Abstract
Prion diseases or transmissible spongiform encephalopathies are fatal neurodegenerative conditions in humans and animals that originate spontaneously, genetically or by infection. Conformational change of the normal (cellular) form of prion protein (PrPc) to a pathological, disease-associated form (PrPTSE) is considered central to pathogenesis and formation of the infectious agent or prion. Neuronal damage is central to clinical manifestation of prion diseases but poorly understood. In this review, we analyze the major pathogenetic pathways that lead to tissue pathology in different forms of disease. Neuropathogenesis of prion diseases evolves in complex ways on several front lines, most but not all of which exist also in other neurodegenerative as well as infectious diseases. Whereas intracellular accumulation of PrP forms might significantly impair cell function and lead to cytopathology, mere extracellular deposition of PrPTSE is questionable as a direct cytotoxic factor. Tissue damage may result from several parallel, interacting, or subsequent pathways. Future studies should clarify the trigger(s) and sequence of these processes and whether, and which, one is dominating or decisive.
Prion diseases are multifaceted disorders affecting the central nervous system (CNS) of multiple species.1 Many aspects of prion disease raise controversies and questions for a broad audience of scientists. Recently, several excellent reviews have summarized basic processes and phenotypic variability.2,3,4,5 In the present review, we aim to analyze major pathogenetic events that lead to detectable tissue pathology in different forms of disease.
It is generally accepted that prion diseases are transmissible—hence the name transmissible spongiform encephalopathy (TSE)—and invariably fatal. There may be exceptions to these rules; on one hand, transmissibility may fail in certain disease forms associated with amyloidogenesis,6 and on the other hand there is increasing recognition of a subclinical carrier state in different species, including humans.7,8 However, forms originating from transmission to and between humans constitute a minority.
In humans sporadic Creutzfeldt-Jakob disease (sCJD) is the most frequent disease form. Acquired forms with suspected or proven external prion exposure include kuru, the historic disease of the Fore tribe in Papua-New Guinea; iatrogenic CJD (iCJD) related to medical interventions with central (eg, in neurosurgery, by deep electrodes) or peripheral (eg, cadaver hypophyseal hormones) inoculation; and variant CJD (vCJD) that relates to bovine spongiform encephalopathy.9,10 Changes in the 253-amino-acid-long human normal (cellular) prion protein (PrPC), encoded by a gene (PRNP) on the short arm of chromosome 20, include point or insertional mutations linked to genetic prion diseases.11 Although at least 15 PRNP polymorphisms are known, only the methionine/valine (M/V) at codon 129 is clearly influential in all disease forms.1,11 Genetic prion diseases include genetic CJD (gCJD), Gerstmann-Sträussler-Scheinker disease, and the thalamic degeneration fatal familial insomnia. Transgenic mouse models of human genetic prion disease have been reported; of these the P101L mutation has been argued to be a susceptibility factor rather than a direct cause of Gerstmann-Sträussler-Scheinker disease (P102L mutation in humans).12,13 Further mutations (T183A and E199K) in mice do not lead to detectable neurodegeneration, whereas a nine-octapeptide repeat insertion in the PRNP is associated with a progressive neurological phenotype without transmissibility.13 It must be noted that not all PRNP mutation-associated disease in humans is transmitted, raising the issue whether transmissible forms should be distinguished from “proteinopathy” forms. It is clear that the PRNP genotype and disease-associated prion protein (PrPTSE) types, including their glycosylation patterns, are major phenotypic determinants, but it is not clear at present how they relate with the heterogeneity of prion diseases. Thus other genetic or epigenetic factors likely modify disease phenotypes.5
In addition to the prototype prion disease of animals (scrapie) that affects sheep and goats, animal diseases include chronic wasting disease of deer and elk, feline and mink spongiform encephalopathy, and the epidemic bovine spongiform encephalopathy (“mad cow disease”).1 Small animals, like hamsters, mice, or bank voles can be infected experimentally. Genetic causation coupled with transmissibility has remained a unique paradigm in biomedicine. Prion diseases are of additional medical and research interest as well-characterized models of neurodegeneration.
Prion Proteins: Multiple Forms
Conformational change distinguishes PrPC from PrPTSE that seems to be the main or only constituent of the infectious agent.1,14 The term PrPTSE was introduced to avoid confusion resulting from complex nomenclatures,15 but it is far from accepted in general use. PrPTSE features a predominantly β-pleated structure, whereas PrPC is α-helix dominant.1 PrPTSE can be distinguished from PrPC by its resistance to protease treatment,1 although a protease-sensitive (PrPSen) but disease-associated transitional form has also been described.16 Accepting PrPTSE as the infectious agent is the basis of the “protein-only” hypothesis. However, belief in other pathogens, which can be summarized as “not-only-protein” hypotheses, has not uniformly diminished. In this context it must be noted that 25-nm virus-like particles were recently demonstrated in cell cultures infected with CJD and scrapie,17 awakening earlier theories. Interestingly, these particles are similar to tubulovesicular structures found in all TSE forms, demanding further clarification of their role.18
The significance of PrPC extends beyond prion diseases. Possible functions comprise roles in neurogenesis and differentiation of neural stem cells, neuritogenesis, involvement and interaction with signal transduction pathways, synaptogenesis, neuronal survival via anti- or pro-apoptotic functions, copper binding, redox homeostasis, long-term renewal of hemopoietic stem cells, activation and development of T cells, differentiation and modulation of phagocytosis of leukocytes, and altering leukocyte recruitment to sites of inflammation.3 A wide range of proteins may act as putative PrP interactors.4 Its up-regulation may occur in inflammatory conditions and may provide an increased substrate for PrPC-PrPTSE conversion.2,19 Based on structural similarity, it has been proposed that PrPC might function as a member of the Bcl-2 family of proteins.20
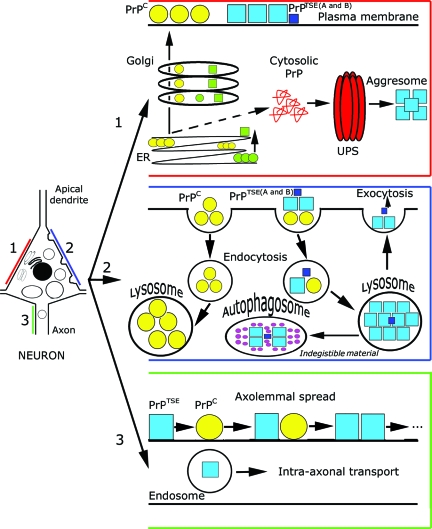
The PrPC polypeptide is synthesized in the endoplasmic reticulum (ER), processed in the Golgi apparatus, and then carried in its mature form to the cell surface where most of it is found in lipid rafts (Figure 1).4 Generation of PrPTSE from PrPC would occur after the arrival of PrPC at the cell surface. PrPC from the plasma membrane or exogenic PrPTSE may be internalized and processed through the endosomal-lysosomal system, which may also be a substrate for their interaction (Figure 1). PrPC can be synthesized in the ER in three topological forms, designated SecPrP, NtmPrP, and CtmPrP. Whereas secretory (SecPrP) molecules are attached to the outer leaflet of the lipid bilayer exclusively by a C-terminal glycosyl-phosphatidylinositol anchor, NtmPrP and CtmPrP molecules span the lipid bilayer with either the N or C terminus.23
Figure 1.
Summary of cytopathology and PrP processing pathways. Pathway 1 (intracellular PrP processing, red): The PrPC polypeptide (yellow circles), including genetic mutants (green circles), is synthesized in the ER, processed in the Golgi apparatus, and then carried in its mature form to the cell surface where most of it is found in lipid rafts. Generation of PrPTSE, consisting of a mixture of dominant and subdominant types (blue and dark blue boxes, A and B), from PrPC would occur after the arrival of PrPC at the cell surface. Another hypothetical pathway would form misfolded cytosolic PrP, associated with neurotoxicity, involving the ubiquitin-proteasome system and forming aggresomes.21,22,51 The same would be promoted by mutant PrPTSE (green quadrangles). Pathway 2 (processing of external PrPC and PrPTSE, blue): PrPC from the plasma membrane is internalized and processed in lysosomes. Exogenic PrPTSE, consisting of a mixture of dominant and subdominant types (blue and dark blue boxes, A and B), leads to conformational change of PrPC before or during internalization via endosomes. Overloading of the endosomal-lysosomal system may lead to accumulation of indigestible material or exocytosis of PrPTSE that forms extracellular aggregated deposits that presumably lack direct neurotoxic effects. This process may be accompanied by an outburst of lysosomal enzymes leading to tissue damage. The detailed delineation of endosomal and lysosomal compartments is omitted for clarity. Pathway 3 (spread of PrPTSE, green): Endosomes may transport PrPTSE in the axons, in addition to domino-like spread of PrPTSE axolemmally.
Invasion and Spread of Prions: Multiple Routes
The routes of infection in naturally acquired prion diseases comprise uptake of prions via the alimentary tract or through scarification of gums (eg, in scrapie), skin, and conjunctiva.24 Experimentally, intracerebral, intraperitoneal, or intravenous inoculation has been successful. In natural diseases like chronic wasting disease, a contagion in the environment or vector has also been suggested, in particular since infectious prions were demonstrated in the saliva of these animals.25 In humans, transmission via intramuscular injections or intracerebral inoculation in iCJD and transfusion of the vCJD agent has been reported. The spread of prions depends on their site of entry, strain, dose, and species as well as the PrP genotype of the host.24
Major involved tissues and pathways following natural infection or experimental peroral challenge have been elucidated, mostly in experimental scrapie, and comprise accumulation of the agent in lymphoid tissue, spread to the peripheral nervous system, ascension to and dissemination within the CNS, and eventual final spread from the CNS to peripheral sites such as muscle. In particular, gut-associated lymphoid tissue and gut-associated lymphoid tissue-draining lymph nodes have a significant role. Prion transport and replication involves microfold cells (M cells), follicle-associated epithelium, follicular dendritic cells, dome and tingible body macrophages, and cells with dendritic cell morphology.24 The complement system and B cells have a supporting role.26,27,28 The vagus and splanchnic nerves are paths for the initial spread to ganglia and to the CNS.24
TSE infectivity has been detected at preclinical and clinical stages of infection in the blood of sheep naturally infected with scrapie or perorally challenged with the bovine spongiform encephalopathy agent.29 In addition, human blood from donors who incubated vCJD also contain the infectious agent.8 Whether hematogenous spread contributes substantially to the infection of the brain in acquired prion diseases is difficult to clarify, due to the rarity of the disease and difficulty of tracing historical routes of transmission.
Even in sporadic prion diseases, PrPTSE may be detected in peripheral organs including the spleen, peripheral nerve (also in Gerstmann-Sträussler-Scheinker disease), and muscle tissue.30,31 Apparently, prions may propagate not only to but also from the CNS via neural pathways. Infection of muscle may happen via efferent projections of motor units to neuromuscular junctions and postsynaptically into muscle fibers, but sensory nerves may provide an additional pathway.24 The possibility of trans-synaptic spread of PrPTSE to the muscle was suggested after detecting PrPTSE subsarcolemmally in myofibers and endoneurally in nerve fibers in muscle tissue of hamsters orally infected with scrapie agent, together with a distribution pattern of PrPTSE resembling the innervation of motor units.32
For prion propagation in the nervous system, axonal transport, passive translocation in perineural lymphatics, spread in neural interspaces, sequential infection of Schwann cells, and a domino-like conversion of PrPC into PrPTSE along neural cell membranes have been proposed (Figure 1).24,33,34 In the diseased human brain, PrPTSE is deposited in diffuse/synaptic, patchy/perivacuolar, perineuronal, and plaque-like patterns.35 PrPTSE may also accumulate in astrocytes and microglia.36 We have found PrPTSE colocalizing with both chemical and electric synapses in the neuronal cell body and dendrites, thus both post- and presynaptically, as well as in intra- and adaxonal localizations.33 This agrees with the finding of PrPTSE in the synaptosomal fraction in CJD brains,37 and with ultrastructural immunogold studies of murine scrapie indicating PrPTSE at the plasmalemma and dendrites.38 Intra-axonal PrPTSE particles are the size of endo-lysosomes and suggest prion trafficking via axonal transport mechanisms33; neuritic transport of prions has also been confirmed in an in vitro model.39 Earlier, we identified PrPTSE within macrophages and vascular-associated dendritic cells in the vessel wall and perivascular area in sCJD.40 Macrophages may take up PrPTSE and sequester infectivity. Dendritic cells, which have close contact to macrophages, are potentially mobile, and can migrate via the blood and across vessel walls,40 thus representing another possibility for prion spread in the body.
Scrapie infection of mice engineered to exhibit nephritis, hepatitis, or pancreatitis with local infiltrates comprising follicular dendritic cells, as well as mastitis of sheep, induces prominent PrPTSE production at the sites of inflammation.2 This has also been demonstrated in a human case of concomitant inclusion body myositis and sCJD.41 Apparently conditions with up-regulation of PrPC (eg, in inflammation or neurogenic lesion of muscle)19 may foster extraneural prion production.
Neurodegeneration in Prion Diseases: Multiple Pathways
A major gap exists in our understanding of how the conformational change of PrPC to PrPTSE ultimately kills neurons. Is neuronal damage caused by a loss of the normal function of PrPC or by gain of toxic property of PrPTSE, or are there additional factors? Moreover, it is not clear which neuronal cell death pathways are crucial and whether other components of tissue pathology (eg, microglial and astroglial responses, inflammation) are primary contributors or secondary events.
PrPs and Neurodegeneration
A Role for Cellular PrP: Loss of Neuroprotective Function as a Neuronal Killer?
PrP deficiency results in resistance from prion infection.42 When neuronal PrPC is depleted in mice with ongoing prion infection, early neuronal loss and histopathological changes are reversed.43 Increased activities of copper/zinc superoxide dismutase and glutathione peroxidase have been observed in neurons expressing higher levels of cellular PrP, and a role of PrPC in the cellular defense against oxidative or other cell stress has been proposed.19,20,44 In experimental modeling of brain damage, Prnp knockout mice have a tendency to exhibit larger infarct size; in this aspect the N-terminal octapeptide region seems to have a lead function in the neuroprotection against oxidative stress.45 PrPC has also been shown to protect against Bax-mediated neuronal apoptosis in vitro.46
A Role for Pathological PrP Deposits: Gain of Function as a Neurotoxic Effect?
Temporal and anatomical correlation between accumulation of PrPTSE and the appearance of neuropathology is generally good and would argue for PrPTSE as being neurotoxic.47 Indeed, a peptide corresponding to amino acid residues 106–126 of the human PrP has a high intrinsic tendency to aggregate into fibrils and is particularly neurotoxic.48 It may be hypothesized that a toxic form of PrP is produced directly from PrPC or as a precursor to pathological PrP.49 However, the link from PrPTSE to neurotoxicity is not straightforward, and apparently the compartment where PrPTSE resides is decisive. The continuing accumulation of PrPTSE in the neuropil of brains of conditional PrP knockout mice with scrapie that reverted early spongiform change and prevented neuronal loss and progression to clinical disease argues against neurotoxicity of extraneuronal PrPTSE.43 Some studies describe pathology in the absence of PrPTSE, but these experiments use either transgenic animals encoding mutant proteins that can adopt a pathogenetic conformation, but are relatively inefficient at forming infectious PrPTSE, or mice expressing PrP molecules with “artificial” sequences or expression levels.49 The recent demonstration of proteinase K-resistant PrP core fragments in non-CJD brains50 on one hand suggests that small quantities of the abnormal form of this protein may not always necessarily be pathogenic and that there might be silent prions lying dormant in normal human brains; on the other hand, it weakens the concept of a direct neurotoxic role of proteinase K-resistant PrP.
Cytosolic PrP, Transmembrane PrP, Anchorless PrP: Further Players?
When PrP accumulates in the cytosol, neurotoxicity and neurodegeneration are observed.51,52 It has been suggested that access of PrP to the cytoplasm is the neurodegenerative trigger in at least some naturally occurring prion diseases,52 although others consider accumulation of PrP in the cytosol as an unlikely general pathogenic mechanism in prion disease.53
CtmPrP has been suggested to be a key pathogenic intermediate in prion diseases by escaping ER-resident quality control mechanisms. Certain mutations within PRNP (eg, A117V) alter the ratio of the topological forms.23 In mice carrying similar mutations, CtmPrP was found to be associated with neurodegeneration. CtmPrP-associated neurodegeneration is dependent on wild-type PrP.54
Some observations support toxicity of PrP located at the plasma membrane. This is based on investigations with PrPΔF, a mutant devoid of octarepeats and the hydrophobic domain of PrP, and of a protein named doppel.52 Doppel’s gene is in downstream position to the PrP gene. Doppel and PrPC have several similarities. Physiologically, neuronal expression of doppel is silenced; however, its ectopic up-regulation leads to loss of cerebellar Purkinje cells.55 Both doppel and PrPΔF can induce apoptotic cell death. A change in the neuroprotective capacity of PrPC, or its interaction with accessory proteins, or affection of intermolecular interactions of PrPC may be the background of neurodegeneration linked to doppel or PrPΔF.52
The glycosyl-phosphatidylinositol anchor is an important component of PrP. Lack of this glycosyl-phosphatidylinositol anchor leads to less infectivity and less neurodegeneration including also a lack of clinical symptoms, despite the presence of more PrPTSE in an amyloid form.56
In sum, none of the aforementioned forms of PrP seems likely as a sufficient single factor for cellular pathology. Genetic ablation of PrP has relatively little phenotypic effect, thus lack of PrPC itself cannot account for neurodegeneration. Rather, a loss-of-function mechanism may be exacerbated by additional toxic gain of function and influenced also by further forms of PrP and their cell biology.4 Nevertheless, most recent studies support the concept of transient non-PrPTSE neurotoxic components since reversible functional impairment occurs before extensive PrPTSE accumulation.57 Further recent work has suggested that the most infectious forms of the PrP may be oligomeric and that much larger aggregates may not necessarily be infectious58 or toxic.
Molecular and Cellular Pathways Leading to Neurodegeneration
In the nervous system, programmed cell death (PCD) includes apoptosis (type I or nuclear PCD), autophagy (type II PCD), or atypical forms like paraptosis (type III or cytoplasmic PCD), calcium-mediated, AIF/ poly(ADP-ribose) polymerase-mediated PCD, and oncosis (ischemic PCD).59 The latter associates with necrosis and is thus unlikely in prion diseases. Pathways that may lead to neuronal death comprise oxidative stress, regulated activation of complement, ubiquitin-proteasome and endosomal-lysosomal systems, synaptic alterations and dendritic atrophy, corticosteroid response, and endoplasmic reticulum stress.
Apoptosis
Apoptosis is characterized ultrastructurally by cell shrinkage, condensation of chromatin (Figure 2a), and formation of apoptotic bodies.63 Many investigators define as apoptotic those pathways that require cysteine protease caspases.64 Demonstration of DNA fragmentation by terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling, also called in situ end-labeling technique (Figure 2b) is frequently used synonymously for apoptosis, but it may rather be used to define “vulnerable” neuronal populations irrespective of the precise death mechanism behind.
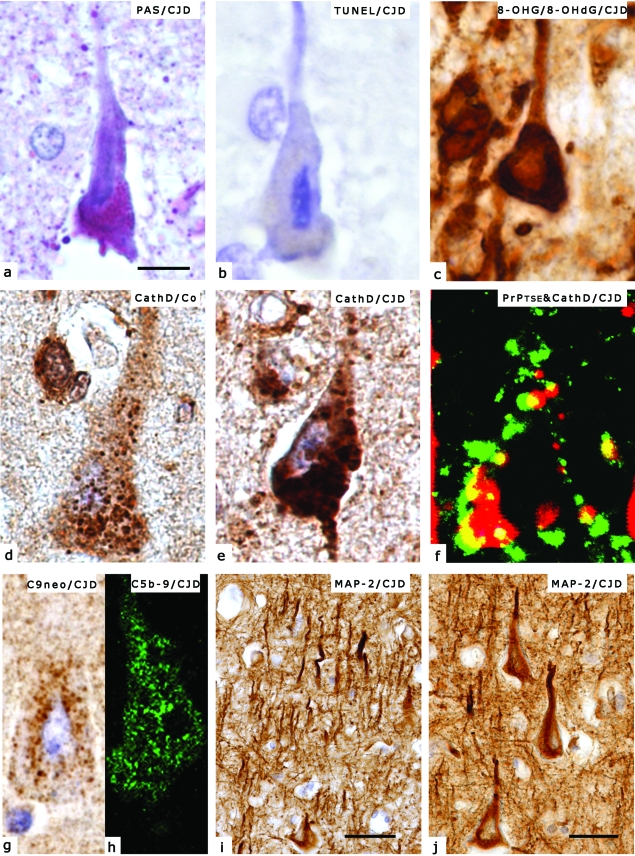
Figure 2.
Histological and immunohistochemical demonstration of pathological alterations discussed in the present review. Technical details are detailed in our previous studies.33,60,61,62 a: Shrinkage of the nucleus in a pyramidal neuron of the frontal cortex accompanied by intense PAS positivity in a representative case with sporadic CJD, suggesting lysosomal overload. b: Terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling positivity, indicated by blue color, of a representative neuronal nucleus in the same case as in Figure 1a, suggesting apoptosis. c: Immunostaining for RNA-derived 8-hydroxy-guanosine (8-OHG) and DNA-derived 8-hydroxydeoxy-guanosine (8-OHdG) in CJD demonstrates predominantly cytoplasmic immunoreactivity. d: Cathepsin D (CathD) immunoreactivity in a normal brain without neurological disease. e: CathD immunopositivity in a representative case of CJD. Note accumulation of enlarged dot-like cytoplasmic immunoreactivity as compared to Figure 1d, suggesting lysosomal overload. f: Intra- and perineuronal colocalization (indicated by yellow color) of PrPTSE (indicated by green color) and CathD (indicated by red color) demonstrated by laser confocal scanning microscopy in CJD, suggesting interaction of PrPTSE with the lysosomal system. g: Immunodeposits of the neoantigen of C9 on a neuron in CJD, suggesting involvement of the terminal complement. h: Neuronal immunoreactivity for the membrane attack complex (C5b-9) in CJD, demonstrated by LCM, supporting the involvement of the terminal complement. i: Immunostaining for the dendritic marker MAP-2 in the frontal cortex in CJD demonstrates distorted and fragmented dendritic arborization. j: MAP-2 immunoreactive atrophic dendrites in the frontal cortex in CJD. Scale bars represent 10 μm for a–h, 40 μm for i, and 20 μm for j.
What evidence do we have for apoptosis in prion diseases? Persistent infection by scrapie prions of a hypothalamic cell line causes morphological features of apoptosis.65 DNA fragmentation, morphological changes typical of apoptosis, and activation of caspases follow the neurotoxic synthetic peptide PrP106–126 in several experiments,47,48 although caspase-3 activation may dissociate from the neurotoxic effect of this peptide.66 In addition, translocation of phosphorylated c-Jun-N-terminal kinase into the nucleus and activation of the nuclear c-Jun transcription factor have been demonstrated.67 Activation of poly(ADP-ribose) polymerase has been found to result from a response to DNA fragmentation rather than as result of a toxic effect.68
DNA fragmentation has been reported in both naturally and experimentally induced prion diseases. Time course experiments indicated that terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling positivity of neurons precedes cell loss. In human brains, some studies confirmed this correlation, whereas others described an inconsistent relationship. No modifications in the expression of Fas, Fas ligand (Fas-L), ERK, MEK, Bcl-2, Bax, N-myc, c-myc, pro-caspase-2 and active caspase-3 were observed in human CJD cerebellum, except for a few cells that displayed dense immunostaining for homogeneous active caspase-3.69 An immunohistochemical and mRNA expression study on Bax and Bcl-2 showed overexpression of Bax, but not caspase-3, in scrapie-affected sheep, whereas no variation of Bcl-2 was observed.70 Also, in the neurological phase of scrapie-infected mice no modifications in the levels of Bcl-2, Bax, Fas, and Fas ligand were observed, although DNA fragmentation and a few caspase-3-immunopositive cells were found.71
In summary, demonstration of morphological features of apoptosis, DNA fragmentation, and activation of caspase-3 supports apoptosis as a relevant cell death pathway in prion disease. However, the variability of results suggests that this is not the exclusive pathway.
Autophagy
Autophagy is a degradative mechanism involved in the recycling and turnover of cytoplasmic components. Autophagic vacuoles have been described in experimentally induced scrapie, CJD, Gerstmann-Sträussler-Scheinker disease, and fatal familial insomnia 63 and may result from intraneuronal accumulation of PrPTSE that overloads the catabolic machinery, followed by eventual bulk removal of damaged neurons.72 It has also been speculated that autophagic vacuoles may precede spongiform change and thus contribute to the overall pathology of prion diseases.
Oxidative Stress
In vivo immunohistochemical studies in experimentally infected mouse brain has demonstrated the presence of nitrotyrosine, heme-oxygenase-1, and lipid oxidation markers, suggesting that Fe-induced oxidative stress might be one mechanism of neuronal loss in scrapie-infected mice.44 In human CJD brains, oxidative damage to nucleic acids (Figure 2c) correlates with disease duration but not with PrPTSE deposition.60
In vitro models provided similar results. Cells permanently infected with scrapie show decreased levels of antioxidants and are more susceptible to cell death induced by free radicals.44 Exposure to the neurotoxic PrP106–126 peptide induces oxidative stress.44 Thus it seems that oxidative stress is a global event in TSEs affecting all neurons, but the most vulnerable subtypes (eg, parvalbumin immunoreactive GABAergic neurons)73 degenerate first.
The Endosomal-Lysosomal System
Ultrastructural, immunohistochemical, enzymatic activity, and gene expression studies have suggested that the endosomal-lysosomal system has a role in the pathogenesis of prion diseases. In cell cultures, the endosomal-lysosomal system is involved in the processing of both PrPC and exogenous PrPTSE. Subcellular subcompartments may, at least in part, be the site of PrPC - PrPTSE transformation as well as of PrPTSE generation.3,36,74,75 Pathogenic PrP oligomers may be released from the host cells to the extracellular space by direct recycling and/or via exosome secretion.74 PrPTSE may also end up in mature lysosomes for degradation. Lysosomal proteases may be involved in the proteolytic processing of PrP forms. Indeed, cysteine protease inhibitors can inhibit PrPTSE accumulation.76 In a recent study we showed that neurons in regions with prominent tissue damage have an increased volume of cathepsin D-immunoreactive lysosomes (Figure 2, d and e); furthermore, cathepsin D colocalizes with PrPTSE (Figure 2f), indicating that affection of the endosomal-lysosomal system correlates with regional pathology.61 Overloading of this system might result in breakdown of lysosomal functions.
Endoplasmic Reticulum Stress in Prion Disease
Neuronal death in neurodegenerative diseases may have its origin in the ER. The ER responds to cellular stress conditions by the activation of adaptive pathways, termed the unfolded protein response. Several ER-related chaperones are up-regulated, and ER-related caspase-12 is activated in vitro in human and experimental prion diseases, thus favoring the hypothesis of ER stress as a key pathogenetic event in prion diseases.77 In another study on human brains, however, PERK, which launches the most immediate response to ER stress, and its downstream effector eIF2α were not found to be activated in human and experimental prion diseases, in contrast to Alzheimer’s disease.78 These conflicting data await resolution.
Ubiquitin-Proteasome System and Aggresomes
The 26S proteasome is a multicatalytic protease complex found in all eukaryotic cells. Its main function is the degradation of misfolded, damaged, and short-lived proteins and some components function in transcription regulation.79,80 Recently, neuronal cells overexpressing PrPC were found to develop cytosolic PrPC aggregates under conditions of mild proteasome inhibition that did not cause cell death; however, neuronal propagation of prions invoked a neurotoxic mechanism with intracellular formation of compartmentalized cytosolic PrPTSE aggresomes that triggered caspase-dependent apoptosis.21 Disease-associated PrP oligomers inhibit the 26S proteasome, suggesting their role in intracellular neurotoxicity.22 In humans cytosolic PrPTSE aggregates supporting the aforementioned process have not yet been observed. However, in sCJD we demonstrated nuclear redistribution and accumulation of ubiquitin-proteasome system components in correlation with regional tissue damage, suggesting their involvement in DNA repair mechanisms and/or cell death machinery.81
Complement Activation in Neurons
Terminal complement activation has been demonstrated in human prion disease (Figure 2, g and h).62 This might be initiated by the disease-specific conformational change of PrP, by free radicals, or alternatively by components associated with infectivity that are yet to be characterized. The role of complement activation in prion disease pathology may be multifactorial; either sublytic levels of C5b-9 may generate oxidative stress and induce apoptotic cell death, or activated complement may directly lead to cell lysis with consecutive tissue damage. Since C5-deficient mice develop clinical scrapie with incubation periods similar to C5-sufficient mice, without a difference in severity of neuropathology, the role of membrane attack complex as a decisive factor for neuronal death is unlikely.82
Synaptic and Dendritic Pathology
Both PrPC and PrPTSE locate to synapses, and thus they have emerged as putative primary targets in prion diseases. Early studies with the Golgi method in human and animal prion diseases and more recent sequential studies have revealed progressive loss of dendritic spines.83 Morphologically, distorted dendritic arborization and dendritic atrophy may be prominent in CJD brains (Figure 2, i and j). Importantly, increased levels of Notch-1 mRNA, which inhibits both dendritic growth and maturation, and translocation of its intracellular domain to the nucleus, correlate with regressive dendritic changes; thus Notch-1 may be a mediator of this process.84
Synaptic degeneration and loss are suggested by immunohistochemical and ultrastructural studies to precede neuronal degeneration. Loss of synapses and dendritic spines from an early stage in the disease process may have the effect of isolating neurons from electrical stimuli and trophic factors, both of which could trigger self-destructive mechanisms.47,83 Inhibition of neuronal apoptosis via Bax deletion fails to rescue a neurological syndrome in mice that is caused by synaptic loss. Thus antiapoptotic therapies are unlikely to work unless associated with pharmacological interventions preventing synaptic damage.85 However, no correlation was detected between decreased synaptic protein expression and cell death via apoptosis following scrapie infection.71
Stress Response: Corticosteroids and Chaperones
Corticosteroids seem to have a role in the pathogenesis of neurodegenerative disorders in general. Indeed, elevated concentrations of corticosterone metabolites have been observed during the last 5 weeks of disease of scrapie-infected mice, as well as a severe disturbance of the circadian periodicity of corticosterone excretion.86 This dysregulation of corticosteroid excretion might act as a further cofactor in the pathogenesis of scrapie, for example by preconditioning nerve cells to neurotoxic stimuli such as oxidative stress and to apoptosis.
Chaperones, including heat shock proteins (Hsps) may have several roles in connection with PrPs. On one hand, they may help to stabilize any of the protein isoforms, thus promoting or inhibiting the formation of the pathogenic conformation. On the other hand, they may play a role in building up a cellular defense response elicited by formation of degradation-resistant PrPTSE aggregates. In CJD brains, the inducible Hsp-72 is markedly up-regulated and highly expressed in cells that show PrPC immunoreactivity as well.87 Elevated levels of the cytoprotective Hsp-72 may contribute to save the neuroprotective function of PrPC.
The yeast protein-based non-Mendelian heritable element [PSI+], a prion-like form of the release factor (Sup35) is a model for the determination of interactions between chaperones and PrPs.88 Hsp-104 is necessary for the cellular maintenance of this heritable non-Mendelian element, but both inactivation and overproduction of the chaperone result in the loss of [PSI+]. Furthermore, in yeast cells overproduction of the Hsp70 analogue Ssa interferes with the effect of overproduced Hsp-104, whereas overproduction of another analogue Ssb has the opposing effect.89 Despite these interesting data from the yeast prion model, the role for chaperones in prion diseases needs to be elucidated.
Astroglia and Microglia in Prion Diseases: Attack or Defense?
Astrocytosis is a prominent feature of prion diseases, and glial fibrillary acidic protein is up-regulated in prion diseases. In addition to glial fibrillary acidic protein, metallothioneins, crystallins, apolipoprotein E, cathepsin D, and lymphokines are up-regulated in scrapie, although lack of glial fibrillary acidic protein or ApoE does not inhibit scrapie infection.90 Up-regulation of astrocytic enzymes precedes the development of neuropathological lesions but follows the rise in PrP, suggesting that the astrocytic response is induced by PrPTSE and may only subsequently play a role in tissue damage.
Microglia activation in prion diseases is confined to regions with spongiform change and PrPTSE deposition as a modified inflammatory response. Microglia respond to the neurotoxic PrP106–126 peptide by producing inflammatory cytokines interleukin-1β and interleukin-6. The neurotoxicity of this PrP fragment seems to depend on the presence of microglia. In contrast, cerebral cytokine gene expression is observed relatively late in murine scrapie, and tumor necrosis factor-α- and interleukin-6-deficient mice are susceptible to prion infection, thus casting doubt on a decisive role of these cytokines.47 In vitro, microglia have been shown to internalize fibrillary PrP106–126 to some extent. In vivo, we have demonstrated microglia to harbor PrPTSE, suggesting that it may be processed or degraded in these cells. Microglia are associated with amyloid plaques and may also contribute to the development of spongiform vacuoles.91
Spongiform Change: The Histopathological Hallmark of Prion Diseases
Spongiform change is characterized by small (<10 μm) round or oval vacuolization of the neuropil. Vacuoles may coalesce (“confluent vacuoles”; size, 10–50 μm), whereas almost complete neuronal loss by various causes is accompanied by “status spongiosus.” These morphological hallmarks must be distinguished from vacuolar clefts in the second cortical layer commonly seen in late stages of various neurodegenerative disorders (“spongy degeneration of the second cortical layer”) and other irregular vacuoles caused by tissue rarefaction or edema. Some disease forms, like Gerstmann-Sträussler-Scheinker disease or fatal familial insomnia in humans, do not feature prominent spongiform change or have it only in very restricted distribution, whereas in others PrPTSE deposits are inconspicuous or even lacking, despite obvious neuronal loss. In murine scrapie ultrastructural vacuoles are found in neurites and less frequently in axon terminals, neuronal perikarya, astrocytes, oligodendrocytes, and myelin.38 In CJD the presence of vacuoles in cell bodies is uncommon.
The exact mechanism is not clear but is likely a result of abnormal membrane permeability and increased water content within neuronal processes. It may be also result from autophagy.63 Interestingly, depleting neuronal PrPC in prion infection reverses spongiosis43 supporting the notion that PrPTSE is not the inducer of spongiform change. In experimental CJD of mice, local dissociation between spongiform change and PrPTSE has been observed.92 Moreover, vacuolization in TSE-infected mink of the Chediak-Higashi genotype, which show abnormality of membrane-bound organelles (including lysosomes) and deficiency of lysosomal enzymes, is apparently suppressed,93 emphasizing the role of the altered endosomal-lysosomal system in tissue pathology.61
Conclusion
Neuropathogenesis of prion diseases evolves in complex ways on several front lines, most but not all of which exist also in other neurodegenerative (eg, Alzheimer disease) as well as infectious diseases. “Seeding” experiments with other amyloidogenic proteins suggest that protein aggregation has a role in ensuing neurodegeneration.94 Whereas intracellular accumulation of PrP forms might significantly impair cell function and lead to cytopathology, mere extracellular deposition of PrPTSE is questionable as a direct cytotoxic factor. Several pathogenetic events may be present and may distinguish disease forms. This is the likely reason for conflicting results reported in different in vitro settings, experimental models, or naturally occurring diseases. Tissue damage may result from several parallel, interacting or subsequent pathways. A simple synthesis of the processing of PrP and pathogenesis, summarized in Figures 1 and 3, could be that (1) a yet unidentified event, either spontaneously occurring or by contact with external prions, or by awakening of silent prions50 initiates conformational change of PrP with potentially reversible functional impairment of neurons. This is followed by (2) pathogenetic effector events outlined in this review, which may develop in parallel or in sequence, leading to (3) histopathologically detectable features of neuronal degeneration, astro- and microgliosis, PrPTSE deposits composed of a mixture of types (“strains”), from which one may be predominant, and spongiform change. Blockade or knockout of one single pathway may be compensated for by another but may occur too late to rescue neurons from damage. Such a working hypothesis may be important for drug design, as targeting of multiple processes might be more likely to succeed. Since the mechanisms of neuronal degeneration seem to be manifold in prion diseases, future studies should clarify the trigger(s) and sequence of these processes and whether, and which, one of the aforementioned pathways is dominating or decisive.
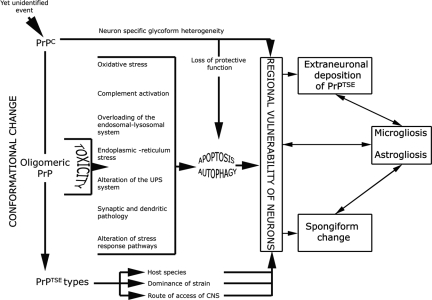
Figure 3.
Diagram of pathogenetic events in prion disease. A yet unidentified event (external prions, spontaneous conversion, or awakening of silent prions50) initiates conformational change of PrPC with potentially reversible functional impairment of neurons. During this process oligomeric forms of PrP are present that may have a direct neurotoxic effect initiating a cascade of events leading to either or both apoptosis and autophagy. This process is potentially influenced by the loss of protective function of PrPC. According to the neuron-specific glycoform heterogeneity,95 the species of host, dominance of PrPTSE type, or route of access of external prions to the CNS, tissue pathology comprising spongiform change, astro- and microgliosis develops in regional variability. Astro- and microgliosis are initiated by the neuronal damage or toxic intermediate forms of PrP, but inversely may also contribute to neuronal death and formation of spongiform change.
Footnotes
Address reprint requests to Professor Herbert Budka, Institute of Neurology, Medical University of Vienna, AKH 4J, Waehringer Guertel 18-20, POB 48, 1097 Vienna, Austria. E-mail: herbert.budka@meduniwien.ac.at.
Supported by the European Union FP6 Network of Excellence NeuroPrion, Subproject PRIOGEN.
References
- Prusiner SB. Prions. Proc Natl Acad Sci USA. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguzzi A, Heikenwalder M. Pathogenesis of prion diseases: current status and future outlook. Nat Rev Microbiol. 2006;4:765–775. doi: 10.1038/nrmicro1492. [DOI] [PubMed] [Google Scholar]
- Caughey B, Baron GS. Prions and their partners in crime. Nature. 2006;443:803–810. doi: 10.1038/nature05294. [DOI] [PubMed] [Google Scholar]
- Westergard L, Christensen HM, Harris DA. The cellular prion protein (PrP(C)): its physiological function and role in disease. Biochim Biophys Acta. 2007;1772:629–644. doi: 10.1016/j.bbadis.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ironside JW, Ritchie DL, Head MW. Phenotypic variability in human prion diseases. Neuropathol Appl Neurobiol. 2005;31:565–579. doi: 10.1111/j.1365-2990.2005.00697.x. [DOI] [PubMed] [Google Scholar]
- Piccardo P, Manson JC, King D, Ghetti B, Barron RM. Accumulation of prion protein in the brain that is not associated with transmissible disease. Proc Natl Acad Sci USA. 2007;104:4712–4717. doi: 10.1073/pnas.0609241104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AF, Collinge J. Subclinical prion infection in humans and animals. Br Med Bull. 2003;66:161–170. doi: 10.1093/bmb/66.1.161. [DOI] [PubMed] [Google Scholar]
- Ironside JW. Variant Creutzfeldt-Jakob disease: risk of transmission by blood transfusion and blood therapies. Haemophilia. 2006;12(Suppl 1):8–15; discussion 26–28. doi: 10.1111/j.1365-2516.2006.01195.x. [DOI] [PubMed] [Google Scholar]
- Kovacs GG, Voigtlander T, Gelpi E, Budka H. Rationale for diagnosing human prion disease. World J Biol Psychiatry. 2004;5:83–91. doi: 10.1080/15622970410029916. [DOI] [PubMed] [Google Scholar]
- Ward HJ, Head MW, Will RG, Ironside JW. Variant Creutzfeldt-Jakob disease. Clin Lab Med. 2003;23:87–108. doi: 10.1016/s0272-2712(02)00068-9. [DOI] [PubMed] [Google Scholar]
- Kovacs GG, Trabattoni G, Hainfellner JA, Ironside JW, Knight RS, Budka H. Mutations of the prion protein gene phenotypic spectrum. J Neurol. 2002;249:1567–1582. doi: 10.1007/s00415-002-0896-9. [DOI] [PubMed] [Google Scholar]
- Manson JC, Jamieson E, Baybutt H, Tuzi NL, Barron R, McConnell I, Somerville R, Ironside JW, Will R, Sy MS, Melton DW, Hope J, Bostock C. A single amino acid alteration (101L) introduced into murine PrP dramatically alters incubation time of transmissible spongiform encephalopathy. EMBO J. 1999;18:6855–6864. doi: 10.1093/emboj/18.23.6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann C, Flechsig E. PrP knock-out and PrP transgenic mice in prion research. Br Med Bull. 2003;66:43–60. doi: 10.1093/bmb/66.1.43. [DOI] [PubMed] [Google Scholar]
- Legname G, Baskakov IV, Nguyen HO, Riesner D, Cohen FE, DeArmond SJ, Prusiner SB. Synthetic mammalian prions. Science. 2004;305:673–676. doi: 10.1126/science.1100195. [DOI] [PubMed] [Google Scholar]
- Brown P, Cervenakova L. A prion lexicon (out of control). Lancet. 2005;365:122. doi: 10.1016/S0140-6736(05)17700-9. [DOI] [PubMed] [Google Scholar]
- Safar J, Wille H, Itri V, Groth D, Serban H, Torchia M, Cohen FE, Prusiner SB. Eight prion strains have PrP(Sc) molecules with different conformations. Nat Med. 1998;4:1157–1165. doi: 10.1038/2654. [DOI] [PubMed] [Google Scholar]
- Manuelidis L, Yu ZX, Barquero N, Mullins B. Cells infected with scrapie and Creutzfeldt-Jakob disease agents produce intracellular 25-nm virus-like particles. Proc Natl Acad Sci USA. 2007;104:1965–1970. doi: 10.1073/pnas.0610999104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberski PP, Brown P. Disease-specific particles without prion protein in prion diseases - phenomenon or epiphenomenon? Neuropathol Appl Neurobiol. 2007;33:395–397. doi: 10.1111/j.1365-2990.2007.00867.x. [DOI] [PubMed] [Google Scholar]
- Kovacs GG, Kalev O, Gelpi E, Haberler C, Wanschitz J, Strohschneider M, Molnar MJ, Laszlo L, Budka H. The prion protein in human neuromuscular diseases. J Pathol. 2004;204:241–247. doi: 10.1002/path.1633. [DOI] [PubMed] [Google Scholar]
- Roucou X, Gains M, LeBlanc AC. Neuroprotective functions of prion protein. J Neurosci Res. 2004;75:153–161. doi: 10.1002/jnr.10864. [DOI] [PubMed] [Google Scholar]
- Kristiansen M, Messenger MJ, Klohn PC, Brandner S, Wadsworth JD, Collinge J, Tabrizi SJ. Disease-related prion protein forms aggresomes in neuronal cells leading to caspase activation and apoptosis. J Biol Chem. 2005;280:38851–38861. doi: 10.1074/jbc.M506600200. [DOI] [PubMed] [Google Scholar]
- Kristiansen M, Deriziotis P, Dimcheff DE, Jackson GS, Ovaa H, Naumann H, Clarke AR, van Leeuwen FW, Menendez-Benito V, Dantuma NP, Portis JL, Collinge J, Tabrizi SJ. Disease-associated prion protein oligomers inhibit the 26S proteasome. Mol Cell. 2007;26:175–188. doi: 10.1016/j.molcel.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Hegde RS, Mastrianni JA, Scott MR, DeFea KA, Tremblay P, Torchia M, DeArmond SJ, Prusiner SB, Lingappa VR. A transmembrane form of the prion protein in neurodegenerative disease. Science. 1998;279:827–834. doi: 10.1126/science.279.5352.827. [DOI] [PubMed] [Google Scholar]
- Beekes M, McBride PA. The spread of prions through the body in naturally acquired transmissible spongiform encephalopathies. FEBS J. 2007;274:588–605. doi: 10.1111/j.1742-4658.2007.05631.x. [DOI] [PubMed] [Google Scholar]
- Mathiason CK, Powers JG, Dahmes SJ, Osborn DA, Miller KV, Warren RJ, Mason GL, Hays SA, Hayes-Klug J, Seelig DM, Wild MA, Wolfe LL, Spraker TR, Miller MW, Sigurdson CJ, Telling GC, Hoover EA. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science. 2006;314:133–136. doi: 10.1126/science.1132661. [DOI] [PubMed] [Google Scholar]
- Klein MA, Frigg R, Flechsig E, Raeber AJ, Kalinke U, Bluethmann H, Bootz F, Suter M, Zinkernagel RM, Aguzzi A. A crucial role for B cells in neuroinvasive scrapie. Nature. 1997;390:687–690. doi: 10.1038/37789. [DOI] [PubMed] [Google Scholar]
- Klein MA, Kaeser PS, Schwarz P, Weyd H, Xenarios I, Zinkernagel RM, Carroll MC, Verbeek JS, Botto M, Walport MJ, Molina H, Kalinke U, Acha-Orbea H, Aguzzi A. Complement facilitates early prion pathogenesis. Nat Med. 2001;7:488–492. doi: 10.1038/86567. [DOI] [PubMed] [Google Scholar]
- Mabbott NA, Bruce ME, Botto M, Walport MJ, Pepys MB. Temporary depletion of complement component C3 or genetic deficiency of C1q significantly delays onset of scrapie. Nat Med. 2001;7:485–487. doi: 10.1038/86562. [DOI] [PubMed] [Google Scholar]
- Hunter N, Foster J, Chong A, McCutcheon S, Parnham D, Eaton S, MacKenzie C, Houston F. Transmission of prion diseases by blood transfusion. J Gen Virol. 2002;83:2897–2905. doi: 10.1099/0022-1317-83-11-2897. [DOI] [PubMed] [Google Scholar]
- Glatzel M, Abela E, Maissen M, Aguzzi A. Extraneural pathologic prion protein in sporadic Creutzfeldt-Jakob disease. N Engl J Med. 2003;349:1812–1820. doi: 10.1056/NEJMoa030351. [DOI] [PubMed] [Google Scholar]
- Hainfellner JA, Budka H. Disease associated prion protein may deposit in the peripheral nervous system in human transmissible spongiform encephalopathies. Acta Neuropathol (Berl) 1999;98:458–460. doi: 10.1007/s004010051109. [DOI] [PubMed] [Google Scholar]
- Thomzig A, Schulz-Schaeffer W, Kratzel C, Mai J, Beekes M. Preclinical deposition of pathological prion protein PrPSc in muscles of hamsters orally exposed to scrapie. J Clin Invest. 2004;113:1465–1472. doi: 10.1172/JCI21083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs GG, Preusser M, Strohschneider M, Budka H. Subcellular localization of disease-associated prion protein in the human brain. Am J Pathol. 2005;166:287–294. doi: 10.1016/S0002-9440(10)62252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzi V, Glatzel M, Nakano MY, Greber UF, Van Leuven F, Aguzzi A. Unhampered prion neuroinvasion despite impaired fast axonal transport in transgenic mice overexpressing four-repeat tau. J Neurosci. 2002;22:7471–7477. doi: 10.1523/JNEUROSCI.22-17-07471.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs GG, Head MW, Hegyi I, Bunn TJ, Flicker H, Hainfellner JA, McCardle L, Laszlo L, Jarius C, Ironside JW, Budka H. Immunohistochemistry for the prion protein: comparison of different monoclonal antibodies in human prion disease subtypes. Brain Pathol. 2002;12:1–11. doi: 10.1111/j.1750-3639.2002.tb00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier JG, Escaig-Haye F, Grigoriev V. Ultrastructural localization of prion proteins: physiological and pathological implications. Microsc Res Tech. 2000;50:76–88. doi: 10.1002/1097-0029(20000701)50:1<76::AID-JEMT11>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Kitamoto T, Shin RW, Doh-ura K, Tomokane N, Miyazono M, Muramoto T, Tateishi J. Abnormal isoform of prion proteins accumulates in the synaptic structures of the central nervous system in patients with Creutzfeldt-Jakob disease. Am J Pathol. 1992;140:1285–1294. [PMC free article] [PubMed] [Google Scholar]
- Jeffrey M, Goodbrand IA, Goodsir CM. Pathology of the transmissible spongiform encephalopathies with special emphasis on ultrastructure. Micron. 1995;26:277–298. doi: 10.1016/0968-4328(95)00004-n. [DOI] [PubMed] [Google Scholar]
- Magalhaes AC, Baron GS, Lee KS, Steele-Mortimer O, Dorward D, Prado MAM, Caughey B. Uptake and neuritic transport of scrapie prion protein coincident with infection of neuronal cells. J Neurosci. 2005;25:5207–5216. doi: 10.1523/JNEUROSCI.0653-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koperek O, Kovacs GG, Ritchie D, Ironside JW, Budka H, Wick G. Disease-associated prion protein in vessel walls. Am J Pathol. 2002;161:1979–1984. doi: 10.1016/S0002-9440(10)64474-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs GG, Lindeck-Pozza E, Chimelli L, Araujo AQ, Gabbai AA, Strobel T, Glatzel M, Aguzzi A, Budka H. Creutzfeldt-Jakob disease and inclusion body myositis: abundant disease-associated prion protein in muscle. Ann Neurol. 2004;55:121–125. doi: 10.1002/ana.10813. [DOI] [PubMed] [Google Scholar]
- Bueler H, Aguzzi A, Sailer A, Greiner RA, Autenried P, Aguet M, Weissmann C. Mice devoid of PrP are resistant to scrapie. Cell. 1993;73:1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- Mallucci G, Dickinson A, Linehan J, Klohn PC, Brandner S, Collinge J. Depleting neuronal PrP in prion infection prevents disease and reverses spongiosis. Science. 2003;302:871–874. doi: 10.1126/science.1090187. [DOI] [PubMed] [Google Scholar]
- Brown DR. Neurodegeneration and oxidative stress: prion disease results from loss of antioxidant defence. Folia Neuropathol. 2005;43:229–243. [PubMed] [Google Scholar]
- Mitteregger G, Vosko M, Krebs B, Xiang W, Kohlmannsperger V, Nölting S, Hamann GF, Kretzschmar HA. The role of the octapeptide region in neuroprotective function of the cellular prion protein. Brain Pathol. 2007;17:174–183. doi: 10.1111/j.1750-3639.2007.00061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bounhar Y, Zhang Y, Goodyer CG, LeBlanc A. Prion protein protects human neurons against Bax-mediated apoptosis. J Biol Chem. 2001;276:39145–39149. doi: 10.1074/jbc.C100443200. [DOI] [PubMed] [Google Scholar]
- Unterberger U, Voigtlander T, Budka H. Pathogenesis of prion diseases. Acta Neuropathol (Berl) 2005;109:32–48. doi: 10.1007/s00401-004-0953-9. [DOI] [PubMed] [Google Scholar]
- Forloni G, Angeretti N, Chiesa R, Monzani E, Salmona M, Bugiani O, Tagliavini F. Neurotoxicity of a prion protein fragment. Nature. 1993;362:543–546. doi: 10.1038/362543a0. [DOI] [PubMed] [Google Scholar]
- Chiesa R, Harris DA. Prion diseases: what is the neurotoxic molecule?. Neurobiol Dis. 2001;8:743–763. doi: 10.1006/nbdi.2001.0433. [DOI] [PubMed] [Google Scholar]
- Yuan J, Xiao X, McGeehan J, Dong Z, Cali I, Fujioka H, Kong Q, Kneale G, Gambetti P, Zou WQ. Insoluble aggregates and protease-resistant conformers of prion protein in uninfected human brains. J Biol Chem. 2006;281:34848–34858. doi: 10.1074/jbc.M602238200. [DOI] [PubMed] [Google Scholar]
- Mironov A, Jr, Latawiec D, Wille H, Bouzamondo-Bernstein E, Legname G, Williamson RA, Burton D, DeArmond SJ, Prusiner SB, Peters PJ. Cytosolic prion protein in neurons. J Neurosci. 2003;23:7183–7193. doi: 10.1523/JNEUROSCI.23-18-07183.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatzelt J, Schatzl HM. Molecular basis of cerebral neurodegeneration in prion diseases. FEBS J. 2007;274:606–611. doi: 10.1111/j.1742-4658.2007.05633.x. [DOI] [PubMed] [Google Scholar]
- Fioriti L, Dossena S, Stewart LR, Stewart RS, Harris DA, Forloni G, Chiesa R. Cytosolic prion protein (PrP) is not toxic in N2a cells and primary neurons expressing pathogenic PrP mutations. J Biol Chem. 2005;280:11320–11328. doi: 10.1074/jbc.M412441200. [DOI] [PubMed] [Google Scholar]
- Stewart RS, Piccardo P, Ghetti B, Harris DA. Neurodegenerative illness in transgenic mice expressing a transmembrane form of the prion protein. J Neurosci. 2005;25:3469–3477. doi: 10.1523/JNEUROSCI.0105-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens A, Aguzzi A. Small is not beautiful: antagonizing functions for the prion protein PrP(C) and its homologue Dpl. Trends Neurosci. 2002;25:150–154. doi: 10.1016/s0166-2236(00)02089-0. [DOI] [PubMed] [Google Scholar]
- Chesebro B, Trifilo M, Race R, Meade-White K, Teng C, LaCasse R, Raymond L, Favara C, Baron G, Priola S, Caughey B, Masliah E, Oldstone M. Anchorless prion protein results in infectious amyloid disease without clinical scrapie. Science. 2005;308:1435–1439. doi: 10.1126/science.1110837. [DOI] [PubMed] [Google Scholar]
- Mallucci GR, White MD, Farmer M, Dickinson A, Khatun H, Powell AD, Brandner S, Jefferys JG, Collinge J. Targeting cellular prion protein reverses early cognitive deficits and neurophysiological dysfunction in prion-infected mice. Neuron. 2007;53:325–335. doi: 10.1016/j.neuron.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Silveira JR, Raymond GJ, Hughson AG, Race RE, Sim VL, Hayes SF, Caughey B. The most infectious prion protein particles. Nature. 2005;437:257–261. doi: 10.1038/nature03989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredesen DE, Rao RV, Mehlen P. Cell death in the nervous system. Nature. 2006;443:796–802. doi: 10.1038/nature05293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guentchev M, Siedlak SL, Jarius C, Tagliavini F, Castellani RJ, Perry G, Smith MA, Budka H. Oxidative damage to nucleic acids in human prion disease. Neurobiol Dis. 2002;9:275–281. doi: 10.1006/nbdi.2002.0477. [DOI] [PubMed] [Google Scholar]
- Kovacs GG, Gelpi E, Ströbel T, Ricken G, Nyengaard JR, Bernheimer H, Budka H. Involvement of the endosomal-lysosomal system correlates with regional pathology in Creutzfeldt-Jakob disease. J Neuropathol Exp Neurol. 2007;66:628–636. doi: 10.1097/nen.0b013e318093ecc7. [DOI] [PubMed] [Google Scholar]
- Kovacs GG, Gasque P, Strobel T, Lindeck-Pozza E, Strohschneider M, Ironside JW, Budka H, Guentchev M. Complement activation in human prion disease. Neurobiol Dis. 2004;15:21–28. doi: 10.1016/j.nbd.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Liberski PP, Sikorska B, Bratosiewicz-Wasik J, Gajdusek DC, Brown P. Neuronal cell death in transmissible spongiform encephalopathies (prion diseases) revisited: from apoptosis to autophagy. Int J Biochem Cell Biol. 2004;36:2473–2490. doi: 10.1016/j.biocel.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Stefanis L. Caspase-dependent and -independent neuronal death: two distinct pathways to neuronal injury. Neuroscientist. 2005;11:50–62. doi: 10.1177/1073858404271087. [DOI] [PubMed] [Google Scholar]
- Schatzl HM, Laszlo L, Holtzman DM, Tatzelt J, DeArmond SJ, Weiner RI, Mobley WC, Prusiner SB. A hypothalamic neuronal cell line persistently infected with scrapie prions exhibits apoptosis. J Virol. 1997;71:8821–8831. doi: 10.1128/jvi.71.11.8821-8831.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sez-Valero J, Angeretti N, Forloni G. Caspase-3 activation by beta-amyloid and prion protein peptides is independent from their neurotoxic effect. Neurosci Lett. 2000;293:207–210. doi: 10.1016/s0304-3940(00)01532-9. [DOI] [PubMed] [Google Scholar]
- Carimalo J, Cronier S, Petit G, Peyrin JM, Boukhtouche F, Arbez N, Lemaigre-Dubreuil Y, Brugg B, Miquel MC. Activation of the JNK-c-Jun pathway during the early phase of neuronal apoptosis induced by PrP106–126 and prion infection. Eur J Neurosci. 2005;21:2311–2319. doi: 10.1111/j.1460-9568.2005.04080.x. [DOI] [PubMed] [Google Scholar]
- Burkle A, Kretzschmar HA, Brown DR. Poly(ADP-ribose) immunostaining to detect apoptosis induced by a neurotoxic fragment of prion protein. Histochem J. 1999;31:711–716. doi: 10.1023/a:1003944314206. [DOI] [PubMed] [Google Scholar]
- Puig B, Ferrer I. Cell death signaling in the cerebellum in Creutzfeldt-Jakob disease. Acta Neuropathol (Berl) 2001;102:207–215. [PubMed] [Google Scholar]
- Lyahyai J, Bolea R, Serrano C, Monlen E, Moreno C, Osta R, Zaragoza P, Badiola JJ, Martn-Burriel I. Correlation between Bax overexpression and prion deposition in medulla oblongata from natural scrapie without evidence of apoptosis. Acta Neuropathol (Berl) 2006;112:451–460. doi: 10.1007/s00401-006-0094-4. [DOI] [PubMed] [Google Scholar]
- Sisó S, Puig B, Varea R, Vidal E, Acín C, Prinz M, Montrasio F, Badiola J, Aguzzi A, Pumarola M, Ferrer I. Abnormal synaptic protein expression and cell death in murine scrapie. Acta Neuropathol (Berl) 2002;103:615–626. doi: 10.1007/s00401-001-0512-6. [DOI] [PubMed] [Google Scholar]
- Jeffrey M, Scott JR, Williams A, Fraser H. Ultrastructural features of spongiform encephalopathy transmitted to mice from three species of bovidae. Acta Neuropathol (Berl) 1992;84:559–569. doi: 10.1007/BF00304476. [DOI] [PubMed] [Google Scholar]
- Guentchev M, Hainfellner JA, Trabattoni GR, Budka H. Distribution of parvalbumin-immunoreactive neurons in brain correlates with hippocampal and temporal cortical pathology in Creutzfeldt-Jakob disease. J Neuropathol Exp Neurol. 1997;56:1119–1124. doi: 10.1097/00005072-199710000-00005. [DOI] [PubMed] [Google Scholar]
- Clarke AR, Jackson GS, Collinge J. The molecular biology of prion propagation. Philos Trans R Soc Lond B Biol Sci. 2001;356:185–195. doi: 10.1098/rstb.2000.0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porto-Carreiro I, Fevrier B, Paquet S, Vilette D, Raposo G. Prions and exosomes: from PrPc trafficking to PrPsc propagation. Blood Cells Mol Dis. 2005;35:143–148. doi: 10.1016/j.bcmd.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Doh-Ura K, Iwaki T, Caughey B. Lysosomotropic agents and cysteine protease inhibitors inhibit scrapie-associated prion protein accumulation. J Virol. 2000;74:4894–4897. doi: 10.1128/jvi.74.10.4894-4897.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz C, Russelakis-Carneiro M, Maundrell K, Castilla J, Soto C. Caspase-12 and endoplasmic reticulum stress mediate neurotoxicity of pathological prion protein. EMBO J. 2003;22:5435–5445. doi: 10.1093/emboj/cdg537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterberger U, Hoftberger R, Gelpi E, Flicker H, Budka H, Voigtlander T. Endoplasmic reticulum stress features are prominent in Alzheimer disease but not in prion diseases in vivo. J Neuropathol Exp Neurol. 2006;65:348–357. doi: 10.1097/01.jnen.0000218445.30535.6f. [DOI] [PubMed] [Google Scholar]
- Bence NF, Sampat RM, Kopito RR. Impairment of the ubiquitin-proteasome system by protein aggregation. Science. 2001;292:1552–1555. doi: 10.1126/science.292.5521.1552. [DOI] [PubMed] [Google Scholar]
- Wojcik C. Regulation of apoptosis by the ubiquitin and proteasome pathway. J Cell Mol Med. 2002;6:25–48. doi: 10.1111/j.1582-4934.2002.tb00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adori C, Kovacs GG, Low P, Molnar K, Gorbea C, Fellinger E, Budka H, Mayer RJ, Laszlo L. The ubiquitin-proteasome system in Creutzfeldt-Jakob and Alzheimer disease: intracellular redistribution of components correlates with neuronal vulnerability. Neurobiol Dis. 2005;19:427–435. doi: 10.1016/j.nbd.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Mabbott NA, Bruce ME. Complement component C5 is not involved in scrapie pathogenesis. Immunobiology. 2004;209:545–549. doi: 10.1016/j.imbio.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Fraser JR. What is the basis of transmissible spongiform encephalopathy induced neurodegeneration and can it be repaired?. Neuropathol Appl Neurobiol. 2002;28:1–11. doi: 10.1046/j.1365-2990.2002.00376.x. [DOI] [PubMed] [Google Scholar]
- Ishikura N, Clever JL, Bouzamondo-Bernstein E, Samayoa E, Prusiner SB, Huang EJ, DeArmond SJ. Notch-1 activation and dendritic atrophy in prion disease. Proc Natl Acad Sci USA. 2005;102:886–891. doi: 10.1073/pnas.0408612101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesa R, Piccardo P, Dossena S, Nowoslawski L, Roth KA, Ghetti B, Harris DA. Bax deletion prevents neuronal loss but not neurological symptoms in a transgenic model of inherited prion disease. Proc Natl Acad Sci USA. 2005;102:238–243. doi: 10.1073/pnas.0406173102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigtlander T, Unterberger U, Touma C, Palme R, Polster B, Strohschneider M, Dorner S, Budka H. Prominent corticosteroid disturbance in experimental prion disease. Eur J Neurosci. 2006;23:2723–2730. doi: 10.1111/j.1460-9568.2006.04801.x. [DOI] [PubMed] [Google Scholar]
- Kovacs GG, Kurucz I, Budka H, Adori C, Muller F, Acs P, Kloppel S, Schatzl HM, Mayer RJ, Laszlo L. Prominent stress response of Purkinje cells in Creutzfeldt-Jakob disease. Neurobiol Dis. 2001;8:881–889. doi: 10.1006/nbdi.2001.0418. [DOI] [PubMed] [Google Scholar]
- Serio TR, Lindquist SL. Protein-only inheritance in yeast: something to get [PSI+]-ched about. Trends Cell Biol. 2000;10:98–105. doi: 10.1016/s0962-8924(99)01711-0. [DOI] [PubMed] [Google Scholar]
- Chernoff YO, Uptain SM, Lindquist SL. Analysis of prion factors in yeast. Methods Enzymol. 2002;351:499–538. doi: 10.1016/s0076-6879(02)51867-x. [DOI] [PubMed] [Google Scholar]
- Liberski PP, Brown P. Astrocytes in transmissible spongiform encephalopathies (prion diseases). Folia Neuropathol. 2004;42(Suppl B):71–88. [PubMed] [Google Scholar]
- Rezaie P, Lantos PL. Microglia and the pathogenesis of spongiform encephalopathies. Brain Res Brain Res Rev. 2001;35:55–72. doi: 10.1016/s0165-0173(01)00042-x. [DOI] [PubMed] [Google Scholar]
- Kordek R, Hainfellner JA, Liberski PP, Budka H. Deposition of the prion protein (PrP) during evolution of experimental Creutzfeldt-Jakob disease. Acta Neuropathol (Berl) 1999;98:597–602. doi: 10.1007/s004010051124. [DOI] [PubMed] [Google Scholar]
- Marsh RF, Sipe JC, Morse SS, Hanson RP. Transmissible mink encephalopathy. Reduced spongiform degeneration in aged mink of the Chediak-Higashi genotype. Lab Invest. 1976;34:381–386. [PubMed] [Google Scholar]
- Gajdusek DC. Spontaneous generation of infectious nucleating amyloids in the transmissible and nontransmissible cerebral amyloidoses. Mol Neurobiol. 1994;8:1–13. doi: 10.1007/BF02778003. [DOI] [PubMed] [Google Scholar]
- DeArmond SJ, Qiu Y, Sanchez H, Spilman PR, Ninchak-Casey A, Alonso D, Daggett V. PrPc glycoform heterogeneity as a function of brain region: implications for selective targeting of neurons by prion strains. J Neuropathol Exp Neurol. 1999;58:1000–1009. doi: 10.1097/00005072-199909000-00010. [DOI] [PubMed] [Google Scholar]