Abstract
The heat stress (HS)-induced increase in occludin protein expression has been postulated to be a protective response against HS-induced disruption of the intestinal epithelial tight junction barrier. The aim of this study was to elucidate the cellular and molecular processes that mediate the HS-induced up-regulation of occludin expression in Caco-2 cells. Exposure to HS (39°C or 41°C) resulted in increased expression of occludin protein; this was preceded by an increase in occludin mRNA transcription and promoter activity. HS-induced activation of heat shock factor-1 (HSF-1) resulted in cytoplasmic-to-nuclear translocation of HSF-1 and binding to its binding motif in the occludin promoter region. HSF-1 activation was associated with an increase in occludin promoter activity, mRNA transcription, and protein expression; which were abolished by the HSF-1 inhibitor quercetin. Targeted HSF-1 knock-down by siRNA transfection inhibited the HSF-1-induced increase in occulin expression and junctional localization of occulin protein. Site-directed mutagenesis of the HSF-1 binding motif in the occludin promoter region inhibited HS-induced binding of HSF-1 to the occludin promoter region and subsequent promoter activity. In conclusion, our data show for the first time that the HS-induced increase in occludin protein expression is mediated by HSF-1 activation and subsequent binding of HSF-1 to the occludin promoter, which initiates a series of molecular and cellular events culminating in increased junctional localization of occludin protein.
The intestinal epithelial barrier consists of apical plasma membrane of the enterocytes that acts as a transcellular barrier and intercellular tight junctions (TJs) that act as a paracellular barrier against intercellular penetration of toxic luminal substances, including bacterial endotoxins, bacterial by-products, digestive enzymes, and food-degradation products.1,2,3,4,5,6 The TJ complex consists of cytoplasmic and transmembrane proteins. The transmembrane proteins, which include occludin, claudin family of proteins, and junctional adhesion molecules, extend from cytoplasmic compartment across the plasma membrane into extracellular compartment to participate in the formation of an extracellular TJ seal.7,8,9,10 The critical role of transmembrane proteins in the formation and maintenance of the TJ barrier is well established; however, the precise protein structure and components and molecular determinants of TJ barrier remain unclear.11
Occludin is an integral transmembrane TJ protein that has been shown to play a crucial role in TJ barrier function and TJ signaling process. Previous studies have shown that overexpression of occludin protein in MDCK cells leads to an enhancement of TJ barrier function.12 Conversely, siRNA knock-down of occludin leads to an increase in TJ permeability to selected paracellular markers.13 Molecular studies have shown that COOH-terminal end of occludin plays a crucial role in the maintenance of paracellular barrier function.14 Additionally, biochemical alteration of occludin phosphorylation has been shown to be an important determinant of TJ localization of occludin protein and enhancement of TJ barrier function.15,16,17 The “pivotal role of occludin in maintenance of TJ barrier function” has also been demonstrated in gene transfection studies after Raf-1-induced depletion of occludin in Pa-4 epithelial cells.18 However, the molecular and cellular mechanisms that regulate occludin gene activation and protein synthesis remain primarily unknown.
Heat stress (HS) causes an increase in intestinal epithelial permeability to luminal antigens including endotoxins.19,20,21,22 Both human and animal studies have shown that HS-induced disruption of intestinal TJ barrier leading to systemic endotoxemia19,20,21,22,23 is an important pathogenic factor contributing to fatality related to heat stroke.24,25 It had been shown that blood circulating endotoxin levels are greater than 1000-fold higher in heat stroke patients compared to normal healthy individuals, and that the degree of endotoxemia is predictive of fatal outcome.26 Therapeutic strategies that eliminate luminal bacteria27 and treatment with anti-endotoxin antibodies before the onset of heat shock24 have been shown to prevent fatality related to heat shock. Thus, therapeutic strategies that maintain intestinal TJ barrier function during HS are being actively pursued as an important therapeutic option in heat stroke.24,25,28 Previous studies from our laboratory indicated that a physiologically relevant increase in temperature (39°C or 41°C) causes an increase in occludin protein expression and an increase in junctional localization.29,30 The increase in junctional localization of occludin has been postulated to be an important protective mechanism against HS-induced disruption of TJ barrier in intestinal epithelial monolayers. The inhibition of HS-induced occludin expression was associated with a marked increase in TJ barrier disruption.29 The intracellular and molecular mechanisms that mediate occludin expression remain unresolved.
The major aim of this study was to elucidate the cellular and molecular processes that mediate the HS-induced increase in occludin protein expression, using Caco-2 intestinal epithelial monolayers as an in vitro intestinal epithelial model system. In this study, we used modest heat exposure as a physiologically relevant inducer of occludin protein expression to gain insight into cellular and molecular mechanisms that regulate occludin protein expression. Our data show that the HS-induced increase in occludin protein expression was regulated by activation of HSF-1. In addition, our studies provide insight into the cellular and molecular mechanisms that mediate HSF-1-induced up-regulation of occludin protein expression during HS.
Materials and Methods
Chemicals
Cell culture media (Dulbecco’s modified Eagle’s medium, DMEM), trypsin, fetal bovine serum (FBS), and related reagents were purchased from Life Technologies (Gaithersburg, MD). Glutamine, penicillin, streptomycin, and phosphate-buffered saline (PBS) were purchased from Life Technologies, Inc. (Grand Island, NY). Anti-occludin antibody was obtained from Zymed Laboratories (South San Francisco, CA). Quercetin, Triton X-100, bovine serum albumin, normal donkey serum, and anti-β-actin antibody were purchased from Sigma (St. Louis, MO). Horseradish peroxidase-conjugated secondary antibodies for Western blot analysis were purchased from Zymed Laboratories. Cy-3 antibodies for immunostaining were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Anti-HSP1 antibodies were purchased from Stressgen Biotechnologies (Victoria, Canada). Tween 20 and nonfat dry milk were purchased from Bio-Rad Laboratories (Hercules, CA). All other chemicals were of reagent grade and were purchased from Sigma, VWR (West Chester, PA), or Fisher Scientific (Pittsburgh, PA).
Cell Culture
Caco-2 cells (passage 18) were purchased from the American Type Culture Collection (Rockville, MD) and maintained at 37°C in a culture medium composed of DMEM with 4.5 mg/ml glucose, 50 U/ml penicillin, 50 U/ml streptomycin, 4 mmol/L glutamine, and 25 mmol/L HEPES, and supplemented with heat-inactivated 10% FBS.31 Culture medium was changed every 2 days. After partial digestion with 0.25% trypsin and 0.9 mmol/L ethylenediaminetetraacetic acid (EDTA) in Ca2+- and Mg2+-free PBS, Caco-2 cells were subcultured on tissue culture plates (Corning, Acton, MA).
Assessment of TJ, HSF-1, and β-Actin Protein Expression by Western Blot Analysis
To study the time-course effect of HS on occludin protein expression, Caco-2 monolayers were exposed to elevated temperatures for varying time periods and analysis of protein expression was performed by Western blot analysis as previously described.29 At the end of the experimental period, Caco-2 monolayers were immediately rinsed with ice-cold PBS, and cells were lysed with lysis buffer (50 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 500 μmol/L NaF, 2 mmol/L EDTA, 100 μmol/L vanadate, 100 μmol/L phenylmethyl sulfonyl fluoride, 1 μg/ml leupeptin, 1 μg/ml pepstatin A, 40 mmol/L paranitrophenyl phosphate, 1 μg/ml aprotinin, and 1% Triton X-100) and scraped, and the cell lysates were placed in microfuge tubes. Cell lysates were centrifuged to yield a clear lysate. Supernatant was collected, and protein measurement was performed using a Bio-Rad protein assay kit. Laemmli gel loading buffer was added to the lysate containing 5 to 10 μg of protein and boiled for 7 minutes, after which proteins were separated on a sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel. Proteins from the gel were transferred to the membrane (Trans-Blot transfer medium, nitrocellulose membrane; Bio-Rad Laboratories) overnight. The membrane was incubated for 2 hours in a blocking solution [5% dry milk in Tris-buffered saline (TBS)-Tween 20 buffer] followed by an incubation (1 to 2 hours) with appropriate primary antibodies in a blocking solution. After being washed in TBS-Tween buffer, the membrane was incubated (1 hour) in appropriate secondary antibodies and developed using the Western blotting luminol reagents (Santa Cruz Biotechnology, Santa Cruz, CA) on the Kodak BioMax MS film (Fisher Scientific).
[35S]Methionine Pulse-Chase Experiments
Caco-2 cells were pulse-labeled with [35S]methionine as previously described.32 Caco-2 monolayers were incubated in methionine-free DMEM medium supplemented with 10% (dialyzed) FBS at 37°C for 60 minutes. Subsequently, Caco-2 cells were pulsed overnight with [35S]methionine by incubation in DMEM medium containing 200 μCi/ml of [35S]methionine at 37°C. The radioactive media was removed, and Caco-2 cells were washed three times with DMEM. The [35S]methionine-labeled Caco-2 cells were then chased by incubation in DMEM media containing 10-fold excess of cold methionine. Caco-2 cells were exposed to 37°C or 41°C temperatures for various time periods. At the end of the chase period, Caco-2 cells were washed three times with cold PBS. Total protein degradation was assessed by counting the radioactivity of [35S]methionine in the sample. Subsequently, occludin protein degradation was assessed by immunoprecipitation of occludin followed by autoradiography. For immunoprecipitation of occludin protein, Caco-2 cell lysate was prepared as described above. In a 1.5-ml microcentrifuge tube, PBS-washed Protein G-Sepharose 4 Fast Flow (Amersham Biosciences Corp, Piscataway, NJ) was combined with occludin antibody and mixed end-over-end for 1 hour at 4°C in a tube rotator. After the incubation time, the beads were washed four times in a wash buffer to remove the unbound antibody. One hundred μg of the protein and 10% bovine serum albumin were added to the tube containing occludin antibody bound to protein G-Sepharose beads and incubated for 2 hours at 4°C while mixing end-over-end in a tube rotator. After the incubation time, the beads were washed four times in a wash buffer to remove the unbound proteins. Twenty μl of Laemmli gel-loading buffer was added to the beads and boiled for 7 minutes, after which proteins were separated on a sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel followed by gel drying in a Gel Dryer Model (Bio-Rad Laboratories) connected to the Universal vacuum system (Savant Instruments, Holbrook, NY). Dried gel was exposed to Kodak BioMax MS film.
Immunostaining of HSF-1 Protein
Cellular localization of HSF-1 was assessed by immunofluorescent antibody labeling. Caco-2 monolayers grown on coverslips were exposed to HS for 1 hour. At the end of the experimental period, Caco-2 monolayers were washed twice in cold PBS (4°C) and fixed with 2% paraformaldehyde for 20 minutes. After being permeabilized with 0.1% Triton X-100 in PBS at room temperature for 20 minutes, Caco-2 monolayers were then incubated in blocking solution composed of bovine serum albumin and normal donkey serum in PBS for 1 hour. Cells were then labeled with primary antibodies in blocking solution overnight at 4°C. After being washed with PBS, the cells were incubated in Cy-3-conjugated secondary antibody for 1 hour at room temperature. Before being mounted on microscope slides (Erie Scientific, Portsmouth, NH), cells were incubated in 4′,6-diamidino-2-phenyindole, dilactate (DAPI) (Sigma). Immunolocalizations of HSF-1 protein were visualized using a Nikon fluorescence microscope (Nikon, Garden City, NY) equipped with a Hamamatsu digital camera (Hamamatsu Photonics, Hamamatsu, Japan). Images were processed with Wasabi software (Hamamatsu Photonics Deutschland, Herrsching, Germany).
Cloning of the Occludin Promoter Region
A 2023-bp occludin promoter region was identified from the human genome database and the occludin promoter region was cloned using the GenomeWalker system (Clontech, Palo Alto, CA). Two gene-specific primers, OCCSEAP-1F (5′-GGGGTACCCGACCCCAAAGGAGAAACAACCC-3′) and OCCSEAP-1R (5′-GATCGCAGATCTCGAGCTGCGTCCTAGACCGGCTC-3′), were designed from human genome sequences upstream of the translation start site of occludin. A 2023-bp DNA fragment (GenBank accession no. DQ264390) was amplified by polymerase chain reaction (PCR). The amplification condition was 1 cycle at 94°C for 2 minutes, followed by 43 cycles at 94°C for 1 minute, 50°C for 1 minute, and 72°C for 2 minutes, 1 cycle at 72°C for 5 minutes. The resultant PCR product was digested with KpnI and XhoI, and inserted into pSEAP2-basic reporter vector (Clontech). The sequence was confirmed by the DNA services at the University of New Mexico.
Transfection of DNA Constructs
DNA construct of occludin promoter (pSEAP2-basic promoter vector, Clontech) were transiently transfected into Caco-2 cells using GeneJuice transfection reagent (EMD Biosciences, San Diego, CA). In brief, Caco-2 cells (5 × 105) were seeded into a six-well plate and grown to confluency. Caco-2 monolayers were then washed with PBS twice and 1.0 ml of Opti-MEM medium was added to each well. One μg of plasmid construct and 2 μl of transfection reagent were preincubated in 250 μl of Opti-MEM in two separate tubes (Invitrogen Corp., Carlsbad, CA). After 5 minutes of incubation, two solutions were mixed and incubated for another 20 minutes, and the mixture was added to each well. After incubation for 3 hours at 37°C, 500 μl of DMEM containing 10% FBS were added to each well to reach a 2.5% final concentration of FBS. Subsequently, media were replaced with normal Caco-2 growth media 16 hours after transfection. HS experiments were performed 48 hours after transfection. Secreted alkaline phosphatase (SEAP) activities were assessed by using the Great EscAPe SEAP chemiluminescence detection kit (BD Biosciences, Palo Alto, CA). Supernatants of the cell culture medium (15 μl) were placed in a separate well of a 96-well plate. Subsequently, the dilution buffer was added and the 96-well plate containing experimental samples was sealed with the adhesive aluminum foil and placed in the incubator (65°C) for 1 hour. Samples were cooled to room temperature. Assay buffer was added to each sample followed by 1.25 mmol/L Chemiluminescence Substrate Phosphatase Detection substrate working dilution. Samples were then incubated at room temperature for 10 minutes. The chemiluminescent signal was detected using a Veritas microplate luminometer (Turner BioSystems, Sunnyvale, CA). SEAP being a heat stable protein33 was used as the reporter protein instead of luciferase, which is heat-sensitive. The experimental values of reporter SEAP activities were normalized to the baseline values that were obtained before the start of the experiments to account for any differences in transfection efficiency between experimental samples.
Nuclear Extracts and Enzyme-Linked Immunosorbent Assay (ELISA)
Caco-2 cells were exposed to heat (41°C) for 1 hour and nuclear extracts were prepared according to the manufacturer’s instruction manual (Active Motif, Carlsbad, CA) with minor modifications. Cells were washed with 2 ml of ice-cold PBS, scraped, and centrifuged at 14,000 rpm for 2 minutes. The cell pellets were resuspended in 1 ml of hypotonic buffer (20 mmol/L HEPES, 5 mmol/L NaF, 10 μmol/L Na2MoO4, 0.1 mmol/L EDTA, pH = 7.5), and incubated on ice for 15 minutes. After the incubation period, 50 μl of 10% Nonidet P-40 was added followed by centrifugation at 14,000 rpm for 30 seconds, pelleted nuclei were resuspended in 40 μl of complete lysis buffer (20 mmol/L HEPES, 20% glycerol, 400 mmol/L NaCl, 10 mmol/L NaF, 0.1 mmol/L EDTA, 10 μmol/L Na2MoO4, 1 mmol/L NaVO3, 10 mmol/L p-nitrophenyl phosphate, and 10 mmol/L β-glycerophosphate, pH = 7.5). Before use 1 μl of 1 mol/L dithiothreitol and 10 μl of protease inhibitor cocktail were added per 1 ml of lysis buffer. After incubation on ice for 30 minutes, the lysates were centrifuged at 14,000 rpm for 10 minutes. The supernatants were stored at −70°C. Protein concentrations were determined using the Bradford method. To demonstrate the HSF-1 binding to the binding motif or heat shock element (HSE) on the occludin promoter, a double-stranded 50-bp oligonucleotide probe (Integrated DNA Technologies, Coralville, IA) encoding the occludin promoter region from −1046 to −997 was synthesized. The oligonucleotide binding reactions was performed according to the Flexi kit instruction manual (Active Motif) with modifications. The binding reactions contained 3 μg of proteins, 1 pmol/μl of biotinylated probe (Integrated DNA Technologies, Inc.) in a total volume of 55 μl of complete binding buffer. After incubation at room temperature for 30 minutes, the reaction mixtures were transferred to an individual well on the plate and incubated for 1 hour. Rabbit HSF-1 antibody was diluted in a total volume of 100 μl of antibody binding buffer (1:2000) and was added to the well to bind HSF-1 from the nuclear extract. After incubation for 1 hour, HSF-1 antibody was removed and 100 μl of anti-rabbit horseradish peroxidase-conjugated IgG (1:5000) were added to the well and incubated for 1 hour. Subsequently, 100 μl of developing solution were added for 2 to 10 minutes, and 100 μl of stop solution were added. The absorbance at 450 nm was determined using the SpectraMax 190 (Molecular Devices, Sunnyvale, CA).
siRNA of HSF-1
To silence HSF-1, ON-TARGETplus SMARTpool (Dharmacon, Inc. Chicago, IL) was used. The sequences for HSF-1 small interfering RNA (siRNA) were: 5′-PUACUUGGGCAUGGAAUGUGUU-3′; 5′-PGUCCAUAGCAUCCAAGUGGUU-3′; 5′-PUAUGUCUUCACUCUUCAGGUU-3′; 5′-PUGAAUCCGGGCUGCUGUUCUU-3′. Caco-2 monolayers were transiently transfected using DharmaFect transfection reagent (Dharmacon, Lafayette, CO). Caco-2 cells were seeded into a six-well plate and grown to confluency. Caco-2 monolayers were then washed with PBS and Opti-MEM medium was added to the well. The plasmid vector containing the siRNA of HSF-1 and DharmaFect reagent were preincubated in Opti-MEM. After 5 minutes of incubation, two solutions were mixed and incubated for another 20 minutes, and the mixture was added to each well. After incubation for 3 hours at 37°C, 500 μl of DMEM containing 10% FBS and no antibiotics were added to cell culture media to reach a 2.5% final concentration of FBS. Heat exposure was performed 6 days after transfection. The siRNA-induced silencing of HSF-1 was confirmed by immunoblot of HSF-1.
RNA Isolation and Reverse Transcription
Caco-2 cells/filter (5 × 105) were seeded into six-well transwell permeable inserts and grown to confluency. Filter-grown Caco-2 cells were exposed to HS for desired time periods. At the end of the experimental period, cells were washed twice with ice-cold PBS. Total RNA was isolated using the RNeasy kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. Total RNA concentration was determined by absorbance at 260/280 nm using SpectrraMax 190 (Molecular Devices). The reverse transcription (RT) was performed using the GeneAmp Gold RNA PCR core kit (Applied Biosystems, Foster city, CA). Two μg of total RNA from each sample were reverse-transcribed into cDNA in a 40-μl reaction containing 1× real-time PCR buffer, 2.5 mmol/L MgCl2, 250 μmol/L of each dNTP, 20 U RNase inhibitor, 10 mmol/L dithiothreitol, 1.25 μmol/L random hexamer, and 30 U multiscribe RT. The RT reactions were performed in a thermocycler (PTC-100; MJ Research, Waltham, MA) at 25°C for 10 minutes, 42°C for 30 minutes, and 95°C for 5 minutes.
Quantification of Gene Expression Using Real-Time PCR
The real-time PCRs were performed using an ABI Prism 7900 sequence detection system and TaqMan universal PCR master mix kit (Applied Biosystems, Branchburg, NJ) as previously described.34,35,36 Each real-time PCR reaction contained 5 μl of RT reaction mix, 25 μl of 2× TaqMan universal PCR master mix, 0.2 μmol/L probe, and 0.6 μmol/L primers. Primer and probe design for the real-time PCR was made with Primer Express version 2 from Applied Biosystems. The primers used in this study are as follows: occludin-specific primer pairs consisted of 5′-CCCCATCTGACTATGTGGAAAGA-3′ (forward), 5′-AAAACCGCTTGTCATTCACTTTG-3′ (reverse); probe specific for occludin consisted of FAM 5′-TGACAGTCCCATGGCATACTCTTCCAATG-3′ TAMRA; the internal control GAPDH-specific primer pairs consisted of 5′-CCACCCATGGCAAATTCC-3′ (forward), 5′-TGGGATTTCCATTGATGACCAG-3′ (reverse); probe specific for GAPDH consisted of JOE 5′-TGGCACCGTCAAGGCTGAGAACG-3′ TAMRA. All runs were performed according to the default PCR protocol (50°C for 2 minutes, 95°C for 10 minutes, 40 cycles of 95°C for 15 seconds, and 60°C for 1 minute). For each sample, real-time PCR reactions were performed in triplicate, and the average threshold cycle (Ct) was calculated. Standard curve was generated to convert the Ct to copy numbers. Expression of occludin mRNA was normalized with GAPDH mRNA expression. The average copy number of occludin mRNA expression in control samples was set to 1.0. The relative expression of occludin mRNA in treated samples was determined as a fold increase compared with control samples.
Site-Directed Mutagenesis
The 11 bp (ATGAAATTTCC) of the HSF-1 binding site (−1085 to −1075) in the occludin promoter were mutated by using the GeneTailor site-directed mutagenesis system (Invitrogen). Briefly, primers were generated that included an 11-bp mutation (CGTCCCGGGAA) flanked by a wild-type sequence on either side. A PCR reaction produced a new complete copy of the plasmid containing the mutation coded for by the primers. The linear PCR product was subsequently transformed into DH5-T1 Escherichia coli, which circularized the PCR product and digested any remaining parent plasmid. DNA sequence was verified by the DNA services at University of New Mexico.
Statistical Analysis
Results are expressed as means ± SE. Statistical significance of differences between mean values was assessed with Student’s t-test for unpaired data. All reported significance levels represent two-tailed P values. A P value of <0.05 was used to indicate statistical significance. All experiments were repeated a minimum of three times to ensure reproducibility.
Results
Mechanism of HS-Induced Up-Regulation of Occludin Protein Expression
The effect of physiologically relevant heat exposure (39°C or 41°C) on occludin protein expression in confluent Caco-2 monolayers was determined by immunoblot analysis. A modest increase in incubation temperature from 37°C to 39°C or 41°C caused a progressive time-dependent increase in occludin protein expression (Figure 1). The heat-induced increase in occludin protein expression appeared to be protein-specific because heat exposure caused a decrease in ZO-1 and did not affect claudin-3 or β-actin (internal control) protein levels (Figure 1b). In the following studies, the intracellular processes that mediated the HS-induced increase in occludin protein expression were investigated. The possibility that the increase in occludin protein expression was attributable to a decrease in rate of protein degradation and/or an increase in protein synthesis or gene transcription was examined. The HS effect on occludin protein degradation was determined by [35S]methionine pulse-chase studies (Figure 2). In these studies, Caco-2 proteins were pulse-labeled with [35S]methionine, and the effect of heat on occludin protein degradation determined by immunoprecipitation of occludin and immunoblot analysis. In the control Caco-2 monolayers (37°C), there was a progressive time-dependent decrease in 35S-labeled occludin protein throughout the 24-hour experimental period. Similar to other studies, the half-life of occludin was ∼10 to 11 hours.37 Heat exposure (41°C) did not affect the rate of occludin protein degradation (Figure 2, a and b). The heat exposure to 41°C also did not affect the total Caco-2 protein degradation as assessed by the total [35S]methionine labeled proteins (Figure 2c). These findings suggested that the increase in occludin protein level was not attributable to a decrease in protein degradation. To determine the role of protein synthesis, Caco-2 protein synthesis was inhibited by a potent translation inhibitor cycloheximide, which inhibits the peptidyl transferase on 60S ribosomal subunit.38 Cycloheximide (10 ng/ml) completely prevented the HS-induced increase in occludin expression (Figure 3a), suggesting that the increase in occludin protein expression was attributable to new protein synthesis. To further investigate the intracellular mechanisms involved, the effect of HS on occludin transcript expression was also examined. Heat exposure (41°C) resulted in a progressive increase in occludin mRNA expression as assessed by real-time PCR (Figure 3b). To confirm the role of occludin transcription in heat-induced increase in occludin expression, the effect of transcription inhibitor actinomycin-D (100 ng/ml) on HS-induced increase in occludin mRNA and protein expression was determined. (Actinomycin-D inhibits RNA polymerase activity and DNA-dependent mRNA transcription.)39 Actinomycin-D treatment did not affect the basal levels of occludin mRNA expression (37°C), but completely prevented the HS-induced increase in occludin mRNA expression (Figure 3c). The actinomycin-D inhibition of mRNA transcription also prevented the heat-induced increase in occludin protein expression (Figure 3d). Together, these results indicated that the increase in occludin protein expression was attributable in part to an increase in mRNA transcription and protein synthesis.
Figure 1.
Time-course effect of heat exposure (39°C or 41°C) on TJ protein expression in Caco-2 monolayers. Caco-2 monolayers were exposed to 39°C or 41°C for increasing time points (0 to 24 hours). Occludin, ZO-1, and claudin-3 protein expressions were determined by Western blot analysis as described in the Materials and Methods. Heat exposure to 39°C (a) or 41°C (b) resulted in a progressive increase in occludin protein expression. b: Exposure to 41°C caused a decrease in ZO-1 and did not affect claudin-3 protein expression during the 24-hour treatment period. β-Actin served as an internal control. All experiments were repeated three to six times to ensure reproducibility.
Figure 2.
The effect of HS on occludin protein degradation. a: Time-course of HS on occludin protein degradation. The HS effect on occludin protein degradation was assessed by [35S]methionine pulse chase as described in the Materials and Methods. After [35S]methionine labeling, Caco-2 monolayers were treated with HS (41°C) for increasing time points (0, 4, 12, and 24 hours). Occludin protein degradation was assessed by immunoprecipitation of occludin and by measuring the 35S radioactivity as detected by gel electrophoresis. There was a progressive time-dependent decrease in 35S-labeled occludin protein throughout the 24-hour experimental period. Heat exposure did not affect the rate of occludin protein degradation. b: Densitometric analysis of occludin bands. c: Time course of HS on total protein degradation. After [35S]methionine labeling, Caco-2 monolayers were treated with HS (41°C) for various time points (0, 4, 12, and 24 hours). Total protein degradation was assessed by measuring of the 35S radioactivity in the whole Caco-2 cell lysate. Experiments were repeated a total of three times.
Figure 3.
The role of protein synthesis and transcription in HS-induced increase in occludin expression. a: Effect of cycloheximide (10 ng/ml) on HS (41°C for 24 hours)-induced increase in occludin expression. b: Time course of HS on occludin mRNA expression. Caco-2 cells were treated with HS for 8 and 16 hours. Heat exposure (41°C) resulted in a progressive increase in occludin mRNA expression. Data represent means ± SE (n = 3); *P < 0.05, **P < 0.001 versus 37°C. c: Actinomycin-D (100 ng/ml) effect on HS (41°C for 16 hours)-induced an increase in occludin mRNA expression. Actinomycin-D treatment inhibited the HS-induced increase in occludin mRNA expression. Expression of occludin mRNA was normalized with GAPDH mRNA expression. Data represent means ± SE (n = 4); *P < 0.01 versus 37°C; **P < 0.01 versus 41°C. d: Actinomycin-D effect on HS (41°C for 24 hours)-induced increase in occludin protein expression. Actinomycin-D treatment inhibited the HS-induced increase in occludin protein expression. All experiments were repeated three to four times.
HS Activates Occludin Gene Expression
Based on the above results, we next examined the possibility that the HS effect on occludin mRNA and protein expression was attributable to an increase in occludin gene activity. To determine the heat effect on occludin gene activity, occludin promoter region was identified using the human genome database and cloned into pSEAP2-Basic plasmid vector (Clontech). Using the Genomatix/Promoter Inspector software, a 2023-bp candidate occludin promoter region upstream of occludin gene was identified (GenBank accession number DQ264390), amplified by PCR, and cloned into pSEAP2-basic reporter vector in the same orientation as the occludin open reading frame. Because SEAP is a heat stable protein,33 SEAP was used as the reporter protein instead of luciferase, which is heat-sensitive and loses activity at 39°C and 41°C. The effect of heat on occludin promoter activity was determined by measuring SEAP activity in Caco-2 cells transfected with plasmid vectors encoding the occludin promoter region. Heat exposure (41°C) resulted in a significant increase in occludin promoter activity (Figure 4), suggesting that the heat-induced increase in occludin mRNA and protein expression was attributable in part to an increase in occludin promoter activity.
Figure 4.
The effect of HS on occludin promoter activity. SEAP vector containing the occludin promoter region was transfected into the Caco-2 cells. Caco-2 cells were exposed to HS (41°C) for 2 hours. The occludin promoter activity was determined by the SEAP assay and expressed as relative SEAP activity. The absolute values for the SEAP activity were 34,777 ± 2470 and 59,471 ± 4093 for 37°C and 41°C groups, respectively. Data represent means ± SE (n = 6); *P < 0.01 versus control. The experiment was repeated a total of four times.
HSF-1 Mediates the Heat-Induced Activation of Occludin Promoter
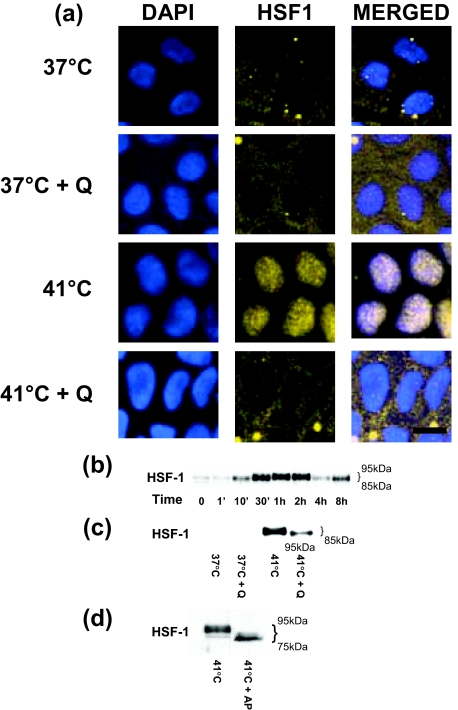
The nuclear transcription factor HSF-1 has been shown to play an important role in mediating HS modulation of various heat response genes.40,41 Thus, in the following series of experiments, we tested the hypothesis that HSF-1 also plays a central role in mediating the heat-induced activation of occludin gene expression. First, the effect of heat on Caco-2 HSF-1 activation was determined by assessing cytoplasmic-to-nuclear translocation of HSF-1 (Figure 5). As shown in Figure 5a, in quiescent Caco-2 cells, HSF-1 is present predominantly in the cytoplasm. After heat exposure, there was a rapid cytoplasmic-to-nuclear translocation (within minutes) of HSF-1, indicating rapid HSF-1 activation. Co-staining with a commonly used nuclear staining agent DAPI (which binds to DNA in the cell nucleus) confirmed localization of HSF-1 in the nucleus after heat exposure. Similarly, immunoblot analysis indicated that in quiescent Caco-2 cells only trace amounts of HSF-1 were present in the nuclear fraction (Figure 5b). On heat exposure, there was a rapid increase in HSF-1 translocation into the nuclear fraction (Figure 5b). HS also caused an increase in HSF-1 phosphorylation as evidenced by the increase in higher molecular weight forms of HSF-1.42,43 HSF-1 inhibitor quercetin42,44 (100 μmol/L) significantly inhibited both the nuclear translocation and phosphorylation of HSF-1 (Figure 5c). Previous studies have shown that the HS-induced increase in higher molecular forms of HSF-1 was attributable to an increase in phosphorylation.41,42 To experimentally validate that the HS-induced increase in high-molecular weight forms of HSF-1 was indeed attributable to phosphorylated HSF-1, protein lysates (after a 1-hour exposure to 41°C) were treated with a dephosphorylating enzyme alkaline phosphatase as previously described.41,42 Consistent with previous studies, alkaline phosphatase treatment resulted in disappearance of higher molecular weight forms of HSF-1 (Figure 5d), confirming that the higher molecular weight forms were attributable to phosphorylation of HSF-1.
Figure 5.
The effect of HS on HSF-1 activation in Caco-2 monolayers. The effect of HS on HSF-1 cytoplasmic-to-nuclear translocation as determined by immunofluorescent antibody labeling as described in Materials and Methods. a: After appropriate treatment, Caco-2 monolayers were fixed and co-stained for HSF1 by using specific antibodies and corresponding secondary antibodies conjugated with fluorescent probes. Nuclei were visualized with DAPI (blue). Heat exposure: 41°C for 1 hour; quercetin (100 μmol/L). b: Time-course effect of HS-induced HSF-1 activation in Caco-2 monolayers. Caco-2 monolayers were treated with HS (41°C) for increasing time points (0 to 8 hours) and changes in the HSF-1 expression in the nuclear fraction were assayed by Western blot analysis. c: Effect of HSF-1 inhibitor quercetin on heat-induced increase in HSF-1 activation. Caco-2 monolayers were treated with 41°C alone or with quercetin (100 μmol/L) for the 1-hour experimental period. Subsequently, HSF-1 protein levels in nuclear fraction were determined by Western blot analysis as described in the Material and Methods. Quercetin treatment at 41°C resulted in an inhibition of HS-induced increase in HSF-1 protein expression. d: Effect of dephosphorylating enzyme alkaline phosphatase on heat-induced increase in HSF-1 phosphorylation. Caco-2 protein lysate (after a 1-hour exposure to 41°C) was treated with alkaline phosphatase. Alkaline phosphatase treatment resulted in disappearance of higher molecular weight forms of HSF-1. The experiment was repeated three to four times. Scale bar = 20 μm.
Next, the role of heat-activated HSF-1 in mediating the HS-induced increase in occludin promoter activity and increase in occludin expression was examined. The inhibition of HS-induced activation of HSF-1 by HSF-1 inhibitor quercetin resulted in inhibition of HS-induced increase in occludin promoter activity (Figure 6a) and mRNA expression (Figure 6b). Quercetin also inhibited the HS-induced increase in occludin protein expression (Figure 6c), indicating that HSF-1 activation was required for the HS modulation of occludin gene activity and protein expression.
Figure 6.
The effect of HSF-1 inhibitor quercetin on HS-induced changes in occludin promoter activity, transcription, and protein expression. a: Caco-2 monolayers were exposed to 41°C for 2 hours in the presence or absence of quercetin (Q) (100 μmol/L). Subsequently, occludin promoter activity was determined by SEAP assay. The absolute values for the SEAP activity were 38,921 ± 3237, 80,105 ± 4031, and 49,505 ± 4185 for 37°C, 41°C, and 41°C + Q group, respectively. Data represent means ± SE (n = 5); *P < 0.05 versus 37°C; **P < 0.01 versus 41°C. b: Caco-2 monolayers were exposed to 41°C for 16 hours in the presence or absence of quercetin (Q) (100 μmol/L). Subsequently, occludin mRNA levels were determined by real-time PCR. Data represent means ± SE (n = 4); *P < 0.001 versus 37°C; **P < 0.001 versus 41°C. c: Caco-2 monolayers were exposed to 41°C for 24 hours in the presence or absence of quercetin (Q) (100 μmol/L). Subsequently, occludin protein expression was determined by Western blot analysis. Treatment with quercetin prevented the heat-induced increase in occludin promoter activity, mRNA, and protein expression. β-Actin served as an internal control. The experiments were repeated three to five times.
To substantiate further the role of HSF-1 in occludin gene transcription and protein expression, HSF-1 expression was selectively knocked-down by HSF-1 siRNA. As shown in Figure 7a, HSF-1 siRNA transfection resulted in a marked depletion of HSF-1 expression in Caco-2 cells. SiRNA depletion of HSF-1 inhibited the heat-induced increase in occludin protein (Figure 7b) and mRNA expression (Figure 7c), confirming the requirement of HSF-1 in mediating the HS-induced increase in occludin mRNA and protein expression.
Figure 7.
Effect of siRNA-targeted knock-down of HSF-1 on heat-induced up-regulation of occludin protein and mRNA expression. a: Effect of HSF-1 siRNA transfection on Caco-2 HSF-1 expression. HSF-1 siRNA caused a significant knock-down of HSF-1 expression. b: Caco-2 monolayers were exposed to 41°C for the 24-hour experimental period. After siRNA transfection HS did not cause an increase in HSF-1 protein expression. Occludin protein expression was up-regulated by heat exposure (41°C for 24 hours) and HSF-1 siRNA prevented that up-regulation. β-Actin served as an internal control. c: Caco-2 monolayers were exposed to 41°C for the 16-hour experimental period with and without pretreatment of HSF-1 siRNA. Subsequently, occludin mRNA levels were determined by real-time PCR. Pretreatment with HSF-1 siRNA prevented HS-induced increase in occludin transcription. Data represent means ± SE (n = 3 to 4); *P < 0.01 versus 37°C; **P < 0.05 versus 41°C. The experiments were repeated three to four times.
We next examined the molecular determinants involved in HSF-1 regulation of occludin promoter activity. In these studies, we tested the hypothesis that HSF-1 activates the occludin promoter by binding to a regulatory site on promoter region. Using Genomatix/Promoter Inspector software, we identified a HSE or HSF-1 binding motif (nGAAnnTTCn)45 on the occludin promoter region (−1085 to −1076 upstream of the start codon for occludin gene). The binding of HS-activated HSF-1 to HSE region on occludin promoter was determined by ELISA-based DNA binding assay. A 50-bp occludin promoter region encoding HSE was synthesized and used as a DNA probe to assess binding of heat-activated HSF-1 (nuclear fraction). HS resulted in a quantitative increase in HSF-1 binding to the DNA probe (Figure 8a). Quercetin inhibited the HS increase in HSF-1 binding to the DNA probe (Figure 8a). As expected, siRNA knock-down of HSF-1 also resulted in a decrease in HSF-1 binding to the DNA probe (Figure 8b). To further investigate the role of HSE as a molecular determinant of occludin promoter activity, HSE motif was mutated via site-directed mutagenesis (Figure 9). As shown in Figure 9, the mutation of HSE motif prevented both the heat-induced increase in binding of HSF-1 to the promoter binding site (Figure 9a) and occludin promoter activity (Figure 9b). These findings confirmed that HSE is the DNA motif that mediates HSF-1 regulation of occludin promoter activity.
Figure 8.
Effect of HSF-1 inhibitor quercetin and HSF-1 siRNA on binding of heat-activated HSF-1 to HSE region on occludin promoter. The 50-pb occludin promoter region encoding HSE was synthesized and used as a DNA probe to assess the binding of heat (41°C for 1 hour)-activated HSF-1 (nuclear fraction). HSF-1 binding was determined by ELISA-based DNA binding assay. a: Heat resulted in a significant increase in HSF-1 binding to the DNA probe encoding HSE; and quercetin inhibition of HSF-1 activation resulted in an inhibition of HS increase in HSF-1 binding to the DNA probe. Data represent means ± SE (n = 4); *P < 0.001 versus 37°C; **P < 0.001 versus 41°C. b: SiRNA knock-down of HSF-1 inhibited the heat-induced HSF-1 binding to the DNA probe. Data represent means ± SE (n = 3); *P < 0.01 versus 37°C; **P < 0.01 versus 41°C. The experiments were repeated three to four times.
Figure 9.
The effect of site-directed mutation of the HSE region on heat-induced modulation of the occludin promoter activity and binding of HSF-1 to HSE on the occludin promoter. a: The 50-pb occludin promoter regions encoding either the wild-type HSE or mutated HSE motif were synthesized and used as a DNA probe to assess the binding of heat (41°C for 1 hour)-activated HSF-1 (nuclear fraction). HSF-1 binding was determined by ELISA-based DNA binding assay. Mutation of the HSE motif inhibited the heat-induced HSF-1 binding to the DNA probe. Data represent means ± SE (n = 3); *P < 0.05 versus 37°C; **P < 0.01 versus 41°C WT. b: The mutant occludin promoter containing the mutation of the HSE region was generated and then transfected into Caco-2 cells as described in the Materials and Methods. Transfected Caco-2 cells were treated with control temperature 37°C or heat 41°C for 2 hours. The mutation of HSE motif prevented the heat-induced increase in occludin promoter activity. The absolute values for the SEAP activity were 105,030 ± 8495, 118,108 ± 11,928, 174,903 ± 6738 and 118,937 ± 8158 for 37°C WT, 37°C Mut, 41°C WT, and 41°C Mut groups, respectively. Data represent means ± SE (n = 4 to 6); *P < 0.001 versus 37°C; **P < 0.001 versus 41°C WT. The experiments were repeated three times.
HSF-1 Mediates the Heat-Induced Increase in Junctional Localization of Occludin
The effect of HS on junctional localization of occludin monolayers was also determined by immunostaining (Figure 10). In the control Caco-2 monolayers (37°C), occludin appeared as a continuous peripheral band encircling the cells at the apical cellular junctions (Figure 10a). Heat exposure (41°C) resulted in a marked increase in intensity of occludin staining at the apical junctional borders (Figure 10c), indicating that the HS-induced increase in occludin protein expression corresponds to an increase in junctional localization. To assess the role of HSF-1 in mediating the increase in junctional localization of occludin, HSF-1 expression was depleted by siRNA transfection. The siRNA depletion of HSF-1 prevented the HS-induced increase in junctional localization of occludin (Figure 10d). Instead, there was a visible decrease in intensity of occludin staining at the apical cellular junctions and the peripheral occludin band was interrupted at various points by discrete areas of low intensity or absent occludin staining. Similarly, quercetin pretreatment also prevented the HS-induced increase in occludin junctional localization (data not shown). These results suggested that the upstream HSF-1 activation has important consequences for the downstream junctional localization of occludin proteins.
Figure 10.
The effect of HSF-1 siRNA on heat-induced junctional localization of occludin. The junctional localization of occludin was assessed by immunofluorescent antibody labeling as described in the Materials and Methods. a: In control Caco-2 monolayers (37°C) (mean relative fluorescent intensity = 256,871 ± 33,578), occludin is localized at the apical junctional regions and seen as a peripheral band encircling the cells. b: The HSF-1 siRNA-transfected Caco-2 monolayers appeared to have a small decrease in intensity of occludin stain (mean relative fluorescent intensity = 233,114 ± 25,327). c: Heat exposure (41°C for 24 hours) resulted in a marked increase in intensity of occludin staining at the apical junctional borders and occludin appeared as a thickened band (mean relative fluorescent intensity = 620,620 ± 102,293). d: The siRNA depletion of HSF-1 prevented the increase in junctional localization of occludin and the overall intensity of occludin staining appeared to be lower than in controls and discrete gaps in occludin staining were present (mean relative fluorescent intensity = 175,547 ± 6683). The experiments were repeated three times. Scale bar = 20 μm.
Discussion
Occludin is an integral transmembrane TJ protein that has been demonstrated to play an important role in TJ barrier function and in TJ signaling.8 However, the transcription factors and molecular determinants that activate occludin gene and protein expression have not been previously reported. Previous studies have concluded that the heat-induced increase in occludin expression and junctional localization represents an important protective response against the TJ barrier disruptive effects of HS.29 In this study, we investigated the cellular and molecular mechanisms that mediate the activation of occludin gene expression and protein synthesis using a physiologically relevant model system.
A modest heat exposure (39°C or 41°C) caused a twofold to fourfold increase in occludin protein expression in confluent Caco-2 intestinal epithelial monolayers (Figure 1). We first examined whether HS-induced increase in occludin protein expression was attributable to a decrease in occludin degradation or an increase in protein synthesis. Our data showed that the increase in occludin protein expression was attributable to an increase in occludin protein synthesis and not a decrease in protein break-down. Our data also indicated that the HS-induced increase in occludin protein expression directly correlated with an increase in occludin transcript expression; and the inhibition of the occludin transcription by actinomycin-D (RNA polymerase inhibitor) prevented both the increase in occludin transcript and protein expression (Figure 3). The HS-induced increase in occludin mRNA and protein expression was also associated with an increase in occludin promoter activity. Thus, our data suggested that the HS-induced increase in occludin protein expression was attributable to activation of occludin gene expression and subsequent increase in protein synthesis.
Nuclear transcription factor HSF-1 has been shown to play a central role in regulation of various HS response genes.40,46,47,48 Under normal conditions, HSF-1 is constitutively expressed in the cytoplasm. After HS, HSF-1 is rapidly activated and translocates to the nucleus.41,49,50,51 There are two sequential steps involved in HSF-1 activation: HSF-1 undergoes trimerization, which enables it to bind to HSE on the promoter region of target genes and HSF-1 becomes inducibly phosphorylated at serine residues52,53 and acquires the transactivation competence.54 It has been shown that both steps of HSF-1 activation are required for optimal transcriptional competence of HSF-1.53,55,56 The physiological role of HSF-1 in mediating various HS responses has been well demonstrated in both in vitro and in vivo studies. For example, targeted disruption of HSF-1 in mouse embryonic fibroblast cells and other cell types have been shown to abolish the thermotolerance response.57 Lethal heat exposure resulted in a significant increase in cell death in HSF-1-deficient cells compared to wild-type cells, indicating that HSF-1 protein was protective against heat-induced cell death. Similarly, HSF-1-deficient mice have been shown to have decreased survival in response to heat, chemical, or other types of lethal stresses.58,59,60
In the present study, we examined the hypothesis that HSF-1 mediates the HS-induced activation of occludin gene activity. Our data showed that HS causes a rapid nuclear translocation and hyperphosphorylation of HSF-1 (Figure 5). The increase in HSF-1 nuclear translocation and hyperphosphorylation correlated with an increase in HSF-1 binding and activation of occludin promoter; and inhibition of HSF-1 activation with HSF-1 inhibitor quercetin42,44 inhibited the HS-induced increase in occludin promoter activity. Quercetin also inhibited the HS-induced increase in occludin mRNA and protein expression. These data suggested that HSF-1 mediated the HS-induced activation of occludin gene expression and subsequent increase in occludin protein synthesis.
Although quercetin is known to be a potent HSF-1 inhibitor, it also has other important biological activities including anti-oxidant effects, inhibition of stress kinase pathways, inhibition of nuclear factor (NF)-κB, and modulation of PI3 kinase pathways. Quercetin has been shown to inhibit lipopolysaccharide-induced expression of proinflammatory cytokines (tumor necrosis factor-α, interleukin-1β, and interleukin-6) and nitric oxide production by inhibition of MAP kinase (ERK and p38) and NF-κB pathways in macrophages61 and neutrophils.62 Similarly, in animal studies, quercetin inhibited dextran sulfate sodium-induced intestinal inflammation and proinflammatory cytokine expression by inhibition of NF-κB pathway.63 Quercetin suppression of NF-κB pathway was also the mechanism by which quercetin inhibited the expression of monocyte chemoattractant protein-1.64 An anti-proliferative effect of quercetin in cancer cells has been shown to be mediated via inhibition of the PI3K-Akt/PKB pathway, leading to caspase-3/7 activation of apoptosis.65,66 Additionally, quercetin anti-oxidant activity has been shown to be mediated in part by activation of ERK pathway.67 Because HS induces a variety of biochemical changes in the heat exposed cells, a possibility exists that the quercetin effect could be attributable to biological effects other than HSF-1 inhibition. Therefore, to provide more direct evidence that HSF-1 mediates the HS-induced modulation of occludin gene activity, HSF-1 expression was selectively knocked-down via siRNA transfection. The HSF-1 siRNA silencing in Caco-2 cells inhibited the HS-induced increase in occludin gene and protein expression (Figure 7), validating the role of HSF-1 as a required transcription factor mediating the HS-induced activation of occludin promoter and subsequent increase in protein expression.
We also investigated the specific molecular determinants on occludin promoter that mediated the HSF-1 regulation of promoter activity. Using the Genomatrix software, we identified a potential HSF-1 binding site (HSE) on occludin promoter (−1085 to −1075 upstream of the start codon). Our studies showed that activated HSF-1 binds to the promoter region containing the HSE motif (Figure 8). The site-directed mutagenesis of the 11-bp HSE motif prevented the HS-induced increase in HSF-1 binding and activation of occludin promoter, indicating that HSE motif on occludin promoter is the active binding site that regulates the activation of occludin promoter activity. Based on our present data, we propose that HS-induced HSF-1 activation is a key regulatory step leading to occludin promoter activation and subsequent increase in protein expression. The increase in occludin protein expression and junctional localization requires HSF-1 activation. A proposed scheme outlining the cellular and molecular processes that mediate the increase in occludin junctional expression is shown in Figure 11.
Figure 11.
The proposed schematic outlining the intracellular processes that mediate HS-induced increase in occludin expression and junctional localization.
In conclusion, our results indicate that the HS-induced increase in occludin protein expression is mediated by activation of transcription factor HSF-1. In a step-wise approach, we show that HS induces activation of HSF-1, which translocates to the nucleus and binds to the HSE motif on occludin promoter, which in turn induces promoter activation and increases in occludin mRNA transcription and protein synthesis (Figure 11). Our data provide new information regarding the role of HSF-1 as a regulator of occludin gene activity and provide important insight into cellular and molecular processes that mediate occludin gene activity and occludin protein expression during physiologically relevant experimental conditions.
Footnotes
Address reprint requests to Thomas Y. Ma, M.D., Ph.D., Internal Medicine–Gastroenterology and Hepatology, MSC10 5550, 1 University of New Mexico, Albuquerque, NM 87131-0001. E-mail: tma@salud.unm.edu.
Supported by a Veterans Affairs (VA) Merit Review grant from the VA Research Service, National Institute of Diabetes and Digestive and Kidney Disease grant RO 1-DK-64165–01, and research funds from University of New Mexico (to T.Y.M.).
References
- Anderson JM, Van Itallie CM. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol. 1995;269:G467–G475. doi: 10.1152/ajpgi.1995.269.4.G467. [DOI] [PubMed] [Google Scholar]
- Baker JW, Deitch EA, Li M, Berg RD, Specian RD. Hemorrhagic shock induces bacterial translocation from the gut. J Trauma. 1988;28:896–906. doi: 10.1097/00005373-198807000-00002. [DOI] [PubMed] [Google Scholar]
- Hollander D. Crohn’s disease—a permeability disorder of the tight junction? Gut. 1988;29:1621–1624. doi: 10.1136/gut.29.12.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma TY. Intestinal epithelial barrier dysfunction in Crohn’s disease. Proc Soc Exp Biol Med. 1997;214:318–327. doi: 10.3181/00379727-214-44099. [DOI] [PubMed] [Google Scholar]
- Madara JL. Loosening tight junctions. Lessons from the intestine. J Clin Invest. 1989;83:1089–1094. doi: 10.1172/JCI113987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning AS, Mitic LL, Anderson JM. Transmembrane proteins in the tight junction barrier. J Am Soc Nephrol. 1999;10:1337–1345. doi: 10.1681/ASN.V1061337. [DOI] [PubMed] [Google Scholar]
- Anderson JM. Molecular structure of tight junctions and their role in epithelial transport. News Physiol Sci. 2001;16:126–130. doi: 10.1152/physiologyonline.2001.16.3.126. [DOI] [PubMed] [Google Scholar]
- Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balda MS, Matter K. Transmembrane proteins of tight junctions. Semin Cell Dev Biol. 2000;11:281–289. doi: 10.1006/scdb.2000.0177. [DOI] [PubMed] [Google Scholar]
- Turner JR. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am J Pathol. 2006;169:1901–1909. doi: 10.2353/ajpath.2006.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy KM, Skare IB, Stankewich MC, Furuse M, Tsukita S, Rogers RA, Lynch RD. Schneeberger EE: Occludin is a functional component of the tight junction. J Cell Sci. 1996;109:2287–2298. doi: 10.1242/jcs.109.9.2287. [DOI] [PubMed] [Google Scholar]
- Yu AS, McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, Lynch RD, Schneeberger EE. Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am J Physiol. 2005;288:C1231–C1241. doi: 10.1152/ajpcell.00581.2004. [DOI] [PubMed] [Google Scholar]
- Chen Y, Merzdorf C, Paul DL, Goodenough DA. COOH terminus of occludin is required for tight junction barrier function in early Xenopus embryos. J Cell Biol. 1997;138:891–899. doi: 10.1083/jcb.138.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke H, Soler AP, Mullin JM. Protein kinase C activation leads to dephosphorylation of occludin and tight junction permeability increase in LLC-PK1 epithelial cell sheets. J Cell Sci. 2000;113:3187–3196. doi: 10.1242/jcs.113.18.3187. [DOI] [PubMed] [Google Scholar]
- Farshori P, Kachar B. Redistribution and phosphorylation of occludin during opening and resealing of tight junctions in cultured epithelial cells. J Membr Biol. 1999;170:147–156. doi: 10.1007/s002329900544. [DOI] [PubMed] [Google Scholar]
- Rao RK, Basuroy S, Rao VU, Karnaky KJ, Jr, Gupta A. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem J. 2002;368:471–481. doi: 10.1042/BJ20011804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Mrsny RJ. Oncogenic Raf-1 disrupts epithelial tight junctions via downregulation of occludin. J Cell Biol. 2000;148:791–800. doi: 10.1083/jcb.148.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathiram P, Gaffin SL, Brock-Utne JG, Wells MT. Time course of endotoxemia and cardiovascular changes in heat-stressed primates. Aviat Space Environ Med. 1987;58:1071–1074. [PubMed] [Google Scholar]
- Gathiram P, Wells MT, Raidoo D, Brock-Utne JG, Gaffin SL. Portal and systemic plasma lipopolysaccharide concentrations in heat-stressed primates. Circ Shock. 1988;25:223–230. [PubMed] [Google Scholar]
- Hall DM, Buettner GR, Oberley LW, Xu L, Matthes RD, Gisolfi CV. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am J Physiol. 2001;280:H509–H521. doi: 10.1152/ajpheart.2001.280.2.H509. [DOI] [PubMed] [Google Scholar]
- Shapiro Y, Alkan M, Epstein Y, Newman F, Magazanik A. Increase in rat intestinal permeability to endotoxin during hyperthermia. Eur J Appl Physiol Occup Physiol. 1986;55:410–412. doi: 10.1007/BF00422742. [DOI] [PubMed] [Google Scholar]
- Brock-Utne JG, Gaffin SL, Wells MT, Gathiram P, Sohar E, James MF, Morrell DF, Norman RJ. Endotoxaemia in exhausted runners after a long-distance race. S Afr Med J. 1988;73:533–536. [PubMed] [Google Scholar]
- Gathiram P, Wells MT, Brock-Utne JG, Gaffin SL. Antilipopolysaccharide improves survival in primates subjected to heat stroke. Circ Shock. 1987;23:157–164. [PubMed] [Google Scholar]
- Gathiram P, Wells MT, Brock-Utne JG, Gaffin SL. Prophylactic corticosteroid increases survival in experimental heat stroke in primates. Aviat Space Environ Med. 1988;59:352–355. [PubMed] [Google Scholar]
- Bouchama A, Parhar RS, el-Yazigi A, Sheth K, al-Sedairy S. Endotoxemia and release of tumor necrosis factor and interleukin 1 alpha in acute heatstroke. J Appl Physiol. 1991;70:2640–2644. doi: 10.1152/jappl.1991.70.6.2640. [DOI] [PubMed] [Google Scholar]
- Gathiram P, Wells MT, Brock-Utne JG, Wessels BC, Gaffin SL. Prevention of endotoxaemia by non-absorbable antibiotics in heat stress. J Clin Pathol. 1987;40:1364–1368. doi: 10.1136/jcp.40.11.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton KD, Wischmeyer PE. Oral glutamine enhances heat shock protein expression and improves survival following hyperthermia. Shock. 2006;25:295–299. doi: 10.1097/01.shk.0000196548.10634.02. [DOI] [PubMed] [Google Scholar]
- Dokladny K, Moseley PL, Ma TY. Physiologically relevant increase in temperature causes an increase in intestinal epithelial tight junction permeability. Am J Physiol. 2006;290:G204–G212. doi: 10.1152/ajpgi.00401.2005. [DOI] [PubMed] [Google Scholar]
- Dokladny K, Wharton W, Lobb R, Ma TY, Moseley PL. Induction of physiological thermotolerance in MDCK monolayers: contribution of heat shock protein 70. Cell Stress Chaperones. 2006;11:268–275. doi: 10.1379/CSC-194R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo IJ, Raub TJ, Borchardt RT. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989;96:736–749. [PubMed] [Google Scholar]
- Wick DA, Seetharam B, Dahms NM. Biosynthesis and secretion of the mannose 6-phosphate receptor and its ligands in polarized Caco-2 cells. Am J Physiol. 1999;277:G506–G514. doi: 10.1152/ajpgi.1999.277.3.G506. [DOI] [PubMed] [Google Scholar]
- Cullen BR, Malim MH. Secreted placental alkaline phosphatase as a eukaryotic reporter gene. Methods Enzymol. 1992;216:362–368. doi: 10.1016/0076-6879(92)16033-g. [DOI] [PubMed] [Google Scholar]
- Ye D, Ma I, Ma TY. Molecular mechanism of tumor necrosis factor-alpha modulation of intestinal epithelial tight junction barrier. Am J Physiol. 2006;290:G496–G504. doi: 10.1152/ajpgi.00318.2005. [DOI] [PubMed] [Google Scholar]
- Overbergh L, Giulietti A, Valckx D, Decallonne R, Bouillon R, Mathieu C. The use of real-time reverse transcriptase PCR for the quantification of cytokine gene expression. J Biomol Tech. 2003;14:33–43. [PMC free article] [PubMed] [Google Scholar]
- Serio KJ, Reddy KV, Bigby TD. Lipopolysaccharide induces 5-lipoxygenase-activating protein gene expression in THP-1 cells via a NF-kappaB and C/EBP-mediated mechanism. Am J Physiol. 2005;288:C1125–C1133. doi: 10.1152/ajpcell.00296.2004. [DOI] [PubMed] [Google Scholar]
- Chen Y, Lu Q, Schneeberger EE, Goodenough DA. Restoration of tight junction structure and barrier function by down-regulation of the mitogen-activated protein kinase pathway in ras-transformed Madin-Darby canine kidney cells. Mol Biol Cell. 2000;11:849–862. doi: 10.1091/mbc.11.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou Elela S, Nazar RN. Role of the 5.8S rRNA in ribosome translocation. Nucleic Acids Res. 1997;25:1788–1794. doi: 10.1093/nar/25.9.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadkins RM, Jovin TM. Actinomycin D and 7-aminoactinomycin D binding to single-stranded DNA. Biochemistry. 1991;30:9469–9478. doi: 10.1021/bi00103a012. [DOI] [PubMed] [Google Scholar]
- Morimoto RI. Cells in stress: transcriptional activation of heat shock genes. Science. 1993;259:1409–1410. doi: 10.1126/science.8451637. [DOI] [PubMed] [Google Scholar]
- Sarge KD, Murphy SP, Morimoto RI. Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress. Mol Cell Biol. 1993;13:1392–1407. doi: 10.1128/mcb.13.3.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai N, Nakai A, Nagata K. Quercetin suppresses heat shock response by down regulation of HSF1. Biochem Biophys Res Commun. 1995;208:1099–1105. doi: 10.1006/bbrc.1995.1447. [DOI] [PubMed] [Google Scholar]
- Kim D, Kim SH, Li GC. Proteasome inhibitors MG132 and lactacystin hyperphosphorylate HSF1 and induce hsp70 and hsp27 expression. Biochem Biophys Res Commun. 1999;254:264–268. doi: 10.1006/bbrc.1998.9840. [DOI] [PubMed] [Google Scholar]
- Hosokawa N, Hirayoshi K, Kudo H, Takechi H, Aoike A, Kawai K, Nagata K. Inhibition of the activation of heat shock factor in vivo and in vitro by flavonoids. Mol Cell Biol. 1992;12:3490–3498. doi: 10.1128/mcb.12.8.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, He H, Yu L, Xia HH, Lin MC, Gu Q, Li M, Zou B, An X, Jiang B, Kung HF, Wong BC. HSF1 down-regulates XAF1 through transcriptional regulation. J Biol Chem. 2006;281:2451–2459. doi: 10.1074/jbc.M505890200. [DOI] [PubMed] [Google Scholar]
- Lis J, Wu C. Protein traffic on the heat shock promoter: parking, stalling, and trucking along. Cell. 1993;74:1–4. doi: 10.1016/0092-8674(93)90286-y. [DOI] [PubMed] [Google Scholar]
- Voellmy R. Transduction of the stress signal and mechanisms of transcriptional regulation of heat shock/stress protein gene expression in higher eukaryotes. Crit Rev Eukaryot Gene Expr. 1994;4:357–401. [PubMed] [Google Scholar]
- Morrison AL, Dinges M, Singleton KD, Odoms K, Wong HR, Wischmeyer PE. Glutamine’s protection against cellular injury is dependent on heat shock factor-1. Am J Physiol. 2006;290:C1625–C1632. doi: 10.1152/ajpcell.00635.2005. [DOI] [PubMed] [Google Scholar]
- Baler R, Dahl G, Voellmy R. Activation of human heat shock genes is accompanied by oligomerization, modification, and rapid translocation of heat shock transcription factor HSF1. Mol Cell Biol. 1993;13:2486–2496. doi: 10.1128/mcb.13.4.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI, Kroeger PE, Cotto JJ. The transcriptional regulation of heat shock genes: a plethora of heat shock factors and regulatory conditions. EXS. 1996;77:139–163. doi: 10.1007/978-3-0348-9088-5_10. [DOI] [PubMed] [Google Scholar]
- Voellmy R. Sensing stress and responding to stress. EXS. 1996;77:121–137. doi: 10.1007/978-3-0348-9088-5_9. [DOI] [PubMed] [Google Scholar]
- Holmberg CI, Hietakangas V, Mikhailov A, Rantanen JO, Kallio M, Meinander A, Hellman J, Morrice N, MacKintosh C, Morimoto RI, Eriksson JE, Sistonen L. Phosphorylation of serine 230 promotes inducible transcriptional activity of heat shock factor 1. EMBO J. 2001;20:3800–3810. doi: 10.1093/emboj/20.14.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotto JJ, Kline M, Morimoto RI. Activation of heat shock factor 1 DNA binding precedes stress-induced serine phosphorylation. Evidence for a multistep pathway of regulation. J Biol Chem. 1996;271:3355–3358. doi: 10.1074/jbc.271.7.3355. [DOI] [PubMed] [Google Scholar]
- Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- Xia W, Voellmy R. Hyperphosphorylation of heat shock transcription factor 1 is correlated with transcriptional competence and slow dissociation of active factor trimers. J Biol Chem. 1997;272:4094–4102. doi: 10.1074/jbc.272.7.4094. [DOI] [PubMed] [Google Scholar]
- Guettouche T, Boellmann F, Lane WS, Voellmy R. Analysis of phosphorylation of human heat shock factor 1 in cells experiencing a stress. BMC Biochem. 2005;6:4. doi: 10.1186/1471-2091-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan DR, Xiao X, Shao L, Graves K, Benjamin IJ. Targeted disruption of heat shock transcription factor 1 abolishes thermotolerance and protection against heat-inducible apoptosis. J Biol Chem. 1998;273:7523–7528. doi: 10.1074/jbc.273.13.7523. [DOI] [PubMed] [Google Scholar]
- Malhotra V, Kooy NW, Denenberg AG, Dunsmore KE, Wong HR. Ablation of the heat shock factor-1 increases susceptibility to hyperoxia-mediated cellular injury. Exp Lung Res. 2002;28:609–622. doi: 10.1080/01902140260426724. [DOI] [PubMed] [Google Scholar]
- Wirth D, Christians E, Li X, Benjamin IJ, Gustin P. Use of Hsf1(−/−) mice reveals an essential role for HSF1 to protect lung against cadmium-induced injury. Toxicol Appl Pharmacol. 2003;192:12–20. doi: 10.1016/s0041-008x(03)00256-4. [DOI] [PubMed] [Google Scholar]
- Xiao X, Zuo X, Davis AA, McMillan DR, Curry BB, Richardson JA, Benjamin IJ. HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. EMBO J. 1999;18:5943–5952. doi: 10.1093/emboj/18.21.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SY, Park SJ, Kwon MJ, Jeong TS, Bok SH, Choi WY, Jeong WI, Ryu SY, Do SH, Lee CS, Song JC, Jeong KS. Quercetin suppresses proinflammatory cytokines production through MAP kinases and NF-kappaB pathway in lipopolysaccharide-stimulated macrophage. Mol Cell Biochem. 2003;243:153–160. doi: 10.1023/a:1021624520740. [DOI] [PubMed] [Google Scholar]
- Liu J, Li X, Yue Y, Li J, He T, He Y. The inhibitory effect of quercetin on IL-6 production by LPS-stimulated neutrophils. Cell Mol Immunol. 2005;2:455–460. [PubMed] [Google Scholar]
- Comalada M, Camuesco D, Sierra S, Ballester I, Xaus J, Galvez J, Zarzuelo A. In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-kappaB pathway. Eur J Immunol. 2005;35:584–592. doi: 10.1002/eji.200425778. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Sugiyama H, Stylianou E, Kitamura M. Bioflavonoid quercetin inhibits interleukin-1-induced transcriptional expression of monocyte chemoattractant protein-1 in glomerular cells via suppression of nuclear factor-kappaB. J Am Soc Nephrol. 1999;10:2290–2296. doi: 10.1681/ASN.V10112290. [DOI] [PubMed] [Google Scholar]
- Gulati N, Laudet B, Zohrabian VM, Murali R, Jhanwar-Uniyal M. The antiproliferative effect of quercetin in cancer cells is mediated via inhibition of the PI3K-Akt/PKB pathway. Anticancer Res. 2006;26:1177–1181. [PubMed] [Google Scholar]
- Braganhol E, Zamin LL, Canedo AD, Horn F, Tamajusuku AS, Wink MR, Salbego C, Battastini AM. Antiproliferative effect of quercetin in the human U138MG glioma cell line. Anticancer Drugs. 2006;17:663–671. doi: 10.1097/01.cad.0000215063.23932.02. [DOI] [PubMed] [Google Scholar]
- Chow JM, Shen SC, Huan SK, Lin HY, Chen YC. Quercetin, but not rutin and quercitrin, prevention of H2O2-induced apoptosis via anti-oxidant activity and heme oxygenase 1 gene expression in macrophages. Biochem Pharmacol. 2005;69:1839–1851. doi: 10.1016/j.bcp.2005.03.017. [DOI] [PubMed] [Google Scholar]