Abstract
Intestinal radiation injury is a dose-limiting factor in radiation therapy for abdominal and pelvic cancers. Because transforming growth factor-β1 is a key mediator involved in radiation-induced damage, we hypothesized that its target gene, plasminogen activator inhibitor type 1 (PAI-1), is an essential mediator of intestinal radiation toxicity. In a model of radiation enteropathy, survival was monitored and intestinal radiation injury was assessed in both wild-type (Wt) and PAI-1 knockout mice. Immunohistochemical labeling of PAI-1 was also assessed in patients treated with preoperative radiotherapy for rectal adenocarcinoma. Finally, the molecular mechanisms involved in radiation-induced PAI-1 expression were investigated. We found that PAI-1 −/− mice exhibited increased survival and better intestinal function compared with Wt mice. Intestinal radiation injury was attenuated in irradiated PAI-1 −/− mice compared with irradiated Wt mice, and irradiation increased blood cell-endothelial cell interactions in Wt but not PAI-1 −/− mice. In vivo, radiation-induced intestinal damage in mice, as well as in patients treated with radiotherapy, was associated with the up-regulation of PAI-1 in the endothelium. In vitro, irradiation increased PAI-1 expression in endothelial cells by a p53/Smad3-dependent mechanism. Together, these data demonstrate that PAI-1 plays a critical role in radiation-induced intestinal damage, suggesting that PAI-1 is an attractive target for preventing or reducing the side effects of radiation therapy.
More than half of cancers are treated with radiation therapy alone or in combination with surgery or chemotherapy or both. Radiation-induced normal tissue injury is a dose-limiting factor in the treatment of cancer with radiotherapy. Early and late side effects not only limit radiation dose escalation but might also affect the patient’s quality of life. Acute radiation-induced bowel damage affects most patients treated by radiotherapy for pelvic cancer.1 Intestinal radiation toxicity is characterized by mucosal injury, inflammation, and vascular activation, followed by development of progressive vascular fibrosis/sclerosis and radiation enteritis. Early phases of radiation-induced fibrogenesis are characterized by an orchestrated wound-healing response initiated by events that include activation of coagulation system, inflammation, mucosal repair, granulation tissue formation, and extracellular matrix remodeling.2 This complex and integrated response involves a large number of molecular pathways activated by pro- and anti-inflammatory cytokines, chemokines, and growth factors. Among these factors, transforming growth factor (TGF)-β1 is considered a key fibrogenic cytokine involved in radiation-induced intestinal damage.3,4 TGF-β1 up-regulates expression of several molecules that could contribute to acute and late radiation damage. Among them, we hypothesized that plasminogen activator inhibitor type-1 (PAI-1) is a critical mediator that triggers pathways leading to acute and late normal tissue lesions after irradiation. PAI-1 belongs to the family of serine protease inhibitors and is the main inhibitor of fibrinolysis. Both plasminogen activators convert plasminogen to plasmin, which degrades insoluble fibrin. PAI-1 inhibits plasminogen activators and thus reduces plasmin generation.5 PAI-1 not only reduces fibrinolysis but also plays a role in extracellular matrix remodeling by reducing plasmin-dependent activation of matrix metalloproteinases.
In pathological conditions, PAI-1 is produced by a variety of cell types such as hepatocytes, adipocytes, smooth muscle cells, platelets, and especially endothelial cells (ECs). The endothelium is known to play a critical role in radiation-induced bowel injury.6 Irradiated ECs acquire a proinflammatory, procoagulant, and prothrombotic phenotype. Up-regulation of EC adhesion molecules after irradiation leads to increased leukocyte-EC interactions and leukocyte transmigration.7,8 Moreover, irradiation increases the interactions of platelets9 with the endothelium and decreases expression of the anticoagulant thrombomodulin.10 This loss of vascular thromboresistance of the endothelium following radiation is a result of increased fibrinogenesis and decreased fibrinolysis.6 In this context, the role of PAI-1 in radiation-induced damage could be crucial.
In this study, we analyzed the effects of PAI-1 genetic deficiency on the intestinal response to radiation exposure. Immunolabeling of PAI-1 was performed in tissues from patients treated with radiotherapy for rectal adenocarcinoma, and molecular mechanisms involved in PAI-1 expression after irradiation in ECs were investigated.
Materials and Methods
Animals and Irradiation Procedure
Experiments were conducted in compliance with legal regulations in France for animal experimentation, and protocols were approved by the ethics committee for animal experimentation of the Institute for Radiological Protection and Nuclear Safety. Intestinal radiation injury was performed after exposure of an intestinal segment to 19 Gy of radiation.4 Briefly, wild-type C57BL/6J (PAI-1 +/+) and PAI-1 −/− mice (Charles River Laboratories, l’Arbresle, France) were anesthetized, and a 3-cm-long intestinal segment (10 cm from the ileocecal valve) was exteriorized and exposed to a single dose of 19 Gy of gamma irradiation (60Co source, 4000 Ci, dose rate 1.4 Gy/minute). Sham irradiation was performed by maintaining the intestinal segment exteriorized without radiation exposure. After radiation exposure or sham irradiation, the exposed segment was returned to the abdominal cavity and peritoneum/abdominal muscles and skin were separately closed with interrupted sutures.
Histology and Immunohistochemistry
The totality of the intestinal segment was assessed for histology. Longitudinal sections of the intestine were fixed in 4% formaldehyde solution and embedded in paraffin. Slides were stained with hematoxylin-eosin-saffron and Sirius red. Radiation injury was determined using a described and validated radiation injury scoring system3,4 administered independently by two authors in a blinded manner, and discrepancies were resolved by discussion. CD45 immunostaining was performed using rat anti-mouse common leukocyte antigen (BD Bioscience, Erembodegem, Belgium). von Willebrand factor (rabbit anti-human von Willebrand factor; Dakocytomation, Trappes, France) and PAI-1 (rabbit anti-mouse PAI-1; Abcam, Trappes, France) immunostaining was performed on frozen sections. Slides were then incubated with goat anti-rabbit IgG tagged with Alexa Fluor 488 (Molecular Probes, Cergy-Pontoise, France) and counterstained with 4,6-diamidino-2-phenylindole.
Ussing Chamber Experiments
Tissue samples were mounted in Ussing chambers and were bathed with a modified Krebs buffer under a 95% O2/5% CO2 atmosphere. Tissue responses to electrical field stimulation and carbachol were measured by clamping the potential difference to 0 mV, under short circuit current (Isc) conditions with a voltage-clamp apparatus (World Precision Instruments, Aston, UK). Electrical field stimulation (35 Hz) was applied with a dual impedance stimulator. Carbachol (final concentration 5.10−5 mol/L) was added on the serosal side. Each tissue was submitted to 35 Hz electrical field stimulation and subsequent carbachol stimulation.
Intravital Microscopy
Intravital microscopy was used to quantify rolling and adhesion of leukocytes and platelets in the mesenteric venules from wild-type (Wt) and PAI-1 −/− mice after an abdominal single exposure of 15 Gy. Twenty-four hours after irradiation, mice were anesthetized by intraperitoneal injection of 2.5% tribromoethanol, and leukocytes and platelets were stained by intravenous administration of 10.4% rhodamine 6G. The mesentery was exteriorized and placed in a 5% gelatin-coated Plexiglas chamber for observation of the mesenteric microcirculation. Venules of 100 to 250 μm diameter were selected for direct observation of leukocyte- and platelet-endothelium interactions as previously described.9 Venules were filmed for 5 to 10 minutes and video recording was resumed for another venule (up to four vessels were analyzed for each mouse). Blood cell-endothelial interactions (rolling and adhesion) were quantified using the software Histolab 4.3.6 (Microvision Instruments, Evry, France). A platelet was considered to be adherent when it was stationary on the endothelium for more than 2 minutes (definitive stops). Leukocyte- and platelet-endothelium interactions were analyzed within a 50 × 50 μm2 square and quantifications were normalized thereafter per 0.01 mm2/minute.
Cell Culture and Irradiation
Human umbilical endothelial cells (HUVECs) were obtained from Cambrex and cultured in EGM-2 MV culture medium (Cambrex, Verviers, Belgium). Cells were used between passages 4 and 6 and were irradiated with a 137Cs source (dose rate 1 Gy/minute).
RNA Isolation and Real-Time PCR
RNA isolation and real-time PCR were performed as previously described.11 The following primers were used: human PAI-1,11 and mouse PAI-1 (gene expression assay Mm 0435860_m1; Applied Biosystems, Courtaboeuf, France). Significant PCR fluorescent signals were normalized to a PCR fluorescent signal obtained from the housekeeping gene GAPDH (glyceraldehyde-3-phosphate dehydrogenase) or ribosomal RNA 18S for each sample.
Plasmids
Expression vectors for 6xMyc-Smad2, 6xMyc-Smad3, 6xMyc-Smad4, Flag-Smad2, Flag-mad3, Flag-Smad7, (CAGA)9-Luc, WtPAI-1 Luc, and Δb123-PAI-1 Luc were previously described.12,13
Reporter Gene Assay
HUVECs were transiently cotransfected with expression vector reporter and pRL-TK plasmids using FuGENE (Roche Diagnostics, Meylan, France) as transfection reagent. Forty-eight hours after transfection, cell media were changed, and cells were irradiated at 2 or 10 Gy. Relative luciferase activity (ratio firefly/Renilla) was measured 24 hours after irradiation. Cell extracts were prepared for the dual-luciferase reporter assay system according to the manufacturer’s instructions (Promega, Charbonnières, France). Relative luciferase activity was measured using a Mithras luminometer (Berthold Technologies, Bad Wildbad, Germany).
Protein Extraction and Immunoblotting
Protein extraction and Western blot were performed as previously described.11 Nuclear protein extracts were prepared using the method of Schreiber.14 The following protein-specific primary antibodies were used: anti-PAI-1 (Novocastra Laboratories Ltd, New Castle, UK), anti-Smad2, anti-Smad3 (Zymed Laboratories, Cergy Pontoise, France), anti-Smad4, anti-p53, anti-Myc (Santa Cruz Biotechnology, Heidelberg, Germany), anti-Phospho Smad3, anti-Phospho Smad2 (Cell Signaling Technology, St. Quentin Falavier, France), anti-FlagM2 (Sigma, St. Quentin, France), and anti-GAPDH (Biodesign, Portland, OR).
Immunoprecipitation
Cells were lysed at 4°C in lysis buffer (10 mmol/L Tris, pH 8.8, 150 mmol/L NaCl, 1 mmol/L EDTA, 1% sodium deoxycholate, 1 mmol/L sodium vanadate, 0.1% SDS, 1% Igepal (Sigma), and protease inhibitor cocktail). Cell lysates were subjected to immunoprecipitation with appropriate antibodies overnight followed by adsorption to Sepharose protein G for 1 hour. Immunoprecipitates were washed several times in lysis buffer and then heated for 5 minutes in Laemmli buffer. Samples were separated by SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. The membranes were blotted with primary antibodies followed by incubation with secondary antibody horseradish peroxidase-conjugated (Amersham, Orsay, France). Blots were developed using the enhanced chemiluminescence method (Amersham).
RNA Interference
The sequence of small-interfering RNAs (siRNAs) designed to specifically target Smad3 is 5′-ACCUAUCCCCGAAUCCGAUdTdT-3′11 and the siRNAs were purchased from Eurogentec (Angers, France). siRNAs targeting human p53 were obtained from Santa Cruz Biotechnology. A nontargeted negative control duplex was used as control (Eurogentec).
Patients, Radiation Injury Score, and Immunohistology
Formalin-fixed, paraffin-embedded tissue samples were obtained following institutional ethics (Gustave Roussy Institute) and French Medical Research Council guidelines. Twenty-five patients treated for rectal adenocarcinoma with preoperative radiotherapy (45 Gy; 2 or 1.8 Gy by fraction) were included in this study. Tumors were surgically resected 5 to 7 weeks post-treatment. For each patient, specimens of normal tissue were taken in the irradiated field adjacent to the tumor and from microscopically normal mucosa distant from the tumor. Slides were stained with hematoxylin-eosin-saffron and radiation injury score was determined independently by two authors as previously described in detail.11 For immunohistochemistry, sections were used to immunolocalize PAI-1 (Novocastra Laboratories Ltd) and phospho-(ser433/435) Smad2/3 (Santa Cruz Biotechnology). Biotinylated rabbit anti-mouse IgG and a streptavidin/biotinylated peroxidase kit (Dakocytomation) were used before staining with the NovaRED substrate kit (Vector) and counterstaining with hematoxylin.
Statistical Analyses
Data are given as means ± SEM. Statistical analyses were performed by analysis of variance or Student’s t-test with a level of significance of P < 0.05. For intravital microscopy analyses, statistical significance was assessed by the Mann-Whitney rank sum test. Mouse survival curves were calculated by the Kaplan-Meier method and compared using the log rank test.
Results
PAI-1 −/− Mice are Protected Against Radiation-Induced Intestinal Damage
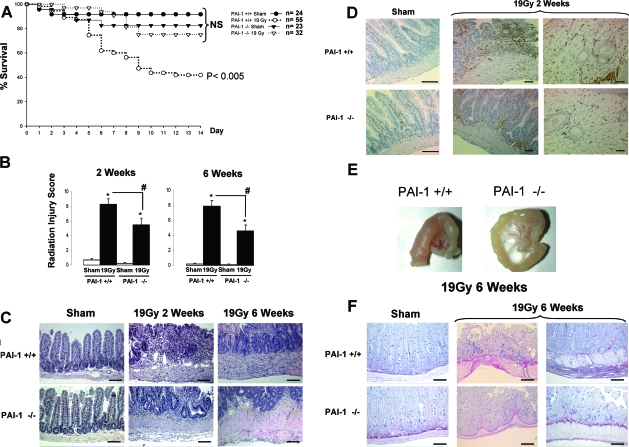
The survival of PAI-1 −/− mice is increased compared with Wt mice following radiation exposure (Figure 1A). Two weeks after irradiation, 75% of PAI-1 −/− mice were alive compared with 41% of Wt mice, and this difference remained the same after six weeks. Irradiation caused significant radiation injury in Wt and PAI-1 −/− mice (Figure 1B). However, acute and late radiation injuries are more severe in Wt mice than in PAI-1 −/− mice (+35% at two weeks and +45% at six weeks, P < 0.05). At two weeks, PAI-1 −/− tissues were characterized by a better epithelial cover associated with numerous hyperproliferating crypts and visibly reduced density of inflammatory cells compared with Wt mice (Figure 1C). The reduced inflammation in mucosal and mesenteric compartments observed in irradiated PAI-1 −/− mice compared with irradiated Wt mice was confirmed by CD45 labeling (Figure 1D). At six weeks, a reduction in fibrosis was observed in PAI-1 −/− mice (Figure 1E). We also observed marked remodeling of extracellular matrix in Wt mice associated with a pronounced intestinal wall fibrosis mainly in muscularis propria infiltrated with collagen and subserosal fibrosis (Figure 1F).
Figure 1.
PAI-1 −/− mice are protected against intestinal irradiation damage. A jejunal segment from PAI-1 +/+ and PAI-1 −/− mice was exposed to a single dose of 19Gy. A: Kaplan-Meier analyses represent the percent survival of PAI-1 +/+ and PAI-1 −/− mice. P < 0.005 versus the three other groups. B: The totality of the intestinal segment was assessed for histology and radiation injury was determined using a radiation injury scoring system. Radiation injury score was measured two and six weeks after irradiation, n = 12 mice per group, *P < 0.05 versus PAI-1 +/+ sham mice, #P < 0.05 versus PAI-1 +/+ irradiated mice. C: Representative microscopic alterations obtained in PAI-1 +/+ and PAI-1 −/− mice are shown. Slides were stained with hematoxylin-eosin-saffron coloration. D: Representative CD45 immunolabeling is shown. E: Representative macroscopic features seen six weeks after irradiation are shown. F: Representative microscopic alterations (Sirius red coloration) seen six weeks after irradiation are shown. Scale bars = 100 μm.
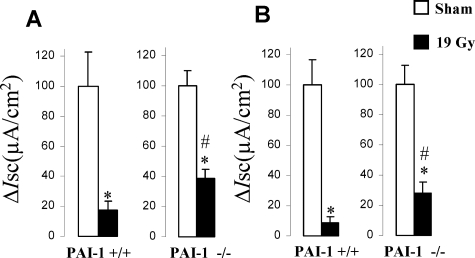
To confirm whether the attenuation of radiation injury in PAI-1 −/− mice is related to better intestinal function, we measured intestinal ion transport capacity three days after irradiation, ie, before the separation of the survival curves. No differences in basal parameters were observed between sham Wt mice and PAI-1 −/− mice. Intestinal ion transport capacity was reduced in both types of mice after irradiation, but intestinal dysfunction was more pronounced in Wt mice (Figure 2). Intestinal ion transport capacity decreased by 83% in Wt mice and 62% in PAI-1 −/− mice following electric stimulation and by 92% in Wt mice and 72% in PAI-1 −/− mice following carbachol stimulation. Two groups without intestinal exteriorization were assessed (Wt and PAI-1 −/− n = 10 per group), and no differences were observed between these groups or compared with the sham groups (data not shown).
Figure 2.
Acute intestinal function is better in PAI-1 −/− than in PAI-1 +/+ mice after irradiation. Ussing chamber experiments were used to evaluate intestinal ion transport capacity in PAI-1 +/+ and PAI-1 −/− mice exposed to a localized dose of 19 Gy. Percent inhibition of Isc in response to (A) electrical field stimulation (35 Hz) or (B) exogenous carbachol stimulation in irradiated tissues from PAI-1 +/+ and PAI-1 −/− mice three days after irradiation. Results are means ± SEM with n = 13 to 17 mice per group, *P < 0.05 versus sham mice; #P < 0.05 versus PAI-1 +/+ irradiated mice.
PAI-1 Expression Is Increased In Vivo After Irradiation
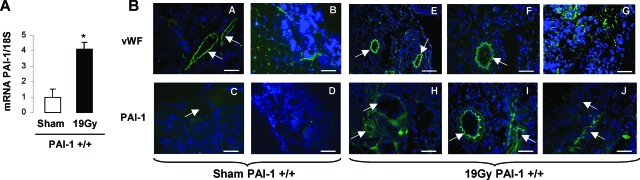
We next examined whether intestinal radiation toxicity is associated with modification of PAI-1 expression in Wt mice. PAI-1 mRNA intestinal level was increased fourfold (P < 0.05) in Wt irradiated mice compared with Wt sham mice (Figure 3A). von Willebrand factor and PAI-1 immunolabeling showed PAI-1 overexpression in endothelial cells from mesenteric vessels (Figure 3B: a, c, e, f, h, and i) and the mucosal microcirculation (Figure 3B: b, d, g, and j).
Figure 3.
PAI-1 expression is increased in vivo after irradiation. A: PAI-1 mRNA expression was measured three days after irradiation (n = 10 mice per group, *P < 0.05). B: Frozen sections of PAI-1 +/+ intestine (three days after irradiation) were stained with antibodies against von Willebrand factor or PAI-1 (green) and counterstained with 4,6-diamidino-2-phenylindole (blue). Shown are endothelial cells from mesenteric vessels (a, c, e, f, h, and i) and the mucosal circulation (b, d, g, and j). Representative images are shown and arrows indicate vessels. Scale bars = 100 μm.
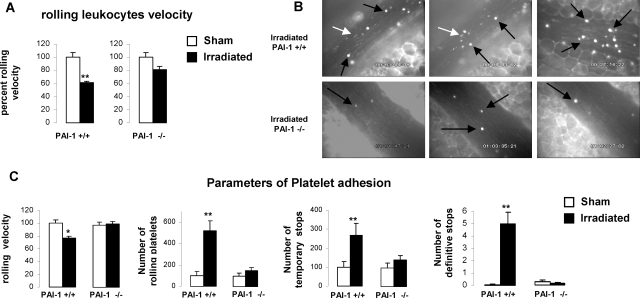
Irradiation Increases Blood Cell/Endothelial Cell Interactions in Wt Mice but Not in PAI-1 −/− Mice
Increased leukocyte-platelet interaction with the endothelium was shown after irradiation.8,9 We hypothesized that PAI-1 could participate in this physiological response following radiation. We investigated blood cell-endothelium interactions in Wt and PAI-1 −/− mice to confirm this hypothesis. To avoid the putative effect of surgery in the model of exteriorized intestinal segment, leukocyte/platelet-endothelium interactions were studied using intravital microscopy 24 hours after abdominal irradiation of 15 Gy. Leukocyte and platelet rolling velocities, as well as number of rolling platelets and number of temporary and permanent arrest of platelets, were determined. Because no statistical differences were observed for any parameters measured between nonirradiated Wt and PAI−/−, we chose to present the results as a percentage of each parameter for each strain except for definitive arrest of platelets. Blood counts (leukocytes and platelets) were determined in nonirradiated and irradiated Wt and PAI-1 −/− mice and no differences were observed (data not shown). Moreover, venular wall shear rate was determined under baseline conditions or after irradiation in both type of mice and no differences were observed (data not shown). Irradiation decreased the rolling velocity of leukocytes and platelets in Wt mice, but not in PAI-1 −/− mice (Figure 4, A–C). A strong increase in the number of rolling platelets and the number of temporary and definitive arrests of platelets was observed in irradiated Wt mice compared with nonirradiated Wt mice (Figure 4C). Interestingly, irradiation has no effect on rolling velocity of platelets or on other parameters of platelet adhesion in PAI-1 −/− mice.
Figure 4.
Irradiation increases blood cell-endothelium interactions in PAI-1 +/+ but not in PAI-1 −/− mice. Intravital microscopy was used to quantify rolling and adhesion of leukocytes and platelets in the mesenteric venules from PAI-1 +/+ and PAI-1 −/− mice (n = 6 mice per group) after an abdominal single exposure of 15 Gy. The mesentery was exteriorized and blood cell-endothelial cell interactions (rolling and adhesion) were quantified 24 hours after irradiation. A: Rolling velocity of leukocytes in PAI-1 +/+ and PAI-1 −/− mice 24 hours after irradiation (n = 6 mice per group). B: Representative images from three irradiated PAI-1 +/+ (top) and three PAI-1 −/− mice (bottom) are shown. The black arrows indicate leukocytes and the white arrows indicate platelets. C: Parameters of platelet adhesion. Results are means ± SEM. Statistical significance was assessed using the Mann-Whitney rank sum test. *P < 0.05, **P < 0.001.
Radiation-Induced Rectal Damage in Humans Is Associated with Overexpression of PAI-1 in the Endothelium
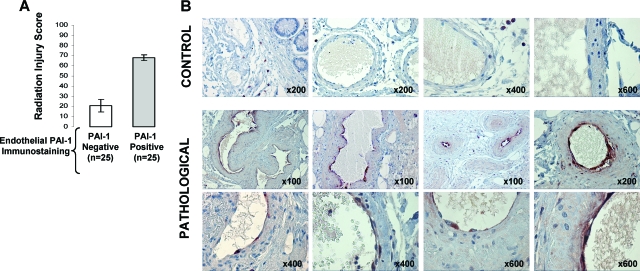
To demonstrate the physiological significance of our results, we undertook a retrospective study in patients. Radiation injury scoring and immunohistochemical staining of PAI-1 were performed for tissue samples from patients treated by radiotherapy for rectal adenocarcinoma. Radiation-induced normal tissue damage was characterized by strong expression of PAI-1 in the endothelium (Figure 5, A–B), suggesting that the endothelial PAI-1 pool plays an important role in rectal damage after radiotherapy.
Figure 5.
Radiation-induced normal tissue damage in humans is associated with overexpression of PAI-1 in the endothelium. A: Radiation injury score was determined in 25 patients treated by radiotherapy for rectal adenocarcinoma. For each patient, tissue specimens were taken in the irradiated field adjacent to the tumor (pathological) and from microscopically normal mucosa distant from the tumor (control). Immunohistochemical staining of PAI-1 was performed and slides were separated according to the positivity of PA1-1 expression in the endothelium. B: Representative microscopic images from control and pathological submucosal vessels are shown.
In Vitro, Irradiation Increases PAI-1 Expression in ECs by a Smad3-Dependent Pathway
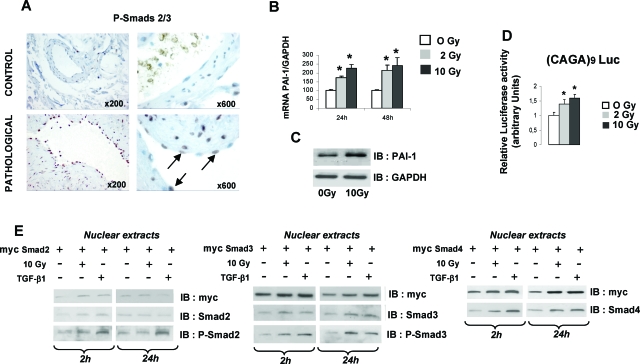
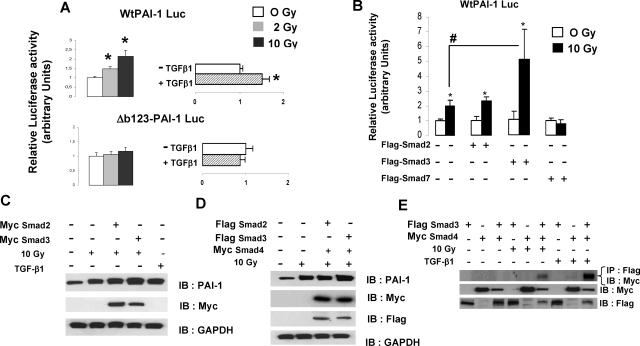
The TGF-β/Smad pathway plays a key role in the control of PAI-1 expression in various cell types under different conditions. Activated Smad2/3 was immunolabeled in tissues from patients treated with radiotherapy (Figure 6A). We observed an increased expression of phospho-Smad2/3 in the endothelium in pathological tissues compared with control tissues suggesting that, in vivo, the Smad pathway may be involved in PAI-1 overexpression in ECs. PAI-1 mRNA and protein levels were increased in HUVECs (Figure 6, B–C). Smad-dependent transcription was performed using TGF-β/Smad-responsive reporter CAGA9-luc, and the results showed that irradiation activates Smad-dependent transcription in ECs (Figure 6D). Nuclear translocation of Smad2, 3, and 4 was followed by immunoblotting using Myc-tagged versions of Smads. Nuclear translocation of Smad2, Smad3, and Smad4 as well as phosphorylated Smad2 and Smad3 was stimulated two hours after irradiation (Figure 6E). However, 24 hours after irradiation, we observed increased nuclear expression of Smad3 and Smad4, but not Smad2. To provide evidence that the Smad pathway is involved in radiation-induced PAI-1 transcription, HUVECs were transfected with Wt human PAI-1-luc reporter or human PAI-1-luc reporter with mutation of the three CAGA boxes (Δb123-PAI-1 Luc).12 Irradiation stimulated luciferase activity in ECs transfected with WtPAI-1-luc, but not with Δb123-PAI-1 Luc, demonstrating that at least one CAGA box is involved in radiation-induced PAI-1 transcription (Figure 7A). To investigate the role of Smad in radiation-induced PAI-1 transcription, ECs were cotransfected with Flag-tagged Smad2, Smad3, or Smad7 and WtPAI-1-luc. In EC-overexpressing Smad7, the radiation-induced PAI-1 transcription was inhibited, demonstrating that this inhibitory Smad abolishes the mechanism (Figure 7B). Moreover, overexpression of Smad3, but not Smad2, strongly stimulated the radiation-induced WtPAI-1-luc luciferase activity, showing that Smad3 influences radiation-induced PAI-1 transcription (Figure 7B). PAI-1 immunoblotting was performed in ECs transfected with Myc-Smad2 or Myc-Smad3. PAI-1 expression was increased in ECs overexpressing Smad3 (Figure 7C), and when we co-expressed Flag-Smad2 and Myc-Smad4 or Flag-Smad3 and Myc-Smad4, only the combination of Smad3/Smad4 led to an increase in radiation-induced PAI-1 expression (Figure 7D). We next examined whether irradiation induces Smad3 to bind Smad4. Co-immunoprecipitation experiments showed that irradiation stimulates the formation of Smad3/Smad4 complexes (Figure 7E).
Figure 6.
Irradiation activates the Smad pathway in EC. A: Immunohistochemical staining of phospho-Smad2/3 was performed in tissues from 10 patients treated by radiotherapy for rectal adenocarcinoma. Representative microscopic images from control and irradiated submucosal vessels are shown. B: PAI-1 mRNA expression was investigated by real-time PCR in HUVECs 24 hours and 48 hours after 2 or 10 Gy irradiation. Irradiation induces PAI-1 mRNA expression. Data are the mean ± SEM of four independent experiments performed in duplicate or triplicate. C: PAI-1 protein expression was measured by Western blot in HUVECs. Irradiation induces PAI-1 protein expression. Representative Western blot of three independent experiments is shown. D: HUVECs were transfected with (CAGA)9-Luc reporter plasmid 48 hours before irradiation. Relative luciferase activity was measured 24 hours after irradiation. Irradiation induces Smad-dependent transcription. Data are the mean ± SEM of three independent experiments (n = 6 by experiment). *P < 0.05 versus control. E: HUVECs were transfected with the indicated expression vector 48 hours before irradiation and nuclear expression of Smad2, Smad3, Smad4, phospho-Smad2, and phospho-Smad3 was measured by Western blot 2 hours and 24 hours after irradiation. HUVECs treated with 10 ng/ml TGF-β1 are shown.
Figure 7.
Irradiation increases PAI-1 expression in ECs by a Smad3-dependent pathway. A: HUVECs were transfected 48 hours before irradiation with WtPAI-1-Luc reporter or CAGA box-mutated PAI-1 Luc reporter (Δb123-PAI-1 Luc) and luciferase activity was measured 24 hours after irradiation. Irradiation stimulates luciferase activity in ECs transfected with WtPAI-1-luc, but not with Δb123-PAI-1 Luc. HUVECs treated with 10 ng/ml TGF-β1 for 24 hours are shown. Data are the mean ± SEM of three independent experiments (n = 6 by experiment). *P < 0.05 versus control. B: HUVECs were transiently cotransfected with the indicated expression vector and WtPAI-1-Luc reporter plasmids. Relative luciferase activity was measured 24 hours after irradiation. In ECs overexpressing Smad7, the radiation-induced PAI-1 transcription was inhibited and overexpression of Smad3, but not Smad2, stimulated the radiation-induced WtPAI-1-luc luciferase activity. C: HUVECs were transfected with the indicated expression vector and PAI-1 protein expression was measured by Western blot 24 hours after irradiation. PAI-1 expression was increased in ECs overexpressing Smad3. D: HUVECs were cotransfected with Myc-Smad4 and Flag-Smad2 or Flag-Smad3, and PAI-1 protein expression was measured by Western blot 24 hours after irradiation. Overexpression of Smad3/Smad4 led to an increase in radiation-induced PAI-1 expression. E: HUVECs were cotransfected with the indicated expression vector. Twenty-four hours after irradiation, cell lysates were immunoprecipitated (IP) with anti-Flag antibody and immunoblotted (IB) with anti-Myc. Results show that irradiation stimulates the formation of Smad3/smad4 complexes.
Smad3 and p53 Are Necessary for Radiation-Induced PAI-1 Expression
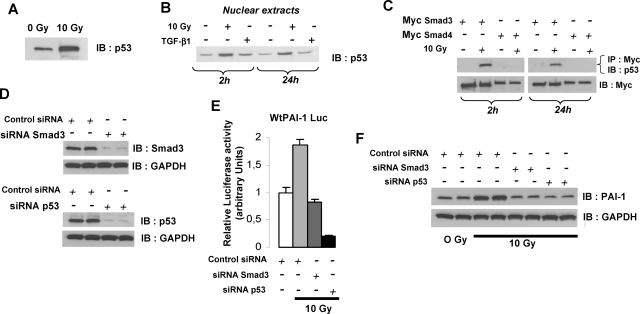
p53 plays a key role in the cell response to ionizing radiation, and a p53 binding element is present in the human PAI-1 promoter. We hypothesized that p53 is involved in PAI-1 expression in ECs after irradiation. Irradiation of ECs stimulated expression and nuclear translocation of p53 (Figure 8, A–B). To investigate the transcriptional cooperation between p53 and Smads, HUVECs were transfected with Myc-Smad3 or Myc-Smad4 and co-immunoprecipitation experiments were performed. Smad3, but not Smad4, co-immunoprecipitated with p53 after irradiation, suggesting that Smad3/p53 transcriptional cooperation could activate PAI-1 transcription (Figure 8C). To prove that Smad3 and p53 were involved, we decided to knockdown their expression using siRNA (Figure 8D). The radiation-induced WtPAI-1-luc luciferase activity was abolished in the presence of siRNA Smad3 or siRNA p53 (Figure 8E), and this effect was confirmed by Western blot (Figure 8F).
Figure 8.
Radiation-induced PAI-1 expression involves Smad3 and p53. A: p53 expression in HUVECs was measured by Western blot 24 hours after irradiation. B: Nuclear expression of p53 in HUVECs was measured by Western blot 2 hours and 24 hours after irradiation. Irradiation induces nuclear translocation of p53. HUVECs treated with 10 ng/ml TGF-β1 are shown. C: HUVECs were transfected with Myc-Smad3 or Myc-Smad4. Two hours and 24 hours after irradiation, the association of p53 with Smad3 or Smad4 was analyzed by blotting the anti-Myc immunoprecipitate with anti-p53. Smad3, but not Smad4, co-immunoprecipitated with p53 after irradiation. D: HUVECs were transfected with siRNA Smad3 or siRNA p53 and the silencing efficiency was determined by Western blot. E: HUVECs were transfected 48 hours before irradiation with WtPAI-1-Luc reporter in the presence or absence of siRNA Smad3 or siRNA p53 and luciferase activity was measured 24 hours after irradiation. The radiation-induced WtPAI-1-luc luciferase activity was abolished by siRNA Smad3- or siRNA p53-tranfected cells. F: HUVECs were transfected with siRNA Smad3 or siRNA p53 48 hours before irradiation. PAI-1 expression was measured 24 hours after irradiation.
Discussion
In this report, we demonstrate for the first time the major role of PAI-1 in radiation-induced intestinal damage. These data supply the proof of concept that PAI-1 may represent a molecular target to limit intestinal injury following radiotherapy.
Ionizing radiation increases PAI-1 expression in NRK52E cells15 and HEPG2 cells.16 Moreover, PAI-1 overexpression has been described in radiation-induced nephrosclerosis17 and in human radiation enteritis,18 suggesting a role of PAI-1 in radiation-induced normal tissue damage. We have clearly demonstrated that PAI-1 plays a crucial role in radiation-induced intestinal lesions following a high single dose of radiation. More than 50% of wild-type mice died in the ten days following radiation, as previously described.4 PAI-1 knockout mice are protected against radiation-induced intestinal damage, with a much higher proportion of surviving mice and a less severe radiation injury score than in irradiated Wt mice. These results show that genetic deficiency of PAI-1 protects against radiation-induced intestinal damage and suggest an early deleterious effect of PAI-1 in Wt mice. PAI-1 expression is rapidly induced in irradiated intestinal segments from Wt mice, and we showed that intestinal function three days after irradiation is more severely affected in Wt mice than in PAI-1 −/− mice. Moreover, PAI-1 up-regulation is observed in the endothelium. Radiation causes loss of vascular thromboresistance partly due to decreased fibrinolysis.19 PAI-1 limits fibrinolysis, and we hypothesized that PAI-1 contributes to the acute response of the endothelium to ionizing radiation. Leukocyte-endothelial cell adhesion is an early event in the leukocyte infiltration involved in the progression of chronic inflammatory diseases of the gastrointestinal tract.20 We show that radiation decreases the rolling velocity of leukocytes and platelets only in Wt mice, as previously described in mice and rats.7,8 Moreover, radiation enhanced platelet adhesion in Wt mice but not in PAI-1 −/− mice. These results show that genetic deficiency of PAI-1 limits the interactions between blood cells and the endothelium following radiation and strongly suggest that PAI-1 plays a crucial role in the vascular response to ionizing radiation. PAI-1 knockout mice are less likely to develop venous thrombi,21 whereas mice overexpressing a human transgene develop spontaneous thromboses.22 Our results suggest that radiation-induced hypofibrinolysis is abolished in PAI-1 −/− mice and consequently protects these mice against the deleterious effects of the loss of thromboresistance of the endothelium.
We observed a reduction of fibrosis in PAI-1 −/− mice six weeks after irradiation, suggesting that early effects could contribute to the delayed effects of radiation injury. A large number of strategies in experimental models show that the reduction of late effects is associated with an improvement in acute effects.23,24,25 Our results suggest that pharmacological strategies aimed to inhibit PAI-1 activity could prevent acute and late effects of radiation-induced normal tissue damage. PAI-1 is up-regulated in fibrotic diseases including hepatic, pulmonary, or renal fibrosis, and PAI-1 knockout mice are protected against fibrosis in various models.26,27,28 Here we demonstrate that radiation-induced intestinal fibrosis is less severe in PAI-1 −/− mice, suggesting that PAI-1 inhibition could be a good anti-fibrotic strategy in different models of radiation-induced fibrosis. By competing with endogenous PAI-1, a mutant noninhibitory PAI-1 was reported to reduce glomerulosclerosis29 and a pharmacological PAI-1 inhibitor protects mice against long-term thrombosis induced by nitric oxide synthase inhibition.30 Our study does not exclude the potential role of other proteases in radiation-induced damages, but the efficiency of pharmacological inhibition of PAI-1 in radiation-induced normal tissue damage is an attractive prospect to emerge from our study.
Intestinal radiation injury is associated with increased TGF-β1 expression.3 We show that PAI-1 is overexpressed in the endothelium of pathological tissue from patients treated with radiotherapy. Interestingly, radiation-induced damage in patients was associated with an increase in phosphorylated Smad 2/3 in the endothelium, suggesting that the Smad pathway may be involved in the overexpression of PAI-1 in ECs in vivo. In vitro, TGF-β1 secretion is increased in ECs after irradiation,11 and an up-regulation of TGF-β in the endothelium was observed in radiation-induced intestinal damage in rats3 and in patients treated with radiotherapy.11 Altogether, these results suggest that radiation-induced TGF-β in the endothelium can activate the Smad pathway via an autocrine loop. PAI-1 expression is increased in ECs following radiation, and we demonstrate that radiation-induced PAI-1 transcription in ECs is Smad3-dependent. These results confirm and support the concept that Smad3-targeted genes are critical mediators of radiation-induced damage in normal tissues. A Smad3 pathway is involved in the fibrogenic phenotype of vascular smooth muscle cells induced by irradiated ECs11 and Smad3 null mice are less susceptible to radiation-induced injury.31 However, it is not clear whether the putative Smad3 genetic deficiency is associated with alteration of PAI-1 expression following radiation, and experiments to answer this question need to be performed.
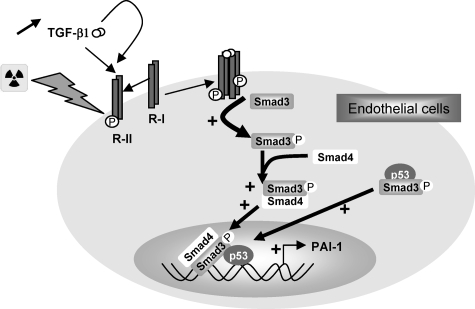
Moreover, our results demonstrate that p53 is necessary for radiation-induced PAI-1 expression in ECs. The tumor suppressor p53 is one of the most frequently mutated genes found in human cancer and plays a key role in the cell response to ionizing radiation. Our study reveals that p53 could contribute to radiation-induced normal tissue toxicity by inducing PAI-1 expression in endothelial cells in cooperation with Smad3. PAI-1 was described as a direct target of p5332 in tumor cells and the mutation of the p53 binding element in the PAI-1 promoter abolished the radiation-induced PAI-1 transcription in HEPG2 cells.16 Radiation and TGF-β cooperate in the radiation-induced PAI-1 transcription, suggesting that a p53/Smad pathway is involved in this mechanism. Moreover, a specific cooperation and physical interaction of Smads with p53 play a key role in embryogenesis.33 p53 is a DNA-binding Smad transcriptional partner that physically binds to R-Smads to control the activation of promoters that contain both p53 and Smad binding elements.33 Our results show that irradiation induces the physical interaction between p53 and Smad3 in ECs, and knockdown experiments demonstrate that both Smad3 and p53 are necessary for the radiation-induced PAI-1 expression. To our knowledge, this is the first report that demonstrates that a p53/Smad3 pathway is involved in the response of normal cells to ionizing radiation. A model of the molecular mechanism involved in radiation-induced PAI-1 overexpression in ECs is shown in Figure 9. Our results also suggest that p53 could contribute to radiation-induced intestinal damage by inducing the expression of PAI-1 in endothelial cells and subsequently contributing to the loss of thromboresistance of endothelium. Further investigations are needed to understand the precise role of the p53/PAI-1 connection in radiation-induced normal tissue damage.
Figure 9.
Proposed model of radiation-induced PAI-1 expression in endothelial cells. The Smad pathway in cooperation with p53 is involved in the radiation-induced overexpression of PAI-1.
In conclusion, our study shows that PAI-1 plays a critical role in radiation-induced intestinal damage. These data contribute to understanding of the normal tissue response after irradiation and especially the role of the Smad3/p53 pathway in radiation-induced PAI-1 expression in the endothelium. Our results indicate that PAI-1 may represent a therapeutic target to prevent and/or reduce side effects of pelvic radiotherapy.
Acknowledgments
We thank Cedric Baudelin for his excellent technical assistance.
Footnotes
Address reprint requests to Dr Fabien Milliat, Laboratory of Radiopathology, Institute for Radiological Protection and Nuclear Safety (IRSN), BP17, 92262 Fontenay-aux-Roses, France. E-mail: fabien.milliat@irsn.fr.
Supported by Electricité de France (EDF).
References
- Andreyev J. Gastrointestinal complications of pelvic radiotherapy: are they of any importance? Gut. 2005;54:1051–1054. doi: 10.1136/gut.2004.062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer. 2006;6:702–713. doi: 10.1038/nrc1950. [DOI] [PubMed] [Google Scholar]
- Wang J, Zheng H, Sung CC, Richter KK, Hauer-Jensen M. Cellular sources of transforming growth factor-beta isoforms in early and chronic radiation enteropathy. Am J Pathol. 1998;153:1531–1540. doi: 10.1016/s0002-9440(10)65741-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Wang J, Koteliansky VE, Gotwals PJ, Hauer-Jensen M. Recombinant soluble transforming growth factor beta type II receptor ameliorates radiation enteropathy in mice. Gastroenterology. 2000;119:1286–1296. doi: 10.1053/gast.2000.19282. [DOI] [PubMed] [Google Scholar]
- Kohler HP, Grant PJ. Plasminogen-activator inhibitor type 1 and coronary artery disease. N Engl J Med. 2000;342:1792–1801. doi: 10.1056/NEJM200006153422406. [DOI] [PubMed] [Google Scholar]
- Hauer-Jensen M, Fink LM, Wang J. Radiation injury and the protein C pathway. Crit Care Med. 2004;32:S325–S330. doi: 10.1097/01.ccm.0000126358.15697.75. [DOI] [PubMed] [Google Scholar]
- Molla M, Gironella M, Miquel R, Tovar V, Engel P, Biete A, Pique JM, Panes J. Relative roles of ICAM-1 and VCAM-1 in the pathogenesis of experimental radiation-induced intestinal inflammation. Int J Radiat Oncol Biol Phys. 2003;57:264–273. doi: 10.1016/s0360-3016(03)00523-6. [DOI] [PubMed] [Google Scholar]
- Panes J, Anderson DC, Miyasaka M, Granger DN. Role of leukocyte-endothelial cell adhesion in radiation-induced microvascular dysfunction in rats. Gastroenterology. 1995;108:1761–1769. doi: 10.1016/0016-5085(95)90138-8. [DOI] [PubMed] [Google Scholar]
- Mouthon MA, Vereycken-Holler V, Van der Meeren A, Gaugler MH. Irradiation increases the interactions of platelets with the endothelium in vivo: analysis by intravital microscopy. Radiat Res. 2003;160:593–599. doi: 10.1667/3068. [DOI] [PubMed] [Google Scholar]
- Wang J, Zheng H, Ou X, Fink LM, Hauer-Jensen M. Deficiency of microvascular thrombomodulin and up-regulation of protease-activated receptor-1 in irradiated rat intestine: possible link between endothelial dysfunction and chronic radiation fibrosis. Am J Pathol. 2002;160:2063–2072. doi: 10.1016/S0002-9440(10)61156-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milliat F, Francois A, Isoir M, Deutsch E, Tamarat R, Tarlet G, Atfi A, Validire P, Bourhis J, Sabourin JC, Benderitter M. Influence of endothelial cells on vascular smooth muscle cells phenotype after irradiation: implication in radiation-induced vascular damages. Am J Pathol. 2006;169:1484–1495. doi: 10.2353/ajpath.2006.060116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennler S, Itoh S, Vivien D, ten Dijke P, Huet S, Gauthier JM. Direct binding of Smad3 and Smad4 to critical TGF beta-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J. 1998;17:3091–3100. doi: 10.1093/emboj/17.11.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo SR, Lallemand F, Ferrand N, Pessah M, L'Hoste S, Camonis J, Atfi A. The novel E3 ubiquitin ligase Tiul1 associates with TGIF to target Smad2 for degradation. EMBO J. 2004;23:3780–3792. doi: 10.1038/sj.emboj.7600398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber E, Matthias P, Muller MM, Schaffner W. Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Spitz DR, Oberley LW, Robbins ME. Redox modulation of the pro-fibrogenic mediator plasminogen activator inhibitor-1 following ionizing radiation. Cancer Res. 2001;61:5537–5543. [PubMed] [Google Scholar]
- Hageman J, Eggen BJ, Rozema T, Damman K, Kampinga HH, Coppes RP. Radiation and transforming growth factor-beta cooperate in transcriptional activation of the profibrotic plasminogen activator inhibitor-1 gene. Clin Cancer Res. 2005;11:5956–5964. doi: 10.1158/1078-0432.CCR-05-0427. [DOI] [PubMed] [Google Scholar]
- Oikawa T, Freeman M, Lo W, Vaughan DE, Fogo A. Modulation of plasminogen activator inhibitor-1 in vivo: a new mechanism for the anti-fibrotic effect of renin-angiotensin inhibition. Kidney Int. 1997;51:164–172. doi: 10.1038/ki.1997.20. [DOI] [PubMed] [Google Scholar]
- Vozenin-Brotons MC, Milliat F, Linard C, Strup C, Francois A, Sabourin JC, Lasser P, Lusinchi A, Deutsch E, Girinsky T, Aigueperse J, Bourhis J, Mathe D. Gene expression profile in human late radiation enteritis obtained by high-density cDNA array hybridization. Radiat Res. 2004;161:299–311. doi: 10.1667/rr3128. [DOI] [PubMed] [Google Scholar]
- Henderson BW, Bicher HI, Johnson RJ. Loss of vascular fibrinolytic activity following irradiation of the liver–an aspect of late radiation damage. Radiat Res. 1983;95:646–652. [PubMed] [Google Scholar]
- Panes J, Granger DN. Leukocyte-endothelial cell interactions: molecular mechanisms and implications in gastrointestinal disease. Gastroenterology. 1998;114:1066–1090. doi: 10.1016/s0016-5085(98)70328-2. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Stassen JM, Schoonjans L, Ream B, van den Oord JJ, De Mol M, Mulligan RC, Collen D. Plasminogen activator inhibitor-1 gene-deficient mice. II. Effects on hemostasis, thrombosis, and thrombolysis. J Clin Invest. 1993;92:2756–2760. doi: 10.1172/JCI116893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson LA, Fici GJ, Lund JE, Boyle TP, Polites HG, Marotti KR. Development of venous occlusions in mice transgenic for the plasminogen activator inhibitor-1 gene. Nature. 1990;346:74–76. doi: 10.1038/346074a0. [DOI] [PubMed] [Google Scholar]
- Wang J, Zheng H, Hauer-Jensen M. Influence of short-term octreotide administration on chronic tissue injury. Transforming growth factor beta (TGF-beta) overexpression, and collagen accumulation in irradiated rat intestine. J Pharmacol Exp Ther. 2001;297:35–42. [PubMed] [Google Scholar]
- Wang J, Zheng H, Ou X, Albertson CM, Fink LM, Herbert JM, Hauer-Jensen M. Hirudin ameliorates intestinal radiation toxicity in the rat: support for thrombin inhibition as strategy to minimize side-effects after radiation therapy and as countermeasure against radiation exposure. J Thromb Haemost. 2004;2:2027–2035. doi: 10.1111/j.1538-7836.2004.00960.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Zheng H, Sung CC, Hauer-Jensen M. The synthetic somatostatin analogue, octreotide, ameliorates acute and delayed intestinal radiation injury. Int J Radiat Oncol Biol Phys. 1999;45:1289–1296. doi: 10.1016/s0360-3016(99)00293-x. [DOI] [PubMed] [Google Scholar]
- Eitzman DT, McCoy RD, Zheng X, Fay WP, Shen T, Ginsburg D, Simon RH. Bleomycin-induced pulmonary fibrosis in transgenic mice that either lack or overexpress the murine plasminogen activator inhibitor-1 gene. J Clin Invest. 1996;97:232–237. doi: 10.1172/JCI118396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori N, Degen JL, Sisson TH, Liu H, Moore BB, Pandrangi RG, Simon RH, Drew AF. Bleomycin-induced pulmonary fibrosis in fibrinogen-null mice. J Clin Invest. 2000;106:1341–1350. doi: 10.1172/JCI10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita K, Hayashi M, Iino S, Kondo T, Inden Y, Iwase M, Kojima T, Hirai M, Ito M, Loskutoff DJ, Saito H, Murohara T, Yamamoto K. Increased expression of plasminogen activator inhibitor-1 in cardiomyocytes contributes to cardiac fibrosis after myocardial infarction. Am J Pathol. 2004;164:449–456. doi: 10.1016/S0002-9440(10)63135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Haraguchi M, Lawrence DA, Border WA, Yu L, Noble NA. A mutant, noninhibitory plasminogen activator inhibitor type 1 decreases matrix accumulation in experimental glomerulonephritis. J Clin Invest. 2003;112:379–388. doi: 10.1172/JCI18038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LH, Dixon JD, Stringham JR, Eren M, Elokdah H, Crandall DL, Washington K, Vaughan DE. Pivotal role of PAI-1 in a murine model of hepatic vein thrombosis. Blood. 2006;107:132–134. doi: 10.1182/blood-2005-07-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanders KC, Sullivan CD, Fujii M, Sowers A, Anzano MA, Arabshahi A, Major C, Deng C, Russo A, Mitchell JB, Roberts AB. Mice lacking Smad3 are protected against cutaneous injury induced by ionizing radiation. Am J Pathol. 2002;160:1057–1068. doi: 10.1016/S0002-9440(10)64926-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz C, Pebler S, Otte J, von der Ahe D. Differential regulation of plasminogen activator and inhibitor gene transcription by the tumor suppressor p53. Nucleic Acids Res. 1995;23:3710–3717. doi: 10.1093/nar/23.18.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordenonsi M, Dupont S, Maretto S, Insinga A, Imbriano C, Piccolo S. Links between tumor suppressors: p53 is required for TGF-beta gene responses by cooperating with Smads. Cell. 2003;113:301–314. doi: 10.1016/s0092-8674(03)00308-8. [DOI] [PubMed] [Google Scholar]