Abstract
M cells, specialized cells within Peyer’s patches (PPs), are reduced in number in chemokine receptor 6 (CCR6)-deficient mice. The pathogenic microorganism Yersinia enterocolitica exploits M cells for the purpose of mucosal tissue invasion exclusively through PPs. The aim of this study was to evaluate the course of yersiniosis in CCR6-deficient mice and to investigate whether these mice might be used as an in vivo model to determine M-cell function. After oral challenge with Y. enterocolitica, control mice suffered from lethal septic infection whereas CCR6-deficient mice showed very limited symptoms of infection. Immunohistochemical analysis demonstrated PP invasion by Y. enterocolitica in control mice whereas no bacteria could be found in CCR6-deficient mice. In addition, a significant induction of proinflammatory cytokines could be found in control mice whereas proinflammatory cytokine levels in CCR6-deficient mice remained unchanged. In contrast, intraperitoneal infection resulted in severe systemic yersiniosis in both mouse groups. Abrogated oral Y. enterocolitica infection in CCR6-deficient mice demonstrates the importance of CCR6 expression in the physiological and pathological immune responses generated within PPs by influencing M-cell differentiation, underscoring the important role of M cells in the process of microbial uptake. CCR6-deficient mice may therefore represent a suitable model for the study of M-cell function in vivo.
The epithelium covering intestinal surfaces provides an effective barrier to the majority of macromolecules and microorganisms. Peyer’s patches (PPs) are major sites of antigen sampling from the intestinal lumen and induce efficient immune responses of the gut-associated lymphoid tissue.1 However, it is known that alternative routes of antigen uptake such as dendritic cells and enterocytes participate in this process. PPs consist of lymphoid follicles covered by an epithelial cell layer called follicle-associated epithelium (FAE). Specialized epithelial cells inside FAE termed M cells are involved in antigen sampling. M cells show a deep invagination of the basolateral membrane forming a large intraepithelial pocket that contains T and B lymphocytes as well as macrophages. Transcytotic vesicle transport absorbs foreign antigens or particles to the intraepithelial pocket where antigens are processed and presented to antigen-presenting cells.2,3
Several pathogens such as Salmonella typhi and typhimurium, Shigella spp., and Yersinia spp. exploit M cells to invade mucosal tissues and spread systemically.4 Although in the case of Salmonella alternative routes of infection are discussed, Y. spp. seems to invade its host exclusively by PPs.5 Y. enterocolitica is pathogenic for humans and rodents. The strain used in this study (serotype O:8) belongs to the highly mouse-pathogenic group of yersiniae.6 After penetration of the intestinal epithelium, Y. enterocolitica colonizes the PPs and can spread systemically through the lymphatics to disseminate to the liver, spleen, and lung.7
The chemokine receptor CCR6 is expressed on a variety of immune cells inside PPs.8 CCR6 was identified in dendritic cells, B cells, and subgroups of CD4+ and CD8+ T cells.9 In contrast to most other chemokine receptors, CCR6 has a single chemokine ligand, Mip-3α (macrophage inflammatory protein-3α)/CCL20),10,11,12 which is specifically expressed by the FAE. Recently, various mouse models of CCR6 deficiency were generated exhibiting size-reduced PPs containing less follicles per PP.13,14 Initial observations in these mice suggested that myeloid dendritic cells (DCs) were absent from the subepithelial dome area underneath the FAE; however, when several independently generated mice were simultaneously studied this observation could not be verified.15 We also found by using an EGFP knock-in model that other CCR6-expressing cells are correctly positioned even in the absence of a functional receptor whereas CD4+CD45Rblow regulatory T cells seem to be specifically absent from PPs of CCR6 KO mice. In addition, we were able to show that the number of UEA-1-positive (rhodamine-conjugated UEA-1 lectin is known to specifically label M cells inside the murine FAE)16 epithelial cells inside the FAE was significantly reduced while the overall microarchitecture of PPs remained intact8 suggesting that the Mip3α-CCR6 interaction mediates lymphoepithelial interaction that indirectly influences the development of M cells. Functionally, these mice do not show any signs of spontaneous intestinal infection, develop a less significant intestinal disease after dextran sulfate sodium challenge17 and also have an impaired humoral immune response to orally administered antigen and to the enteropathic virus rotavirus.13 However, the mechanisms involved in this process remain obscure.
Various data support the hypothesis that M cells are generated from epithelial cells inside the FAE by lymphoepithelial interaction, however, the precise mechanisms still remain obscure.18 Although an in vitro model of M cells could be generated by co-culturing Caco-2 cells and B lymphocytes,19,20 a suitable model to study M-cell function in vivo is still not available. Here, we report that targeted genetic deletion of the CCR6 gene dramatically protects mice against infection with Y. enterocolitica. Although wild-type (WT) mice suffered from severe yersiniosis, there was no evidence of systemic infection in CCR6-deficient mice. In contrast, no differences were observed after Citrobacter rodentium challenge. These results suggest that interaction of CCR6 with its ligand Mip3α is essential for functionality and integrity of M cells inside the FAE of PPs. Therefore, this model might serve for studying M-cell function in vivo, eg, for pharmacological or vaccination studies.
Materials and Methods
Mice
The in vivo study was conducted according to the Organisation for Economic Cooperation and Development principles of good laboratory practice. The study was approved by the institutional review board of the Animal Care and Use Committee. All mice were kept under sterile conditions in microisolator cages in appropriate animal facilities (Institute of Medical Microbiology, University of Muenster) with unlimited access to food and water. Moribund mice were sacrificed.
The gene-targeting strategy used to generate CCR6 EGFP knock-in mice was described in a previous report.9 The homozygous CCR6-deficient mice were back-crossed eight to nine times to C57BL/6. WT C57BL/6 control mice and homozygous CCR6-deficient mice shared the same background. Genotyping of the individual offspring was performed by a three-primer polymerase chain reaction (PCR) approach.9 Comparisons of CCR6-deficient and WT control mice were performed using mice between 8 and 9 weeks of age.
Bacterial Strains and Growth Conditions
The Y. enterocolitica strain used in this study, JB580v, is a restriction − derivative of a virulent American strain, 8081v, serogroup O:8, biotype 1B, Nalr.21 JB580v was grown at 26 to 28°C overnight with aeration in Luria-Bertani (LB) media supplemented with 20 μg/ml of nalidixic acid (Sigma, Heidelberg, Germany). The number of colony-forming units (CFU) was determined by plating 100-μl aliquots of serial dilutions on LB media in duplicate.
Induction of Bacterial Infection and Monitoring of Bacterial Infection
Mice were orally gavaged with 2.5 × 107 CFU of Y. enterocolitica. Intraperitoneal injection was performed by 1 × 106 CFU. Fecal specimens (two to three per mouse) were collected on days 2 and 6, weighed, and 1.0 ml/0.2 g stool of sterile phosphate-buffered saline (PBS) was added. Specimens were homogenized and 100-μl aliquots of serial dilutions were cultivated on LB agar at 28°C. Spleens were removed from euthanized mice, pressed through a sieve, centrifuged, resuspended in 2 ml of PBS, and 100-μl aliquots of serial dilutions were plated on LB agar. To determine the number of bacteria inside PPs, the gut was washed with a solution containing gentamicin (100 μg/ml) and dithiothreitol (20 mmol/L) for 1 hour to eliminate Yersinia adherence to mucus. After washing the PP was cut, pressed through a sieve, centrifuged, and plated on LB agar as described before. The number of CFUs was determined the following day.
Cytokine Enzyme-Linked Immunosorbent Assay and Reverse Transcription Real-Time PCR
On day 6 after infection spleen cells were isolated as described previously.8 Isolated cells were plated on mouse anti-CD3 T-cell activation plates (BD Biosciences, Heidelberg, Germany) and cultured for 48 hours. The supernatants were collected and an interleukin (IL)-4, IL-12, tumor necrosis factor (TNF)-α, and interferon-γ enzyme-linked immunosorbent assay (BD Biosciences) was performed according to the manufacturer’s instructions.
For real time analysis total cellular RNA from PPs was isolated with Trizol RNA isolation reagent (Life Technologies, Gaithersburg, MD). After random hexamer-primed first-strand cDNA synthesis (Superscript II; Invitrogen, San Diego, CA), real-time PCR was performed in an ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA) for IL-4, IL-6, IL-12, transforming growth factor (TGF)-β, TNF-α, and IL-1β. Forward and reverse cytokine-specific primers are seen in Table 1. β-Actin-specific primers were used as positive control. The amplification of target genes in PPs of infected WT and CCR6-deficient mice was calculated by first normalizing to the amplification of β-actin and then expressing the normalized values as fold increase over the value obtained with PPs in uninfected mice.
Table 1.
Cytokine-Specific Primers Used in RT-PCR Assay
| Target cDNA | Forward primer | Reverse primer |
|---|---|---|
| IL-4 | 5′-ACAGGAGAAGGGACGCCAT-3′ | 5′-GAAGCCCTACAGACGAGCTCA-3′ |
| IL-6 | 5′-GAGGATACCACTCCCAACAGACC-3′ | 5′-AAGTGCATCATCGTTGTTCATACA-3′ |
| IL-12 | 5′-GGAAGCACGGCAGCAGAATA-3′ | 5′-AACTTGAGGGAGAAGTAGGAATGG-3′ |
| TGF-β | 5′-CGGGGCGACCTGGGCACCATCCATGAC-3′ | 5′-CTGCTCCACCTTGGGCTTGCGACCCAC-3′ |
| TNF-α | 5′-ATGAGCACAGAAAGCAGTATC-3′ | 5′-TACAGGCTTGTCACTCGAATT-3′ |
| IL-1β | 5′-GAAGTTGACGGACCCCAAAAG-3′ | 5′-ACAGCTTCTCCACAGCCACAA-3′ |
| β-Actin | 5′-GAGGGAAATCGTGCGTGACA-3′ | 5′-GAACCGCTCGTTGCCAATAG-3′ |
Scoring of Disease Activity
To evaluate severity of infection with Y. enterocolitica, we developed a scoring system based on the following parameters: general condition, involvement of organs, condition of small intestine, PPs, and MLNs. General condition: 0, healthy; 1, limited vitality; 2, obvious symptoms, hump, shaggy, uveitis; 3, apathy. Involvement of organs: 0, no organ; 1, one organ; 2, two organs; 3, three or more organs. Small intestine: 0, normal; 1, mild inflammation of the small intestine; 2, moderate inflammation, reddening of peritoneum; 3, severe inflammation including stomach and/or colon; 4, abscesses inside diaphragm and/or mesentery. PPs: 0, normal; 1, swollen; 2, abscesses; 3, disrupted. Mesenteric lymph nodes (MLNs): 0, normal;1, swollen; 2, abscesses. Based on these parameters, the following scoring system was developed: no infection, 0; mild infection, 1 to 5; moderate infection, 6 to 9; severe infection, 10 to 15.
Histopathology
Tissue was fixed in 2% (v/v) paraformaldehyde solution (Sigma), embedded in paraffin, and subsequently sectioned. Hematoxylin and eosin (H&E) staining was used for general assessment of intestinal inflammation.
Immunohistochemistry
PPs from WT and CCR6-deficient mice were embedded in O.C.T. (Tissue-Tek; Sakura, Zoeterwonde, The Netherlands) compound and snap-frozen in liquid nitrogen. Cryostat sections (5 μm) were air-dried and fixed in acetone at −20°C for 10 minutes. Nonspecific binding was inhibited using TNB blocking buffer (0.5 mol/L Tris/HCl, 0.15 mol/L NaCl, 0.5% blocking reagent; Perkin Elmer, Boston, MA) for 1 hour at room temperature. A polyclonal rabbit anti-Yersinia invasin antibody (1:1000) was added to the slides in TNB blocking buffer and incubated overnight at 4°C. This was followed by the addition of biotinylated goat anti-rabbit (2.5 μg/ml) (Pharmingen, Heidelberg, Germany) and streptavidin Alexa Fluor 546 (10 μg/ml) (Molecular Probes, Eugene, OR) for 1 hour each.
C. Rodentium Infection
C. rodentium infection was performed as described previously.22 In brief, bacteria were grown overnight in LB broth at 37°C, harvested by centrifugation, and resuspended in fresh LB broth at a concentration of 2.5 × 109/ml. Adult (>10 weeks) mice were infected with 200 μl of the bacterial suspension (5 × 108 bacteria) by oral gavage. To determine bacterial numbers in the stool, fecal pellets were collected from individual mice throughout a 2-hour period, weighed, and homogenized in 5 ml of PBS. For determining bacterial numbers in the spleen, the organ was removed and homogenized in 2 ml of sterile PBS. Serial dilutions of the homogenates were plated onto MacConkey agar, and the number of CFUs was determined after overnight incubation at 37°C. The detection limit of the CFU assay was 103 colonies per g of feces or per organ for bacterial counts in the stool and <101 colonies/spleen. The identity of representative colonies was verified by PCR analysis. Individual colonies were picked with a sterile pipette tip and resuspended in 50 μl of water, of which 5 μl was added directly to a standard PCR, containing the primers 5′-AAGTCTGTCAATACCGCCTC-3′ (sense) and 5′-AATGTGCCAACTGTCTCATC-3′ (antisense). These primers amplify a 95-bp PCR product of the C. rodentium espB gene.23 The amplification profile was 35 cycles of 1 minute of denaturation at 95°C and 2.5 minutes of annealing and extension at 53°C. To statistically analyze the histological changes after Citrobacter infection the crypt depth was measured microscopically.
Statistics
Results are generally expressed as the mean ± SD, unless stated otherwise. The statistical significance of differences between groups was evaluated by Wilcoxon rank sum test.
Results
CCR6-Deficient Mice Are Resistant to Oral Infection with Y. Enterocolitica
To determine the clinical course of yersiniosis WT and CCR6-deficient mice were orally infected with 2.5 × 107 CFU Y. enterocolitica. Two and six days after infection mice were sacrificed. At day 2 after infection particularly PPs in the distal ileum and MLNs of WT mice showed significant swelling, whereas all other tissues appeared still normal (data not shown). In contrast, CCR6-deficient mice showed limited signs of infection. Both WT and CCR6-deficient mice were in a good general condition and appeared healthy. This dramatically changed at day 6 after infection (Figure 1A). At this time, WT mice showed clinically severe symptoms of disease, were humpbacked and shaggy, some appeared even apathic. PPs became greatly enlarged and suppurative (Figure 1A, black arrow and detailed figure of PP). PPs in the distal ileum were even disrupted. The MLNs were also swollen and showed suppuration. Small intestine and stomach were extremely bloated by gas (Figure 1A, green and red arrows) and mice showed symptoms of uveitis. Some mice had abscesses in spleen and liver. Clinically, these mice had all characteristics known to occur with yersiniosis. In contrast, CCR6-deficient mice appeared healthy at day 6 after infection and showed no significant symptoms of infection (Figure 1B). PPs, MLNs, small intestines, stomachs, spleens, and livers in these mice did not demonstrate any lesions. Even at higher concentrations using up to 5 × 108 CFU of Y. enterocolitica CCR6-deficient mice did not develop any signs of systemic infection. In addition, no signs of an intestinal infection developed at a later time point (2 weeks, data not shown). To evaluate severity of infection with Y. enterocolitica, we developed a scoring system based on following parameters: general condition, involvement of organs, condition of small intestine, PPs, and MLNs. CCR6-deficient mice orally infected with Y. enterocolitica showed both at day 2 and day 6 only mild infection (Figure 1C). In contrast, WT mice exhibited mild infection at day 2 and severe infection at day 6 compared with CCR6-deficient mice.
Figure 1.
Development of yersiniosis in C57BL/6 WT and CCR6-deficient mice orally infected with 2.5 × 107 CFU of Y. enterocolitica. A: Six days after infection with Y. enterocolitica, WT mice showed severe symptoms of yersiniosis. Stomach and small intestine were swollen (green arrows) and extremely bloated by gas (red arrows). PPs became greatly enlarged and suppurative (black arrow and detailed figure of PP). PPs in the distal ileum were swollen and sometimes disrupted. B: In contrast to WT mice CCR6-deficient mice showed no signs of disease 6 days after oral infection with Y. enterocolitica. PPs in CCR6 KO mice were markedly reduced and do not show any signs of infection (see detailed figure of PP). The figure shows representative pathology from an experiment using five mice in each group. The experiment was repeated five times. C: CCR6-deficient mice orally infected with 2.5 × 107 Y. enterocolitica showed mild infection both at day 2 and day 6. In contrast, WT mice suffered from more severe disease at both days. The figure shows representative pathology from an experiment using five mice in each group. The experiment was repeated five times.
Histopathological Analysis of Infection Caused by Y. Enterocolitica
Histological analysis of PPs of WT and CCR6-deficient mice 6 days after infection with Y. enterocolitica also revealed significant differences. PPs of WT mice showed massive aggregations of neutrophils. In some cases the normal architecture of PPs had been completely destroyed (Figure 2A). In contrast to these findings, PPs of CCR6-deficient mice appeared normal (Figure 2B). Histological analysis of MLNs yielded the same results (data not shown). Although WT mice showed suppuration inside MLNs, MLNs of CCR6-deficient mice appeared unaltered. Figure 2 shows a representative histology from an experiment using five mice in each group.
Figure 2.
Histology and immunohistochemistry of PPs and MLNs from WT and CCR6-deficient mice 6 days after oral infection with 2.5 × 107 CFU Y. enterocolitica. A: PPs of WT mice showed dramatic signs of infection. PPs of WT mice became suppurative and showed massive infiltrates of neutrophils. The normal architecture of PPs appears to be completely destroyed. B: PPs of CCR6-deficient mice showed normal morphology. B-cell follicles were covered by the FAE and flanked by T-cell zones. In correlation to the macroscopic findings CCR6-deficient mice revealed no signs of infection histologically. C–F: An inv antibody was used to determine Y. enterocolitica in PPs (C, D) and MLNs (E, F) from WT mice and CCR6-deficient mice 6 days after infection. Y. enterocolitica could only be detected in WT tissues, especially in abscesses of PPs (C) and MLNs (E). In contrast, CCR6-deficient mice showed no infiltration of Y. enterocolitica neither in PPs (D) nor in MLNs (F). Figures show a representative histology from five independent experiments using five mice in each group.
Resistance of CCR6-Deficient Mice to Y. Enterocolitica Infection Depends on Oral Infection and M-Cell Uptake
Y. enterocolitica once inside the intestinal tract attach to and invade the PPs. Subsequently, if systemic disease is established, Y. enterocolitica can disseminate to deeper tissues such as MLN, spleen, liver, and lung. To prove the lack of invasiveness of Y. enterocolitica in CCR6-deficient mice the expression of invasin was analyzed within PPs after Y. enterocolitica challenge. Invasin is one of the main invasion proteins of Y. enterocolitica that promote internalization. Immunohistological staining of PPs and MLNs from WT and CCR6-deficient mice with an invasin antibody showed an accumulation of Y. enterocolitica particularly in abscesses of PPs and MLNs of WT mice (Figure 2, C and E) 6 days after infection whereas no bacteria could be observed inside PPs and MLNs of CCR6-deficient mice (Figure 2, D and F).
To bypass the routing of Y. enterocolitica through PPs and to exclude an altered immune response to this pathogen in the absence of CCR6, mice were infected intraperitoneally. After intraperitoneal infection with 1 × 106 CFU bacteria both C57BL/6 WT and CCR6 KO mice showed severe symptoms of yersiniosis already 3 days after infection (Figure 3, A and B). Liver, spleen, MLN, mesentery, and diaphragm were infiltrated by abscesses. PPs of WT mice showed only minor changes regarding size and infection status (Figure 3A) whereas PPs of CCR6-deficient mice appeared normal (Figure 3B). However, no significant differences of disease activity could be determined between both groups. C57BL/6 WT as well as CCR6-deficient mice showed severe symptoms of disease and had a disease activity between 10 and 12 already 3 days after infection (Figure 3C). As well as disease activity, no differences between WT and CCR6-deficient mice of colonization of stool after intraperitoneal infection with Y. enterocolitica could be observed. Both WT mice and CCR6-deficient mice showed similar bacterial excretion in the stool of 1.5 × 109 CFU Y. enterocolitica/g on day 2 and 3.5 × 1011 (WT) or 3 × 1011 (CCR6-deficient mice) on day 3 (Figure 3D).
Figure 3.
No difference in the severity of yersiniosis between WT and CCR6 KO mice intraperitoneally infected with Y. enterocolitica. A and B: To bypass the routing of Y. enterocolitica through PPs, mice were infected intraperitoneally. After intraperitoneal infection with 1 × 106 CFU bacteria both C57BL/6 WT and CCR6-deficient mice showed 3 days after infection severe symptoms of yersiniosis. Liver, spleen, MLN, mesentery, and diaphragm were infiltrated by abscesses. PPs of WT showed only minor changes regarding size and infection status. PPs of CCR6-deficient mice appeared normal. Spleen and kidneys of mice in both groups were obviously swollen. Furthermore, small intestine and stomach were bloated by gas. C: In contrast to oral infection mice of both groups infected with Y. enterocolitica intraperitoneally exhibited only minor differences. C57BL/6 WT as well as CCR6 KO mice showed severe symptoms of disease and had a disease activity between 10 and 12 already 3 days after infection. D: Both WT mice and CCR6-deficient mice showed similar bacterial excretion in the stool of 1.5 × 109 CFU Y. enterocolitica/g on day 2 and 3.5 × 1011 (WT) or 3 × 1011 (CCR6-deficient mice) on day 3.
Colonization of C57BL/6 WT and CCR6 Knockout Mice after Oral Infection with Y. Enterocolitica
To determine whether the difference between both groups of mice may reflect a delayed infection of Y. enterocolitica a kinetic analysis of stool excretion and colonization in PPs and spleens of WT and CCR6-deficient mice was performed. In this study, stool and tissues were analyzed for their bacterial load on day 2 and day 6 after oral infection with 2.5 × 107 CFU Y. enterocolitica (Figure 4). Bacterial levels on day 2 showed a moderate bacterial excretion of 2.5 × 1010 CFU Y. enterocolitica/g stool of WT mice (Figure 4A) and 4.5 × 107 CFU/g tissue in PPs (Figure 4B). CCR6-deficient mice exhibited bacterial loads of 2.5 × 109 CFU/g in stool but no bacterial load inside PPs. Six days after infection Y. enterocolitica was detectable neither in stool nor in PPs of CCR6-deficient mice. In contrast, bacterial loads of stool and PPs in WT mice were dramatically increased and showed 1.5 × 1014 CFU/g in stool and 7.9 × 1010 CFU/g tissue in PPs.
Figure 4.
Colonization of C57BL/6 WT and CCR6 KO mice after oral infection with Y. enterocolitica. A and B: Bacterial levels on day 2 after infection revealed a moderate bacterial excretion of 2.5 × 1010 CFU Y. enterocolitica/g in the stool of WT mice and 4.5 × 107 CFU/g tissue in PPs.CCR6-deficient mice showed similar bacterial loads of 2.5 × 109 CFU/g in stool but no bacteria inside PPs. Six days after infection Y. enterocolitica was detectable neither in stool nor MLNs or PPs of CCR6-deficient mice. In contrast, bacterial loads of stool and PPs in WT mice were dramatically increased and showed 1.5 × 1014 CFU/g in stool and 7.9 × 1010 CFU/g tissue in PPs. Results are representative for three independent experiments including five mice in each group giving comparable results.
Up-Regulation of Inflammatory Cytokines in PPs and Spleen in WT Mice Compared to CCR6−/− Mice
Bacterial infections such as yersiniosis induce a rapid production of proinflammatory cytokines within the host. Because WT and CCR6-deficient mice exhibit a significantly different clinical pattern of infection we measured cytokine expression of splenic T cells after infection by enzyme-linked immunosorbent assay. Spleen cells of WT mice showed a 15-fold increased production of interferon-γ after infection with Y. enterocolitica compared with CCR6-deficient mice (Figure 5B). In addition, levels of other proinflammatory cytokines in the spleen such as IL-12 and TNF-α were also increased.
Figure 5.
Diminished up-regulation of inflammatory cytokines in CCR6-deficient mice after infection with Yersinia enterocolitica. A: Real-time analysis of total cellular RNA from PPs of WT and CCR6-deficient mice 6 days after infection. Whereas IL-4, IL-12, and TGF-β were moderately increased in WT mice, IL-6, TNF-α, and particularly IL-1β showed an up to 128-fold increased level in expression in WT mice compared with ratios to uninfected controls. B: Isolated cells from spleen 6 days after infection were plated on mouse anti-CD3 T-cell activation plates and cultured for 48 hours. Protein expression of different cytokines (IL-4, IL-12, TNF-α, interferon-γ) was determined by enzyme-linked immunosorbent assay. Spleens of WT mice showed a 50-fold increased production of interferon-γ after infection with Y. enterocolitica compared to CCR6-deficient infected and uninfected mice. Additionally, in WT mice other proinflammatory cytokines such as IL-12 or TNF-α and regulatory cytokines such as IL-4 were also increased after infection with Y. enterocolitica. Boxes represent medians of experiments including at least four (WT) or three (KO) individual mice (lines). *P < 0.01, **P < 0.05.
In addition, we determined cytokine expression of PP leukocytes by real-time PCR (Figure 5A). Whereas IL-4, IL-12, and TGF-β were moderately increased in WT mice, IL-6, TNF-α, and IL-1β showed an up to 128-fold increased level of expression in WT mice compared with CCR6-deficient mice. PPs of infected CCR6-deficient mice showed comparable cytokine levels like PPs of uninfected WT and CCR6-deficient mice.
C. Rodentium-Induced Colitis
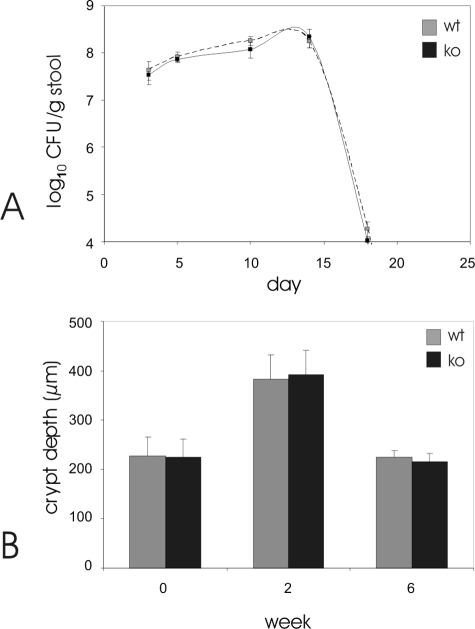
To determine whether CCR6 deficiency also influences other bacterial infections C. rodentium-induced transmissible murine colitis was investigated in CCR6-deficient and control mice.22 Oral infection of normal C57BL/6 mice with C. rodentium led to a high level of bacterial colonization of the colon within 1 week, with >107 CFU in the entire colon at that time and peaked ∼10 to 15 days after infection at >108 CFU/g of feces (Figure 6). Subsequently, fecal C. rodentium numbers declined below the detection limit of the CFU assay by week 4. In contrast to infection with Yersinia, CCR6-deficient mice showed similar kinetics of bacterial colonization and were also able to eliminate the pathogen completely by week 4. No deaths were observed during the infections, and no bacteria could be found within the spleen at week 2 and at week 6. The measurement of crypt hyperplasia as a histological hallmark of citrobacter infection also showed comparable hyperplasia at week 2 with a complete restitution by week 6. Ulcerations or severe transmural inflammation were not observed in both types of mice.
Figure 6.
C. rodentium infection. A: CCR6-deficient mice (closed line) and C57BL/6 controls (open line) were orally infected with 5 × 108 C. rodentium, and bacterial numbers (CFU) in the stool were determined every 5 days. Data are means ± SE (n = 5 mice per time point). P > 0.1 relative to C57BL/6 controls at all time points. Paraffin sections from uninfected (week 0) and C. rodentium-infected (weeks 2 and 6 after infection, p.i.) control (C57BL/6, open bars) and CCR6-deficient mice (closed bars) were stained with H&E. Crypt depths in colon were determined microscopically showing no statistical differences (P > 0.1) between both types of mice. Data are means ± SE (n = 5 mice per time point).
Discussion
The interaction of CCR6 with its ligand Mip3α is essential for the regular development of the organized lymphoid tissue inside the gut. The deletion of CCR6 inhibits the development of PPs as well as isolated lymphoid follicles and reduces the number of UEA-1-positive M cells within the FAE whereas it conserves the PP microarchitecture.8 In this study we could show that CCR6-deficient mice might serve as a model for inhibition of M-cell-specific antigen processing because the deletion of CCR6 almost completely abrogates Y. enterocolitica infection within these mice. Missing infection of CCR6-deficient hosts appears rather to be a consequence of missing uptake through M cells than an immunologically mediated alteration in CCR6-deficient mice because intraperitoneal injection of Y. enterocolitica and challenge with C. rodentium in CCR6 knockouts results in the same degree of infection as in WT mice. To our knowledge this model is unique in its ability to study M-cell tropic functions because the formation of other lymphoid organs (spleen, MLNs) appear unaffected by the mutation. It also proves a functional relevance of M cells in microbial uptake.
M cells are known as specialized cells inside the FAE involved in antigen sampling. They are exploited by a range of pathogens such as Salmonella spec., Shigella flexneri, and Yersinia spp. as a route of host invasion.24 Despite their suggested importance in immunity and infection, the origin, function, and developmental pathways of these specialized cells still remain primarily unknown. In particular, the lack of appropriate in vitro and in vivo M-cell models excluded intensive investigation to further understand the role of this cell population within the gut. Most studies on M cells used electron microscopy or an in vitro model that was developed by Kernéis and colleagues.19,20 In this model lymphocytes were co-cultured with Caco-2 cells to reproduce the main characteristics of M cells. Even though co-cultured epithelial cells derived within this model develop various properties reminding of M cells, it is still unclear whether this artificial model represents all characteristics of M cells and might be used as an appropriate M-cell model. In addition, other mechanisms of antigen uptake via intestinal epithelial cells and dendritic cells have been described that interfere with M-cell-directed functions in vivo.25Therefore, the significance of M-cell-directed targeting in vivo has been discussed controversially.
Y. enterocolitica is known to specifically exploit M cells to invade mucosal tissues and subsequently spread systemically. MLNs are draining PPs by means of afferent lymphoid vessels and are connected by efferent lymphoid vessels to the thoracic duct that disembogue into the bloodstream. This spreading of bacteria through the lymphoid system is reflected in successive colonization of organs such as PPs, MLNs, spleen, liver, and lung resulting in a septic and lethal infection. In contrast to that, CCR6-deficient mice did not show colonization of different organs and developed only limited signs of intestinal infection. In contrast, CCR6-deficient mice developed similar symptoms after parenteral infection and exhibited a comparable infection after challenge with C. rodentium. This pathogen is a noninvasive bacterium that causes enteropathogenic E. coli-like lesions in the murine colon.26 In contrast to Y. enterocolitica, C. rodentium does not invade M cells. After oral application of C. rodentium, WT as well as CCR6-deficient mice show typical symptoms of infection without any significant differences between both strains. These results suggest that the deletion of CCR6 inhibits M-cell tropic infection with Y. enterocolitica but does not significantly alter the systemic immune response nor does it enhance unspecific antimicrobial defense and leave the host susceptible to other enteropathogenic bacteria.
During the last years, a variety of knockout models with defects in organized gut-associated lymphoid tissue development have been described. Several gene-deficient mice present with missing or reduced numbers of PPs such as LTα −/−,27,28 LTβ −/−,29 IL7R −/−,30 Relb −/−,31 and Light −/−32 among several others have been described or mice with gestational LTβRIgG treatment. However, even though there are different other models that lack M cells, most of these models have the disadvantage of markedly impaired mucosal and systemic immune responses. LT-α-deficient mice lack lymph nodes and PPs and their spleens have indistinct follicular marginal zones.27 In addition, LTα −/− mice have a T/B cell disorganization and fail to form B-cell follicles, follicular dendritic networks, and germinal centers.28 We have previously demonstrated a critical role of LTαβ/LTβR interactions in oral infection with the noninvasive pathogen C. rodentium.33 Like in many other experimental diseases34 the course of Y. enterocolitica infection in LTα−/− mice was more severe with systemic spread of bacteria to the spleen, MLN, and other organs. After oral infection with Y. enterocolitica, bacterial dissemination to the spleen was evident in LTα-deficient mice.35 Obviously, a defect in the immune system other than the absence of PPs and MLNs caused severe barrier dysfunction leading to systemic colonization with Y. enterocolitica in these mice. In consequence, these models are not suitable to determine M-cell function in vivo. In contrast, CCR6 knockout mice, despite their markedly reduced size of PPs still exhibit normal anatomical features with appropriate mucosal immune responses. CCR6 knockout mice therefore appear as a suitable model to determine M-cell function for pharmacological or oral vaccination studies in the future.
Recently, Salazar-Gonzalez and colleagues36 showed that CCR6 expression by dendritic cells is crucial for the activation of pathogen-specific T cells within PPs. In this study, mice were orally infected with Salmonella typhimurium supposed to enter the host via M cells; however, this pathogen is known to use different pathways such as dendritic and villus epithelial cells as well. In this model, the amount of bacteria detected inside PPs and MLNs was comparable between WT and CCR6-deficient mice although PPs of CCR6-deficient mice are significantly size-reduced when compared to WT controls. The data indicate that pathogens might exploit M cells inside the FAE, however, the significance of this interaction might be substantially different between various pathogens. The fact that the systemic bacterial burden after S. typhimurium infection is even higher in spleen and liver of CCR6-deficient mice might also indicate that this pathogen is capable of exploiting other routes than M cells. In addition, this study indicates that the deletion of CCR6 might not solely inhibit M-cell development but also affect other effector pathways such as T-cell activation. On initial description CCR6-deficient mice were found to have markedly fewer antigen-specific antibody-producing cells after oral administration of keyhole limpet hemocyanin13; this observation might be interpreted as a reduction of antigen uptake via M cells, but other mechanisms such as defective T-cell response inside PPs cannot be excluded.
Finally, the physiological role of CCR6 expression still remains to be further elucidated. Under noninflammatory condition, PPs of CCR6-deficient mice appear size-reduced with a regular microarchitecture and even correctly positioned CCR6 expressing T-, B-, and dendritic cells. However, the FAE of PPs lack M cells and the progression of cryptopatches to mature isolated lymphoid follicles is impaired.37 Under inflammatory conditions (eg, after challenge with S. thyphimurium) a different mechanism including T-cell stimulation by CCR6-expressing DCs is present within PPs. However, the role of cryptopatches and isolated lymphoid follicles as sites of high expression of CCR6 for intestinal immune responses is primarily unknown. It should be noted that particularly inside cryptopatches CCR6 is not expressed by DCs. In conclusion, CCR6 seems to determine physiological and pathological immune responses generated within PPs particularly by influencing M-cell formation and DC-dependent T-cell stimulation. Because these structures are potential sites for initial abnormalities found in Crohn’s disease,38,39 the influence of CCR6 expression with inflammatory bowel disease pathology needs to be further investigated.
Acknowledgments
We thank Sonja Dufentester and Elke Weber for expert technical support.
Footnotes
Address reprint requests to Torsten Kucharzik, M.D., University of Muenster, Department of Medicine B, Albert-Schweitzer Strasse 33, D-48149 Muenster, Germany. E-mail: torsten.kucharzik@ukmuenster.de.
Supported by the Interdisciplinary Center for Clinical Research (grant Kuc2/018/06) and the Deutsche Forschungsgemeinschaft (grant LU 816/2-1).
S.W. and A.L. contributed equally to this study.
References
- Neutra MR. Current concepts in mucosal immunity. V Role of M cells in transepithelial transport of antigens and pathogens to the mucosal immune system. Am J Physiol. 1998;274:G785–G791. doi: 10.1152/ajpgi.1998.274.5.G785. [DOI] [PubMed] [Google Scholar]
- Niedergang F, Kweon MN. New trends in antigen uptake in the gut mucosa. Trends Microbiol. 2005;13:485–490. doi: 10.1016/j.tim.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Rimoldi M, Rescigno M. Uptake and presentation of orally administered antigens. Vaccine. 2005;23:1793–1796. doi: 10.1016/j.vaccine.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Vazquez-Torres A, Fang FC. Cellular routes of invasion by enteropathogens. Curr Opin Microbiol. 2000;3:54–59. doi: 10.1016/s1369-5274(99)00051-x. [DOI] [PubMed] [Google Scholar]
- Jang MH, Kweon MN, Iwatani K, Yamamoto M, Terahara K, Sasakawa C, Suzuki T, Nochi T, Yokota Y, Rennert PD, Hiroi T, Tamagawa H, Iijima H, Kunisawa J, Yuki Y, Kiyono H. Intestinal villous M cells: an antigen entry site in the mucosal epithelium. Proc Natl Acad Sci USA. 2004;101:6110–6115. doi: 10.1073/pnas.0400969101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trülzsch K, Sporleder T, Igwe EI, Russmann H, Heesemann J. Contribution of the major secreted yops of Yersinia enterocolitica O:8 to pathogenicity in the mouse infection model. Infect Immun. 2004;72:5227–5234. doi: 10.1128/IAI.72.9.5227-5234.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis G, Laroche Y, Balligand G, Sory MP, Wauters G. Yersinia enterocolitica, a primary model for bacterial invasiveness. Rev Infect Dis. 1987;9:64–87. doi: 10.1093/clinids/9.1.64. [DOI] [PubMed] [Google Scholar]
- Lügering A, Floer M, Westphal S, Maaser C, Spahn TW, Schmidt MA, Domschke W, Williams IR, Kucharzik T. Absence of CCR6 inhibits CD4+ regulatory T-cell development and M-cell formation inside Peyer’s patches. Am J Pathol. 2005;166:1647–1654. doi: 10.1016/S0002-9440(10)62475-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharzik T, Hudson JT, III, Waikel RL, Martin WD, Williams IR. CCR6 expression distinguishes mouse myeloid and lymphoid dendritic cell subsets: demonstration using a CCR6 EGFP knock-in mouse. Eur J Immunol. 2002;32:104–112. doi: 10.1002/1521-4141(200201)32:1<104::AID-IMMU104>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Greaves DR, Wang W, Dairaghi DJ, Dieu MC, Saint-Vis B, Franz-Bacon K, Rossi D, Caux C, McClanahan T, Gordon S, Zlotnik A, Schall TJ. CCR6, a CC chemokine receptor that interacts with macrophage inflammatory protein 3alpha and is highly expressed in human dendritic cells. J Exp Med. 1997;186:837–844. doi: 10.1084/jem.186.6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba M, Imai T, Nishimura M, Kakizaki M, Takagi S, Hieshima K, Nomiyama H, Yoshie O. Identification of CCR6, the specific receptor for a novel lymphocyte-directed CC chemokine LARC. J Biol Chem. 1997;272:14893–14898. doi: 10.1074/jbc.272.23.14893. [DOI] [PubMed] [Google Scholar]
- Power CA, Church DJ, Meyer A, Alouani S, Proudfoot AE, Clark-Lewis I, Sozzani S, Mantovani A, Wells TN. Cloning and characterization of a specific receptor for the novel CC chemokine MIP-3alpha from lung dendritic cells. J Exp Med. 1997;186:825–835. doi: 10.1084/jem.186.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DN, Prosser DM, Forster R, Zhang J, Kuklin NA, Abbondanzo SJ, Niu XD, Chen SC, Manfra DJ, Wiekowski MT, Sullivan LM, Smith SR, Greenberg HB, Narula SK, Lipp M, Lira SA. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity. 2000;12:495–503. doi: 10.1016/s1074-7613(00)80201-0. [DOI] [PubMed] [Google Scholar]
- Varona R, Villares R, Carramolino L, Goya I, Zaballos A, Gutierrez J, Torres M, Martinez AC, Marquez G. CCR6-deficient mice have impaired leukocyte homeostasis and altered contact hypersensitivity and delayed-type hypersensitivity responses. J Clin Invest. 2001;107:R37–R45. doi: 10.1172/JCI11297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Sato A, Dela Cruz CS, Linehan M, Luegering A, Kucharzik T, Shirakawa AK, Marquez G, Farber JM, Williams I, Iwasaki A. CCL9 is secreted by the follicle-associated epithelium and recruits dome region Peyer’s patch CD11b+ dendritic cells. J Immunol. 2003;171:2797–2803. doi: 10.4049/jimmunol.171.6.2797. [DOI] [PubMed] [Google Scholar]
- Clark MA, Jepson MA, Simmons NL, Booth TA, Hirst BH. Differential expression of lectin-binding sites defines mouse intestinal M-cells. J Histochem Cytochem. 1993;41:1679–1687. doi: 10.1177/41.11.7691933. [DOI] [PubMed] [Google Scholar]
- Varona R, Cadenas V, Flores J, Martinez AC, Marquez G. CCR6 has a non-redundant role in the development of inflammatory bowel disease. Eur J Immunol. 2003;33:2937–2946. doi: 10.1002/eji.200324347. [DOI] [PubMed] [Google Scholar]
- Nicoletti C. Unsolved mysteries of intestinal M cells. Gut. 2000;47:735–739. doi: 10.1136/gut.47.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernéis S, Bogdanova A, Kraehenbuhl JP, Pringault E. Conversion by Peyer’s patch lymphocytes of human enterocytes into M cells that transport bacteria. Science. 1997;277:949–952. doi: 10.1126/science.277.5328.949. [DOI] [PubMed] [Google Scholar]
- Kernéis S, Caliot E, Stubbe H, Bogdanova A, Kraehenbuhl J, Pringault E. Molecular studies of the intestinal mucosal barrier physiopathology using cocultures of epithelial and immune cells: a technical update. Microbes Infect. 2000;2:1119–1124. doi: 10.1016/s1286-4579(00)01266-1. [DOI] [PubMed] [Google Scholar]
- Kinder SA, Badger JL, Bryant GO, Pepe JC, Miller VL. Cloning of the YenI restriction endonuclease and methyltransferase from Yersinia enterocolitica serotype O8 and construction of a transformable R-M+ mutant. Gene. 1993;136:271–275. doi: 10.1016/0378-1119(93)90478-l. [DOI] [PubMed] [Google Scholar]
- Maaser C, Housley MP, Iimura M, Smith JR, Vallance BA, Finlay BB, Schreiber JR, Varki NM, Kagnoff MF, Eckmann L. Clearance of Citrobacter rodentium requires B cells but not secretory immunoglobulin A (IgA) or IgM antibodies. Infect Immun. 2004;72:3315–3324. doi: 10.1128/IAI.72.6.3315-3324.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JV, Zabel BA, Jha SS, Schauer DB. Citrobacter rodentium espB is necessary for signal transduction and for infection of laboratory mice. Infect Immun. 1999;67:6019–6025. doi: 10.1128/iai.67.11.6019-6025.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MA, Jepson MA. Intestinal M cells and their role in bacterial infection. Int J Med Microbiol. 2003;293:17–39. doi: 10.1078/1438-4221-00242. [DOI] [PubMed] [Google Scholar]
- Kelsall BL, Strober W. Distinct populations of dendritic cells are present in the subepithelial dome and T cell regions of the murine Peyer’s patch. J Exp Med. 1996;183:237–247. doi: 10.1084/jem.183.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald TT, Frankel G, Dougan G, Goncalves NS, Simmons C. Host defences to Citrobacter rodentium. Int J Med Microbiol. 2003;293:87–93. doi: 10.1078/1438-4221-00247. [DOI] [PubMed] [Google Scholar]
- Banks TA, Rouse BT, Kerley MK, Blair PJ, Godfrey VL, Kuklin NA, Bouley DM, Thomas J, Kanangat S, Mucenski ML. Lymphotoxin-alpha-deficient mice. Effects on secondary lymphoid organ development and humoral immune responsiveness. J Immunol. 1995;155:1685–1693. [PubMed] [Google Scholar]
- De Togni P, Goellner J, Ruddle NH, Streeter PR, Fick A, Mariathasan S, Smith SC, Carlson R, Shornick LP, Strauss-Schoenberger J, Russell JH, Karr R, Chaplint DD. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- Fütterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity. 1998;9:59–70. doi: 10.1016/s1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- Adachi S, Yoshida H, Honda K, Maki K, Saijo K, Ikuta K, Saito T, Nishikawa SI. Essential role of IL-7 receptor alpha in the formation of Peyer’s patch anlage. Int Immunol. 1998;10:1–6. doi: 10.1093/intimm/10.1.1. [DOI] [PubMed] [Google Scholar]
- Yilmaz ZB, Weih DS, Sivakumar V, Weih F. RelB is required for Peyer’s patch development: differential regulation of p52-RelB by lymphotoxin and TNF. EMBO J. 2003;22:121–130. doi: 10.1093/emboj/cdg004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheu S, Alferink J, Potzel T, Barchet W, Kalinke U, Pfeffer K. Targeted disruption of LIGHT causes defects in costimulatory T cell activation and reveals cooperation with lymphotoxin beta in mesenteric lymph node genesis. J Exp Med. 2002;195:1613–1624. doi: 10.1084/jem.20020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spahn TW, Maaser C, Eckmann L, Heidemann J, Lugering A, Newberry R, Domschke W, Herbst H, Kucharzik T. The lymphotoxin-beta receptor is critical for control of murine Citrobacter rodentium-induced colitis. Gastroenterology. 2004;127:1463–1473. doi: 10.1053/j.gastro.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Spahn TW, Eugster HP, Fontana A, Domschke W, Kucharzik T. Role of lymphotoxin in experimental models of infectious diseases: potential benefits and risks of a therapeutic inhibition of the lymphotoxin-beta receptor pathway. Infect Immun. 2005;73:7077–7088. doi: 10.1128/IAI.73.11.7077-7088.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley SA, Newberry RD, Miller VL. Yersinia enterocolitica invasin-dependent and invasin-independent mechanisms of systemic dissemination. Infect Immun. 2005;73:8453–8455. doi: 10.1128/IAI.73.12.8453-8455.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar-Gonzalez RM, Niess JH, Zammit DJ, Ravindran R, Srinivasan A, Maxwell JR, Stoklasek T, Yadav R, Williams IR, Gu X, McCormick BA, Pazos MA, Vella AT, Lefrancois L, Reinecker HC, McSorley SJ. CCR6-mediated dendritic cell activation of pathogen-specific T cells in Peyer’s patches. Immunity. 2006;24:623–632. doi: 10.1016/j.immuni.2006.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald KG, McDonough JS, Williams I, Kucharzik T, Newberry R. Cc chemokine receptor 6 is essential for isolated lymphoid follicle formation. Gastroenterology. 2005;128:A21. doi: 10.2353/ajpath.2007.060817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuvelier CA, Quatacker J, Mielants H, De Vos M, Veys E, Roels HJ. M-cells are damaged and increased in number in inflamed human ileal mucosa. Histopathology. 1994;24:417–426. doi: 10.1111/j.1365-2559.1994.tb00550.x. [DOI] [PubMed] [Google Scholar]
- Fujimura Y, Kamoi R, Iida M. Pathogenesis of aphthoid ulcers in Crohn’s disease: correlative findings by magnifying colonoscopy, electron microscopy, and immunohistochemistry. Gut. 1996;38:724–732. doi: 10.1136/gut.38.5.724. [DOI] [PMC free article] [PubMed] [Google Scholar]