6, three sequences matching for chymotrypsin C (caldecrin, calcium-decreasing factor) were seen.
Figure 9.
Characterization of complexed form of HD-5 in ileostomy fluid of CD patient CD21. A: AU-PAGE Western blot analysis of RP-HPLC 59% acetonitrile fraction containing HD-5 from CD ileostomy fluid (Figure 8B). After AU-PAGE, the 59% acetonitrile fraction (lane 3), synthetic mature HD-5 (lane 2), and acid extract of ileal crypt epithelial cells (lane 1) were transferred to PVDF membrane for immunoblotting using anti-HD-5 antibody. In the 59% acetonitrile fraction of ileostomy fluid, a single HD-5-positive band was seen (lane 3), with electrophoretic mobility slower than that for pro-HD-5 in ileal crypt epithelial cell extract (lane 1). Amido black staining of the same membrane revealed a prominent protein band in the same position as the HD-5-immunoreactive band. N-terminal sequencing of this protein band revealed two sequences, IVNGEDAV and CGVPAIH, which match for chymotrypsinogen and chymotrypsin chain B, respectively. B: SDS-PAGE Western blot analysis of RP-HPLC 59% acetonitrile fraction containing HD-5 from CD ileostomy fluid (Figure 8B). After SDS-PAGE and transfer to PVDF membrane of the same samples as in A, immunoblotting showed that the HD-5-positive band now had electrophoretic mobility similar to that of synthetic mature HD-5. Amido black staining of the same membrane showed a prominent protein band at ∼27.5 kDa. Immunoblotting of a similar PVDF membrane using anti-chymotrypsin antibody also showed a prominent band at ∼27.5 kDa. C: Amino acid sequence of chymotrypsinogen (amino acids 19 to 263) and its signal sequence (amino acids 1 to 18). Cleavage for activation leads to chymotrypsin chain A (amino acids 19 to 33) and chymotrypsin chain B (amino acids 34 to 164). Sequences in bold were identified after ES-QToF MS analysis of HD-5-positive 59% acetonitrile fraction. Edman degradation revealed the underlined sequences. D: MS analysis of HD-5-positive 59% acetonitrile fraction revealed a mass of 3580 Da, consistent with predicted mass of mature HD-5 (see Figure 1).
Results of AU-PAGE and SDS-PAGE Western blot analyses using HD-5-positive RP-HPLC fraction eluting at 59% acetonitrile, from ileostomy fluid of CD patient CD21 are shown in Figure 9, A and B. HD-5 AU-PAGE Western (Figure 9A) showed a single-positive band that migrated more slowly than pro-HD-5 (isolated from normal ileal crypts). Amido black staining of the same membrane showed a single band in the same region as the HD-5-immunoreactive band. N-terminal sequencing of this band (Edman degradation) revealed two sequences, CGVPAIH and IVNGEDAV, that match chymotrypsinogen and chymotrypsin (chain B), respectively (Figure 9C). HD-5 SDS-PAGE Western under reducing/denaturing conditions (Figure 9B) showed a single-positive band with electrophoretic mobility approximately similar to that for the molecular weight 4-kDa marker and in similar position to synthetic mature HD-5. Amido black staining of the membrane showed a positive band at 27.5 kDa. Chymotrypsin SDS-PAGE Western (under reducing/denaturing conditions) showed that the main positive band had electrophoretic mobility similar to the 27.5-kDa molecular weight marker (with weaker, small bands below).
ES-QToF MS data confirmed the presence of sequences matching chymotrypsinogen/chymotrypsin (chain B) (Figure 9C). These studies also showed the presence of a mass (3580 Da) consistent with that for mature HD-5 (Figure 9D). Thus, in this HPLC-purified fraction, HD-5 is present in its mature form (processed from precursor form but remaining associated with protease complex). Comparison with Figure 7E suggests that the HD-5-positive band with the slowest electrophoretic mobility in this figure is eluting at 59% acetonitrile and contains both chymotrypsinogen/chymotrypsin and trypsin. Immunoblotting studies also showed that α1-anti-trypsin is present in the RP-HPLC fraction eluting at 59% acetonitrile, from ileostomy fluid of all five patients with CD studied (patients CD1, CD2, CD6, CD11, and CD21; Figure 10). Taken together, the above studies suggest that a complex of mature HD-5, chymotrypsinogen/chymotrypsin (chain B), trypsin, and α1-anti-trypsin migrate together on AU-PAGE and elute together at 59% acetonitrile on RP-HPLC, but become dissociated from each other under reducing/denaturing conditions of SDS-PAGE.
Figure 10.
α1-Anti-trypsin immunoreactivity in dot blots of acid extract of normal small intestinal crypt epithelial cells (a), cation exchange-concentrated ileostomy fluid from control patient 1(b), cation-exchange concentrated ileostomy fluid from a patient with CD (CD21) (c), and HD-5-containing 59% acetonitrile RP-HPLC fraction from patients with CD (d: CD1; e: CD2; f: CD6; g: CD11; and h: CD21). Samples applied to PVDF membranes contained varying amounts of total protein.
Lack of an Association between HD-5 and Chymotrypsin/Trypsin in Crypt Cell Extracts
Crypt cell extracts and postoperative ileostomy fluid from the same patient with CD (CD6, Figures 3 and 6) were studied (Figure 11A). As expected for ileostomy fluid, AU-PAGE Westerns showed that the two slowest HD-5-immunoreactive bands had similar electrophoretic mobility as trypsin-positive bands. The only chymotrypsin-immunoreactive band had an electrophoretic mobility similar to that for the slowest migrating HD-5- and trypsin-positive bands. By contrast, crypt cell extracts applied to the same AU-PAGE gels as ileostomy fluid showed distinct electrophoretic mobility profiles for HD-5-, chymotrypsin-, and trypsin-immunoreactive bands. Moreover, pro-HD-5 purified from crypt cell extracts (eluting at 39% acetonitrile) did not contain any chymotrypsin or trypsin when analyzed by dot blots (Figure 11B). The above studies imply that HD-5, chymotrypsin, and trypsin are not associated with each other in crypt cell extracts (as is the case in ileostomy fluid).
Figure 11.
Lack of an association between HD-5 and chymotrypsin/trypsin in crypt cell extracts. A: AU-PAGE Westerns of crypt epithelial extract and ileostomy fluid concentrate from same patient. Ileal crypt epithelial acid extract obtained from resection specimen of patient CD6 (lanes 1, 3, and 5) was separated by AU-PAGE in same gels as concentrated ileostomy fluid from the same patient (obtained 5 weeks after operation; lanes 2, 4, and 6). After transfer to PVDF membranes, immunoblotting was performed using antibodies to chymotrypsin (i), HD-5 (ii), and trypsin (iii). Chymotrypsin- and trypsin-immunoreactive bands in ileostomy fluid (lanes 2 and 6, respectively) have similar electrophoretic mobility as two HD-5 immunoreactive bands (dashed arrows, lane 4). By contrast, for ileal crypt epithelial cell extracts, electrophoretic mobility profiles of immunoreactive bands for chymotrypsin (lane 1), HD-5 (lane 3), and trypsin (lane 5) do not show any similarity. For HD-5 in ileostomy fluid (lane 4), the findings are consistent with the presence of the peptide in free mature form (solid arrow), and in a complex with trypsin and chymotrypsin (dashed arrows). B: Absence of chymotrypsin and trypsin in preparations of pro-HD-5 purified from small intestinal crypt extracts. RP-HPLC-purified pro-HD-5 from acid extracts of normal small intestinal crypt epithelial cells (lane 1) and acid extracts of crypt epithelial cells from two separate Crohn’s small intestinal resection specimens (lanes 2 and 3) were studied by dot blot analysis. Purified pro-HD-5 preparations from all three crypt extracts were immunoreactive for HD-5, but not trypsin or chymotrypsin.
Chymotrypsin Is Expressed by Paneth Cells in the Small Intestine
Immunohistochemical studies showed chymotrypsin immunoreactivity in Paneth cells in CD and normal small intestinal mucosal samples (Figure 12). Immunostaining on the apical surface of villus enterocytes in some sections implies significant luminal secretion of this enzyme.
Figure 12.
Immunohistochemistry of normal ileal mucosa (A, B) and Crohn’s ileal mucosa (C, D) using mouse anti-human chymotrypsin monoclonal antibody. Immunoreactivity is seen within Paneth cells in both normal and Crohn’s tissue, indicating the presence of chymotrypsin in these cells. In the normal sections, immunoreactive material is seen to coat the villi, in keeping with the secretion of chymotrypsin from Paneth cells.
Discussion
Studies by immunohistochemistry showed expression of HD-5 in Paneth cells of normal ileum and that affected by CD. In addition, in CD sections, a few strongly HD-5-immunoreactive intermediate cells were also seen. Such cells have morphological features of Paneth and goblet cells24 and were only occasionally seen in the normal ileum.9 Occasional HD-5-positive cells with morphological features of absorptive enterocytes were also seen in the crypt. We have recently seen such cells in the jejunum of patients after gastric bypass surgery, associated with luminal secretion of HD-5.28 It remains to be determined whether such cells synthesize HD-5 or have taken it up from the lumen after secretion by Paneth cells. Significant luminal HD-5 immunoreactivity in sections with active CD implies active secretion via Paneth cell degranulation.
Paneth cells in the normal small intestine have previously been shown to store HD-5 in precursor form.9,18 To determine whether these cells process pro-HD-5 in the presence of inflammation, HD-5 was isolated from crypts of small intestine affected by CD. We confirmed previous findings9,18 regarding the presence of precursor form of HD-5 in Paneth cells of normal small intestine. HD-5 stored in Paneth cells of inflamed small intestine of patients with CD was also found to be present almost exclusively in the precursor form. Similar studies using metaplastic Paneth cell-containing colonic mucosal samples from patients with ulcerative colitis and CD also showed the presence of only the precursor form of HD-5 (unpublished results). This suggests that HD-5 in the normal and inflamed intestine is processed by Paneth cells either during secretion, or in the lumen.
We could easily detect HD-5 immunoreactivity (by dot blot analysis) in ileostomy fluid of controls and patients with CD. We found that HD-5 concentrations in ileostomy fluid of patients with CD were lower than in controls, especially those with active disease (as assessed by modified Harvey Bradshaw index) and those with homozygote (SNP13, n = 3)/compound heterozygote (SNP8/SNP13, n = 1) mutations in the NOD2 gene. It is possible that in some patients the modified Harvey Bradshaw index mainly reflects increased ileostomy output. This, together with reduced total Paneth cell mass attributable to intestinal ulceration could be responsible for the reduction in the intraluminal concentration of HD-5. However, low concentrations of HD-5 in the four patients with homozygous/compound heterozygous mutations in the NOD2 gene is of interest because a recent study has reported reduced expression of HD-5 mRNA transcripts and protein levels in Paneth cells of CD patients with SNP13 mutations in the NOD2 gene.29 A recent study also postulates that the process of disulfide bridge formation may be disturbed in Paneth cells of patients with CD.30
We believe the most interesting findings of our study relate to the context in which HD-5 is present in the small intestinal lumen of patients with CD. In contrast to controls (in whom we confirmed previous reports of the presence of only the mature form of HD-518), HD-5 in the lumen of patients with CD existed in three forms: mature, precursor, and that associated in a complex with trypsin and chymotrypsinogen/chymotrypsin. The latter form of HD-5 migrated more slowly in AU-PAGE gel than the precursor form present inside Paneth cells. This complex also had a different elution profile on RP-HPLC (eluting at 59% acetonitrile), compared to free mature (eluted at 50% acetonitrile) and free precursor (eluted at 39% acetonitrile) forms of HD-5. The complex showed immunoreactivity for trypsin and chymotrypsin and analysis by ES-QToF MS and N-terminal sequencing confirmed the presence of amino acid sequences for trypsin and chymotrypsinogen/chymotrypsin. When analyzed by SDS-PAGE Western blot analysis, under reducing/denaturing conditions, the complex dissociated to reveal the presence of HD-5 with characteristics similar to the mature form and the two enzymes (trypsin and chymotrypsinogen/chymotrypsin). Thus, the complex appears to be stable during AU-PAGE and RP-HPLC but becomes dissociated in the presence of a reducing agent and boiling, before SDS-PAGE. Comparison of HD-5 AU-PAGE and SDS-PAGE Westerns of the same CD ileostomy fluid samples (Figure 7, A and B), suggests that the complex may contain both the precursor and mature forms of HD-5, together with trypsin and chymotrypsinogen/chymotrypsin. Although only the mature form of HD-5 is present in control ileostomy fluid, the presence of immunoreactive chymotrypsin (Figure 7D) and trypsin (not shown) suggests that the processing of pro-HD-5 in the normal small intestine occurs in a similar complex containing trypsin and chymotrypsinogen/chymotrypsin.
Isoforms of trypsin, pancreatic trypsin inhibitor (PSTI), and α1-anti-trypsin have previously been shown to be present in Paneth cells.18,20,21,31 We have found strong chymotrypsin immunoreactivity in Paneth cells by immunohistochemistry and also by AU-PAGE in crypt cell extract. Thus, Paneth cells appear to be a significant extra-pancreatic source of trypsin and chymotrypsin. It is known that chymotrypsinogen (from Paneth cells/pancreas), requires cleavage by trypsin for activation.32,33 Such activation may occur in a complex that also involves a precursor form of HD-5, which would be processed by trypsin.18 Because neither trypsin nor chymotrypsinogen/chymotrypsin were associated with pro-HD-5 purified from crypt extracts, it is likely that the precursor form of HD-5 is enzymatically processed in the lumen, after secretion by Paneth cells. Our previous studies showed that processing to mature form of HD-5 did not occur after stimulation of Paneth cell secretion in vitro.9 We believe therefore that Paneth cells in the normal and inflamed small intestine secrete mainly the precursor form of HD-5, which subsequently becomes associated with Paneth cell-derived trypsin and chymotrypsinogen, with subsequent processing to mature HD-5 and chymotrypsin.
The persistence of the luminal complex of mature/pro-HD-5, trypsin, and chymotrypsinogen/chymotrypsin was seen in all seven patients with CD studied (Figure 7A). This implies a CD-specific disturbance in the normal processing of the precursor form of HD-5. None of five CD patients studied further had evidence of overtly active small intestinal disease, implying an underlying abnormality in the processing of pro-HD-5 in CD. Persistence of the luminal complex of HD-5, trypsin, and chymotrypsinogen/chymotrypsin in CD could be attributable to the presence of excess luminal anti-proteases. This concept is supported by the presence of immunoreactive α1-anti-trypsin in the complex. Indeed, increased small intestinal epithelial expression of α1-anti-trypsin has been reported in CD.31 The proposed mechanism for impaired HD-5 processing in CD is depicted in Figure 13.
Figure 13.
Schematic diagram for proposed incomplete/impaired processing of pro-HD-5 after secretion from Paneth cells in CD small intestinal lumen.
The identification of chymotrypsin C (caldecrin, calcium-decreasing factor) in one 59% acetonitrile sample from a patient with CD suggests that the luminal complex may contain other proteins and further studies are required to characterize them. In our limited in vitro studies, HD-5 in complexed form (RP-HPLC 59% acetonitrile fraction) did express antimicrobial activity (data not shown). This may reflect the fact that, although not fully dissociating it, the purification procedure undertaken does affect the complex such that free mature HD-5 is readily released during the antimicrobial assay. It may also reflect the antimicrobial activity of pro-HD-5 from within the complex.
Our studies indicate that the majority of HD-5 in Crohn’s small intestinal fluid exists in a complex. It is possible that the persistence of HD-5 in a complex with trypsin, chymotrypsinogen/chymotrypsin, and α1-anti-trypsin, leads to impaired intraluminal function of this antimicrobial peptide in vivo, which may allow increased exposure of the intestinal mucosa to luminal microorganisms and their products. Although NOD2 protein has been shown to be expressed by Paneth cells, we found persistence of luminal HD-5 in complex form in patients with homozygote/compound heterozygote mutations in the NOD2 gene as well as those with the wild-type NOD2 gene.
Acknowledgments
We thank Mr. M. Weldon, Department of Biochemistry, Cambridge University, UK, for performing N-terminal sequencing; Dr. P. Tighe for assistance in NOD2 genotyping; and our clinical colleagues for facilitating collection of samples from patients.
Footnotes
Address reprint requests to Y.R. Mahida, Institute of Infection, Immunity, and Inflammation, C Floor, West Block, Queen’s Medical Centre, Nottingham, NG7 2UH, UK. E-mail: yash.mahida@nottingham.ac.uk.
Supported by the University of Nottingham and the Medical Research Council (UK).
References
- Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- Strober W, Murray PJ, Kitani A, Watanabe T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol. 2006;6:9–20. doi: 10.1038/nri1747. [DOI] [PubMed] [Google Scholar]
- Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P, Gower-Rousseau C, Macry J, Colombel JF, Sahbatou M, Thomas G. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, Achkar JP, Brant SR, Bayless TM, Kirschner BS, Hanauer SB, Nunez G, Cho JH. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- Hampe J, Cuthbert A, Croucher PJ, Mirza MM, Mascheretti S, Fisher S, Frenzel H, King K, Hasselmeyer A, MacPherson AJ, Bridger S, van Deventer S, Forbes A, Nikolaus S, Lennard-Jones JE, Foelsch UR, Krawczak M, Lewis C, Schreiber S, Mathew CG. Association between insertion mutation in NOD2 gene and Crohn’s disease in German and Br populations. Lancet. 2001;357:1925–1928. doi: 10.1016/S0140-6736(00)05063-7. [DOI] [PubMed] [Google Scholar]
- Inohara N, Chamaillard M, McDonald C, Nunez G. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu Rev Biochem. 2005;74:355–383. doi: 10.1146/annurev.biochem.74.082803.133347. [DOI] [PubMed] [Google Scholar]
- Lala S, Ogura Y, Osborne C, Hor SY, Bromfield A, Davies S, Ogunbiyi O, Nunez G, Keshav S. Crohn’s disease and the NOD2 gene: a role for Paneth cells. Gastroenterology. 2003;125:47–57. doi: 10.1016/s0016-5085(03)00661-9. [DOI] [PubMed] [Google Scholar]
- Ogura Y, Lala S, Xin W, Smith E, Dowds TA, Chen FF, Zimmermann E, Tretiakova M, Cho JH, Hart J, Greenson JK, Keshav S, Nunez G. Expression of NOD2 in Paneth cells: a possible link to Crohn’s ileitis. Gut. 2003;52:1591–1597. doi: 10.1136/gut.52.11.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunliffe RN, Rose FR, Keyte J, Abberley L, Chan WC, Mahida YR. Human defensin 5 is stored in precursor form in normal Paneth cells and is expressed by some villous epithelial cells and by metaplastic Paneth cells in the colon in inflammatory bowel disease. Gut. 2001;48:176–185. doi: 10.1136/gut.48.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson JC, Watson SH. Paneth cell metaplasia in ulcerative colitis. Am J Pathol. 1961;38:243–249. [PMC free article] [PubMed] [Google Scholar]
- Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elphick DA, Mahida YR. Paneth cells: their role in innate immunity and inflammatory disease. Gut. 2005;54:1802–1809. doi: 10.1136/gut.2005.068601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005;6:551–557. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr. 2002;22:283–307. doi: 10.1146/annurev.nutr.22.011602.092259. [DOI] [PubMed] [Google Scholar]
- Simon GL, Gorbach SL. Intestinal flora in health and disease. Gastroenterology. 1984;86:174–193. [PubMed] [Google Scholar]
- Ahmad T, Armuzzi A, Bunce M, Mulcahy-Hawes K, Marshall SE, Orchard TR, Crawshaw J, Large O, de Silva A, Cook JT, Barnardo M, Cullen S, Welsh KI, Jewell DP. The molecular classification of the clinical manifestations of Crohn’s disease. Gastroenterology. 2002;122:854–866. doi: 10.1053/gast.2002.32413. [DOI] [PubMed] [Google Scholar]
- Jones DE, Bevins CL. Paneth cells of the human small intestine express an antimicrobial peptide gene. J Biol Chem. 1992;267:23216–23225. [PubMed] [Google Scholar]
- Ghosh D, Porter E, Shen B, Lee SK, Wilk D, Drazba J, Yadav SP, Crabb JW, Ganz T, Bevins CL. Paneth cell trypsin is the processing enzyme for human defensin-5. Nat Immunol. 2002;3:583–590. doi: 10.1038/ni797. [DOI] [PubMed] [Google Scholar]
- Porter EM, van Dam E, Valore EV, Ganz T. Broad-spectrum antimicrobial activity of human intestinal defensin 5. Infect Immun. 1997;65:2396–2401. doi: 10.1128/iai.65.6.2396-2401.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohe M, Borgstrom A, Lindstrom C, Ohlsson K. Pancreatic endoproteases and pancreatic secretory trypsin inhibitor immunoreactivity in human Paneth cells. J Clin Pathol. 1986;39:786–793. doi: 10.1136/jcp.39.7.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senegas-Balas FO, Figarella CG, Amouric MA, Guy-Crotte OM, Bertrand CA, Balas DC. Immunocytochemical demonstration of a pancreatic secretory protein of unknown function in human duodenum. J Histochem Cytochem. 1991;39:915–919. doi: 10.1177/39.7.1865108. [DOI] [PubMed] [Google Scholar]
- Porter EM, Poles MA, Lee JS, Naitoh J, Bevins CL, Ganz T. Isolation of human intestinal defensins from ileal neobladder urine. FEBS Lett. 1998;434:272–276. doi: 10.1016/s0014-5793(98)00994-6. [DOI] [PubMed] [Google Scholar]
- Wilson CL, Ouellette AJ, Satchell DP, Ayabe T, Lopez-Boado YS, Stratman JL, Hultgren SJ, Matrisian LM, Parks WC. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 1999;286:113–117. doi: 10.1126/science.286.5437.113. [DOI] [PubMed] [Google Scholar]
- Kamal M, Wakelin D, Ouellette AJ, Smith A, Podolsky DK, Mahida YR. Mucosal T cells regulate Paneth and intermediate cell numbers in the small intestine of T. spiralis-infected mice. Clin Exp Immunol. 2001;126:117–125. doi: 10.1046/j.1365-2249.2001.01589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;1:514. doi: 10.1016/s0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- Best WR. Predicting the Crohn’s disease activity index from the Harvey-Bradshaw Index. Inflamm Bowel Dis. 2006;12:304–310. doi: 10.1097/01.MIB.0000215091.77492.2a. [DOI] [PubMed] [Google Scholar]
- Mahida YR, Galvin AM, Gray T, Makh S, McAlindon ME, Sewell HF, Podolsky DK. Migration of human intestinal lamina propria lymphocytes, macrophages and eosinophils following the loss of surface epithelial cells. Clin Exp Immunol. 1997;109:377–386. doi: 10.1046/j.1365-2249.1997.4481346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundbom M, Elphick D, Mahida Y. Alteration in human defensin-5 expression following gastric bypass surgery. J Clin Pathol. 2007;60:1029–1034. doi: 10.1136/jcp.2006.041871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehkamp J, Salzman NH, Porter E, Nuding S, Weichenthal M, Petras RE, Shen B, Schaeffeler E, Schwab M, Linzmeier R, Feathers RW, Chu H, Lima H, Jr, Fellermann K, Ganz T, Stange EF, Bevins CL. Reduced Paneth cell alpha-defensins in ileal Crohn’s disease. Proc Natl Acad Sci USA. 2005;102:18129–18134. doi: 10.1073/pnas.0505256102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe H, Ayabe T, Maemoto A, Ishikawa C, Inaba Y, Sato R, Moriichi K, Okamoto K, Watari J, Kono T, Ashida T, Kohgo Y. Denatured human alpha-defensin attenuates the bactericidal activity and the stability against enzymatic digestion. Biochem Biophys Res Commun. 2007;358:349–355. doi: 10.1016/j.bbrc.2007.04.132. [DOI] [PubMed] [Google Scholar]
- Molmenti EP, Perlmutter DH, Rubin DC. Cell-specific expression of alpha 1-antitrypsin in human intestinal epithelium. J Clin Invest. 1993;92:2022–2034. doi: 10.1172/JCI116797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krehbiel A, Kassell B, Laskowski M., Sr Activation of chymotrypsinogen B. Biochim Biophys Acta. 1964;92:312–318. doi: 10.1016/0926-6569(64)90188-9. [DOI] [PubMed] [Google Scholar]
- Balls AK, Ryan CA. Tryptic activation of acetylated chymotrypsinogen. Proc Natl Acad Sci USA. 1964;51:151–155. doi: 10.1073/pnas.51.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]