Abstract
Idiopathic pulmonary fibrosis (IPF) is a severely debilitating disease associated with a dismal prognosis. There are currently no effective therapies for IPF, thus the identification of novel therapeutic targets is greatly needed. The receptor for advanced glycation end products (RAGE) is a member of the immunoglobulin superfamily of cell surface receptors whose activation has been linked to various pathologies. In healthy adult animals, RAGE is expressed at the highest levels in the lung compared to other tissues. To investigate the hypothesis that RAGE is involved in IPF pathogenesis, we have examined its expression in two mouse models of pulmonary fibrosis and in human tissue from IPF patients. In each instance we observed a depletion of membrane RAGE and its soluble (decoy) isoform, sRAGE, in fibrotic lungs. In contrast to other diseases in which RAGE signaling promotes pathology, immunohistochemical and hydroxyproline quantification studies on aged RAGE-null mice indicate that these mice spontaneously develop pulmonary fibrosis-like alterations. Furthermore, when subjected to a model of pulmonary fibrosis, RAGE-null mice developed more severe fibrosis, as measured by hydroxyproline assay and histological scoring, than wild-type controls. Combined with data from other studies on mouse models of pulmonary fibrosis and human IPF tissues indicate that loss of RAGE contributes to IPF pathogenesis.
Idiopathic pulmonary fibrosis (IPF) is a debilitating disease with a dismal prognosis. Mean survival time after biopsy-confirmed diagnosis is 3 to 5 years.1,2 Traditional therapy involves the use of corticosteroids as nonspecific anti-inflammatory agents. This treatment produces an objective response in only 10 to 20% of patients and has a minimal effect on the fatal course of IPF.2,3,4 Thus, the need for new therapeutic modalities is evident.
The receptor for advanced glycation end products (RAGE) is a member of the immunoglobulin super family of cell surface receptors.5 In most healthy adult animal tissues, RAGE is expressed at low to undetectable levels.6,7 Activation of membrane-bound RAGE (mRAGE) by its ligands (including advanced glycation end products, HMGB1/amphoterin, S100/calgranulins, and amyloid-β peptide) often leads to proinflammatory signaling as well as up-regulation of RAGE itself.8 This signaling by mRAGE is believed to play an important role in disease progression for several nonpulmonary diseases, including various diabetic complications, chronic inflammation, and Alzheimer’s disease, among others.9,10
In contrast to other healthy adult tissues, RAGE mRNA and sRAGE protein are highly expressed in normal adult lungs.6,7,11 Most recently it has been suggested that RAGE is a marker of type I alveolar epithelial cells12 and type II alveolar epithelial cell transdifferentiation, a component of pulmonary re-epithelialization and repair.13,14 Although RAGE expression is high in the lung compared to other tissues there have been few studies examining its role in pulmonary diseases such as IPF. Notably, there are several reasons to suggest that there may be an important role for RAGE in the pathogenesis of pulmonary fibrosis: 1) mRAGE ligation by AGEs in rat kidney epithelial cells results in myofibroblast metaplasia15; 2) AGE levels are increased in pulmonary fibrotic lungs16; 3) mRAGE signaling results in transforming growth factor-β and collagen synthesis in renal cells17; 4) mRAGE ligation leads to oxidant generation in endothelial cells18; and 5) RAGE expression is essential for cellular adherence and spreading in type I alveolar epithelial cells.19 Thus, RAGE signaling has the capacity to activate most of the components believed to play a role in the pathogenesis of pulmonary fibrosis, but whether or not RAGE has profibrotic responses in the lung has not been determined. Furthermore, RAGE expression is essential for type I alveolar epithelial cell morphology and transdifferentiation of type II cells, both of which are believed to play a role in the maintenance of normal lung physiology and repair.13,14,19,20 Therefore, we investigated the hypothesis that RAGE plays a central role in IPF pathogenesis by using animal models of pulmonary fibrosis, human IPF tissues, and RAGE-null mice.
Materials and Methods
Asbestos Mouse Model for Pulmonary Fibrosis
C57BL/6 and RAGE-null mice were treated with 0.1 mg of crocidolite asbestos or titanium dioxide (inert particulate control) intratracheally as previously described.21 A 24-gauge feeding needle was inserted endotracheally under anesthesia and a 70-μl suspension containing 1.43 mg/ml of either asbestos or titanium dioxide was then introduced. Mice were sacrificed at indicated time points.
Genotyping of RAGE-Null Mice
Genotyping of RAGE−/− mice was performed by Southern blot according to standard methods. In brief, DNA isolated from tail tip was digested with KpnI (Promega, Heidelberg, Germany) and blotted on nitrocellulose membranes (GE Health Care, München, Germany) before genotyping as described.22
Tissue Homogenate Preparation
Soluble lung homogenates were prepared as described previously.7 Lungs were homogenized in an isotonic buffer (50 mmol/L Tris-HCl, 150 mmol/L NaCl, pH 7.4) to obtain all soluble proteins. The supernatant containing the soluble portion was removed and the insoluble portion was then pelleted by centrifugation and resuspended in a buffer containing CHAPS detergent (50 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 10 mmol/L CHAPS), rocked for 2 hours at 4°C, then centrifuged at 20,000 × g for 20 minutes at 4°C. The membrane fraction supernatant was removed and stored at −80°C until use.
Soluble RAGE Purification from Bovine Lung
Fresh bovine lungs were obtained from McDonald’s Meats (Girard, PA) and used as a starting material. One kg of lung tissue was homogenized in 2 L of homogenization buffer (50 mmol/L potassium phosphate, pH 7.4, 0.3 mol/L potassium bromide, 3 mmol/L diethylenetriamine-pentaacetic acid, 0.5 mmol/L phenylmethyl sulfonyl fluoride). Fast protein liquid chromatography was performed as previously described.23 Purified sRAGE was dialyzed into phosphate-buffered saline (PBS) and assayed for endotoxin using the Kinetic-QCL kinetic chromogenic assay (Lonza Group, Basel, Switzerland). sRAGE containing less than 0.05 ng/ml was stored at −20°C until use.
Mouse Treatment with sRAGE
RAGE knockout mice were treated daily by intraperitoneal injection with 50 μg of bovine sRAGE or bovine serum albumin control.24,25 Treatment with sRAGE was initiated 1 day before intratracheal injection of asbestos.
Western Blotting/Antibodies
Twenty μg of each protein sample was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electroblotted as described previously.7 Mouse mRAGE/sRAGE was detected using an anti-RAGE antibody previously described7; for human samples, anti-RAGE H-300 (Santa Cruz Biotechnology, Santa Cruz, CA) was used. Mouse aquaporin-5 was used as a marker of type I alveolar epithelial cells and was detected using anti-AQP5 antibody (Sigma, St. Louis, MO). Mouse anti-β-actin (Sigma) and mouse anti-β-tubulin (Sigma) were used for loading controls. To visualize antibody binding, enhanced chemiluminescence was used (ECL, Amersham Biosciences, Buckinghamshire, UK; and ECL Plus, GE Health Care). Image capture and densitometric analysis were performed on a Kodak Gel Logic 2200 imaging system and Kodak molecular imaging software, respectively (Eastman Kodak, Rochester, NY).
Human Lung Tissue Samples
Samples were obtained from the tissue bank of the Department of Pathology at the University of Pittsburgh. The use of archived tissue has been approved by the institutional review board. Diagnosis of IPF was supported by history, physical examination, pulmonary function studies, chest high-resolution computed tomography, and corroborated by open lung biopsy. The morphological diagnosis of IPF was based on typical microscopic findings consistent with this disease.26 The patients fulfilled the criteria of the American Thoracic Society and European Respiratory Society.27 Control samples included histologically normal lung samples resected from patients with lung cancer obtained from the University of Pittsburgh Department of Pathology Tissue Bank.
Oligonucleotide Microarrays
Total RNA was extracted from 13 IPF samples (surgical remnants of biopsies or lungs explanted from patients with IPF that underwent pulmonary transplant) and 11 control samples (normal histology lung samples resected from patients with lung cancer) and was used for microarray analysis according to manufacturer’s recommendations (CodeLink UniSet Human I Bioarray, Amersham Biosciences) and as previously described.28,29 Fragmented cRNA was hybridized to CodeLink UniSet Human I Bioarray (Amersham Biosciences) slides. After hybridization, arrays were washed and stained with streptavidin-Alexa Fluor 647. The arrays were scanned using a Genepix 4000B microarray scanner. Images were analyzed using CodeLink Expression II Analysis Suite (Amersham Biosciences). They were visually inspected for visual defects and QC parameters as recommended by the manufacturer. Data files were imported into a microarray database and linked with updated gene annotations using SOURCE (http://genome-www5.stanford.edu/cgi-bin/SMD/source/sourceSearch) and then median scaled. Statistical analysis was performed using Scoregene gene expression package (http://www.cs.huji.ac.il/labs/compbio/scoregenes), and data visualization was performed using Genexpress (http://genexpress.stanford.edu) and Spotfire Decision Suite 8.0 (Spotfire Inc., Göteborg, Sweden). The complete set of gene array data has been deposited in the Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo) with GEO serial accession number GSE2052. The general approach to analysis has been previously described.28 The oligo probe designed to detect human RAGE expression recognizes both mRAGE and sRAGE transcripts.
Histology
Sirius Red was used to stain collagen and reticulin fibers as previously described.30 Sections of lung were deparaffinized with xylene and hydrated with an ethanol series, washed in tap water, and stained with a Sirius Red/fast green solution for 30 minutes at room temperature. They were washed again and dehydrated in an ethanol series and xylene.
Immunohistochemistry
Paraffin sections (4 μm thick) layered on silane-coated slides were used for immunohistochemical staining with primary antibodies against RAGE (Merck and Dhome, Cramlington, UK) (1:4000) and collagen type I (Quartett, Germany) (1:10). Before immunostaining using the Vectastain Elite Kit (Vector/Alexis, Grunberg, Germany), the sections were treated with 10 mmol/L sodium citrate buffer (pH 6.0) in a microwave (750 W, 2 × 5 minutes). Endogenous peroxidase in the tissue was blocked by incubation using 1% H2O2 in PBS, pH 7.4. Nonspecific sites were blocked with 10% goat serum in PBS (15 minutes at room temperature). After the incubation with primary antibody (1 hour, 37°C), the sections were washed with PBS, then incubated with the corresponding biotinylated link antibody (30 minutes, 37°C) and with the ABC complex (30 minutes, 37°C). After further washes with PBS, bound ABC complexes were detected with 0.06% diaminobenzidine tetrahydrochloride in PBS. The sections were counterstained with hematoxylin. Controls for immunospecificity were included in all experiments by omission of the primary antibody and its replacement by phosphate-buffered saline and matching concentrations of normal mouse, rabbit, or goat serum (data not illustrated).
Hydroxyproline Analysis
Lungs were dried at 110°C for at least 24 hours, then acid hydrolyzed using 6 N hydrochloric acid in the absence of oxygen. Vials were then vacuum-sealed and incubated at 110°C for 24 hours. Hydroxyproline content was quantified as described previously.21,31,32
Histological Scoring
Standard hematoxylin and eosin (H&E) staining was performed on 5-μm-thick lung sections as described previously.32 The sections were scored by a pathologist (T.D.O.) who was blinded to both treatment and strain as previously described.21 Individual fields were examined with a light microscope at ×200 magnification. Every field in the entire lung was scored, starting peripherally. Each field had to contain >50% alveolar tissue/terminal bronchioles to be counted. Scoring was based on the extent of interstitial fibrosis according to the following scale: 0 = no fibrosis, 1 = 0 to 25%, 2 = 25 to 50%, 3 = 50 to 75%, and 4 = 75 to 100%. The pathological index score was then reported as ratio of the sum of all of the scores divided by the total number of fields counted for each sample. Group scores were averaged for statistical analyses.
Quantitative Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
RT reactions were performed on 1 μg of RNA using 5 mmol/L MgCl2, PCR buffer II, 1 mmol/L nucleotide mix (Promega, Madison, WI), 1.0 U RNAsin, 2.5 U MuLV reverse transcriptase enzyme, and 3 μg of random primers. RT reactions were performed in a thermocycler programmed for 42°C for 40 minutes, 99°C for 5 minutes, and 5°C for 5 minutes. Quantitative PCR was performed by adding Universal PCR buffer and TaqMan primer/probe assay reagent for RAGE according to the manufacturer’s protocol. Primers/probe for GAPDH were used as a loading control for normalization. The default program was performed on an ABI Prism 7300 (Applied Biosystems, Foster City, CA) and consisted of 50°C for 2 minutes, 95°C for 10 minutes, and 40 cycles of 95°C for 15 seconds, and 60°C for 1 minute. Sequence Detection Software Version 1.4 (Applied Biosystems) was used to analyze the data and obtain relative quantities of mRNA expression for each sample based on the crossing threshold. The relative expressions for each treatment group were then averaged and the expression of the TiO2 treated controls was set to 100% for relative expression comparison.
Statistical Analyses
Paired samples were analyzed using Student’s t-test. Variances of paired samples were analyzed by an F-test. When variances were determined to be significantly different, analysis was performed by t-test with Welch’s correction. Analysis of variance followed by a Tukey test were used for multiple comparisons. Values are reported ±SEM, and P values <0.05 were considered significant.
Results
Membrane RAGE Is Highly Expressed in Adult Mouse Lungs
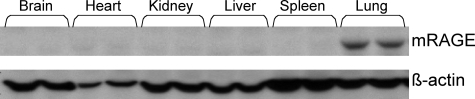
Although it has been shown that RAGE mRNA and sRAGE protein expression are highest in the lung among normal adult tissues,6,7 it is important to determine whether mRAGE is also expressed at high levels in the lung because this is the isoform of RAGE that mediates signaling. mRAGE expression was examined in membrane preparations from several untreated mouse tissues by Western blot analysis (Figure 1). Similar to RAGE mRNA and sRAGE protein, mRAGE protein is found at the highest levels in the lung. The relatively abundant expression of pulmonary mRAGE and sRAGE in the normal lung observed by us as well as others6,7,33 suggests that it plays a homeostatic role unique to the lung. Thus, alterations in mRAGE and sRAGE expression may be a factor in pulmonary diseases such as pulmonary fibrosis.
Figure 1.
mRAGE is highly expressed in the lung. Membrane fractions from each of the indicated tissues were prepared from untreated wild-type C57BL/6 mice and analyzed by Western blot for mRAGE expression. The PVDF membrane was then stripped and reprobed for β-actin expression as a loading control. Note that mRAGE expression is highest in the lungs.
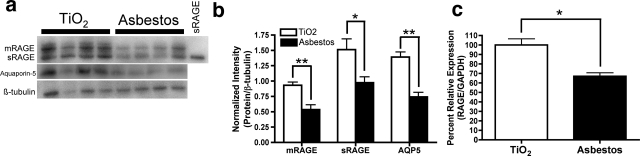
Pulmonary mRAGE and sRAGE Are Down-Regulated after Asbestos Injury
As indicated above, RAGE contributes to the pathogenesis of many nonpulmonary diseases. In such diseases, mRAGE expression has been found to be up-regulated. To investigate the regulation of mRAGE and sRAGE expression in pulmonary fibrosis, we used both the bleomycin and the asbestos mouse models of pulmonary fibrosis.21,32 Because mouse sRAGE is not a product of alternative splicing,23 it is necessary to look at the protein level to examine differential expression of these two isoforms. The asbestos model recapitulates many of the salient features of IPF. In this model, we found that pulmonary mRAGE and sRAGE levels decrease significantly by 24 hours after asbestos injury (not illustrated) and remain depressed through 14 days (Figure 2, a and b). This loss corresponds with a 1.5-fold decrease in mRNA expression of the RAGE gene after 14 days of asbestos treatment as compared to a 24-hour titanium dioxide treatment (Figure 2c). Previous experiments have shown that titanium dioxide has no effect on the lungs at any time point.21
Figure 2.
mRAGE and sRAGE expression are decreased after asbestos injury. C57BL/6 mice were treated with 0.1 mg of crocidolite asbestos or titanium dioxide (TiO2) as an inert particulate control. Fourteen days later, lungs were extracted to obtain both membrane and soluble protein fractions. a: mRAGE, sRAGE, and aquaporin-5 expression were analyzed by Western blot analysis. PVDF membranes were stripped and reprobed for β-tubulin as a loading control. b: Shown to the right is the densitometric analysis, where the protein of interest band intensities are normalized to β-tubulin band intensities for each lane. RNA was isolated from the lungs of mice 14 days after they were treated with asbestos and compared with normal lungs from mice treated with titanium dioxide for 24 hours. RAGE mRNA expression was measured by real-time PCR and normalized to GAPDH. c: Results are reported as a percent relative quantity compared to the TiO2-treated group. A 1.5-fold decrease in RAGE expression was seen after asbestos treatment. **P < 0.01 and *P < 0.05 compared to TiO2-treated controls.
Both mRAGE and sRAGE were also significantly depleted in response to bleomycin injury (not illustrated), a model commonly used to study pulmonary fibrosis. mRAGE was significantly decreased 24 hours after injury whereas sRAGE was significantly depleted 3 days after injury. Both mRAGE and sRAGE remained significantly lower up to 7 days after bleomycin injury, consistent with our previous report demonstrating a significant loss of mRAGE and sRAGE 7 days after bleomycin injury.7
The loss of RAGE in the asbestos model corresponded with a similar loss of aquaporin-5, a marker of type I alveolar epithelial cells (Figure 2b). This suggests that the loss of RAGE in response to fibrotic injury might correspond with a loss of type I epithelial cells. However, it is also possible that both proteins are being down-regulated in response to the injury. Previous studies using bleomycin-induced pulmonary fibrosis demonstrate a similar finding for aquaporin-5 expression.34 Thus, in contrast to the nonpulmonary diseases studied in which RAGE expression is elevated in disease tissues,9,10 we found that pulmonary expression of mRAGE and sRAGE is significantly decreased in two models of pulmonary fibrosis and that this depletion is an early event after injury.
Loss of mRAGE/sRAGE Expression in Human IPF Lungs
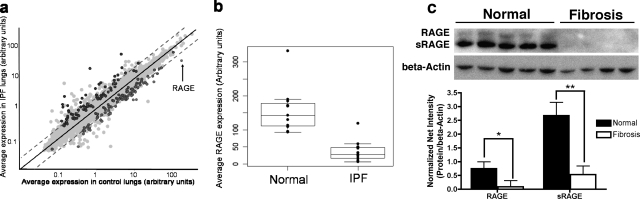
To determine whether observations from the animal models also occur in human IPF, we obtained IPF tissue samples from diagnostic biopsies or patients undergoing lung transplantation (thus representing late stages of IPF pathogenesis) and control lung specimens from histologically normal lung samples resected from patients with lung cancer. Microarray analysis performed on these human lung samples indicated that RAGE is the most down-regulated gene transcript in IPF lungs compared to control lungs, demonstrating a 4.65-fold reduction in IPF lungs (Figure 3, a and b).
Figure 3.
RAGE transcripts and mRAGE/sRAGE protein are down-regulated in IPF lungs. a and b: Microarray analysis was performed on human IPF lung samples or control lung samples to compare expression of RAGE transcripts between IPF and control lungs. a: Summary of microarray data. Each point represents a single gene, plotted by its average expression in control lungs versus its average expression in IPF lungs. Points in color represent genes whose difference in expression in control versus IPF lungs reached significance. The point representing RAGE transcripts is indicated. b: Plot demonstrating the RAGE transcript level in each patient sample (represented by a single point). RAGE transcript levels are significantly decreased in IPF lungs compared to control lungs (P = 0.0000054). c: Western blot analysis on a different set of patient samples demonstrates a reduction in mRAGE (*P < 0.05) and sRAGE (**P < 0.01) protein levels in fibrotic areas of IPF lungs. Densitometry is shown with band intensities of mRAGE and sRAGE normalized to β-actin as a loading control.
To confirm the findings from the microarray experiments, protein expression in another set of human IPF and control lung specimens was examined. These studies demonstrate that pulmonary mRAGE and sRAGE protein levels are reduced in IPF lungs compared to control lungs (Figure 3, c and d). Taken together, these data indicate a depletion of RAGE expression in pulmonary fibrosis pathogenesis; the mouse models suggest this depletion is an early event after disease onset, whereas the human data demonstrate that it is sustained through late stages of IPF.
Absence of mRAGE/sRAGE Expression Leads to Pulmonary Fibrosis-Like Alterations
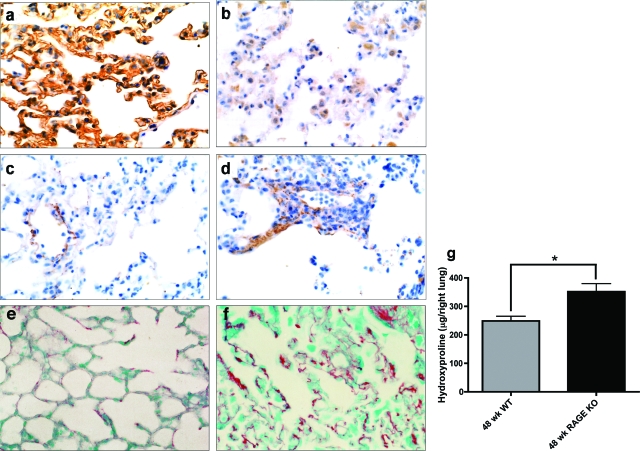
To determine whether mRAGE and sRAGE loss contributes to pulmonary fibrosis pathogenesis, RAGE knockout mice22,35,36,37 were examined (Figure 4, a and b, confirms loss of RAGE protein expression). RAGE knockout mice were allowed to age without any injury. These aged knockout mice were found to spontaneously develop fibrosis-like alterations in their lungs. Immunohistochemical staining demonstrated increased staining in the lung parenchyma for collagen type I in 64% (18 of 28) of the RAGE knockout mice studied compared to 10% (2 of 20) of wild-type mice (Figure 4, c and d). Similar results were found using Sirius Red staining, which also showed qualitative visually apparent increases in collagen staining in the knockout mice as compared to wild-type mice as shown in Figure 4, e and f.
Figure 4.
Aged RAGE knockout (KO) mouse lungs spontaneously develop features of pulmonary fibrosis. Lung sections from 19- to 24-month-old wild-type (a, c, e) and RAGE KO (b, d, f) mice were subjected to immunohistochemical analysis for RAGE (a, b) and collagen type I (c, d) as well as Sirius Red staining for total collagen (e, f). RAGE knockout lungs lack immunoreactivity for RAGE (b) and show increased staining for collagen (d, f). g: Right lungs from 48-week-old wild-type (n = 3) or RAGE knockout mice (n = 4) were subjected to hydroxyproline analysis as an indicator of the extent of fibrosis. Forty-eight-week-old RAGE KO mice have significantly increased levels of hydroxyproline compared to age-matched wild-type controls (*P < 0.05).
As another means of assessing fibrosis in the lungs of RAGE-null mice, hydroxyproline quantification was performed on the entire right lung from 48-week-old RAGE knockout mice or age-matched wild-type controls. Because hydroxyproline is a posttranslational modification found abundantly in collagen, hydroxyproline levels reflect the extent of collagen deposition/fibrosis in a tissue. Notably, hydroxyproline levels in the right lungs from RAGE knockout mice are significantly higher than those from control mice (Figure 4g). In addition, no differences in hydroxyproline levels were seen between 8- and 12-week-old RAGE knockout and wild-type mice. Hydroxyproline quantification was performed on total lungs (right and left) from 6-month-old RAGE knockout and wild-type mice (n = 5 for both groups). A similar increase in collagen content of the lung was noted with an average hydroxyproline content of 260 μg/total lung in the wild-type versus 367 μg/total lung in the RAGE knockouts (P < 0.0001). This suggests that the increase in fibrosis in the RAGE knockout mice is age-related. Taken together, these data indicate that the lack of mRAGE/sRAGE expression is sufficient to result in fibrotic alterations in the lung.
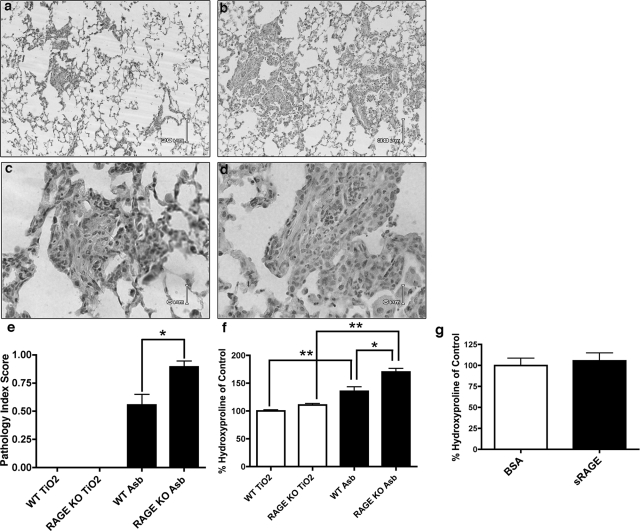
Absence of mRAGE Expression Increases the Severity of Pulmonary Fibrosis
To further examine the role of mRAGE and sRAGE in pulmonary fibrosis, 8-week-old RAGE-null mice and age-matched wild-type controls were treated with asbestos and the lungs were examined 14 days after this injury. These studies demonstrate that RAGE-null mice develop more severe pulmonary fibrosis as measured by histological scoring (Figure 5, a–c) and supported by total lung hydroxyproline quantification (Figure 5d). To determine whether responses seen in the RAGE-null mice were a result of the absence of sRAGE or mRAGE, RAGE-null mice were treated with asbestos and given sRAGE by daily intraperitoneal injections. Notably, we were able to detect sRAGE by Western blot analysis in the lung homogenates 3 days after the start of treatment (not illustrated) indicating that sRAGE was being delivered to the lung. There was no difference in the degree of fibrosis as measured by relative hydroxyproline quantification between those treated with sRAGE and those treated with bovine serum albumin as a control (Figure 5e).
Figure 5.
RAGE knockout (KO) mice develop more severe fibrosis after asbestos treatment. RAGE KO mice and C57BL/6 wild-type mice were treated with 0.1 mg of crocidolite asbestos or titanium dioxide (TiO2) as an inert particulate control. Fourteen days later, lungs were either inflation-fixed with 10% formalin for histology (n = 3 per group) or removed and dried for hydroxyproline quantification (n = 5 per group). Light microscopic analysis demonstrates increased alveolar thickening in the RAGE-null mice (b, d) compared to wild-type (a, c). e: Histological scoring by a pathologist blinded to genotype and treatment demonstrates more severe fibrosis in the asbestos-treated RAGE KO group compared to wild-type controls. No fibrosis was appreciated in either of the TiO2-treated groups. f: Asbestos treatment resulted in increased fibrosis in the RAGE KO mice as determined by hydroxyproline levels compared to wild-type mice. g: RAGE KO mice were subjected to asbestos treatment along with intraperitoneal injections of either bovine sRAGE or bovine serum albumin (50 μg/day). There was no difference in the degree of fibrosis between the groups (n = 5 per group) as measured by total lung hydroxyproline. **P < 0.01 and *P < 0.05 compared to TiO2-treated controls. Scale bars: 30 μm (a, b); 6 μm (c, d).
Discussion
RAGE has been shown to contribute to the pathogenesis of many nonpulmonary diseases. In such diseases, RAGE expression is up-regulated in response to the disease. In contrast to these nonpulmonary diseases, the current studies demonstrate that pulmonary expression of RAGE and sRAGE are significantly decreased in two models of pulmonary fibrosis and that this depletion is an early event after injury. In addition, the data from human IPF tissue indicate that mRAGE and sRAGE loss is sustained through later stages of pulmonary fibrosis.
Data from the current study suggests a protective role of pulmonary mRAGE in pulmonary fibrosis. This sharply contrasts with the view of RAGE as a propagator of pathology, a role that has been described in several other diseases. For example, in the diabetic vasculature that is prone to atherosclerosis, RAGE expression is up-regulated and interacts with advanced glycation end products and S100/calgranulins to initiate proinflammatory signaling that exacerbates plaque formation and degree of complexity.25,38,39,40 In the Alzheimer’s diseased brain, RAGE is up-regulated and serves to activate microglia as well as mediate amyloid-β transport across the blood-brain barrier.41,42,43 In contrast, in the lungs, RAGE expression is relatively high in healthy adults. However, as shown here in both mouse models and human IPF lungs, mRAGE and sRAGE expression is significantly down-regulated after profibrotic pulmonary injury. It is possible that this loss of RAGE corresponds with a loss of type I alveolar epithelial cells and not a decrease in expression in these cells. However, the absence of mRAGE/sRAGE expression in RAGE knockout mice leads to an age-related pulmonary fibrosis that displays many of the same features as human IPF. Additionally, the absence of mRAGE and sRAGE in knockout mice also leads to enhanced fibrosis in a mouse model of asbestos-induced pulmonary fibrosis. Notably, treatment of the RAGE knockout mice with sRAGE offered no protection against asbestos-induced pulmonary fibrosis despite seeing the protein in the lung. This is in contrast to other RAGE-related disease processes, including atherosclerosis25 and wound healing,44 in which this dosage of sRAGE prevented disease. This suggests that the absence of mRAGE in the knockout mice may be the primary mediator contributing to pulmonary fibrosis in these mice. Thus, these studies reveal a novel aspect of RAGE biology because it appears to have a potential beneficial role in both normal lung homeostasis and protecting the lungs from fibrosis after injury.
A potential mechanism for which pulmonary RAGE loss is pathogenic may involve alveolar epithelial type I cell morphology. Demling and colleagues11 and Fehrenbach and colleagues12 have recently reported that RAGE expression enhances adherence of alveolar epithelial type I cells to the extracellular matrix and is essential for cellular spreading. RAGE also induces spreading of these cells on a collagen type IV surface.11 These observations are interesting given that thin spreading on the basement membrane, which is composed of collagen type IV, is essential for normal lung physiology. Additionally, differentiation of alveolar type II cells to alveolar type I cells has been shown to be an important mechanism by which the lung repairs itself. RAGE expression has been shown to correlate with this differentiation.13 Thus, loss of RAGE expression may result in decreased binding of the basement membrane and increased susceptibility to alveolar injury and/or prevent the proper re-epithelialization of alveoli during IPF pathogenesis.
Although there is always the possibility that genetic manipulations performed to knockout the expression of a gene may have effects independent of the actual loss of expression of the gene, these studies do suggest, that the high normal expression of RAGE in the lung does have a normal homeostatic function. This finding, although contrary to findings observed in other tissues/organs, may actually not be too surprising. It might be more unusual for a tissue to express a protein in very high amounts under normal conditions if its expression is detrimental to the organ. In summary, these studies demonstrate that decreased mRAGE/sRAGE expression is an early and sustained event in animal models of pulmonary fibrosis. mRAGE/sRAGE expression is also significantly decreased in patients with IPF. Although previous reports indicate that RAGE signaling is important in the propagation of several nonpulmonary diseases, we found that the absence of mRAGE/sRAGE expression results in age-related fibrotic changes in the lung and that mRAGE loss contributes to a more severe fibrosis in an animal model of pulmonary fibrosis. These findings suggest that the normally high expression of mRAGE in the lungs plays an important protective/homeostatic role, and that decreased expression of RAGE contributes to the pathogenesis of pulmonary fibrosis.
Acknowledgments
We thank Lara Chensny and John Igwe for assistance with the experiments and Charleen T. Chu for constructive comments during manuscript preparation.
Footnotes
Address reprint requests to Tim D. Oury, 200 Lothrop St., W-957 BST, Pittsburgh, PA 15261. E-mail: tdoury@pitt.edu.
Supported by the American Heart Association (grants 702359 to T.D.O. and 0415412U to L.E.H.), the National Institutes of Health (grants R01HL063700, R01HL110166, and R21ES013986 to T.D.O.; R01HL073745 to N. K.; and F30ES013621 to R.J.T.), the Deutche Forschungsgemeinschaft (grant DFG/SFB 405 to P.P.N.), the European Foundation for the Study of Diabetes (to A.B.), and by a generous donation from the Simmons family.
References
- Crystal RG, Bitterman PB, Rennard SI, Hance AJ, Keogh BA. Interstitial lung diseases of unknown cause. Disorders characterized by chronic inflammation of the lower respiratory tract. N Engl J Med. 1984;310:235–244. doi: 10.1056/NEJM198401263100406. [DOI] [PubMed] [Google Scholar]
- Collard HR, King TE., Jr Demystifying idiopathic interstitial pneumonia. Arch Intern Med. 2003;163:17–29. doi: 10.1001/archinte.163.1.17. [DOI] [PubMed] [Google Scholar]
- Turner-Warwick M, Burrows B, Johnson A. Cryptogenic fibrosing alveolitis: response to corticosteroid treatment and its effect on survival. Thorax. 1980;35:593–599. doi: 10.1136/thx.35.8.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- Schmidt AM, Vianna M, Gerlach M, Brett J, Ryan J, Kao J, Esposito C, Hegarty H, Hurley W, Clauss M, Wang F, Pan Y-CE, Tsang TC, Stern D. Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J Biol Chem. 1992;267:14987–14997. [PubMed] [Google Scholar]
- Brett J, Schmidt AM, Yan SD, Zou YS, Weidman E, Pinsky D, Nowygrod R, Neeper M, Przysiecki C, Shaw A, Migheli A, Stern D. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am J Pathol. 1993;143:1699–1712. [PMC free article] [PubMed] [Google Scholar]
- Hanford LE, Fattman CL, Shaefer LM, Enghild JJ, Valnickova Z, Oury TD. Regulation of receptor for advanced glycation end products during bleomycin-induced lung injury. Am J Respir Cell Mol Biol. 2003;29:S77–S81. [PubMed] [Google Scholar]
- Li J, Schmidt AM. Characterization and functional analysis of the promoter of RAGE, the receptor for advanced glycation end products. J Biol Chem. 1997;272:16498–16506. doi: 10.1074/jbc.272.26.16498. [DOI] [PubMed] [Google Scholar]
- Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108:949–955. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AM, Yan SD, Yan SF, Stern DM. The biology of the receptor for advanced glycation end products and its ligands. Biochim Biophys Acta. 2000;1498:99–111. doi: 10.1016/s0167-4889(00)00087-2. [DOI] [PubMed] [Google Scholar]
- Demling N, Ehrhardt C, Kasper M, Laue M, Knels L, Rieber EP. Promotion of cell adherence and spreading: a novel function of RAGE, the highly selective differentiation marker of human alveolar epithelial type I cells. Cell Tissue Res. 2006;323:475–488. doi: 10.1007/s00441-005-0069-0. [DOI] [PubMed] [Google Scholar]
- Fehrenbach H, Kasper M, Tschernig T, Shearman MS, Schuh D, Muller M. Receptor for advanced glycation endproducts (RAGE) exhibits highly differential cellular and subcellular localisation in rat and human lung. Cell Mol Biol. 1998;44:1147–1157. [PubMed] [Google Scholar]
- Shirasawa M, Fujiwara N, Hirabayashi S, Ohno H, Iida J, Makita K, Hata Y. Receptor for advanced glycation end-products is a marker of type I lung alveolar cells. Genes Cells. 2004;9:165–174. doi: 10.1111/j.1356-9597.2004.00712.x. [DOI] [PubMed] [Google Scholar]
- Dahlin K, Mager EM, Allen L, Tigue Z, Goodglick L, Wadehra M, Dobbs L. Identification of genes differentially expressed in rat alveolar type I cells. Am J Respir Cell Mol Biol. 2004;31:309–316. doi: 10.1165/rcmb.2003-0423OC. [DOI] [PubMed] [Google Scholar]
- Oldfield MD, Bach LA, Forbes JM, Nikolic-Paterson D, McRobert A, Thallas V, Atkins RC, Osicka T, Jerums G, Cooper ME. Advanced glycation end products cause epithelial-myofibroblast transdifferentiation via the receptor for advanced glycation end products (RAGE). J Clin Invest. 2001;108:1853–1863. doi: 10.1172/JCI11951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuse T, Ohga E, Teramoto S, Fukayama M, Nagai R, Horiuchi S, Ouchi Y. Immunohistochemical localisation of advanced glycation end products in pulmonary fibrosis. J Clin Pathol. 1998;51:515–519. doi: 10.1136/jcp.51.7.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JH, Wang W, Huang XR, Oldfield M, Schmidt AM, Cooper ME, Lan HY. Advanced glycation end products induce tubular epithelial-myofibroblast transition through the RAGE-ERK1/2 MAP kinase signaling pathway. Am J Pathol. 2004;164:1389–1397. doi: 10.1016/S0002-9440(10)63225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol. 2001;280:E685–E694. doi: 10.1152/ajpendo.2001.280.5.E685. [DOI] [PubMed] [Google Scholar]
- Demling N, Ehrhardt C, Kasper M, Laue M, Knels L, Rieber EP. Promotion of cell adherence and spreading: a novel function of RAGE, the highly selective differentiation marker of human alveolar epithelial type I cells. Cell Tissue Res. 2006;323:475–488. doi: 10.1007/s00441-005-0069-0. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Humpert PM, Morcos M, Wendt T, Chavakis T, Arnold B, Stern DM, Nawroth PP. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med. 2005;83:876–886. doi: 10.1007/s00109-005-0688-7. [DOI] [PubMed] [Google Scholar]
- Tan RJ, Fattman CL, Watkins SC, Oury TD. Redistribution of pulmonary EC-SOD after exposure to asbestos. J Appl Physiol. 2004;97:2006–2013. doi: 10.1152/japplphysiol.00480.2004. [DOI] [PubMed] [Google Scholar]
- Constien R, Forde A, Liliensiek B, Grone HJ, Nawroth P, Hammerling G, Arnold B. Characterization of a novel EGFP reporter mouse to monitor Cre recombination as demonstrated by a Tie2 Cre mouse line. Genesis. 2001;30:36–44. doi: 10.1002/gene.1030. [DOI] [PubMed] [Google Scholar]
- Hanford LE, Enghild JJ, Valnickova Z, Petersen SV, Schaefer LM, Schaefer TM, Reinhart TA, Oury TD. Purification and characterization of mouse soluble receptor for advanced glycation end products (sRAGE). J Biol Chem. 2004;279:50019–50024. doi: 10.1074/jbc.M409782200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt TM, Tanji N, Guo J, Kislinger TR, Qu W, Lu Y, Bucciarelli LG, Rong LL, Moser B, Markowitz GS, Stein G, Bierhaus A, Liliensiek B, Arnold B, Nawroth PP, Stern DM, D'Agati VD, Schmidt AM. RAGE drives the development of glomerulosclerosis and implicates podocyte activation in the pathogenesis of diabetic nephropathy. Am J Pathol. 2003;162:1123–1137. doi: 10.1016/S0002-9440(10)63909-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ, Jr, Chow WS, Stern D, Schmidt AM. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med. 1998;4:1025–1031. doi: 10.1038/2012. [DOI] [PubMed] [Google Scholar]
- Katzenstein AL, Myers JL. Idiopathic pulmonary fibrosis: clinical relevance of pathologic classification. Am J Respir Crit Care Med. 1998;157:1301–1315. doi: 10.1164/ajrccm.157.4.9707039. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- Kaminski N, Friedman N. Practical approaches to analyzing results of microarray experiments. Am J Respir Cell Mol Biol. 2002;27:125–132. doi: 10.1165/ajrcmb.27.2.f247. [DOI] [PubMed] [Google Scholar]
- Pardo A, Gibson K, Cisneros J, Richards TJ, Yang Y, Becerril C, Yousem S, Herrera I, Ruiz V, Selman M, Kaminski N. Up-regulation and profibrotic role of osteopontin in human idiopathic pulmonary fibrosis. PLoS Med. 2005;2:e251. doi: 10.1371/journal.pmed.0020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper M, Seidel D, Knels L, Morishima N, Neisser A, Bramke S, Koslowski R. Early signs of lung fibrosis after in vitro treatment of rat lung slices with CdCl2 and TGF-beta1. Histochem Cell Biol. 2004;121:131–140. doi: 10.1007/s00418-003-0612-6. [DOI] [PubMed] [Google Scholar]
- Woessner JF. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- Fattman CL, Chu CT, Kulich SM, Enghild JJ, Oury TD. Altered expression of extracellular superoxide dismutase in mouse lung after bleomycin treatment. Free Radic Biol Med. 2001;31:1198–1207. doi: 10.1016/s0891-5849(01)00699-2. [DOI] [PubMed] [Google Scholar]
- Katsuoka F, Kawakami Y, Arai T, Imuta H, Fujiwara M, Kanma H, Yamashita K. Type II alveolar epithelial cells in lung express receptor for advanced glycation end products (RAGE) gene. Biochem Biophys Res Commun. 1997;238:512–516. doi: 10.1006/bbrc.1997.7263. [DOI] [PubMed] [Google Scholar]
- Gabazza EC, Kasper M, Ohta K, Keane M, D'Alessandro-Gabazza C, Fujimoto H, Nishii Y, Nakahara H, Takagi T, Menon AG, Adachi Y, Suzuki K, Taguchi O. Decreased expression of aquaporin-5 in bleomycin-induced lung fibrosis in the mouse. Pathol Int. 2004;54:774–780. doi: 10.1111/j.1440-1827.2004.01754.x. [DOI] [PubMed] [Google Scholar]
- Schwenk F, Baron U, Rajewsky K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 1995;23:5080–5081. doi: 10.1093/nar/23.24.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liliensiek B, Weigand MA, Bierhaus A, Nicklas W, Kasper M, Hofer S, Plachky J, Grone HJ, Kurschus FC, Schmidt AM, Yan SD, Martin E, Schleicher E, Stern DM, Hammerling GG, Nawroth PP, Arnold B. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J Clin Invest. 2004;113:1641–1650. doi: 10.1172/JCI18704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierhaus A, Haslbeck KM, Humpert PM, Liliensiek B, Dehmer T, Morcos M, Sayed AA, Andrassy M, Schiekofer S, Schneider JG, Schulz JB, Heuss D, Neundorfer B, Dierl S, Huber J, Tritschler H, Schmidt AM, Schwaninger M, Haering HU, Schleicher E, Kasper M, Stern DM, Arnold B, Nawroth PP. Loss of pain perception in diabetes is dependent on a receptor of the immunoglobulin superfamily. J Clin Invest. 2004;114:1741–1751. doi: 10.1172/JCI18058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kislinger T, Tanji N, Wendt T, Qu W, Lu Y, Ferran LJ, Jr, Taguchi A, Olson K, Bucciarelli L, Goova M, Hofmann MA, Cataldegirmen G, D'Agati V, Pischetsrieder M, Stern DM, Schmidt AM. Receptor for advanced glycation end products mediates inflammation and enhanced expression of tissue factor in vasculature of diabetic apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol. 2001;21:905–910. doi: 10.1161/01.atv.21.6.905. [DOI] [PubMed] [Google Scholar]
- Schmidt AM, Yan SD, Wautier JL, Stern D. Activation of receptor for advanced glycation end products: a mechanism for chronic vascular dysfunction in diabetic vasculopathy and atherosclerosis. Circ Res. 1999;84:489–497. doi: 10.1161/01.res.84.5.489. [DOI] [PubMed] [Google Scholar]
- Naka Y, Bucciarelli LG, Wendt T, Lee LK, Rong LL, Ramasamy R, Yan SF, Schmidt AM. RAGE axis: animal models and novel insights into the vascular complications of diabetes. Arterioscler Thromb Vasc Biol. 2004;24:1342–1349. doi: 10.1161/01.ATV.0000133191.71196.90. [DOI] [PubMed] [Google Scholar]
- Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt AM. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, Zhu H, Ghiso J, Frangione B, Stern A, Schmidt AM, Armstrong DL, Arnold B, Liliensiek B, Nawroth P, Hofman F, Kindy M, Stern D, Zlokovic B. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- Mackic JB, Stins M, McComb JG, Calero M, Ghiso J, Kim KS, Yan SD, Stern D, Schmidt AM, Frangione B, Zlokovic BV. Human blood-brain barrier receptors for Alzheimer’s amyloid-beta 1-40. Asymmetrical binding, endocytosis, and transcytosis at the apical side of brain microvascular endothelial cell monolayer. J Clin Invest. 1998;102:734–743. doi: 10.1172/JCI2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wear-Maggitti K, Lee J, Conejero A, Schmidt AM, Grant R, Breitbart A. Use of topical sRAGE in diabetic wounds increases neovascularization and granulation tissue formation. Ann Plast Surg. 2004;52:519–522. doi: 10.1097/01.sap.0000122857.49274.8c. [DOI] [PubMed] [Google Scholar]