Abstract
Diabetic kidney disease is associated with monocyte chemoattractant CC chemokine ligand 2 (CCL2)-dependent glomerular and interstitial macrophage recruitment. In addition, nephropathy is delayed in Ccl2 mutant diabetic mice. However, whether the late onset of therapeutic Ccl2 blockade modulates the progression of advanced diabetic nephropathy remains unknown. We addressed this question by antagonizing Ccl2 with mNOX-E36–3′PEG, an anti-Ccl2 L-enantiomeric RNA aptamer (ie, a Spiegelmer), which binds murine Ccl2 and blocks the recruitment of ex vivo-labeled macrophages to the kidneys of db/db mice with type 2 diabetes. We injected mNOX-E36–3′PEG subcutaneously at a dose of 50 mg/kg three times per week into uninephrectomized (1K) db/db mice with advanced glomerulopathy from 4 to 6 months of age. mNOX-E36–3′PEG reduced the number of glomerular macrophages by 40% compared with nonfunctional (control) Spiegelmer-treated 1K db/db mice. This result was associated with protection from diffuse glomerulosclerosis and significantly improved the glomerular filtration rate. mNOX-E36–3′PEG also reduced renal Ccl2 mRNA and protein expression compared with control Spiegelmer-treated 1K db/db mice of the same age. Together, the late onset of therapeutic Ccl2 blockade, eg, with specific Spiegelmers, offers protection from diffuse glomerulosclerosis in type 2 diabetic db/db mice and, thus, may represent a novel therapeutic strategy for advanced glomerulosclerosis.
Glomerulosclerosis, eg, in diabetes, remains a leading cause of end-stage renal disease because targeting the angiotensin-dependent pathomechanisms does not always prevent disease progression.1,2,3,4 Hence, other treatment strategies are required to add on to the therapeutic armament for glomerulosclerosis. Data from recent experimental studies relate the progression of glomerulosclerosis in diabetic mice and humans to intrarenal inflammation.5,6,7,8 For example, mycophenolate mofetil, methotrexate, or irradiation reduce urinary albumin excretion, and glomerulosclerosis in rats with streptozotocin-induced diabetes.9,10 Yet, the molecular and cellular mechanisms of intrarenal inflammation in diabetic nephropathy remain poorly characterized. Patients with diabetic nephropathy have increased serum levels of acute phase markers of inflammation but this may not represent intrarenal inflammation.11,12 Patients with diabetic nephropathy excrete high levels of the CC-chemokine monocyte chemoattractant protein 1 (CCL2) in the urine that may be more specific for intrarenal inflammation.13,14,15 In fact, CCL2 is expressed by human mesangial cells exposed to either high glucose concentrations or advanced glycation end products.16,17 CCL2 is involved in the complex multistep process of leukocyte recruitment from intravascular to extravascular compartments, ie, glomeruli and the renal interstitium.18 In fact, macrophage infiltrates are a common finding in human and experimental glomerulosclerosis and tubulointerstitial injury.19,20,21 Ccl2-deficient type 1 or type 2 diabetic mice have lower glomerular macrophage counts, which are associated with less glomerular injury.22,23 In these studies the functional role of CCL2 for glomerular pathology of type 1 and type 2 diabetes was also demonstrated. Chow and colleagues23 induced type 1 diabetes by injection of streptozotocin into Ccl2-deficient mice and observed improvement of glomerular and interstitial macrophage accumulation, glomerulosclerosis, and interstitial fibrosis. In addition, backcrossing Ccl2-deficient mice into obese db/db mice with type 2 diabetes demonstrated that Ccl2 is important for the progression of renal injury.22 Hence, CCL2 may represent a potential therapeutic target for glomerulosclerosis in diabetes, and suitable CCL2 antagonists with favorable pharmacokinetic profiles should be validated in this disease context.
The biological functions of proteins can be blocked by aptamers, ie, nucleic acid structures designed to bind to target molecules like antibodies.24,25 The therapeutic use of nonmodified aptamers is limited by their susceptibility to the ubiquitous nucleases. A very elegant strategy to generate biostable oligonucleotides is represented by the Spiegelmer technology. Spiegelmers are l-enantiomeric RNA or DNA oligonucleotides in which the mirror-image configuration of the nucleotides prevents nuclease degradation.26 Furthermore, Spiegelmers do not induce type I interferons in dendritic cells, as recently described for certain natural and synthetic RNAs.27,28,29,30,31 Hence, Spiegelmers are immunologically inert and show excellent biostability without any further chemical modifications that renders Spiegelmers very well suited for in vitro and in vivo applications.31,32,33,34 In fact, we have recently shown that mNOX-E36, an anti-Ccl2-Spiegelmer, is effective in reducing glomerular and interstitial macrophage recruitment in MRLlpr/lpr mice.31 In this study we found that pegylation of the Spiegelmer significantly increased its plasma levels and was more effectively protected from progressive lupus nephritis.31 Therefore, we hypothesized that the pegylated anti-Ccl2 Spiegelmer mNOX-E36–3′PEG would be suitable for the treatment of glomerulosclerosis.
Materials and Methods
Ccl2 Antagonistic Spiegelmer mNOX-E36
The ribonucleotide sequence of the Spiegelmer mNOX-E36 (5′-GGCGACAUUGGUUGGGCAUGAGGCGAGGCCCUUUGAUGAAUCCGCGGCCA-3′ has been identified as previously described.31 mNOX-E36 binds specifically to murine Ccl2 (mCcl2) and inhibits the biological effects of mCcl2 in vitro at low nanomolar concentrations. For in vivo application, mNOX-E36 and the nonfunctional control Spiegelmer PoC (5′-UAAGGAAACUCGGUCUGAUGCGGUAGCGCUGUGCAGAGCU-3′) were terminally modified with 40-kDa polyethylene glycol (PEG).
Animal Studies
Male 5-week-old C57BLKS db/db or C57BLKS wild-type mice were obtained from Taconic (Ry, Denmark) and housed in filter top cages with a 12-hour dark/light cycle and unlimited access to food and water for the duration of the study. At the age of 6 weeks uninephrectomy (1K mice) or sham surgery (2K mice) was performed through a 1-cm flank incision as previously described in db/db and wild-type mice.35 In mice of the sham surgery groups the kidney was left in situ. At the age of 4 months, 1K db/db mice were divided into three groups that received three times per week subcutaneous injections with either 50 mg/kg of mNOX-E36 pegylated at the 3′ end (mNOX-E36–3′PEG) in 50 μl of 5% glucose or 50 mg/kg of PoC-PEG, a Spiegelmer with a nonspecific scrambled sequence in 5% glucose or glucose only (vehicle). Treatment was continued for 8 weeks when tissues were obtained for histopathological evaluation 3 to 4 hours after last Spiegelmer injection. Blood and urine samples were obtained at monthly intervals for the analysis of blood glucose levels (Accu Check sensor; Roche, Mannheim, Germany), urinary albumin (ELISA; Bethyl Labs, Montgomery, TX), and urinary creatinine (Jaffé reaction; DiaSys Diagnostic Systems, Holzheim, Germany). The plasma levels of mNOX-E36–3′PEG were determined 12 hours after injection as described.36 White blood and platelet counts were performed by Coulter counter. All experimental procedures had been approved by the local government authorities.
Immunohistochemistry
All immunohistological studies were performed on paraffin-embedded sections as described.35 The following rat and rabbit antibodies were used as primary antibodies: rat anti-Mac2 (glomerular macrophages, 1:50; Cedarlane, Burlington, Canada), anti-Ki-67 (cell proliferation, 1:25; Dianova, Hamburg, Germany), and anti-Ccl2 (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA).
Test for Spiegelmer-Specific Antibodies
Plasma from mNOX-E36-treated db/db mice was tested for the presence of mNOX-E36-specific antibodies by immobilizing biotinylated Spiegelmers to streptavidin-coated microwell plates. Diluted sera (1:100 and 1:500) were incubated with an anti-mouse-IgG-horseradish peroxidase conjugate and detection of horseradish peroxidase with a fluorogenic substrate.
Histopathological Evaluation
From each mouse parts of the kidneys were fixed in 10% formalin in phosphate-buffered saline and embedded in paraffin. Three-μm sections were stained with periodic acid-Schiff reagent or silver following the instructions of the supplier (Bio-Optica, Milano, Italy). Glomerular sclerotic lesions were assessed using a semiquantitative score by a blinded observer as follows: 0 = no lesion, 1 = <25% sclerotic, 2 = 25 to 49% sclerotic, 3 = 50 to 74% sclerotic, 4 = 75 to 100% sclerotic, respectively. Fifteen glomeruli were analyzed per section. The indices for interstitial volume and tubular dilatation were determined by superimposing a grid of 100 points on 10 nonoverlapping cortical fields as described previously.35 Interstitial cell counts were determined in 15 high-power fields (hpfs, ×400) by a blinded observer.
Laser Capture Microdissection of Paraffin-Embedded Renal Tissue
A robot MicroBeam system (P.A.L.M., Wolfratshausen, Germany) was used to isolate glomeruli from formaldehyde-fixed and paraffin-embedded renal sections. After deparaffination with 100% xylene and rehydration in 100%, 90%, and 70% ethanol ∼100 glomeruli per animal were isolated under direct visual control by the focused nitrogen laser beam from the surrounding tissue, as recently described.37 For harvesting of the samples the energy of the laser was increased and the microdissected glomerulus was catapulted with a single laser shot. The detached glomeruli were collected in a microfuge cap coated with mineral oil (Fluka Sigma-Aldrich, Deisenhofen, Germany). Samples were stored in liquid nitrogen until being further processed.
RNA Preparation and Real-Time Quantitative (TaqMan) Real-Time Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Microdissected glomeruli were incubated successively in xylol, 100%, 90%, and 70% ethanol. Deparaffinized glomeruli were incubated in lysing buffer (10 mmol/L Tris-HCl, 0.1 mmol/L ethylenediaminetetraacetic acid, 2% sodium dodecyl sulfate, and 20 μg/ml proteinase K) for 16 hours at 60°C before phenol-chloroform-based RNA extraction was performed. Glomerular RNA was dissolved in 10 μl of RNase-free water. RNA isolation from organs of db/db mice was performed using standard methods as described.35 RT and real-time RT-PCR from total organ and glomerular RNA was performed as described.35,37 Controls consisting of ddH2O were negative for target and housekeeper genes. Oligonucleotide primer (300 nmol/L) and probes (100 nmol/L) for mCcl2, mCcr2, mTgf-β, Gapdh, and 18s rRNA were predeveloped with a TaqMan assay reagent from PE Biosystems, Weiterstadt, Germany.
Glomerular Filtration Rate (GFR)
GFR was determined by clearance kinetics of plasma fluorescein isothiocyanate-inulin (Sigma-Aldrich, Steinheim, Germany) 5, 10, 15, 20, 35, 60, and 90 minutes after a single intravenous bolus injection.38 Fluorescence was determined with 485-nm excitation and read at 535-nm emission. GFR was calculated based on a two-compartment model using a nonlinear regression curve-fitting software (GraphPad Prism; GraphPad Software Inc., San Diego, CA).
In Vivo Assay of Renal Macrophage Recruitment
Mac2-positive macrophages were prepared by immunomagnetic selection from spleens of db/db mice as previously described.39 Purity of isolated cells was verified by flow cytometry. Separated cells were labeled with PKH26 (red fluorescent cell linker kit; Sigma-Aldrich Chemicals, Steinheim, Germany) and labeling efficacy was assessed by flow cytometry. Mac2 macrophages (2 × 105) in 200 μl of isotonic saline were injected into the tail vein of 5-month-old db/db mice that had received a single dose of either mNOX-E36–3′PEG, PoC-PEG, or vehicle 3 hours before injection. Renal tissue was obtained 3 hours after injection of cells, snap-frozen, and prepared for fluorescence microscopy. The number of interstitial fluorescent cells was determined in 15 glomeruli and high-power fields, respectively.
Cell Culture Experiments
J774 murine macrophages (American Type Culture Collection, Rockville, MD) were grown in RPMI 1640 medium (Gibco/Invitrogen, Carlsbad, CA) containing 10% heat-inactivated fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C supplied with 5% CO2/air). A murine mesangial cell line was maintained in Dulbecco’s modified Eagle’s medium (Biochrom KG, Berlin, Germany) supplemented with 2.5% bovine serum (Serum Supreme; BioWhittaker, Walkersville, MD) and 1% penicillin-streptomycin, 100 U/ml and 100 μg/ml, as described.40 Cells were kept in medium with or without fetal calf serum 24 hours before incubation with the Spiegelmers 10, 50, 100, or 200 μg/ml. Proliferation of J774 murine macrophages and mesangial cells was assessed after 36 hours using CellTiter 96 proliferation assay by adding 20 μl of CellTiter 96 Aqueous One solution to each well and kept for 1.5 hours at 37°C (Promega, Mannheim, Germany). The optical density was measured at 492 nm.
Statistical Analysis
Data are presented as mean ± SEM. Comparison of groups was performed using analysis of variance and posthoc Bonferroni’s correction was used for multiple comparisons. A value of P < 0.05 was considered to indicate statistical significance.
Results
Uninephrectomy Increases Renal Ccl2 mRNA Levels in db/db Mice
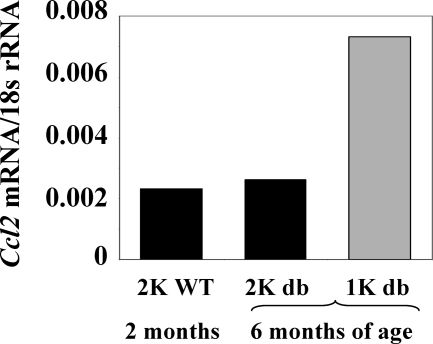
We have previously shown that early uninephrectomy accelerates the onset of kidney disease in db/db mice.35 We questioned whether the uninephrectomy-related increase in albuminuria may be related with an increase in renal Ccl2 expression. Hence, we examined the renal expression of Ccl2 mRNA in wild-type BKS mice and in 1K or 2K db/db mice by real-time RT-PCR (Figure 1). Kidneys of 2- and 6-month-old 2K db/db mice showed low Ccl2 mRNA expression. By contrast, early uninephrectomy was associated with a marked increase in renal Ccl2 mRNA expression in db/db mice at 6 months of age.
Figure 1.
Effect of uninephrectomy on albuminuria and renal Ccl2 expression of db/db mice. Quantitative real-time RT-PCR analysis was performed on total cDNA derived from kidneys of 2- or 6-month-old 2K db/db mice (black bars) or 6-month-old 1K db/db mice (white bars). The cDNA was amplified using primers specific for mCcl2 for 40 PCR cycles. The data shown are derived from pooled cDNA samples from six mice of each group and are expressed as ratio to the respective 18s rRNA expression.
mNOX-E36–3′PEG Reduces Recruitment of Macrophages to Kidneys of 1K db/db Mice
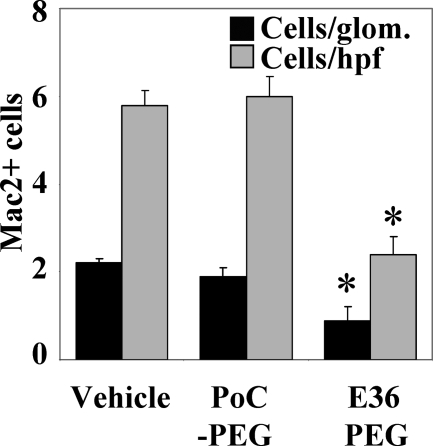
Chemokines and chemokine receptors have compartment-specific functions in the mouse kidney. Thus, we tested whether the Ccl2 antagonist mNOX-E36–3′PEG can block macrophage recruitment to the glomerular and renal interstitial compartment of 1K db/db mice. We performed cell transfer studies with ex vivo fluorescently labeled Mac2-positive macrophages into 5-month-old 1K db/db mice. Three hours after injection Mac2 cells were found to localize to glomeruli and to the tubulointerstitial compartment of 1K db/db mice. Pretreatment with a single dose of mNOX-E36–3′PEG significantly reduced the numbers of labeled Mac2 cells that infiltrated into glomeruli and into the tubulointerstitium of 1K db/db mice (Figure 2). These data provide the rationale for using mNOX-E36–3′PEG to block renal macrophage recruitment in 1K db/db mice.
Figure 2.
mNOX-E36–3′PEG blocks recruitment of macrophages into the kidney of db/db mice. Five-month-old male 1K db/db mice were injected intravenously with PKH26-labeled Mac2+ macrophages isolated from spleens of donor db/db mice. Recipient mice were pretreated with a single dose of either PoC-PEG or mNOX-E36–3′PEG before injection and kidneys were obtained 3 hours after injection and examined by fluorescence microscopy. Cell counts for glomerular and tubulointerstitial Mac2+ cells were determined by fluorescence microscopy from 15 high-power fields (hpfs) or 10 glomeruli. Black bars represent cells per glomerulus, gray bars represent cells per hpf. Values are means ± SEM. *P < 0.001.
mNOX-E36–3′PEG Reduces Glomerular Macrophage Counts and Global Glomerulosclerosis in 1K db/db Mice
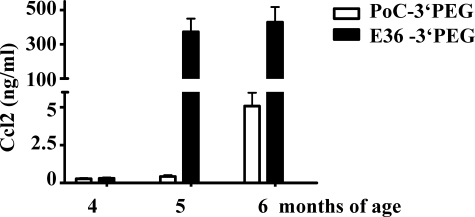
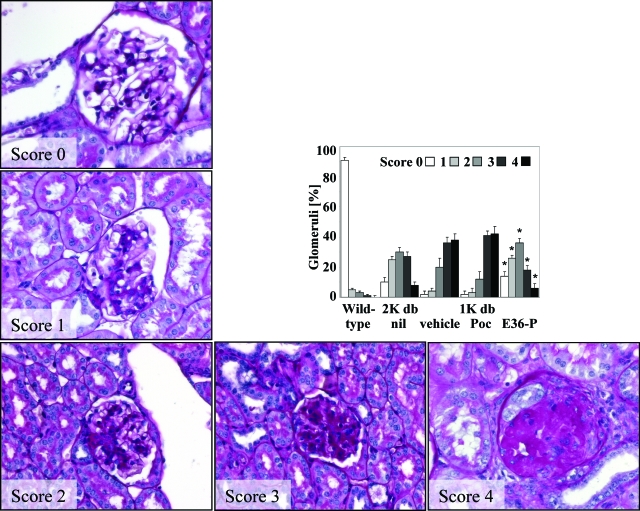
When lack of functional Ccl2 is associated with decreased glomerular macrophage recruitment in db/db mice22 and mNOX-E36–3′PEG is able to block Ccl2-mediated macrophage recruitment in vitro and in vivo mNOX-E36–3′PEG should impair renal macrophage recruitment in db/db mice with advanced type 2 diabetic nephropathy. To test this hypothesis we initiated subcutaneous injections with mNOX-E36–3′PEG or PoC-PEG (50 mg/kg, three times per week) at the age of 4 months in 1K db/db mice. Treatment was continued for 8 weeks when urine samples and tissues were collected for the assessment of nephropathy. At 20 and 24 weeks mNOX-E36–3′PEG plasma concentrations were found to be 2.1 ± 0.6 μmol/ml and 2.1 ± 0.1 μmol/ml 12 hours after injection. During that period mNOX-E36–3′PEG treatment did not significantly affect white blood or platelet counts, blood glucose levels, or body weight that were both markedly elevated in all groups of db/db mice as compared to nondiabetic BKS mice (data not shown). Interestingly, mNOX-E36–3′PEG increased the serum levels of Ccl2 in 1K db/db mice, indicating that the Ccl2 antagonist retains Ccl2 in the circulation (Figure 3). None of the mNOX-E36–3′PEG-treated animals revealed specific IgGs indicating that Spiegelmers are not immunogenic (data not shown). Consistent with our hypothesis mNOX-E36–3′PEG significantly reduced the number of glomerular macrophages by 40% as compared to PoC-PEG- or vehicle-treated db/db mice (Table 1). This was associated with lower numbers of Ki-67-positive proliferating cells within the glomerulus in mNOX-E36–3′PEG-treated db/db mice (Table 1). These findings were associated with a significant improvement of global diabetic glomerulosclerosis in 1K db/db mice (Figure 4). In fact, mNOX-E36–3′PEG treatment reduced glomerulosclerosis in 1K db/db mice to the extent of glomerulosclerosis present in age-matched 2K db/db mice (Figure 4). These findings show that delayed blockade of Ccl2-dependent glomerular macrophage recruitment with mNOX-E36–3′PEG prevents global glomerulosclerosis in type 2 diabetic db/db mice.
Figure 3.
Serum Ccl2 levels in Spiegelmer-treated db/db mice. Serum Ccl2 levels were determined in the groups of PoC-PEG- (white bars) and mNOX-E36–3′PEG-treated 1K db/db mice (black bars) by ELISA at different time points as indicated. Data are means ± SEM. *P < 0.05 mNOX-E36–3′PEG versus PoC-PEG.
Table 1.
Immunostaining on Renal Sections of 6-Month-Old db/db Mice
| Immunostaining | 2K
|
1K
|
|||
|---|---|---|---|---|---|
| Wild type + nil | db/db + nil | db/db + nil | db/db + PoC-PEG | db/db + E36-PEG | |
| Glomerular cells (cells/glomerulus) | |||||
| Mac-2+ | 0.3 ± 0.1 | 1.8 ± 0.2 | 5.0 ± 0.7* | 5.9 ± 0.4 | 3.5 ± 0.3# |
| Ki-67+ | 0.7 ± 0.1 | 0.9 ± 0.2 | 2.4 ± 0.2* | 3.1 ± 0.3 | 1.1 ± 0.2# |
| Interstitial cells (cells/hpf) | |||||
| Mac-2+ | 3.2 ± 0.3 | 8.6 ± 1.0 | 19.2 ± 2.8* | 23.8 ± 3.3 | 12.3 ± 1.2# |
Data are means ± SEM.
P < 0.05 1K db/db versus 2K db/db,
P < 0.05 mNOX-E36–3′PEG versus PoC-PEG.
Figure 4.
Glomerulosclerosis in 6-month-old db/db mice. Renal sections from mice of the different groups were stained with periodic acid-Schiff and scored for the extent of glomerulosclerosis as described in the Materials and Methods. Images show representative glomeruli graded to the respective scores as indicated. From each mouse 15 glomeruli from one renal section were graded by that score. The graph illustrates the mean percentage of each score ± SEM from all mice in each group (n = 7 to 10). Note that uninephrectomy was associated with a shift toward higher scores of glomerulosclerosis and that PoC-PEG had no effect on glomerulosclerosis as compared to vehicle-treated 1K db/db mice. *P < 0.05 mNOX-E36–3′PEG versus PoC-PEG-treated 1K db/db mice. Original magnifications, ×400.
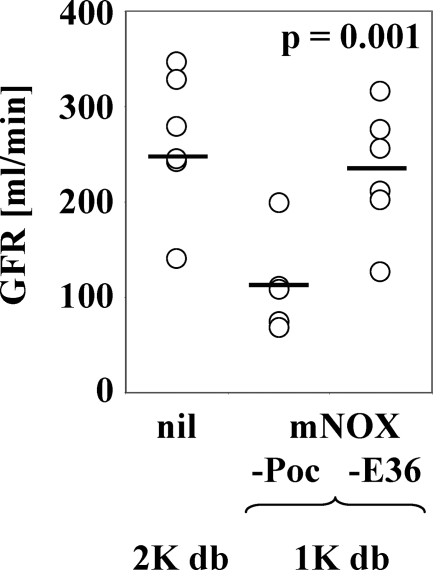
mNOX-E36–3′PEG Improves GFR in 1K db/db Mice
The beneficial effects of mNOX-E36–3′PEG treatment on diabetic glomerulosclerosis in 1K db/db mice should be associated with a better GFR. We analyzed fluorescein isothiocyanate-inulin clearance kinetics as a marker of GFR in db/db mice.38 As compared to a normal GFR of ∼260 ml/minute in sham-operated db/db mice and as reported by others,38 we found a reduced GFR of 112 ± 23 ml/minute in 6-month-old 1K db/db mice injected with PoC-PEG (Figure 5). mNOX-E36–3′PEG treatment significantly improved the GFR to 231 ± 30 ml/minute in 1K db/db mice (P < 0.001) suggesting that blocking Ccl2-dependent glomerular macrophage recruitment can also improve renal function in type 2 diabetic mice.
Figure 5.
GFR in 6-month-old untreated 2K db/db mice and 1K db/db mice treated with mNOX-E36–3′PEG or PoC-PEG. GFR was determined by fluorescein isothiocyanate-inulin clearance kinetics in the groups of untreated 2K db/db mice, PoC-PEG-treated and mNOX-E36–3′PEG-treated 1K db/db mice at the end of the study as described in the Materials and Methods. Note that mNOX-E36–3′PEG significantly improved the GFR.
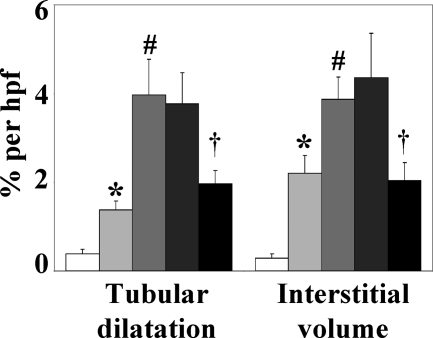
mNOX-E36–3′PEG Reduces Interstitial Macrophage Counts and Tubulointerstitial Injury in 1K db/db Mice
Advanced diabetic nephropathy in humans is associated with significant numbers of interstitial macrophages and tubulointerstitial injury.19 In 2K db/db mice interstitial macrophage infiltrates and significant tubulointerstitial injury does not occur before 8 months of age.21 Early uninephrectomy accelerates the development of tubulointerstitial pathology in db/db mice,35 thus we quantified interstitial macrophages, tubular dilatation, and interstitial volume as markers of tubulointerstitial damage in mice of all groups at 6 months of age. At this time point 1K db/db mice revealed increased numbers of interstitial macrophages and significant elevations of tubular dilatation and interstitial volume as compared to 2K db/db mice (Table 1, Figure 6). mNOX-E36–3′PEG treatment reduced the numbers of interstitial macrophages by 53% as well as tubular dilatation and interstitial volume in 1K db/db mice (Table 1, Figure 6). Thus, blocking Ccl2-dependent renal macrophage recruitment prevents tubulointerstitial injury in diabetic db/db mice.
Figure 6.
Tubular atrophy and interstitial volume of 6-month-old db/db mice. Renal sections from mice of all groups were stained with silver. Morphometric analysis of cortical renal sections was performed as described in the Materials and Methods. Values represent means ± SEM of the respective index from 7 to 10 mice in each group. 2K BKS wild-type mice (white bars), 2K db/db mice (light gray bars), and 1K db/db mice (vehicle, medium gray bars; PoC-PEG, dark gray bars; mNOX-E36–3′PEG, black bars). *P < 0.05 2K db/db versus BKS wild-type mice, #P < 0.05 1K versus 2K db/db mice, †P < 0.05 mNOX-E36–3′PEG- versus PoC-PEG-treated 1K db/db mice.
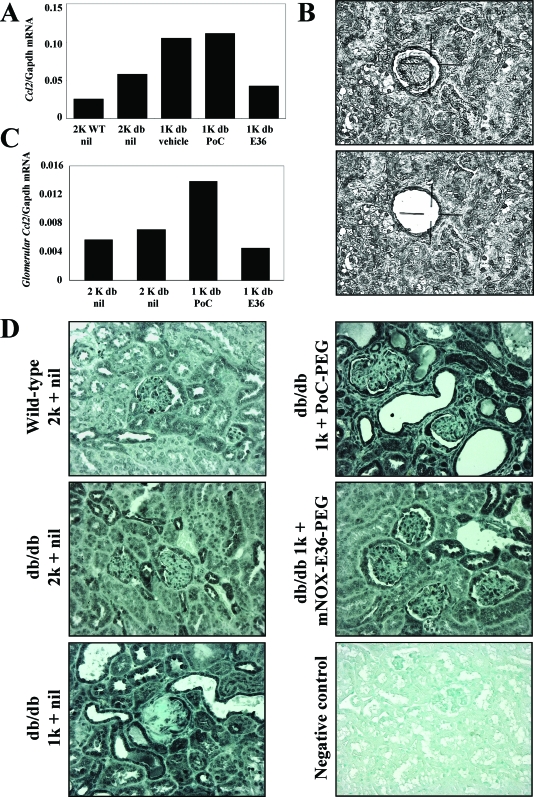
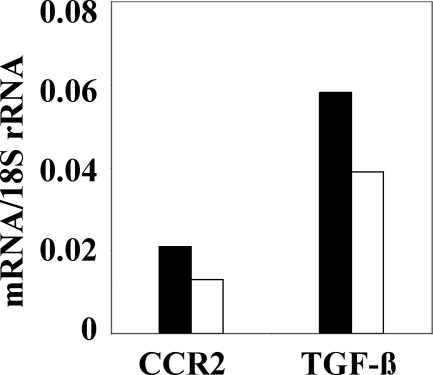
mNOX-E36–3′PEG Reduces Glomerular Expression of Ccl2, Ccr2, and TGF-β in 1K db/db Mice
Macrophage infiltrates amplify inflammatory responses in tissue injury, eg, local Ccl2 expression. We therefore hypothesized that the mNOX-E36–3′PEG-related decrease in renal macrophages would be associated with less renal Ccl2 expression. We used real-time RT-PCR to quantify the total kidney mRNA expression of Ccl2 in db/db mice. mNOX-E36–3′PEG reduced the mRNA levels of Ccl2 in 6-month-old 1K db/db mice as compared to age-matched PoC-PEG-treated mice (Figure 7A). Does this finding relate to Ccl2 expression in the glomerular compartment? We used laser capture microdissection to answer this question by collecting glomerular tuft tissue samples from paraffin-embedded section of high purity, which were not contaminated by extraglomerular cells (Figure 7B). Real-time RT-PCR for Ccl2 revealed that mNOX-E36–3′PEG decreased the glomerular expression of Ccl2 in 6-month-old 1K db/db mice compared to PoC-PEG or nil-treated controls (Figure 7C). To further assess the spatial expression of Ccl2 we performed immunostaining for Ccl2 protein on renal sections. In 1K db/db mice the expression of Ccl2 was markedly enhanced in glomeruli, tubuli, and interstitial cells as compared to 2K db/db or 2K wild-type mice (Figure 7D). mNOX-E36–3′PEG markedly reduced the staining for Ccl2 in all these compartments as compared to vehicle- or PoC-PEG-treated 1K db/db mice. These data indicate that blocking Ccl2-dependent renal macrophage recruitment with mNOX-E36–3′PEG reduces the local expression of Ccl2 in 1K db/db mice. Local Ccl2 expression recruits macrophages via the chemokine receptor Ccr2 and renal macrophages produce the profibrotic cytokine TGF-β, which contributes to glomerulosclerosis and to interstitial fibrogenesis. mNOX-E36–3′PEG treatment reduced the mRNA expression of both factors in kidneys of db/db mice consistent with reduced renal macrophage counts (Figure 8), suggesting that therapeutic Ccl2 blockade has a positive effect on renal fibrogenesis.
Figure 7.
Renal Ccl2 expression in db/db mice. A: mRNA expression for renal Ccl2 was determined by real-time RT-PCR using total renal RNA pooled from 6 to 10 mice of each group. mRNA levels for each group of mice are expressed per respective Gapdh expression. B: Laser capture microdissection of glomeruli from paraffin-embedded sections as described in Materials and Methods. Images illustrate a representative renal section before and after laser capture microdissection. C: mRNA expression for glomerular Ccl2 was determined by real-time RT-PCR using a pool of 600 glomerular isolates from 6 to 10 mice of each group. mRNA levels for each group of mice are expressed per respective Gapdh expression. D: Spatial Ccl2 expression in kidneys of db/db mice was determined by immunostaining as described in Materials and Methods. Images illustrate representative sections of kidneys from 6-month-old mice of the respective groups as indicated. Original magnifications, ×200.
Figure 8.
Renal Ccr2 and Tgf-β expression in db/db mice. Renal mRNA expression levels for Ccr2 and Tgf-β were determined by real-time RT-PCR using total renal RNA pooled from 6 to 10 mice of each group. mRNA levels for each group of mice are expressed per respective 18S rRNA expression. Black bars: PoC-treated mice; white bars: E36-treated mice.
mNOX-E36–3′PEG Does Not Directly Affect Macrophage or Mesangial Cell Proliferation
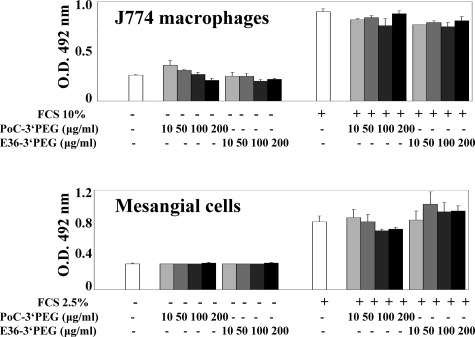
Our finding that mNOX-E36–3′PEG reduced the number of glomerular proliferating cells raises the question whether mNOX-E36–3′PEG elicits direct effects on these cells. We used J774 cells, a murine monocyte/macrophage cell line, and a murine mesangial cell line40 to study the impact of mNOX-E36–3′PEG on the proliferation rate of these cells. Proliferation of both cell lines was low within 36 hours in the absence of fetal calf serum but markedly increased when fetal calf serum was added to the culture dishes (Figure 9). Adding mNOX-E36–3′PEG or the control Spiegelmer at different doses had no effect on the proliferation rate of both cell types. These data suggest that mNOX-E36–3′PEG does not directly affect macrophage or mesangial cell proliferation.
Figure 9.
mNOX-E36–3′PEG and the proliferation of J774 macrophages, mesangial cells, and tubular epithelial cells. The proliferation of cultured J774 macrophages and mesangial cells was assessed after 72 hours using the CellTiter 96 proliferation assay as described in Materials and Methods. Data are expressed at means ± SEM of the optical density (O.D.) read at a wavelength of 492 nm. *P < 0.05 versus medium plus 10% fetal calf serum (FCS).
Discussion
Aptamers are structured oligonucleotides that can be used to neutralize biological functions of target molecules. Unlike aptamers, Spiegelmer-based oligonucleotides are nuclease-resistant and thus biostable without further modifications.26 The Spiegelmer mNOX-E36 binds with high affinity to murine Ccl2 and blocks its biological function at low nanomolar concentrations. We have previously shown that mNOX-E36 also lacks interferon-α induction in dendritic cells via innate RNA recognition receptors.27,30,31 Spiegelmer treatment was associated with a massive increase of plasma Ccl2 levels in our previous as well as in the present study.31 One may argue that retaining Ccl2 may only affect the chemokine gradient required for macrophage transmigration into the kidney rather than blocking Ccl2 function. This potential mechanism was ruled out by chemokine migration assay experiments in which Ccl2 in the presence of Spiegelmer could not trigger macrophage migration (unpublished data). Hence, we considered mNOX-E36 well suited for in vivo antagonism of Ccl2.
Evidence for a pathogenic role of Ccl2 for glomerulosclerosis in experimental diabetes already exists. Ccl2-deficient mice were protected from glomerular pathology of streptozotocin-induced type 1 diabetes23 and Ccl2-deficient type 2 diabetic db/db mice were protected from the progression of glomerulosclerosis.22 Although, the phenotype of Ccl2-deficient diabetic mice supports a role of Ccl2 for the pathogenesis of glomerulosclerosis these data cannot predict whether Ccl2 blockade will be also protective when initiated after onset of disease. It is therefore necessary to block Ccl2 after glomerulosclerosis has established using suitable Ccl2 antagonists, like mNOX-E36–3′PEG.
Ccl2 is known to mediate macrophage recruitment to sites of tissue inflammation18 and in both of the aforementioned studies lack of Ccl2 was also associated with a reduction of glomerular macrophages. Here we now provide experimental evidence that Ccl2 blockade with mNOX-E36–3′PEG effectively blocks macrophage recruitment into the glomerular and interstitial compartments of the kidney. Hence, we hypothesized that mNOX-E36–3′PEG treatment may reduce the numbers of renal macrophages in type 2 diabetic db/db mice. We used early uninephrectomy to aggravate glomerular hyperfiltration during the development of glomerulosclerosis in db/db mice. In fact, 1K db/db mice showed a significant aggravation of glomerulosclerosis and tubulointerstitial pathology at 6 months of age as compared to sham-operated 2K db/db mice. Consistent with our hypothesis injections with mNOX-E36–3′PEG for 8 weeks started from 4 months of age reduced the numbers of glomerular and interstitial macrophages at 6 months of age. This was associated with less renal Ccr2 expression, the Ccl2 receptor, which represents an indirect marker of renal macrophage numbers. Furthermore, we observed less proliferating glomerular cells. Our in vitro studies exclude direct effects of mNOX-E36–3′PEG on the proliferation of macrophages and mesangial cells. Hence, less proliferation within the glomerular compartment may rather be related to a reduction of macrophage-dependent mesangial cell proliferation and glomerular inflammation.41 This concept is supported by our finding that glomerular expression of Ccl2 mRNA was markedly reduced with mNOX-E36–3′PEG treatment. Furthermore, lower numbers of glomerular macrophages and glomerular proliferating cells in mNOX-E36–3′PEG-treated 1K db/db mice were associated with protection from global glomerulosclerosis. The beneficial effects of mNOX-E36–3′PEG on glomerular pathology and GFR in db/db mice are consistent with those studies that have used other Ccl2 antagonists in other models of glomerular injury.42,43,44,45,46,47 Remarkably, delayed onset of Ccl2 blockade also reduced the numbers of interstitial macrophages being associated with less tubulointerstitial pathology in 1K db/db mice.
These data indicate that the presence of glomerular and interstitial macrophages contribute to renal injury in db/db mice, a mechanism that may be referred to as inflammation.5,6,7 For example, macrophages produce proinflammatory mediators, eg, Ccl2, which add to the mediators produced by renal cells, ie, in a positive amplification loop.20,48 This observation made in nondiabetic types of kidney disease is likely to apply also to diabetic nephropathy in humans, because interstitial macrophage infiltrates are common in diabetic nephropathy19 and patients with diabetic nephropathy excrete high levels of Ccl2 into the urine, itself indicating intrarenal inflammation.13,49 Chemokine expression involves activation of protein kinase C in renal cells as well as immune cell infiltrates. Therapeutic intervention targeting protein kinase C can disrupt this positive amplification loop by reducing renal chemokine expression, subsequent recruitment of immune cells, and tubular injury in experimental and human diabetic nephropathy.50,51 Based on these data the contribution of inflammation to the progression of diabetic nephropathy becomes increasingly anticipated.8 The relevance of these experimental data for human disease was recently supported by transcriptome analysis of human renal biopsy samples from patients with diabetic nephropathy that identified a specific NF-κB promoter-dependent inflammatory stress response in progressive diabetic nephropathy.52 Together, these data validate Ccl2 as a promising therapeutic target for glomerulosclerosis in diabetes and suggest that initiating Ccl2 blockade, eg, with mNOX-E36–3′PEG, even at an advanced stage of the disease may still be protective.
Acknowledgments
We thank Dan Draganovici, Jana Mandelbaum, and Claudia Schmidt for expert technical support.
Footnotes
Address reprint requests to PD Dr. Hans-Joachim Anders, Medizinische Poliklinik, Klinikum der Universität München-Innenstadt, Pettenkoferstr. 8a, 80336 Munchen, Germany. E-mail: hjanders@med.uni-muenchen.de.
Supported by the Else-Kroener-Fresenius Foundation (to H.J.A. and S.S.); the European Union (EU integrated project “INNOCHEM” FP6-518167 to H.J.A); and the Faculty of Medicine, University of Munich (to S.C.).
V.N. and S.C. contributed equally to the results of this project.
Parts of this project were prepared as a doctoral thesis at the Faculty of Medicine, University of Munich by S.C.
References
- Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- Ritz E, Rychlik I, Locatelli F, Halimi S. End-stage renal failure in type 2 diabetes: a medical catastrophe of worldwide dimensions. Am J Kidney Dis. 1999;34:795–808. doi: 10.1016/S0272-6386(99)70035-1. [DOI] [PubMed] [Google Scholar]
- United States Renal Data System Annual data report: incidence and prevalence 2004. Am J Kidney Dis. 2004;45:S77–S80. [Google Scholar]
- Svensson M, Sundkvist G, Arnqvist HJ, Bjork E, Blohme G, Bolinder J, Henricsson M, Nystrom L, Torffvit O, Waernbaum I, Ostman J, Eriksson JW. Diabetes Incidence Study in Sweden (DISS): signs of nephropathy may occur early in young adults with diabetes despite modern diabetes management: results from the nationwide population-based Diabetes Incidence Study in Sweden (DISS). Diabetes Care. 2003;26:2903–2909. doi: 10.2337/diacare.26.10.2903. [DOI] [PubMed] [Google Scholar]
- Galkina E, Ley K. Leukocyte recruitment and vascular injury in diabetic nephropathy. J Am Soc Nephrol. 2006;17:368–377. doi: 10.1681/ASN.2005080859. [DOI] [PubMed] [Google Scholar]
- Mora C, Navarro JF. The role of inflammation as a pathogenic factor in the development of renal disease in diabetes. Curr Diabetes Rep. 2005;5:399–401. doi: 10.1007/s11892-005-0044-x. [DOI] [PubMed] [Google Scholar]
- Meyer TW. Immunosuppression for diabetic glomerular disease? Kidney Int. 2003;63:377–378. doi: 10.1046/j.1523-1755.2003.00747.x. [DOI] [PubMed] [Google Scholar]
- Tuttle KR. Linking metabolism and immunology: diabetic nephropathy is an inflammatory disease. J Am Soc Nephrol. 2005;16:1537–1538. doi: 10.1681/ASN.2005040393. [DOI] [PubMed] [Google Scholar]
- Yozai K, Shikata K, Sasaki M, Tone A, Ohga S, Usui H, Okada S, Wada J, Nagase R, Ogawa D, Shikata Y, Makino H. Methotrexate prevents renal injury in experimental diabetic rats via anti-inflammatory actions. J Am Soc Nephrol. 2005;16:3326–3338. doi: 10.1681/ASN.2004111011. [DOI] [PubMed] [Google Scholar]
- Utimura R, Fujihara CK, Mattar AL, Malheiros DM, Noronha IL, Zatz R. Mycophenolate mofetil prevents the development of glomerular injury in experimental diabetes. Kidney Int. 2003;63:209–216. doi: 10.1046/j.1523-1755.2003.00736.x. [DOI] [PubMed] [Google Scholar]
- Dalla Vestra M, Mussap M, Gallina P, Bruseghin M, Cernigoi AM, Saller A, Plebani M, Fioretto P. Acute-phase markers of inflammation and glomerular structure in patients with type 2 diabetes. J Am Soc Nephrol. 2005;16(Suppl 1):S78–S82. doi: 10.1681/asn.2004110961. [DOI] [PubMed] [Google Scholar]
- Navarro JF, Mora C, Maca M, Garca J. Inflammatory parameters are independently associated with urinary albumin in type 2 diabetes mellitus. Am J Kidney Dis. 2003;42:53–61. doi: 10.1016/s0272-6386(03)00408-6. [DOI] [PubMed] [Google Scholar]
- Morii T, Fujita H, Narita T, Shimotomai T, Fujishima H, Yoshioka N, Imai H, Kakei M, Ito S. Association of monocyte chemoattractant protein-1 with renal tubular damage in diabetic nephropathy. J Diabetes Complications. 2003;17:11–15. doi: 10.1016/s1056-8727(02)00176-9. [DOI] [PubMed] [Google Scholar]
- Tashiro K, Koyanagi I, Saitoh A, Shimizu A, Shike T, Ishiguro C, Koizumi M, Funabiki K, Horikoshi S, Shirato I, Tomino Y. Urinary levels of monocyte chemoattractant protein-1 (MCP-1) and interleukin-8 (IL-8), and renal injuries in patients with type 2 diabetic nephropathy. J Clin Lab Anal. 2002;16:1–4. doi: 10.1002/jcla.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebayashi K, Matsumoto S, Aso Y, Inukai T. Association between circulating monocyte chemoattractant protein-1 and urinary albumin excretion in nonobese type 2 diabetic patients. J Diabetes Complications. 2006;20:98–104. doi: 10.1016/j.jdiacomp.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Ihm CG, Park JK, Hong SP, Lee TW, Cho BS, Kim MJ, Cha DR, Ha H. A high glucose concentration stimulates the expression of monocyte chemotactic peptide 1 in human mesangial cells. Nephron. 1998;79:33–37. doi: 10.1159/000044988. [DOI] [PubMed] [Google Scholar]
- Yamagishi S, Inagaki Y, Okamoto T, Amano S, Koga K, Takeuchi M, Makita Z. Advanced glycation end product-induced apoptosis and overexpression of vascular endothelial growth factor and monocyte chemoattractant protein-1 in human-cultured mesangial cells. J Biol Chem. 2002;277:20309–20315. doi: 10.1074/jbc.M202634200. [DOI] [PubMed] [Google Scholar]
- Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- Bohle A, Wehrmann M, Bogenschutz O, Batz C, Muller CA, Muller GA. The pathogenesis of chronic renal failure in diabetic nephropathy. Investigation of 488 cases of diabetic glomerulosclerosis. Pathol Res Pract. 1991;187:251–259. doi: 10.1016/s0344-0338(11)80780-6. [DOI] [PubMed] [Google Scholar]
- Furuta T, Saito T, Ootaka T, Soma J, Obara K, Abe K, Yoshinaga K. The role of macrophages in diabetic glomerulosclerosis. Am J Kidney Dis. 1993;21:480–485. doi: 10.1016/s0272-6386(12)80393-3. [DOI] [PubMed] [Google Scholar]
- Chow FY, Ozols E, Nikolic-Paterson DJ, Atkins RC, Tesch GH. Macrophages in mouse type 2 diabetic nephropathy: correlation with diabetic state and progressive renal injury. Kidney Int. 2004;65:116–128. doi: 10.1111/j.1523-1755.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- Chow FY, Nikolic-Paterson DJ, Ma FY, Ozols E, Rollins BJ, Tesch GH. Monocyte chemoattractant protein-1-induced tissue inflammation is critical for the development of renal injury but not type 2 diabetes in obese db/db mice. Diabetologica. 2007;50:471–480. doi: 10.1007/s00125-006-0497-8. [DOI] [PubMed] [Google Scholar]
- Chow FY, Nikolic-Paterson DJ, Ozols E, Atkins RC, Rollin BJ, Tesch GH. Monocyte chemoattractant protein-1 promotes the development of diabetic renal injury in streptozotocin-treated mice. Kidney Int. 2006;69:73–80. doi: 10.1038/sj.ki.5000014. [DOI] [PubMed] [Google Scholar]
- Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346:818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- Klussmann S, Nolte A, Bald R, Erdmann VA, Furste JP. Mirror-image RNA that binds D-adenosine. Nat Biotechnol. 1996;14:1112–1115. doi: 10.1038/nbt0996-1112. [DOI] [PubMed] [Google Scholar]
- Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- Hornung V, Ellegast J, Kim S, Brzozka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann KK, Schlee M, Endres S, Hartmann G. 5′-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- Hornung V, Guenthner-Biller M, Bourquin C, Ablasser A, Schlee M, Uematsu S, Noronha A, Manoharan M, Akira S, de Fougerolles A, Endres S, Hartmann G. Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat Med. 2005;11:263–270. doi: 10.1038/nm1191. [DOI] [PubMed] [Google Scholar]
- Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa C, Matsuura Y, Fujita T, Akira S. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- Kulkarni O, Pawar RD, Purschke W, Eulberg D, Selve N, Buchner K, Ninichuk V, Segerer S, Vielhauer V, Klussmann S, Anders HJ. Spiegelmer inhibition of CCL2/MCP-1 ameliorates lupus nephritis in MRL-(Fas)lpr mice. J Am Soc Nephrol. 2007;18:2350–2358. doi: 10.1681/ASN.2006121348. [DOI] [PubMed] [Google Scholar]
- Purschke WG, Eulberg D, Buchner K, Vonhoff S, Klussmann S. An L-RNA-based aquaretic agent that inhibits vasopressin in vivo. Proc Natl Acad Sci USA. 2006;103:5173–5178. doi: 10.1073/pnas.0509663103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlotzka B, Leva S, Eschgfaller B, Burmeister J, Kleinjung F, Kaduk C, Muhn P, Hess-Stumpp H, Klussmann S. In vivo properties of an anti-GnRH Spiegelmer: an example of an oligonucleotide-based therapeutic substance class. Proc Natl Acad Sci USA. 2002;99:8898–8902. doi: 10.1073/pnas.132067399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmling S, Maasch C, Eulberg D, Buchner K, Schroder W, Lange C, Vonhoff S, Wlotzka B, Tschop MH, Rosewicz S, Klussmann S. Inhibition of ghrelin action in vitro and in vivo by an RNA-Spiegelmer. Proc Natl Acad Sci USA. 2004;101:13174–13179. doi: 10.1073/pnas.0404175101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninichuk V, Khandoga AK, Segerer S, Loetscher P, Schlapbach A, Revesz L, Khandoga A, Krombach F, Nelson PJ, Schlöndorff, Anders HJ. The role of interstitial macrophages in nephropathy of type 2 diabetes. Am J Pathol. 2007;170:1267–1276. doi: 10.2353/ajpath.2007.060937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drolet DW, Nelson J, Tucker CE, Zack PM, Nixon K, Bolin R, Judkins MB, Farmer JA, Wolf JL, Gill SC, Bendele RA. Pharmacokinetics and safety of an anti-vascular endothelial growth factor aptamer (NX1838) following injection into the vitreous humor of rhesus monkeys. Pharm Res. 2000;17:1503–1510. doi: 10.1023/a:1007657109012. [DOI] [PubMed] [Google Scholar]
- Cohen CD, Gröne HJ, Gröne EF, Nelson PJ, Schlöndorff D, Kretzler M. Laser microdissection and gene expression analysis on formaldehyde-fixed archival tissue. Kidney Int. 2002;61:125–132. doi: 10.1046/j.1523-1755.2002.00112.x. [DOI] [PubMed] [Google Scholar]
- Qi Z, Whitt I, Mehta A, Jin J, Zhao M, Harris RC, Fogo AB, Breyer MD. Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am J Physiol. 2004;286:F590–F596. doi: 10.1152/ajprenal.00324.2003. [DOI] [PubMed] [Google Scholar]
- Ninichuk V, Gross O, Reichel C, Khandoga A, Pawar RD, Ciubar R, Segerer S, Belemezova E, Radomska E, Luckow B, de Lema GP, Murphy PM, Gao JL, Henger A, Kretzler M, Horuk R, Weber M, Krombach F, Schlondorff D, Anders HJ. Delayed chemokine receptor 1 blockade prolongs survival in collagen 4A3-deficient mice with Alport disease. J Am Soc Nephrol. 2005;16:977–985. doi: 10.1681/ASN.2004100871. [DOI] [PubMed] [Google Scholar]
- Satriano JA, Banas B, Luckow B, Nelson PJ, Schlöndorff D. Regulation of RANTES and ICAM-1 expression in murine mesangial cells. J Am Soc Nephrol. 1997;8:596–603. doi: 10.1681/ASN.V84596. [DOI] [PubMed] [Google Scholar]
- Ikezumi Y, Hurst LA, Masaki T, Atkins RC, Nikolic-Paterson DJ. Adoptive transfer studies demonstrate that macrophages can induce proteinuria and mesangial cell proliferation. Kidney Int. 2003;63:83–95. doi: 10.1046/j.1523-1755.2003.00717.x. [DOI] [PubMed] [Google Scholar]
- Lloyd CM, Minto AW, Dorf ME, Proudfoot A, Wells TN, Salant DJ, Gutierrez-Ramos JC. RANTES and monocyte chemoattractant protein-1 (MCP-1) play an important role in the inflammatory phase of crescentic nephritis, but only MCP-1 is involved in crescent formation and interstitial fibrosis. J Exp Med. 1997;185:1371–1380. doi: 10.1084/jem.185.7.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa H, Kohno M, Sasaki M, Inoue A, Ito MR, Terada M, Hieshima K, Maruyama H, Miyazaki J, Yoshie O, Nose M, Fujita S. Antagonist of monocyte chemoattractant protein 1 ameliorates the initiation and progression of lupus nephritis and renal vasculitis in MRL/lpr mice. Arthritis Rheum. 2003;48:2555–2566. doi: 10.1002/art.11231. [DOI] [PubMed] [Google Scholar]
- Tang WW, Qi M, Warren JS. Monocyte chemoattractant protein 1 mediates glomerular macrophage infiltration in anti-GBM Ab GN. Kidney Int. 1996;50:665–671. doi: 10.1038/ki.1996.363. [DOI] [PubMed] [Google Scholar]
- Wenzel U, Schneider A, Valente AJ, Abboud HE, Thaiss F, Helmchen UM, Stahl RA. Monocyte chemoattractant protein-1 mediates monocyte/macrophage influx in anti-thymocyte antibody-induced glomerulonephritis. Kidney Int. 1997;51:770–776. doi: 10.1038/ki.1997.108. [DOI] [PubMed] [Google Scholar]
- Fujinaka H, Yamamoto T, Takeya M, Feng L, Kawasaki K, Yaoita E, Kondo D, Wilson CB, Uchiyama M, Kihara I. Suppression of anti-glomerular basement membrane nephritis by administration of anti-monocyte chemoattractant protein-1 antibody in WKY rats. J Am Soc Nephrol. 1997;8:1174–1178. doi: 10.1681/ASN.V871174. [DOI] [PubMed] [Google Scholar]
- Schneider A, Panzer U, Zahner G, Wenzel U, Wolf G, Thaiss F, Helmchen U, Stahl RA. Monocyte chemoattractant protein-1 mediates collagen deposition in experimental glomerulonephritis by transforming growth factor-beta. Kidney Int. 1999;56:135–144. doi: 10.1046/j.1523-1755.1999.00543.x. [DOI] [PubMed] [Google Scholar]
- Chow FY, Nikolic-Paterson DJ, Ozols E, Atkins RC, Tesch GH. Intercellular adhesion molecule-1 deficiency is protective against nephropathy in type 2 diabetic db/db mice. J Am Soc Nephrol. 2005;16:1711–1722. doi: 10.1681/ASN.2004070612. [DOI] [PubMed] [Google Scholar]
- Erwig LP, Kluth DC, Rees AJ. Macrophage heterogeneity in renal inflammation. Nephrol Dial Transplant. 2003;18:1962–1965. doi: 10.1093/ndt/gfg313. [DOI] [PubMed] [Google Scholar]
- Kelly DJ, Chanty A, Gow RM, Zhang Y, Gilbert RE. Protein kinase Cbeta inhibition attenuates osteopontin expression, macrophage recruitment, and tubulointerstitial injury in advanced experimental diabetic nephropathy. J Am Soc Nephrol. 2005;16:1654–1660. doi: 10.1681/ASN.2004070578. [DOI] [PubMed] [Google Scholar]
- Tuttle KR, Bakris GL, Toto RD, McGill JB, Hu K, Anderson PW. The effect of ruboxistaurin on nephropathy in type 2 diabetes. Diabetes Care. 2005;28:2686–2690. doi: 10.2337/diacare.28.11.2686. [DOI] [PubMed] [Google Scholar]
- Schmid H, Boucherot A, Yasuda Y, Henger A, Brunner B, Eichinger F, Nitsche A, Kiss E, Bleich M, Grone HJ, Nelson PJ, Schlondorff D, Cohen CD, Kretzler M, European Renal cDNA Bank (ERCB) Consortium Modular activation of nuclear factor-kappaB transcriptional programs in human diabetic nephropathy. Diabetes. 2006;55:2993–3003. doi: 10.2337/db06-0477. [DOI] [PubMed] [Google Scholar]